S100A9 Differentially Modifies Phenotypic States of Neutrophils, Macrophages, and Dendritic Cells: Implications for Atherosclerosis and Adipose Tissue Inflammation (original) (raw)
. Author manuscript; available in PMC: 2012 Mar 22.
Abstract
Background
S100A9 is constitutively expressed in neutrophils, dendritic cells, and monocytes, is associated with acute and chronic inflammatory conditions, and is implicated in obesity and cardiovascular disease in humans. Most of the constitutively secreted S100A9 is derived from myeloid cells. A recent report demonstrated that mice deficient in S100A9 exhibit reduced atherosclerosis compared to controls, and suggested that this effect was due in large to loss of S100A9 in bone marrow-derived cells.
Methods and Results
In order to directly investigate the role of bone marrow-derived S100A9 in atherosclerosis and insulin resistance, LDL receptor (LDLR)-deficient S100A9-deficient bone marrow chimeras were generated. Neither atherosclerosis nor insulin resistance was reduced in S100A9-deficient chimeras fed a diet rich in fat and carbohydrates. In order to investigate the reason for this lack of effect, myeloid cells were isolated from the peritoneal cavity or bone marrow. S100A9-deficient neutrophils exhibited a reduced secretion of cytokines in response to toll-like receptor (TLR) 4-stimulation. In striking contrast, S100A9-deficient dendritic cells showed an exacerbated release of cytokines following TLR-stimulation. Macrophages rapidly lost S100A9 expression during maturation; hence S100A9-deficiency did not affect the inflammatory status of macrophages.
Conclusions
S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells. The effect of S100A9-deficiency on atherosclerosis, as well as other inflammatory diseases, is therefore predicted to depend on the relative contribution of these cell types at different stages of disease progression. Furthermore, S100A9 expression in non-myeloid cells is likely to contribute to atherosclerosis.
Keywords: Atherosclerosis, Immunology, Macrophage, S100 proteins
S100A9, and its binding partner S100A8, are members of the S100 family of proteins, and are promising novel markers of cardiovascular risk in humans1. Recent studies on mice demonstrate that S100A8/A9 promote atherosclerosis2. Thus, S100A9 appears to be both a marker and a mediator of atherosclerosis. Furthermore, circulating levels of S100A8/A9 are increased in a number of autoimmune and pro-inflammatory states, including type 1 diabetes3-4 and obesity5, characterized by increased cardiovascular risk. Studies on S100A9-deficient mice suggest that S100A8/A9 has pro-inflammatory actions, e.g. in sepsis6-7 and pancreatitis8.
S100A8/A9 are abundantly and constitutively expressed in neutrophils and monocytes. Expression of S100A8/A9 is lost during differentiation of monocytes into macrophages9 yet some expression is sustained in dendritic cells (DCs)10. S100A8/A9 promote migration of neutrophils through increased CD11b expression11. The effects of S100A9-deficiency in neutrophils might be due to the combined loss of S100A8 and S100A9, because S100A9-deficient neutrophils express S100A8 mRNA but not S100A8 protein, suggesting that S100A9 stabilizes S100A811,12. S100A8/A9 are secreted from phagocytes through a tubulin-dependent mechanism,13 and extracellular functions include signaling through TLR4 and the receptor for advanced glycation end-products (RAGE)6,14, resulting in pro-inflammatory effects15. However, S100A8/A9 expression is also induced by anti-inflammatory signals16,17, and overexpression of S100A9 blocks maturation of DCs18. Thus, S100A8/A9 might exert inhibitory or anti-inflammatory actions.
Because of the abundance of S100A8/A9 in myeloid cells compared to non-myeloid cells, myeloid-derived S100A8/A9 is believed to mediate the effects of whole-body S100A9-deficiency. However, S100A8 and S100A9 can be induced by inflammatory stimuli in non-hematopoietic cells, e.g. endothelial cells12,19,20. The goal of this study, therefore, was to investigate whether myeloid-derived S100A8/A9 contribute to atherosclerosis. Insulin resistance was analyzed as a secondary end-point.
Our results demonstrate that bone marrow-derived S100A9 is not sufficient to promote atherosclerosis or insulin resistance in LDLR-deficient (LDLR−/−) mice. Furthermore, we have uncovered disparate effects of S100A9 on the inflammatory phenotype of neutrophils, macrophages, and DCs. These findings suggest that the biological effects of S100A9 depend on the relative abundance of different immune cells and non-myeloid cells in different disease states.
Methods
S100A9-deficient mice
SV129;C57BL/6J S100A9−/− mice11 were backcrossed 10 generations onto the C57BL/6J background, and a colony of S100A9+/− mice was used to generate S100A9 wild-type (S100A9+/+) and S100A9−/− offspring. S100A9−/− mice were viable, with normal myelopoietic properties, consistent with previous studies9,10. Male and female mice, 10-15 weeks of age, were used for cell isolation. In vivo experiments included studies on male whole-body S100A9−/− mice, and bone marrow transplants from S100A9−/− and littermate S100A9+/+ donors into male LDLR−/− recipients. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Washington.
Cell isolation
Peritoneal neutrophils and macrophages were collected 4 h and 5 days after thioglycollate injection, respectively. Bone marrow-derived macrophages were differentiated in 30% L-conditioned medium (a source of M-CSF), and DCs were differentiated in 3-10 ng/mL recombinant GM-CSF for 5-7 days. In some experiments, monocyte enrichment from bone marrow was achieved by using a negative selection monocyte-enrichment kit (Easy Sep, Vancouver, BC, Canada). Classical activation of DCs and macrophages was induced by a 24 h exposure to 5 ng/mL LPS plus 12 ng/mL IFNγ. To examine TLR activation, CD11+ DCs were exposed to varying concentrations of zymosan, LPS, CpG-B, and Pam3CSK4 overnight. T-cells from spleen and peripheral lymph nodes were collected with subsequent immunomagnetic depletion of contaminating cells, as described in the online supplement. More than 95% of these cells were CD4+ or CD8+, as evaluated by flow cytometry.
Atheroscleros is and insulin resistance studies
Male C57BL/6 whole-body S100A9+/+ and S100A9−/− mice (10-12 weeks old) were fed a chow diet or a diabetogenic diet rich in fat and carbohydrates with 0.15% w/w cholesterol (DDC; BioServ No.F4997) for 12 weeks. Insulin resistance was evaluated by glucose tolerance (GTT) and insulin tolerance tests (ITT) at 10 and 12 weeks, respectively. The mice were then saline perfused and epididymal fat was dissected, weighed, and fixed in paraformaldehyde.
Following lethal irradiation, male LDLR-deficient mice (12-14 weeks old) received intravenous bone marrow transplants from S100A9+/+ or S100A9−/− mice. After a 3-week recovery period, the mice were fed chow or DDC for 24 weeks. Body weights were measured weekly, and blood glucose and blood cholesterol were determined at the beginning and end of the study. There were no differences in these measurements between the groups at baseline. GTT and ITT were performed at 20 and 22 weeks, respectively. At the end of the 24-week study, mice were saline perfused under physiological pressure. Epididymal fat pads, heart, and brachiocephalic artery (BCA) were dissected, fixed, embedded, and serial sectioned. Every 4th section was stained using a Movat's pentachrome stain21. Adjacent sections were stained with antibodies against S100A9, Ly6G, Mac-2, and MHC class II. Maximal atherosclerotic lesion area was determined in a masked fashion. Plasma IL-6 was analyzed using ELISA, and triglycerides were determined using a kit from Sigma Aldrich.
Additional methods
General laboratory methods and a more detailed description of methods are provided in the online supplement.
Statistical analyses
Prism4 (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis. Unpaired Student's t-test was used when comparing two groups. One- or two-way ANOVA was used to compare more than 2 groups or parameters with Tukey's and Bonferroni post hoc tests, respectively. Probabilities of less than 0.05 were considered statistically significant. All p-values are based on two-tailed analyses.
Results
Bone marrow S100A9-deficiency does not diminish atherosclerosis in LDLR-deficient mice
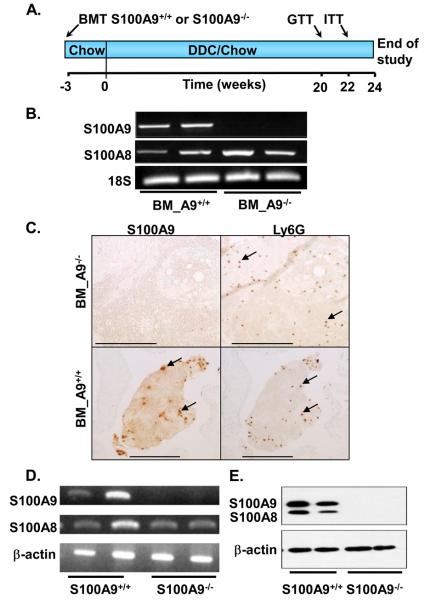
Whole-body S100A9-deficiency reduces atherosclerosis in ApoE−/− mice through reduced arterial macrophage accumulation2. We therefore asked whether bone marrow-specific deletion of S100A9 would recapitulate the effect of whole-body S100A9-deficiency on atherosclerosis. Bone marrow from S100A9+/+ and S100A9−/− mice was transplanted into irradiated male LDLR−/− mice. The mice were fed chow or DDC for 24 weeks to allow for development of atherosclerosis and insulin resistance (Fig. 1A). Successful chimerism was confirmed by several different methods. Thus, S100A9 mRNA was undetectable in circulating blood cells from mice that had received S100A9-deficient bone marrow, whereas S100A8 mRNA levels were unchanged (Fig. 1B). Accordingly, blood clots from LDLR−/− S100A9−/− bone marrow chimeras exhibited no detectable S100A9 immunoreactivity, despite the presence of neutrophils, identified by the neutrophil marker Ly6G, whereas clear S100A9-positive cells were identified in the wild-type controls (Fig. 1C). Bone marrow cells from S100A9-deficient mice differentiated in the presence of GM-CSF or M-CSF also showed loss of S100A9 mRNA, and loss of both S100A9 and S100A8 protein (Fig. 1D-E and Supplemental Fig. 1), consistent with published results.11,12
Figure 1. Bone marrow S100A9-deficiency results in loss of S100A9 in leukocytes.
Irradiated male LDLR−/− mice received wild type (BM_A9+/+) or S100A9-deficient (BM_A9−/−) bone marrow. Three weeks later, some mice were switched to DDC, and maintained for 24 weeks (A). Blood was collected 27 weeks after transplant, erythrocytes were lysed, and leukocyte RNA was extracted. S100A8 and S100A9 mRNA was determined using semi-quantitative RT-PCR and normalized to 18S (B). Blood clots were stained for Ly6G (neutrophils) and S100A9 to determine chimerism (C). Representative S100A9-positive and Ly6G-positive cells are indicated by arrows; bars are 250 μm. Bone marrow cells were differentiated in 10 ng/mL GM-CSF for 5 days. S100A8 and S100A9 mRNA and protein levels were analyzed by semi-quantitative RT-PCR (D) and Western blot (E). The experiments were repeated several times with similar results.
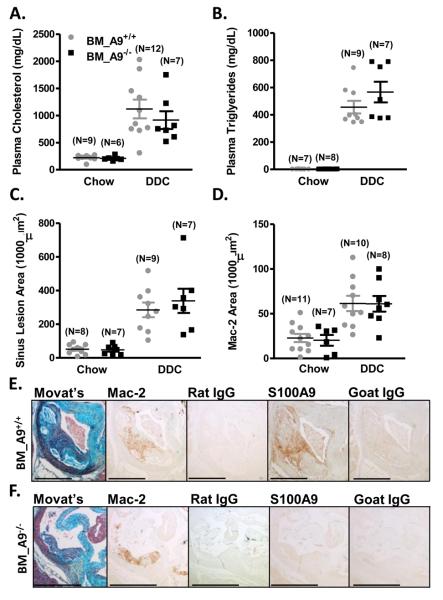
DDC-fed LDLR−/− mice exhibited significant increases in plasma cholesterol and triglycerides compared to chow-fed controls, and there was no effect of bone marrow S100A9-deficiency on these parameters (Fig. 2A-B). Atherosclerosis was analyzed in Movat's pentachrome-stained cross-sections from the aortic sinus and the BCA. DDC-fed mice demonstrated larger aortic sinus and BCA lesions than chow-fed mice, confirming previous reports (Fig. 2C; Supplemental Fig. 2A)22. However, bone marrow S100A9-deficiency did not modulate lesion area (Fig. 2C; Supplemental Fig. 2A). Immunohistochemistry was used to evaluate macrophage, DC, neutrophil, and T-cell accumulation in atherosclerotic tissue. The Mac-2-positive area (identifying both macrophages and DCs; LB, MMA, KEB, JWH; unpublished observations) in the aortic sinus and BCA was significantly elevated in DDC-fed mice compared to chow-fed mice, with no effect of bone marrow S100A9-deficiency (Fig 2D; Supplemental Fig. 2B). In BCA lesions, neutrophils were detected in only 1 out of 18 DDC-fed mice (Supplemental Fig. 3), suggesting that neutrophils do not play a significant role in this model, at least not at this time point. Likewise, no CD3+ T-cells were detected in sinus lesions (data not shown). Importantly, S100A9−/− bone marrow chimeras had undetectable S100A9 immunoreactivity in Mac-2-positive areas in sinus lesions (Fig. 2E-F), confirming successful chimerism. Finally, there were no differences in necrotic core size or other lesion morphological features between mice with S100A9+/+ and S100A9−/− bone marrow (data not shown).
Figure 2. Bone marrow S100A9-deficiency does not reduce atherosclerosis.
Male LDLR−/− mice were transplanted with wild type (BM_A9+/+) or S100A9-deficient (BM_A9−/−) bone marrow and fed chow or DDC for 24 weeks. Plasma cholesterol (A) and triglycerides (B) were measured at the end of the study. Total aortic sinus lesion area (C) and Mac-2 positive area (D) was quantified. The results are presented as scatter plots, with mean ± SEM indicated by horizontal lines and vertical lines, respectively. Two-way ANOVA, followed by Bonferroni post-hoc tests, was used to evaluate the effect of diet (chow versus DDC) and genotype (BM_A9+/+ versus BM_A9−/−) on (A) plasma cholesterol (mg/dL), (B) plasma triglycerides (mg/dL), (C) sinus lesion area (1000 μm2), and (D) Mac-2-positive lesion area (1000 μm2). DDC-feeding significantly increased plasma cholesterol, plasma triglycerides, sinus lesion area, and Mac-2 area (all p<0.001 versus chow), but bone marrow S100A9-deficiency had no effect on any of these parameters (p>0.05). Representative BM_A9+/+ (E) and BM_A9−/− (F) aortic sinus sections were stained with a Movat's pentachrome method, anti-Mac-2, anti-Ly6G, anti-S100A9, or relevant negative control IgGs. Bar represents 500 μm
Thus, S100A9-deficieny in bone marrow-derived cells is not sufficient to reduce atherosclerosis or accumulation of macrophages/DCs in LDLR−/− mice.
S100A9-deficiency does not affect insulin resistance
Adipose tissue inflammation, with accumulation of immune cells, is believed to play a causal role in insulin resistance,23 and TLR4 expression on hematopoietic cells promotes this effect24. Male DDC-fed LDLR−/− mice develop insulin resistance, obesity, systemic inflammation, and macrophage infiltration into epididymal adipose tissue.22 We therefore investigated whether S100A9-deficiency would affect these parameters.
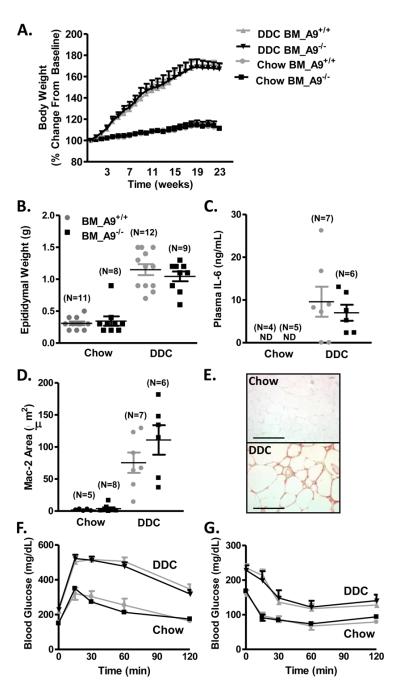
Chow-fed LDLR−/− mice showed stable body weight while DDC-fed mice gained a significant amount of weight during the 24-week study (Fig. 3A), and exhibited increased epididymal fat pad weight (Fig. 3B). There was, however, no difference in weight gain or fat pad mass between LDLR−/− mice with S100A9+/+ and S100A9−/− bone marrow (Fig. 3A-B). Systemic inflammation, as detected by circulating IL-6, was increased in DDC-fed mice, but bone marrow S100A9-deficiency had no significant effect (Fig. 3C). The epididymal Mac-2-positive area was significantly increased in DDC-fed mice as compared to chow-fed mice (Fig. 3D-E). However, there was no significant difference in mice with S100A9+/+ and S100A9−/− bone marrow (Fig. 3D).
Figure 3. Bone marrow S100A9-deficiency does not affect insulin resistance.
Male LDLR−/− mice were transplanted with wild-type (BM_A9+/+) or S100A9-deficient (BM_A9−/−) bone marrow, and fed chow or DDC for 24 weeks. Body weights were normalized to starting body weight (A). Two-way ANOVA was used to evaluate the effect of diet (chow versus DDC) over time (weeks) on body weight (% change from baseline), followed by Bonferroni post-hoc analysis to compare the effect of S100A9-deficiency (BM_A9+/+ versus BM_A9−/−). DDC-feeding significantly increased body weight (p<0.001) compared to chow-feeding in both BM_A9+/+ and BM_A9−/− mice, but there were no differences between BM_A9+/+ mice and BM_A9−/− mice fed chow or DDC (p>0.05). N=6 chow-fed BM_A9+/+ mice; N=6 chow-fed BM_A9−/−; N=12 DDC-fed BM_A9+/+; N=9 DDC-fed BM_A9−/− mice. Epididymal fat pad weight (B). Twoway ANOVA was used to evaluate the effect of diet (chow versus DDC) and genotype (BM_A9+/+ versus BM_A9−/− mice) on epididymal weight (g), followed by Bonferroni post-hoc analysis. DDC-feeding significantly increased epididymal weight (p<0.001), but bone marrow S100A9-deficiency had no effect (p>0.05). Plasma IL-6 was measured by ELISA (C). Statistical analysis was performed using unpaired two-tailed Student's _t_-test on IL-6 levels (ng/mL) in DDC-fed mice. Bone marrow S100A9-deficiency had no effect (p>0.05). Mac-2-positive area was quantified in 3 epididymal fat sections/animal (D). Two-way ANOVA was used to evaluate the effect of diet (chow versus DDC) and genotype (BM_A9+/+ versus BM_A9−/− mice) on Mac-2 area (μm2), followed by Bonferroni post-hoc analysis. DDC-feeding significantly increased Mac-2 area (p<0.001), but bone marrow S100A9-deficiency had no effect (p>0.05). Representative sections of anti-Mac-2 stained epididymal sections (E). GTT (F) and ITT (G) were conducted after 20 and 22 weeks on diet, respectively. Two-way ANOVA was used to evaluate the effect of diet (chow versus DDC) as a function of area under the curve on blood glucose (mg/dL), followed by Bonferroni post-hoc analysis. DDC-feeding significantly increased the area under the curve for both ITT and GTT (both p<0.001), but bone marrow S100A9-deficiency had no effect (p>0.05). N=11 chow-fed BM_A9+/+ mice; N=11 chow-fed BM_A9−/−; N=14 DDC-fed BM_A9+/+; N=14 DDC-fed BM_A9−/− mice. The results are presented as mean + SEM, (A, F, G) or as scatter plots, with mean ± SEM indicated by horizontal lines and vertical lines, respectively (B-D). Bar, 200 μm in E; ND, non-detectable
In order to evaluate insulin resistance, we conducted GTT and ITT. DDC-fed mice had significantly elevated baseline glucose and increased insulin resistance compared to chow-fed mice (Fig. 3F-G). Similar to the lack of difference in adipose inflammation and body weight, there was no effect of bone marrow S100A9-deficiency on GTT or ITT (Fig. 3F-G).
Although S100A8 and S100A9 are primarily expressed in myeloid cells, evidence suggests that inflammatory stimuli induce their expression in non-myeloid cells, including endothelial cells19,20. Accordingly, in isolated mouse heart endothelial cells, S100A8 mRNA expression was increased following TNF-α treatment compared to controls (Supplemental Fig. 4). This suggests that under pro-inflammatory conditions, such as those present in diet-induced obesity, non-hematopoietic cells may express increased levels of S100A8/A9. Secreted S100A8/A9 from unstimulated or TNF-α-stimulated endothelial cells was below detection (data not shown), indicating that endothelial cells secrete lower amounts of these proteins than do myeloid cells, or do not release these proteins unless damaged.
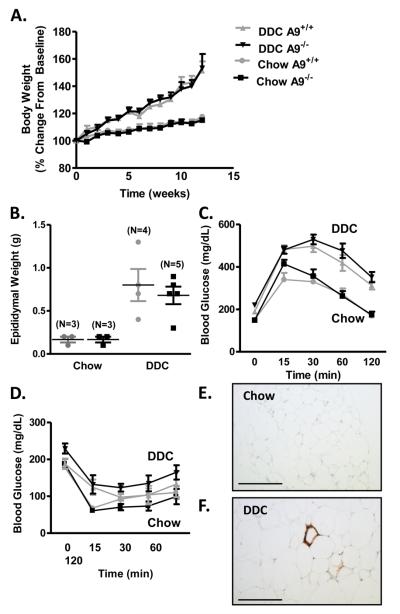
In order to assess the potential role of S100A8/A9 in non-hematopoietic cells, whole-body S100A9+/+ and S100A9−/− C57BL/6J male mice were fed chow or DDC for 12 weeks. DDC-fed animals gained a significant amount of weight compared to chow-fed mice, with no effect of S100A9-deficiency on body weight or fat pad weight (Fig. 4A-B). After 11-12 weeks on DDC, the mice displayed glucose intolerance, yet whole-body S100A9-deficiency had no significant effect on insulin- or glucose tolerance (Fig. 4C-D). Although DDC-fed mice exhibited increased accumulation of Mac-2-positive cells in epididymal adipose tissue (Fig. 4E-F), S100A9-deficiency had no effect (data not shown). Together, these results demonstrate that neither bone marrow S100A9-deficiency, nor whole-body S100A9-deficiency affects adipose tissue inflammation, obesity, or diet-induced insulin resistance.
Figure 4. Whole-body S100A8/A9 deficiency does not improve insulin resistance.
Whole-body S100A9+/+ (A9+/+) and S100A9−/− (A9−/−) male mice were fed chow or DDC for 12 weeks. Body weights were normalized to starting body weight (A). Two-way ANOVA was used to evaluate the effect of diet (chow versus DDC) on body weight (% change from baseline) as a function of time (weeks), followed by Bonferroni post-hoc analysis. DDC-feeding significantly increased body weight (p<0.001) in A9+/+ and A9−/− mice, but S100A9-deficiency had no effect (p>0.05). N=3 chow-fed A9+/+ mice; N=3 chow-fed A9−/−; N=4 DDC-fed A9+/+; N=7 DDC-fed A9−/− mice. Epididymal fat pad weight was measured (B). Two-way ANOVA was used to evaluate the effect of diet (chow versus DDC) on epididymal weight (g), followed by Bonferroni post-hoc analysis. DDC-feeding significantly increased epididymal weight (p<0.001), but bone marrow S100A9-deficiency had no effect (p>0.05). GTT (C) and ITT (D) were conducted after 10 and 12 weeks on diet, respectively. Two-way ANOVA was used to evaluate the effect of diet (chow versus DDC) as a function of time (min) on blood glucose (mg/dL), followed by Bonferroni post-hoc analysis. DDC-feeding significantly increased area under the curve from the GTT (p<0.001), but S100A9-deficiency had no effect (p>0.05). DDC-feeding did not significantly increase area under the curve for ITT (p>0.05), nor was there an effect of S100A9-deficiency. N=3 chow-fed A9+/+ mice; N=3 chow-fed A9−/−; N=4 DDC-fed A9+/+; N=4 (C) or N=7 (D) DDC-fed A9−/− mice. The results are presented as mean + or ± SEM (A, C-D), or as scatter plots, with mean ± SEM indicated by horizontal lines and vertical lines, respectively (B). Representative Mac-2-stained epididymal sections from chow-fed (E) and DDC-fed (F) S100A9+/+ mice. Bar, 200 μm in E-F
Neutrophils, early differentiation stages of macrophages and DCs, and more mature DCs express significantly more S100A9 than do macrophages
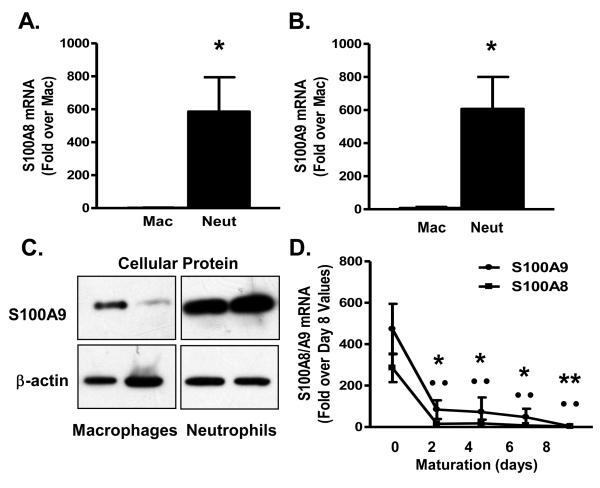
In order to investigate the reason(s) for the unexpected lack of effect of bone marrow S100A9-deficiency on atherosclerosis, we performed a number of studies. First, expression levels of S100A9 in different myeloid cell populations were determined. S100A8 and S100A9 mRNA levels were analyzed in neutrophils, immature macrophages and DCs, and more mature macrophages and DCs in wild-type mice. S100A8 and S100A9 mRNA levels were markedly higher in thioglycollate-elicited neutrophils as compared to thioglycollate-elicited macrophages (Fig. 5A-B), consistent with previous studies9. S100A9 protein levels were also higher in neutrophils compared to macrophages (Fig. 5C). S100A8 and S100A9 mRNA levels declined rapidly during macrophage and DC differentiation (Fig. 5D and data not shown), as expected9.
Figure 5. Neutrophils and monocytes express more S100A8 and S100A9 than macrophages.
Thioglycollate-elicited neutrophils and macrophages were obtained from C57BL/6 mice. S100A8 (A, N=6) and S100A9 (B, N=6) mRNA levels were measured by real-time PCR. Cellular S100A9 protein and β-actin levels were measured in macrophages and neutrophils. Statistical analysis to compare levels in macrophages and neutrophils was performed by unpaired Student's _t_-test (*p<0.05). A representative Western blot is shown; images are from the same blot (C). Bone marrow from C57BL/6 mice was collected and enriched for monocytes. Monocytes were cultured in 30% L-conditioned medium. S100A8 and S100A9 mRNA was expressed relative to day 8 (D, N=3). One-way ANOVA was used to evaluate S100A9 and S100A8 mRNA as a function of time (days), followed by Tukey posthoc test (*p<0.05, **p<0.01 for S100A9 mRNA versus S100A9 mRNA day 0; ··p<0.01 for S100A8 mRNA versus S100A8 mRNA at day 0). The results are presented as mean + or ± SEM (A-B, D). Mac, macrophages; Neut, neutrophils
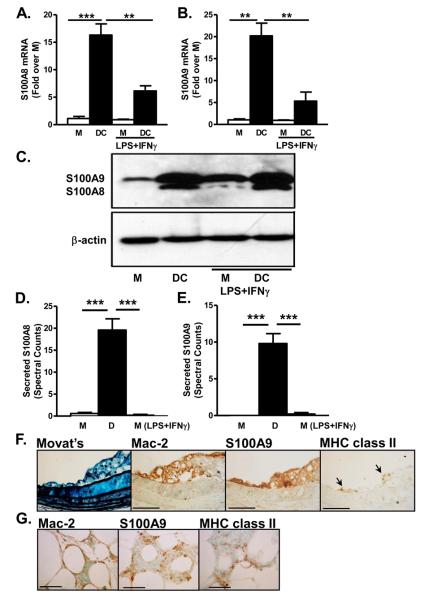
Next we investigated the levels of S100A8/A9 in bone marrow-derived DCs, differentiated in the presence of GM-CSF, as compared to M-CSF-differentiated macrophages. The DC population expressed higher levels of the DC markers CD11c and MHC class II (Supplemental Fig. 5). DCs expressed more S100A8 and S100A9 mRNA and protein (Fig. 6A-C) as compared to macrophages. In parallel experiments, the relative levels of secretion of S100A8 and S100A9 were evaluated by mass spectrometry. Both S100A8 and S100A9 were secreted at significantly elevated levels from DCs as compared to macrophages (Fig. 6D-E).
Figure 6. Dendritic cells express and secrete more S100A8 and S100A9 than macrophages.
Bone marrow-derived macrophages or DCs were cultured in L-conditioned medium as a source of M-CSF (macrophages) or GM-CSF (DCs). Some cells were stimulated with LPS plus IFNγ during the last 24 h. S100A8 (A; N=3) and S100A9 (B; N=3) mRNA levels were measured by real-time-PCR. Cells from the same mice were used in (A) and (B). Statistical analysis was performed by repeated measures one-way ANOVA for S100A8 and S100A9 mRNA (fold over M), followed by Tukey posthoc test. M versus DC ***p<0.001 in (A) and **p<0.01 in (B); DC versus M LPS+IFNγ ***p<0.001; DC versus DC LPS+IFNγ **p<0.01. Other comparisons; p>0.05. Cellular S100A8, S100A9, and #$actin levels were evaluated by Western blot (C). (D-E) Macrophages were differentiated for 7 days followed by LPS+IFNγ activation for 24 h. DCs were cultured for 9 days. Conditioned media were collected, and secreted S100A8 (D; N=5) and S100A9 (E; N=5) was analyzed by tandem mass-spectrometry. Statistical analysis was performed by one-way ANOVA for secreted S100A8 and S100A9 (spectral counts) followed by Tukey posthoc test. M versus DC ***p<0.001; DC versus M LPS+IFNγ ***p<0.001. Other comparisons; p>0.05. The results are presented as mean + SEM (A-B, D-E). (F) BCA representative adjacent sections of atherosclerotic lesions from LDLR−/− mice stained with Movat's pentachrome stain, anti-Mac-2, anti-S100A9, and anti-MHC class II (F). Epididymal adipose tissue from male LDLR−/− mice fed DDC for 24 weeks (G). Representative sections of anti-Mac-2, anti-S100A9, and anti-MHC class II stained tissue from the same animal are shown. Lines indicate 100 μm in F and 50 μm in G. M, macrophage; D, dendritic cell
LPS plus IFNγ was used to address the expression changes of S100A9 in macrophages and DCs subjected to inflammatory stimuli. In macrophages, LPS/IFNγ had no effect on S100A8 and S100A9 mRNA (Fig. 6A-B) or secretion of S100A8 and S100A9 (Fig. 6D-E), although there was some increase in cellular S100A8 and S100A9 protein (Fig. 6C and Supplemental Fig. 2). The levels of S100A8 and S100A9 in macrophages stimulated with LPS/IFNγ were consistently lower than those of DCs (Fig. 6A-E). DCs stimulated with LPS plus IFNγ, agents known to result in maturation of DCs, demonstrated reduced S100A8 and S100A9 mRNA levels compared to those of non-stimulated DCs (Fig. 6A-B), but cellular S100A8/A9 protein levels (Fig. 6C) and secretion of S100A9 remained high (Supplemental Fig. 6). Thus, classical activation of macrophages by LPS/IFNγ fails to induce S100A8 and S100A9 to levels seen in DCs. Furthermore, DDC-feeding did not induce S100A8 or S100A9 mRNA in peritoneal macrophages from LDLR−/− hypercholesterolemic insulin resistant mice (Supplemental Fig. 7), and a high-fat diet also did not affect secretion of S100A8 or S100A9 from peritoneal macrophages.34
In atherosclerotic lesions, S100A9 immunoreactivity was frequently present primarily in areas positive for Mac-2 (Fig. 6F), a marker of macrophages and DCs (LB, MMA, KEB, JWH; unpublished observations). The S100A9-positive area also contained occasional cells positive for MHC class II (Fig. 6F). Few lesions (5% of lesions analyzed) showed occasional cells positive for the neutrophil marker Ly6G. Thus, it is unlikely that neutrophils represent a major source of S100A9 in lesions from LDLR−/− mice. A similar pattern of S100A9 immunoreactivity was apparent in the crown-like structures of inflamed adipose tissue, whereas adipocytes themselves were negative for S100A9 (Fig. 6G).
Thus, S100A9 is expressed at higher levels in neutrophils and early differentiation stages of macrophages and DCs than in mature DCs, which in turn show higher expression and secretion of S100A8 and S100A9 than mature resting or activated macrophages. Immunohistochemical analyses indicate that early differentiation stages of macrophages and DCs, or mature DCs are likely sources of S100A9 in lesions of atherosclerosis and inflamed adipose tissue.
S100A9-deficient neutrophils have a blunted inflammatory response and show reduced accumulation in inflamed adipose tissue
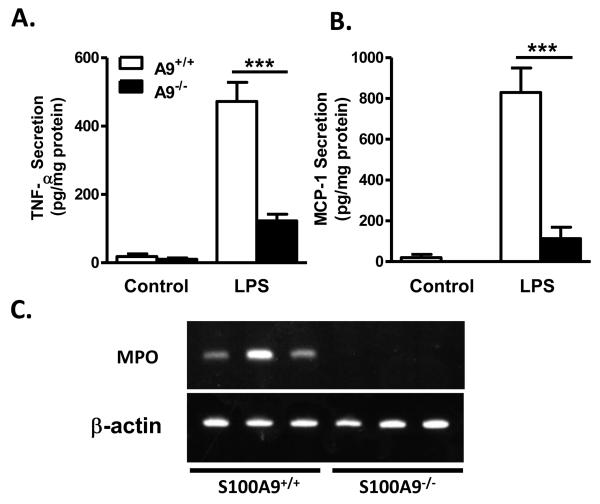
We next asked whether S100A9-deficiency have similar effects in these different myeloid cell populations. S100A9-deficient neutrophils elicited from the peritoneal cavity and then stimulated with LPS released significantly less TNF-α and MCP-1 than wild-type neutrophils (Fig. 7A-B). Thus, S100A9, and/or S100A8, has a pro-inflammatory role in neutrophils.
Figure 7. S100A9 -deficient neutrophils secrete less TNF-α and MCP-1.
Thioglycollate-elicited peritoneal neutrophils were obtained from S100A9+/+ and S100A9−/− mice, and stimulated with 250 ng/mL LPS overnight. TNF-α (A) and MCP-1 (B) were measured in conditioned media by ELISA. The results are presented as mean + SEM (A-B). Statistical analysis was performed by one-way ANOVA for TNF-α and MCP-1 secretion (pg/mg protein) followed by Tukey posthoc test. A9+/+ and A9−/− control versus A9+/+ LPS ***p<0.001; A9+/+ LPS versus A9−/− LPS ***p<0.001. Other comparisons; p>0.05. N=3 except for LPS-stimulated cells in (A; N=4). (C) Epididymal adipose tissue harvested from three S100A9+/+ and S100A9−/− mice after a 2-week regimen on DDC. Levels of MPO mRNA were evaluated by RT-PCR in each mouse.
Neutrophils infiltrate adipose tissue in mice within the first two weeks of fat-feeding25. We therefore investigated neutrophil accumulation in epididymal fat in DDC-fed whole-body S100A9+/+ and S100A9−/− mice. Myeloperoxidase (MPO) mRNA was used as a sensitive neutrophil marker. After 14 days of DDC-feeding, MPO mRNA was detectable in adipose tissue from wild-type mice, but not in adipose tissue from S100A9−/− mice (Fig. 7C). The number of neutrophils in S100A9+/+ mice was low; less than one neutrophil/cross section was identified (data not shown). Conversely, no differences in CD68 mRNA, used as a macrophage and dendritic cell marker, were observed between S100A9+/+ and S100A9−/− adipose tissue (data not shown). This suggests that S100A9-deficiency inhibits neutrophil infiltration into adipose tissue, corroborating evidence that S100A9 plays an important role in promoting neutrophil migration as well as their inflammatory status.
S100A9-deficient DCs display an enhanced inflammatory response to TLR2 and TLR4 ligands
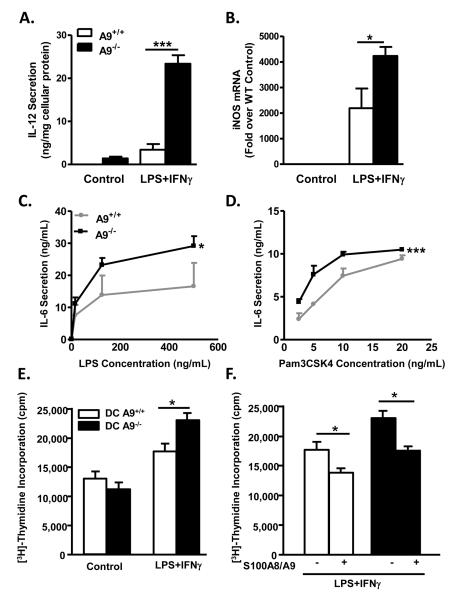
In contrast to neutrophils, S100A9−/− bone marrow-derived DCs stimulated with LPS plus IFNγ exhibited significant increases in IL-12p40 secretion and iNOS mRNA levels (Fig. 8A-B), two markers of inflammatory status, compared to S100A9+/+ DCs. The enhanced pro-inflammatory response of S100A9−/− DCs was confirmed in bone marrow DCs purified by CD11c+ selection. In the CD11c+ DC population, LPS and Pam3CSK4, TLR4 and TLR2/1 agonists, respectively, induced a significantly higher release of IL-6 from S100A9-deficient DCs compared to wild-type DCs (Fig. 8C-D). Zymosan, another TLR2 ligand, elicited a similarly enhanced effect on IL-12p70 release in S100A9−/− DCs (Supplemental Fig. 8A).
Figure 8. S100A9-deficient DCs display increased pro-inflammatory properties.
Bone marrow-derived DCs from S100A9+/+ or S100A9−/− mice were activated with LPS and IFNγ. Secreted IL-12p40 was measured by ELISA (A) and iNOS mRNA was measured with real-time PCR (B). Statistical analysis was performed by oneway ANOVA for IL-12 secretion (ng/mg cellular protein) or iNOS mRNA (fold over wildtype control), followed by Tukey posthoc test. In (A), A9−/− control cells versus A9−/− LPS-stimulated cells, and A9−/− LPS-stimulated cells versus A9+/+ control cells or A9+/+ LPS-stimulated cells ***p<0.001. In (B), A9−/− and A9+/+ control cells versus A9−/− LPS-stimulated cells, A9+/+ control cells versus A9+/+ LPS-stimulated cells, and A9+/+ LPS-stimulated cells versus A9−/− control cells or A9−/− LPS-stimulated cells *p<0.05. Other comparisons; p>0.05. CD11c+-purified DCs were stimulated with LPS (C) or Pam3CSK4 (D). IL-6 secretion was measured by ELISA. Two-way ANOVA was used to evaluate the effect of S100A9-deficiency on IL-6 secretion (ng/mL) as a function of LPS and Pam3CSK4 concentration (ng/mL). LPS and Pam3CSK4 significantly increased IL-6 secretion (p<0.001). S100A9-deficiency significantly increased IL-6 secretion in response to LPS compared to wildtype controls (*p<0.05) and Pam3CSK4 (***p<0.01). CD4+/CD8+ T lymphocytes from BALB/c mice were mixed with control DCs and stimulated DCs obtained from S100A9+/+ and S100A9−/− mice in the absence (E) or presence (F) of 10 mg/mL exogenous S100A8/A9. T-cell proliferation was estimated by [3H]-thymidine incorporation. Statistical analysis was performed by unpaired Student's t-test between the groups indicated by lines (*p<0.05). The results are presented as mean + SEM; N=3.
Furthermore, S100A9−/− DCs activated with LPS plus IFNγ significantly increased T-cell proliferation compared to wild-type DCs (Fig. 8E). Addition of exogenous recombinant S100A8 and S100A9 did not affect T-cell proliferation in the presence of unstimulated DCs (data not shown), yet in both wild-type and S100A9−/− DCs activated by LPS and IFNγ, exogenous S100A8/A9 significantly reduced T-cell proliferation (Fig. 8F). Thus, S100A8 and S100A9 have inhibitory functions in activated DCs, an effect due in part to secreted S100A8/A9 acting in an autocrine fashion.
The inflammatory response of S100A9-deficient macrophages was also investigated. Neither bone marrow-derived macrophages, nor thioglycollate-elicited S100A9-deficient peritoneal macrophages showed a difference in LPS-induced pro-inflammatory gene expression or in TNF-α, IL-12p40, or MCP-1 release, or in zymosan-induced IL-12p40 release compared to S100A9+/+ macrophages (Supplemental Fig. 8BD and data not shown).
Thus, DCs from S100A9-deficient mice exhibit an increased pro-inflammatory response and an increased ability to induce T-cell proliferation, whereas S100A9-deficiency in macrophages has no effect on their inflammatory response to TLR ligands.
Discussion
We demonstrate that despite of the majority of S100A9 being derived from circulating myeloid cells, bone marrow-specific S100A9-deficiency is not sufficient to reduce systemic inflammation, insulin resistance, or atherosclerosis in fat-fed LDLR-deficient mice. We propose that the lack of a net effect of S100A9-deficiciency in bone marrow-derived cells is explained by our findings that three different myeloid cell populations - neutrophils, macrophages, and DCs - respond in distinctly different ways following inflammatory activation in the setting of S100A9-deficiency. We further propose that the relative involvement of distinct myeloid cell populations is likely to explain the inconsistent effects of S100A9-deficiency in inflammatory diseases2,18,26. A limitation of our study is that myeloid cell populations from adipose tissue and the artery wall of insulin resistant hyperlipidemic mice were not evaluated, due to the difficulty of obtaining large enough quantities of well-characterized myeloid cell populations from tissues. Such studies would provide further insight into the role of S100A9 in vivo. Another limitation is that small significant differences might have been detected with larger groups of mice.
S100A8 and S100A9 play important roles in neutrophil migration11 and NADPH oxidase activation27. S100A9-deficiency inhibits inflammation in models of sepsis and pancreatitis6-8. These disorders are partially mediated by neutrophils, consistent with our current findings, which demonstrate reduced neutrophils in inflamed adipose tissue of fat-fed S100A9-deficient mice. Together, these findings suggest that S100A9-deficieincy reduces neutrophil-mediated inflammation. Conversely, S100A9 inhibits DC maturation18, and our data suggests that S100A9-deficiency increases the inflammatory response of DCs. We propose that S100A9-deficiency in diseases in which DCs play a more prominent role will result in increased tissue inflammation.
S100A8/A9 have been shown to predict cardiovascular events1,28, are highly expressed in rupture-prone lesions29 and at the site of coronary occlusion29-30, and whole-body S100A9-deficiency inhibits atherosclerosis in ApoE−/− mice2. Therefore, S100A8/A9 play important roles in atherosclerosis. However, we show that LDLR−/− mice transplanted with S100A9-deficient bone marrow do not exhibit reduced atherosclerosis or macrophage accumulation in lesions. A potential reason for this difference is that the previous report2 used mice with whole-body S100A9-deficiency in contrast to our bone marrow transplant study. Several reports have identified inducible expression of S100A8 and/or S100A9 in non-myeloid cells19-20. S100A8/A9 can induce chemotactic factor expression in endothelial cells31, impair endothelial integrity32, and promote smooth muscle proliferation33. Given that S100A8/A9 have extracellular actions6,14-15,31, non-myeloid cell-derived S100A8/A9 may also act in a paracrine fashion on artery wall cells. In addition, ApoE itself has significant effects on macrophage function34 and ApoE mice show a significant number of neutrophils in the shoulder regions of atherosclerotic lesions35. Interestingly, DCs contribute to lesion development in LDLR−/− mice36. Thus, part of the difference between the present study and that of Croce et al. 2 could be due to differences in lesion myeloid cell composition between ApoE−/− and LDLR−/− mice.
In conclusion, S100A8/A9 differentially modulate the inflammatory responses of myeloid cells in diametrically opposed manners. S100A8/A9 are pro-inflammatory in neutrophils, have no detectable inflammatory effects in macrophages, and are anti-inflammatory in DCs. The main source of S100A9 in lesions of atherosclerosis, at least in LDLR-deficient mice, appears to be early macrophages or DCs. Further study is needed to fully understand the functions of S100A8/A9 in specific cell populations and disease states before S100A8/A9 are considered as therapeutic targets.
CLINICAL PERSPECTIVE.
It has previously been demonstrated that elevated plasma levels of S100A9 (also known as myeloid related protein-14; MRP-14) in complex with its binding partner S100A8 (MRP-8) predict increased risk of future cardiovascular events in healthy postmenopausal women, as well as recurrent events in patients with acute coronary syndromes. Furthermore, apolipoprotein E-deficient mice that are also deficient in S100A9 exhibit reduced atherosclerosis. These important findings suggest that S100A9 is both a biomarker and a mediator of atherosclerosis and cardiovascular events. Most of the constitutively secreted S100A9 is believed to be derived from myeloid cells. We demonstrate that LDL receptor-deficient mice that lack S100A9 in bone marrow-derived cells, including myeloid cells, are not protected against diet-induced atherosclerosis or insulin resistance. Furthermore, S100A9-deficiency differentially modifies phenotypic states of myeloid cell populations. S100A9-deficient neutrophils exhibit a reduced secretion of cytokines, whereas S100A9-deficient dendritic cells show an exacerbated release of cytokines. The effect of S100A9-deficiency on atherosclerosis, as well as other inflammatory diseases, is therefore predicted to depend on the relative contribution of these cell types at different stages of disease progression. Furthermore, S100A9 expression in non-myeloid cells is likely to contribute to atherosclerosis. Further study is needed to fully understand the functions of S100A8/A9 in specific cell populations and disease states before S100A8/A9 are considered as therapeutic targets.
Supplementary Material
1
Acknowledgments
We are grateful to Drs. Hiroaki Ito for help with TLR stimulation, and Wolfgang Nacken for providing S100A9-deficient breeders.
Funding sources
This study was supported by NIH grants HL062887, HL092969, and HL097365 (KEB), HL079382 (RCL), AI073441 (JAH), HL030086 and HL092969 (JWH), and “Interdisziplinäres Zentrum für Klinische Forschung”, University of Muenster, project Ker3/086/04, and “Deutsche Forschungsgemeinschaft”, projects KE 820/6-1 and KE 820/2-4 (CK). MMA was supported by a Cardiovascular Postdoctoral Training Grant (T32 HL07828).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113:2278–2284. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- 2.Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RR, Hogg N, Libby P, Simon DI. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–436. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouma G, Coppens JM, Lam-Tse WK, Luini W, Sintnicolaas K, Levering WH, Sozzani S, Drexhage HA, Versnel MA. An increased mrp8/14 expression and adhesion, but a decreased migration towards proinflammatory chemokines of type 1 diabetes monocytes. Clin Exp Immunol. 2005;141:509–517. doi: 10.1111/j.1365-2249.2005.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frosch M, Vogl T, Seeliger S, Wulffraat N, Kuis W, Viemann D, Foell D, Sorg C, Sunderkotter C, Roth J. Expression of myeloid-related proteins 8 and 14 in systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2003;48:2622–2626. doi: 10.1002/art.11177. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen OH, Nielsen AR, Erikstrup C, Plomgaard P, Fischer CP, Krogh-Madsen R, Lindegaard B, Petersen AM, Taudorf S, Pedersen BK. Calprotectin--a novel marker of obesity. PLoS One. 2009;4:e7419. doi: 10.1371/journal.pone.0007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and mrp14 are endogenous activators of toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 7.van Zoelen MA, Vogl T, Foell D, Van Veen SQ, van Till JW, Florquin S, Tanck MW, Wittebole X, Laterre PF, Boermeester MA, Roth J, van der Poll T. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am J Respir Crit Care Med. 2009;180:1098–1106. doi: 10.1164/rccm.200810-1552OC. [DOI] [PubMed] [Google Scholar]
- 8.Schnekenburger J, Schick V, Kruger B, Manitz MP, Sorg C, Nacken W, Kerkhoff C, Kahlert A, Mayerle J, Domschke W, Lerch MM. The calcium binding protein S100A9 is essential for pancreatic leukocyte infiltration and induces disruption of cell-cell contacts. J Cell Physiol. 2008;216:558–567. doi: 10.1002/jcp.21433. [DOI] [PubMed] [Google Scholar]
- 9.Lagasse E, Weissman I. Mouse mrp8 and mrp14, two intracellular calcium-binding proteins associated with the development of the myeloid lineage. Blood. 1992;79:1907–1915. [PubMed] [Google Scholar]
- 10.Kumar A, Steinkasserer A, Berchtold S. Interleukin-10 influences the expression of mrp8 and mrp14 in human dendritic cells. Int Arch Allergy Immunol. 2003;132:40–47. doi: 10.1159/000073263. [DOI] [PubMed] [Google Scholar]
- 11.Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schonlau F, Roth J, Sorg C, Nacken W. Loss of S100A9 (mrp14) results in reduced interleukin-8-induced cd11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23:1034–1043. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyette J, Geczy CL. Inflammation-associated S100 proteins: New mechanisms that regulate function. Amino Acids. 2010 doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 13.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (mrp) 8 and mrp14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–9502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 14.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res. 2008;102:1239–1246. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 15.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S, Makino H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa b and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K, Yen T, Geczy CL. Il-10 up-regulates macrophage expression of the S100 protein S100A8. J Immunol. 2001;166:6358–6366. doi: 10.4049/jimmunol.166.10.6358. [DOI] [PubMed] [Google Scholar]
- 17.Hsu K, Passey RJ, Endoh Y, Rahimi F, Youssef P, Yen T, Geczy CL. Regulation of S100A8 by glucocorticoids. J Immunol. 2005;174:2318–2326. doi: 10.4049/jimmunol.174.4.2318. [DOI] [PubMed] [Google Scholar]
- 18.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yen T, Harrison CA, Devery JM, Leong S, Iismaa SE, Yoshimura T, Geczy CL. Induction of the S100 chemotactic protein, cp-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood. 1997;90:4812–4821. [PubMed] [Google Scholar]
- 20.Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, Chait A, Bornfeldt KE. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest. 2004;114:659–668. doi: 10.1172/JCI17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, 3rd, Kirk EA, O'Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Rampersad RR, Esserman D, McGinnis MW, Lee DM, Patel DD, Tarrant TK. S100A9 is not essential for disease expression in an acute (k/bxn) or chronic (cia) model of inflammatory arthritis. Scand J Rheumatol. 2009;38:445–449. doi: 10.3109/03009740902895743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and rac-2. FASEB J. 2005;19:467–469. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- 28.Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, Pradhan AD, Healy AM, Buros J, McCabe CH, Libby P, Cannon CP, Braunwald E, Simon DI. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the pravastatin or atorvastatin evaluation and infection therapy: Thrombolysis in myocardial infarction (prove it-timi 22) trial. Am Heart J. 2008;155:49–55. doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ionita MG, Vink A, Dijke IE, Laman JD, Peeters W, van der Kraak PH, Moll FL, de Vries JP, Pasterkamp G, de Kleijn DP. High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2009;29:1220–1227. doi: 10.1161/ATVBAHA.109.190314. [DOI] [PubMed] [Google Scholar]
- 30.Altwegg LA, Neidhart M, Hersberger M, Muller S, Eberli FR, Corti R, Roffi M, Sutsch G, Gay S, von Eckardstein A, Wischnewsky MB, Luscher TF, Maier W. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: A novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007;28:941–948. doi: 10.1093/eurheartj/ehm078. [DOI] [PubMed] [Google Scholar]
- 31.Ehlermann P, Eggers K, Bierhaus A, Most P, Weichenhan D, Greten J, Nawroth PP, Katus HA, Remppis A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi: 10.1186/1475-2840-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viemann D, Barczyk K, Vogl T, Fischer U, Sunderkotter C, Schulze-Osthoff K, Roth J. Mrp8/mrp14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood. 2007;109:2453–2460. doi: 10.1182/blood-2006-08-040444. [DOI] [PubMed] [Google Scholar]
- 33.Inaba H, Hokamura K, Nakano K, Nomura R, Katayama K, Nakajima A, Yoshioka H, Taniguchi K, Kamisaki Y, Ooshima T, Umemura K, Murad F, Wada K, Amano A. Upregulation of S100 calcium-binding protein A9 is required for induction of smooth muscle cell proliferation by a periodontal pathogen. FEBS Lett. 2009;583:128–134. doi: 10.1016/j.febslet.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Becker L, Gharib SA, Irwin AD, Wijsman E, Vaisar T, Oram JF, Heinecke JW. A macrophage sterol-responsive network linked to atherogenesis. Cell Metab. 2010;11:125–135. doi: 10.1016/j.cmet.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Björkström NK, Malmberg KJ, Lindbom L, Eriksson EE. Distinct infiltration of neutrophils in lesion shoulders in ApoE−/− mice. Am J Pathol. 2010;177:493–500. doi: 10.2353/ajpath.2010.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1