Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria (original) (raw)
Abstract
New evidence and consensus has led to further revision of the McDonald Criteria for diagnosis of multiple sclerosis. The use of imaging for demonstration of dissemination of central nervous system lesions in space and time has been simplified, and in some circumstances dissemination in space and time can be established by a single scan. These revisions simplify the Criteria, preserve their diagnostic sensitivity and specificity, address their applicability across populations, and may allow earlier diagnosis and more uniform and widespread use. Ann Neurol 2011
Diagnostic criteria for multiple sclerosis (MS) include clinical and paraclinical laboratory assessments1,2 emphasizing the need to demonstrate dissemination of lesions in space (DIS) and time (DIT) and to exclude alternative diagnoses. Although the diagnosis can be made on clinical grounds alone, magnetic resonance imaging (MRI) of the central nervous system (CNS) can support, supplement, or even replace some clinical criteria,3–9 as most recently emphasized by the so-called McDonald Criteria of the International Panel on Diagnosis of MS.8,9 The McDonald Criteria have resulted in earlier diagnosis of MS with a high degree of both specificity and sensitivity,10–13 allowing for better counseling of patients and earlier treatment.
Since the revision of the McDonald Criteria in 2005, new data and consensus have pointed to the need for their simplification to improve their comprehension and utility and for evaluating their appropriateness in populations that differ from the largely Western Caucasian adult populations from which the Criteria were derived. In May 2010 in Dublin, Ireland, the International Panel on Diagnosis of MS (the Panel) met for a third time to examine requirements for demonstrating DIS and DIT and to focus on application of the McDonald Criteria in pediatric, Asian, and Latin American populations.
Considerations Related to Revisions to the McDonald Criteria
The Panel reviewed published research related to the diagnosis of MS and to the original and revised McDonald Criteria, gathered from literature searches of English language publications containing the terms multiple sclerosis and diagnosis, and from specific recommendations of relevant papers by Panel members. The Panel concluded that most recent research supports the utility of the McDonald Criteria in a typical adult Caucasian population seen in MS centers, despite only limited research and practical experience in general neurology practice populations.
In its discussions, the Panel stressed that the McDonald Criteria should only be applied in those patients who present with a typical clinically isolated syndrome (CIS) suggestive of MS or symptoms consistent with a CNS inflammatory demyelinating disease, because the development and validation of the Criteria have been limited to patients with such presentations. CIS presentations can be monofocal or multifocal, and typically involve the optic nerve, brainstem/cerebellum, spinal cord, or cerebral hemispheres.
In applying the McDonald Criteria, it remains imperative that alternative diagnoses are considered and excluded. Differential diagnosis in MS has been the subject of previous data- and consensus-driven recommendations that point to common and less common alternative diagnoses for MS and identify clinical and paraclinical red flags that should signal particular diagnostic caution.14,15 In its current review, the Panel focused specifically on the often-problematic differential diagnosis for MS of neuromyelitis optica (NMO) and NMO spectrum disorders. There is increasing evidence of relapsing CNS demyelinating disease characterized by involvement of optic nerves (unilateral or bilateral optic neuritis), often severe myelopathy with MRI evidence of longitudinally extensive spinal cord lesions, often normal brain MRI (or with abnormalities atypical for MS), and serum aquaporin-4 (AQP4) autoantibodies.16,17 There was agreement that this phenotype should be separated from typical MS because of different clinical course, prognosis, and underlying pathophysiology and poor response to some available MS disease-modifying therapies.18 The Panel recommends that this disorder should be carefully considered in the differential diagnosis of all patients presenting clinical and MRI features that are strongly suggestive of NMO or NMO spectrum disorder, especially if (1) myelopathy is associated with MRI-detected spinal cord lesions longer than 3 spinal segments and primarily involving the central part of the spinal cord on axial sections; (2) optic neuritis is bilateral and severe or associated with a swollen optic nerve or chiasm lesion or an altitudinal scotoma; and (3) intractable hiccough or nausea/vomiting is present for >2 days with evidence of a periaqueductal medullary lesion on MRI.19,20 In patients with such features, AQP4 serum testing should be used to help make a differential diagnosis between NMO and MS to help avoid misdiagnosis and to guide treatment.
Correct interpretation of symptoms and signs is a fundamental prerequisite for diagnosis.21 The Panel considered again what constitutes an attack (relapse, exacerbation) and defined this as patient-reported symptoms or objectively observed signs typical of an acute inflammatory demyelinating event in the CNS, current or historical, with duration of at least 24 hours, in the absence of fever or infection. Although a new attack should be documented by contemporaneous neurological examination, in the appropriate context, some historical events with symptoms and evolution characteristic for MS, but for which no objective neurological findings are documented, can provide reasonable evidence of a prior demyelinating event. Reports of paroxysmal symptoms (historical or current) should, however, consist of multiple episodes occurring over not less than 24 hours. There was consensus among the Panel members that before a definite diagnosis of MS can be made, at least 1 attack must be corroborated by findings on neurological examination, visual evoked potential (VEP) response in patients reporting prior visual disturbance, or MRI consistent with demyelination in the area of the CNS implicated in the historical report of neurological symptoms.
The Panel concluded that the underlying concepts of the original (2001) and revised (2005) McDonald Criteria8,9 are still valid, including the possibility of establishing a diagnosis of MS based on objective demonstration of dissemination of lesions in both space and time on clinical grounds alone or by careful and standardized integration of clinical and MRI findings. However, the Panel now recommends key changes in the McDonald Criteria related to the use and interpretation of imaging criteria for DIS and DIT as articulated by the recently published work from the MAGNIMS research group.22–24 Such changes are likely to further increase diagnostic sensitivity without compromising specificity, while simplifying the requirements for demonstration of both DIS and DIT, with fewer required MRI examinations. The Panel also makes specific recommendations for application of the McDonald Criteria in pediatric and in Asian and Latin American populations.
Recommended Modifications to the McDonald Criteria: The 2010 Revisions
Magnetic Resonance Imaging Criteria for DIS
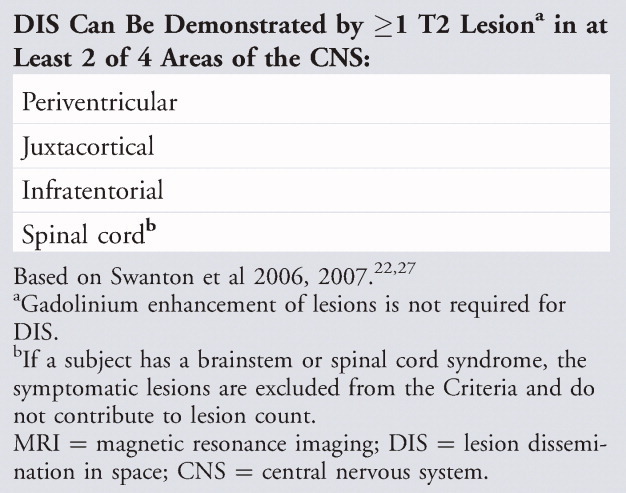
In past versions of the McDonald Criteria, DIS demonstrated by MRI was based on the Barkhof/Tintoré criteria.4,6 Despite having good sensitivity and specificity, these criteria have been difficult to apply consistently by nonimaging specialists.25,26 The European MAGNIMS multicenter collaborative research network, which studies MRI in MS, compared the Barkhof/Tintoré criteria for DIS4,6 with simplified criteria developed by Swanton and colleagues.22,27 In the MAGNIMS work, DIS can be demonstrated with at least 1 T2 lesion in at least 2 of 4 locations considered characteristic for MS and as specified in the original McDonald Criteria (juxtacortical, periventricular, infratentorial, and spinal cord), with lesions within the symptomatic region excluded in patients with brainstem or spinal cord syndromes. In 282 CIS patients, the Swanton-based DIS criteria were shown to be simpler and slightly more sensitive than the original McDonald Criteria for DIS, without compromising specificity and accuracy.22 The Panel accepted these MAGNIMS DIS Criteria, which can simplify the diagnostic process for MS while preserving specificity and improving sensitivity (Table 1).
TABLE 1.
2010 McDonald MRI Criteria for Demonstration of DIS
Magnetic Resonance Imaging Criteria for DIT
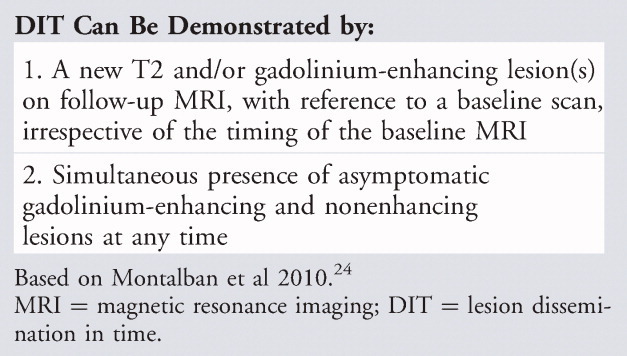
The 2005 revision of the McDonald Criteria simplified the MRI evidence required for DIT, basing it on the appearance of a new T2 lesion on a scan compared to a reference or baseline scan performed at least 30 days after the onset of the initial clinical event.9 In clinical practice, however, there is reason not to postpone a first MRI until after 30 days of clinical onset, which would result in an extra MRI scan to confirm a diagnosis. Abandoning the requirement for an extra reference MRI after 30 days does not compromise specificity,28 and therefore the Panel, in its current revision of the McDonald Criteria, allows a new T2 lesion to establish DIT irrespective of the timing of the baseline MRI.
More recently, the MAGNIMS group confirmed earlier studies29,30 by showing that, in patients with typical CIS, a single brain MRI study that demonstrates DIS and both asymptomatic gadolinium-enhancing and non-enhancing lesions is highly specific for predicting early development of clinically definite MS (CDMS) and reliably substitutes for prior imaging criteria for DIT.23,24 After review of these data, the Panel accepted that the presence of both gadolinium-enhancing and nonenhancing lesions on the baseline MRI can substitute for a follow-up scan to confirm DIT (Table 2), as long as it can be reliably determined that the gadolinium-enhancing lesion is not due to non-MS pathology.
TABLE 2.
2010 McDonald MRI Criteria for Demonstration of DIT
By using the recommended simplified MAGNIMS criteria to demonstrate DIS22 and allowing DIT to be demonstrated by a scan containing both enhancing and nonenhancing lesions in regions of the CNS typical for MS,23 a diagnosis of MS can be made in some CIS patients based on a single MRI.24 The Panel felt this is justified because it simplifies the diagnostic process without reducing accuracy. However, a new clinical event or serial imaging to show a new enhancing or T2 lesion will still be required to establish DIT in those patients who do not have both gadolinium-enhancing and nonenhancing lesions on their baseline MRI.
The Value of Cerebrospinal Fluid Findings in Diagnosis
The Panel reaffirmed that positive cerebrospinal fluid (CSF) findings (elevated immunoglobulin G [IgG] index or 2 or more oligoclonal bands) can be important to support the inflammatory demyelinating nature of the underlying condition, to evaluate alternative diagnoses, and to predict CDMS.15,31 In the 2001 and 2005 McDonald Criteria, a positive CSF finding could be used to reduce the MRI requirements for reaching DIS criteria (requiring only 2 or more MRI-detected lesions consistent with MS if the CSF was positive).8,9 However, when applying the simplified MAGNIMS imaging criteria for DIS and DIT,24 the Panel believes that even further liberalizing MRI requirements in CSF-positive patients is not appropriate, as CSF status was not evaluated for its contribution to the MAGNIMS criteria for DIS and DIT.22,24 Prospective studies using widely available standardized techniques and the most sensitive methods of detection of oligoclonal bands in the CSF together with the new imaging requirements are needed to confirm the additional diagnostic value of CSF.32,33
Making a Diagnosis of Primary Progressive Multiple Sclerosis
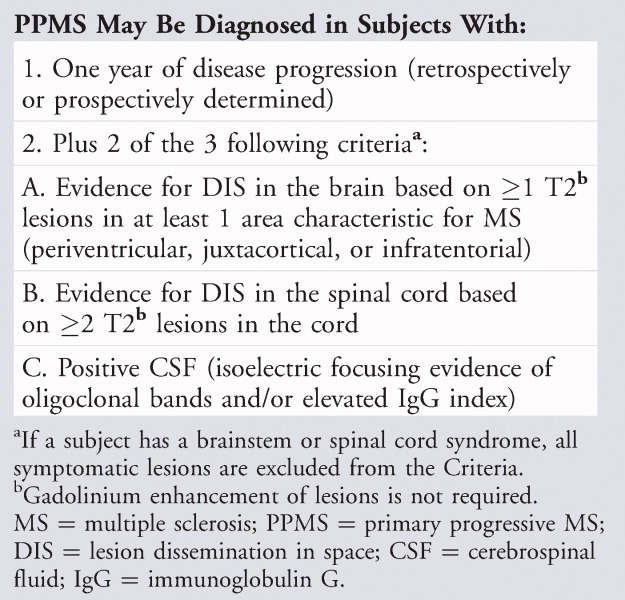
In 2005, the Panel recommended revising the McDonald Criteria for diagnosis of primary progressive multiple sclerosis (PPMS) to require, in addition to 1 year of disease progression, 2 of the following 3 findings: positive brain MRI (9 T2 lesions or 4 or more T2 lesions with positive VEP); positive spinal cord MRI (2 focal T2 lesions); or positive CSF. These criteria reflected the special role of both CSF examination and spinal cord MRI in PPMS, have been found to be practical and are generally well accepted by the neurological community,34 and have been used as inclusion criteria for PPMS clinical trials.35 To harmonize MRI criteria within the diagnostic criteria for all forms of MS, while recognizing the special diagnostic needs for PPMS, the Panel recommends that the McDonald Criteria requirement of fulfilling 2 of 3 MRI or CSF findings be maintained for PPMS, with replacement of the previous brain imaging criterion with the new MAGNIMS brain imaging criterion for DIS (2 of 3 of the following: ≥1 T2 lesions in at least 1 area characteristic for MS [periventricular, juxtacortical, or infratentorial]; ≥2 T2 lesions in the cord; or positive CSF [isoelectric focusing evidence of oligoclonal bands and/or elevated IgG index]) (Table 3). This consensus-based recommendation is justified by comparing diagnostic criteria for PPMS36 and by a subsequent reanalysis of these data (X. Montalban, personal communication). Use of MAGNIMS-based imaging criteria for PPMS with or without associated CSF evaluation should be supported by additional data further documenting the sensitivity and specificity of the criteria in this population.
TABLE 3.
2010 McDonald Criteria for Diagnosis of MS in Disease with Progression from Onset
Applicability of the McDonald Criteria in Pediatric, Asian, and Latin American Populations
The McDonald Criteria were developed with data gathered largely from adult Caucasian European and North American populations, and their applicability has been questioned for other populations, particularly pediatric cases,37,38 Asians,39,40 and Latin Americans.41
Pediatric MS
Over 95% of pediatric MS patients have an initial relapsing–remitting disease course, whereas PPMS is exceptional in children and should prompt detailed consideration of alternative diagnoses.42–45 About 80% of pediatric cases, and nearly all adolescent onset cases, present with attacks typical for adult CIS, with a similar or greater total T2 lesion burden.46–48 In children younger than 11 years, lesions are larger and more ill-defined than in teenagers.49 Imaging criteria for demonstrating DIS in pediatric MS show high sensitivity and/or specificity.38,50,51
The Panel's consensus was that the proposed MAGNIMS-based MRI revisions for DIS will also serve well for most pediatric MS patients, especially those with acute demyelination presenting as CIS, because most pediatric patients will have >2 lesions and are very likely to have lesions in 2 of the 4 specified CNS locations (periventricular, brainstem-infratentorial, juxtacortical, or spinal cord). The frequency of spinal cord lesions in pediatric MS patients is currently unreported, but the appearance of cord lesions in pediatric MS patients with spinal cord symptoms appears generally similar to that of adults.52
However, approximately 15 to 20% of pediatric MS patients, most aged <11 years, present with encephalopathy and multifocal neurological deficits difficult to distinguish from acute disseminated encephalomyelitis (ADEM).43,50 Current operational international consensus criteria for MS diagnosis in children with an ADEM-like first attack require confirmation by 2 or more non-ADEM like attacks, or 1 non-ADEM attack followed by accrual of clinically silent lesions.53 Although children with an ADEM-like first MS attack are more likely than children with monophasic ADEM to have 1 or more non-enhancing T1 hypointense lesions, 2 or more periventricular lesions, and the absence of a diffuse lesion pattern,54 these features are not absolutely discriminatory. Furthermore, MRI scans of children with monophasic ADEM typically demonstrate multiple variably enhancing lesions (often >2) typically located in the juxtacortical white matter, infratentorial space, and spinal cord. Thus, application of the revised MAGNIMS-based criteria for DIS and DIT on initial MRI would be inappropriate for such patients, and serial clinical and MRI observations are required to confirm a diagnosis of MS. In this young age group, there can be marked lesion resolution following an initial attack49 prior to emergence over time of new lesions and attacks leading to a diagnosis of MS.
MS in Asian and Latin American Populations
Among Asian patients with CNS inflammatory demyelinating disease, a phenotype characterized by NMO, longitudinally extensive spinal cord lesions, and positive AQP4 autoantibody seropositivity19 has been relatively more common than in Western populations.55–57 The Panel solicited input on use of the McDonald Criteria in Asia and Latin America, where there is evidence of a similar phenotype distinction.41 Although the McDonald Criteria are widely used in these parts of the world, there is some uncertainty, especially in Asia, about whether MS and NMO are distinct and if so, how they should be distinguished.39 As currently applied, the term opticospinal MS appears to be an admixture of conventional MS and NMO. Confusion has arisen (1) because of the recognition that most cases of NMO are relapsing; (2) because AQP4 autoantibody testing has facilitated the diagnosis of NMO and permitted inclusion of individuals with symptomatic brain lesions who would previously have been excluded; and (3) because of the recognition that selective involvement of optic nerve and spinal cord alone does not differentiate NMO from MS.58 It is insufficient to make a diagnosis of NMO in the absence of the required specificity criteria of the revised Wingerchuk Criteria for “definite” NMO, which recommend presence of optic neuritis, acute myelitis, and at least 2 of 3 supportive paraclinical assessments (a contiguous spinal cord lesion at least 3 segments in length, brain MRI at onset that is nondiagnostic for MS, or NMO-IgG seropositivity).59 These criteria are successful in most instances to distinguish NMO from MS in patients with optic neuritis and myelitis, but the spectrum of NMO includes recurrent myelitis and optic neuritis, NMO syndromes with symptomatic brain lesions at presentation, and NMO associated with systemic autoimmune diseases.60 Failure to make the correct diagnosis in patients with NMO may impact treatment.20
The Panel recommends testing for AQP4 autoantibodies with validated assays in patients who are suspected of having NMO or NMO spectrum disorders, especially in patients with Asian or Latin American genetic background because of the higher prevalence of the disease in these populations. Such testing may be less important in those subjects presenting with conventional Western type MS. Although not all patients with an NMO-like presentation will be AQP4 antibody positive, the majority are, whereas those with MS are more likely to be AQP4 antibody negative.16,56,61 Current evidence suggests that once NMO and NMO spectrum disorders have been excluded, Western type MS in Asia or Latin America is not fundamentally different from typical MS in the Caucasian population, and that the MAGNIMS MRI criteria would apply for such patients, although confirmatory studies should be done.
The McDonald Criteria: 2010 Revisions
Application of the Criteria
The Panel recommends revisions to the McDonald Criteria for diagnosis of MS (Table 4) focusing specifically on requirements to demonstrate DIS, DIT, and on diagnosis of PPMS. These 2010 revisions to the McDonald Criteria are likely to be applicable in pediatric, Asian, and Latin American populations once careful evaluation for other potential explanations for the clinical presentation is made. The predictive validity of DIS and DIT based on a single first scan in children with CIS needs to be confirmed in prospective studies. The McDonald Criteria have not yet been validated in Asian and Latin American populations, and studies need to be done to confirm the sensitivity and specificity of the Criteria in such patients. Care must be taken to exclude NMO as a differential diagnosis, which can be confounded by the imperfect sensitivity of AQP-4 autoantibody assays, the presence of brain lesions in NMO, and the difficulty of detecting long spinal cord lesions in immunosuppressed patients.
TABLE 4.
The 2010 McDonald Criteria for Diagnosis of MS

Future Directions
Potential Added Value of Biomarkers
Although increased IgG index or the presence of oligoclonal bands in the CSF support an MS diagnosis, and AQP4 antibody assays can help in the differential diagnosis process, there are still no specific biomarkers to confirm the diagnosis. Several blood and CSF biomarkers may be promising,62–65 and high-resolution spectral domain optical coherence tomography might be as good as VEP in assessing visual involvement.66 The diagnostic utility of such markers needs to be validated and tested prospectively.
Refinements in Imaging Criteria
The McDonald Criteria were based on detection of lesions generally using 1.5T magnet strength in noncortical regions of the brain and spinal cord. However, a large proportion of MS lesions are in the cortex67,68 and can be detected using double inversion recovery imaging.69–74 The presence of at least 1 intracortical lesion in subjects with CIS may help identify subjects at high risk for developing CDMS.75 Magnet strengths >1.5T with tailored acquisition protocols76–79 may also enhance diagnosis, with improvements in image resolution, signal-to-noise ratio, and chemical shift. Scans at 7.0T showed lesions in the white and gray matter with enhanced in vivo detection of pathological hallmarks of MS lesions.80–83 Finally, MRI techniques such as magnetic transfer imaging allow the detection of damage outside focal lesions (for instance, in normal-appearing brain tissues) not present in conditions such as ADEM and NMO.15,84,85 The utility of these scanning technologies for MS diagnosis in patients with CIS remains a matter for future research and validation.
Many individuals with high lesion loads may have had a protracted subclinical disease course prior to their first clinical event. As a consequence, occasional individuals investigated by MRI for indications unrelated to MS have incidental findings of brain lesions with appearance and topography consistent with MS. Detection of this presymptomatic phase, or radiologically isolated syndrome, is increasingly common. Some of these individuals followed clinically and by serial imaging will develop DIT by MRI, and some have clinical disease-defining events after several years.86–89 However, in the absence of supportive research findings, the Panel concluded that a firm diagnosis of MS based on incidental findings on MRI alone, even with additional supportive findings on evoked potentials or typical CSF findings in the absence of MS-relevant clinical symptoms, is problematic. A future definite diagnosis of MS, however, cannot be excluded and may be likely, depending on the evolution of neurologic symptoms and signs.
Conclusions
The 2010 revisions to the McDonald Criteria will in some instances allow a more rapid diagnosis of MS, with equivalent or improved specificity and/or sensitivity compared with past Criteria and will in many instances clarify and simplify the diagnostic process with fewer required MRI examinations. A proportion of patients with nonspecific symptoms (eg, fatigue, weakness, or dizziness) and nonspecific MRI findings are referred to secondary and tertiary MS centers in the developed world for a second opinion and do not in fact have MS.90 These revised McDonald Criteria for MS diagnosis should therefore be applied only when patients have experienced a typical CIS (or progressive paraparesis/cerebellar/cognitive syndrome in the case of suspected PPMS).
The Panel acknowledges that using these refined diagnostic criteria may change some of the outcomes of patients in natural history studies and clinical trials, when original expectations for outcomes may be based on subjects whose diagnosis was made using past, somewhat different criteria.91 Most of the currently recommended revisions are based upon new data generated since the 2005 revisions. However, there remains a need for further testing in prospective and retrospective datasets of many of these criteria, especially in populations of patients typical of those seen in general neurology practices, both to further assess their value and utility and to provide suggestions for further refinements in the future.
Potential Conflicts of Interest
C.H.P.: consultancy, Actelion, Biogen Idec, Bayer Schering, Teva, Merck-Serono, Novartis, Glaxo SK, UCB, Roche, Antisense Ther; expert testimony, Biogen Idec; grants/grants pending, Biogen Idec, Bayer Schering, Teva, Merck-Serono, Novartis, Glaxo SK, UCB. S.C.R.: travel support, US NMSS, ECTRIMS, Multiple Sclerosis International Federation, MS Ireland; payment for writing or reviewing manuscript, US NMSS, ECTRIMS; consultancy, US NMSS, ECTRIMS, Sanofi-Aventis, Bayer Schering Pharma, BioMarin, EMD Merck Serono, Mt Sinai College of Medicine (New York, NY), European Committee for Treatment and Research in MS, Eisai, INC Research, Eli Lilly Inc, Isis Pharmaceuticals Inc, MediciNova, Cleveland Clinic Foundation, Free University Amsterdam, Genentech/F. Hoffmann-LaRoche, Synthon BV, Antisense Therapeutics Ltd, BaroFold, Protein Design Laboratories; royalties, Demos Medical Publishers (New York, NY). B.B.: travel support, US NMSS, ECTRIMS, MSIF, MS Ireland; consultancy, Biogen Idec, Genzyme; grants/grants pending, Multiple Sclerosis Society of Canada, Canadian Institutes of Health Research; paid educational presentations, honoraria for symposia at the American Academy of Neurology. M.C.: board membership, GENMAB; consultancy, Biogen, Genzyme; grants/grants pending, Bayer Schering, Biogen Elan, Novartis, Merck Serono, Sanofi-Aventis, Teva. J.A.C.: travel expenses, US NMSS; consultancy, Biogen Idec, Lilly, Novartis, Serono, Teva; grants/grants pending, Department of Defense, NIH, US NMSS; speaking fees, Biogen Idec, Novartis, Sanofi-Aventis, Waterfront Media. M.F.: travel expenses, US NMSS, ECTRIMS, MSIF, MS Ireland; board membership, Teva Pharmaceutical Industries Ltd, Genmab A/S; consultancy, Bayer Schering Pharma, Biogen-Dompé AG, Genmab A/S, Merck Serono, Pepgen Corporation, Teva Pharmaceutical Industries Ltd; grants/grants pending, Bayer-Schering, Biogen-Dompé AG, Genmab A/S, Merck Serono, Teva Pharmaceutical Industries Ltd, Fondazione Italiana Sclerosi Multipla, Fondazione Mariani; speaking fees, Bayer Schering Pharma, Biogen-Dompé AG, Genmab A/S, Merck Serono, Teva Pharmaceutical Industries Ltd; travel expenses, Teva, Biogen-Dompé AG, Merck-Serono, Sanofi-Aventis, Genmab, Bayer Schering. K.F.: travel expenses, US NMSS, ECTRIMS, MSIF, MS Ireland; consultancy, Bayer Schering Pharma, Biogen Idec, Merck Serono; grants/grants pending, Bayer Schering Pharma, Biogen Idec, Asahi Kasei Kuraray Medical Co Ltd, Chemo-Sero-Therapeutic Research Institute, Mitsubishi Tanabe Pharma, Teijin Pharma, Theva Pharmaceutical, Eisai Inc, Kowa Pharmaceutical, Ministry of Education, Science, and Technology of Japan, Ministry of Health, Labor, and Welfare of Japan; speaking fees, Bayer Schering Pharma, Biogen Idec, Eisai Inc, Mitsubishi Tanabe Pharma, Astellas Pharma, Takeda Pharmaceutical Company Ltd, Asahi Kasei Kuraray Medical Co; paid manuscript preparation, Cosmic Corporation; royalties, Bunkodo. E.H.: travel expenses, US NMSS, ECTRIMS, MSIF, MS Ireland; consultancy, Biogen Idec, Genzyme, Merck Serono, Novartis, Grifols; grants/grants pending, Biogen Idec; speaking fees, Biogen Idec, Genzyme, Merck Serono, Novartis, Bayer Healthcare, Sanofi-Aventis; paid educational presentations, Novartis. M.H.: consultancy, Biogen Idec; grant/grants pending, Health Research Board Ireland; speaking fees, Biogen Idec. L.K.: travel expenses, US NMSS; board membership, Editorial Board of Multiple Sclerosis; grants/grants pending, National Research Foundation Switzerland, Rubatto Foundation, Swiss MS society, European Union, Roche Foundation, Novartis Foundation; speaking fees, various companies involved in development of MS therapeutics; paid educational presentations, Neurostatus System for Standardized Neurological Assessment. F.D.L.: travel support, US NMSS; consultancy, Novartis, Bayer, Biogen Idec, EMD Serono, Genentech, Teva Neuroscience, Genmab, Medicinova, Actelion, Allozyne, Sanofi-Aventis, Acorda, Questcor, Avanir, Roche, Celgene, Abbott, Pfizer, Morphosys; grants/grants pending, NIH, NMSS, Acorda, Biogen Idec, Teva, Novartis, Sanofi-Aventis; speaking fees, Genzyme, Teva, EMD Serono; paid educational presentations, various continuing medical education services; stock/stock options, cognition pharmaceuticals. X.M.: travel expenses, US NMSS; consultancy, Bayer Schering Pharma, Biogen Idec, EMD Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceuticals, Almirall; grants/grants pending, Bayer Schering Pharma, Biogen Idec, EMD Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceuticals, Almirall; speaking fees, Bayer Schering Pharma, Biogen Idec, EMD Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceuticals, Almirall. M.S.-W.: travel support, US NMSS, ECTRIMS, MSIF, MS Ireland; fees for review activities, Genentech, Merck Serono, Roche; board membership, Board of Directors of Active Biotech, Sweden; consultancy, Elan, Merck Serono; speaking fees, Bayer Health Care, Merck Serono, Serono Symposia International Foundation, Sanofi-Aventis, Swedish Bank SEB. A.J.T.: travel support, US NMSS, ECTRIMS, MSIF, MS Ireland; board membership, National Hospital Development Foundation, Patrick Berthoud Charitable Trust; consultancy, Weleda AG/Society for Clinical Research, Medical Research Council, MS Society of Great Britain, Merck Serono, Biogen Idec, DIGNA Biotech, Novartis, Eisai London Research Laboratories, Teva Pharmaceuticals; grants/grants pending, National Institute for Health Research, MS Society of Great Britain; speaking fees, Serono Symposia, Sanofi-Aventis; travel expenses, MS International Federation, US NMSS, Biogen Idec; honoraria, Editor-in-Chief of Multiple Sclerosis. E.W.: consultancy, Roche, Actelion; grants/grants pending, US NMSS, NIH; speaking fees, Teva; received free drug for a trial given by Sanofi-Aventis and Biogen Idec. B.W.: travel support, US NMSS; European Committee for Treatment of MS; MS International Foundation; MS Ireland; consultancy, Novartis, Biogen Idec; employment, Mayo Clinic; royalties, RSR Ltd. J.S.W.: travel support, US NMSS, ECTRIMS, MSIF, MS Ireland; board membership, Antisense Therapeutics Ltd, BCDecker, Novartis Pharmaceuticals, Sanofi-Aventis, Teva Pharmaceuticals, Eli Lilly, UCB; consultancy, Genentech, Novartis Pharmaceuticals, Sanofi-Aventis, Teva Neuroscience, Teva Pharmaceuticals, Acorda, Acetilon, Bayer HealthCare, Facet Biotech, Peptimmune; grants/grants pending, NIH, Sanofi-Aventis, Clayton Foundation for Research, US NMSS; honoraria for lectures, Consortium MS Centers, Sanofi-Aventis New Zealand, Sterling Meeting Services, USF Health Professionals, Texas Neurological Society, Teva Pharmaceuticals, Lone Star Chapter NMSS, ICHE, Pfizer EMD Serono, SUNY, Stony Brook Foundation, UTMB, Medscape CME, University of Buffalo, Serono Symposia International Foundation, University of Utah; royalties, Millipore (Chemicon International) Corporation.
Acknowledgments
The work of the Panel was supported by the US National Multiple Sclerosis Society (NMSS), the European Committee for Treatment and Research in Multiple Sclerosis, the Multiple Sclerosis International Federation, and MS Ireland.
The Panel thanks Drs T. Saida, M. Lana-Peixoto, D. Callegaro, and C. Oehninger for help in gaining perspective on the use of the McDonald Criteria in Asia and Latin America.
This work is dedicated to the memory of Dr W. Ian McDonald, who chaired the original Panel and whose continuing inspiration has driven the work for the second and third revisions to the Criteria that now bear his name.
References
- 1.Schumacher FA, Beeve GW, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 1965;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. [DOI] [PubMed] [Google Scholar]
- 2.Poser CM, Paty DW, Scheinberg LC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;1:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 3.Paty DW, Oger JJ, Kastrukoff LF, et al. MRI in the diagnosis of MS: a prospective study with comparison of clinical evaluation, evoked potentials, oligoclonal banding and CT. Neurology. 1988;38:180–185. doi: 10.1212/wnl.38.2.180. [DOI] [PubMed] [Google Scholar]
- 4.Barkhof F, Filippi M, Miller DH, et al. Comparison of MR imaging criteria at first presentation to predict conversion to clinically definite MS. Brain. 1997;120:2059–2069. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- 5.Fazekas F, Offenbacher H, Fuchs S, et al. Criteria for an increased specificity of MRI interpretation in elderly subjects with suspected multiple sclerosis. Neurology. 1988;38:1822–1825. doi: 10.1212/wnl.38.12.1822. [DOI] [PubMed] [Google Scholar]
- 6.Tintoré M, Rovira A, Martinéz M, et al. Isolated demyelinating syndromes: comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. Am J Neuroradiol. 2000;21:702–706. [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson AJ, Montalban X, Barkhof F, et al. Diagnostic criteria for primary progressive multiple sclerosis: a position paper. Ann Neurol. 2000;6:831–835. [PubMed] [Google Scholar]
- 8.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 9.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 10.Dalton CM, Brex PA, Miszkiel KA, et al. Application of the new McDonald criteria to patients with clinically isolated syndromes suggestive of multiple sclerosis. Ann Neurol. 2002;52:47–53. doi: 10.1002/ana.10240. [DOI] [PubMed] [Google Scholar]
- 11.CHAMPS Study Group. MRI predictors of early conversion to clinically definite MS in the CHAMPS placebo group. Neurology. 2002;59:998–1005. doi: 10.1212/wnl.59.7.998. [DOI] [PubMed] [Google Scholar]
- 12.Barkhof F, Rocca M, Francis G, et al. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon beta-1a. Ann Neurol. 2003;53:718–724. doi: 10.1002/ana.10551. [DOI] [PubMed] [Google Scholar]
- 13.Tintoré M, Rovira A, Rio J, et al. New diagnostic criteria for multiple sclerosis. Application in first demyelinating episode. Neurology. 2003;60:27–30. doi: 10.1212/wnl.60.1.27. [DOI] [PubMed] [Google Scholar]
- 14.Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation.”. Lancet Neurol. 2006;10:841–852. doi: 10.1016/S1474-4422(06)70572-5. [DOI] [PubMed] [Google Scholar]
- 15.Miller DH, Weinshenker BG, Filippi M, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler. 2008;14:1157–1174. doi: 10.1177/1352458508096878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2012. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 17.Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu J, Hatanaka Y, Hasegawa M, et al. IFNβ-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology. 2010;75:1423–1427. doi: 10.1212/WNL.0b013e3181f8832e. [DOI] [PubMed] [Google Scholar]
- 19.Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
- 20.Carroll WM, Fujihara K. Neuromyelitis optica. Curr Treat Options Neurol. 2010;12:244–255. doi: 10.1007/s11940-010-0071-z. [DOI] [PubMed] [Google Scholar]
- 21.Hawkes CH, Giovannoni G. The McDonald Criteria for multiple sclerosis: time for clarification. Mult Scler. 2010;16:566–575. doi: 10.1177/1352458510362441. [DOI] [PubMed] [Google Scholar]
- 22.Swanton JK, Rovira A, Tintoré M, et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a multicentre retrospective study. Lancet Neurol. 2007;6:677–686. doi: 10.1016/S1474-4422(07)70176-X. [DOI] [PubMed] [Google Scholar]
- 23.Rovira A, Swanton J, Tintoré M, et al. A single, early magnetic resonance imaging study in the diagnosis of multiple sclerosis. Arch Neurol. 2009;5:287–292. doi: 10.1001/archneurol.2009.49. [DOI] [PubMed] [Google Scholar]
- 24.Montalban X, Tintoré M, Swanton J, et al. MRI criteria for MS in patients with clinically isolated syndromes. Neurology. 2010;74:427–434. doi: 10.1212/WNL.0b013e3181cec45c. [DOI] [PubMed] [Google Scholar]
- 25.McHugh JC, Galvin PL, Murphy RP. Retrospective comparison of the original and revised McDonald Criteria in a general neurology practice in Ireland. Mult Scler. 2008;14:81–85. doi: 10.1177/1352458507081169. [DOI] [PubMed] [Google Scholar]
- 26.Albertyn C, O'Dowd S, McHugh J, Murphy R. Compliance with McDonald criteria and red flag recognition in a general neurology practice in Ireland. Mult Scler. 2010;16:678–684. doi: 10.1177/1352458510368688. [DOI] [PubMed] [Google Scholar]
- 27.Swanton JK, Fernando K, Dalton CM, et al. Modification of MRI criteria for multiple sclerosis in patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry. 2006;77:830–833. doi: 10.1136/jnnp.2005.073247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tur C, Tintoré M, Rovira A, et al. Very early scans for demonstrating dissemination in time in multiple sclerosis. Mult Scler. 2008;14:631–635. doi: 10.1177/1352458507087327. [DOI] [PubMed] [Google Scholar]
- 29.Heun R, Kappos L, Bittkau S, et al. Magnetic resonance imaging and early diagnosis of MS. Lancet. 1988;2:1202–1203. doi: 10.1016/s0140-6736(88)90277-2. [DOI] [PubMed] [Google Scholar]
- 30.Tas MW, Barkhof F, van Walderveen MA, et al. The effect of gadolinium on the sensitivity and specificity of MR in the initial diagnosis of multiple sclerosis. Am J Neuroradiol. 1995;16:259–264. [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman MS, Thompson EJ, Deisenhammer F, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol. 2005;62:865–870. doi: 10.1001/archneur.62.6.865. [DOI] [PubMed] [Google Scholar]
- 32.Masiuan J, Alvarez-Cermeño JC, García-Barragán N, et al. Clinically isolated syndromes: a new oligoclonal band test accurately predicts conversion to MS. Neurology. 2006;66:576–578. doi: 10.1212/01.wnl.0000198253.35119.83. [DOI] [PubMed] [Google Scholar]
- 33.Tintoré M, Rovira A, Río J, et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology. 2008;70:1079–1083. doi: 10.1212/01.wnl.0000280576.73609.c6. [DOI] [PubMed] [Google Scholar]
- 34.Polman CH, Wolinsky JS, Reingold SC. Multiple sclerosis diagnostic criteria: three years later. Mult Scler. 2005;11:5–12. doi: 10.1191/1352458505ms1135oa. [DOI] [PubMed] [Google Scholar]
- 35.Hawker K, O'Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 36.Montalban X, Sastre-Garriga J, Filippi M, et al. Primary progressive multiple sclerosis diagnostic criteria: a reappraisal. Mult Scler. 2009;15:1459–1465. doi: 10.1177/1352458509348422. [DOI] [PubMed] [Google Scholar]
- 37.Absinta MA, Rocca MA, Moiola L, et al. Brain macro- and microscopic damage in patients with pediatric MS. J Neuro Neurosurg Psychiatry. 2010;81:1357–1362. doi: 10.1136/jnnp.2010.205682. [DOI] [PubMed] [Google Scholar]
- 38.Callen DJA, Shroff MM, Branson HM, et al. MRI in the diagnosis of pediatric multiple sclerosis. Neurology. 2009;72:961–967. doi: 10.1212/01.wnl.0000338629.01627.54. [DOI] [PubMed] [Google Scholar]
- 39.Chong HT, Kira J, Tsai CP, et al. Proposed modifications to the McDonald criteria for use in Asia. Mult Scler. 2009;15:887–888. doi: 10.1177/1352458509104587. [DOI] [PubMed] [Google Scholar]
- 40.Chong HT, Li PCK, Singhal BJ, et al. Proposed modification to McDonald diagnostic criteria for Asians with multiple sclerosis. Neurol Asia. 2006;11:87–90. [Google Scholar]
- 41.Lana-Piexoto MA. Devic's neuromyelitis optica: a critical review. Arq Neuropsiquiatr. 2008;66:120–138. doi: 10.1590/s0004-282x2008000100034. [DOI] [PubMed] [Google Scholar]
- 42.Yeh EA, Chitnis T, Krupp L, et al. Pediatric multiple sclerosis. Nat Rev Neurol. 2009;5:621–631. doi: 10.1038/nrneurol.2009.158. [DOI] [PubMed] [Google Scholar]
- 43.Banwell B, Ghezzi A, Bar-Or A, et al. Multiple sclerosis in children: clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007;6:887–902. doi: 10.1016/S1474-4422(07)70242-9. [DOI] [PubMed] [Google Scholar]
- 44.Venkateswaran S, Banwell B. Pediatric multiple sclerosis. Neurologist. 2010;16:92–105. doi: 10.1097/NRL.0b013e3181c923d5. [DOI] [PubMed] [Google Scholar]
- 45.Hahn JS, Pohl D, Rensel M, et al. Differential diagnosis and evaluation in pediatric multiple sclerosis. Neurology. 2007;68(suppl 2):S13–S22. doi: 10.1212/01.wnl.0000259403.31527.ef. [DOI] [PubMed] [Google Scholar]
- 46.Ghassemi R, Antel SB, Narayanan S, et al. Lesion distribution in children with clinically isolated syndromes. Ann Neurol. 2008;63:401–405. doi: 10.1002/ana.21322. [DOI] [PubMed] [Google Scholar]
- 47.Waubant E, Chabas D, Okuda DT, et al. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch Neurol. 2009;66:967–971. doi: 10.1001/archneurol.2009.135. [DOI] [PubMed] [Google Scholar]
- 48.Yeh EA, Weinstock-Guttman B, Ramanathan M, et al. Magnetic resonance imaging characteristics of children and adults with paediatric-onset multiple sclerosis. Brain. 2009;132(pt 12):3392–3400. doi: 10.1093/brain/awp278. [DOI] [PubMed] [Google Scholar]
- 49.Chabas D, Castillo-Trivino T, Mowry EM, et al. Vanishing MS T2-bright lesions before puberty. Neurology. 2008;71:1090–1093. doi: 10.1212/01.wnl.0000326896.66714.ae. [DOI] [PubMed] [Google Scholar]
- 50.Mikaeloff Y, Adamsbaum C, Husson B, et al. MRI prognostic factors for elapse after acute CNS inflammatory demyelination in childhood. Brain. 2004;127:1942–1947. doi: 10.1093/brain/awh218. [DOI] [PubMed] [Google Scholar]
- 51.Ketelslegers IA, Neuteboom RF, Boon M, et al. A comparison of MRI criteria for diagnosing pediatric ADEM and MS. Neurology. 2010;74:1412–1415. doi: 10.1212/WNL.0b013e3181dc138b. [DOI] [PubMed] [Google Scholar]
- 52.Verhey L, Branson HM, Makhija M, et al. Magnetic resonance imaging features of the spinal cord in pediatric multiple sclerosis: a preliminary study. Neuroradiology. 2010;52:1153–1162. doi: 10.1007/s00234-010-0755-9. [DOI] [PubMed] [Google Scholar]
- 53.Krupp LB, Banwell B, Tenembaum S, et al. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(suppl 2):S7–S12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 54.Callen DJA, Shroff MM, Branson HM, et al. Role of MRI in the differentiation of ADEM from MS in children. Neurology. 2009;72:968–973. doi: 10.1212/01.wnl.0000338630.20412.45. [DOI] [PubMed] [Google Scholar]
- 55.Matsuoka T, Matsushita T, Kawano Y, et al. Heterogeneity of aquaporin-4 autoimmunity and spinal cord lesions in Japanese. Brain. 2007;130:1206–1223. doi: 10.1093/brain/awm027. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 57.Osoegawa M, Kira J, Fukazawa T, et al. Temporal changes and geographic differences in multiple sclerosis phenotypes in Japanese: nationwide survey results over 30 years. Mult Scler. 2009;15:159–173. doi: 10.1177/1352458508098372. [DOI] [PubMed] [Google Scholar]
- 58.Nakashima I, Fukazawa T, Ota K, et al. Two subtypes of optic-spinal form of multiple sclerosis in Japan: clinical and laboratory features. J Neurol. 2007;254:488–492. doi: 10.1007/s00415-006-0400-z. [DOI] [PubMed] [Google Scholar]
- 59.Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 60.Sato D, Fujihara K. NMO without typical opticospinal phenotype. Mult Scler. 2010;16:1154–1155. doi: 10.1177/1352458510377908. [DOI] [PubMed] [Google Scholar]
- 61.Smith CH, Waubant E, Langer-Gould A. Absence of neuromyelitis optica IgG antibody in an active relapsing-remitting multiple sclerosis population. J Neuroopthalmol. 2009;29:104–106. doi: 10.1097/WNO.0b013e3181a63606. [DOI] [PubMed] [Google Scholar]
- 62.Awad A, Hemmer B, Hartung HP, et al. Analysis of cerebrospinal fluid in the diagnosis and monitoring of multiple sclerosis. J Neuroimmunol. 2010;219:1–7. doi: 10.1016/j.jneuroim.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Brettschneider J, Jaskowski TD, Tumani H, et al. Serum anti-GAGA4 IgM antibodies differentiate relapsing remitting and secondary progressive multiple sclerosis from primary progressive multiple sclerosis and other neurological diseases. J Neuroimmunol. 2009;217:95–101. doi: 10.1016/j.jneuroim.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 64.Quintana FJ, Farez MF, Viglietta V, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci U S A. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keller A, Leidinger P, Lange J, et al. Multiple sclerosis: microRNA expression profiles accurately differentiate patients with relapsing-remitting disease from healthy controls. PLoS One. 2009;4:e7440. doi: 10.1371/journal.pone.0007440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frohman EM, Balcer LJ, Calabresi PA. Multiple sclerosis: can retinal imaging accurately detect optic neuritis? Nat Rev Neurol. 2010;6:125–126. doi: 10.1038/nrneurol.2010.13. [DOI] [PubMed] [Google Scholar]
- 67.Bø L, Vedeler CA, Nyland H, et al. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 68.Bø L, Vedeler CA, Nyland HI, et al. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62:723–732. doi: 10.1093/jnen/62.7.723. [DOI] [PubMed] [Google Scholar]
- 69.Geurts JJ, Pouwels PJ, Uitdehaag BM, et al. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology. 2005;236:254–260. doi: 10.1148/radiol.2361040450. [DOI] [PubMed] [Google Scholar]
- 70.Calabrese M, De Stefano N, Atzori M, et al. Detection of cortical inflammatory lesions by double inversion recovery magnetic resonance imaging in patients with multiple sclerosis. Arch Neurol. 2007;64:1416–1422. doi: 10.1001/archneur.64.10.1416. [DOI] [PubMed] [Google Scholar]
- 71.Nelson F, Poonawalla A, Hou P, et al. 3D MPRAGE improves classification of cortical lesions in multiple sclerosis. Mult Scler. 2008;14:1214–1219. doi: 10.1177/1352458508094644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelson F, Poonawalla A, Hou P, et al. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. Am J Neuroradiol. 2007;28:1645–1649. doi: 10.3174/ajnr.A0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calabrese M, Rocca MA, Atzori M, et al. Cortical lesions in primary progressive multiple sclerosis: a 2-year longitudinal MR study. Neurology. 2009;72:1330–1336. doi: 10.1212/WNL.0b013e3181a0fee5. [DOI] [PubMed] [Google Scholar]
- 74.Calabrese M, Rocca MA, Atzori M, et al. A 3-year magnetic resonance imaging study of cortical lesions in relapsing-onset MS. Ann Neurol. 2010;67:376–383. doi: 10.1002/ana.21906. [DOI] [PubMed] [Google Scholar]
- 75.Filippi M, Rocca MA, Calabrese M, et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology. 2010;75:1988–1994. doi: 10.1212/WNL.0b013e3181ff96f6. [DOI] [PubMed] [Google Scholar]
- 76.Wattjes MP, Harzheim M, Lutterbey GG, et al. Does high field MRI allow an earlier diagnosis of multiple sclerosis? J Neurol. 2008;255:1159–1163. doi: 10.1007/s00415-008-0861-3. [DOI] [PubMed] [Google Scholar]
- 77.Wattjes MP, Barkhof F. High field MRI in the diagnosis of multiple sclerosis: high field-high yield? Neuroradiology. 2009;51:279–292. doi: 10.1007/s00234-009-0512-0. [DOI] [PubMed] [Google Scholar]
- 78.Stankiewicz JM, Neema M, Alsop DC, et al. Spinal cord lesions and clinical status in multiple sclerosis: a 1.5 T and 3T MRI study. J Neurol Sci. 2009;279:99–105. doi: 10.1016/j.jns.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wattjes MP, Lutterbey GG, Gieseke J, et al. Double inversion recovery brain imaging at 3T: diagnostic value in the detection of multiple sclerosis. Am J Neuroradiol. 2007;28:54–59. [PMC free article] [PubMed] [Google Scholar]
- 80.Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64:707–713. doi: 10.1002/ana.21582. [DOI] [PubMed] [Google Scholar]
- 81.Mainero C, Benner T, Radding A, et al. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology. 2009;73:941–948. doi: 10.1212/WNL.0b013e3181b64bf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kangarlu A, Bourekas EC, Ray-Chaudhury A, Rammohan KW. Cerebral cortical lesions in multiple sclerosis detected by MR imaging at 8 Tesla. Am J Neuroradiol. 2007;28:262–266. [PMC free article] [PubMed] [Google Scholar]
- 83.Kollia K, Maderwald S, Putzki N, et al. First clinical study on ultra-high-field MR imaging in patients with multiple sclerosis: comparison of 1.5T and 7T. Am J Neuroradiol. 2009;30:699–702. doi: 10.3174/ajnr.A1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Filippi M. In-vivo tissue characterization of multiple sclerosis and other white matter diseases using magnetic resonance based techniques. J Neurol. 2001;248:1019–1029. doi: 10.1007/s004150170020. [DOI] [PubMed] [Google Scholar]
- 85.Rocca M, Agosta F, Sormani MP, et al. A three-year, multi-parametric MRI study in patients at presentation with CIS. J Neurol. 2008;255:683–691. doi: 10.1007/s00415-008-0776-z. [DOI] [PubMed] [Google Scholar]
- 86.LeBrunn C, Bensa C, Debouverie M, et al. Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid and visual evoked potential. Arch Neurol. 2009;66:841–846. doi: 10.1001/archneurol.2009.119. [DOI] [PubMed] [Google Scholar]
- 87.Sellner J, Schirmer L, Hemmer B, Mühlau M. The radiologically isolated syndrome: take action when the unexpected is uncovered? J Neurol. 2010;257:1602–1611. doi: 10.1007/s00415-010-5601-9. [DOI] [PubMed] [Google Scholar]
- 88.Okuda DT, Mowr EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009;72:800–805. doi: 10.1212/01.wnl.0000335764.14513.1a. [DOI] [PubMed] [Google Scholar]
- 89.Siva A, Saip S, Altintas A, et al. Multiple sclerosis risk in radiologically uncovered asymptomatic possible inflammatory-demyelinating disease. Mult Scler. 2009;15:918–927. doi: 10.1177/1352458509106214. [DOI] [PubMed] [Google Scholar]
- 90.Carmosino MJ, Brosseau KM, Arciniegas DB, Corboy JR. Initial evaluations for multiple sclerosis in a university multiple sclerosis center: outcomes and role of magnetic resonance imaging in referral. Arch Neurol. 2005;62:585–590. doi: 10.1001/archneur.62.4.585. [DOI] [PubMed] [Google Scholar]
- 91.Sormani MP, Tintoré M, et al. The Will Rogers phenomenon in multiple sclerosis. Ann Neurol. 2008;64:428–433. doi: 10.1002/ana.21464. [DOI] [PubMed] [Google Scholar]