Bovine Milk as a Source of Functional Oligosaccharides for Improving Human Health (original) (raw)
Abstract
Human milk oligosaccharides are complex sugars that function as selective growth substrates for specific beneficial bacteria in the gastrointestinal system. Bovine milk is a potentially excellent source of commercially viable analogs of these unique molecules. However, bovine milk has a much lower concentration of these oligosaccharides than human milk, and the majority of the molecules are simpler in structure than those found in human milk. Specific structural characteristics of milk-derived oligosaccharides are crucial to their ability to selectively enrich beneficial bacteria while inhibiting or being less than ideal substrates for undesirable and pathogenic bacteria. Thus, if bovine milk products are to provide human milk–like benefits, it is important to identify specific dairy streams that can be processed commercially and cost-effectively and that can yield specific oligosaccharide compositions that will be beneficial as new food ingredients or supplements to improve human health. Whey streams have the potential to be commercially viable sources of complex oligosaccharides that have the structural resemblance and diversity of the bioactive oligosaccharides in human milk. With further refinements to dairy stream processing techniques and functional testing to identify streams that are particularly suitable for enriching beneficial intestinal bacteria, the future of oligosaccharides isolated from dairy streams as a food category with substantiated health claims is promising.
Introduction
Human milk contains a wide variety of bioactive molecules, including Ig and nucleotides. Recently, human milk oligosaccharides (HMO)3 are being recognized as a new class of potent bioactive molecules. HMO consist of a lactose core extensively elongated by β1–3 or β1–6 linkages to lactosamine units and further decorated with fucose or sialic acid residues in terminal positions connected with α1–2, –3, and –4 and α2–3 and –6 linkages, respectively (1–4). The diversity of monosaccharide combinations and linkages results in a structurally complex array of linear and branched oligosaccharide structures. At present, the only source of oligosaccharide structures with the structural complexity of HMO is human milk, a fact that limits the applicability of these protective oligosaccharides in population groups other than breast-fed infants. Presumably, many of the health benefits that milk oligosaccharides provide for infants could also be available to humans of all ages if the same structures and functions could be provided in the diet.
The advantages of HMO are likely related to the structural and functional diversity of multiple components that act in synergy and confer protection to infants. The neutral HMO (containing the monomers _N_-acetylglucosamine and fucose) are considered to be the most relevant factors for the development of the intestinal microbiota typical for breast-fed infants (2) as well as for direct effects on the immune system (5). In fact, the structural specificity for the consumption preferences of different intestinal bacteria has been described (6–8). Recently, Bifidobacterium longum ssp. Infantis (B. infantis), a bifidobacterium enriched in the gastrointestinal tracts of healthy breast-fed infants, was found to have a unique gene cassette that allows it to transport and metabolize the specific oligosaccharide structures found in human milk (9), arguing for a specific coevolutionary relationship between this unique bacterium and the infant (10). The acidic oligosaccharides (decorated with the monomer sialic acid), on the other hand, play an important role in the prevention of adhesion of pathogenic bacteria to the epithelial surface (11) and have recently also been found to be metabolized by B. infantis (8).
Various strategies have been used to mimic the structural complexity of HMO; much simpler structures, including fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS), so far have been used in dietary products. These simple structures possess a stimulating prebiotic effect that increases the bacterial counts of bifidobacteria and lactobacilli; however, this effect has been inconsistent, with 16 studies showing a bifidogenic effect and 4 studies showing no effect in infants [reviewed in (12)]. FOS and GOS also mimic some but not all of the other functions of HMO, including SCFA production, pathogen blocking, and immune modulation [reviewed in (12)]. Additionally, these simpler oligosaccharides do not contain the structural complexity and diversity of HMO; thus, better sources of complex oligosaccharides that more closely mimic the structures and functions of HMO are needed to improve upon the existing supplementation strategy.
Recent research has demonstrated that bovine milk contains oligosaccharides that are analogous to HMO, suggesting a similar protective role (13–15). The oligosaccharides found in bovine milk (BMO) are structurally similar to those in human milk, but their concentration in milk is low, particularly in mature milk compared with early milk (i.e. colostrum) (15). Both human and bovine milk contain large amounts of the acidic oligosaccharides known as sialyloligosaccharides, especially at the early lactation stage (15, 16). Because mature bovine milk contains only trace amounts of these valuable components, up to now it has not been considered a viable source of oligosaccharides for human supplementation. In addition, the evaluation of BMO as a substitute for HMO has been hindered by the lack of precise analytical methods to accurately characterize and quantify these oligosaccharides. Recently, a high-throughput strategy to annotate the human and bovine milk glycomes by using high accuracy MS has been developed (17). Using these novel techniques, dairy streams including whey permeate from cheese production have been identified as novel sources of oligosaccharides that mimic HMO (18).
This review will describe the current status of knowledge of the effects of HMO on human health, give a brief overview of the structures and available sources of oligosaccharides, and then focus on bovine milk and dairy streams as a source for functional oligosaccharides that mimic the beneficial effects of HMO.
Analysis of oligosaccharides
As a result of the heterogeneity of oligosaccharides, technologies for their characterization have lagged far behind technologies for nucleic acids and proteins. In recent years, MS has become an essential tool for the analysis of oligosaccharides because of the breadth of information obtained with high resolution and sensitive instrumentation. The use of Fourier transform ion cyclotron resonance (FT-ICR) in the detection of oligosaccharides recently has been reviewed (19). Several features of FT-ICR make it ideal for oligosaccharide analysis: the high resolution and mass accuracy readily yields the composition in terms of numbers of fucose, glucose or galactose, _N_-acetylglucosamine, and sialic acid without the need for exhaustive and expensive derivatization methods. The 2 most useful ionization techniques for analyzing oligosaccharides, electrospray ionization and matrix-assisted laser desorption/ionization, can now be performed in FT-ICR, allowing for maximum detection of both neutral and acidic oligosaccharides. The availability of reliable tandem MS techniques such as collision-induced dissociation and infrared multiphoton dissociation combined with any ionization method in FT-ICR make it a vital toolset in acquiring detailed information about glycan structures (19).
The systematic examination of oligosaccharides in bovine milk and dairy streams can also now be accomplished using newly introduced methodologies such as microchip liquid chromatography separation and high performance MS techniques, including time-of-flight and quadrupole time-of-flight analyzers (3, 4). Currently only a few commercial standards are available for bovine oligosaccharides; therefore, to date, the highest number of oligosaccharides identified in both human milk (over 70 fully annotated HMO) (3, 4) and bovine milk (40 BMO) (14, 15, 18) has been obtained using a combination of FT-ICR MS, enzymatic digestion, and HPLC-chip/time-of-flight MS techniques.
Current status of knowledge
Benefits of HMO
Because they pass undigested through the upper intestine, HMO arrive intact in the colon, where they act as prebiotics by supporting the growth and establishment of commensal or beneficial gastrointestinal microbiota (2, 6, 9, 20). HMO may also be absorbed to a small extent in the gastrointestinal tract (2, 21), and a range of immunomodulatory effects have been proposed in mucosal and metabolic tissues: oligosaccharides inhibit immune cell recruitment in the lung (22), reduce allergic reaction in the skin (23), inhibit immune cell recruitment and adhesion in endothelial cells (24), and stimulate the production of cytokines in blood-borne immune cells (5). Oligosaccharides can also be taken up directly from the gut lumen by dendritic cells (25), with many potential downstream effects due to the many known immune-regulating functions of dendritic cells (26). HMO interact directly with gut epithelial cells (27) and enteroendocrine cells. Finally, HMO have known antipathogenic effects and prevent infection and adhesion of both pathogenic bacteria such as enterotoxigenic Escherichia coli (28) and viruses such as HIV (29). Thus, HMO provide a spectrum of protective and immuno-modulatory functions mediated via either their prebiotic role in enriching specific beneficial microbiota or direct interaction with the epithelia and the immune system.
Milk oligosaccharides as prebiotics
The importance of prebiotics, or ingredients that are selectively fermented by and modify the intestinal microbiota, in maintaining gut health and human health in general has gained increasing interest (30). Prebiotics enhance the growth of bifidobacteria and lactobacilli at the expense of other groups of potentially harmful bacteria such as clostridia, enterococci, eubacteria, enterobacteria, and others. Therefore, a diet with significant amounts of selective prebiotics would selectively feed the saccharolytic bacteria (bifidobacteria and lactobacilli), allowing them to dominate the gut and compete with potentially harmful bacteria by creating an acidic environment that is less favorable to pathogens (31–34).
The use of dietary prebiotics is a practical and efficient way to manipulate the gut microbiota. The microbial colonization of the human intestine commences at birth, when the newborn is exposed to an external environment rich in different bacteria. The first bacteria to colonize the infant’s gut are important in determining the ultimate gut microbial composition (35). These colonizing bacteria affect the immune response (36), making the intestinal environment more favorable to their own survival and less favorable to competing microbial species (37). Evidence is increasingly assembling that a healthy infant microbiota is mainly composed of bifidobacteria and lactobacilli; this composition has been proposed to be important for the development of a fully functional immune system (38). Alterations in the infant gut microbiota from this composition have been associated with allergy development and other diseases. There have been several reports of a direct correlation between exposure to Staphylococcus aureus and clostridia in early infancy and allergy development by the age of 2 y (39–41).
The intestinal microbiota of infants exclusively fed breast milk contains up to 90% bifidobacteria, whereas the microbiota of formula-fed infants resembles that of adults (32, 33, 42). How a human milk-specific microbiota dominated by bifidobacteria and lactobacilli is developed and maintained has been a subject of extensive research. HMO fermentation requires the enzymatic ability to degrade the complex HMO structures into their constituent monosaccharides, as well as the ability to transport both residual intact oligosaccharides and the resulting monosaccharides (9, 43, 44). Recent studies suggest that the neutral fraction of HMO containing fucose could play an important role in the development of the typical breast-fed infant microbiota (45).
Protection by milk oligosaccharides against pathogen infection
The intestinal mucosa is the largest surface of the human body and it is among the most heavily glycosylated tissues (39). The mucosa of the intestine is covered with complex glycans, including glycoproteins, glycolipids, mucins, and others (46). The principal function of these glycans is thought to be the mediation of communication with the extracellular environment, including cell-cell communication, molecular discrimination, barrier functions, and diverse signaling actions. To overcome this barrier, the first step of bacterial and viral infection is to recognize and bind specific cell surface glycans of the intestinal mucosa, where sialylated and fucosylated oligosaccharides are the primary targets (12). Because many milk oligosaccharides contain structural units that are homologous to these carbohydrate structures, it has been suggested that they act as soluble receptor analogs that inhibit the adhesion of pathogens, thus preventing infection (47). In fact, HMO are synthesized by the same glycosyl- and fucosyltranferases, enzymes responsible for the formation of glycans present on different cell types (48, 49). Fucosylated and sialylated milk oligosaccharides inhibit the binding of pathogenic bacteria by blocking bacteria from attaching to target oligosaccharides on the intestinal mucosal surface (20, 33). Milk oligosaccharides have adhesion-inhibiting activity for both Gram-negative and Gram-positive bacteria (50).
Plant-based and synthetic sources of oligosaccharides
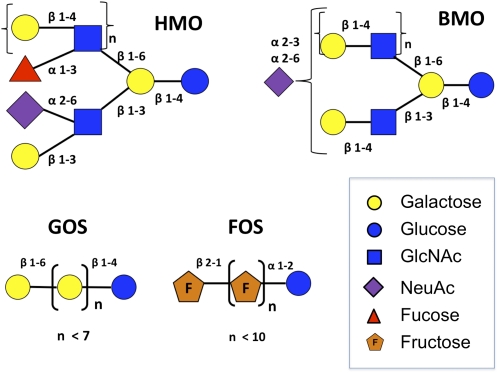
In the absence of a commercial source of the complex structures found in HMO, the infant formula industry uses inulin, FOS, GOS, lactulose, and acidic oligosaccharides derived from pectin as prebiotic oligosaccharides (12). The wide availability of these simple structures has enabled numerous in vitro, human, and animal studies on their putative prebiotic effects. Comprehensive information about FOS and GOS and their clinical and prebiotic dossiers are available in reviews by Roberfroid (30) and Boehm and Moro (12). Although these structures have been shown to be effective in a number of functional endpoints, including reduced rates of atopic dermatitis in supplemented formula-fed infants (23), FOS and GOS are structurally very different from HMO and BMO (Fig. 1). Whereas FOS and GOS are linear chains, HMO and BMO are branched structures and contain the structural elements fucose, sialic acid, and _N_-acetylglucosamine that are completely absent in FOS and GOS. Furthermore, they do not exhibit the inherent structural complexity that is characteristic of HMO and BMO that results from the large number of possible β and α glycosidic linkages present in these oligosaccharides.
Figure 1.
Schematics of representative structures of HMO, BMO, GOS, and FOS. Linkages between monosaccharides are also shown and include the following: α1–2, α1–3, α2–3, α2–6, β1–3, β1–4, β1–6, and β2–1. The HMO structures can be elongated by repeating units of lactosamine (GlcNAc and galactose) and further decorated by sialic acid and fucose. The BMO structures can be further elongated with residues of GlcNAc, galactose, and sialic acids. Whereas characteristic HMO and BMO structures are branched and display a variety of α and β linkages, GOS and FOS are linear chains containing repeating units of galactose and fructose, respectively (depicted in brackets with a subscript of n < 7 and n < 10 repeating units, respectively).
BMO
Bovine milk, and in particular colostrum, is a source of simple as well as complex oligosaccharides that resemble HMO (14–16) (Fig. 1). Twenty-four different acidic structures containing _N_-acetylhexosamine, _N_-acetylneuraminic acid (NeuAc), and _N_-glycolylneuraminic acid (NeuGc) or sialic acid, as well as 16 neutral oligosaccharide structures, have been identified in the colostrum of Holstein-Friesian cows (16).
Because fluid bovine milk contains only trace amounts of these valuable components, the use of dairy streams, in particular whey permeate, for large-scale extraction has been the subject of recent investigation. The attraction of specific whey fractions stems from their wide availability and low cost compared to other dairy streams. Whey permeate is a by-product obtained when cheese whey is passed through an ultrafiltration membrane to concentrate whey protein. Whey proteins are retained by the membrane, whereas smaller molecules such as lactose and salts pass through the membrane making up the whey permeate. The composition of a variety of neutral and acidic oligosaccharides in whey permeate, of which many had identical composition to HMO, has recently been described (18). Of the 15 acidic oligosaccharide structures identified in whey permeate, 3 are also found in HMO, and of the 8 neutral oligosaccharide structures identified, 4 are also found in HMO (18).
One further differentiating factor of oligosaccharides derived from whey permeate compared with bovine milk is that whereas bovine milk was found to contain 7 different NeuGc-containing oligosaccharides, whey permeate only contained 1 such structure (18). One possible reason for this difference in acidic component distribution is the specific lactation stage of the milk used in the analysis. Recent literature has shown that acidic oligosaccharides decrease markedly immediately after the first milking and continue to decrease in the following milkings (16, 51, 52). Tao et al. (15) reported that mature milk (120 d lactation) contained only traces of NeuGc (0.2% of total oligosaccharides), whereas the total neutral oligosaccharide content increased compared with colostrum.
The sialic acid NeuGc is found in all mammals except humans. The lack of this sugar is due to a mutation in the enzyme cytidine monophosphate-NeuAc hydroxylase that occurred in the hominid lineage subsequent to its divergence from the lineage of the great ape about 3 million y ago (53). There is evidence that the lack of production of NeuGc may be involved in resistance to infection by certain microbial pathogens, that immune system receptors that recognize sialic acid residues may be differentially modulated by NeuGc compared with NeuAc, and that dietary sources of NeuGc may lead to its accumulation and immune recognition in certain inflammatory conditions (53). Although there are no clear associations between the consumption of NeuGc and disease risk or incidence, it may be beneficial to reduce NeuGc-containing oligosaccharides in certain sensitive populations. Whey, being the by-product of cheesemaking that uses mature milk, is naturally low in NeuGc.
Preliminary evidence indicates that whey permeate that is further processed by membrane filtration may have as much as 10-fold higher total concentrations of free oligosaccharides than bovine milk. This concentration is considerably higher compared to the current literature in which it is stated that bovine milk only contains a trace amount of oligosaccharides. The concentration of BMO in whey streams can be increased 4-fold compared to whey permeate by removing residual lactose. These enabling discoveries provide promising alternatives for industrial production of oligosaccharides that are functionally parallel to those found in human milk.
Potential health implications of new sources of BMO that mimic the beneficial functions of HMO
A major challenge in isolating oligosaccharides from bovine milk and dairy streams is to enrich the milk oligosaccharides while simultaneously reducing the content of lactose and other simple sugars that do not possess the desired specific prebiotic activities. The residual content of mineral salts may also constitute an obstacle to the application of these ingredients in the infant formula sector and in other health applications. The smallest milk oligosaccharides are now available as a result of improvements in synthetic methods (54), but commercial sources that reproduce the breadth of the variety of milk oligosaccharides are not yet available.
One argument supporting the need for more complex and well-defined oligosaccharide structures than those that are already commercially available (i.e. FOS and GOS) is that the structure-function relationships involved in mediating crucial health effects are highly structure specific. For example, in a recent study that compared the gastrointestinal epithelial transfer and immuno-modulatory properties of acidic oligosaccharides from human milk, cow milk, and pectin, although all of the structures were transported across epithelial cells, only the human milk-derived structures induced atopy-suppressing cytokine production in cord blood-derived monocytes (55). In a mouse model of infection, the specific structures lacto-_N_-fucopentaose III and lacto-_N_-neotetraose abundant in HMO induced production of IL-10, an antiinflammatory cytokine (56). The lacto-_N_-neotetraose structure has also been detected in significant concentrations (10% of total oligosaccharides) in mature bovine milk (15).
Bacterial growth studies in which detailed structural analyses of oligosaccharide consumption were performed by MS analysis revealed strain-specific differences in growth and consumption patterns even among very closely related species. For example, B. infantis reached 4-fold higher cellular density when grown exclusively on purified HMO compared with its close relatives B. breve and B. longum bv. longum (6). Furthermore, whereas B. infantis consumed 64% of the total HMO, including 5 of the most abundant shorter-chain HMO and 3 longer-chain HMO, B. breve and B. longum bv. longum consumed 35 and 24%, respectively, and only one oligosaccharide, lacto-_N_-tetraose (6). Thus, the potential of BMO to serve as a new source of health-promoting functional ingredients that can act as selective prebiotics is enticing precisely because it can provide a mixture of oligosaccharides that mimics the structural complexity of HMO.
Conclusions
There is an unmet need for alternative sources from which to obtain a diverse mixture of complex human milk-like oligosaccharides. The development of commercially viable methods to produce oligosaccharides that mimic the structures and biological functions of HMO presents both a health value and an opportunity to capture value in currently underutilized dairy products. Further processing, enrichment, and purification methods will need to be developed to optimize the oligosaccharide content of bovine milk and bovine dairy streams such that specific structures found in human milk are maximized, whereas those that are not found in human milk are minimized or excluded and additional ingredients such as lactose, minerals, and salts are reduced or eliminated. The evidence to date described in this review highlights the opportunities and challenges for BMO to become a novel source for HMO-mimetic oligosaccharides with the structural diversity and specificity that underlies the numerous health benefits delivered by human milk.
Acknowledgments
D.B. and A.M.Z. wrote the paper. Both authors have primary responsibility for final content.
Footnotes
1
Supported by the California Dairy Research Foundation (10 GEB-02 NH); the University of California Discovery Program (05GEB01NHB); the National Institute of Environmental Health Sciences (P42ES004699); the Dairy Research Institute; and the CHARGE study (P01 ES11269).
2
Author disclosures: A. M. Zivkovic and D. Barile received research funding from the Dairy Research Institute and the California Dairy Research Foundation.
3
Abbreviations used: B. infantis, Bifidobacterium longum ssp. Infantis; BMO, bovine milk oligosaccharide; FOS, fructo-oligosaccharide; FT-ICR, Fourier transform ion cyclotron resonance; GOS, galacto-oligosaccharide; HMO, human milk oligosaccharide; NeuAc, _N_-acetylneuraminic acid; NeuGc, _N_-glycolylneuraminic acid.
Literature Cited
- 1.German JB, Freeman SL, Lebrilla CB, Mills DA. Human milk oligosaccharides: evolution, structures and bioselectivity as substrates for intestinal bacteria. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:205–18, discussion 218–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722 [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res. 2011;10:856–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9:4138–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eiwegger T, Stahl B, Schmitt J, Boehm G, Gerstmayr M, Pichler J, Dehlink E, Loibichler C, Urbanek R, et al. Human milk-derived oligosaccharides and plant-derived oligosaccharides stimulate cytokine production of cord blood T-cells in vitro. Pediatr Res. 2004;56:536–40 [DOI] [PubMed] [Google Scholar]
- 6.LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB, Mills DA, German JB. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem. 2007;55:8914–9 [DOI] [PubMed] [Google Scholar]
- 7.Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. 2010;58:5334–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sela DA, Li Y, Lerno L, Wu S, Marcobal AM, German JB, Chen X, Lebrilla CB, Mills DA. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J Biol Chem. Epub 2011 Feb 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA. 2008;105:18964–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zivkovic AM, German JB, Lebrilla CB, Mills DA. Microbes and Health Sackler Colloquium: human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci USA. Epub 2010 Aug 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guggenbichler JP, De Bettignies-Dutz A, Meissner P, Schellmoser S, Jurenitsch J. Acidic oligosaccharides from natural sources block adherence of Escherichia coli on uroepithelial cells. Pharm Pharmacol Lett. 1997;7:35–8 [Google Scholar]
- 12.Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. J Nutr. 2008;138:S1818–28 [DOI] [PubMed] [Google Scholar]
- 13.Gopal PK, Gill HS. Oligosaccharides and glycoconjugates in bovine milk and colostrum. Br J Nutr. 2000;84 Suppl 1:S69–74 [DOI] [PubMed] [Google Scholar]
- 14.Tao N, DePeters EJ, Freeman S, German JB, Grimm R, Lebrilla CB. Bovine milk glycome. J Dairy Sci. 2008;91:3768–78 [DOI] [PubMed] [Google Scholar]
- 15.Tao N, DePeters EJ, German JB, Grimm R, Lebrilla CB. Variations in bovine milk oligosaccharides during early and middle lactation stages analyzed by high-performance liquid chromatography-chip/mass spectrometry. J Dairy Sci. 2009;92:2991–3001 [DOI] [PubMed] [Google Scholar]
- 16.Barile D, Marotta M, Chu C, Mehra R, Grimm R, Lebrilla CB, German JB. Neutral and acidic oligosaccharides in Holstein-Friesian colostrum during the first 3 days of lactation measured by high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. J Dairy Sci. 2010;93:3940–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ninonuevo MR, Lebrilla CB. Mass spectrometric methods for analysis of oligosaccharides in human milk. Nutr Rev. 2009;67 Suppl 2:S216–26 [DOI] [PubMed] [Google Scholar]
- 18.Barile D, Tao N, Lebrilla CB, Coisson JD, Arlorio M, German JB. Permeate from cheese whey ultrafiltration is a source of milk oligosaccharides. Int Dairy J. 2009;19:524–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park Y, Lebrilla CB. Application of Fourier transform ion cyclotron resonance mass spectrometry to oligosaccharides. Mass Spectrom Rev. 2005;24:232–64 [DOI] [PubMed] [Google Scholar]
- 20.Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci. 2009;87:26–34 [DOI] [PubMed] [Google Scholar]
- 21.Gnoth MJ, Rudloff S, Kunz C, Kinne RK. Investigations of the in vitro transport of human milk oligosaccharides by a Caco-2 monolayer using a novel high performance liquid chromatography-mass spectrometry technique. J Biol Chem. 2001;276:34363–70 [DOI] [PubMed] [Google Scholar]
- 22.Sonoyama K, Watanabe H, Watanabe J, Yamaguchi N, Yamashita A, Hashimoto H, Kishino E, Fujita K, Okada M, et al. Allergic airway eosinophilia is suppressed in ovalbumin-sensitized Brown Norway rats fed raffinose and alpha-linked galactooligosaccharide. J Nutr. 2005;135:538–43 [DOI] [PubMed] [Google Scholar]
- 23.Gruber C, van Stuijvenberg M, Mosca F, Moro G, Chirico G, Braegger CP, Riedler J, Boehm G, Wahn U. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol. 2010;126:791–7 [DOI] [PubMed] [Google Scholar]
- 24.Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost. 2004;92:1402–10 [DOI] [PubMed] [Google Scholar]
- 25.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8 [DOI] [PubMed] [Google Scholar]
- 26.Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. J Clin Invest. 2009;119:2441–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braunstein J, Qiao L, Autschbach F, Schurmann G, Meuer S. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut. 1997;41:215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Sosa S, Martin MJ, Hueso P. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J Nutr. 2002;132:3067–72 [DOI] [PubMed] [Google Scholar]
- 29.Hong P, Ninonuevo MR, Lee B, Lebrilla C, Bode L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN). Br J Nutr. 2009;101:482–6 [DOI] [PubMed] [Google Scholar]
- 30.Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137:S830–7 [DOI] [PubMed] [Google Scholar]
- 31.Bezkorovainy A. Probiotics: determinants of survival and growth in the gut. Am J Clin Nutr. 2001;73:S399–405 [DOI] [PubMed] [Google Scholar]
- 32.Rivero-Urgell M, Santamaria-Orleans A. Oligosaccharides: application in infant food. Early Hum Dev. 2001;65 Suppl:S43–52 [DOI] [PubMed] [Google Scholar]
- 33.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58 [DOI] [PubMed] [Google Scholar]
- 34.Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J Nutr. 2005;135:1304–7 [DOI] [PubMed] [Google Scholar]
- 35.Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25 [DOI] [PubMed] [Google Scholar]
- 36.Martino DJ, Currie H, Taylor A, Conway P, Prescott SL. Relationship between early intestinal colonization, mucosal immunoglobulin A production and systemic immune development. Clin Exp Allergy. 2008;38:69–78 [DOI] [PubMed] [Google Scholar]
- 37.Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008;138:S1796–800 [DOI] [PubMed] [Google Scholar]
- 38.Seifert S, Watzl B. Inulin and oligofructose: review of experimental data on immune modulation. J Nutr. 2007;137:S2563–7 [DOI] [PubMed] [Google Scholar]
- 39.Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol. 2001;1:59–67 [DOI] [PubMed] [Google Scholar]
- 40.Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–6 [DOI] [PubMed] [Google Scholar]
- 41.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–34 [DOI] [PubMed] [Google Scholar]
- 42.Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68:219–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LoCascio RG, Desai P, Sela DA, Weimer B, Mills DA. Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization. Appl Environ Microbiol. 2010;76:7373–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007;137:2420–4 [DOI] [PubMed] [Google Scholar]
- 46.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61:2–8 [DOI] [PubMed] [Google Scholar]
- 47.Urashima T, Odaka G, Asakuma S, Uemura Y, Goto K, Senda A, Saito T, Fukuda K, Messer M, et al. Chemical characterization of oligosaccharides in chimpanzee, bonobo, gorilla, orangutan, and siamang milk or colostrum. Glycobiology. 2009;19:499–508 [DOI] [PubMed] [Google Scholar]
- 48.Newburg DS. Are all human milks created equal? Variation in human milk oligosaccharides. J Pediatr Gastroenterol Nutr. 2000;30:131–3 [DOI] [PubMed] [Google Scholar]
- 49.Erney RM, Malone WT, Skelding MB, Marcon AA, Kleman-Leyer KM, O'Ryan ML, Ruiz-Palacios G, Hilty MD, Pickering LK, et al. Variability of human milk neutral oligosaccharides in a diverse population. J Pediatr Gastroenterol Nutr. 2000;30:181–92 [DOI] [PubMed] [Google Scholar]
- 50.Hakkarainen J, Toivanen M, Leinonen A, Frangsmyr L, Stromberg N, Lapinjoki S, Nassif X, Tikkanen-Kaukanen C. Human and bovine milk oligosaccharides inhibit Neisseria meningitidis pili attachment in vitro. J Nutr. 2005;135:2445–8 [DOI] [PubMed] [Google Scholar]
- 51.McJarrow P, van Amelsfort-Schoonbeek J. Bovine sialyl oligosaccharides: seasonal variations in their concentrations in milk, and a comparison of the colostrums of Jersey and Friesian cows. Int Dairy J. 2004;14:571–9 [Google Scholar]
- 52.Nakamura T, Kawase H, Kimura K, Watanabe Y, Ohtani M, Arai I, Urashima T. Concentrations of sialyloligosaccharides in bovine colostrum and milk during the prepartum and early lactation. J Dairy Sci. 2003;86:1315–20 [DOI] [PubMed] [Google Scholar]
- 53.Varki A. Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol. 2001; Suppl 33:54–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roussel F, Takhi M, Schmidt RR. Solid-phase synthesis of a branched hexasaccharide using a highly efficient synthetic strategy. J Org Chem. 2001;66:8540–8 [DOI] [PubMed] [Google Scholar]
- 55.Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, Urbanek R, Szepfalusi Z. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol. 2010;21:1179–88 [DOI] [PubMed] [Google Scholar]
- 56.Velupillai P, Harn DA. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T-cell subsets. Proc Natl Acad Sci USA. 1994;91:18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]