Vaginal and rectal mucosal transmission of R5 and X4 tropic HIV-1 in humanized Rag2−/−γc−/− (RAG-hu) mice (original) (raw)
. Author manuscript; available in PMC: 2011 May 11.
Abstract
Studies on HIV-1 mucosal transmission to evaluate early events in pathogenesis and the development of effective preventive/prophylactic methods have thus far been hampered by the lack of a suitable animal model susceptible to HIV-1 infection by either vaginal and/or rectal routes. In this regard, while primate-SIV/SHIV and FIV-cat models provided useful surrogate platforms to derive comparative data, these viruses are distinct and different from that of HIV-1. Therefore an optimal model that permits direct study of HIV-1 transmission via mucosal routes is highly desirable. The new generation of humanized NOD/SCID BLT, NOD/SCIDγc−/−, and Rag2−/−γc−/− mouse models show great promise to achieve this goal. Here, we show that humanized Rag2−/−γc−/− mice (RAG-hu) engrafted with CD34 hematopoietic progenitor cells harbor HIV-susceptible human cells in the rectal and vagina mucosa and are susceptible to HIV-1 infection when exposed to cell free HIV-1 either via vagina or rectum. Infection could be established without any prior hormonal conditioning or mucosal abrasion. Both R5 and X4 tropic viruses were capable of mucosal infection resulting in viremia and associated helper T cell depletion. There was systemic spread of the virus with infected cells detected in different organs including the intestinal mucosa. R5 virus was highly efficient in mucosal transmission by both routes whereas X4 virus was relatively less efficient in causing infection. HIV-1 infection of RAG-hu mice by vaginal and rectal routes as shown here represents the first in vivo model of HIV-1 transmission across intact mucosal barriers and as such may prove very useful for studying early events in HIV-1 pathogenesis in vivo, as well as the testing of microbicides, anti-HIV vaccines/therapeutics, and other novel strategies to prevent HIV-1 transmission.
Keywords: HIV/AIDS pathogenesis, HIV vaginal and rectal transmission, Microbicides, Humanized mice, HIV-1 mucosal transmission/infection, Animal disease models, Hematopoietic stem cells, SCID-hu mice, Rag2−/−γc−/− mice, RAG-hu mice
Background
Infection via the mucosal surfaces constitutes the predominant natural mode of transmission of HIV/AIDS (Pope and Haase, 2003). This most commonly involves transmission of the virus by the heterosexual vaginal route or the rectal route during homosexual contact. Detailed studies on the early dynamic interactions between HIV-1 target cells, namely the macrophages and dendritic cells during initial infection with the commonly transmitted R5 tropic virus and its subsequent dissemination in the body have so far been hampered by the lack of a suitable animal model that permits HIV-1 infection via mucosal routes (Hladik et al., 2007). In this regard, the rhesus macaque model using SHIV and SIV or the cat model involving FIV have been useful in deriving valuable comparative pathogenesis data and in evaluation of a number of vaccine candidates and vaginal/rectal microbicides (Burkhard and Dean, 2003; Miller et al., 1989; Spira et al., 1996). However, SIV and FIV are distinct viruses from that of HIV-1 and differ in terms of their viral genetic make up and viral-host interactions. Thus, animal model systems that employ HIV-1 itself to study pathogenesis and treatment modalities are continuously being sought.
Humanized mice have filled this gap somewhat by providing a platform in which human target cells are evaluated in terms of viral susceptibility and associated helper T cell loss. During nearly the last two decades, two conventional human-mouse chimeric models, namely the hu-PBL-SCID mouse with transplanted adult human PBLs, and the SCID-hu mouse model with transplanted human thymus liver tissues played a key role in pathogenesis studies using a human hemato-lymphoid system (Jamieson, Aldrovandi, and Zack, 1996; Mosier, 1996; Shultz, Ishikawa, and Greiner, 2007). However, infection of these mice was achieved by injection into the peritoneal cavity or into the thy/liv organ. Attempts to transmit the virus by mucosal routes by either using cell-free virus or infected cells resulted in inconsistent infection rates. However prior hormonal conditioning to dimish the mucosal thickness resulted in increased infection rates (D'Cruz and Uckun, 2007; Khanna et al., 2002; Olmsted et al., 2005). Furthermore, the nature and breadth of the resident human cell types and their tissue distribution in the intact host in these models was less than optimal due to the absence of continual de novo hematopoiesis and skewed production of certain cells such as primarily T cells as seen in the SCID-hu mouse model. Although HIV-1 infection of the T cells and monocytes/macrophages ensues, infection kinetics predominantly represent an acute mode with a consequent rapid helper T cell loss. Thus the chronic nature and prolonged T cell loss as seen in a natural human HIV-1 infection are not adequately mimicked in these models in addition to lack of efficient mucosal viral transmission.
Recent exciting advancements have resulted in the production of improved humanized mice. Transplantation of human hematopoietic stem cells (CD34+ cells) into novel immunodeficient mice with additional mutations and much lower innate immunity have permitted higher engraftment levels and sustained multilineage human hematopoiesis. These new models include the NOD/SCIDγc−/− and Rag2−/−γc−/− strains reconstituted with human CD34+ cells. Additionally, an innovative improvement of the standard SCID-hu model involved transplantation of thymic and liver tissues under the kidney capsule of NOD-SCID mice followed by reconstitution with autologous human CD34+ cells (BLT mice). Multilineage hematopoiesis with the generation of HIV-susceptible CD4+ T cells, macrophages, monocytes, and dendritic cells in addition to B cells with a capacity for primary human immune responses distinguish these newer humanized mouse models (Ito et al., 2002; Melkus et al., 2006; Traggiai et al., 2004). A number of groups including ours have demonstrated the utility of these humanized mice as improved models for HIV-1 infection by showing chronic viremia lasting several months by both R5 and X4 tropic viral strains, virus replication in a variety of lymphoid and non-lymphoid organs including thymus, lymph nodes, spleen, lung, gut-associated lymphoid tissue, and male and female reproductive tracts. Viral infection leads to gradual CD4+ T cell depletion. Furthermore, humoral immune responses against HIV-1 could also be seen to some extent (An et al., 2007; Baenziger et al., 2006; Berges et al., 2006; Gorantla et al., 2006; Sun et al., 2007; Watanabe et al., 2007; Zhang, Kovalev, and Su, 2006).
However, a major deviation in these ground-breaking studies from that of natural infection was that the different routes used for infection, namely, the intraperitoneal injection or intravenous injection, bypassed the mucosal barriers. While these studies have conclusively demonstrated efficient infection by HIV-1, the inoculation methods used are relatively rare routes of exposure in humans. As pointed out above, the most common route of HIV-1 infection is across the mucosa during vaginal or rectal intercourse (Pope and Haase, 2003). In a recent attempt to introduce HIV-1 by a natural route, studies of Sun et al demonstrated the presence of HIV-1 susceptible cells in the mucosa of humanized BLT mice. They achieved successful HIV-1 infection via rectal exposure. Sustained viremia with associated productive infection of lymphoid cells in different organs and a consequent helper T cell loss was demonstrated. However, it was necessary to cause intentional abrasion of the rectal epithelium prior to viral exposure resulting in an artificial break of mucosal barriers (Sun et al., 2007). To truly mimic the natural and predominant mode of HIV-1 infection, one must demonstrate a successful infection via vaginal or rectal routes without overt epithelial abrasion. Thus far as pointed out above, this has been achieved only with HIV-1-related viruses: SIV or SHIV infection of the rhesus macaque and FIV infection of the cat. Although good substitutes, inherent limitations also exist with the currently employed in vitro models of HIV-1 infection of human vaginal tissue explants (Collins et al., 2000). The availability of a small animal model to directly study HIV-1 mucosal transmission combined with human mucosal immunity would greatly facilitate the understanding of the transmission mechanism and could thereby lead to the development of novel strategies to protect against mucosal infection.
With these criteria in mind, we evaluated RAG-hu mice for their susceptibility to HIV-1 infection via the mucosal route. Here, we show that these humanized mice are engrafted with human T cells and macrophages/dendritic cells in the vaginal and rectal mucosa. Further, they are readily infected by R5 tropic HIV-1 introduced into either the vaginal or rectal cavities, with viremia detected within a week post-infection. Interestingly, we also show that X4 tropic virus can cause successful infection through the vaginal and rectal routes but not as efficiently as R5 tropic virus. This system can be potentially very valuable for pathogenesis as well as prevention studies involving microbicides and therapeutic vaccines.
Results
R5 tropic HIV-1 infection/exposure of RAG-hu mice by vaginal and rectal mucosal routes leads to productive infection and sustained viremia
Humanized mice were prepared as described previously by injecting CD34+ cells into conditioned neonatal Rag2−/−γc−/− mice (Berges et al., 2006). The mean human cell engraftment level in peripheral blood for mice used in these experiments was 65% as assessed by FACS for the CD45 marker (summarized in Table 1 and Table 2). Humanized mice with both high and low engraftment levels were utilized to determine their susceptibility to infection via mucosal routes.
Table 1.
Summary of human cell engraftment in mice used for R5 tropic HIV-1 mucosal infection.
| Vaginal infection | Rectal infection | ||||||
|---|---|---|---|---|---|---|---|
| Mouse | Gender | Peripheral blood engraftment | CD4:CD8 | Mouse | Gender | Peripheral blood engraftment | CD4:CD8 |
| 213 | F | 74.4% | 2.4:1 | 234 | M | 63.8% | 3.3:1 |
| 217 | F | 54.2% | 2.3:1 | 235 | M | 71.2% | 6.1:1 |
| 238 | F | 77.6% | 3.4:1 | 236 | M | 77.6% | 2.3:1 |
| 258 | F | 69.4% | 2.2:1 | 257 | M | 73.7% | 1.2:1 |
| 259 | F | 58.6% | 1.3:1 | 260 | F | 88.2% | 1.7:1 |
| 264 | F | 77.4% | 3.5:1 | 261 | F | 86.9% | 2:1 |
| 265 | F | 89.0% | 2.0:1 | 262 | M | 82.2% | 1.8:1 |
| 266 | F | 80.7% | 3.5:1 | 263 | M | 73.7% | 2.4:1 |
| 200 | F | 8.2% | 1.4:1 | 216 | F | 36.5% | 1.0:1 |
| Mean+/−SD | 65.5+/−24.0% | 72.6+/−15.6% |
Table 2.
Summary of human cell engraftment in mice used for X4 tropic HIV-1 mucosal infection.
| Vaginal infection | Rectal infection | ||||||
|---|---|---|---|---|---|---|---|
| Mouse | Gender | Peripheral blood engraftment | CD4:CD8 | Mouse | Gender | Peripheral blood engraftment | CD4:CD8 |
| 300 | F | 68.4% | 1.6:1 | 296 | M | 18.3% | 1.2:1 |
| 301 | F | 77.1% | 1.3:1 | 297 | M | 41.6% | 1.2:1 |
| 318 | F | 83.3% | 1.1:1 | 310 | F | 81.6% | 1.3:1 |
| 319 | F | 88.9% | 1.5:1 | 313 | F | 31.0% | 1.0:1 |
| 320 | F | 85.7% | 3.9:1 | 315 | F | 7.4% | 1.0:1 |
| Mean+/−SD | 80.7+/−8.1% | 36.0+/−28.6% |
We first determined if the cells targeted by HIV-1 were present in the RAG-hu mouse mucosal tissues. Specifically, vaginal, rectal, and gut tissues were stained for the presence of human leukocytes (CD45), T cells (CD3), macrophages/dendritic cells (CD68), and for the cells bearing the primary HIV-1 receptor CD4 (Fig. 1). All of the above tissues were positive for cells staining for the pan-leukocyte marker CD45, thus indicating the presence of human leukocytes (Fig.1A–C). The presence of human T cells was revealed by positive staining for CD3 (Fig. 1 D–F) and CD68+ macrophages and/or dendritic cells were also detected (Fig. 1 G–I). Finally, CD4+ cells were also detected in each tissue (Fig. 1 J–L). Unengrafted tissues were negative for human CD45 or any other human stains (Fig. 1 M–O and data not shown). In addition, no staining was evident when primary antibodies were omitted.
Figure 1. RAG-hu mouse mucosal tissues are populated by HIV-1-susceptible human cells.
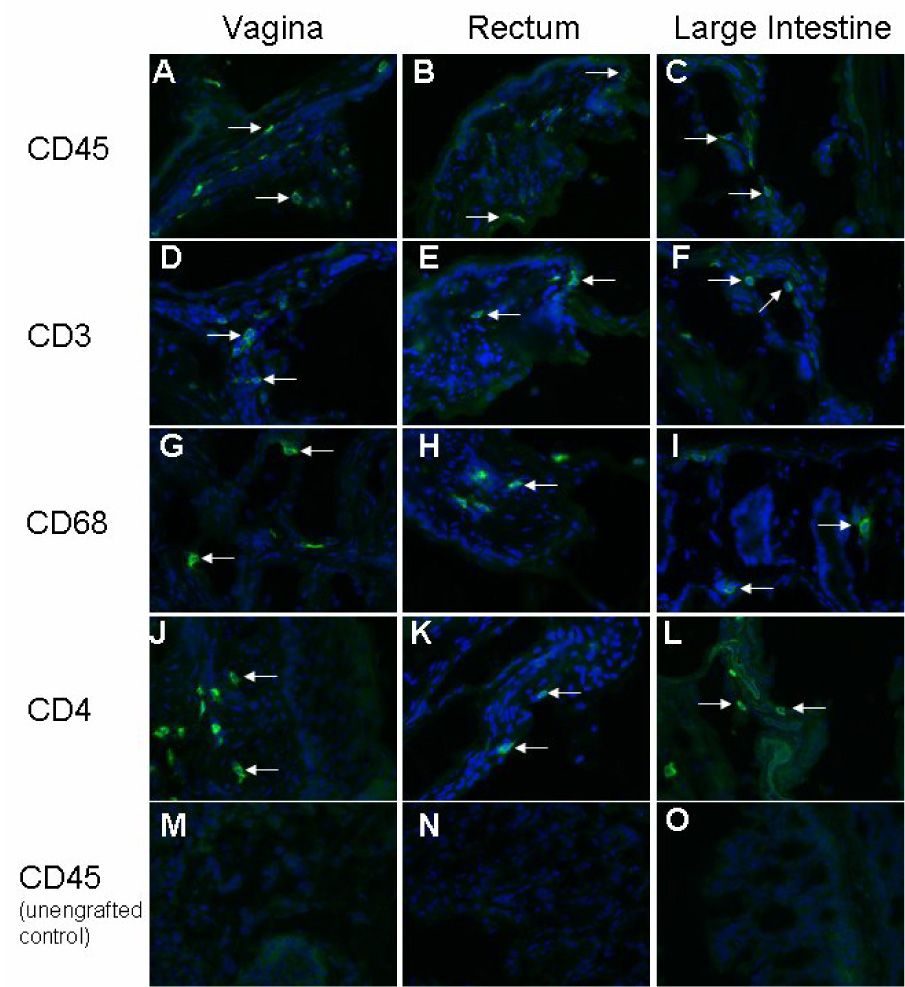
Engrafted and unengrafted mice were sacrificed and vagina, rectum, and large intestines were dissected and frozen. Tissue sections from engrafted mice were immunostained with fluorescent-labeled antibodies for the presence of the human cell markers CD45 (A–C), CD3 (D–F), CD68 (G–I), and CD4 (J–L). Unengrafted tissues were negative for the human cell markers CD45 (M–O), as well as CD3, CD4, and CD68 (data not shown).
In the first series of experimental infections, we began with R5 tropic HIV-1 infection of these mice by both vaginal and rectal routes (n=9 each) as described in methods. We initially used the R5 tropic HIV-1 strain BaL-1 as it represents the class of HIV-1 which is most readily transmitted and most commonly detected following initial infection in humans (Berger, Murphy, and Farber, 1999). The human cell engraftment levels of the mice chosen for R5 viral infection averaged 65.5+/−24.0% for the vaginal route and 72.6+/−15.6% for the rectal route (summarized in Table 1). Mice were bled at weekly intervals and plasma viremia was measured by Q-RT-PCR. All the mice infected by either route (including female mice infected via the rectal route) with R5 tropic HIV-1 became viremic by the first week post-infection and this persisted throughout the 10 week observation period (Fig. 2 A), thus indicating successful infection and viral maintenance in vivo. Two control, uninfected mice exhibited undetectable viral load. As can be seen, viral load peaked during 7–8 weeks in vaginally infected mice whereas the peak viral load was observed at an earlier stage between 5–7 weeks in the rectally infected mice. At later time points, viral loads fell to the levels seen during early weeks post-infection. The highest viral titers reached were 6×107 RNA copies/ml at 7 weeks for a vaginally infected mouse and 2×107 at 7 weeks for a rectally infected mouse. In addition, virus was successfully re-isolated from 6 out of 6 vaginally infected mice (4 weeks post-infection) by co-culture, further establishing that the circulating virus detected by Q-RT-PCR was indeed a viable, infectious virus (data not shown).
Figure 2. HIV-1 viral load in peripheral blood following mucosal infection.
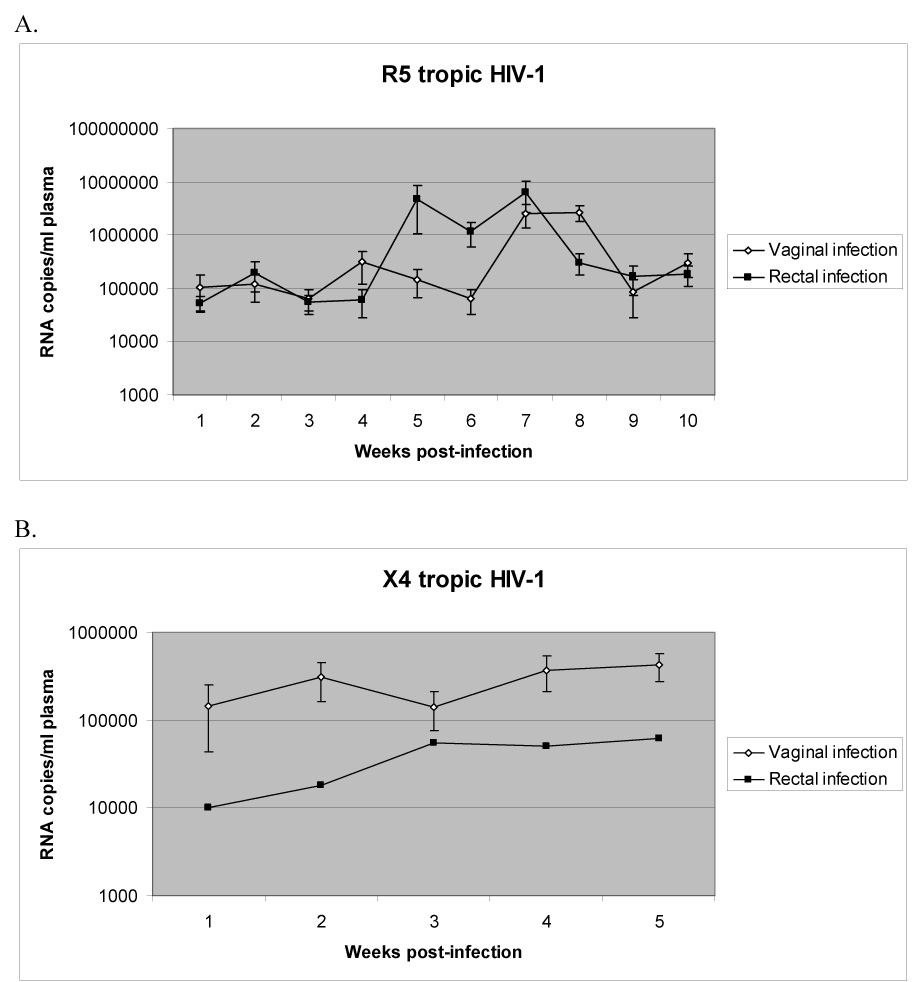
Infected mouse plasma was collected weekly and RNA was extracted. Viral load (in RNA copies/ml plasma) was determined by Q-RT-PCR as described in methods. A. R5 virus infection. B. X4 virus infection. Mice that failed to become viremic are not included.
Mucosal infection of RAG-hu mice with HIV-1 leads to systemic viral dissemination and replication
Initial infection with HIV-1 at the portal of entry is believed to be amplified locally and carried to local lymph nodes first, then followed by systemic dissemination to target cells in various organ systems. Therefore, to provide additional evidence of widespread infection following mucosal transmission of HIV-1, a viremic mouse was sacrificed at 4 weeks post-infection. Mesenteric lymph node, spleen, and intestinal tissue sections were subjected to RNA in situ hybridization to detect HIV-1 sequences in infected cells (Fig. 3). Numerous HIV+ cells were apparent in the lymph nodes (Fig. 3 B), small intestines (Fig. 3 D), and spleen (Fig. 3 F), indicating viral spread and productive infection of distant target cells. On the contrary, no positive hybridization signal was detectable in uninfected tissues (Fig. 3 A,C,E) or on any tissues hybridized with a sense probe (data not shown.
Figure 3. HIV-1 replication in proximal and distal organs following mucosal infection.
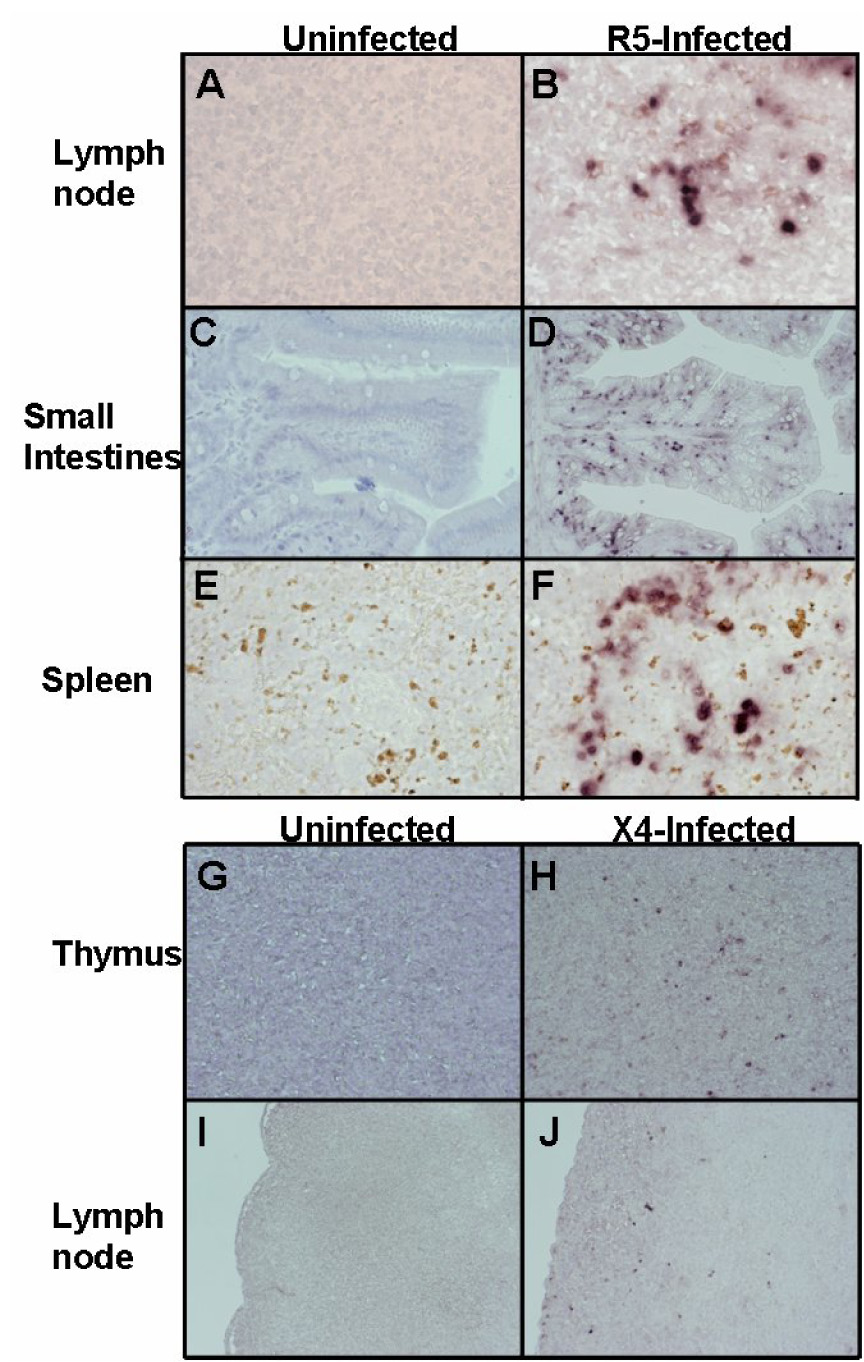
Mucosally-infected mice were sacrificed and organs were collected and probed for HIV-1 RNA using an env riboprobe by in situ hybridization. Mouse 262 was sacrificed at 4 weeks following rectal infection with HIV-1 BaL-1, and mouse 320 was sacrificed at 8 weeks following rectal infection with HIV-1 NL4-3. (A, C, E): uninfected tissue sections from mesenteric lymph node, small intestines, and spleen, respectively. (B, D, F): R5 virus-infected tissue sections from mesenteric lymph node, small intestines, and spleen, respectively. (G, I): uninfected tissue sections from thymus and lymph node, respectively. (H, J): X4 virus-infected tissue sections from thymus and lymph node, respectively. Panels A, B, E, F: original magnification 600X. Panels C, D, G, H, I, J: original magnification 200X.
X4 tropic HIV-1 can also cause productive infection of RAG-hu mice by vaginal and rectal routes
R5 tropic viruses are assumed to have the greatest capacity for infection via mucosal routes as these are the strains that are generally isolated during the primary infection stage (Berger, Murphy, and Farber, 1999). On the contrary, it is not clear if X4 tropic viruses can efficiently spread via the mucosal route. Therefore, we evaluated if X4 viruses can cause productive infection of RAG-hu mice by either vaginal or rectal exposure. Similar protocols as above were used for infection of humanized mice with the X4-tropic HIV-1 strain NL4-3. We found that 4 out of 5 female mice infected by the vaginal route were positive for viremia during the first 5 weeks post-infection, with the highest viral load (9×104) appearing at 5 weeks post-infection (Fig. 2 B). In mice receiving rectal infection with X4 virus, 2 out of 5 mice were positive for viremia during the first 5 weeks, with the highest viral load (9×104) appearing at 5 weeks (Fig. 2B). In all of the above X4 viral infections, mice were either consistently positive for viremia or never positive for viremia throughout the time course. To verify systemic dissemination of the virus, thymus and mesenteric lymph node tissue sections obtained from a viremic mouse at 8 weeks post-infection were subjected to RNA in situ hybridization to detect HIV-1 sequences (Fig. 3). HIV+ cells were detected in both thymus and lymph nodes (Fig. 5 H,J) while the uninfected sections were negative (Fig. 3 G,I). Thus, X4-tropic virus can also infect across both vaginal and rectal routes and produce a disseminated infection.
Both R5 and X4 HIV-1 strains can cause CD4+ T cell depletion following infection via mucosal routes
CD4+ T cell loss is a hallmark of HIV-1 infection in the human. In a typical case, helper T cell levels fall transiently during the acute stage of infection followed by a rebound to a set point for several months to years with an eventual depletion leading to AIDS. To assess depletion of CD4+ T cells, peripheral blood was collected from mice mucosally infected with R5 or X4 virus and stained with CD3 and CD4 antibodies and assayed by FACS as a ratio of CD4+CD3+ cells to CD4−CD3+ cells (Fig. 4). CD4+. T cell levels in mice infected with R5 virus first began to drop at 7 weeks post-infection and the greatest depletion was at 9 weeks, followed by a rebound to normal levels by week 11 (Fig. 4A). Representative FACS plots are shown in Fig. 4B. CD4+ T cell depletion was more pronounced following vaginal infection (~25% decrease) than for rectal infection (~10% decrease). Significant CD4+ T cell loss amounting up to a 75% decrease was also seen following X4 viral infections in vaginally-infected mice (Fig. 4C).
Figure 4. CD4+ T cell depletion following mucosal infection with HIV-1.
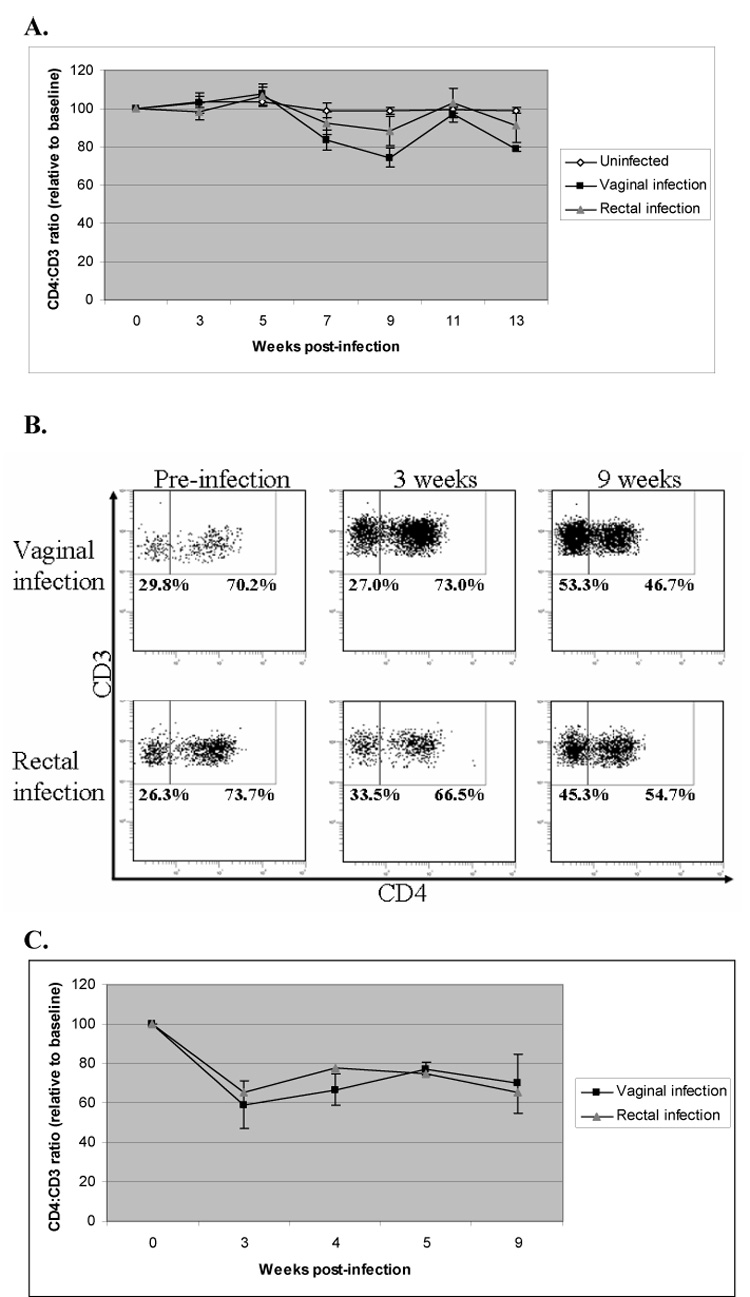
Mice mucosally infected with HIV-1 were screened for CD4+ T cell depletion by FACS analysis of peripheral blood cells stained with CD3 and CD4. The CD4:CD3 ratio reflects the percentage of CD3+ cells that were also CD4+ relative to total CD3+ cells. Since individual mice have varying CD4:CD3 ratios, all data shown is standardized to the baseline values obtained prior to infection. A. Mucosal infection with R5 tropic virus. Vaginal infection, n=7. Rectal infection, n=9. Uninfected mice, n=2. B. FACS dot plots from representative R5 virus-infected mice infected either vaginally or rectally. C. Mucosal infection with X4 tropic virus. Vaginal infection, n=4. Rectal infection, n=2. Key: V=vaginal infection, R=rectal infection.
Discussion
Improved humanized mouse models with a capacity for sustained multilineage hematopoiesis have opened up many new exciting avenues of HIV-1 research which until recently were not possible. Here we have shown that Rag2−/−γc−/− mice reconstituted with human CD34+ cells (RAG-hu mice) can be efficiently infected with R5 tropic HIV-1, and remarkably also with R4 tropic HIV-1 across both the intact vaginal and rectal mucosa, thus representing the first efficient in vivo model of HIV-1 mucosal transmission to human cells.
In previous efforts with a small animal model, studies on mucosal transmission in conventional hu-PBL-SCID mice showed inefficient viral transmission (D'Cruz and Uckun, 2007). In a recent report, studies of Sun et al using an improved humanized mouse model, namely the BLT mouse, demonstrated successful intra-rectal transmission of HIV-1. Although this represents a significant advancement over the previous models, the rectum had to be manipulated to create abrasions before exposure to CXCR4-tropic HIV-1 for successful viral transmission. Furthermore, vaginal transmission was not attempted, and these studies were confined to transmission with an X4 tropic virus not known to be the major transmitted virus by the mucosal route (Sun et al., 2007). In contrast, in our present studies successful HIV-1 transmission was obtained via both vaginal and rectal routes without causing any artificial abrasions. Infection with R5 tropic virus was highly efficient with all the HIV-1 exposed mice becoming viremic within the first week. This substantiates the previous notion that R5 tropic viruses are the major strains transmitted by the natural mucosal route. Surprisingly, we also found that even X4 tropic viruses can be transmitted by natural routes. However, not all mice exposed to the X4 tropic virus were successfully infected, indicating that transmission is not as efficient as that achieved by R5 tropic virus. Further, infection across the rectal mucosa with X4 tropic virus was less efficient (40%, n=5) than across the vaginal mucosa (80%, n=5), indicating that an intact rectal mucosa is not as efficiently traversed by the X4 virus. A possible reason may be that target T cells are not as abundant in the rectal mucosa; this will require further investigation.
We also have shown that mucosally transmitted virus is spread to systemic organs such as thymus, spleen and lymph nodes in addition to the small intestines. Numerous infected cells were seen similar to that in human infections. Characteristic CD4+ T cell depletion is also evident in these mucosally infected mice. A comparison of previous data on mice infected via the intraperitoneal route with the present vaginal/rectal infections revealed similarities as well as minor differences: viral loads were fairly similar, while CD4+ T cell depletion in peripheral blood varied both in terms of the kinetics and the severity of depletion following R5 virus infection (less severe following mucosal infection). The more rapid and severe CD4+ T cell depletion following intraperitoneal infection might be due to more immediate viral access to large numbers of susceptible target cells compared to mucosal infection, wherein the virus gains access to systemic spread in a more gradual way. However, larger numbers of mice need to be studied before further conclusions can be drawn. For mucosal infection, CD4 T cell depletion was more pronounced in mice infected with X4 tropic virus, even though viral loads were comparable. This is consistent with previous studies in RAG-hu mice which showed that CD4 T cell depletion following infection with R5 strains is less robust compared to that observed with X4 viral infection, even though viral loads are similar (Baenziger et al., 2006; Zhang, Kovalev, and Su, 2006).
To date, the best surrogate model for HIV-1 mucosal transmission involved vaginal SIV and SHIV infections in non-human primates (Miller et al., 1989; Spira et al., 1996), although FIV infection of the cat (Burkhard and Dean, 2003) and in vitro models of HIV-1 transmission across the human mucosal tissue explants have also been valuable (Collins et al., 2000). However, there are important differences and limitations between HIV-1 transmission to humanized mice and SIV transmission models in macaques. In addition to SIV, SHIV and HIV-1 being different viruses, the vaginal transmission model of SIV in macaques is expensive and the use of large numbers of animals is prohibitive. In contrast, large numbers of RAG-hu mice can be generated at a fraction of the cost of similar numbers of monkeys. Furthermore, RAG-hu mice are also permissive to X4 tropic viral infection via the mucosal route thereby broadening the scope of future intervention studies. Thus, the present RAG-hu mouse model of mucosal HIV-1 transmission shows exquisite sensitivity to infection.
Many novel studies on HIV-1 pathogenesis using human cells can now ensue with the advent of this new RAG-hu mouse model of mucosal infection. For example, recent elegant in vitro work using explant cultures of vaginal mucosa showed that the initial R5 viral infection targeted cells such as Langerhans cells before further viral spread (Hladik et al., 2007). The current model will permit a more dynamic physiologic study of these initial viral-cell interactions at the site of viral entry as well as later spread to the draining lymph node in an intact animal. Prophylactic measures involving anti-HIV-1 drugs can now be tested using this model to evaluate protection from mucosal transmission.
Vaginal and rectal microbicides can now be easily evaluated in vivo on HIV-1 (McGowan, 2006; Veazey et al., 2005) itself in a cost-efficient manner using large number of animals before proceeding with further testing in more expensive monkey models. In addition to conventional microbicides approaches, novel molecules such as siRNAs are also currently being investigated. In one recent study, the efficacy of an siRNA-based microbicide was demonstrated against lethal vaginal challenge of mice with herpes simplex virus (Palliser et al., 2006). With regard to HIV-1, our laboratory has characterized several effective siRNAs targeted to the HIV-1 co-receptors CXCR4 and CCR5 (Anderson and Akkina, 2005; Anderson and Akkina, 2007). These await testing against mucosal HIV-1 transmission.
Although the RAG-hu mouse model has not yet proved to be ideal to demonstrate anti-HIV-1 humoral and cellular immune responses, a recent report from our laboratory described the production of neutralizing antibodies following challenge of RAG-hu mice with Dengue virus (Kuruvilla et al., 2007), indicating that specific immune responses can be generated to other viruses. In the case of HIV-1, at the least, passive antibody mediated protection against mucosal infection can now be examined using a natural route of challenge, thus permitting testing of broadly neutralizing antibodies for their protective effects.
Although each novel humanized mouse model is unique, the RAG-hu model of HIV-1 mucosal transmission offers many technical and practical advantages over the recently described BLT mouse model of rectal HIV-1 transmission (Sun et al., 2007). First, it permits both vaginal and rectal mucosal transmission across an intact mucosal barrier with a much lower input virus. Second, preparation of RAG-hu mice is not as technically intensive since no surgical procedure is required as is necessary to generate BLT mice. Third, RAG-hu mice have a longer life span compared to BLT mice (NOD mice experience a high incidence of lymphomas), thus permitting long-term studies. For example, the effect of microbicides on the mucosal epithelia during long-term application can now be evaluated and whether or not a microbicide which showed short-term protection can be used long-term safely. Furthermore, for any large scale testing, a sufficient cohort number of humanized mice are needed. In this regard, greater numbers of RAG-hu mice can be generated per human tissue donor versus BLT mice. Although these proof-of-concept studies have established that efficient mucosal infection is feasible in the humanized RAG-hu mice, many questions remain and need to be further evaluated. Among these: What is the minimal dose required to achieve consistent HIV-1 infection? Which cell type(s) are first infected in the mucosa and how does the virus reach the regional lymph nodes for subsequent systemic dissemination?
In summary, RAG-hu mice capable of multilineage human hematopoiesis are exquisitely susceptible to mucosal transmission of HIV-1. Both R5 and X4 viruses can initiate mucosal infection followed by viremia and associated T cell depletion. Thus, this model will permit many new pathogenesis, prophylactic and therapeutic studies on HIV-1.
Methods
Generation of humanized Rag2−/−γc−/− mice (RAG-hu mice)
Humanized BALB/c-Rag2−/−γc−/− mice were prepared as described (Traggiai et al., 2004) with the minor modification of using human fetal liver-derived CD34+ cells. Briefly, neonatal mice were conditioned with 350 rads and then injected intrahepatically with 0.5−1×106 human CD34+ cells. Approximately 12 weeks post-reconstitution, mice were screened for human cell engraftment. Blood was collected by tail bleed, and red blood cells were lysed using the Whole Blood Erythrocyte Lysing Kit (R&D Systems, Minneapolis, MN). The white blood cell fraction was stained with antibodies against the human pan-leukocyte marker CD45 (Caltag) and FACS analyzed as described (Berges et al., 2006). Mice with varying human cell reconstitution levels were used to determine mucosal HIV-1 infection susceptibility.
HIV-1 infection of RAG-hu mice
Humanized mice were infected with either cell-free HIV-1 strain BaL-1 (R5 tropic) or NL4-3 (X4 tropic) contained in the original media used to produce the virus (RPMI 1640 medium supplemented with 10% fetal bovine serum). Mice were allowed to defecate prior to rectal inoculation so as to prevent immediate expulsion of virus. Vaginal infections were performed in a volume of 50µl (156 TCID50 of BaL-1 virus or 203 TCID50 of NL4-3) and rectal infections were performed in a volume of 20µl (BaL-1 virus at 62 TCID50; NL4-3 virus at 81 TCID50). Sterile P200 tips that had been previously heated over a flame to smooth any abrasive surfaces were used to deliver the virus. Mice were held in an inverted position for four minutes post-inoculation to allow virus to adsorb and to prevent immediate discharge of virus. Mice were monitored daily.
Cell culture and virus re-isolation
To re-isolate the circulating virus from infected mice by co-culture, 150µl of heparinized blood was collected by tail-bleed and red blood cells were lysed. White blood cells were cultured in Iscove’s modified Dulbecco’s medium (Invitrogen, Grand Island, NY) supplemented with 10% FBS, PHA (1µg/ml) and IL-2 (1µg/ml) for 48 hours, then co-cultured with 1×106 freshly isolated and stimulated human PBMCs. Co-cultures were maintained in IMDM with 10% FBS, PHA (1µg/ml) and IL-2 (1µg/ml). After 1 week of co-culture, supernatants were assayed for the presence of p24 by ELISA (Beckman Coulter, Fullerton, CA).
Measurement of viral load in plasma
To detect cell-free HIV-1 by RT-PCR, RNA was extracted from 25–50µl of EDTA-treated plasma using the QIAamp Viral RNA kit (Qiagen, Valencia, CA). cDNAs were produced with Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) using a primer set specific for the HIV-1 LTR sequence, and Q-PCR was performed with the same primer set and a LTR-specific probe (Rouet et al., 2005) using Supermix UDG (Invitrogen, Carlsbad, CA).
Flow cytometry
Whole blood was collected and red blood cells lysed as reported previously (Berges et al., 2006). Peripheral blood cells were stained for hCD3-PE and hCD4-PECy5 (Caltag) markers and analyzed using a Coulter EPICS XL-MCL FACS analyzer (Beckman Coulter, Fullerton, CA). CD4+ T cell levels were calculated as a ratio of the entire CD3 population (CD4+CD3+:CD4−CD3+). To establish baseline CD4+ T cell ratios, all mice were analyzed prior to infection.
In situ hybridization for HIV-1 RNA
In situ hybridization was performed on 4-µm formalin-fixed, paraffin-embedded tissue sections from thymus, mesenteric lymph nodes, spleen, and gut tissue from HIV-1 infected and uninfected RAG-hu mice. At least three sections were evaluated from each tissue. Hybridization was performed using digoxigenin-labeled antisense and sense riboprobes specific for HIV-1 env (nt 6411–6752) and detected using nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) as reported previously (Berges, Wolfe, and Fraser, 2005). Sections were lightly counterstained with hematoxylin to enable visualization of nuclei.
Immunostaining of mucosal tissues
Four micron frozen sections were fixed in 1% paraformaldehyde in Phosphate Buffered Saline for 20 min., washed in Tris Buffered Saline (TBS), pH 7.6 (50 mM TRIS Hydrochloride, 150 mM Sodium Chloride) and blocked for 1 hour with Vector Mouse Ig Blocking Reagent (Vector Laboratories, Burlingame, CA). After washing in TBS, slides were incubated 30 min. with primary antibodies diluted in TBS with 1% Bovine Serum Albumin and 0.1% Sodium Azide (antibody diluent), followed by three 10 min. washes in TBS and a 15 min. incubation with Alexa Fluor® 488 Goat anti-Mouse IgG (Molecular Probes, Eugene, OR) diluted in antibody diluent. After three washes in TBS, sections were coverslipped using SlowFade® Gold antifade reagent with DAPI (Molecular Probes). Slides were viewed and imaged on a Leica DM5000B microscope (Leica, Cambridge, England) equipped with epifluorescence and digital monochrome camera (Leica DFC350FX) using Leica FW4000 software. Primary antibodies that were determined to not cross-react with mouse antigens and used for staining were: CD3 clone UCHT1 (BD Biosciences Pharmingen, San Jose, CA), CD4 clone RPA-T4 (BD Biosciences Pharmingen), CD45 LCA, clone 2B11 + PD7/26 (DakoCytomation, Denmark) and CD68 clone EBM11 (DakoCytomation).
Acknowledgements
Work reported here was supported by NIH RO1 grants AI50492, AI057066 and AI073255 to R.A. This work has also been facilitated by the infrastructure and resources provided by the Colorado Center for AIDS Research Grant P30 AI054907. We thank Leila Remling and Jes Kuruvilla for assistance in RAG-hu mouse production. We thank Irving Weissman of Stanford University for supplying the Rag mice breeding stock and NIH AIDS Research and Reference Reagents Program for HIV-1 related reagents used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An DS, Poon B, Fang RHT, Weijer K, Blom B, Spits H, Chen ISY, Uittenbogaart CH. The human immune system (HIS) Rag2−/−γc−/− mouse, a novel chimeric mouse model for HIV-1 infection. Clinical Vaccine Immunology. 2007;14:391–396. doi: 10.1128/CVI.00403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Akkina R. CXCR4 and CCR5 shRNA transgenic CD34+ cell derived macrophages are functionally normal and resist HIV-1 infection. Retrovirology. 2005;2:53–64. doi: 10.1186/1742-4690-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J, Akkina R. Complete knockdown of CCR5 by lentiviral vector-expressed siRNAs and protection of transgenic macrophages against HIV-1 infection. Gene Ther. 2007;14:1287–1297. doi: 10.1038/sj.gt.3302958. [DOI] [PubMed] [Google Scholar]
- Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, Behnke S, Frey J, Oxenius A, Joller H, Aguzzi A, Manz MG, Speck RF. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−{gamma}c−/− mice. Proc Natl Acad Sci USA. 2006;103:15951–15956. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Ann Rev Imm. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Berges BK, Wheat WH, Palmer BE, Connick E, Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gc−/− (RAG-hu) mouse model. Retrovirology. 2006;3:76. doi: 10.1186/1742-4690-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges BK, Wolfe JH, Fraser NW. Stable levels of long-term transgene expression driven by the latency-associated transcript promoter in a herpes simplex virus type 1 vector. Mol Ther. 2005;12:1111–1119. doi: 10.1016/j.ymthe.2005.06.478. [DOI] [PubMed] [Google Scholar]
- Burkhard MJ, Dean GA. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr HIV Res. 2003;1:15–29. doi: 10.2174/1570162033352101. [DOI] [PubMed] [Google Scholar]
- Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- D'Cruz OJ, Uckun FM. Limitations of the Human-PBL-SCID Mouse Model for Vaginal Transmission of HIV-1. Am J Reprod Immunol. 2007;57:353–360. doi: 10.1111/j.1600-0897.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Gorantla S, Sneller H, Walters L, Sharp JG, Pirruccello SJ, West JT, Wood C, Dewhurst S, Gendelman HE, Poluektova L. HIV-1 pathobiology studied in humanized Balb/c-Rag2−/−{gamma}c−/− mice. J Virol. 2006;81:2700–2712. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- Jamieson BD, Aldrovandi GM, Zack JA. The SCID-hu mouse: an in-vivo model for HIV-1 pathogenesis and stem cell gene therapy for AIDS. Sem in Immun. 1996;8:215–221. doi: 10.1006/smim.1996.0027. [DOI] [PubMed] [Google Scholar]
- Khanna KV, Whaley KJ, Zeitlin L, Moench TR, Mehrazar K, Cone RA, Liao Z, Hildreth JE, Hoen TE, Shultz L, Markham RB. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J Clin Invest. 2002;109:205–211. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized Rag2−/−γc−/− (RAG-hu) mice. Virol. 2007 doi: 10.1016/j.virol.2007.06.005. (in press) [DOI] [PubMed] [Google Scholar]
- McGowan I. Microbicides: a new frontier in HIV prevention. Biologicals. 2006;34:241–255. doi: 10.1016/j.biologicals.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Alexander NJ, Sutjipto S, Lackner AA, Gettie A, Hendrickx AG, Lowenstine LJ, Jennings M, Marx PA. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier DE. Human immunodeficiency virus infection of human cells transplanted to severe combined immunodeficient mice. Adv Immun. 1996;63:79–125. doi: 10.1016/s0065-2776(08)60855-x. [DOI] [PubMed] [Google Scholar]
- Olmsted SS, Khanna KV, Ng EM, Whitten ST, Johnson ONr, Markham RB, Cone RA, Moench TR. Low pH immobilizes and kills human leukocytes and prevents transmission of cell-associated HIV in a mouse model. BMC Infect Dis. 2005;5:79. doi: 10.1186/1471-2334-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439:89–94. doi: 10.1038/nature04263. [DOI] [PubMed] [Google Scholar]
- Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–852. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- Rouet F, Ekouevi DK, Chaix ML, Burgard M, Inwoley A, Tony TD, Danel C, Anglaret X, Leroy V, Msellati P, Dabis F, Rouzioux C. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol. 2005;43:2709–2717. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Cellular Targets of Infection and Route of Viral Dissemination after an Intravaginal Inoculation of Simian Immunodeficiency Virus into Rhesus Macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, Melkus MW, Padgett-Thomas A, Zupancic M, Haase AT, Garcia JV. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204:705–714. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, Dewan MZ, Yu Z, Ito M, Morio T, Shimizu N, Honda M, Yamamoto N. Hematopoietic stem cell-engrafted NOD/SCID/IL2R{gamma}null mice develop human lymphoid system and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–218. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kovalev GI, Su L. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood. 2006;109:2978–2981. doi: 10.1182/blood-2006-07-033159. [DOI] [PMC free article] [PubMed] [Google Scholar]