Emergence of Oxacillinase-Mediated Resistance to Imipenem in Klebsiella pneumoniae (original) (raw)
Abstract
Klebsiella pneumoniae strain 11978 was isolated in Turkey in 2001 and was found to be resistant to all β-lactams, including carbapenems. Cloning and expression in Escherichia coli identified five β-lactamases, including two novel oxacillinases. The β-lactamase OXA-48 hydrolyzed imipenem at a high level and was remotely related (less than 46% amino acid identity) to the other oxacillinases. It hydrolyzed penicillins and imipenem but not expanded-spectrum cephalosporins. The bla_OXA-48 gene was plasmid encoded and not associated with an integron, in contrast to most of the oxacillinase genes. An insertion sequence, IS_1999, was found immediately upstream of _bla_OXA-48. Another plasmid that encoded a second oxacillinase gene, _bla_OXA-47, located inside a class 1 integron was identified in K. pneumoniae 11978. OXA-47 had a narrow spectrum of hydrolysis activity and did not hydrolyze ceftazidime or imipenem, as is found for the β-lactamase (OXA-1) to which it is related. In addition, β-lactamases TEM-1 and SHV-2a were expressed from the same K. pneumoniae isolate. Analysis of the outer membrane proteins of this isolate revealed that it lacked a porin of ca. 36 kDa. Thus, the high-level resistance to β-lactams of this clinical isolate resulted from peculiar β-lactamases and modification of outer membrane proteins.
Acquired resistance to expanded-spectrum cephalosporins has been reported in Klebsiella pneumoniae and is mainly related to the production of Ambler class A extended-spectrum β-lactamases (ESBLs) (20) and plasmid-encoded AmpC cephalosporinases (28). A few reports have described β-lactamase-mediated resistance to carbapenems in K. pneumoniae related to Ambler class B metalloenzymes, i.e., IMP-1 (15) and IMP-8 (38) from Southeast Asia. The plasmid-mediated Ambler class A β-lactamase KPC-1 has also been reported to be a source of carbapenem resistance in a K. pneumoniae isolate from North Carolina (39). In this case, modification of outer membrane proteins (OMPs) was shown to play an additional role in carbapenem resistance, as is found with AmpC-type enzymes (2, 7, 18, 19).
Among the Ambler class D enzymes, rare β-lactamases with weak carbapenem-hydrolyzing activities have been characterized. These are OXA-24, OXA-25, OXA-26, OXA-27, and OXA-40 from Acinetobacter baumannii isolates (1, 6, 12), whereas OXA-23 was found in A. baumannii and Proteus mirabilis (5, 10, 26). The OXA-23 and OXA-27 β-lactamases share 99% amino acid identity, whereas they share 60% identity with the amino acid sequences of a second group of these oxacillinases, consisting of OXA-24, OXA-25, OXA-26, and OXA-40, which differ by a few amino acid substitutions (12, 34).
In the present study we have characterized the β-lactamase content of a K. pneumoniae isolate that was resistant to all available β-lactams. Thus, we have identified a novel carbapenem-hydrolyzing oxacillinase with strong carbapenem-hydrolyzing activity.
MATERIALS AND METHODS
Bacterial strains and plasmids.
K. pneumoniae 11978 was a clinical isolate from the Istanbul Faculty Hospital, Istanbul, Turkey. It was identified with the API 32GN system (bioMérieux, Marcy l'Etoile, France). Escherichia coli reference strain DH10B and plasmid pBK-CMV (Stratagene, Amsterdam, The Netherlands) were used for cloning experiments. K. pneumoniae CIP53153 and E. coli K-12 (a gift from P. Plésiat) were used as reference strains in endonuclease I-_Ceu_I digestion experiments. K. pneumoniae CIP53153 was also used in transformation experiments.
Antimicrobial agents and MIC determinations.
The antimicrobial agents and their sources have been described elsewhere (29). Antibiotic-containing disks were used for detection of antibiotic susceptibility with Mueller-Hinton agar plates by a disk diffusion assay (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France). MICs were determined by an agar dilution technique, as reported previously (31), and the results were interpreted in accordance with the guidelines of the National Committee for Clinical Laboratory Standards (24).
Cloning experiments.
Whole-cell DNA of K. pneumoniae 11978 was extracted as described previously (29). Cloning experiments were performed with _Bam_HI- or _Bss_HII-digested DNA of K. pneumoniae 11978 and _Bam_HI- or Bss_HII-restricted plasmid pBK-CMV, followed by expression of recombinant plasmids in E. coli DH10B, as described previously (31). Antibiograms were performed with E. coli DH10B harboring recombinant plasmids, and the sizes of the plasmid inserts were determined by restriction analysis (35). Recombinant plasmids pVT-1 to pVT-5 were retained for further analysis. In addition, since part of the IS_1999 element was present in pVT-1, it was amplified from whole-cell DNA of K. pneumoniae 11978 by PCR with primers IS1999B and OXA-TKext (Table 1).
TABLE 1.
Sequences of primers designed for this study
| Primer | Sequence (5′→3′)a | Location |
|---|---|---|
| KPC-1A | CGTTCTTGTCTCTCATGGCC | _bla_KPC-1 |
| KPC-1B | CCTCGCTGTGCTTGTCATCC | _bla_KPC-1, reverse primer |
| OXA-48A | TTGGTGGCATCGATTATCGG | _bla_OXA-48 |
| OXA-48B | GAGCACTTCTTTTGTGATGGC | _bla_OXA-48, reverse primer |
| OXA-TKext1 | AATACACGCATAACGTCCCC | _bla_OXA-48, 5′ reverse primer |
| OXA-1A | TCAACTTTCAAGATCGCA | _bla_OXA-47 |
| OXA-1B | GTGTGTTTAGAATGGTGA | _bla_OXA-47, reverse primer |
| OXA-9A | TTCGTTTCCGCCACTCTCCC | _bla_OXA-9 |
| OXA-9B | ACGAGAATATCCTCTCGTGC | _bla_OXA-9, reverse primer |
| SWSHV-A | AAGATCCACTATCGCCAGCAG | _bla_SHV |
| SWSHV-B | ATTCAGTTCCGTTTCCCAGCGG | _bla_SHV, reverse primer |
| TEMalpha | GAGTATTCAACATTTCCGTGTC | _bla_TEM |
| TEMbeta | TAATCAGTGAGGCACCTATCTC | _bla_TEM reverse primer |
| IS1999A | CAGCAATTCTTTCTCCGTG | IS1999 tnpA |
| IS1999B | CAAGCACAACATCAAGCGC | IS1999 tnpA, reverse primer |
| A | AGAGTTTGATCHTGGYTYAGA | 16S RNA gene |
| B | ACGGYTACCTTGTTACGACTTC | 16S RNA gene |
| 5′CS | GGCATCCAAGCAGCAAG | attI recombination site |
| 3′CS | AAGCAGACTTGACCTGA | qacE_Δ_1, 5′ end, reverse primer |
DNA sequencing and protein analysis.
Both strands of the cloned DNA fragments were sequenced with an Applied Biosystems sequencer (ABI 373). The nucleotide and deduced protein sequences were analyzed with software available over the Internet at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov).
Plasmid analysis and hybridizations.
Extraction of plasmid DNA from K. pneumoniae 11978 and the electroporants was performed by the method of Kieser (14). Analysis was performed on a 0.7% agarose gel with ethidium bromide staining after migration. Southern transfer was performed on a nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Orsay, France) as described previously (33). The plasmid extract of K. pneumoniae 11978 was also used for transformation experiments with a Gene Pulser II electroporator (Bio-Rad, Ivry-sur-Seine, France) and selection on amoxicillin (100 μg/ml)-containing plates.
The endonuclease I-_Ceu_I (New England Biolabs, Saint-Quentin-en-Yvelines, France), which digests a 26-bp sequence in rrn genes for 23S large-subunit rRNA (17), was used to identify the genetic locations of the β-lactamase genes. After digestion, the DNA fragments were separated by pulsed-field gel electrophoresis (PFGE) with a CHEF-DRII apparatus (Bio-Rad) (11). The sizes of the endonuclease I-_Ceu_I-generated fragments were determined by comparison with those generated from E. coli K-12 (17). The I-_Ceu_I-generated DNA fragments of K. pneumoniae 11978 and CIP53513 and plasmid DNA of K. pneumoniae 11978 and the transformants analyzed by PFGE were transferred onto a nylon membrane by the Southern technique and subsequently UV cross-linked (Stratalinker; Stratagene) for 2 min. The membranes were successively hybridized with several probes, including a 743-bp PCR-generated probe specific for the _bla_OXA-48 gene (primers OXA-48A and OXA-48B [Table 1]), a 609-bp PCR-generated probe specific for the _bla_OXA-47 gene (primers OXA-1A and OXA-1B [Table 1]), a 300-bp PCR-generated probe specific for _bla_SHV genes (primers SWSHV-A and SWSHV-B [Table 1]), a 850-bp PCR-generated probe specific for the bla_TEM-1 gene (primers TEMalpha and TEMbeta [Table 1]), a 985-bp PCR-generated probe specific for the IS_1999 transposase gene (primers IS1999A and IS1999B [Table 1]), and a 1,504-bp PCR-generated probe specific for 16S and 23S rRNA genes (primers A and B [Table 1]). Southern hybridizations were performed with the ECL nonradioactive labeling and detection kit (Amersham Pharmacia Biotech) as described by the manufacturer.
IEF analysis.
Isoelectric focusing (IEF) analysis was performed with an ampholine polyacrylamide gel (pH 3.5 to 9.5) as described previously (31) with culture extracts of K. pneumoniae 11978 and E. coli DH10B harboring recombinant plasmids.
β-Lactamase purification.
A culture of E. coli DH10B harboring recombinant plasmid pVT-1 that produced OXA-48 was grown overnight at 37°C in 4 liters of tryptic soy broth containing 100 μg of amoxicillin per ml and 30 μg of kanamycin per ml. The protein extracts obtained were purified as described previously (32). Briefly, the extracts were subjected to purification steps, including ion-exchange chromatography with Q-Sepharose and 20 mM Tris-HCl buffer (pH 9.0), followed by chromatography with S-Sepharose columns and 100 mM sodium phosphate buffer (pH 5.8). Elution of the β-lactamase was performed with a K2SO4 gradient in order to prevent any potential inhibition by NaCl. Peaks of β-lactamase activity were concentrated by using Centrisart-C30 spin columns (Sartorius, Göttingen, Germany) and were dialyzed with 100 mM phosphate buffer (pH 7.0).
Kinetic studies.
Purified β-lactamases were used for kinetic measurements, which were determined at 30°C in 100 mM sodium phosphate (pH 7.0) (34). The _k_cat and Km values were determined by analyzing β-lactam hydrolysis under initial-rate conditions with a UV spectrophotometer, as described previously (31). The 50% inhibitory concentrations (IC50s) of clavulanic acid, tazobactam, sulbactam, and NaCl were determined as described previously (31). The specific activities of the protein extracts and purified β-lactamase from the culture of E. coli DH10B(pVT-1) were determined as described previously (3). The protein purification rate was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The protein content was measured by the Bio-Rad DC protein assay. The specific activity of the β-lactamase for imipenem was also determined by UV spectrophotometry (wavelength, 297 nm) with a culture extract of K. pneumoniae 11978 from 10 ml of tryptic soy broth with 100 μM imipenem, as described previously (3). One unit of enzyme activity was defined as the activity that hydrolyzed 1 μmol of imipenem per min per mg of protein.
OMP analysis.
OMPs were isolated by sarcosyl extraction of total membrane preparations, as described previously (13). Briefly, cell cultures were harvested in logarithmic phase and lysed by sonication. OMPs were obtained after treatment of the cell membranes with sodium lauryl sarcosylate (Sigma, Saint-Quentin-Fallavier, France) and subsequent ultracentrifugation. The proteins were examined by SDS-PAGE on 10% polyacrylamide gels.
Nucleotide sequence accession numbers.
The nucleotide sequences of _bla_OXA-48 and _bla_OXA-47 reported in this paper have been submitted to the EMBL/GenBank nucleotide sequence database and have been given accession nos. AY236073 and AY237830, respectively.
RESULTS
Origin of K. pneumoniae isolate and preliminary antibiotic susceptibility testing.
K. pneumoniae 11978 was isolated in September 2001 at the Istanbul Faculty Hospital from the urinary tract of a 54-year-old man with a urinary tract infection and skin burns. An antibiotic regimen consisting of vancomycin and meropenem had been given for 1 month before strain isolation. Antibiotic susceptibility testing suggested an uncommon mechanism of resistance since the isolate was resistant to all β-lactams tested (Table 2). Addition of clavulanic acid and tazobactam did not significantly decrease the MICs of ticarcillin, piperacillin, and ceftazidime (Table 2). K. pneumoniae 11978 was also resistant to aminoglycosides, chloramphenicol, nalidixic acid, rifampin, sulfonamides, and tetracycline and of intermediate susceptibility to ciprofloxacin (data not shown). Culture extracts of K. pneumoniae 11978 analyzed by IEF gave five β-lactamases with pIs of 5.4, 7.2, 7.4, 7.6, and 7.7 (data not shown). To determine whether resistance to carbapenems was due to at least one of these β-lactamases, preliminary hydrolysis experiments were performed with culture extracts of K. pneumoniae 11978, which showed significant imipenem-hydrolyzing activity (22 mU/mg of protein).
TABLE 2.
MICs of β-lactams for K. pneumoniae 11978; K. pneumoniae CIP53153 harboring natural plasmid pA-1; E. coli DH10B harboring recombinant plasmids pVT-1, pVT-2, and pVT-3; E. coli DH10B harboring natural plasmid pA-1 alone or with pA-2; and E. coli DH10B and K. pneumoniae CIP53153 reference strains
| β-Lactam(s)a | MIC (μg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K. pneumoniae 11978 | K. pneumoniae CIP53153 (pA-1) (OXA-48) | K. pneumoniae CIP53153 | E. coli DH10B (pA-1) (OXA-48) | E. coli DH10B (pA-1, pA-2) (TEM-1, SHV-2a, OXA-47, OXA-48) | E. coli DH10B (pVT-1) (OXA-48) | E. coli DH10B (pVT-2) (OXA-47) | E. coli DH10B (pVT-3) (SHV-2a) | E. coli DH10B | |
| Amoxicillin | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 4 |
| Amoxicillin + CLA | >512 | 128 | 4 | >512 | >512 | >512 | 128 | 128 | 4 |
| Ticarcillin | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 4 |
| Ticarcillin + CLA | >512 | 128 | 2 | >512 | >512 | >512 | 128 | 256 | 4 |
| Piperacillin | >512 | 8 | 2 | 64 | 256 | 128 | 32 | >512 | 2 |
| Piperacillin + TZB | 512 | 4 | 0.5 | 64 | 128 | 128 | 16 | 128 | 2 |
| Cephalothin | >512 | 0.5 | 0.5 | 8 | 512 | 64 | 2 | >512 | 4 |
| Cefuroxime | 256 | 0.12 | 0.12 | 8 | 8 | 8 | 4 | 512 | 4 |
| Cefoxitin | 128 | 2 | 2 | 4 | 8 | 8 | 1 | 32 | 4 |
| Ceftazidime | 512 | 0.12 | 0.12 | 0.12 | 64 | 0.12 | 0.06 | >512 | 0.06 |
| Ceftazidime + CLA | 256 | 0.12 | 0.12 | 0.12 | 2 | 0.12 | 0.06 | 2 | 0.06 |
| Cefotaxime | 64 | 0.06 | 0.06 | 0.25 | 2 | 0.25 | 0.06 | 128 | 0.06 |
| Cefepime | 32 | 0.06 | 0.06 | 0.25 | 0.25 | 0.06 | 0.06 | 32 | 0.06 |
| Cefpirome | 128 | 0.12 | 0.06 | 0.5 | 0.5 | 0.25 | 0.12 | 32 | 0.06 |
| Moxalactam | 64 | 0.06 | 0.06 | 4 | 4 | 2 | 0.06 | 1 | 0.06 |
| Aztreonam | 512 | 0.06 | 0.06 | 0.06 | 64 | 0.06 | 0.06 | >512 | 0.12 |
| Imipenem | 64 | 2 | 0.06 | 2 | 2 | 2 | 0.06 | 0.25 | 0.06 |
| Meropenem | 64 | 0.25 | 0.06 | 0.25 | 0.25 | 0.25 | 0.06 | 0.06 | 0.06 |
Cloning and sequencing of β-lactamase content.
Cloning of _Bss_HII- or _Sau_3AI-restricted DNA of K. pneumoniae 11978 into pBK-CMV and expression in E. coli DH10B gave recombinant plasmids expressing different β-lactam resistance phenotypes. E. coli DH10B with recombinant plasmid pVT-1 had an oxacillinase (pI 7.2) phenotype, consistent with a carbapenem-hydrolyzing activity, which confers resistance to most penicillins and reduced susceptibility to imipenem (Table 2).
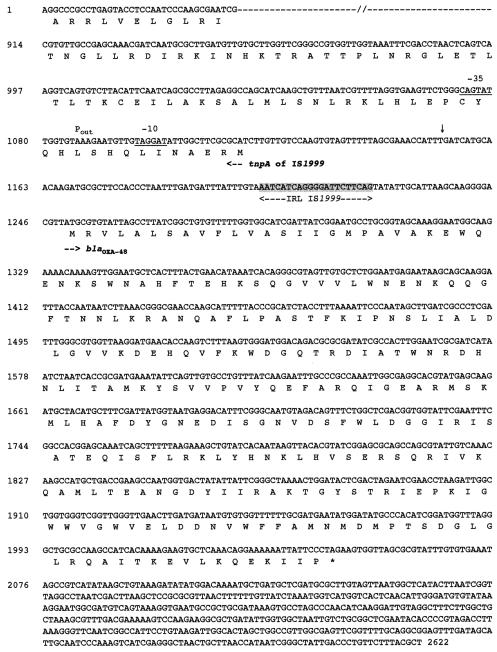
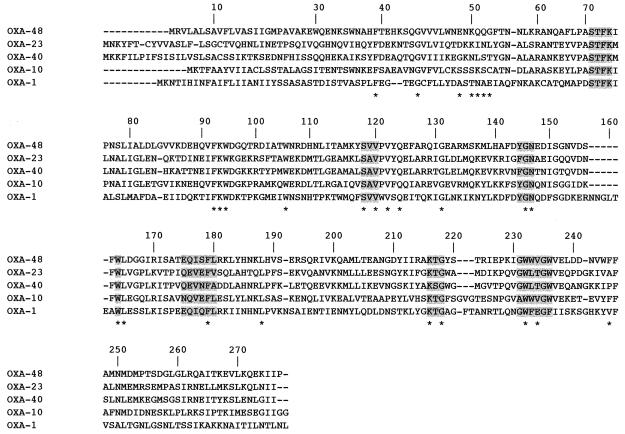
DNA sequence analysis of the 1.5-kb insert of pVT-1 identified a 798-bp open reading frame (ORF) for _bla_OXA-48 that encoded a 265-amino-acid protein (Fig. 1). A serine-threonine-phenylalanine-lysine tetrad (S-T-F-K) was found at positions 70 to 73 in the deduced protein encoded by this ORF. All four structural elements characteristic of Ambler class D β-lactamases (DBLs) were found: W-X-X-X-X-X-X-I-X at DBL positions 164 to 172, Q-X-X-X-L at DBL positions 176 to 180, K-T-G at positions 216 to 218, and YGN at DBL positions 144 to 146 (8, 16, 21). A K-T-G element (positions 216 to 218) was found, as in the carbapenem-hydrolyzing OXA-23 and OXA-27 β-lactamases, whereas it was replaced by a K-S-G motif in the second group of carbapenem-hydrolyzing oxacillinases, consisting of OXA-24, OXA-25, OXA-26, and OXA-40. The fourth structural element of oxacillinases, YGN, was not replaced by an FGN motif in OXA-48 as it was in the other carbapenem-hydrolyzing oxacillinases (34). OXA-48 was weakly related to other oxacillinases and shared 46, 36, 32, and 21% amino acid identities with OXA-10; OXA-23 and OXA-27; a cluster consisting of OXA-24, OXA-25, OXA-26, and OXA-40; and OXA-1, respectively (Fig. 2).
FIG. 1.
Nucleotide sequence of a 2,622-bp fragment of recombinant plasmid pA-1 containing the bla_OXA-48 gene. The deduced amino acid sequence is designated in single-letter code below the nucleotide sequence. The start codons of the ORFs are indicated by horizontal arrows. The left inverted repeat (IRL) of IS_1999 is shaded in gray, and the putative −35 and −10 promoter sequences (Pout) provided by the insertion sequence element are underlined. The tnpA transposase gene is indicated. The asterisk indicates the stop codon, and the vertical arrow indicates the _Sau_3AI cloning insertion site in pVT-1.
FIG. 2.
Comparison of the amino acid sequence of OXA-48 to those of OXA-23, OXA-40, OXA-1, and OXA-10. The shading indicates conserved residues of the oxacillinases, and asterisks indicate highly conserved residues. The numbering of the β-lactamases is according to DBL (8).
Part of insertion sequence element IS_1999_ was found upstream of the _bla_OXA-48 gene in pVT-1 (Fig. 1). Since only 96 bp upstream of the bla_OXA-48 was cloned in pVT-1, PCR experiments were performed with combinations of primers specific for the IS_1999 transposase gene and for the 5′ part of bla_OXA-48 and with whole-cell DNA of K. pneumoniae 11978. An entire IS_1999 element was found. This element is also found upstream of the class A ESBL bla_VEB-1 gene of Pseudomonas aeruginosa isolates from Thailand (23). The IS_1999 element that was located 26 bp upstream of the start codon of bla_OXA-48 likely provided promoter sequences. Indeed, DNA sequences typical of −35 and −10 enterobacterial promoter regions were found inside the IS_1999 transposase-coding sequence (Fig. 1; Pout). No transposon or integron structure was found downstream of the _bla_OXA-48 gene. Thus, unlike most of the oxacillinase genes (21, 34), _bla_OXA-48 was not in the form of a gene cassette.
A second recombinant strain, E. coli DH10B(pVT-2), expressed another oxacillinase (pI 7.4) phenotype and was resistant to penicillins and β-lactamase inhibitors and susceptible to most cephalosporins and carbapenems (Table 2). Analysis of part of the 8-kb cloned fragment of pVT-2 identified the _bla_OXA-47 gene encoding a novel oxacillinase that had seven amino acid substitutions compared to the sequence of OXA-1, i.e., Pro34Gln, Thr65Ala, Thr98Ile, Lys191Arg, Glu200Asp, Asp208Glu, and Asn254Ser (DBL numbering [8]). None of these changes was located inside the conserved motifs of DBLs (16, 21). The _bla_OXA-47 gene was in the form of a gene cassette in a class 1 integron structure. The 59-bp-element sequence associated with this β-lactamase gene was 108 bp long and had a 1-bp change compared to the sequence of the oxa-1 cassette (25). The oxa-47 gene cassette was located downstream of an _aadA_-like gene cassette that was truncated in recombinant plasmid pVT-2 and downstream of a qacF gene cassette identical to that previously reported from Enterobacter aerogenes (30).
Another recombinant plasmid, pVT-3, gave an ESBL (pI 7.7) phenotype to E. coli DH10B due to the encoded β-lactamase SHV-2a. E. coli DH10B(pVT-3) was resistant to penicillins and expanded-spectrum cephalosporins but not to carbapenems (Table 2). Its activity was inhibited by clavulanic acid and tazobactam (Table 2). As is observed in P. aeruginosa RP-1 (22), the bla_SHV-2a gene was preceded by an IS_26 insertion sequence element that gave rise to a likely hybrid promoter with a −35 promoter sequence provided by the insertion sequence element and a −10 promoter sequence of the _bla_SHV-2a gene (data not shown).
E. coli DH10B harboring recombinant plasmid pVT-4 had a penicillinase (pI 5.4) phenotype corresponding to TEM-1. The _bla_TEM-1 gene was preceded by a tnpR resolvase gene, which was preceded by the bla_OXA-9 β-lactamase gene (data not shown). This structure was identical to that of Tn_1331, a plasmid-located transposon that has also been identified in K. pneumoniae (36, 37). However, a single base substitution inside the coding sequence for _bla_OXA-9 resulted in a stop codon that truncated the last 163 carboxy-terminal amino acids. This observation was consistent with the β-lactam resistance phenotype of E. coli DH10B(pVT-4), which was susceptible to β-lactamase inhibitors (the lack of expression of oxacillinase OXA-9 likely had no effect on the β-lactam resistance phenotype).
The last recombinant plasmid, pVT-5, expressed a penicillinase (pI 7.6) phenotype in E. coli DH10B that corresponded to SHV-1. This β-lactamase gene corresponded to the naturally occurring SHV enzyme found in K. pneumoniae (4).
Biochemical properties of OXA-48 β-lactamase.
After purification from culture extracts of E. coli DH10B(pVT-1), the specific activity of the OXA-48 β-lactamase against cephalothin was 17 U/mg of protein, and its purification factor was 75-fold. Protein purity was estimated to be >90% by SDS-PAGE analysis (data not shown). The OXA-48 β-lactamase had a narrow-spectrum hydrolysis profile that included penicillins, cephalothin, and imipenem and, to a lesser extent, cefotaxime, ceftazidime, cefepime, and cefpirome (Table 3). The catalytic activity (_k_cat/Km) of OXA-48 for imipenem (Table 3) was 10- and 3-fold higher than those reported for OXA-40 and the class A carbapenemase KPC-1, respectively (39). Hydrolysis of meropenem was detectable at a very low level, as is the case for the carbapenem-hydrolyzing β-lactamases OXA-24, OXA-25, and OXA-26 (1, 6). OXA-48 had hydrolytic activity against ceftazidime, although at a lower level than that of OXA-40 (Table 3). Oxacillin was significantly hydrolyzed by OXA-48, whereas it was slightly hydrolyzed by the other carbapenem-hydrolyzing oxacillinases, including OXA-40 (Table 3) (12).
TABLE 3.
Kinetic parameters for purified β-lactamase OXA-48 from K. pneumoniae 11978 compared to those for OXA-40 from A. baumannii CLA-1a
| Substrate | OXA-48 | OXA-40b | ||||
|---|---|---|---|---|---|---|
| _k_cat (s−1) | Km (μM) | _k_cat/Km (mM−1 · s−1) | _k_cat (s−1) | Km (μM) | _k_cat/Km (mM−1 · s−1) | |
| Benzylpenicillin | 245 | 40 | 6,100 | 5 | 23 | 220 |
| Ampicillin | 340 | 5,200 | 65 | 5 | 220 | 20 |
| Ticarcillin | 45 | 55 | 820 | 1 | 60 | 20 |
| Piperacillin | 75 | 410 | 180 | 1 | 23 | 50 |
| Cephalothin | 3 | 20 | 150 | 3 | 72 | 50 |
| Cephaloridine | 2 | 27 | 75 | 5 | 1,000 | 5 |
| Cefotaxime | 10 | 190 | 60 | NDc | —d | — |
| Ceftazidime | 4 | 5,100 | 1 | 20 | 2,500 | 10 |
| Cefepime | 1 | 160 | 6 | ND | — | — |
| Cefpirome | 8 | 390 | 20 | ND | — | — |
| Oxacillin | 25 | 30 | 850 | 2 | 876 | 3 |
| Aztreonam | ND | — | — | ND | — | — |
| Imipenem | 2 | 14 | 145 | 0.1 | 6.5 | 15 |
| Meropenem | 0.1 | 200 | 0.5 | ND | — | — |
Studies of activity inhibition, as measured by determination of IC50s, showed that OXA-48 was weakly inhibited by clavulanic acid (IC50, 16 μM), tazobactam (IC50, 1.7 μM), and sulbactam (IC50, 50 μM), as has been found for most of the oxacillinases (24). Nevertheless, these IC50s were lower than those reported for OXA-40 (12). OXA-48 activity was inhibited by NaCl (IC50, 7 mM), which is a property commonly observed for oxacillinases except those possessing an FGN motif at DBL positions 144 to 146 (12).
Genetic support of the β-lactamase genes.
Analysis of plasmid extracts of K. pneumoniae 11978 identified a 70-kb plasmid (pA-1) and a 140-kb plasmid (pA-2) (data not shown). After electroporation into E. coli DH10B, three β-lactam-resistant phenotypes were obtained. Transformants contained either pA-1 or pA-2, or both. E. coli DH10B(pA-1) expressed an oxacillinase phenotype with reduced susceptibility to imipenem (Table 2), since pA-1 had the _bla_OXA-48 gene. E. coli DH10B(pA-2) expressed an ESBL phenotype that corresponded to SHV-2a (data not shown). PCR experiments indicated that E. coli DH10B(pA-2) possessed the _bla_SHV-2a, _bla_OXA-47, and _bla_TEM-1 genes and a nonexpressed _bla_OXA-9 gene. Transformants harboring pA-1 and pA-2 expressed a broad-spectrum β-lactam resistance phenotype, including resistance to ceftazidime and aztreonam and reduced susceptibility to imipenem (Table 1).
Although most of the β-lactamase genes identified were located on plasmids, the I-_Ceu_I digestion technique was used to search the K. pneumoniae 11978 chromosome for an additional chromosomal location of these novel _bla_OXA-48 and _bla_OXA-47 genes. Whereas a DNA probe specific for rRNA genes hybridized with five of six fragments of whole-cell DNA of K. pneumoniae 11978 generated with I-_Ceu_I, the _bla_OXA-48- and _bla_OXA-47-specific probes did not hybridize with any of these fragments, indicating that these genes are located only on plasmids.
OMPs of K. pneumoniae 11978.
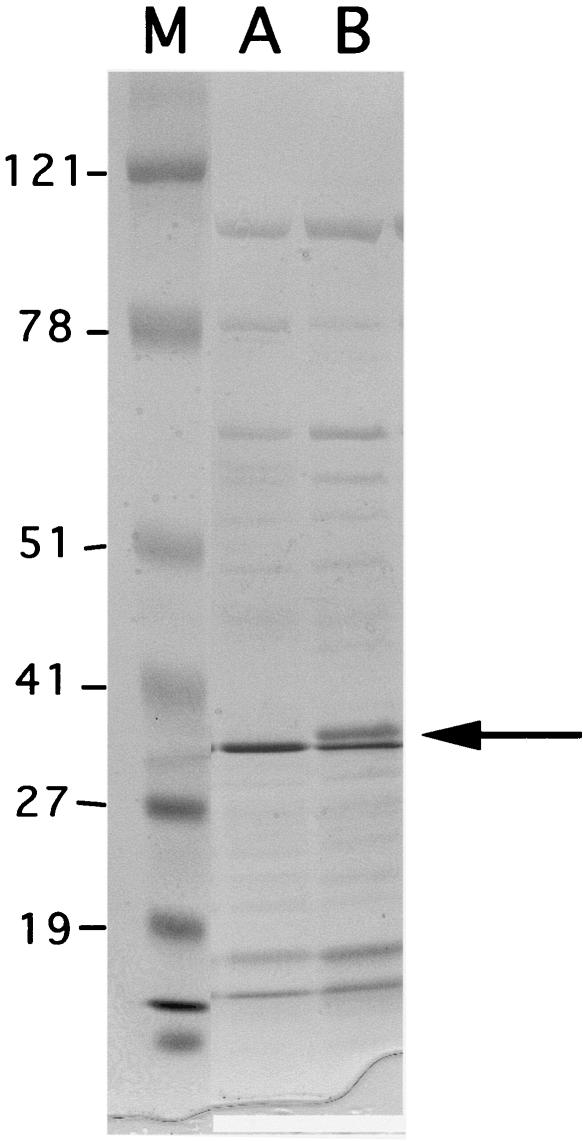
Because natural plasmid pAT-1 conferred decreased susceptibility to imipenem once it was transformed into E. coli DH10B, this plasmid was also used to transform reference strain K. pneumoniae CIP53153 to evaluate its effect on the susceptibility of a wild-type K. pneumoniae strain. Similar β-lactam MICs were obtained for the reference strain, indicating that the type of species of the family Enterobacteriaceae by itself does not explain the high imipenem MIC for K. pneumoniae 11978 (Table 2). OMP analysis of K. pneumoniae 11978 was performed, and the results were compared to that for a K. pneumoniae reference strain. K. pneumoniae 11978 lacked a 36-kDa porin, whereas the 35- and 37 kDa porins were found in cell extracts of both strains in the form of a doublet (Fig. 3).
FIG. 3.
OMP profiles of K. pneumoniae 11978 (lane A) and reference strain K. pneumoniae CIP53153 (lane B). Lane M, molecular size marker, with sizes (in kilodaltons) indicated on the left. The arrow indicates the position of the 36-kDa protein that was missing from K. pneumoniae 11978, whereas the 35- and 37-kDa proteins likely appeared in the form of a doublet.
DISCUSSION
A novel carbapenem-hydrolyzing oxacillinase, OXA-48, that was weakly related to other oxacillinases, including those with carbapenem-hydrolyzing activities, was identified. The OXA-48 β-lactamase significantly hydrolyzed carbapenems at a level much higher than those of the other carbapenem-hydrolyzing oxacillinases and at a level similar to that of the class A carbapenem-hydrolyzing β-lactamase KPC-1 (39).
Unlike OXA-23 to OXA-27 and OXA-40, OXA-48 possesses the classical YGN motif of oxacillinases instead of an FGN motif at DBL positions 144 to 146, confirming that a Phe residue at DBL position 144 is not required by itself to provide carbapenem hydrolytic activity (12). The inhibition of OXA-48 activity by NaCl confirmed that this inhibition property is related to a Tyr residue at DBL position 144, whereas the other carbapenem-hydrolyzing oxacillinases, which have a Phe residue at this position, are resistant to inhibition by NaCl (12).
The plasmid-encoded bla_OXA-48 gene was located just downstream of IS_1999, which likely provided promoter sequences and which may also have played a role in the process of mobilization of this β-lactamase gene. This is the first report of a tight association between an insertion sequence element and an oxacillinase gene. This observation raises the question of the origin of IS_1999_, which has so far been found in association with the class A ESBL _bla_VEB-1 gene in P. aeruginosa in Thailand and A. baumannii in France (23; personal communication). Like the other carbapenem-hydrolyzing oxacillinase genes, _bla_OXA-48 was not present in the form of a gene cassette in a class 1 integron, as opposed to most of the oxacillinase genes. This result again raises the question of the origin of these peculiar oxacillinase genes.
Five additional β-lactamase genes were identified in the same K. pneumoniae isolate, providing further confirmation that a single isolate can carry multiple β-lactamase genes. Two studies reported that expression of the SHV-2 ESBL in K. pneumoniae, which was associated with reduced porin production, may contribute to cephalosporin resistance and reduced susceptibility to imipenem (9, 18). On the other hand, Martinez-Martinez et al. (19) did not observe a significant increase in the imipenem MIC when the SHV-2, SHV-3, or SHV-5 ESBL was expressed in a K. pneumoniae mutant from which OmpK36 was deleted, whereas the imipenem MIC increased significantly for a mutant from which OmpK36 was deleted and which carried an AmpC β-lactamase. Although OXA-48 contributed to the carbapenem resistance in K. pneumoniae 11978, OMP deficiency may also have participated by further raising the level of carbapenem resistance.
This study is the second description of a carbapenem-hydrolyzing oxacillinase in a member of the family Enterobacteriaceae, after that of OXA-23 in P. mirabilis (5). Interestingly, the level of imipenem resistance conferred by OXA-48 in E. coli is similar to that reported for several class B enzymes. It would be interesting to estimate the prevalence of this novel β-lactamase gene in Turkey, where the OXA-48-positive isolate originated, since gram-negative bacteria in Turkey have a high level of resistance to β-lactams (27).
Acknowledgments
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, Paris France.
REFERENCES
- 1.Afzal-Shah, M., N. Woodford, and D. M. Livermore. 2001. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D β-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 45**:**583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardanuy, C., J. Linares, M. A. Dominguez, S. Hernandez-Alles, V. J. Benedi, and L. Martinez-Martinez. 1998. Outer membrane profiles of clonally related Klebsiella pneumoniae isolates from clinical samples and activities of cephalosporins and carbapenems. Antimicrob. Agents Chemother. 42**:**1636-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, D., L. Poirel, J. Chevalier, S. Léotard, J. M. Pagès, and P. Nordmann. 2001. Oxacillinase-mediated resistance to cefepime and susceptibility to ceftazidime in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45**:**1615-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthélémy, M., J. Péduzzi, and R. Labia. 1988. Complete amino acid sequence of p453-plasmid-mediated PIT-2 β-lactamase (SHV-1). Biochem. J. 251**:**73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet, R., H. Marchandin, C. Chanal, D. Sirot, R. Labia, C. De Champs, E. Jumas-Bilak, and J. Sirot. 2002. Chromosome-encoded class D β-lactamase OXA-23 in Proteus mirabilis. Antimicrob. Agents Chemother. 46**:**2004-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bou, G., A. Oliver, and J. Martinez-Beltran. 2000. OXA-24, a novel class D β-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents Chemother. 44**:**1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradford, P. A., C. Urban, N. Mariano, S. J. Projan, J. J. Rahal, and K. Bush. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob. Agents Chemother. 41**:**563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couture, F., J. Lachapelle, and R. C. Levesque. 1992. Phylogeny of LCR-1 and OXA-5 with class A and class D β-lactamases. Mol. Microbiol. 6**:**1693-1705. [DOI] [PubMed] [Google Scholar]
- 9.Crowley, B., V. J. Benedi, and A. Domenech-Sanchez. 2002. Expression of SHV-2 β-lactamase and of reduced amounts of OmpK36 porin in Klebsiella pneumoniae results in increased resistance to cephalosporins and carbapenems. Antimicrob. Agents Chemother. 46**:**3679-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donald, H. M., W. Scaife, S. G. Amyes, and H. K. Young. 2000. Sequence analysis of ARI-1, a novel OXA β-lactamase, responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrob. Agents Chemother. 44**:**196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girlich, D., T. Naas, A. Leelaporn, L. Poirel, M. Fennewald, and P. Nordmann. 2002. Nosocomial spread of the integron-located _veb-1_-like cassette encoding an extended-spectrum β-lactamase in Pseudomonas aeruginosa in Thailand. Clin. Infect. Dis. 34**:**603-611. [DOI] [PubMed] [Google Scholar]
- 12.Héritier, C., L. Poirel, D. Aubert, and P. Nordmann. 2003. Genetic and functional analysis of the chromosome-encoded carbapenem-hydrolyzing oxacillinase OXA-40 of Acinetobacter baumannii. Antimicrob. Agents Chemother. 47**:**268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez-Alles, S., S. Alberti, D. Alvarez, A. Domenech-Sanchez, L. Martinez-Martinez, J. Gil, J. M. Tomas, and V. J. Benedi. 1999. Porin expression in clinical isolates of Klebsiella pneumoniae. Microbiology 145**:**673-679. [DOI] [PubMed] [Google Scholar]
- 14.Kieser, T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12**:**19-36. [DOI] [PubMed] [Google Scholar]
- 15.Koh, T. H., G. S. Babini, N. Woodford, L. H. Sng, L. M. Hall, and D. M. Livermore. 1999. Carbapenem-hydrolysing IMP-1 β-lactamase in Klebsiella pneumoniae from Singapore. Lancet 353**:**2162. [DOI] [PubMed] [Google Scholar]
- 16.Ledent, P., X. Raquet, B. Joris, J. Van Beeumen, and J.-M. Frère. 1993. A comparative study of class-D β-lactamases. Biochem. J. 292**:**555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90**:**6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacKenzie, F. M., K. J. Forbes, T. Dorai-John, S. G. Amyes, and I. M. Gould. 1997. Emergence of a carbapenem-resistant Klebsiella pneumoniae. Lancet 350**:**783. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Martinez, L., A. Pascual, S. Hernandez-Alles, D. Alvarez-Diaz, A. I. Suarez, J. Tran, V. J. Benedi, and G. A. Jacoby. 1999. Roles of β-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob. Agents Chemother. 43**:**1669-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medeiros, A. A. 1997. Evolution and dissemination of β-lactamases accelerated by generation of β-lactam antibiotics. Clin. Infect. Dis. 24(Suppl. 1)**:**S19-S45. [DOI] [PubMed] [Google Scholar]
- 21.Naas, T., and P. Nordmann. 1999. OXA-type β-lactamases. Curr. Pharm. Design 5**:**865-879. [PubMed] [Google Scholar]
- 22.Naas, T., L. Philippon, L. Poirel, E. Ronco, and P. Nordmann. 1999. An SHV-derived extended-spectrum beta-lactamase in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43**:**1281-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naas, T., L. Poirel, A. Karim, and P. Nordmann. 1999. Molecular characterization of In_50_, a class 1 integron encoding the gene for the extended-spectrum β-lactamase VEB-1 in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 176**:**411-419. [DOI] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing. Eleventh informational supplement. M100-S11. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Ouellette, M., L. Bissonnette, and P. H. Roy. 1987. Precise insertion of antibiotic resistance determinants into Tn_21_-like transposons: nucleotide sequence of the OXA-1 β-lactamase gene. Proc. Natl. Acad. Sci. USA 84**:**7378-7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paton, R., R. S. Miles, J. Hood, and S. G. B. Amyes. 1993. ARI-1: β-lactamase mediated imipenem resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2**:**81-88. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., V. Korten, R. N. Jones, G. V. Doern, et al. 1999. Multicenter evaluation of the antimicrobial activity for seven broad-spectrum β-lactams in Turkey using the Etest method. Diagn. Microbiol. Infect. Dis. 35**:**65-73. [DOI] [PubMed] [Google Scholar]
- 28.Philippon, A., G. Arlet, and G. A. Jacoby. 2002. Plasmid-determined AmpC-type β-lactamases. Antimicrob. Agents Chemother. 46**:**1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philippon, L. N., T. Naas, A.-T. Bouthors, V. Barakett, and P. Nordmann. 1997. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41**:**2188-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ploy, M. C., P. Courvalin, and T. Lambert. 1998. Characterization of In_40_ of Enterobacter aerogenes BM2688, a class 1 integron with two new gene cassettes, cmlA2 and qacF. Antimicrob. Agents Chemother. 42**:**2557-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poirel, L., D. Girlich, T. Naas, and P. Nordmann. 2001. OXA-28, an extended-spectrum variant of OXA-10 β-lactamase from Pseudomonas aeruginosa and its plasmid- and integron-located gene. Antimicrob. Agents Chemother. 45**:**447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poirel, L., C. Héritier, I. Podglajen, W. Sougakoff, L. Gutmann, and P. Nordmann. 2003. Emergence in Klebsiella pneumoniae of a chromosome-encoded SHV β-lactamase that compromises the efficacy of imipenem. Antimicrob. Agents Chemother. 47**:**755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poirel, L., T. Naas, M. Guibert, E. B. Chaibi, R. Labia, and P. Nordmann. 1999. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum beta-lactamase encoded by an Escherichia coli integron gene. Antimicrob. Agents Chemother. 43**:**573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poirel, L., and P. Nordmann. 2002. Acquired carbapenem-hydrolyzing β-lactamases and their genetic support. Curr. Pharm. Biotechnol. 3**:**117-127. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Sarno, R., G. McGillivary, D. J. Sherratt, L. A. Actis, and M. E. Tolmasky. 2002. Complete nucleotide sequence of Klebsiella pneumoniae multiresistance plasmid pJHCMW1. Antimicrob. Agents Chemother. 46**:**3422-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolmasky, M. E., and J. H. Crosa. 1993. Genetic organization of antibiotic resistance genes (aac(6′)-Ib, aadA, and oxa9) in the multiresistance transposon Tn_1331._ Plasmid 29**:**31-40. [DOI] [PubMed] [Google Scholar]
- 38.Yan, J. J., W. C. Ko, and J. J. Wu. 2001. Identification of a plasmid encoding SHV-12, TEM-1, and a variant of IMP-2 metallo-β-lactamase, IMP-8, from a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45**:**2368-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yigit, H., A. M. Queenan, G. J. Anderson, A. Domenech-Sanchez, J. W. Biddle, C. D. Steward, S. Alberti, K. Bush, and F. C. Tenover. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45**:**1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]