LiGluR Restores Visual Responses in Rodent Models of Inherited Blindness (original) (raw)
Abstract
Inherited retinal degeneration results from many different mutations in either photoreceptor-specific or nonphotoreceptor-specific genes. However, nearly all mutations lead to a common blinding phenotype that initiates with rod cell death, followed by loss of cones. In most retinal degenerations, other retinal neuron cell types survive for long periods after blindness from photoreceptor loss. One strategy to restore light responsiveness to a retina rendered blind by photoreceptor degeneration is to express light-regulated ion channels or transporters in surviving retinal neurons. Recent experiments in rodents have restored light-sensitivity by expressing melanopsin or microbial opsins either broadly throughout the retina or selectively in the inner segments of surviving cones or in bipolar cells. Here, we present an approach whereby a genetically and chemically engineered light-gated ionotropic glutamate receptor (LiGluR) is expressed selectively in retinal ganglion cells (RGCs), the longest-surviving cells in retinal blinding diseases. When expressed in the RGCs of a well-established model of retinal degeneration, the rd1 mouse, LiGluR restores light sensitivity to the RGCs, reinstates light responsiveness to the primary visual cortex, and restores both the pupillary reflex and a natural light-avoidance behavior.
Introduction
Retinal degeneration patients suffering from diseases such as retinitis pigmentosa (RP) and Leber's congenital amaurosis, become gradually blind as photoreceptors die.1,2 These diseases arise from mutations in photoreceptor or nonphotoreceptor specific genes.3,4 Gene-replacement and pharmaceutical therapies to slow photoreceptor cell death are in development.5 However, there are currently no approved treatments available to patients that would restore light sensitivity to the retina after photoreceptor cells are lost.
Following the blinding loss of the photoreceptors, much of the inner retinal architecture remains intact in these conditions.6,7 This opens the possibility of restoring vision by imparting light sensitivity to surviving retinal neurons. One strategy that has been successful in human studies is to use electrode arrays to stimulate remaining retinal neurons, allowing subjects to discriminate large, high-contrast images, and letters.8,9 However, the resolution of electrical implants is currently limited by the small number of electrode channels (~1,200).9,10 Another strategy involves in vivo expression of light-activated channels in surviving retinal neurons using adeno-associated viral vectors (AAV).11 AAV vectors have successfully introduced replacement RPE65 genes into the retinas of Leber's congenital amaurosis patients in three clinical trials.12,13,14 In late-stage retinal degeneration, AAV gene therapy could be used to transfer exogenous light sensors to surviving retinal neurons.
AAV vectors of defined serotypes carrying cell-specific promoters can target expression of a light sensor to specific retinal cell classes. In early stages of retinal disease, restoring light sensitivity to photoreceptors may recapture much of the normal retinal processing. In late-stage degeneration, after the photoreceptors are lost, bipolar, amacrine, and ganglion cells could be a viable target for light restoration strategies.15,16 However, it is known that the connectivity and the expression of ion channels and receptors in bipolar cells changes as the degeneration progresses.6,17,18 Therefore, targeting the retinal ganglion cells (RGCs) would be the most viable therapeutic option for long-term vision restoration.
Recently, Channerhodopsin2 (ChR2) and melanopsin were successfully delivered to the retina with AAV, restoring light sensitivity to rodent models of blindness such as rd1 mice and RCS rats.19,20,21,22,23 Electroporation of ChR2 into ON-bipolar cells of rd1 mouse retina rescued light-sensitivity and light-dependent behaviors.15 Vision restoration has also been achieved by AAV-targeted expression of halorhodopsin (NpHR) in photoreceptor inner segments, which survive in a nonlight sensitive state after loss of the light-sensing outer segments.24 In sum, these studies suggest that introduction of light-sensitive channels to surviving retinal neurons can restore visual function.
An alternative to opsins is the use of endogenous channels rendered light sensitive by chemical modification.25,26,27 Engineered mammalian channels and receptors have been used in vitro and in vivo to modulate behavior and probe neuronal circuits.27,28 One such protein is the light-gated ionotropic glutamate receptor (LiGluR). LiGluR consists of iGluR6 with an introduced cysteine in position 439 (L439C) for the covalent attachment of a photoisomerable molecule (“photoswitch”) that reversibly activates the receptor.25,29 The LiGluR photoswitch has a maleimide linked to a glutamate by a photoisomerizable azobenzene linker (maleimide-azobenzene-glutamate (MAG)). At one wavelength, MAG places the glutamate into the binding pocket, opening the ion channel and at a second wavelength, it withdraws it to close the channel, thus enabling the channel to be turned on and off with light.
We used intravitreal delivery of AAV2-LiGluR to restore light responsiveness to the RGCs of adult rd1 mice. This successfully restored light sensitivity to RGCs in retina whole mounts, the in vivo light response to the primary visual cortex, and the pupillary reflex and a natural light-avoidance behavior. Our results demonstrate that viral delivery of LiGluR to RGCs can restore vision in disease models of blindness.
Results
AAV2-LiGluR delivered in vivo restores light responses to RGCs
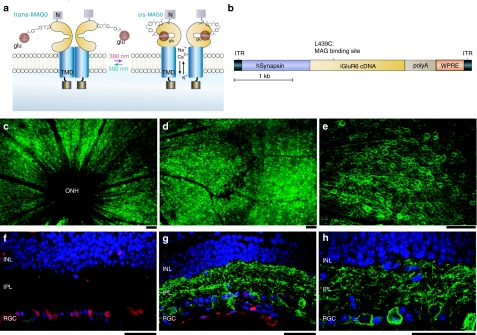
We used adult rd1 mice (age 3 months or older), a well-characterized model of human RP that carry a null mutation in PDE6 that leads to rapid loss of all photoreceptors,30 in our experiments. We expressed LiGluR (Figure 1a) in adult rd1 mice RGCs by intravitreal injection of AAV2 containing a synapsin promoter driving LiGluR (AAV2-hSyn-LiGluR) (Figure 1b). LiGluR expression plateaued 4–6 weeks after injection and was observed throughout the retina (Figure 1c–e; Supplementary Figure S1), exclusively in the RGC layer (Figure 1g,h). Using antibodies against iGluR6 and ChAT (to label starburst amacrine cells) we estimated that 54–87% of the RGCs expressed LiGluR (Supplementary Figure S1). No labeling of bipolar cells or interneurons was observed (Figure 1g,h) and no LiGluR labeling was observed in the uninjected eye (Figure 1f).
Figure 1.
Light-gated glutamate receptor (LiGluR) expression is selectively targeted to retinal ganglion cells in rd1 mouse retinas. (a) The LiGluR opens and closes upon the reversible photoisomerization of the tethered agonist maleimide-azobenzene-glutamate (MAG) between its trans (500 nm) and cis configuration (380 nm). The maleimide moiety of MAG binds covalently to the cysteine on the ligand-binding domain of the receptor and photoswitching occurs through reversible binding of the glutamate moiety of MAG, in and out of the ligand-binding pocket. MAG binding activates the receptor allowing cations to flow, resulting in membrane depolarization. TMD, transmembrane domain; C, C- terminus; N, N-terminus; glu, glutamate. (b) AAV-2 construct with the human Synapsin promoter (hSyn) driving iGLuR6 cDNA with a L439C mutation for MAG conjugation. A WPRE (woodchuck hepatitis post-transcriptional regulatory element) follows the LiGluR coding region for enhanced expression. (c–e, g–h) fluorescence images of rd1 mouse retinas 12 weeks post AAV2-hSyn-iGluR injection, stained with antibodies to iGluR6 (in green) and counterstained with NeuN antibodies (in red) in the cross-sections. Retinal ganglion cells (RGCs) were strongly labelled in both the rd1 (c–e) and triple knockout mice (TKO) retinas, as observed in top (c–e) and cross-sectional (g–h) views. Strong LiGluR expression was observed around the optic nerve head where RGCs cell bodies are dense (ONH, optic nerve head) (c), extending to the periphery (d) with clear membrane labeling of most RGCs (e). Cross-sections show LiGluR expression is restricted to RGCs (g–h). No detectable labeling was found in uninjected rd1 retinas (f). Blue, 4',6-diamidino-2-phenylindole-stained cell nuclei. Bars = 50 µm. INL, inner nuclear layer; IPL, inner plexiform layer.
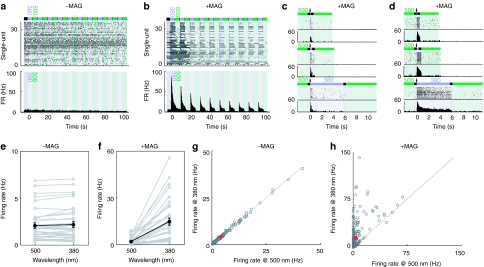
We tested the ability of LiGluR to drive light responses in RGCs by multi-electrode array recordings from retinal whole mounts. Retinas were isolated from adult rd1 mice injected with AAV2-hSyn-LiGluR 45 days earlier and incubated with the MAG photoswitch prior to recording. In the absence of LiGluR expression, RGC activity in rd1 retinas was not modulated by light (Supplementary Figure S2). LiGluR expressing rd1 retinas not pretreated with MAG showed no response to the LiGluR activating wavelength (380 nm, Figure 2a) and no difference between flashes at 380 nm and at the LiGluR-deactivating wavelength of 500 nm (Figure 2a,e,g). In these same retinas, MAG exposure consistently imparted light responsiveness. Stimulation with 380 nm light pulses (5 seconds duration) triggered short-latency spike trains [median latency: 20 ms, range (5–98); n = 261] across a large area of the retina, with dozens of RGCs activated (Figure 1b,f,h). The firing frequency of the RGCs peaked at ~50 Hz (50.74 ± 3.46 Hz, n = 261) within 100 ms of the light pulse and then declined. Within the same retina, some cells presented a rapid (hundreds of ms) decline in firing rate (Figure 2c), while in others, it was much slower (seconds) (Figure 2d). Across the population of recorded cells, 14.84% presented such “transient” responses. Similar “transient” and “sustained” responses have been observed in WT retinas driven by a flash of white light and in experiments where ChR2 was electroporated into rd1 bipolar cells in vivo.15
Figure 2.
Light-gated glutamate receptor (LiGluR) restores light responsiveness to rd1 retinas in the presence of the photoswitch maleimide-azobenzene-glutamate (MAG). (a) Example multiunit recording from a retina from an adult LiGluR-rd1 mouse in the absence of MAG. Top, light stimulation protocol (5 seconds 380 nm alternating with 5 seconds 500 nm). Middle, raster plot showing spikes for all recorded retinal ganglion cells (RGCs). Bottom, peri-stimulus time histogram (PSTH) for the population of recorded neurons. (b) Example of a typical multiunit recording from a retina of an adult LiGluR-rd1 mouse in the presence of MAG (same retina as a). Top, light stimulation protocol (5 seconds 380 nm alternating with 5 seconds 500 nm). Middle, raster plot showing spikes for all recorded RGCs. Bottom, PSTH for the population of recorded neurons. (c,d) Example raster plots of LiGluR-mediated light responses in both a transient (c) and a more sustained (d) ganglion cell for three different stimuli durations (380 nm). Top, 50 ms. Middle, 300 ms. Bottom, 5 s. Arrow indicates the onset of the 380 nm light pulse. (e) Comparison of the mean firing rates at 380 and 500 nm for the RGCs recorded in a. Gray lines, single units. Black lines, population mean. (f) Comparison of the mean firing rates at 380 and 500 nm for the RGCs recorded in b. Gray lines, single units. Black lines, population mean. (g,h) Scatter plot of the mean firing rates of all recorded RGCs (n = 6 retinas) in response to a 5 seconds pulse of 380 or 500 nm light in the absence (g) or presence (h) of MAG. Gray dots, individual cells. Red dot, population mean. Error bars, SEM.
While activation of LiGluR expressing RGCs with 380 nm light resulted in a significant increase in spiking, illumination with 500 nm light did not (Figure 1b,f,h), consistent with the spectral sensitivity of LiGluR.29 When the retina was illuminated with alternating 5-second pulses of 380 or 500 nm light (Figure 2), 94% of cells showed significant light-modulated spiking (n = 33/35 cells, n = 10 trials, P < 0.05, see Materials and Methods section). Across the population of RGCs, the mean firing rate increased from 1.94 ± 0.43 Hz to 14.88 ± 2.26 Hz (n = 35 cells, P < 10−6, averaged over 5 seconds). No light-modulation of RGC spiking was observed in the same retina without MAG with this illumination protocol (Figure 2a,c; n = 36 cells, P = 0.13), confirming the loss of photoreceptors at this age. Across all retinas, transduction of RGCs with LiGluR resulted in significant light-mediated modulation of RGC firing rate [median number of significant cells/retina: 91.50%, range (29.40–100), n = 6 retinas, n = 261 cells, P < 10−39; control: n = 6 retinas, n = 212 cells, P = 0.21; light pulse: 5 seconds). Similar results were obtained for 50 ms and 300 ms light pulses (Supplementary Figure S2).
LiGluR in RGCs restores light responses to the visual cortex
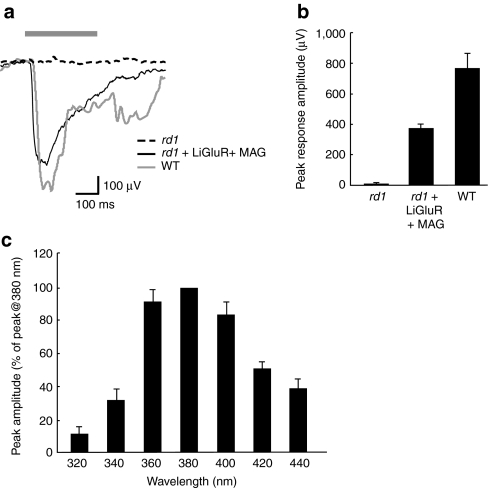
We conducted visually-evoked field recordings (VEPs) from primary visual cortex of LiGluR expressing rd1 mice (LiGluR-rd1), rd1, and wild-type mice (WT) to assess LiGluR function_in vivo_ (Figure 3a). Recordings were made at a 400 µm depth, corresponding to the highest amplitude VEP peak and cortical layer 4.31 Whereas WT mice had large VEPs, rd1 mice had no measurable responses (Figure 3a,b), consistent with the lack of photosensitivity of the isolated rd1 retinas (Figure 2a; Supplementary Figure S2). Expression of LiGluR in the RGCs of the rd1 mice restored the VEPs to 50% of WT (371.75 ± 35.70 µV, n = 13 versus 766.00 ± 100.51 µV, n = 7, respectively) (Figure 3a,b).
Figure 3.
Light-gated glutamate receptor (LiGluR) expression restores visually-evoked potentials (VEPs) in the primary visual cortex of rd1 mice. (a) Example VEPs recorded from the visual cortex in an rd1, LiGluR-rd1 (+ maleimide-azobenzene-glutamate (MAG)) and wild type mouse in response to a 300 ms pulse of 380 nm light (see Materials and Methods section). Bar shows the timing of the light stimulus. (b) Mean peak VEP amplitude for each experimental group (rd1, n = 6; LiGluR-rd1, n = 13; WT, n = 7). (c) Wavelength tuning of VEPs. Mean peak VEP amplitude is expressed as a percentage of the VEP at 380 nm (n = 4–6 for each wavelength tested). Error bars are SEM. WT, wild-type mice.
Interestingly, the latency to onset of the VEP was significantly shorter in LiGluR-rd1 than in WT (28.58 ± 2.11 ms versus 45.14 ± 4.03 ms, respectively; P < 0.005, Mann–Whitney-U test) (Figure 3a). This is consistent with expectations of the LiGluR-rd1 response originating in the RCGs without the phototransduction, photoreceptor, and bipolar cell synaptic delays. LiGluR-mediated visual responses peaked at 380 nm (Figure 3c),with a tuning curve consistent with the MAG photoswitch itself and LiGluR in vitro.29 Although smaller, the response remained substantial in the visible range, with about 80% of the visual response being present at 400 nm. There were no differences in the electroretinograms of mice injected intravitreally with phosphate-buffered saline (PBS), dimethyl sulfoxide (DMSO), or MAG, highlighting the safety of this approach (Supplementary Figure S3).
Restoration of the pupillary reflex
We tested whether LiGluR could restore a simple behavioral response to light: the pupillary reflex. Normally, this behavior is mediated by a small subset of intrinsically light-sensitive RGCs that express melanopsin.32 Experiments were conducted in triple knockout (transducin (tra−), the cone cyclic nucleotide gated channel (cnga3−) and melanopsin (opn4−) mice, which lack phototransduction and pupillary reflexes.33
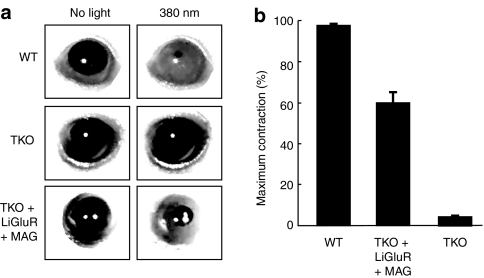
AAV2-hSyn-LiGluR was intravitreally injected into TKO mice. Six weeks after LiGluR transduction, MAG was injected intravitreally into TKO-LiGluR animals and the pupillary reflex was assessed at different time points. Twenty-four hours after MAG injection, illumination with 380 nm light elicited a pupillary response in both WT (97.4 ± 0.3%, n = 5) and LiGluR-TKOs (59.8 ± 5.3%, n = 7), but not in the control (uninjected) TKOs (4.1 ± 0.9%, n = 5) (Figure 4a,b). Thus, across the population, LiGluR restored ~60% of the pupillary response, a significant improvement over control TKOs (P < 0.005). One week after application of the photoswitch, 67% of the LiGluR-TKO animals retained the pupillary reflex. Fifty percent of the animals showed persistent pupillary responses 2 weeks postinjection, reflecting the stability of the system.
Figure 4.
Restoration of pupillary reflex in triple knockout mice lacking phototransduction and melanopsin expression by transduction with light-gated glutamate receptor (LiGluR). (a) Representative infrared images of the pupil area taken in the dark (left) and during exposure to 380 nm (right) for WT, TKO, and TKO-LiGluR(+MAG) animals. (b) Mean maximal pupillary constriction across the population (WT, n = 5; TKO-LiGluR, n = 7; TKO, n = 5). Error bars are SEM. MAG, maleimide-azobenzene-glutamate; TKO, triple knockout mice; WT, wild-type mice.
Restoration of light-avoidance behavior by LiGluR
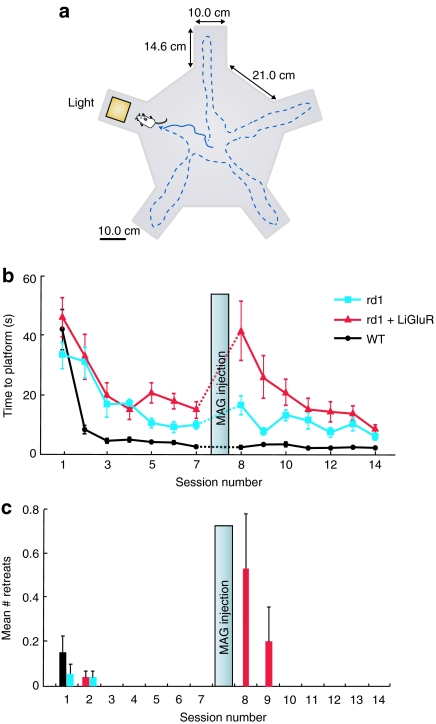
Can LiGluR restore basic intrinsic behavioral responses to light in rd1 mice? We used a behavioral test to assess light-avoidance behavior, since bright light is a known aversive stimulus to rodents.34 We modified a five-arm water maze (Figure 5a) and required animals to follow a visual cue present in only one arm to identify it as the arm containing a target platform. We measured the time to enter the arm containing the lighted platform and the number of times the mouse approached the illuminated platform only to retreat from it. The retreats reflect the light-avoidance behavior.
Figure 5.
Light-gated glutamate receptor (LiGluR) restores a water-maze based light-avoidance behavior to adult rd1 mice. (a) Diagram of the behavioral setup (see Material and Methods section). (b) Time to reach platform for the three experimental groups (rd1, LiGluR-rd1, WT, n = 10/group, mean ± SEM). (c) Mean number of retreats from the illuminated arm (which contained the platform) for the three experimental groups (rd1, LiGluR-rd1, WT, n = 10/group, mean ± SEM). Mice were run continuously for 7 days (one session/day). At day 8, animals were intravitreally injected with MAG. Testing continued for 7 more days. MAG, maleimide-azobenzene-glutamate.
WT, control rd1, and LiGluR-rd1 animals that had not been injected with MAG were trained in the water maze, one session per day for 7 days. For all animals, we observed a decrease in the time to locate the platform during the first days of training (Figure 5b). The rate of learning was faster in WT animals, likely due to their visually guided exploration. Although slower, the blind rd1 animals and LiGluR-rd1 mice also learned aspects of the task and decreased their search time for the platform, perhaps due to changes in exploration strategy. The time to locate the platform stabilized between days 3 and 5 for all groups. On day 8, the WT, rd1, and LiGluR-rd1 mice were all bilaterally intravitreally injected with MAG. On day 9, we observed a significant increase in the latency to enter the illuminated platform-containing arm in the LiGluR-rd1 group (n = 10, P < 0.01), but not in the control rd1 or the WT group (WT: n = 10, P = 0.30; control rd1: n = 10, P = 0.20) (Figure 5b). There was also a significant increase in the number of retreats from the illuminated platform-containing arm for the LiGluR-rd1 group (n = 10, P < 0.005), but not for the other experimental groups (Figure 5c), accounting for at least part of the increase in latency of the LiGluR-rd1 group. This suggests that MAG-conjugated LiGluR in RGCs restored light perception. In addition, it suggests that MAG alone has no appreciable behavioral effect. With repeated trials over several days the time to find the platform (Figure 5b) and the number of retreats (Figure 5c) for the MAG-conjugated LiGluR-rd1 mice decreased, indicating that, as with WT mice, the aversion to light could be counterbalanced by the aversion to water, which motivates the search for the platform. The results indicate that MAG-conjugated LiGluR in RGCs restores light perception and light-avoidance behavior to the rd1 mouse.
Discussion
Retinal degeneration begins with rod cell death, followed by cone loss, typically sparing other retinal neurons for long periods after blindness. Introduction of opsin channels, receptors and pumps to surviving retinal neurons can restore light-sensitivity.15,19,20,21,22,24 We show here that the selective expression of LiGluR in RGCs (the retinal output cells and longest-surviving cells in retinal blinding diseases) restores light sensitivity to the blind rd1 mouse retina and the TKO mouse retina. We found that the restored visual information mediates a simple visual function (the pupillary reflex), is conveyed to the primary visual cortex, and mediates a more complex light-avoidance based behavior.
Selecting the best cell target for the light-activated channels in retinal degeneration will depend upon the stage of the disease. In early stages of disease, the retina is rendered light insensitive due to apoptosis of rods and loss of cone outer segments. At this stage, imparting light sensitivity to surviving cone inner segments may be a viable strategy.24 In later stages, photoreceptors are lost, and targeting the most upstream remaining cells, the bipolars, may take advantage of their on and off pathways, and center-surround organization.7,17,35 Persistent expression of light-gated channels in bipolar cells has not yet been accomplished. Studies targeting bipolar cells using electroporation resulted in limited areas of transduction and transient expression (weeks to months). Moreover, in rodent models of retinitis pigmentosa, bipolar cells eventually lose their metabotropic glutamate receptor 6 and iGluR currents, undergo dendritic sprouting and retraction of synapses, suggesting functional disruption.6,14,15 In contrast, amacrine cells and RGCs retain their iGluR-mediated responses, suggesting integrity of the furthest downstream circuit.17 In mouse models of retinitis pigmentosa, photoreceptor loss progresses to loss of other retinal layers, with the RGC layer surviving the longest.35 In humans, retinal remodelling is less well understood than in rodents, with limited histological observations of donor retinas or in vivo imaging.36,37 However, clinical electrophysiological and anatomical observations suggest that the RGC layer remains morphologically intact in late-stage retinal degeneration and retains connectivity to other visual areas. Thus, while there may be a transient benefit to endowing photoreceptors or bipolar cells with light sensitivity, longer term benefit would likely be obtained from targeting RGCs.
A recent study testing whether endowing RGCs with light sensitivity could restore vision by expressing ChR2 exclusively in RGCs of transgenic mice did not show improved light-guided behavior.38 Our study delivered the light-gated channel via AAV2 vectors, a method currently in use for gene delivery in several clinical trials.12,13,14 As an alternative to the microbial opsin ChR2, we used the mammalian iGluR6-based LiGluR. We obtained high-level expression, exclusively in RGCs, broadly across the retina. The restored retinal response evoked robust, reproducible field responses in the primary visual cortex at half the amplitude of WT mice. The reduced amplitudes may be due to changes in cortical neuronal excitability resulting from increased spontaneous activity observed in the retina of rd1 mice,39 or to simultaneous expression in both the on and off functional subsets of RGCs.
In addition to restoring light responsiveness to the image-forming pathway (retina to lateral geniculate nucleus to primary visual cortex), LiGluR in the RGCs restored the light drive to a parallel pathway mediating the pupillary reflex. Although the success rate of restoration was high, the pupillary constriction was only 60% that of WT, suggesting that the special classes of RGCs mediating this nonvisual reflex may not be as effectively activated by LiGluR as they are by their native melanopsin. Alternatively, it may be that not all RGCs of this class expressed LiGluR. It may also be that the expression of the LiGluR in both the “on” and “off” pathways reduced the net response of the system.
The restoration of light-evoked activity to the visual system by LiGluR in RGCs was sufficient to restore light-avoidance behavior. In our behavioral setup, the light on the platform-containing arm of the maze initially repels WT mice, yet over time, they become habituated to the light and their time to enter the lighted arm decreases. The initial decrease in latency to platform therefore reflects both the learning of the task and the habituation to the aversive stimulus. The other two experimental groups contained adult rd1 mice, which were unable to detect the light and, therefore, were not repelled. Upon MAG injection, rd1 mice lacking LiGluR showed no behavioral change, whereas LiGluR-rd1 mice showed a significant increase in latency to reach the platform and an increase in the number of retreats from the lighted arm. This suggests that after MAG conjugation to LiGluR these mice become able to detect the light for the first time and retreat from it in a typical photophobic response. As with WT mice, the LiGluR-rd1 mice that have been rendered light responsive by MAG habituate to light with repeated trials and their latency to platform returns to baseline levels.
Together, our results indicate that in severe retinal pathologies, where the photoreceptors are lost, vision can be restored by expression of a light-gated excitatory channel in RGCs. The acuity of the restored vision remains to be determined. The positive responses following AAV2 mediated expression of LiGluR in RGCs compared to the lack of response from RGC expression of ChR2 in transgenic mice37 suggests that either the rd1 strain used in our experiments had a more complete loss of light response or that LiGluR evokes larger photo-currents. This difference may be due to higher expression levels of LiGluR or because of the ~25 to 200-fold larger single channel conductance of the iGluR6 we used compared to ChR2.39 Recent improvements in macroscopic ChR2 current,21,40,41 may narrow this difference.
Two immediate issues arise for clinical application of LiGluR to treat blinding diseases.42 First, unlike retinal, the chromophore for microbial opsins, which is available in the eye, MAG must be supplied. MAG attaches covalently to the cysteine-substituted iGluR6 channels, and without MAG replenishment, the light response will decrease as iGluR channels are turned over. We found the pupillary light response to persist for 1–2 weeks after MAG injection. For longer treatments, MAG would have to be supplied repeatedly or via slow release, as for intravitreally injected agents used to treat retinal neovascularization.43 Second, it would be beneficial if RGCs that are rendered light-sensitive in order to take over for missing photoreceptor cells were to respond to visible light. While MAG, like its conventional azobenzene photoisomerizable core, is activated in the long-wave UV to blue range, azobenzene can be red-shifted,44,45 which would enable LiGluR to respond to more natural stimuli.
In conclusion, our study shows that the introduction of a light-gated excitatory ion channel into RGCs using an intravitreal injection of an AAV2 vector can restore several fundamental light responses of the visual system, including the retinal light response, the pupillary response, light-driven activity in the visual cortex and a basic visual behavior. This provides a promising approach for restoring light perception in blindness due to loss of photoreceptor cells and in advanced retinal degenerations that disrupt secondary retinal neurons but leave the retinal output cells intact.
Materials and Methods
Animals. The University of California Animal Care and Use Committee approved all animal procedures. Wild-type C57BL/6J, C3H/HeNCrl (rd1) mice (Charles River, Wilmington, MA) and triple knockout mice (TKO) (tra−/−, cnga3−/−, onp4−/−) lacking all phototransduction (gift of King-Wai Yau) were housed on a 12-hour light-dark cycle, with food and water ad libitum.
Generation of rAAV vectors. Adeno-associated virus was produced by standard methods.46 Vector was titered for DNase-resistant vector genomes by real-time PCR relative to a woodchuck hepatitis post-transcriptional regulatory element standard.
Intraocular vector and photoswitch administration. Before injection, animals were anesthetized with IP ketamine (72 mg/kg) and xylazine (64 mg/kg) and pupils were dilated with tropicamide (1%). An incision was made through the sclera, below the ora serrata with a 30 ga. needle and 2 µl of PBS, MAG0, DMSO, or 5 × 1012–5 × 1013 vg/ml of AAV-2 LiGluR were injected into the vitreous with a blunt ended 32 ga. Hamilton syringe.
Photoswitch preparation. Twenty millimolar MAG0 suspended in 100% DMSO was illuminated for 30 minutes in UV to facilitate labeling of LiGluR 29. MAG0 was diluted 1/100 in sterile PBS and mixed thoroughly. Two microliter of 200 µmol/l MAG0 (1% DMSO) were injected into the eye of animals that had been previously (>3 weeks) injected with AAV2-LiGluR. Comparison of electroretinograms from WT animals injected with PBS (1×), DMSO (1%), or MAG (200 µmol/l in 1% DMSO) showed no deleterious effect of MAG on retinal responses (Supplementary Figure S3).
Flatmounts. Retinal flatmounts were prepared by gently detaching the retina from the RPE in PBS using a small brush. Once removed, radial cuts were made to flatten the retinal tissue.
Immunolabeling and histology. Eye cups were cryoprotected in 30% sucrose, embedded in OCT (Tissue-Tek, Torrance, CA), and cut in 10 µm transverse cryosections. Sections were incubated with a monoclonal antibody against ionotropic glutamate receptors 6 and 7 (Upstate Solutions, Billerica, MA) at 1:500 in blocking solution overnight at 4 °C. Secondary anti-rabbit Alexa-488 antibody was used (Invitrogen, Carlsbad, CA) at 1:2,000 in blocking solution for 2 hours at RT. Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI) and retinas were examined by confocal microscopy (LSM5; Carl Zeiss, Göttingen, Germany).
Quantification of RGC labeling. We enucleated eyes from five animals and stained the retinas for LiGluR (iGluR6). All 10 retinas showed widespread expression of the protein across the retina (Supplementary Figure S1). In addition, we took two of these retinas and imaged them using confocal microscopy for more detailed analysis and cell counting (Supplementary Figure S1b). To calculate the fraction of LiGluR expressing RGCs, we examined four fields of 1024 × 1024 pixels (approximately 425 × 425 µm), one from each retinal quadrant, ~ 800 µm from the optic nerve (Supplementary Figure S1a). The AAV construct contained a promoter specific for neurons (hSyn), and expression was observed only in RGCs and amacrine cells in the ganglion cell layer. Approximately 75% of all amacrine cells in the mouse retina are cholinergic starburst amacrine cells, and are major component of the displaced amacrine cell population in the RGC layer. We costained for these cells using ChAT antibody to eliminate starburst amacrine cells from our RGC counts.
Electroretinography. Mice were dark-adapted for 4 hours, anesthetized, and pupils dilated. Corneal contact lens electrodes were used with subcutaneous reference electrodes and a ground electrode in the tail. Electroretinograms were recorded with a Espion E2 (Diagnosys, Lowell, MA) over eight flash intensities from 0.0001–1(cd-s)/m2 presented in series of three. Data were analyzed with MatLab (v7.7; Mathworks, Natick, MA).
Multi-electrode array recordings. LiGluR expressing and uninjected control rd1 mice were killed and enucleated. The extracted retinas were placed into Ringer's solution (119 mmol/l NaCl, 2.5 mmol/l KCl, 1 mmol/l KH2PO4, 1.3 mmol/l MgCl2, 2.5 mmol/l CaCl2, 26.2 mmol/l NaHCO3, 20 mmol/l -glucose) bubbled with 95% O2 and 5% CO2 at 25 °C. Flatmounts were placed ganglion side down, on a 60-channel multi-electrode array (Multi-Channel Systems, Tubingen, Germany). Retinas were secured in place with a weighted dialysis membrane, and continuously perfused with 37 °C Ringer's solution bubbled with 95% O2 and 5% CO2 at 1 ml/minute. Retinas expressing LiGluR were labelled with previously illuminated (UV) 50 µmol/l MAG0.
The multi-electrode array has 60, 30 µm diameter electrodes in an 8 × 8-grid with an inter-electrode distance of 200 µm. Data was sampled at 20 kHz and recorded for off-line analysis. Voltage traces were filtered with a 200 Hz high band-pass filter. Spikes that crossed the 5 SD threshold were sorted using Offline Sorter (Plexon, Dallas, TX) to isolate single units. Principal component analysis was performed on all of the unsorted waveforms, and the values of the principal component scores for all the waveforms were plotted. Clusters of points in the principal component space allowed for the isolation of single units. Typically, each electrode recorded from 1–3 units. To ensure accurate sorting by Offline Sorter, all spike clusters were manually inspected in principal component space. The histograms were binned at 100 ms. Data were analyzed with MatLab. Illumination for photoswitching was provided by a 100 W mercury arc lamp illuminator (USH-102DH, Ushio America, Cypress, CA) with band-pass filters (D380xv2; ChromaTech, Rockingham, VT and FF01-497/16-25; Semrock, Rochester, NY). We measured light output using a hand-held optical power meter (840-C; Newport, Irvine, CA). at the plane of the retina through a ×20 objective. The light path was controlled using a Lambda 10-3 optical filter changer with SmartShutter (LB10-3, LB10-NWIQ; Sutter Instruments, Novato, CA). The incident light intensity was 2.56 × 1,016 photons cm−2 s−1 (13.4 mW/cm2) and 2.77 × 1016 photons cm−2 s−1 (11.0 mW/cm2) for 380 nm and 500 nm light, respectively, which is similar to the light intensity used for ChR2 and NpHR stimulation in the retina.15,24
The latency for response onset was estimated from the peri-stimulus time histogram of each RGC by setting a threshold equal to the sum of the mean firing rate and two standard deviations of the activity preceding light onset.
To determine whether the LiGluR-mediated light-modulation of RGC firing rate was significant, we compared the distribution of firing rates for each cell at 500 nm and at 380 nm across ten trials using a nonparametric _t_-test (Mann–Whitney U-test). Cells that showed significantly different firing rate distributions across both wavelength, using a _P_-value <0.05, were considered to be significantly modulated by light.
Visually-evoked potential recording. Adult mice were anesthetized with ketamine (100 mg/kg, intraperitoneally) and xylazine (10 mg/kg, intraperitoneally). Pupils were dilated, skin and connective tissue over the skull were retracted, and a small headplate was attached. The animal was supported from the headplate during the experiments. Anaesthesia was supplemented with 0.5% isofluorane, as necessary. Body temperature was maintained at 36°–37 °C via a heating blanket (Harvard Apparatus, Holliston, MA). A small craniotomy and durotomy (~1 mm2) were performed over the primary visual cortex (2 mm lateral to the midline, 0.5 mm anterior to lambda). Electrodes were pulled with a horizontal puller (Sutter Instruments) from unfilamented borosilicate glass (1.5 mm OD; 1.16 mm ID; Warner Instruments) to tips of 1–2 µm. The electrodes (resistance ~0.5–3 Mω) containing saline solution were lowered to 0.4 mm below the surface of the cortex contralateral to the stimulated eye. Recordings were made with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). Sweeps were filtered at 2 kHz, sampled at 10 kHz by a 12 bit acquisition board (National Instruments, Austin, TX), and analyzed with custom software in Matlab. Eye drops were used to prevent corneal dehydration and cataract.
Illumination (1.5–2 mW/mm2 intensity, measured with a hand-held meter, 840-C, Newport) was applied using a Polychrome monochromator through a 1.5 mm diameter fiber optic placed 0.5 cm away from the eye. Flash wavelength and duration were controlled though TTL inputs to the monochromator. Visual stimulation consisted of 20–300 ms pulses of 380 nm light preceded and followed by 3 seconds of 500 nm light presented at 0.2 Hz for 40–50 repeats (Polychrome V; Till Photonics, Gräfelfing, Germany). Recording experiments were conducted 24 to 48 hours post MAG injection.
Pupillary reflex. Mice were dark-adapted for 1 hour before the recordings. Pupillary light responses were recorded under infrared conditions with an infrared sensitive CCD camera and IR filter. An LED source (385 nm, 5.4–5.6 mW/mm2) was used for all light stimuli. Maximum pupillary contraction was calculated by comparing the area of the pupil before and during illumination using ImageJ software. Measurements commenced 24 hours after MAG injection and were repeated daily for at least 1 week.
Water-maze behavior. Adult mice (3–5 months) were trained to approach a 380 nm light source in modified radial arm maze. The opaque Plexiglas maze (Figure 5a) consisted of five swim alley arms (10 cm width × 14.5 cm length × 22 cm high) radiating from the vertices of a pentagonal center pool (33.4 cm apothem). The maze was filled with water (26 °C) until the top of the escape platform (6.5 cm wide × 12 cm high) was 1 cm below the water surface. A 385 nm LED guide light serving as the aversive stimulus was mounted on the arm containing the platform, oriented towards the entrance of the arm. The intensity of the light at the entrance of the arm was < 1 mW/cm2. The study room was dark except for a dim red lamp over the maze and a dim red headlamp for the experimenter.
In each trial, a mouse was released at the center of the maze and its behavior monitored for 120 seconds. Entering the arm containing the platform and stepping on the platform or the passing of 120 seconds resulted in the termination of the trial. Subsequently, animals were kept on the platform under the illumination of the LED for an additional 30 seconds to reinforce the light/platform contingency. Animals were then removed, dried, and placed in a warm chamber. The location of the platform with the LED was varied randomly between trials. All animals performed between 3–5 trials per experimental session, one session a day. Water maze sessions were conducted over the course of 15 consecutive days except for day 8, when the mice were injected intravitreally with MAG (see intraocular vector and photoswitch administration). The latency to reach the platform was recorded for each trial. We also recorded the number of “retreat events,” which were events in which the mouse commenced to enter the target arm and retreated into the maze without reaching the platform.
The experimenter remained always in the same position with respect to the maze and this was not correlated with the position of the platform.
Statistical analysis. Microelectrode array and VEP data were analyzed using Wilcoxon signed rank's test unless otherwise stated. Pupillary reflexes and behavioral assays were analyzed using the Mann–Whitney U-test for paired comparisons.
SUPPLEMENTARY MATERIAL Figure S1. LiGluR is expressed in the majority of RGCs, throughout the retina. (A) High resolution assembly of confocal Z-stack projections at 10x through the RGC layer of a LiGluR expressing flat-mount. Immunocytochemistry with antibodies against iGluR6 (in green) shows abundant expression in many RGCs in the central and peripheral retina. 425x425 μm regions (red squares 1-4) in each quadrant were imaged at higher magnification (20x objective) for retinal cell counts. High magnification images (1a-4b) show staining for nuclei with DAPI (1a-4a) and for iGluR6 (green) and ChAT (red) (1b-4b). (B) Summary of cells counted per quadrant. The bar graph represents the mean counts in each of the four areas. Error bars represent SEM. The table shows cell counts per quadrant. Scale bars are 500 μm and 100 μm, respectively.Figure S2. LiGluR restores light responsiveness to short light pulses to rd1 retinas in the presence of the photoswitch MAG. (A) Mean firing rate (Hz) across all recorded RGCs in response to a 300ms flash of 380 nm light. In the absence of LiGluR (left four bars), there was no modulation of RGC firing rate by the 380 nm pulse, regardless of the treatment with MAG. In contrast, in retinas expressing LiGluR, treatment with MAG resulted in a highly significant change in the RGC firing upon illumination with 380nm light (p< 10−39, Wilcoxon Signed Rank Test). (**B**) Mean firing rate (Hz) across all recorded RGCs in response to a 50ms flash of 380nm light. In the absence of LiGluR (left four bars), there was no modulation of RGC firing rate by the 380nm pulse, regardless of the treatment with MAG (p>0.4, Wilcoxon Signed Rank Test). In contrast, in retinas expressing LiGluR, treatment with MAG resulted in a highly significant change in the RGC firing upon illumination with 380nm light (p< 10−29, Wilcoxon Signed Rank Test).**Figure S3.** Comparison of ERGs between WT animals injected intravitreally with MAG, DMSO or PBS. (**A**) Mean amplitude of the maximum A-wave recorded from WT animals after intravitreal injection of 2 μl of DMSO (1%), MAG (200μM in 1% DMSO) or PBS(1X). ERGs were conducted 24hs, 48hs and 1 week post-injection. Comparison of A-wave values at 1 week showed no significant difference between any of the experimental groups (Wilcoxon Ranked Sum test, p>0.4, n=5-10). Error bars are SEM. (B) Mean amplitude of the maximum B-wave recorded from WT animals after intravitreal injection of 2 μl of DMSO (1%), MAG (200μM in 1% DMSO) or PBS(1X). ERGs were conducted 24hs, 48hs and 1 week post-injection. Comparison of B-wave values at 1 week showed no significant difference between any of the experimental groups (Wilcoxon Ranked Sum test, p>0.5, n=5-10). Error bars are SEM.
Acknowledgments
The authors would like to thank M. Visel and S. Wiese for technical assistance, H. Aaron for help with microscopy, Support for this work was from the National Institutes of Health Nanomedicine Development Center for the Optical Control of Biological Function (PN2EY018241), The Foundation for Fighting Blindness (individual grant to J.G.F.)
Supplementary Material
Figure S1.
LiGluR is expressed in the majority of RGCs, throughout the retina. (A) High resolution assembly of confocal Z-stack projections at 10x through the RGC layer of a LiGluR expressing flat-mount. Immunocytochemistry with antibodies against iGluR6 (in green) shows abundant expression in many RGCs in the central and peripheral retina. 425x425 μm regions (red squares 1-4) in each quadrant were imaged at higher magnification (20x objective) for retinal cell counts. High magnification images (1a-4b) show staining for nuclei with DAPI (1a-4a) and for iGluR6 (green) and ChAT (red) (1b-4b). (B) Summary of cells counted per quadrant. The bar graph represents the mean counts in each of the four areas. Error bars represent SEM. The table shows cell counts per quadrant. Scale bars are 500 μm and 100 μm, respectively.
Figure S2.
LiGluR restores light responsiveness to short light pulses to rd1 retinas in the presence of the photoswitch MAG. (A) Mean firing rate (Hz) across all recorded RGCs in response to a 300ms flash of 380 nm light. In the absence of LiGluR (left four bars), there was no modulation of RGC firing rate by the 380 nm pulse, regardless of the treatment with MAG. In contrast, in retinas expressing LiGluR, treatment with MAG resulted in a highly significant change in the RGC firing upon illumination with 380nm light (p< 10−39, Wilcoxon Signed Rank Test). (**B**) Mean firing rate (Hz) across all recorded RGCs in response to a 50ms flash of 380nm light. In the absence of LiGluR (left four bars), there was no modulation of RGC firing rate by the 380nm pulse, regardless of the treatment with MAG (p>0.4, Wilcoxon Signed Rank Test). In contrast, in retinas expressing LiGluR, treatment with MAG resulted in a highly significant change in the RGC firing upon illumination with 380nm light (p< 10−29, Wilcoxon Signed Rank Test).
Figure S3.
Comparison of ERGs between WT animals injected intravitreally with MAG, DMSO or PBS. (A) Mean amplitude of the maximum A-wave recorded from WT animals after intravitreal injection of 2 μl of DMSO (1%), MAG (200μM in 1% DMSO) or PBS(1X). ERGs were conducted 24hs, 48hs and 1 week post-injection. Comparison of A-wave values at 1 week showed no significant difference between any of the experimental groups (Wilcoxon Ranked Sum test, p>0.4, n=5-10). Error bars are SEM. (B) Mean amplitude of the maximum B-wave recorded from WT animals after intravitreal injection of 2 μl of DMSO (1%), MAG (200μM in 1% DMSO) or PBS(1X). ERGs were conducted 24hs, 48hs and 1 week post-injection. Comparison of B-wave values at 1 week showed no significant difference between any of the experimental groups (Wilcoxon Ranked Sum test, p>0.5, n=5-10). Error bars are SEM.
REFERENCES
- Humphries P, Kenna P., and, Farrar GJ. On the molecular genetics of retinitis pigmentosa. Science. 1992;256:804–808. doi: 10.1126/science.1589761. [DOI] [PubMed] [Google Scholar]
- Sung CH, Davenport CM, Hennessey JC, Maumenee IH, Jacobson SG, Heckenlively JR.et al. (1991Rhodopsin mutations in autosomal dominant retinitis pigmentosa Proc Natl Acad Sci USA 886481–6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL., and, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Léveillard T., and, Sahel JA. Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling. Sci Transl Med. 2010;2:26ps16. doi: 10.1126/scitranslmed.3000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Kornacker K., and, Cepko CL. Stimulation of the insulin/mTOR pathway delays cone death in a mouse model of retinitis pigmentosa. Nat Neurosci. 2009;12:44–52. doi: 10.1038/nn.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes E, Seeliger M, Michalakis S, Biel M, Humphries P., and, Haverkamp S. Morphological characterization of the retina of the CNGA3(-/-)Rho(-/-) mutant mouse lacking functional cones and rods. Invest Ophthalmol Vis Sci. 2004;45:2039–2048. doi: 10.1167/iovs.03-0741. [DOI] [PubMed] [Google Scholar]
- Mazzoni F, Novelli E., and, Strettoi E. Retinal ganglion cells survive and maintain normal dendritic morphology in a mouse model of inherited photoreceptor degeneration. J Neurosci. 2008;28:14282–14292. doi: 10.1523/JNEUROSCI.4968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chader GJ, Weiland J., and, Humayun MS. Artificial vision: needs, functioning, and testing of a retinal electronic prosthesis. Prog Brain Res. 2009;175:317–332. doi: 10.1016/S0079-6123(09)17522-2. [DOI] [PubMed] [Google Scholar]
- Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel VP.et al. (2011Subretinal electronic chips allow blind patients to read letters and combine them to words Proc Biol Sci 2781489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun MS, Dorn JD, Ahuja AK, Caspi A, Filley E, Dagnelie G.et al. (2009Preliminary 6 month results from the Argus II epiretinal prosthesis feasibility study Conf Proc IEEE Eng Med Biol Soc 20094566–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Li Q, Raisler B, Timmers AM, Berns KI, Flannery JG.et al. (2004Range of retinal diseases potentially treatable by AAV-vectored gene therapy Novartis Found Symp 255179–188; discussion 188. [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K.et al. (2008Effect of gene therapy on visual function in Leber's congenital amaurosis N Engl J Med 3582231–2239. [DOI] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL.et al. (2010Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration Mol Ther 18643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL.et al. (2009Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year Hum Gene Ther 20999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagali PS, Balya D, Awatramani GB, Münch TA, Kim DS, Busskamp V.et al. (2008Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration Nat Neurosci 11667–675. [DOI] [PubMed] [Google Scholar]
- Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA.et al. (2011Virally delivered Channelrhodopsin-2 Safely and Effectively Restores Visual Function in Multiple Mouse Models of Blindness Mol Therepub ahead of print). [DOI] [PMC free article] [PubMed]
- Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH.et al. (2007Neural reprogramming in retinal degeneration Invest Ophthalmol Vis Sci 483364–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzibasi E, Calamusa M, Novelli E, Domenici L, Strettoi E., and, Cellerino A. Age-dependent remodelling of retinal circuitry. Neurobiol Aging. 2009;30:819–828. doi: 10.1016/j.neurobiolaging.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM.et al. (2006Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration Neuron 5023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Sugano E, Yawo H, Ishizuka T, Isago H, Narikawa S.et al. (2007Restoration of visual response in aged dystrophic RCS rats using AAV-mediated channelopsin-2 gene transfer Invest Ophthalmol Vis Sci 483821–3826. [DOI] [PubMed] [Google Scholar]
- Lin B, Koizumi A, Tanaka N, Panda S., and, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci USA. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ivanova E, Bi A., and, Pan ZH. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J Neurosci. 2009;29:9186–9196. doi: 10.1523/JNEUROSCI.0184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM., and, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S.et al. (2010Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa Science 329413–417. [DOI] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY., and, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD.et al. (2007Remote control of neuronal activity with a light-gated glutamate receptor Neuron 54535–545. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, Gaudry Q.et al. (2008Photochemical control of endogenous ion channels and cellular excitability Nat Methods 5331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H.et al. (2009Optogenetic dissection of a behavioural module in the vertebrate spinal cord Nature 461407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza P, Volgraf M, Numano R, Szobota S, Trauner D., and, Isacoff EY. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc Natl Acad Sci USA. 2007;104:10865–10870. doi: 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DB, Flannery JG, Bowes-Rickman C. The rd mouse story: seventy years of research on an animal model of inherited retinal degeneration. Prog Ret Eye Res. 1994;13:31–64. [Google Scholar]
- Porciatti V, Pizzorusso T., and, Maffei L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision Res. 1999;39:3071–3081. doi: 10.1016/s0042-6989(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Güler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW.et al. (2008Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision Nature 453102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW.et al. (2003Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice Nature 42476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Sanabria F, Lasswell A, Thrailkill EA, Pawlak AP., and, Killeen PR. Brief light as a practical aversive stimulus for the albino rat. Behav Brain Res. 2010;214:402–408. doi: 10.1016/j.bbr.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK., and, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol Vis. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- Milam AH, Li ZY., and, Fariss RN. Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res. 1998;17:175–205. doi: 10.1016/s1350-9462(97)00012-8. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Jacobson SG, Khanna H, Sumaroka A, Aguirre GK.et al. (2007Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis Hum Mutat 281074–1083. [DOI] [PubMed] [Google Scholar]
- Thyagarajan S, van Wyk M, Lehmann K, Löwel S, Feng G., and, Wässle H. Visual function in mice with photoreceptor degeneration and transgenic expression of channelrhodopsin 2 in ganglion cells. J Neurosci. 2010;30:8745–8758. doi: 10.1523/JNEUROSCI.4417-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasheff SF. Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. J Neurophysiol. 2008;99:1408–1421. doi: 10.1152/jn.00144.2007. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K., and, Hegemann P. Ultrafast optogenetic control. Nat Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, Bamann C.et al. (2011Ultra light-sensitive and fast neuronal activation with the Ca²(+)-permeable channelrhodopsin CatCh Nat Neurosci 14513–518. [DOI] [PubMed] [Google Scholar]
- Jacobson SG., and, Cideciyan AV. Treatment possibilities for retinitis pigmentosa. N Engl J Med. 2010;363:1669–1671. doi: 10.1056/NEJMcibr1007685. [DOI] [PubMed] [Google Scholar]
- Donahue SP, Recchia F., and, Sternberg P., Jr Bevacizumab vs ranibizumab for age-related macular degeneration: early results of a prospective double-masked, randomized clinical trial. Am J Ophthalmol. 2010;150:287; author reply 287. doi: 10.1016/j.ajo.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chi L, Sadovski O., and, Woolley GA. A blue-green absorbing cross-linker for rapid photoswitching of peptide helix content. Bioconjug Chem. 2006;17:670–676. doi: 10.1021/bc050363u. [DOI] [PubMed] [Google Scholar]
- Sadovski O, Beharry AA, Zhang F., and, Woolley GA. Spectral tuning of azobenzene photoswitches for biological applications. Angew Chem Int Ed Engl. 2009;48:1484–1486. doi: 10.1002/anie.200805013. [DOI] [PubMed] [Google Scholar]
- Grieger JC, Choi VW., and, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
LiGluR is expressed in the majority of RGCs, throughout the retina. (A) High resolution assembly of confocal Z-stack projections at 10x through the RGC layer of a LiGluR expressing flat-mount. Immunocytochemistry with antibodies against iGluR6 (in green) shows abundant expression in many RGCs in the central and peripheral retina. 425x425 μm regions (red squares 1-4) in each quadrant were imaged at higher magnification (20x objective) for retinal cell counts. High magnification images (1a-4b) show staining for nuclei with DAPI (1a-4a) and for iGluR6 (green) and ChAT (red) (1b-4b). (B) Summary of cells counted per quadrant. The bar graph represents the mean counts in each of the four areas. Error bars represent SEM. The table shows cell counts per quadrant. Scale bars are 500 μm and 100 μm, respectively.
Figure S2.
LiGluR restores light responsiveness to short light pulses to rd1 retinas in the presence of the photoswitch MAG. (A) Mean firing rate (Hz) across all recorded RGCs in response to a 300ms flash of 380 nm light. In the absence of LiGluR (left four bars), there was no modulation of RGC firing rate by the 380 nm pulse, regardless of the treatment with MAG. In contrast, in retinas expressing LiGluR, treatment with MAG resulted in a highly significant change in the RGC firing upon illumination with 380nm light (p< 10−39, Wilcoxon Signed Rank Test). (**B**) Mean firing rate (Hz) across all recorded RGCs in response to a 50ms flash of 380nm light. In the absence of LiGluR (left four bars), there was no modulation of RGC firing rate by the 380nm pulse, regardless of the treatment with MAG (p>0.4, Wilcoxon Signed Rank Test). In contrast, in retinas expressing LiGluR, treatment with MAG resulted in a highly significant change in the RGC firing upon illumination with 380nm light (p< 10−29, Wilcoxon Signed Rank Test).
Figure S3.
Comparison of ERGs between WT animals injected intravitreally with MAG, DMSO or PBS. (A) Mean amplitude of the maximum A-wave recorded from WT animals after intravitreal injection of 2 μl of DMSO (1%), MAG (200μM in 1% DMSO) or PBS(1X). ERGs were conducted 24hs, 48hs and 1 week post-injection. Comparison of A-wave values at 1 week showed no significant difference between any of the experimental groups (Wilcoxon Ranked Sum test, p>0.4, n=5-10). Error bars are SEM. (B) Mean amplitude of the maximum B-wave recorded from WT animals after intravitreal injection of 2 μl of DMSO (1%), MAG (200μM in 1% DMSO) or PBS(1X). ERGs were conducted 24hs, 48hs and 1 week post-injection. Comparison of B-wave values at 1 week showed no significant difference between any of the experimental groups (Wilcoxon Ranked Sum test, p>0.5, n=5-10). Error bars are SEM.