Revisiting Human IL-12Rβ1 Deficiency: A Survey of 141 Patients From 30 Countries (original) (raw)
. Author manuscript; available in PMC: 2011 Nov 1.
Published in final edited form as: Medicine (Baltimore). 2010 Nov;89(6):381–402. doi: 10.1097/MD.0b013e3181fdd832
Abstract
Interleukin-12 receptor β1 (IL-12Rβ1) deficiency is the most common form of Mendelian susceptibility to mycobacterial disease (MSMD). We undertook an international survey of 141 patients from 102 kindreds in 30 countries. Among 102 probands, the first infection occurred at a mean age of 2.4 years. In 78 patients, this infection was caused by Bacille Calmette-Guérin (BCG; n = 65), environmental mycobacteria (EM; also known as atypical or nontuberculous mycobacteria) (n = 9) or Mycobacterium tuberculosis (n = 4). Twenty-two of the remaining 24 probands initially presented with nontyphoidal, extraintestinal salmonellosis. Twenty of the 29 genetically affected sibs displayed clinical signs (69%); however 8 remained asymptomatic (27%). Nine nongenotyped sibs with symptoms died. Recurrent BCG infection was diagnosed in 15 cases, recurrent EM in 3 cases, recurrent salmonellosis in 22 patients. Ninety of the 132 symptomatic patients had infections with a single microorganism. Multiple infections were diagnosed in 40 cases, with combined mycobacteriosis and salmonellosis in 36 individuals. BCG disease strongly protected against subsequent EM disease (p = 0.00008). Various other infectious diseases occurred, albeit each rarely, yet candidiasis was reported in 33 of the patients (23%). Ninety-nine patients (70%) survived, with a mean age at last follow-up visit of 12.7 years ± 9.8 years (range, 0.5-46.4 yr). IL-12Rβ1 deficiency is characterized by childhood-onset mycobacteriosis and salmonellosis, rare recurrences of mycobacterial disease, and more frequent recurrence of salmonellosis. The condition has higher clinical penetrance, broader susceptibility to infections, and less favorable outcome than previously thought.
INTRODUCTION
Mendelian susceptibility to mycobacterial disease (MSMD; MIM 209950) is a clinical syndrome, probably first described in 1951 (45), that predisposes otherwise apparently healthy individuals to infections caused by weakly virulent mycobacteria, such as Bacille Calmette-Guérin (BCG) and environmental mycobacteria (EM; also known as atypical or nontuberculous mycobacteria).[9] Since 1996, MSMD-causing mutations have been identified in 6 genes.[4,25] Five of these genes are autosomal and encode the 2 chains of the interferon (IFN)-γ receptor (IFNGR1 and IFNGR2), the signal transducer and activator of transcription factor 1 (STAT1), the p40 subunit of interleukin (IL)-12 and IL-23 (IL12B), and the β1 chain shared by the IL-12 and IL-23 receptors (IL12RB1), whereas the sixth gene is X-linked and encodes nuclear factor-κB essential modulator (NEMO).[25] These defects impair IFN-γ-mediated immunity. The allelic heterogeneity is such that mutations in these 6 genes define up to 13 different genetic traits, with some genes associated with recessive or dominant inheritance, complete or partial defects, and loss of expression or the expression of nonfunctional molecules.[4,25] Patients with MSMD are also susceptible to the more virulent species Mycobacterium tuberculosis, and IL-12Rβ1 deficiency was the first identified Mendelian genetic etiology of pediatric tuberculosis in children with normal resistance to BCG and EM.[3,6,8,48] These defects also predispose patients to Salmonella infections.[25,43] A few other infections have been diagnosed, but mostly in smaller numbers of patients, making it difficult to draw firm conclusions about the relationship between these infections and the underlying genetic defects.[25]
The most common genetic etiology of MSMD is autosomal recessive IL-12Rβ1 deficiency, first reported in 1998.[5,16] NK and T cells from patients with this condition do not respond to IL-12 and produce low levels of IFN-γ. To our knowledge, the first large series of patients was reported in 2003 and included 41 patients from 29 unrelated families in 17 countries.[24] This survey described 5 key clinical features of IL-12Rβ1 deficiency, differentiating this deficiency from other genetic etiologies of MSMD, such as IFN-γR1 deficiency[18]: 1) infections typically appeared in childhood, with no adult onset of disease; 2) the recurrence of mycobacterial disease was exceedingly rare, with BCG disease protecting against subsequent EM disease; 3) clinical penetrance was incomplete, with up to 45% of genetically affected sibs remaining asymptomatic; 4) patients displayed broad resistance to infectious agents other than Mycobacterium and Salmonella; and 5) the outcome was favorable in most cases, with a mortality rate of only 15%.
Individual case reports and small series have since brought the number of reported patients with this deficiency to 78.[2,6-8,11,13-15,19,20,22,23,27,28,30,33-37,40,42,46-49,54-60,63-67,69,70,72] However, improvements in the description of this disorder are required. These improvements require a decrease in ascertainment bias, through description of the clinical phenotype of a larger number of patients with diverse genetic backgrounds exposed to different microbial flora, including, in particular, genetically affected sibs of index cases. We report here the molecular, cellular, and clinical features of a series of 141 patients (including 63 unpublished patients) with IL-12Rβ1 deficiency from 102 kindreds in 30 countries.
PATIENTS AND METHODS
Subjects and Kindreds
Patients (and their families) were recruited into the current study through a large, worldwide network of collaborating clinicians and immunologists. These patients presented with a history of unusual infections, such as disseminated and/or recurrent disease caused by weakly virulent mycobacteria and/or Salmonella, corresponding to the description of MSMD and other similar conditions. Patients with severe, disseminated forms of tuberculosis were also studied. The study was conducted in accordance with the Helsinki Declaration, with informed consent obtained from each patient or the patient's family, as requested and approved by the institutional review boards of the various institutions involved, including the Necker Medical School.
Whole-Blood Activation
Whole-blood activation was used as the first-line screening for the possible mutation in the IL-12/IFN-γaxis. Venous blood samples were collected in tubes containing heparin and were transported at room temperature by express mail to the Laboratory of Human Genetics of Infectious Diseases in Paris for analysis. Blood was diluted 1:2 in RPMI 1640 medium (Invitrogen Life Technologies, Paisley, UK). Aliquots of diluted blood were dispensed into the wells of a 48-well plate and incubated in 4 sets of conditions: with medium alone, with live BCG (Mycobacterium bovis BCG, Pasteur strain, MOI 20:1), with BCG plus IFN-γ (5000 IU/mL; Imukin Boehringer Ingelheim, Reims, France), or with BCG plus IL-12p70 (20 ng/mL; R & D Systems, Minneapolis, MN), the final volume within each well being 1 mL.[22] Supernatants were collected after 48 hours and centrifuged at 1000 g for 5 minutes. All supernatants were stored at −20°C until analysis.
Determination of Cytokine Levels
IL-12p40, IL-12p70, and IFN-γ levels (48-hour culture supernatants) were determined by enzyme-linked immunosorbent assay (ELISA). We used the capture antibodies, detection antibodies, and standards supplied in the R & D Systems kits for IL-12p40 and IL-12p70 (Quantikine SP400) and in the Sanquin kit (CLB, Amsterdam, The Netherlands) for IFN-γ (M9333), diluted in HPE dilution buffer (M1940, Sanquin, CLB). Milk was used for blocking, and antibody binding was detected with streptavidin horseradish peroxidase (M2032, Sanquin, CLB) and TMB microwell peroxidase substrate (50-76-00, KPL, Gaithersburg, MD). The reaction was stopped by adding H2SO4 (1.8 M). Optical density was determined with an MRX microplate reader (Thermolab Systems, Saint Herblain, France). Quantitative analysis with a nonlinear, 4-parameter logistic (4PL) calibration model was carried out with inhouse software based on the Microsoft Excel application language developed for this purpose (gift from Max Feinberg). Results for each cytokine are expressed in pg/mL/106 peripheral blood mononuclear cells.[(22)] Whole-blood activation and subsequent ELISA were repeated only in cases when the blood arrived in poor condition due to long travel. The results of this assay, when performed with optimal conditions, were strictly consistent for the same patient as well as between patients.
Cell Culture
Epstein-Barr virus-transformed lymphoblastoid cell lines (EBV-B cell lines) were cultured in RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen Life Technologies). Saimiri herpesvirus-transformed T cells were cultured in a 1:2 mixture of RPMI 1640 medium/Panserin 401 medium (Pan Biotech, Aidenbach, Germany) with 10% FBS, 2 mM L-glutamine (Invitrogen Life Technologies), 10 U/mL IL-2 (Roche, Mannheim, Germany), and 100 μg/mL gentamycin. For the production of phytohemagglutinin (PHA)-activated T cells, peripheral blood mononuclear cells were purified by centrifugation on a Ficoll-Hypaque gradient (GE Healthcare, Saclay, France), resuspended in RPMI medium supplemented with 10% FBS and activated by incubation with 1/700 PHA (Becton Dickinson, Sparks, MD) for 72-96 hours. PHA-T-cell blasts were then stimulated by incubation for 48 hours with IL-2 (50 IU/mL; Proleukin, Chiron, Amsterdam, The Netherlands) and cultured at a density of 2 × 105 cells/mL in Panserin 401 medium (Pan Biotech) with 10% FBS and 2 mM L-glutamine (Invitrogen Life Technologies). All cells were incubated at 37°C, under an atmosphere containing 5% CO2.
Transfection
Saimiri herpesvirus-transformed T cells were transfected with a wildtype pEGFPN1-IL12RB1 vector or with 1 of the various missense mutants. We transfected 5 × 106 cells with 2 μg of DNA, using the Cell Line Nucleofector Kit V (VCA-1003, Amaxa, Paris, France) and Y-001. We assessed receptor expression at the cell surface and IL-12 binding 48 hours after transfection.
Flow Cytometry
PHA-T-cell blasts or EBV-B cell lines were washed in phosphate-buffered saline (PBS) and dispensed into a 96-well plate for labeling. The cells were incubated with an anti-IL-12Rβ1 antibody (1:100 dilution of the 2.4E6 or 2B10 clone, Becton Dickinson) or an equivalent concentration of isotype-matched control mAb (MOPC-21 and/or R35-95, Becton Dickinson) in 2% FBS in PBS, on ice, for 20 minutes. The cells were then washed twice with cold 2% FBS in PBS and incubated on ice for 20 minutes with Alexa Fluor 488-conjugated goat antimouse or goat antirat antibody (A-11029 or A-11006, Invitrogen Life Technologies). Cells were then washed twice with 2% FBS in PBS and analyzed with a FACScan machine, using Cellquest software (Becton Dickinson).
Fluorescent IL-12 Binding
IL-12 fluorescence-binding experiments were performed as follows: 5 × 105 transfected cells were incubated in 25 μL of PBS with or without 50 ng/mL IL-12p70 (R & D Systems) for 30 minutes at 4°C, and then with mouse antihuman IL-12p40-p70 IgG1 (Pharmingen, San Diego, CA) and, finally, with PE-conjugated goat anti-mouse antibody (Invitrogen Life Technologies). Stained cells were analyzed with a FACScan machine, using Cellquest software (Becton Dickinson, Maryland, USA).
Genetic Analysis
Human genomic DNA was isolated from the pellets obtained after the Ficoll-Paque Plus gradient purification of peripheral blood mononuclear cells, or from whole blood or cell lines. The cells were lysed in extraction buffer (10 mM Tris, pH 7.4, 0.1 M EDTA, 0.5% SDS, and 10 mg/mL proteinase K) and incubated overnight at 37°C. The DNA was isolated by phenol/chloroform extraction, precipitated in ethanol, and resuspended in 10 mM Tris, pH 7.4, 1 mM EDTA. RNA was isolated from EBV-B cell lines or PHA-T-cell blasts with Trizol reagent (Invitrogen Life Technologies), according to the manufacturer's instructions. RNA was reverse transcribed by Superscript II reverse transcriptase (Invitrogen Life Technologies) with oligo-dT. The first-strand cDNA was stored at −20°C. Polymerase chain reaction (PCR) amplification was carried out with the AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA) and the GeneAmp PCR system 9700 (Applied Biosystems). The primers and conditions used for PCR amplification of the coding exons, including the flanking intron sequences, or the cDNA of IL12RB1 are available upon request. Amplified PCR products were checked by electrophoresis in a 1% agarose gel and were purified by centrifugation through Sephadex G-50 Superfine resin (GE Healthcare) on multiscreen MAHV-N45 (Millipore, Molsheim, France) filter plates. PCR products were sequenced by dideoxynucleotide termination, with the BigDye Terminator kit v1.1 (Applied Biosystems) and appropriate primers. Sequencing products were purified by centrifugation through Sephadex G-50 Superfine resin and analyzed on an ABI Prism 3100 or 3130xl apparatus (Applied Biosystems). Sequences files and chromatograms were analyzed with GENALYS software (CNG, France; http://software.cng.fr).[62]
Statistical Methods
Infection-free status, survival, and penetrance curves as a function of age were estimated by the Kaplan-Meier method, and, when necessary, curves were compared by log-rank tests. Penetrance curves for IL-12Rβ1 deficiency were obtained from the data for the sibs of index cases, through the use of 2 strategies. The first strategy was based on the use of data for sibs with identified IL-12Rβ1 mutations only (n = 29). The second strategy was based on the assumption that nongenotyped sibs suffering from MSMD-related infections were also IL-12Rβ1-deficient, leading to the inclusion of these sibs with relevant information in the estimation of penetrance (n = 8). However, we avoided the bias that would result from the addition of clinically affected sibs only, by also including nongenotyped healthy sibs as follows: 1) we calculated the proportion of genotyped healthy sibs with genetically confirmed IL-12Rβ1 deficiency: 0.08 (8/100); 2) we assumed that the same proportion of the 57 nongenotyped healthy sibs would be IL-12Rβ1-deficient (that is, 5 sibs); 3) we randomly selected 5 follow-up periods for the 57 healthy sibs, such that the mean duration of follow-up for these 5 sibs did not differ significantly from the overall mean follow-up period for all 57 healthy sibs (that is, 12.78 ± 13.70 yr). All calculations were carried out and curves plotted with R software (http://cran.r-project.org/).
RESULTS
Clinical Features and Mutation Analysis in 102 Index Cases
Children and young adults with clinical disease caused by BCG or EM or those with salmonellosis or tuberculosis and suspected IL-12Rβ1 deficiency were referred to our laboratory. By sequencing the 17 IL12RB1 coding exons and flanking intron regions, we identified 102 IL-12Rβ1-deficient index cases from 30 countries (Figure 1; Table 1). We identified 54 mutant alleles, including nonsense (n = 11), missense (n = 14), and splice (n = 10) mutations, small insertions (n = 2), small deletions (n = 9), large deletions (n = 3), deletions/insertions (n = 4), and 1 duplication (Figure 2; Table 1). Missense mutations were not found among the polymorphisms reported in the National Center for Biotechnology Information (NCBI) and Ensembl databases. Furthermore, none of the missense mutations was found in 50 healthy control individuals. All predicted splice mutations had a major impact on the structure of the IL12RB1 mRNA, with no full-length mRNAs detected, as determined by RT-PCR (data not shown). All but 2 of the deletions and insertions resulted in frame shifts, and the 14 missense mutations tested compromised protein expression (see below). The index cases were typically homozygous (n = 87) or, in rare cases, compound heterozygous (n = 14).
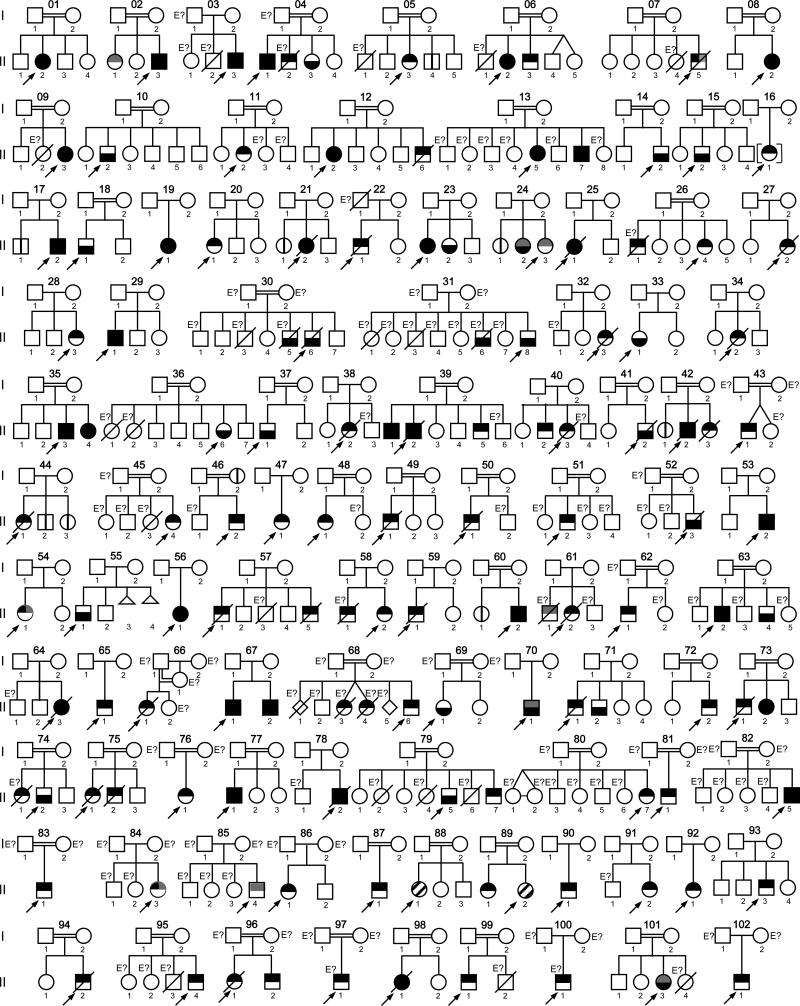
fig. 1.
Pedigrees of 102 families with IL-12Rβ1 deficiency. Each kindred is designated by an integer (1–102), each generation is designated by a roman numeral (I–II), and each individual is designated by an arabic numeral (each individual studied is identified by these 3 numbers, organized from left to right). The double lines connecting the parents, and in 1 case parent and offspring, indicate presumed consanguinity. Symbols are divided into 2 by a horizontal line. The upper part of the symbol indicates mycobacterial infection status (in black, patients with BCG disease or atypical mycobacteriosis; in gray, patients with tuberculosis); the lower part of the symbol indicates salmonellosis status, black indicating that the patient has had salmonellosis. The probands are indicated by an arrow. Proband 88.II.1 had granulomatous disease of unknown origin; proband 89.II.2 presented with nocardiosis and klebsiellosis. Individuals whose genetic status could not be evaluated are indicated by the symbol “E?”. Asymptomatic individuals carrying 2 mutant IL12RB1 alleles are represented by a vertical line. Kindreds 11 and 63 were related, as were kindreds 12, 13, 36 and 80, kindreds 25 and 30, and kindreds 48 and 51.
TABLE 1.
Genetic and Clinical Features of Patients With IL-12Rβ1 Deficiency
| Patient | Kindred | Code | Country of Origin* | Mutation | Age/Sex (yr) | Follow-Up Outcome | BCG† | EM‡ | Mtb§ | Salmonella¶ | Candida** | Other Diseases†† | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | II.2 | Morocco | K305X | 29.3/F | Alive | nk | - | - | Stm | na | na | 9,11,44 |
| 2 | 2 | II.1 | Morocco | R213W | 28.0/F | Alive | R | - | Mtb | - | na | na | 3,11 |
| 3 | 2 | II.3 | Morocco | R213W | 16.6/M | Alive | nk | - | - | Sen | na | na | 3,11 |
| 4 | 3 | II.3 | Cameroon | Y367C | 8.2/M | Alive | D | - | - | Sd, Sh | - | - | 11 |
| 5 | 4 | II.1 | Cyprus | 1623_1624delinsTT | 39.0/M | Alive | R | Ma, Mt, Mg | - | - | - | 9,11,33,37 | |
| 6 | 4 | II.2 | Cyprus | NA | 7.5/M | Deceased | R | Ma | - | - | Ca | - | 9,11,33,37 |
| 7 | 4 | II.3 | Cyprus | 1623_1624delinsTT | 27.1/F | Alive | R | - | - | S. spp | - | - | 9,11,33,37 |
| 8 | 5 | II.3 | Turkey (Kurds) | 783+1G>A | 21.8/F | Alive | D | - | - | - | - | - | 9,11,32 |
| 9 | 5 | II.4 | Turkey (Kurds) | 783+1G>A | 17.4/M | Alive | nv | - | - | - | - | - | 9,11,32 |
| 10 | 6 | II.2 | Turkey (Kurds) | 783+1G>A | 20.3/F | Alive | D | - | - | Se | - | - | 11 |
| 11 | 6 | II.3 | Turkey (Kurds) | 783+1G>A | 15.0/M | Alive | D | - | - | - | - | - | 11 |
| 12 | 7 | II.5 | Turkey | R173P | 17.4/M | Deceased | R | Ma, Mfc | Mtb | - | Ca | - | 11,14 |
| 13 | 8 | II.2 | Turkey | R173P | 14.8/F | Alive | L | Mc | - | Se | - | Leukocytoclastic vasculitis | 11,25 |
| 14 | 9 | II.3 | Turkey | 557_563delins8 | 18.0/F | Alive | L | - | - | Se, Stm, St | Ca | Vasculitis | 11 |
| 15 | 10 | II.2 | Israel (Bedouin Arab) | 700+362_1619-944del | 9.9/M | Alive | nv | - | - | SD | Ca | - | 11,18,19,44 |
| 16 | 11 | II.2 | KSA | 1190-1G>A | 8.4/F | Alive | D | - | - | - | - | - | 11 |
| 17 | 12 | II.2 | Qatar | C186S | 13.0/F | Alive | L | - | - | Se | - | - | 11 |
| 18 | 12 | II.6 | Qatar | C186S | 3.4/M | Deceased | D | - | - | - | - | - | - |
| 19 | 13 | II.5 | Qatar | C186S | 10.6/F | Alive | R | M. spp | - | SD | - | - | 11 |
| 20 | 13 | II.7 | Qatar | C186S | 9.1/M | Alive | R | M. spp | - | S. spp | - | - | 11 |
| 21 | 14 | II.2 | Iran | 1791+2T>G | 13.5/M | Alive | nv | - | - | Se | - | - | 11,16 |
| 22 | 15 | II.2 | Pakistan | S321X | 24.2/M | Alive | R | - | - | Se | - | - | 11 |
| 23 | 16 | II.1 | Sri Lanka | 1791+2T>G | 23.9/F | Alive | D | - | - | - | - | - | 11 |
| 24 | 17 | II.1 | France | [Q32X]+[1623_1624delinsT] | 18.8/M | Alive | R | - | - | - | na | na | 11 |
| 25 | 17 | II.2 | France | [Q32X]+[1623_1624delinsT] | 13.8/M | Alive | nv | Mg | - | Stm | na | na | 11 |
| 26 | 18 | II.1 | France | Q376X | 30.8/M | Alive | R | - | - | Sd | Ca | - | 11 |
| 27 | 19 | II.1 | France | [1745 1746delinsCA]+[1483+182_1619-1073del] | 37.2/F | Alive | nk | - | - | S. spp, Sd | Ca | Toxoplasma retinitis | 11,44 |
| 28 | 20 | II.1 | France | Q32X | 11.7/F | Alive | nk | - | - | - | na | na | 11,44 |
| 29 | 21 | II.1 | Belgium | Q32X | 21.5/F | Alive | nv | - | - | - | na | na | 11,44 |
| 30 | 21 | II.2 | Belgium | Q32X | 7.2/F | Deceased | nv | Ma | - | Se | Ca | - | 11,34 |
| 31 | 22 | II.1 | Germany | 1623_1624delinsTT | 3.5/M | Deceased | nv | Ma | - | - | na | na | 11 |
| 32 | 23 | II.1 | Germany | 1623_1624delinsTT | 14.9/F | Alive | D | - | - | Se | - | - | 11 |
| 33 | 23 | II.2 | Germany | 1623_1624delinsTT | 11.9/F | Alive | nv | - | - | S. spp | - | - | 11 |
| 34 | 24 | II.1 | Spain | 1791+2T>G | 21.5/F | Alive | nv | - | - | - | - | - | 5,11 |
| 35 | 24 | II.2 | Spain | 1791+2T>G | 11.5/F | Alive | nv | - | Mtb | - | - | - | 5,11 |
| 36 | 24 | II.3 | Spain | 1791+2T>G | 18.9/F | Alive | nv | - | Mtb | Se | - | - | 5,11 |
| 37 | 25 | II.1 | Spain | 1791+2T>G | 7.5/F | Deceased | nv | Ma | - | Se | Ca | - | 11 |
| 38 | 26 | II.1 | Bosnia and Herzegovina | NA | 3.8/M | Deceased | D | - | - | - | - | - | 11 |
| 39 | 26 | II.4 | Bosnia and Herzegovina | 549+2T>C | 12.0/F | Alive | nv | M. spp | - | - | - | - | 11 |
| 40 | 27 | II.2 | Slovakia | [1440_1447delins16]+[Q171P] | 2.4/F | Deceased | D | - | - | - | - | - | 11,20 |
| 41 | 28 | II.3 | Slovakia | [1007_1008delinsG]+[Q171P] | 9.0/F | Alive | D | - | - | - | - | - | 11,20 |
| 42 | 29 | II.1 | Brazil | L77P | 29.8/M | Alive | L | - | - | Stm | - | Paracoccidioides brasiliensis, Toxoplasma gondii chorioretinitis | 11,21 |
| 43 | 30 | II.5 | Spain | NA | 6.9/M | Deceased | na | - | - | Se | Ca | - | - |
| 44 | 30 | II.6 | Spain | 1791+2T>G | 30.2/M | Deceased | nv | - | - | Se, Sp | Ca | Esophageal carcinoma | - |
| 45 | 31 | II.6 | Mexico | NA | na/M | Deceased | na | - | - | S. spp | - | - | - |
| 46 | 31 | II.8 | Mexico | 1791+2T>G | 34.0/M | Alive | R | - | - | SB | - | - | - |
| 47 | 32 | II.2 | Belgium | [1623_1624delinsTT]+[65delCTGC] | 13.7/F | Deceased | nv | Ma | - | - | - | - | 46,49 |
| 48 | 33 | II.1 | France | [C196Y]+[1483+182_1619-1073del] | 27.8/F | Alive | R | - | - | Stm | na | na | 31 |
| 49 | 34 | II.2 | Poland | [I369T]+[1623_1624delinsTT] | 5.9/F | Deceased | D | - | - | - | - | - | - |
| 50 | 35 | II.3 | KSA | Y88X | 12.1/M | Alive | D | - | - | SD | - | - | - |
| 51 | 35 | II.4 | KSA | Y88X | 6.1/F | Alive | D | - | - | SD | - | Citrobacter freundii | - |
| 52 | 36 | II.6 | Qatar | C186S | 8.5/F | Alive | R | - | - | SD | - | Ataxia-telangiectasia | 48 |
| 53 | 37 | II.1 | Turkey | R173P | 14.3/M | Alive | R | - | - | Se | - | - | 44 |
| 54 | 38 | II.2 | Turkey | 711insC | 2.0/F | Deceased | D | - | - | - | Ca | - | 26 |
| 55 | 39 | II.1 | Turkey | 628-644dup | 10.7/M | Alive | L | - | - | S. spp | - | - | - |
| 56 | 39 | II.2 | Turkey | 628-644dup | 4.9/M | Deceased | L | Ma | - | S. spp | Ca | - | 26 |
| 57 | 39 | II.5 | Turkey | 628-644dup | 2.5/M | Alive | L | - | - | - | Ca | - | - |
| 58 | 40 | II.2 | KSA | 1336delC | 7.8/M | Alive | L | - | - | - | - | - | - |
| 59 | 40 | II.3 | KSA | 1336delC | 3.8/F | Deceased | D | - | - | - | - | - | - |
| 60 | 41 | II.2 | Turkey | 783+1G>A | 3.1/M | Deceased | R | - | - | Se | Ca | - | 26 |
| 61 | 42 | II.1 | Israel (Arabic) | 700+362_1619-944del | 11.5/F | Alive | nv | - | - | - | - | - | - |
| 62 | 42 | II.2 | Israel (Arabic) | 700+362_1619-944del | 9.4/M | Deceased | nv | Ma | - | Stm | - | - | 29 |
| 63 | 42 | II.3 | Israel (Arabic) | 700+362_1619-944del | 2.1/F | Deceased | nv | Ma | - | - | - | - | - |
| 64 | 43 | II.1 | Turkey | R486X | 4.0/M | Alive | L | - | - | - | - | - | 50 |
| 65 | 44 | II.1 | Mexico | 1791+2T>G | 3.6/F | Deceased | D | - | - | - | Ca | - | - |
| 66 | 44 | II.2 | Mexico | 1791+2T>G | 2.2/M | Alive | nv | - | - | - | - | - | - |
| 67 | 44 | II.3 | Mexico | 1791+2T>G | 8 mo/F | Alive | nv | - | - | - | - | - | - |
| 68 | 45 | II.4 | Iran | 1791+2T>G | 8.6/F | Alive | D | - | - | - | - | - | - |
| 69 | 46 | I.2 | Iran | 580+1G>A | na/F | Alive | na | - | - | - | na | na | - |
| 70 | 46 | II.2 | Iran | 580+1G>A | 4.1/M | Alive | D | - | - | - | - | - | - |
| 71 | 47 | II.1 | Brazil | [983_999del] + [R173W] | 5.8/F | Alive | D | - | - | - | - | - | - |
| 72 | 48 | II.1 | KSA | Y88X | 3.4/F | Alive | D | - | - | - | - | - | - |
| 49 | II.1 | Turkey | 783+1G>A | 1.9/M | Deceased | D | - | - | - | Ca | - | - | |
| 74 | 50 | II.1 | Brazil | 1791+2T>G | 2.0/M | Deceased | D | - | - | - | - | - | 36 |
| 75 | 51 | II.2 | KSA | Y88X | 5.1/M | Alive | D | Ms | - | - | - | - | - |
| 76 | 52 | II.3 | Venezuela | R173W | 13.7/M | Deceased | R | - | - | Se | Ca | - | - |
| 77 | 53 | II.2 | Ukraine | [1189+2T>A]+[1791+2T>G] | 9.1/M | Alive | D | M. spp | - | Stm, Se | - | - | - |
| 78 | 54 | II.1 | India | R521X | 8.5/F | Alive | D | - | Mtb | - | - | Histoplasmosis | - |
| 79 | 55 | II.1 | Taiwan | R211P | 23.5/M | Alive | R | - | - | Se | - | - | 22,47 |
| 80 | 56 | II.1 | Poland | R173W | 16.6/F | Alive | D | - | - | Se | - | - | - |
| 81 | 57 | II.1 | Mexico | 1791+2T>G | 16.5/M | Deceased | D | - | - | - | Ca | - | - |
| 82 | 57 | II.5 | Mexico | 1791+2T>G | 3.3/M | Deceased | D | - | - | - | - | - | - |
| 83 | 58 | II.1 | China | NA | 1.2/M | Deceased | D | - | - | - | - | - | 30 |
| 84 | 58 | II.2 | China | 1791+2T>G | 3.8/F | Alive | D | - | - | - | - | - | 30 |
| 85 | 59 | II.1 | Chile | [169delA]+[C62G] | 2.1/M | Deceased | D | - | - | - | - | - | - |
| 86 | 60 | II.1 | Turkey | C198R | 7.9/F | Alive | R | - | - | - | - | - | - |
| 87 | 60 | II.2 | Turkey | C198R | 3.7/M | Alive | L | - | - | Stm | - | - | - |
| 88 | 61 | II.1 | China | NA | 10.6/M | Deceased | nv | - | Mtb | - | - | - | 30 |
| 89 | 61 | II.2 | China | Q285X | 2.0/F | Deceased | D | - | - | - | - | - | 30 |
| 90 | 62 | II.1 | Iran | R521X | 7.5/M | Alive | D | - | - | - | - | - | - |
| 91 | 63 | II.2 | KSA | 1190-1G>A | 13.1/M | Alive | D | - | - | Stm | - | - | 44 |
| 92 | 63 | II.4 | KSA | 1190-1G>A | 9.1/M | Alive | R | - | - | SD | - | - | 44 |
| 93 | 64 | II.2 | Netherlands | Q376X | 28.4/F | Deceased | nv | Ma | - | Stm | Ca | - | 10,37 |
| 94 | 65 | II.1 | Argentina | [E67X]+[1623_1624delinsTT] | 3.1/M | Alive | D | - | - | - | - | - | 49 |
| 95 | 66 | II.1 | UK | 1623_1624delinsTT | 6.8/F | Deceased | nv | MAIc | - | - | - | Klebsiella pneumoniae | - |
| 96 | 67 | II.1 | Ukraine | E480X | 11.6/M | Alive | L | - | - | Stm | - | - | - |
| 97 | 67 | II.2 | Ukraine | E480X | 2.7/M | Alive | D | - | - | Stm | - | - | - |
| 98 | 68 | II.3 | Turkey | NA | 4.4/F | Deceased | D | - | - | - | na | - | - |
| 99 | 68 | II.4 | Turkey | NA | 5.0/F | Deceased | D | - | - | - | na | - | - |
| 100 | 68 | II.6 | Turkey | R175W | 2.6/M | Alive | L | - | - | - | - | - | - |
| 101 | 69 | II.1 | Turkey | R175W | 6.0/F | Alive | R | - | - | Se | - | - | 27 |
| 102 | 70 | II.1 | French West Indies | 1765delG | 32.0/M | Alive | M. spp | M. spp | M. spp | SD | - | - | - |
| 103 | 71 | II.1 | Turkey | 467_483del | 5.0/M | Deceased | D | - | - | - | - | - | 17 |
| 104 | 71 | II.2 | Turkey | 467_483del | 8.0/M | Alive | nv | - | - | SD | - | Leishmania | 17,23,28 |
| 105 | 72 | II.2 | Turkey | C198R | 15.7/M | Alive | D | - | - | - | - | - | 17 |
| 106 | 73 | II.1 | Turkey | 783+1G>A | 4.0/M | Deceased | D | - | - | - | Ca | - | 17 |
| 107 | 73 | II.2 | Turkey | 783+1G>A | 10.2/F | Alive | nv | M. spp | M. spp | SB, SD | Ca | Leukocytoclastic vasculitis | 17 |
| 108 | 74 | II.1 | Turkey | NA | 4.2/F | Deceased | D | - | - | - | - | - | 35 |
| 109 | 74 | II.2 | Turkey | 783+1G>A | 11.1/M | Alive | nv | - | - | SD | - | - | 23 |
| 110 | 75 | II.1 | Turkey | 783+1G>A | 7.0/F | Deceased | D | - | - | - | Ca | - | 17 |
| 111 | 75 | II.2 | Turkey | 783+1G>A | 4.0/M | Deceased | D | - | - | - | Ca | - | 17 |
| 112 | 76 | II.1 | Turkey | 783+1G>A | 6.4/F | Alive | L | - | - | - | - | - | - |
| 113 | 77 | II.1 | Turkey | R173P | 16.8/M | Alive | L | - | - | Se, St, Spt | - | - | 23 |
| 114 | 78 | II.2 | Mexico | R486X | 4.7/M | Deceased | D | - | - | S. spp | Ca | K. pneumoniae | - |
| 115 | 79 | II.5 | KSA | Y88X | 5.7/F | Alive | R | - | - | SB | - | Klebsiella spp. | - |
| 116 | 79 | II.6 | KSA | Y88X | 1.3/M | Alive | L | - | - | - | - | - | - |
| 117 | 80 | II.7 | Qatar | C186S | 1.6/F | Alive | D | - | - | - | - | - | - |
| 118 | 81 | II.1 | Turkey | 64+2T>G | 4.2/M | Alive | D | - | - | - | Ca | - | - |
| 119 | 82 | II.5 | Turkey | 1425delC | 3.0/M | Alive | D | - | - | Se | Ca | - | - |
| 120 | 83 | II.1 | Turkey | 783+1G>A | 3.9/M | Alive | D | - | - | - | Ca | - | - |
| 121 | 84 | II.3 | Iran | G569D | 5.5/F | Alive | D | - | Mtb | - | - | - | - |
| 122 | 85 | II.4 | Iran | T355del | 32.4/M | Alive | R | - | Mtb | - | - | - | - |
| 123 | 86 | II.1 | KSA | 1791+2T>G | 2.0/F | Alive | D | - | - | - | - | - | - |
| 124 | 87 | II.1 | Turkey | 64+2T>G | 1.7/M | Alive | L | - | - | - | - | - | - |
| 125 | 88 | II.1 | UK | Q32X | 46.4/F | Alive | R | - | - | - | - | - | - |
| 126 | 89 | II.1 | Turkey | 1791+2T>G | 3.9/F | Alive | L | - | - | - | Ca | - | |
| 127 | 89 | II.2 | Turkey | 1791+2T>G | 1.4/F | Alive | nv | - | - | - | - | Nocardia nova and K. pneumoniae | - |
| 128 | 90 | II.1 | Argentina | 1623_1624delinsTT | 4.6/M | Alive | D | - | - | - | - | - | 49 |
| 129 | 91 | II.2 | Argentina | W531X | 10.3/F | Alive | D | - | - | - | - | - | - |
| 130 | 92 | II.1 | Argentina | [1623 1624delinsTT]+[DelEx4] | 8.2/F | Alive | D | - | - | - | - | - | 49 |
| 131 | 93 | II.3 | Argentina | 1623_1624delinsTT | 19.8/M | Alive | D | - | - | - | - | - | 24,49 |
| 132 | 94 | II.2 | Japan | R213W | 37.7/M | Deceased | R | Ma | - | - | - | - | 13 |
| 133 | 95 | II.4 | Tunisia | 64+2T>G | 10.9/M | Alive | D | - | - | - | Ca | - | - |
| 134 | 96 | II.1 | Tunisia | 1386_1387delGT | 6 mo/F | Deceased | D | - | - | - | Ca | - | - |
| 135 | 96 | II.2 | Tunisia | 1386_1387delGT | 6 mo/M | Alive | D | - | - | - | - | - | - |
| 136 | 97 | II.1 | Tunisia | NA | 27.6/M | Alive | R | M. spp | - | - | Ca | - | - |
| 137 | 98 | II.1 | Tunisia | 550-2A>G | 7.8/F | Deceased | D | - | - | S. spp | - | K. pneumoniae | 15 |
| 138 | 99 | II.1 | Tunisia | 64+5G>A | 2.5/M | Alive | D | - | - | - | Ca | - | 15 |
| 139 | 100 | II.1 | Argentina | [1623_1624delinsTT]+[nk] | 7 mo/M | Alive | D | - | - | - | - | - | - |
| 140 | 101 | II.2 | Turkey | 1021+1G>C | 15.0/F | Alive | R | - | Mtb | S. spp | - | - | 6 |
| 141 | 102 | II.1 | China | [I369T]+[R211P] | 3.1/M | Alive | D | - | - | - | na | na | - |
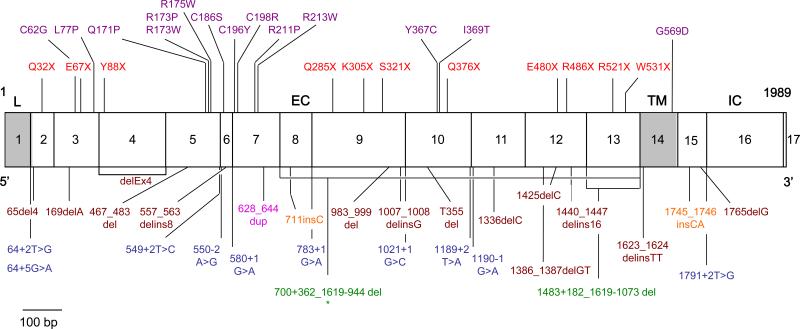
fig. 2.
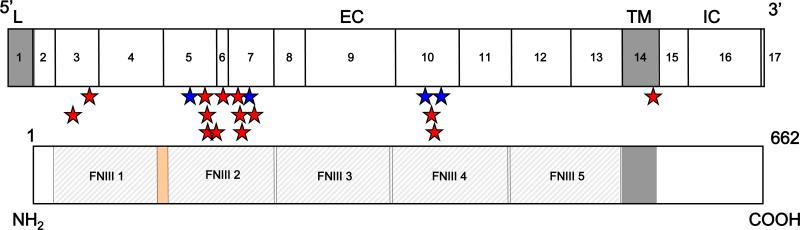
Mutated alleles of IL12RB1 genes. Schematic representation of the coding region of the IL-12Rβ1 chain containing 17 coding exons encoding a 662-amino acid protein with a peptide leader sequence (exon 1, L), extracellular domain (exons 2-13, EC), transmembrane domain (exon 14, TM) and an intracellular cytoplasmic domain (exons 15-17, IC). Missense mutations are shown in purple, nonsense mutations are shown in red, and complex mutations are shown in brown. Splicing mutations are shown in blue, large deletions are shown in green, insertions are shown in orange, and duplication in magenta. *The 700+362_1619-944del mutation is the only mutation resulting in protein expression at the cell surface.
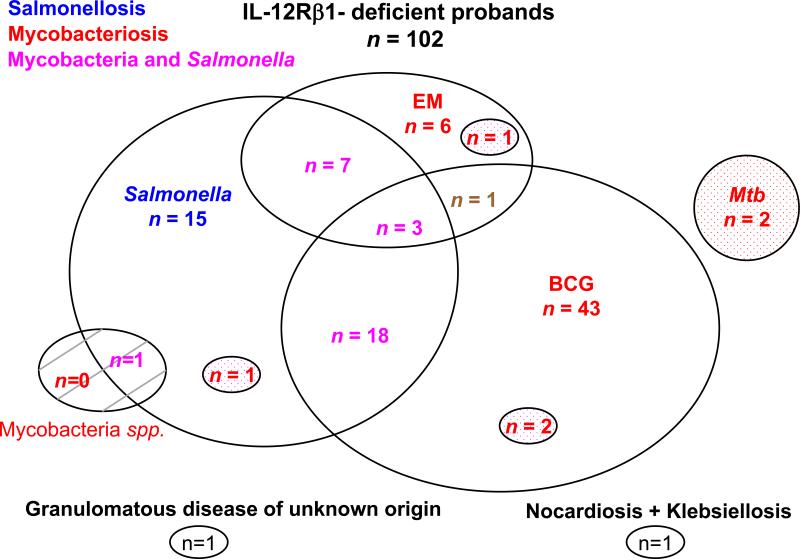
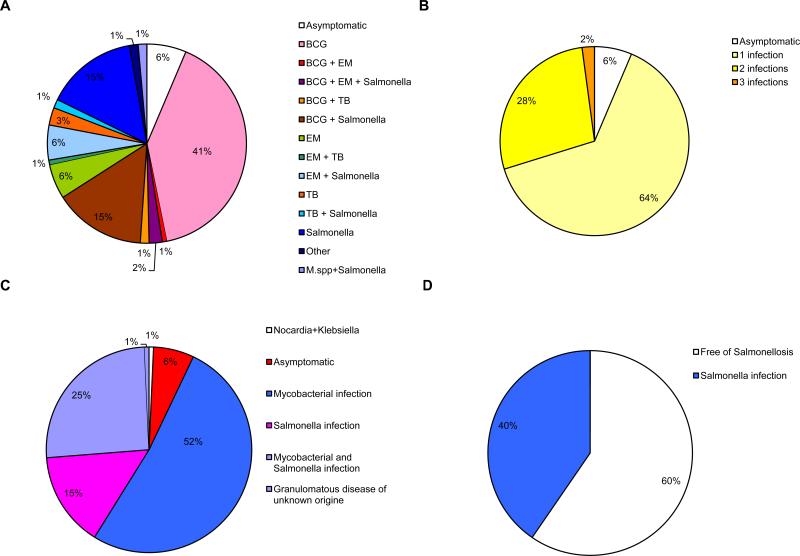
The overall clinical spectrum of infectious diseases in index cases was as follows: isolated BCG disease was present in 43 patients, isolated salmonellosis in 15 patients, isolated EM disease in 6 patients, and isolated tuberculosis in 2 patients. A combination of BCG disease and salmonellosis was reported in 18 cases, BCG and EM disease in only 1 case, BCG disease and tuberculosis in 2 cases, and BCG and EM disease plus salmonellosis in 3 cases. A combination of salmonellosis and EM disease was diagnosed in 7 cases, and salmonellosis and tuberculosis in 1 case. One of the 4 remaining probands had EM disease and tuberculosis, the second had nocardiosis and klebsiellosis, the third had granulomatous disease of unknown origin, and the fourth had salmonellosis and a mycobacterial disease of unknown origin (Figure 3). Among the 102 index cases, the first clinical infections typically occurred in childhood (mean age, 2.38 yr, SD ± 4.86 yr; range, 2 wk to 31.72 yr). Sixty-seven of the 86 BCG-vaccinated probands developed BCG disease (78%).
fig. 3.
Distribution of clinical phenotypes for IL-12Rβ1-deficient index cases. Each patient is classified as a function of his or her status for mycobacterial infections (in red, “BCG” for BCG disease, “=EM” for environmental or nontuberculous mycobacteria, “Mtb” for tuberculosis) and Salmonella infections (in blue, “Salmonella” for Salmonella disease). Patients with both mycobacterial infection and salmonellosis are shown in purple. Tuberculosis is represented as a dotted circle in each group. Infection with unidentified mycobacterial species is presented as a hatched circle.
Abolition of Cellular Responses to IL-12 and IL-23
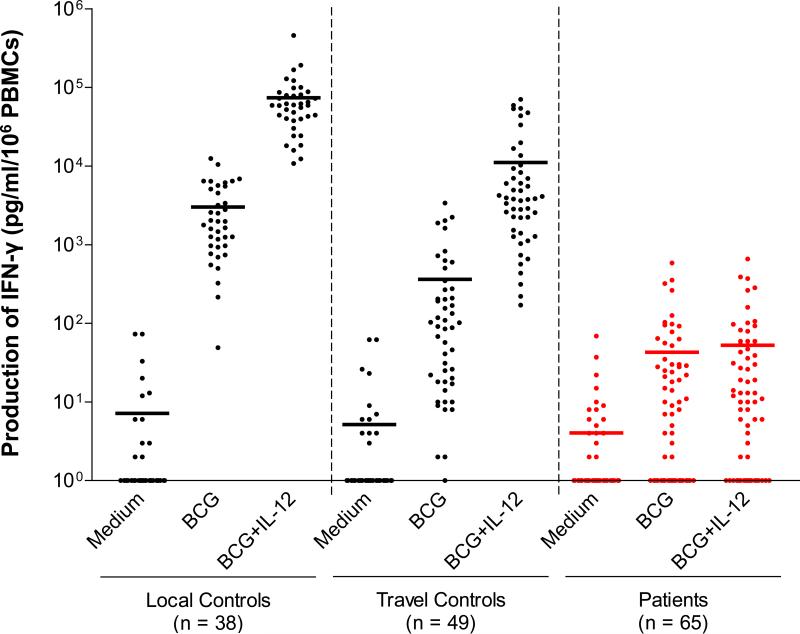
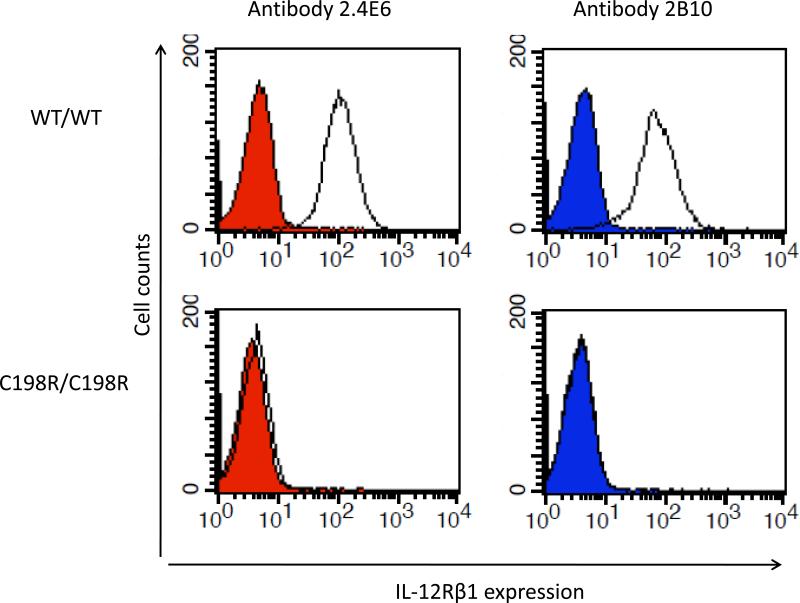
Whole-blood responses to IL-12 were investigated in 65 patients carrying 2 mutant IL12RB1 alleles (47 index cases and 18 relatives; see below). We measured the production of IFN-γ in whole blood in response to stimulation with BCG alone (partly resulting from BCG-dependent, endogenous IL-12 production) and in response to BCG plus exogenous recombinant IL-12, as previously described.[22,24] All patients tested had an impaired response to IL-12 in this assay (Figure 4). The whole-blood phenotype of the patients was, therefore, functional IL-12Rβ1 deficiency. We then assessed IL-12Rβ1 expression on the surface of T-cell blasts and/or EBV-B cell lines, by flow cytometry with 2 specific antibodies recognizing different epitopes on the extracellular domain of IL-12Rβ1. No IL-12Rβ1 molecules were detected on the surface of cells from patients carrying 47 alleles tested, except for 4 patients from 2 Israeli families (kindreds 10 and 43) carrying the same, large, in-frame deletion 700+362_1619-944del as described in a previous study.[23] This deletion led to the generation of a truncated IL12Rβ1 protein, which was present at the cell surface but was nonfunctional, resulting in complete IL-12Rβ1 deficiency. The C198R mutation has been described elsewhere and is thought to confer residual responsiveness to IL-12.[42] We have identified another patient with the C198R mutation. However, neither cell surface IL-12Rβ1 expression on the patient's PHA-T-cell blasts (Figure 5) nor IFN-γ production by these cells in response to IL-12 stimulation was detected (data not shown). Four of the remaining 6 homozygous alleles not tested by flow cytometry abolished IFN-γ production in response to the IL-12 stimulation of whole blood. The other 2 alleles were predicted to result in a loss of expression due to the creation of a premature stop codon.
fig. 4.
Impaired cellular response to IL-12. Production of IFN-γ by whole blood cells from 38 healthy “local” positive controls (fresh blood), from 49 healthy “travel” positive controls, and from 65 patients, either unstimulated (−) or stimulated with BCG alone or with BCG plus recombinant IL-12p70. The horizontal bars indicate the mean. Individual responses are not indicated because of the large number of patients studied.
fig. 5.
Impaired IL12Rβ1 expression on PHA-T-cell blast carrying C198R mutation. Flow cytometry staining for IL12Rβ1 molecules expressed on the surface of PHA-T-cell blasts from a healthy control (WT/WT) and a patient carrying the homozygous C198R mutation. In the right column, the 2.4E6 antibody specific for IL-12Rβ1 was used. In the left column, the 2B10 antibody specific for IL-12Rβ1 was used. Specific antibodies are indicated by a solid black line; isotype control antibodies are shown in red and blue, respectively.
Finally, we have shown that IL-12Rβ1-deficient patients do not respond to IL-23 in terms of IFN-γ production by T-cell blasts.[23] Consistent with these data, we have subsequently shown that IL-12Rβ1-deficient patients had a smaller proportion of IL-17-producing T cells ex vivo, and that their T-cell blasts did not express IL-17 in response to stimulation with IL-23 in vitro.[14] Overall, the blood cells from the patients tested displayed an impaired response to both IL-12 and IL-23,[14] strongly suggesting that these patients had complete IL-12Rβ1 deficiency.
Missense Mutations Responsible for IL-12Rβ1 Deficiency
Missense mutations were common (14 of the 54 alleles, 26%; 27 of 102 probands, 26%). Unlike mutations causing a premature termination of translation, it is difficult to predict whether missense and other in-frame mutations (n = 2, found in 3 index cases) are intrinsically deleterious. They may be in linkage disequilibrium with a causal mutation elsewhere, particularly in the broad IL12RB1 regulatory regions not sequenced in the patients. The 14 IL12RB1 missense mutations found are not polymorphisms, as they were not found in a panel of 50 healthy controls studied. Most are clustered in fibronectin domain 2 (FD2, 9 mutations), although some are found in FD1 (2 mutations), FD4 (2 mutations), and the transmembrane domain (TM, 1 mutation). The 4 known missense polymorphisms are also found in FD2 and FD4 (Figure 6).
fig. 6.
Missense mutations affecting IL-12Rβ1. Localization of polymorphism (n = 4, blue stars) and missense mutations (n = 14, red stars) in the IL-12Rβ1 gene.
We predicted the impact of the 18 amino acid substitutions with the PolyPhen tool, which classifies the impact as benign, possibly damaging, or probably damaging (http://genetics.bwh.harvard.edu/pph/).[53] Three of the 4 polymorphisms were predicted to have a benign impact, with only R156H being possibly damaging. By contrast, 12 of the 14 rare mutations were classified as probably damaging, and 2 were classified as possibly damaging (Table 2). ClustalX assessments of the phylogenic conservation of IL12RB1 in several species revealed considerable variation in IL12RB1, particularly in FD2 (http://bips.u-strasbg.fr/fr/Documentation/ClustalX/).[32] However, 10 of the 14 rare mutations affected conserved residues, whereas the residues affected by the 4 polymorphisms were not conserved residues (data not shown).
TABLE 2.
Impact of the IL12RB1 Polymorphisms and Missense Mutations*
| Variation | PolyPhen-Predicted Possible Impact of an AA Substitution on the Structure and Function of a Human Protein | Extracellular Expression | Binding IL-12 | |
|---|---|---|---|---|
| Ab 2.4E6 | Ab 2B10 | |||
| GFP | - | - | - | |
| WT | + | + | + | |
| C62G | 3.410 - Probably damaging | - | - | ND |
| L77P | 2.110- Probably damaging | - | + | - |
| R156H | 1.908 - Possibly damaging | ND | ND | ND |
| Q171P | 2.146- Probably damaging | - | - | - |
| R173P | 2.270- Probably damaging | - | - | - |
| R173W | 2.495 - Probably damaging | - | - | - |
| R175W | 2.495 - Probably damaging | - | - | - |
| C186S | 2.690 - Probably damaging | - | - | - |
| C196Y | 3.185 - Probably damaging | - | - | - |
| C198R | 3.410 - Probably damaging | - | - | - |
| R211P | 2.270 - Probably damaging | - | - | - |
| R213W | 2.495 - Probably damaging | - | - | - |
| Q214R | 0.402 - Benign | ND | ND | ND |
| M365T | 0.675 - Benign | ND | ND | ND |
| Y367C | 2.616 - Probably damaging | - | - | - |
| I369T | 1.673 - Possibly damaging | - | - | - |
| G378R | 0.542 - Benign | ND | ND | ND |
| G569D | 1.947 - Possibly damaging | ND | ND | ND |
We further investigated the function of 13 of the 14 rare missense alleles by transient transfection of an IL-12Rβ1-deficient Saimiri herpesvirus-transformed T cell line. We assessed the cell-surface expression of IL-12Rβ1 with 2 antibodies recognizing different epitopes.[24] We also assessed the ability of the encoded receptors to bind IL-12 in the same assay, because these missense mutations might affect the epitope recognized by the antibodies. Neither the surface expression of IL-12Rβ1 nor IL-12 binding was detected for the 13 missense alleles tested (see Table 2). Cells from patients carrying the remaining missense mutation were unresponsive to IL-12 (see above). Thus, the rare missense IL12RB1 alleles found in our patients resulted in a loss of both expression and function.
Relatives of the Index Cases
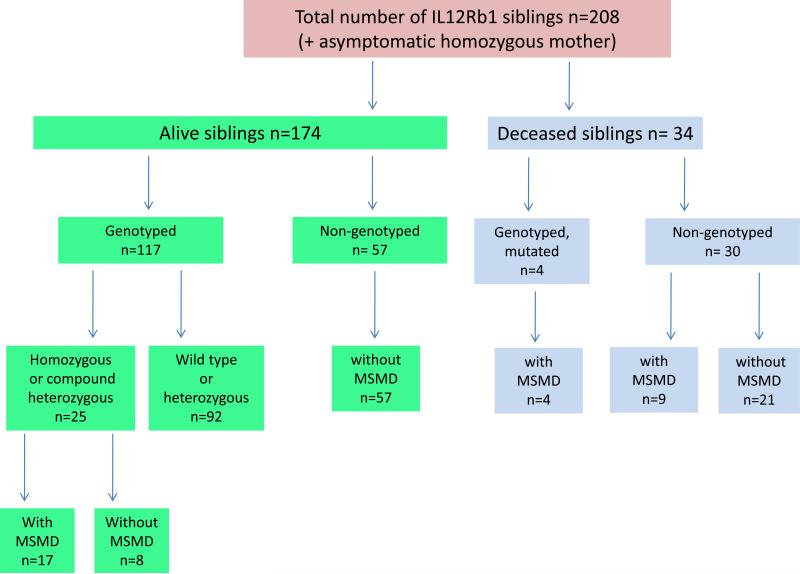
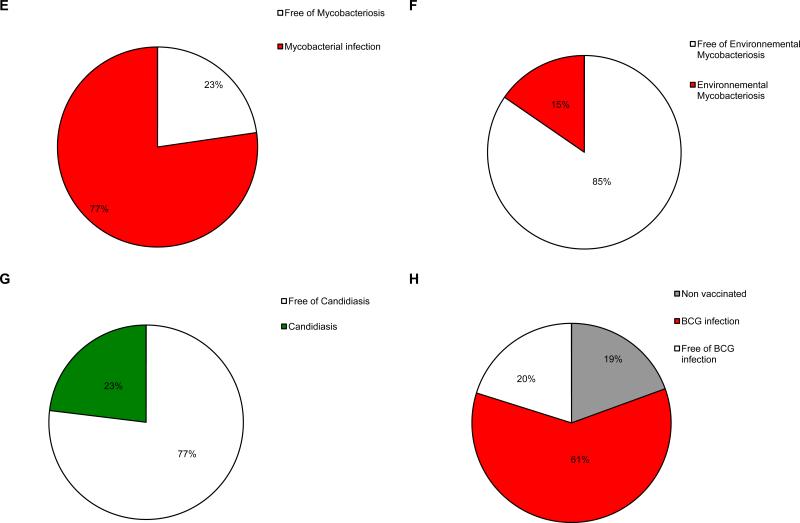
The 102 probands had a total of 208 sibs, 174 of whom were alive and 34 of whom had died (Figure 7). Genotyping was carried out for 117 of the 174 living sibs. The other 57 sibs were not genotyped. We found that 92 of the genotyped sibs were wildtype or heterozygous for the IL12RB1 mutation, whereas another 25 sibs carried mutations in both alleles. Seventeen of these sibs with mutations in both alleles presented unusual infections, whereas the remaining 8 were asymptomatic. Fifty-seven nongenotyped living sibs had not had diseases caused by mycobacteria or Salmonella. Thirty of the 34 sibs who had died had not been genotyped. The other 4 dead sibs had carried homozygous mutations in IL12RB1 and had died from BCG (n = 3) and EM (n = 1) diseases. Nine of the 30 nongenotyped dead sibs had died from infections caused by mycobacteria or Salmonella (see below). No unusual infections were reported for the remaining nongenotyped sibs who had died (see Figure 7). We identified 2 IL12RB1 null alleles in 29 of the 38 sibs affected clinically or genetically. The molecular defect was considered probable but not documented in 9 of the sibs with symptoms who had died. These 9 sibs died from BCGosis (n = 5, 26.II.1, 58.II.1, 68.II.3, 68.II.4, 74.II.1), S. enteritidis disease (n = 2, 30.II.5, 31.II.6), M. avium disease (n = 1, 4.II.2), and disseminated tuberculosis (n = 1, 61.II.1). Eight of the 29 genetically identified sibs displayed no known MSMD infectious phenotype at last follow-up. This group of sibs lacking MSMD symptoms presented the same cellular phenotype as their clinically affected IL-12Rβ1-deficient sibs. Sixteen of the 21 genetically affected symptomatic sibs had been vaccinated with BCG and 12 developed BCG disease, which was the first clinical manifestation of MSMD in all of these cases (n = 11 BCG alone, and n = 1 BCG plus Salmonella). In the other 4 vaccinated patients, salmonellosis was the first clinical manifestation in 2 cases, with EM disease and tuberculosis being the first clinical manifestation in 1 individual each. The 5 remaining genetically affected sibs who had not been vaccinated with BCG developed salmonellosis (n = 2), EM disease (n = 1), disease due to Mycobacterium species and salmonellosis (n = 1), and tuberculosis and salmonellosis (n = 1) (see Figure 7). Age at first infection could be evaluated in only 26 of the 30 symptomatic patients, and did not differ from that of index cases (mean, 2.4 yr ± 4 yr; range, 5 d to 18 yr). The duration of follow-up for these sibs was also similar to that for the index cases (mean, 7.91 yr ± 6.92 yr; range, 0.51-28 yr). The infectious phenotype of these 30 sibs was thus similar to that of the 102 index cases, in terms of the nature of the infectious diseases suffered and the age at which they occurred (Figure 8). In total, 161 parents of index cases were genotyped. One mother (46.I.2) was found to be homozygous for an IL12RB1 mutation, but neither she nor any of the other parents had any symptoms. In total, at least 141 individuals from 102 kindreds, including 132 individuals actually carrying 2 IL12RB1 mutant alleles and 9 related individuals identified on the basis of their clinical presentation, probably had autosomal recessive IL-12Rβ1 deficiency (see Figure 1; Table 1). One of the major conclusions to be drawn from the analysis of the genetically affected relatives is that the clinical penetrance of IL-12Rβ1 deficiency is incomplete.
fig. 7.
Description of all sibs of IL-12Rβ1-deficient index cases, according to vital status, genotyping status, and the presence or absence of clinical symptoms of MSMD.
fig. 8.
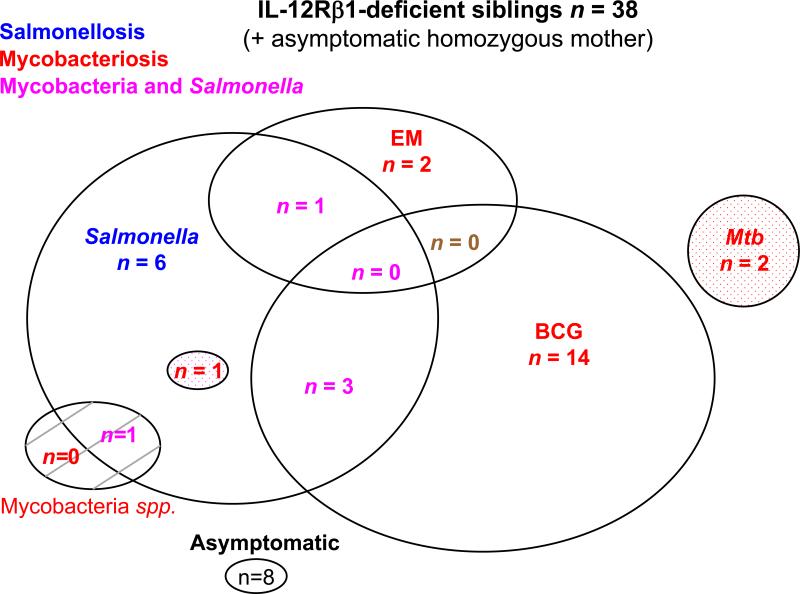
Distribution of the clinical phenotypes of IL-12Rβ1-deficient sibs. Each patient is classified as a function of his or her status for mycobacterial infections (in red, “BCG” for BCG disease, “EM” for environmental or nontuberculous mycobacteria, “Mtb” for tuberculosis) and Salmonella infections (in blue, “Salmonella” for Salmonella disease). Patients with both mycobacterial infection and salmonellosis are shown in purple. Tuberculosis is represented as a dotted circle in each group. Infection with unidentified mycobacterial species is presented as a hatched circle.
Mycobacterial Diseases in 132 Symptomatic Patients
Mycobacterial diseases were the most frequent infections, diagnosed in 109 of the 132 symptomatic patients (83%) (Figure 9). We first analyzed the individuals developing case-definition opportunistic infections caused by weakly virulent mycobacteria (BCG and EM). We found that 108 of the 132 patients had been vaccinated with BCG and 84 patients developed BCG disease (localized, n = 17; disseminated, n = 63; not known, n = 4). By contrast, only 21 of the 132 patients developed EM disease due to M. avium (n = 10); M. avium, M. triplex, and M. genavense (n = 1); M. genavense (n = 1); M. avium, M. chelonae, and M. fortuitum (n = 1); M. chelonae (n = 1), M. simiae (n = 1), M. avium intracellulare (n = 1), and undefined Mycobacterium species (n = 5). Two of these patients had multiple EM diseases, with 1 patient in particular presenting successive infections with M. avium, M. triplex, and M. genavense (n = 1). Another patient had infection with M. chelonae and M. fortuitum, followed by an infection with M. avium (n = 1). One patient had both BCG and EM disease, and 3 patients had infections with BCG, EM, and Salmonella. Two patients had mycobacterial infections caused by unidentified Mycobacterium species and Salmonella infection. In total, 9 patients had tuberculosis, with 4 of these patients developing disease due to M. tuberculosis alone; 1 patient developing disease due to M. tuberculosis in combination with M. avium, M. chelonae, and M. fortuitum; and 2 patients developing disease due to M. tuberculosis and Salmonella. The remaining 2 cases of tuberculosis occurred in combination with BCG disease. Thus, 109 of the 132 symptomatic patients had mycobacterial disease, but 2 different mycobacterial species were involved in only 9 of these cases (8%). We found that multiple mycobacterial diseases are rare, consistent with our previous observations.[24] Finally, 36 of the 42 deaths (among 132 patients) could be attributed to mycobacterial disease (BCG = 23, EM = 11, tuberculosis = 1, EM and Salmonella = 1; see below).
fig. 9.
Distribution of clinical phenotypes of all IL-12Rβ1-deficient patients (n = 141). A) Overall distribution of clinical phenotypes. B) Proportion of multiple infections due to 1 or more different families of infectious agents. C) Distribution of mycobacterial and Salmonella diseases. D) Distribution of salmonellosis. E) Distribution of mycobacterial diseases. F) Distribution of environmental mycobacterial diseases (nontuberculous mycobacteria). G) Distribution of candidiasis. H) Distribution of nonvaccinated individuals resistant to BCG, and BCG diseases.
We defined recurrence as a subsequent episode of infection with the same microorganism after a period free from clinical symptoms and treatment. However, reliable systematic data on the complete absence of clinical symptoms and treatment, and bacteriologic identification of the pathogen responsible for the new clinical episode were often lacking. Based on the available data, recurrent BCG infection was diagnosed in 15 cases (18% of all patients with BCG disease), and recurrent EM in only 3 cases (14% of all patients with EM infections).
Impact of BCG Vaccination on Other Mycobacterial Diseases
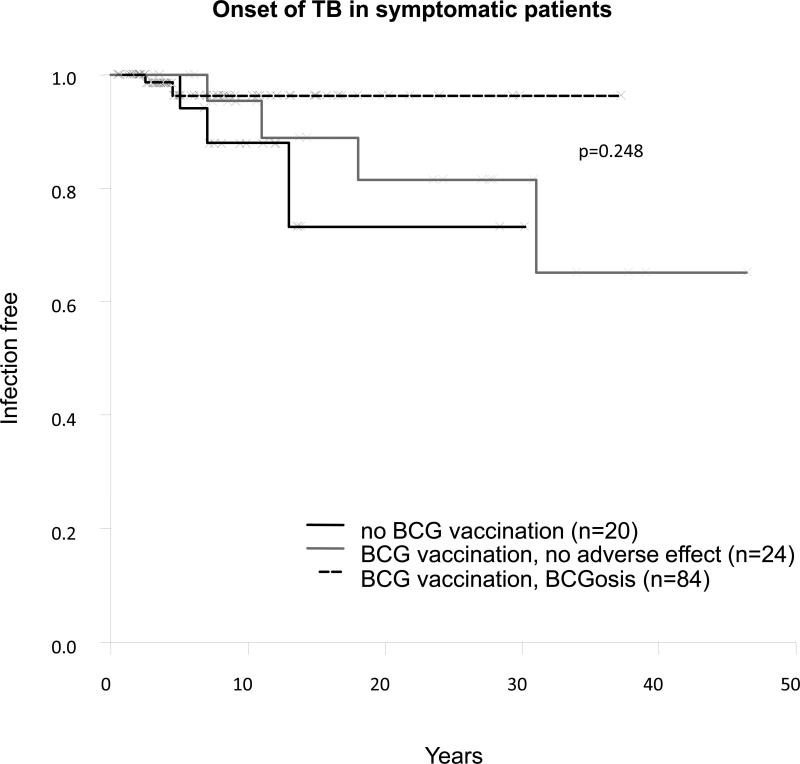
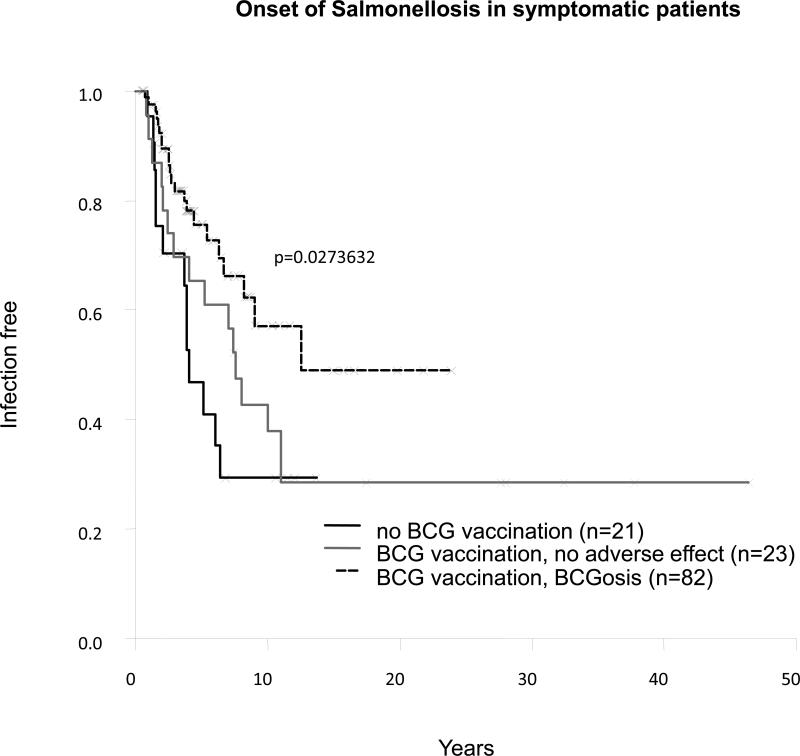
The rarity of multiple mycobacterial diseases (8%) was also consistent with the rarity of recurrence (18% for BCG and 14% for EM). This finding may reflect the protective role of primary infection against the reactivation of a latent organism or secondary infection. The protective effects of primary infections with EM and tuberculosis are difficult to assess, but precise information about BCG vaccination was available for most patients. We determined the impact of BCG vaccination and BCG disease on the clinical phenotype of 129 patients. Only 4 of the 84 patients with BCG disease developed EM diseases, with a mean age of EM disease onset at 4.5 years, suggesting that BCG vaccination may prevent EM disease. Seven of the 24 patients resistant to BCG (vaccinated with BCG but without BCG infection) had EM disease, with late onset of the disease (mean age, 10.83 ± 12.02 yr; range, 5 d to 31.72 yr). By contrast, 10 of the 21 symptomatic patients (48%) who had not been vaccinated with BCG had EM diseases, with an early onset of EM disease (mean age, 5.08 ± 3.48 yr; range, 0.83-12.05 yr). The difference in incidence of EM disease between the 3 groups of patients (patients with both BCG and EM disease, patients resistant to BCG with EM disease, and patients not vaccinated with BCG with EM disease) was highly significant (p = 8 × 10−5) (Figure 10). This difference in EM disease incidence was also significant if patients with BCG disease were compared with patients not inoculated with BCG (p = 1.55 × 10−5). The difference in incidence between patients resistant to BCG and nonvaccinated patients was also statistically significant (p = 0.045). Finally, the difference in EM disease incidence between BCG-vaccinated (with or without BCG disease) and nonvaccinated patients was highly significant (p = 4.83 × 10−5). However, this pattern was not observed for tuberculosis (p = 0.25) (Figure 11). The difference in the onset of salmonellosis was barely significant between these 3 groups (p = 0.03) (Figure 12).
fig. 10.
Onset of environmental mycobacterial disease (nontuberculous mycobacteria) in symptomatic patients.
fig. 11.
Onset of tuberculosis disease in symptomatic patients.
fig. 12.
Onset of salmonellosis in symptomatic patients.
These data confirm our previous description of a strong protective effect of BCG vaccination,[24] preventing EM disease in IL-12Rβ1-deficient patients. This observation can probably be extended to account for the rarity of recurrences and multiple mycobacterial diseases in patients. Human IL-12Rβ1 seems to be essential for protective immunity to primary infection, but not to secondary infection or reactivation by mycobacteria.
Salmonellosis in the 132 Symptomatic Patients
Salmonellosis occurred in 57 of the 132 symptomatic patients (43%) (see Figures 3, 8, 9), and was the only infectious disease in 21 patients (16%). The remaining 36 patients with salmonellosis also had tuberculosis (n = 2), EM disease (n = 8), BCG disease (n = 21), EM and BCG disease (n = 3), or mycobacterial disease caused by unidentified Mycobacterium species (n = 2). Four patients died from salmonellosis (10%). Various serotypes of nontyphoidal Salmonella (S. enteritidis, S. typhimurium, S. dublin, S. hadar, S. typhi O and typhi H, S. group B and D, S. portland, S. paratyphi) were isolated from the 57 patients. Two patients were diagnosed with typhoid fever (patients 9.II.3 and 77.II.1), caused by S. typhi and/or S. paratyphi. Six of the 57 patients (11%) had salmonellosis caused by 2 or more serotypes (3.II.3, 9.II.3, 30.II.6, 53.II.2, 73.II.2 and 77.II.1). Multiple salmonellosis was more frequent than multiple mycobacteriosis (8% vs. 11%, respectively); however, not all clinical episodes were confirmed bacteriologically. Recurrent salmonellosis was diagnosed in 22 patients (39% of all patients with salmonellosis), but the Salmonella species responsible for the recurrence was not identified bacteriologically. Recurrent Salmonella infection was more frequent than recurrent infection with BCG and EM (18% and 14%, respectively), consistent with our previous findings suggesting that IL-12 and IL-23 are required to mount an efficient immune response to primary, latent, and secondary Salmonella infections.[24]
Infections Caused by Other Agents
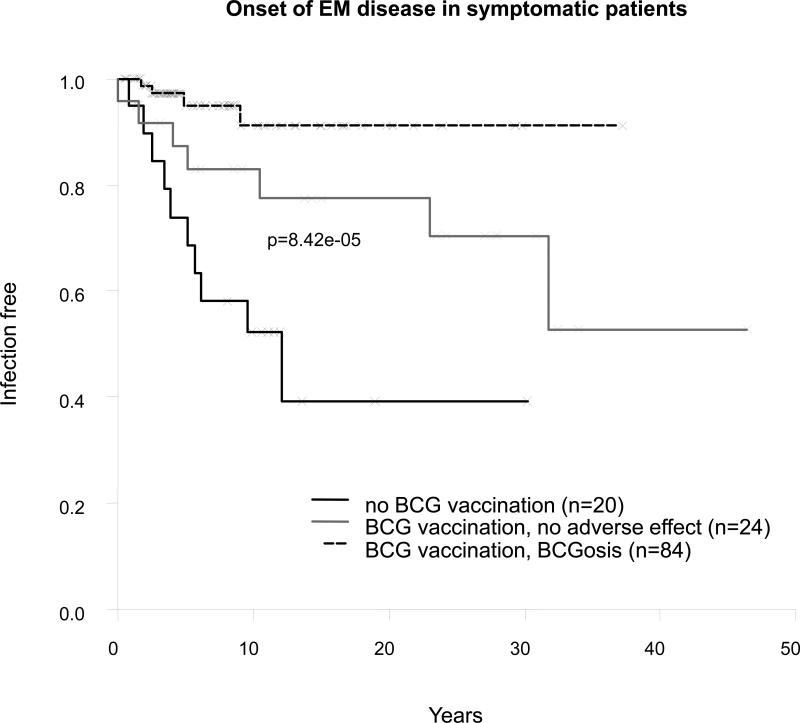
We recently found that 32 (24%) of the 132 symptomatic patients for whom information was available presented mucocutaneous disease caused by Candida albicans (see Figure 9). The vast majority of patients had recurrent thrush, even in the absence of antibiotic treatment. The clinical features of candidiasis in IL-12Rβ1-deficient patients will be reported elsewhere (Rodriguez-Gallego et al, unpublished data). One patient developed recurrent visceral leishmaniasis at the age of 5 years (71.II.2).[57] Another had disseminated paracoccidioidomycosis at the age of 21 years (29.II.1).[47] Two patients had posterior uveitis due to toxoplasmosis (19.II.1 and 29.II.1). One patient had disseminated histoplasmosis at the age of 5 years (54.II.1). Five patients had Klebsiella pneumoniae infection (66.II.1, 78.II.2, 79.II.5, 89.II.2 and 98.II.1). One patient developed sepsis and meningitis due to Citrobacter freundii at 3 months of age, recovering fully with treatment (35.II.4). Patient 89.II.2 presented with simultaneous Klebsiella pneumoniae and Nocardia nova infections in the absence of mycobacterial or Salmonella infection (Picard et al, unpublished data). The occurrences of klebsiellosis and salmonellosis may be linked, because these 2 species are phylogenetically related.[44] Symptoms of vasculitis were reported in 3 patients. Vasculitis was considered secondary to S. enteritidis or mycobacterial infection in 2 patients,[34,58] but no histologic examination was available for the third patient, so other causes of vasculitis could not be ruled out.
IL-12Rβ1-deficient patients seem to be susceptible to Candida and Klebsiella, and to intracellular microbes with pathogenesis and immune control similar to those of mycobacteria, such as Nocardia, Paracoccidioidomyces, Histoplasma, and Leishmania. Patients with unusually severe disease caused by these and, possibly, other microorganisms should be investigated for IL-12Rβ1 deficiency. This is particularly important for children with disseminated disease.
Age at Onset of Infection in the 132 Symptomatic Patients
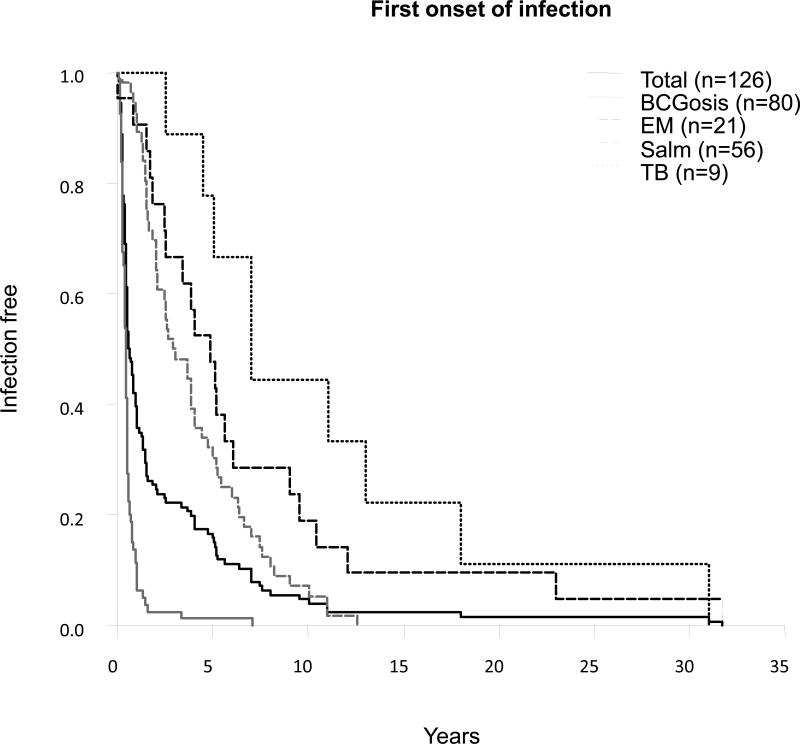
We then focused our analysis on the 126 symptomatic patients for whom relevant information was available: 100 index cases and 26 sibs. The age at onset of the first infection was typically in early childhood. The mean age at onset of first infection was 2.4 years (range, 1 wk to 31.7 yr; SD 4.9 yr) (Figure 13). In most cases, the first infection was due to live BCG (regional BCGitis or disseminated BCGosis). It occurred at ages between 2 weeks and 7.1 years, with a mean of 0.6 years ± 0.9 years (from 1 wk to 3.2 yr after vaccination, with a mean at 0.4 yr after vaccination ± 0.4 yr). In 75 cases (96%), BCG disease occurred within a year of vaccination. Salmonellosis (range, 3 mo to 12.5 yr; mean, 4 yr, SD 3 yr) and EM disease (range, 1 wk to 31.7 yr; mean, 6.9 yr, SD 7.7 yr) occurred at a similar age. Tuberculosis occurred later, at ages of 2.5 to 31 years, with a mean age at tuberculosis onset of 11 years ± 8.9 years. The earlier onset of BCG, EM, and Salmonella disease than of tuberculosis may be accounted for by earlier exposure to these microorganisms.
fig. 13.
First onset of infection.
Survival Analysis of IL-12Rβ1-Deficient Patients
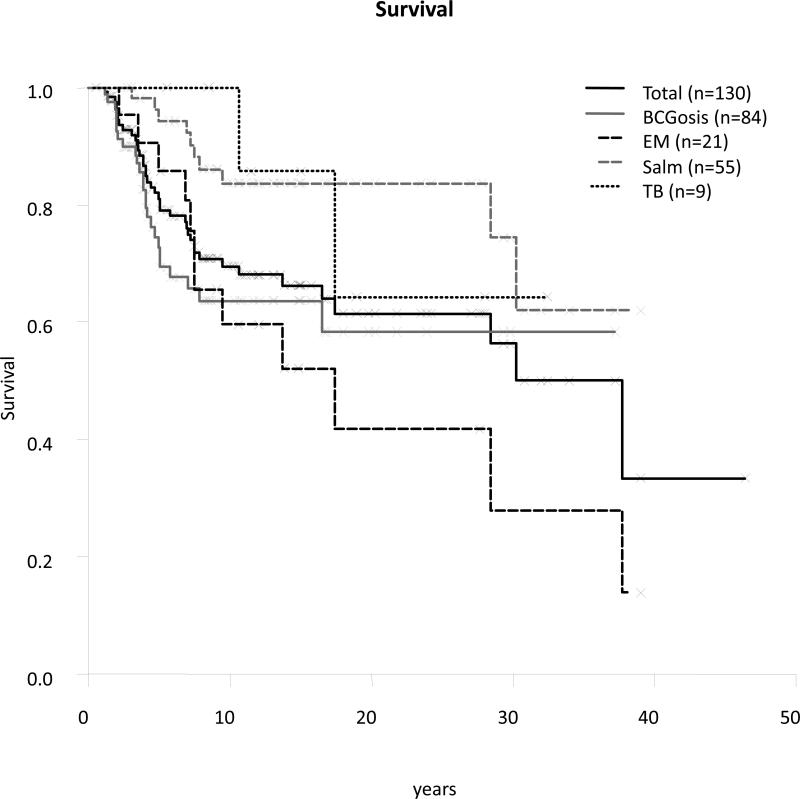
The mortality rate among symptomatic patients was 32% (42 of the 132 symptomatic patients) (see Table 1), which is somewhat higher than the rate of 15% previously reported for a series of 41 patients.[24] Global mortality, including asymptomatic patients, was 30.[24] The date of birth and date of death were known for 40 of the 42 patients who died. The mean age at death was 7.5 years in the 40 patients who died (range, 1.2-37.7 yr, SD 8.1 yr). The cause of death was BCGosis (n = 23, 27% of patients with BCG disease) in most of the patients who died, with smaller numbers of patients dying from EM disease (n = 11, 52% of patients with EM disease), tuberculosis (n = 1, 11% of patients with tuberculosis), or salmonellosis (n = 4, only 7% of patients with salmonellosis). One patient died from concurrent M. avium and Salmonella infections (patient 39.II.2), and another patient died from a severe electrolyte disorder following diarrhea. However, it is unknown whether the diarrhea was related to salmonellosis in this patient. One patient died from esophageal carcinoma (patient 30.II.6) (Rodriguez-Gallego et al, unpublished data).
Clinical outcome depends largely on the infectious agent concerned, with mortality ranging from 7% (Salmonella) to 52% (EM) (Figure 14). However, there is an ascertainment bias, as discussed above, with fewer asymptomatic sibs investigated than in the previous study. The clinical outcome of this defect is directly related to the therapeutic approach used. IL-12Rβ1-deficient individuals were commonly treated with prolonged courses of antibiotics and exogenous IFN-γ. More rarely, they underwent surgical resection of the affected areas (abdominal in particular), and, in very rare cases, hematopoietic stem cell transplantation was carried out. However, there are no comprehensive data available to evaluate the impact of treatment options at the moment. We are currently collecting data about both the treatment and preventive management of these patients.
fig. 14.
Survival.
Incomplete Clinical Penetrance
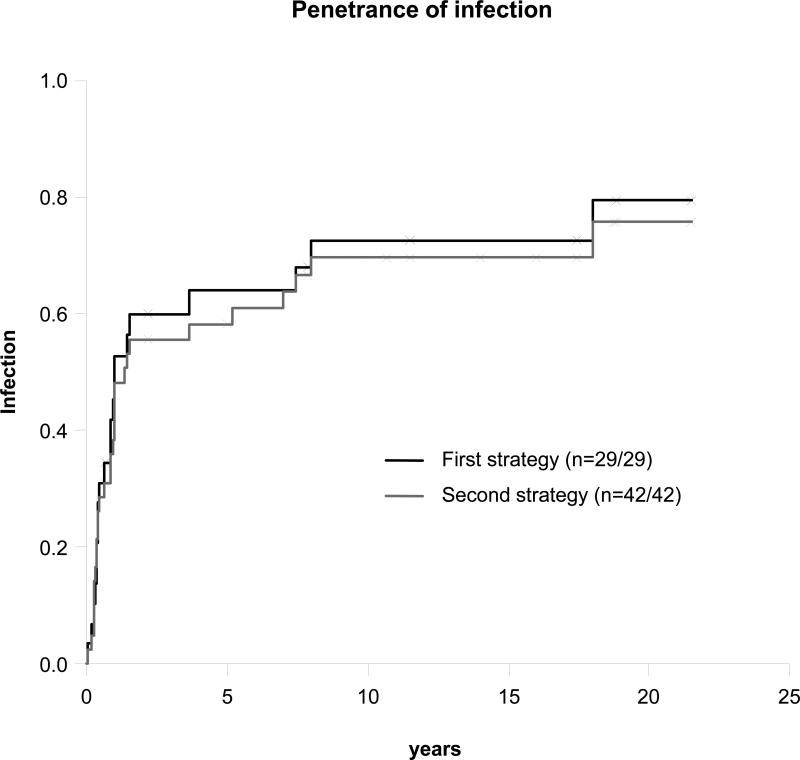
Eight of the 29 known genetically affected sibs were free of MSMD-related or other unusual infections at the last follow-up visit. We first estimated clinical penetrance by focusing on these 21 symptomatic (follow-up range, 0.5-28 yr; mean, 8.8 yr, SD 7.8 yr) and 8 asymptomatic (follow-up range, 0.7-21.5 years; mean, 12.7 yr, SD 8.4 yr) genetically affected sibs. The overall penetrance of infections was found to increase rapidly to 0.64 (95% confidence interval [CI], 0.41-0.78) at the age of 5 years, increasing slowly thereafter to reach 0.79 (95% CI, 0.52-0.91) by the age of 20 years (Figure 15). Twelve (75%) of the 16 BCG-vaccinated sibs developed BCGosis. EM disease, salmonellosis, and tuberculosis occurred in 2 (7%), 10 (35%), and 2 (7%) genetically affected sibs, respectively. These proportions are higher than those reported in the previous series.[24] However, 57 (33%) of the 174 living sibs had not been genotyped, whereas only 9% of the living sibs had not been genotyped in the smaller series studied in 2003.[24] We therefore also estimated clinical penetrance by including 13 nongenotyped symptomatic and healthy sibs (as described in Patients and Methods section). This second curve provided estimates of penetrance very similar to those for the first curve generated from data for genotyped sibs only. We cannot rule out the possibility of an ascertainment bias, with genetically affected asymptomatic relatives being underdiagnosed, but both estimation strategies indicate that IL-12Rβ1 deficiency may have a higher penetrance than initially thought.
fig. 15.
Penetrance of infection.
DISCUSSION
We describe here 141 patients with IL-12Rβ1 deficiency. The patients originate from 30 countries in the Americas, Europe, Africa, and Asia, and comprise individuals from various ethnic groups (Africans, Amerindians, Arabs, Chinese, Europeans, Indians, Iranians, Japanese, Jews, and Turks).[41] Consistent with the considerable geographic and ethnic heterogeneity of the patients, we also observed substantial genetic heterogeneity, with up to 54 mutant alleles in 102 kindreds. In all but 2 kindreds,[23,59] the patients had IL-12Rβ1 deficiency with no expression of the receptor on the cell surface. The cells of all patients have an impaired response to IL-12 and IL-23, resulting in the impaired production of IFN-γ and IL-17. A diagnosis of partial, as opposed to complete, IL-12Rβ1 deficiency was proposed in a child homozygous for the C198R mutation.[42] However, we detected no IL-12Rβ1 expression at the surface of the patient's PHA-T-cell blasts, and no IFN-γ was produced upon IL-12 stimulation. Similarly, an IL-12Rβ1-independent T-cell response to IL-12 has been proposed as a general compensatory mechanism,[66] but this hypothesis was not confirmed in our assays.[8,23,24,48,60,65,PR] In all patients tested, including patients with IL-12Rβ1 expression on the cell surface, no cellular response to IL-12 was detected in our whole-blood assay.[22] Despite the varying clinical presentation as well as substantial genetic heterogeneity, the cellular defect was complete.
In any event, the large number of kindreds from different ethnic groups, bearing different mutant alleles, identified in this study strongly suggests that IL-12Rβ1 deficiency will be diagnosed in many other families worldwide, particularly as awareness of the clinical features of MSMD and IL-12Rβ1 deficiency increases. Furthermore, many of these patients come from countries with high prevalence of consanguinity, as well as with the national coverage of BCG vaccination during the first days of life. The latter increases the probability for this autosomal recessive defect to occur, whereas the former accounts for the high prevalence of BCG infection in such patients. Low consanguinity and more restricted BCG vaccination policy may explain why there were no North American and Australian patients diagnosed. It is difficult to speculate on whether the lack of patients diagnosed in Africa, where BCG is widely used, can be attributed to under-reporting, early death from infectious disease, or some other yet unrecognized factor. The current study should help to increase awareness of these conditions, thereby improving their diagnosis and the clinical management of these patients worldwide, and should help to incite the critical reappraisal of risks and benefits of the BCG vaccination in a global context.
The uniform cellular phenotype is associated with a highly heterogeneous clinical phenotype, ranging from early death in infancy to an asymptomatic course until adulthood, and reflects the natural course of this condition in 141 patients originating from highly diverse ethnic backgrounds[41] and exposed to highly diverse microbial flora.[26] Mycobacterial infections predominated in these patients, affecting 83% of symptomatic patients and 77% of all patients. The high proportion of mycobacterial diseases, and of infections due to BCG and EM disease in particular, may reflect an ascertainment bias, as most of the subjects studied for defects in the IL-12Rβ1 chain are patients with MSMD. However, similar proportions of mycobacterial diseases were obtained for affected relatives of probands (80% of symptomatic relatives).
We also report 4 cases in which tuberculosis was the sole clinical manifestation.[6,8,48] The IL12RB1 gene may be considered the first Mendelian gene for susceptibility to tuberculosis to be discovered.[3] The prevalence of tuberculosis in IL-12Rβ1-deficient patients is lower than that of disease due to BCG or EM infection in these patients, probably because patients are less frequently exposed to M. tuberculosis than to BCG vaccines (85% vaccination coverage worldwide) and the almost ubiquitous EM. It is less likely to be due to an initial mycobacterial infection protecting against tuberculosis. Indeed, 2 patients presented with BCGosis and tuberculosis. In IL-12Rβ1-deficient patients, BCG seems to confer greater protection against EM disease than against tuberculosis, despite the close phylogenetic relationship between BCG and M. tuberculosis, presumably because M. tuberculosis is more virulent than EM. In any event, children with severe forms of tuberculosis should be tested for IL-12Rβ1 deficiency.
Salmonellosis is the second most common infection in these patients, affecting 43% of symptomatic IL-12Rβ1-deficient patients. It was the only infection in 37% of salmonellosis cases (21/57) and in 15% of all 141 patients. The remaining patients had both mycobacteriosis and salmonellosis. The current study highlights the need to consider IL-12Rβ1 deficiency in patients with a pure phenotype of salmonellosis, particularly in cases of extraintestinal nontyphoidal salmonellosis (typhoid fever was diagnosed in only 2 patients). We suspect that more patients may have had Salmonella infection, but the complex, “noisy” clinical setting of concomitant mycobacterial infection and the use of broad-spectrum antibiotics for treatment may have led to underdiagnosis. Furthermore, salmonellosis was not accompanied by an overt inflammatory syndrome in some patients. Infections other than those caused by mycobacteria and Salmonella are increasingly being diagnosed in these patients. Klebsiellosis has been diagnosed in 5 IL-12Rβ1 patients, Klebsiella being closely related phylogenetically to Salmonella.[44] Toxoplasmosis was diagnosed in 2 patients, and histoplasmosis, paracoccidioidomycosis, leishmaniasis, and nocardiosis were each diagnosed in 1 patient. These organisms are intramacrophagic pathogens, consistent with a possible role of IL-12Rβ1 deficiency in the pathogenesis of these infections. Moreover, 1 child with nocardiosis has been reported to suffer from IL-12p40 deficiency[51], and a patient with IFN-γR1 deficiency and histoplasmosis has been reported.[73] These findings tend to implicate IL-12Rβ1 deficiency in these infections, but the diagnosis of a larger number of cases is required to confirm this hypothesis.
More surprisingly, mild forms of chronic mucocutaneous candidiasis have been diagnosed in 33 patients (23%) (Rodriguez-Gallego et al, unpublished data). Over the last few years, IL-12Rβ1 has been implicated in the human IL-23-IL-17 axis,[1,10,21,68] as initially described in mice (reviewed in references 17, 61). Mice with impaired IL-17 immunity are susceptible to Candida.[12,31,38] It has been demonstrated that patients with IL-12Rβ1 deficiency display impaired development of IL-17-producing T cells, although this impairment is less pronounced than that in STAT3-deficient patients.[14] The high proportion of patients with candidiasis may therefore reflect changes in the IL-23-IL-17 axis. The impairment of IL-23-IL-17 immunity may also account for the higher frequency of salmonellosis in IL-12p40- and IL-12Rβ1-deficient patients (43%) than in IFN-γreceptor-deficient patients (7%),[43] and the small number of cases of klebsiellosis reported here (Pedraza et al, unpublished data). Indeed, in mice and primates, the IL-23-IL-17 circuit is important for immunity to Salmonella and Klebsiella.[29,52,71] In any event, the infectious phenotype of IL-12Rβ1-deficient patients appears to be broader than initially thought.
We confirm that the penetrance of MSMD in IL-12Rβ1 deficiency is not complete for either BCG or EM disease. The penetrance of susceptibility to salmonellosis also seems to be incomplete, although it is difficult to determine which patients have been exposed to Salmonella. This problem also makes it difficult to assess the penetrance of susceptibility to tuberculosis, as it is likely that only a small fraction of patients have been exposed to M. tuberculosis. The larger number of patients in the current study compared with our 2003 survey[24] (141 vs. 41) resulted in a higher penetrance of MSMD (including salmonellosis) in this study (72%) than in the previous study (45%) at the age of 20 years. If we include tuberculosis, global penetrance reaches 79% at this age. However, this revised penetrance value is probably overestimated, because the proportion of asymptomatic sibs tested was much lower than in the 2003 study. Penetrance may vary between countries, as a function of BCG vaccination policy, tuberculosis burden, and the likelihood of being exposed to Salmonella. The virulence and abundance of EM may also vary with geographic region. Thus, even healthy sibs of probands and their more distant relatives in consanguineous kindreds should be investigated.
We also confirm here that IL-12Rβ1 deficiency mostly begins in childhood. Only 3 of the 141 patients had a clinical onset after the age of 13 years. Our findings also reveal that the prognosis of IL-12Rβ1 deficiency is not as good as initially thought. Consistent with the higher penetrance, the outcome is much poorer than that observed in 2003 in a study of fewer patients.[24] The overall mortality rate for IL-12Rβ1-deficient patients was estimated at 32%, compared with only 15% in 2003. There does not seem to be a correlation between mortality rate and country of origin, but the type of infection has a detectable impact, EM disease being associated with a poorer prognosis. Only 6 deaths were recorded in patients over the age of 13 years. However, the revised mortality rates obtained in this study may reflect the underdiagnosis of asymptomatic sibs. IL-12Rβ1 deficiency is often, but not always, symptomatic. It typically begins in childhood and is lethal in up to a third of patients, particularly in patients with EM disease, and its prognosis seems to improve with age.
Given the wide range of clinical presentation, ranging from overwhelming lethal disease in childhood to a completely asymptomatic life in adulthood, it is difficult to delineate rigidly the clinical criteria that should lead to a suspicion of IL-12Rβ1 deficiency. IL-12Rβ1 deficiency should probably be suspected in any patient with unusual infections by intracellular bacteria, such as mycobacteria and Salmonella, even in the absence of parental consanguinity. Both curative and preventive treatment of IL-12Rβ1 deficiency, based on prolonged courses of antibiotics, exogenous IFN-γ treatment, and, in rare cases, surgical resection of affected areas, may influence clinical outcome in these patients. However, we were unable to explore the effects of treatment on clinical outcome in the present study because the information available was too limited. We are currently collecting data on the treatments administered to our patients. The description of IL-12Rβ1 deficiency, like that of IL-12p40 deficiency, is essential, not only to improve patient care, but also to improve the quality and safety of monitoring for potential adverse effects, including infectious diseases in particular, in other patients treated with antibodies blocking IL-12p40 or IL-12Rβ1, which are currently used to treat various clinical conditions.[39,50]
ACKNOWLEDGMENTS
We would particularly like to thank the patients and their families, whose trust, support, and cooperation were essential for the collection of the data used in this study. We thank all the members of the Laboratory of Human Genetics of Infectious Diseases for helpful discussions and critical reading of our manuscript. We thank Martine Courat, Catherine Bidalled, Michele N'Guyen, Tony Leclerc, Maya Chrabieh, Sylvanie Fahy, and Guy Brami for secretarial and technical assistance. We thank Jerôme Flatot and Max Feinberg for assistance with computing.
Financial support: The Laboratory of Human Genetics of Infectious Diseases is supported by grants from The Rockefeller University Center for Clinical and Translational Science grant number 5UL1RR024143-03, and The Rockefeller University, ANR, PHRC, EU (LHSP-CT-2005-018736), BNP Paribas Foundation, the Dana Foundation, and the March of Dimes. LDB is supported by the Fondation pour la Recherche Médicale as part of the PhD program of Pierre et Marie Curie University (Paris, France). JLC was an International Scholar of the Howard Hughes Medical Institute.
Abbreviations
BCG
Bacille Calmette-Guérin
CI
confidence interval
EBV-B cell lines
Epstein-Barr virus-transformed lymphoblastoid cell lines
ELISA
enzyme-linked immunosorbent assay
EM
environmental mycobacteria (nontuberculous mycobacteria)
FBS
fetal bovine serum
FD
fibronectin domain
IFNGR
interferon-γ receptor
IL12B
interleukin-12B
IL12Rb1
interleukin-12 receptor beta 1 chain
MSMD
Mendelian susceptibility to mycobacterial disease
NEMO
nuclear factor-κB essential modulator
PBS
phosphate-buffered saline
PCR
polymerase chain reaction
PHA
phytohemagglutinin
PR
present report
STAT1
Signal transducer and activator of transcription 1
Footnotes
Conflicts of interest: LDB is a consultant for Janssen-Cilag. The other authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 2.Aksu G, Tirpan C, Cavusoglu C, Soydan S, Altare F, Casanova JL, Kutukculer N. Mycobacterium fortuitum-chelonae complex infection in a child with complete interleukin-12 receptor beta 1 deficiency. Pediatr Infect Dis J. 2001;20:551–553. doi: 10.1097/00006454-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Alcais A, Fieschi C, Abel L, Casanova JL. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med. 2005;202:1617–1621. doi: 10.1084/jem.20052302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Muhsen S, Casanova JL. The genetic heterogeneity of Mendelian susceptibility to mycobacterial diseases. J Allergy Clin Immunol. 2008;122:1043–1051. doi: 10.1016/j.jaci.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 6.Altare F, Ensser A, Breiman A, Reichenbach J, Baghdadi JE, Fischer A, Emile JF, Gaillard JL, Meinl E, Casanova JL. Interleukin-12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J Infect Dis. 2001;184:231–236. doi: 10.1086/321999. [DOI] [PubMed] [Google Scholar]
- 7.Asilsoy S, Bilgili G, Turul T, Dizdarer C, Kalkan S, Yasli H, Can D, Genel F, Sanal O. Interleukin-12/-23 receptor beta 1 deficiency in an infant with draining BCG lymphadenitis. Pediatr Int. 2009;51:310–312. doi: 10.1111/j.1442-200X.2009.02818.x. [DOI] [PubMed] [Google Scholar]
- 8.Caragol I, Raspall M, Fieschi C, Feinberg J, Larrosa MN, Hernandez M, Figueras C, Bertran JM, Casanova JL, Espanol T. Clinical tuberculosis in 2 of 3 siblings with interleukin-12 receptor beta1 deficiency. Clin Infect Dis. 2003;37:302–306. doi: 10.1086/375587. [DOI] [PubMed] [Google Scholar]
- 9.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Tato CM, Muul L, Laurence A, O'Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleary AM, Tu W, Enright A, Giffon T, Dewaal-Malefyt R, Gutierrez K, Lewis DB. Impaired accumulation and function of memory CD4 T cells in human IL-12 receptor beta 1 deficiency. J Immunol. 2003;170:597–603. doi: 10.4049/jimmunol.170.1.597. [DOI] [PubMed] [Google Scholar]
- 12.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa FF, Castro G, Andrade J, Jesus Ade R, de Almeida RP, Nascimento-Carvalho CM. Resistant Mycobacterium bovis disseminated infection. Pediatr Infect Dis J. 2006;25:190. doi: 10.1097/01.inf.0000200103.69932.4c. [DOI] [PubMed] [Google Scholar]
- 14.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, Fieschi C, Stephan JL, Boileau C, Lyonnet S, Jondeau G, Cormier-Daire V, Le Merrer M, Hoarau C, Lebranchu Y, Lortholary O, Chandesris MO, Tron F, Gambineri E, Bianchi L, Rodriguez-Gallego C, Zitnik SE, Vasconcelos J, Guedes M, Vitor AB, Marodi L, Chapel H, Reid B, Roifman C, Nadal D, Reichenbach J, Caragol I, Garty BZ, Dogu F, Camcioglu Y, Gulle S, Sanal O, Fischer A, Abel L, Stockinger B, Picard C, Casanova JL. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer T, van Dissel JT, Kuijpers TW, Rimmelzwaan GF, Kroon FP, Ottenhoff TH. Influenza virus vaccination induces interleukin-12/23 receptor beta 1 (IL-12/23R beta 1)-independent production of gamma interferon (IFN-gamma) and humoral immunity in patients with genetic deficiencies in IL-12/23R beta 1 or IFN-gamma receptor I. Clin Vaccine Immunol. 2008;15:1171–1175. doi: 10.1128/CVI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, Casanova JL, Ottenhoff TH. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 17.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 18.Dorman SE, Picard C, Lammas D, Heyne K, van Dissel JT, Baretto R, Rosenzweig SD, Newport M, Levin M, Roesler J, Kumararatne D, Casanova JL, Holland SM. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet. 2004;364:2113–2121. doi: 10.1016/S0140-6736(04)17552-1. [DOI] [PubMed] [Google Scholar]
- 19.Ehlayel M, de Beaucoudrey L, Fike F, Nahas SA, Feinberg J, Casanova JL, Gatti RA. Simultaneous presentation of 2 rare hereditary immunodeficiencies: IL-12 receptor beta1 deficiency and ataxia-telangiectasia. J Allergy Clin Immunol. 2008;122:1217–1219. doi: 10.1016/j.jaci.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Elloumi-Zghal H, Barbouche MR, Chemli J, Bejaoui M, Harbi A, Snoussi N, Abdelhak S, Dellagi K. Clinical and genetic heterogeneity of inherited autosomal recessive susceptibility to disseminated Mycobacterium bovis bacille calmette-guerin infection. J Infect Dis. 2002;185:1468–1475. doi: 10.1086/340510. [DOI] [PubMed] [Google Scholar]
- 21.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinberg J, Fieschi C, Doffinger R, Feinberg M, Leclerc T, Boisson-Dupuis S, Picard C, Bustamante J, Chapgier A, Filipe-Santos O, Ku CL, de Beaucoudrey L, Reichenbach J, Antoni G, Balde R, Alcais A, Casanova JL. Bacillus Calmette Guerin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur J Immunol. 2004;34:3276–3284. doi: 10.1002/eji.200425221. [DOI] [PubMed] [Google Scholar]
- 23.Fieschi C, Bosticardo M, de Beaucoudrey L, Boisson-Dupuis S, Feinberg J, Santos OF, Bustamante J, Levy J, Candotti F, Casanova JL. A novel form of complete IL-12/IL-23 receptor {beta}1 deficiency with cell surface-expressed nonfunctional receptors. Blood. 2004;104:2095–2101. doi: 10.1182/blood-2004-02-0584. [DOI] [PubMed] [Google Scholar]
- 24.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, Altare F, Baretto R, Le Deist F, Kayal S, Koch H, Richter D, Brezina M, Aksu G, Wood P, Al-Jumaah S, Raspall M, Da Silva Duarte AJ, Tuerlinckx D, Virelizier JL, Fischer A, Enright A, Bernhoft J, Cleary AM, Vermylen C, Rodriguez-Gallego C, Davies G, Blutters-Sawatzki R, Siegrist CA, Ehlayel MS, Novelli V, Haas WH, Levy J, Freihorst J, Al-Hajjar S, Nadal D, De Moraes Vasconcelos D, Jeppsson O, Kutukculer N, Frecerova K, Caragol I, Lammas D, Kumararatne DS, Abel L, Casanova JL. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. J Exp Med. 2003;197:527–535. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C, Casanova JL. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, Bresolin N, Sironi M. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206:1395–1408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guia S, Cognet C, de Beaucoudrey L, Tessmer MS, Jouanguy E, Berger C, Filipe-Santos O, Feinberg J, Camcioglu Y, Levy J, Al Jumaah S, Al-Hajjar S, Stephan JL, Fieschi C, Abel L, Brossay L, Casanova JL, Vivier E. A role for interleukin-12/23 in the maturation of human natural killer and CD56+ T cells in vivo. Blood. 2008;111:5008–5016. doi: 10.1182/blood-2007-11-122259. [DOI] [PubMed] [Google Scholar]
- 28.Haerynck F, Holland SM, Rosenzweig SD, Casanova JL, Schelstraete P, De Baets F. Disseminated Mycobacterium avium infection in a patient with a novel mutation in the interleukin-12 receptor-beta1 chain. J Pediatr. 2008;153:721–722. doi: 10.1016/j.jpeds.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 29.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoeve MA, de Boer T, Langenberg DM, Sanal O, Verreck FA, Ottenhoff TH. IL-12 receptor deficiency revisited: IL-23-mediated signaling is also impaired in human genetic IL-12 receptor beta1 deficiency. Eur J Immunol. 2003;33:3393–3397. doi: 10.1002/eji.200324343. [DOI] [PubMed] [Google Scholar]
- 31.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 32.Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 33.Jeppsson O, Petrini B, Andersson J, Heurlin N, Malm G. Defective handling of mycobacteria. Lancet. 1988;2:570. doi: 10.1016/s0140-6736(88)92693-1. [DOI] [PubMed] [Google Scholar]
- 34.Kutukculer N, Genel F, Aksu G, Karapinar B, Ozturk C, Cavusoglu C, Casanova JL, Fieschi C. Cutaneous leukocytoclastic vasculitis in a child with interleukin-12 receptor beta-1 deficiency. J Pediatr. 2006;148:407–409. doi: 10.1016/j.jpeds.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Lachaux A, Descos B, Loras-Duclaux I, Souillet G, Floret D, Brunet J, Freney J, Hermier M. [Recurrent infections caused by Salmonella typhimurium]. Arch Fr Pediatr. 1988;45:337–339. [PubMed] [Google Scholar]
- 36.Lee PP, Jiang LP, Wang XC, Chan KW, Tu WW, Lau YL. Severe mycobacterial infections in two pairs of Chinese siblings with interleukin-12 receptor beta1 deficiency. Eur J Pediatr. 2008;167:231–232. doi: 10.1007/s00431-007-0430-2. [DOI] [PubMed] [Google Scholar]
- 37.Lee WI, Huang JL, Lin TY, Hsueh C, Wong AM, Hsieh MY, Chiu CH, Jaing TH. Chinese patients with defective IL-12/23-interferon-gamma circuit in Taiwan: partial dominant interferon-gamma receptor 1 mutation presenting as cutaneous granuloma and IL-12 receptor beta1 mutation as pneumatocele. J Clin Immunol. 2009;29:238–245. doi: 10.1007/s10875-008-9253-9. [DOI] [PubMed] [Google Scholar]
- 38.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 39.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 40.Levin M, Newport MJ, D'Souza S, Kalabalikis P, Brown IN, Lenicker HM, Agius PV, Davies EG, Thrasher A, Klein N, et al. Familial disseminated atypical mycobacterial infection in childhood: a human mycobacterial susceptibility gene? Lancet. 1995;345:79–83. doi: 10.1016/s0140-6736(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 41.Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, Ramachandran S, Cann HM, Barsh GS, Feldman M, Cavalli-Sforza LL, Myers RM. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319:1100–1104. doi: 10.1126/science.1153717. [DOI] [PubMed] [Google Scholar]
- 42.Lichtenauer-Kaligis EG, de Boer T, Verreck FA, van Voorden S, Hoeve MA, van de Vosse E, Ersoy F, Tezcan I, van Dissel JT, Sanal O, Ottenhoff TH. Severe Mycobacterium bovis BCG infections in a large series of novel IL-12 receptor beta1 deficient patients and evidence for the existence of partial IL-12 receptor beta1 deficiency. Eur J Immunol. 2003;33:59–69. doi: 10.1002/immu.200390008. [DOI] [PubMed] [Google Scholar]
- 43.MacLennan C, Fieschi C, Lammas DA, Picard C, Dorman SE, Sanal O, MacLennan JM, Holland SM, Ottenhoff TH, Casanova JL, Kumararatne DS. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis. 2004;190:1755–1757. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 44.Martinez J, Martinez L, Rosenblueth M, Silva J, Martinez-Romero E. How are gene sequence analyses modifying bacterial taxonomy? The case of Klebsiella. Int Microbiol. 2004;7:261–268. [PubMed] [Google Scholar]
- 45.Mimouni J. [Our experiences in three years of BCG vaccination at the center of the O.P.H.S. at Constantine; study of observed cases (25 cases of complications from BCG vaccination).]. Alger Medicale. 1951;55:1138–1147. [PubMed] [Google Scholar]
- 46.Miro F, Nobile C, Blanchard N, Lind M, Filipe-Santos O, Fieschi C, Chapgier A, Vogt G, de Beaucoudrey L, Kumararatne DS, Le Deist F, Casanova JL, Amigorena S, Hivroz C. T cell-dependent activation of dendritic cells requires IL-12 and IFN-gamma signaling in T cells. J Immunol. 2006;177:3625–3634. doi: 10.4049/jimmunol.177.6.3625. [DOI] [PubMed] [Google Scholar]
- 47.Moraes-Vasconcelos D, Grumach AS, Yamaguti A, Andrade ME, Fieschi C, de Beaucoudrey L, Casanova JL, Duarte AJ. Paracoccidioides brasiliensis disseminated disease in a patient with inherited deficiency in the beta1 subunit of the interleukin (IL)-12/IL-23 receptor. Clin Infect Dis. 2005;41:e31–37. doi: 10.1086/432119. [DOI] [PubMed] [Google Scholar]
- 48.Ozbek N, Fieschi C, Yilmaz BT, de Beaucoudrey L, Bikmaz YE, Feinberg J, Casanova JL. Interleukin-12 receptor beta 1 chain deficiency in a child with disseminated tuberculosis. Clin Infect Dis. 2005;40:e55–58. doi: 10.1086/427879. [DOI] [PubMed] [Google Scholar]
- 49.Ozen M, Ceyhan M, Sanal O, Bayraktar M, Mesci L. Recurrent Salmonella bacteremia in interleukin-12 receptor beta1 deficiency. J Trop Pediatr. 2006;52:296–298. doi: 10.1093/tropej/fml001. [DOI] [PubMed] [Google Scholar]
- 50.Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S, Dooley LT, Reich K. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 51.Picard C, Fieschi C, Altare F, Al-Jumaah S, Al-Hajjar S, Feinberg J, Dupuis S, Soudais C, Al-Mohsen IZ, Genin E, Lammas D, Kumararatne DS, Leclerc T, Rafii A, Frayha H, Murugasu B, Wah LB, Sinniah R, Loubser M, Okamoto E, Al-Ghonaium A, Tufenkeji H, Abel L, Casanova JL. Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am J Hum Genet. 2002;70:336–348. doi: 10.1086/338625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenzweig SD, Yancoski J, Bernasconi A, Krasovec S, Marciano BE, Casimir L, Berberian G, Simboli N, Rousseau M, Calle G. Thirteen years of culture-positive M. bovis-BCG infection in an IL-12Rbeta1 deficient patient: treatment and outcome. J Infect. 2006;52:e69–72. doi: 10.1016/j.jinf.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Sakai T, Matsuoka M, Aoki M, Nosaka K, Mitsuya H. Missense mutation of the interleukin-12 receptor beta1 chain-encoding gene is associated with impaired immunity against Mycobacterium avium complex infection. Blood. 2001;97:2688–2694. doi: 10.1182/blood.v97.9.2688. [DOI] [PubMed] [Google Scholar]
- 56.Sanal O, Morgan G, Gocmen A, Novelli V, Klein N, Tezcan I, Ersoy F, Berkel AI, Yel L. Isolated cutaneous response to granulocyte-monocyte colony stimulating factor in fatal idiopathic disseminated Bacillus-Calmette-Guerin infection. Eur J Pediatr. 2000;159:149–152. doi: 10.1007/s004310050039. [DOI] [PubMed] [Google Scholar]
- 57.Sanal O, Turkkani G, Gumruk F, Yel L, Secmeer G, Tezcan I, Kara A, Ersoy F. A case of interleukin-12 receptor beta-1 deficiency with recurrent leishmaniasis. Pediatr Infect Dis J. 2007;26:366–368. doi: 10.1097/01.inf.0000258696.64507.0f. [DOI] [PubMed] [Google Scholar]
- 58.Sanal O, Turul T, De Boer T, Van de Vosse E, Yalcin I, Tezcan I, Sun C, Memis L, Ottenhoff TH, Ersoy F. Presentation of interleukin-12/-23 receptor beta1 deficiency with various clinical symptoms of Salmonella infections. J Clin Immunol. 2006;26:1–6. doi: 10.1007/s10875-006-7830-3. [DOI] [PubMed] [Google Scholar]
- 59.Scheuerman O, de Beaucoudrey L, Hoffer V, Feinberg J, Casanova JL, Garty BZ. Mycobacterial disease in a child with surface-expressed non-functional interleukin-12Rbeta1 chains. Isr Med Assoc J. 2007;9:560–561. [PubMed] [Google Scholar]
- 60.Staretz-Haham O, Melamed R, Lifshitz M, Porat N, Fieschi C, Casanova JL, Levy J. Interleukin-12 receptor beta1 deficiency presenting as recurrent Salmonella infection. Clin Infect Dis. 2003;37:137–140. doi: 10.1086/375229. [DOI] [PubMed] [Google Scholar]
- 61.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi M, Matsuda F, Margetic N, Lathrop M. Automated identification of single nucleotide polymorphisms from sequencing data. J Bioinform Comput Biol. 2003;1:253–265. doi: 10.1142/s021972000300006x. [DOI] [PubMed] [Google Scholar]
- 63.Tanir G, Dogu F, Tuygun N, Ikinciogullari A, Aytekin C, Aydemir C, Yuksek M, Boduroglu EC, de Beaucoudrey L, Fieschi C, Feinberg J, Casanova JL, Babacan E. Complete deficiency of the IL-12 receptor beta1 chain: three unrelated Turkish children with unusual clinical features. Eur J Pediatr. 2006;165:415–417. doi: 10.1007/s00431-005-0078-8. [DOI] [PubMed] [Google Scholar]
- 64.Tuerlinckx D, Vermylen C, Brichard B, Ninane J, Cornu G. Disseminated Mycobacterium avium infection in a child with decreased tumour necrosis factor production. Eur J Pediatr. 1997;156:204–206. doi: 10.1007/s004310050583. [DOI] [PubMed] [Google Scholar]
- 65.Ulrichs T, Fieschi C, Nevicka E, Hahn H, Brezina M, Kaufmann SH, Casanova JL, Frecerova K. Variable outcome of experimental interferon-gamma therapy of disseminated Bacillus Calmette-Guerin infection in two unrelated interleukin-12Rbeta1-deficient Slovakian children. Eur J Pediatr. 2005;164:166–172. doi: 10.1007/s00431-004-1599-2. [DOI] [PubMed] [Google Scholar]
- 66.Verhagen CE, de Boer T, Smits HH, Verreck FA, Wierenga EA, Kurimoto M, Lammas DA, Kumararatne DS, Sanal O, Kroon FP, van Dissel JT, Sinigaglia F, Ottenhoff TH. Residual type 1 immunity in patients genetically deficient for interleukin 12 receptor beta1 (IL-12Rbeta1): evidence for an IL- 12Rbeta1-independent pathway of IL-12 responsiveness in human T cells. J Exp Med. 2000;192:517–528. doi: 10.1084/jem.192.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verreck FA, de Boer T, Hoeve M, van Dissel JT, Sanal O, Kurimoto M, Ottenhoff TH. Human host defense and cytokines in mycobacterial infectious diseases: interleukin-18 cannot compensate for genetic defects in the interleukin-12 system. Clin Infect Dis. 2002;35:210–212. doi: 10.1086/341314. [DOI] [PubMed] [Google Scholar]
- 68.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 69.Wood PM, Fieschi C, Picard C, Ottenhoff TH, Casanova JL, Kumararatne DS. Inherited defects in the interferon-gamma receptor or interleukin-12 signalling pathways are not sufficient to cause allergic disease in children. Eur J Pediatr. 2005;164:741–747. doi: 10.1007/s00431-005-1745-5. [DOI] [PubMed] [Google Scholar]
- 70.Yancoski J, Rocco C, Bernasconi A, Oleastro M, Bezrodnik L, Vratnica C, Haerynck F, Rosenzweig SD. A 475 years-old founder effect involving IL12RB1: a highly prevalent mutation conferring Mendelian susceptibility to mycobacterial diseases in European descendants. Infect Genet Evol. 2009;9:574–580. doi: 10.1016/j.meegid.2009.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 72.Yu HR, Chen RF, Hong KC, Bong CN, Lee WI, Kuo HC, Yang KD. IL-12-independent Th1 polarization in human mononuclear cells infected with varicella-zoster virus. Eur J Immunol. 2005;35:3664–3672. doi: 10.1002/eji.200526258. [DOI] [PubMed] [Google Scholar]
- 73.Zerbe CS, Holland SM. Disseminated histoplasmosis in persons with interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2005;41:e38–41. doi: 10.1086/432120. [DOI] [PubMed] [Google Scholar]