Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling (original) (raw)
Abstract
Drosophila epithelia acquire a planar cell polarity (PCP) orthogonal to their apical-basal axes. Frizzled (Fz) is the receptor for the PCP signal, and Dishevelled (Dsh) transduces the signal. Here, I demonstrate that unipolar relocalization of Dsh to the membrane is required to mediate PCP, but not Wingless (Wg) signaling. Dsh membrane localization reflects the activation of Fz/PCP signaling, revealing that the initially symmetric signal evolves to one that displays unipolar asymmetry, specifying the cells' ultimate polarity. This transition from symmetric to asymmetric Dsh localization requires Dsh function, and reflects an amplification process that generates a steep intracellular activity gradient necessary to determine PCP.
Keywords: Planar cell polarity, Dishevelled, Frizzled, membrane localization, signal amplification
Cells secreting the adult cuticle of Drosophila produce trichomes, or hairs; the planar cell polarity (PCP) signal polarizes these hairs within the plane of the epithelium, forming regular, parallel arrays (Shulman et al. 1998). Frizzled (Fz) is the receptor for the PCP signal (Vinson and Adler 1987; Vinson et al. 1989) and, in addition, functions redundantly with Dfrizzled2 (DFz2) as a receptor for Wingless (Wg) (Bhat 1998; Kennerdell and Carthew 1998). Dishevelled (Dsh) is the most receptor-proximal known component in both of these pathways (Klingensmith et al. 1994; Noordermeer et al. 1994; Siegfried et al. 1994; Thiesen et al. 1994). The readout of the PCP pathway is an asymmetric organization of the cytoskeleton within the plane of the epithelium. In the wing, Fz/PCP signaling directs the location of prehair assembly to the distal vertex of each cell, resulting in a distally oriented hair (Wong and Adler 1993).
The identity of the extracellular PCP signal is not known, nor is it understood how the signal is distributed (Shulman et al. 1998). Graded overexpression of Fz reorients polarity, suggesting that differential Fz signaling levels control PCP (Adler et al. 1997). However, a Wnt (or other) PCP ligand has not been identified, and little is known about how a presumptively asymmetric extracellular signal is converted to a polarized subcellular response. Control of Dsh subcellular localization has been implicated in the regulation of various Wnt-mediated signaling events (Steitz et al. 1996; Yang-Snyder et al. 1996; Axelrod et al. 1998; Miller et al. 1999; Rothbacher et al. 2000; Torres and Nelson 2000; Umbhauer et al. 2000; Wallingford et al. 2000). A heterologous assay, in frog animal caps, was used to demonstrate that Fz but not DFz2 directs recruitment of Dsh to the membrane (Axelrod et al. 1998). These, and additional genetic data, led to the prediction that, in vivo, Dsh may localize to the membrane as a response to Fz signaling through the PCP, but not the β-catenin-dependent Wg pathway (Axelrod et al. 1998). An asymmetric Fz/PCP signal might therefore produce asymmetric localization of Dsh, marking one side of the cell as the location for prehair assembly. Furthermore, specificity between the polarity and Wg signaling activities of Fz could be explained, at least in part, by differential recruitment of Dsh to the membrane.
Recently, asymmetric distribution of two PCP signaling components has been reported. Flamingo (Fmi; also known as Starry night; Chae et al. 1999), a seven-pass transmembrane cadherin family member, has been proposed to localize on both the proximal and distal ends of wing cells (Usui et al. 1999), whereas Fz has been shown to localize to the distal end of these cells (Strutt 2001). Here, I show that translocation of Dsh from the cytoplasm to the cell cortex is required to mediate the PCP signal. Furthermore, although Dsh localization is initially essentially symmetric, Dsh subsequently adopts a unipolar asymmetry similar to that seen for Fz. Dsh localization to the membrane and Dsh function are necessary to mediate this process, which reflects the activity of a feedback amplification system also involving Fmi.
Results and Discussion
Dsh relocalizes from the cytoplasm to the cell cortex and evolves from a nearly symmetric to an asymmetric pattern
To investigate a possible role for Dsh membrane association during Fz/PCP signaling in vivo, I examined Dsh subcellular localization during PCP signaling in the developing wing. Because I was unable to obtain satisfactory results by using existing anti-Dsh antibodies, transgenes were produced that express a Dsh::green fluorescent protein (GFP) C-terminal fusion, driven by native dsh regulatory sequences. One or two copies of these transgenes rescue dshv26 null mutants to viability and produce wild-type PCP (not shown), indicating that they fully replace the function of endogenous Dsh in both Wg and PCP signaling.
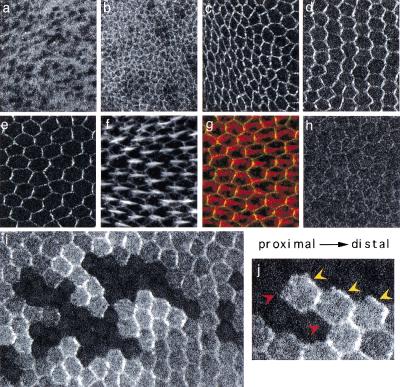
Previous work using temperature-sensitive alleles of both fz and another PCP gene, inturned, has suggested that signaling is active after puparium formation (apf), and culminates just before the initiation of prehair morphogenesis (32–34 h apf) (Adler et al. 1994a; Adler et al. 1994b). In wings, a dynamic pattern of subcellular localization of tagged Dsh protein was observed during this period. Consistent with published reports, Dsh is observed predominantly in the cytoplasm of embryonic epidermis (not shown) and third-instar wing discs (Fig. 1a). Some weak, perimembranous enrichment of Dsh is observed in apicolateral regions throughout third-instar wing development (Fig. 1b, and not shown). This component of the pattern is stable in formaldehyde fixation, but significantly diminished in methanol fixation (and is fz independent; see following). However, at or shortly after the white prepupal stage, Dsh strongly associates with the membrane, accumulating in an apical circumferential ring, with an apparent simultaneous decrease in cytoplasmic levels (Fig. 1c). This pattern is stable in both formaldehyde and methanol fixation. Through 18 h apf, the ring is approximately symmetric; however, by 24 h apf (not shown), and most pronounced by 30 h apf, Dsh is seen to accumulate preferentially at proximal–distal boundaries, and is depleted at anterior–posterior boundaries (Fig. 1d). Viewed en face, this produces a pattern of parallel zigzags similar to that seen for Fmi (Usui et al. 1999) and Fz (Strutt 2001). By 32–34 h apf, Dsh is often seen in discrete patches that appear to be at the distal surface of each cell, corresponding to the site of nascent actin-rich prehair emergence (Fig. 1e–g). In a wild-type wing, clones of cells lacking the tagged transgene reveal that Dsh does indeed accumulate solely at the distal edge (Fig. 1i–j). Dsh subcellular localization therefore evolves into a pattern showing unipolar asymmetry within the plane of the epithelium, prefiguring the distal position of prehair assembly. The unipolar distribution of the PCP effector protein Dsh reflects the proximal–distal polarity vector, and strongly suggests that its distal localization is required to determine PCP.
Figure 1.
Subcellular localization of Dishevelled (Dsh). In panels c to j, proximal is left, distal is right. (a) Localization of Dsh::green fluorescent protein (GFP) in late third-instar subapical section. (b) Late third-instar apical section (including the dorsal-ventral boundary). A similar result was observed in fzR52 mutant wing discs (not shown, but see Fig. 3c). (c) Two-hours after-puparium-formation (apf) apical sections. (d) Thirty-hour apf apical sections. (e) Thirty-four-hour apf apical section. (e–g) Double label of 34-h apf wing with Dsh::GFP (e), phalloidin (actin, f), and overlay (g) (Dsh::GFP, green; phalloidin, red). In panels a to g, the Dsh::GFP transgene was expressed in a dshv26 null mutant background. The result was identical when expressed in a wild-type background (not shown). (h) Dsh1::GFP subcellular localization in a dsh1 mutant background at 30 h apf. The dsh1 allele carries a point mutation in the DEP domain, and is a strong allele for Frizzled (Fz)/planar cell polarity (PCP) signaling (Perrimon and Mahowald 1987; Axelrod et al. 1998). Similar results were obtained in dshv26 null and in wild-type backgrounds (not shown). (i–j) Clones of cells lacking the Dsh::GFP transgene in 30-h apf wings appear as holes in the GFP pattern, and were also marked with anti-βgal (not shown). All cells express endogenous dsh+, and thus are genetically wild type. Note that in cells abutting the clones, Dsh::GFP accumulates at the distal edges (yellow arrowheads), but not at the proximal edges (red arrowheads). Twinspots of the clones have two copies of Dsh::GFP and show enhanced fluorescence compared with heterozygous tissue. Panels a to h are of the same magnification.
Membrane association of Dsh is required for PCP signaling
To demonstrate the importance of Dsh membrane localization for Fz/PCP signaling, I enlisted the dsh1 allele. Dsh1 is specifically compromised in its ability to transduce the PCP, but not the Wg signal (Perrimon and Mahowald 1987). The lesion in dsh1 maps to its DEP domain, which was required for its membrane localization in the frog animal cap assay (Axelrod et al. 1998). A dsh1::GFP fusion, otherwise identical to the wild-type construct, rescues dsh null mutant flies to viability, and the flies exhibit the dsh1 mutant phenotype (not shown). In the absence of wild-type Dsh (in either a dsh1 or a dshv26 null mutant background), apical membrane association of Dsh1::GFP was severely reduced, and the Dsh1 protein instead remained almost entirely in the cytoplasm throughout pupal development (Fig. 1h, and data not shown). Therefore, introducing a lesion into Dsh blocks its localization to the membrane in vivo and its ability to signal. Similarly, in the presence of wild-type Dsh, Dsh1::GFP remains in the cytoplasm (not shown). Because wild-type Dsh does not induce the relocalization of Dsh1::GFP, it is unlikely that Dsh acts as a multimer during PCP signaling.
Codependence of Dsh and Fmi
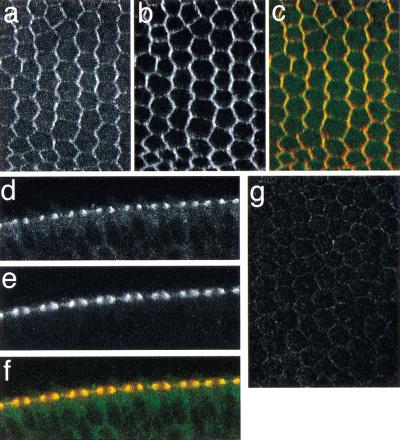
The pattern of Dsh localization observed in pupal wings is reminiscent of that for Fmi, a seven-pass transmembrane cadherin required for PCP signaling (Usui et al. 1999). By 30 h apf, both are seen at the proximal–distal boundaries, though Dsh is strictly distal, whereas Fmi was proposed to be at both proximal and distal edges (Usui et al. 1999). Double labeling for Fmi and Dsh reveals significant colocalization in 30-h apf pupal wings, each demonstrating a zigzag pattern (Fig. 2a–c). However, at later times, the Dsh asymmetry persists (Fig. 1e–g), whereas the Fmi asymmetry decays (Usui et al. 1999). Transverse sections taken in wing-edge cells indicate that both are located at the most apical region of cell–cell contact, and that low levels of Dsh are also seen throughout the cytoplasm (Fig. 2d–f). Fmi localization was previously shown to depend on both Fz and Dsh, but not on Multiple wing hairs (Mwh), suggesting that Fmi functions downstream of Dsh (Usui et al. 1999). To study this relationship further, I examined Dsh localization in fmi mutant wings. At 30 h apf, little Dsh is associated with the membrane in fmi mutant wings (Fig. 2i). This reveals a reciprocal dependence between Dsh and Fmi for persistent membrane association, and suggests that Fmi does not simply function downstream of Dsh.
Figure 2.
Relationship of Dishevelled (Dsh)::green fluorescent protein (GFP) and Flamingo (Fmi) localization. (a–e) A 30-h after puparium formation (apf) pupal wing showing Dsh::GFP (a), Fmi (b), and the overlay of Dsh::GFP (green) and Fmi (red) (c). (d–f) Tangential section of a double-labeled wing taken through edge cells: Dsh::GFP (d), Fmi (e), overlay, as in c (f). Much of the Dsh colocalizes with Fmi, and some remains in the cytoplasm. (g) Dsh::GFP does not show significant association with the membrane in a 30-h apf fmi45/fmi59 mutant wing.
Recently, Fz and Fmi were shown to colocalize at proximal–distal boundaries at 30 h apf (Strutt 2001). Furthermore, the asymmetric pattern of Fz localization depends on Fmi, whereas the asymmetric pattern of Fmi localization depends on Fz (Usui et al. 1999). Taken together, these data are consistent with the possibility that Fz, Dsh, and Fmi function together, perhaps in a complex, during PCP signaling, with both Fz and Dsh localizing to the distal edge, and Fmi apparently localizing to both the proximal and distal edges of the cell. A mutual dependence for asymmetric localization exists between these three proteins.
Membrane localization of Dsh and asymmetry of Dsh require Fz function
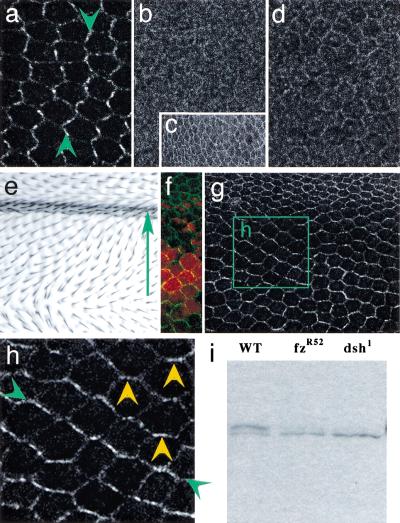
I next asked whether Dsh localization depends on upstream signaling through the Fz/PCP pathway by examining Dsh localization in a fz mutant background. In a fzR52 null mutant, Dsh fails to accumulate at the membrane at 30 h apf (Fig. 3b). At 2 h apf, only the weak, perimembranous, methanol-sensitive enrichment of Dsh, reminiscent of that seen in wild-type third-instar discs, remains (compare Figs. 3c, 1a). Absence of membrane-associated Dsh from around 2 h apf through 30 h apf indicates that both the earlier, symmetric phase of Dsh-membrane association, as well as the late, asymmetric phase, are Fz dependent. Nearly identical results were obtained with the fzJ22 missense allele (Fig. 3d). fzJ22 produces a normal amount of protein that migrates normally on SDS gels, yet fails to signal (Jones et al. 1996). Therefore, Dsh-membrane association depends not simply on the presence of Fz protein, but also on its ability to signal. Disrupting the ability to localize Dsh to the membrane, either by mutating Dsh (dsh1) or by blocking Fz function (fzR52 or fzJ22), produces a mutant PCP phenotype. Dsh-membrane association is therefore necessary to transduce the polarity signal.
Figure 3.
Frizzled (Fz) loss and gain of function alters Dishevelled (Dsh) localization. In a to f, proximal is left, distal is right. Dsh localization in 30-h after-puparium-formation (apf) wild type (a), fzR52 (b), and fzJ22 (d) mutant wings, and in a 2-h apf fzR52 mutant wing (c). (e) Ectopic Fz overexpressed in a gradient in the dpp domain produces hairs pointing down the Fz gradient. The arrow points from high toward low ectopic Fz expression. (f) In an equivalent region of a 30-h apf pupal wing in which dpp-GAL4 drives both UAS-fz and UAS-lacZ (red), the ectopic Fz gradient is visualized (anti-βgal, red) along with a reorganized Dsh::green fluorescent protein (GFP) pattern (green). (g) A 30-h pupal wing from a region equivalent to that in e, showing only the reorganized Dsh::GFP localization. (h) Enlarged view of the region marked by the box in g. Note that Dsh::GFP accumulates in a zigzag pattern that is perpendicular to the pattern of hairs in this region (green arrowheads; compare with a) and includes anterior–posterior boundaries (yellow arrowheads). (i) A Western blot of wild-type (WT), fzR52, and dsh1 pupal wings, probed with anti-Dsh. The slower migrating isoform seen in WT corresponds to a previously identified hyperphosphorylated form (Yanagawa et al. 1995) and is severely reduced in the mutants. Equal loading between lanes was assured by Ponceau staining before hybridization (not shown).
To determine whether Fz signaling is sufficient to produce the asymmetric localization of Dsh, I examined its localization in a Fz expression gradient that alters the polarity pattern on the wing. Consistent with previous demonstrations, graded expression of ectopic Fz in the dpp (Fig. 3e,f) or dll (not shown) expression domains reorients hairs from high to low levels of Fz expression. In these wings, asymmetric Dsh localization realigns according to the Fz gradient (Fig. 3f–h). Therefore, both the membrane localization of Dsh and its asymmetry are dependent on signaling through Fz. In contrast, Dsh localization is normal in a mwh mutant (not shown), consistent with previous arguments placing Dsh upstream of Mwh in the polarity signaling pathway.
PCP signaling activity is required to generate unipolar asymmetry
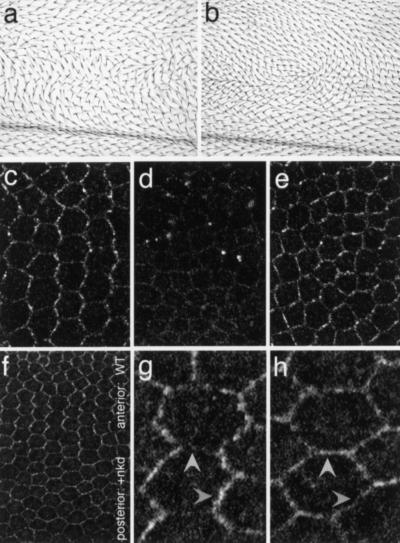
I next investigated the mechanism of the transition from a nearly symmetric to an asymmetric pattern of Dsh localization. Several maneuvers to interfere with Dsh function were performed, and the effect on Dsh localization assayed. I first tested how expression of two dominant negative, truncated Dsh constructs might modify the localization of full-length (tagged) Dsh. Expression of a form containing the DEP domain, but lacking the PDZ domain [Dsh(ΔbPDZ); Axelrod et al. 1998] produces a polarity defect (Fig. 4a; Axelrod et al. 1998), and in pupal wings, causes a failure of Dsh::GFP to localize to the membrane (Fig. 4d). Similar results were obtained by expressing Dsh(DEP+), a form containing only the DEP domain (not shown). It is likely that the DEP domain in the truncated proteins competes with the DEP domain in Dsh::GFP (and presumably with endogenous Dsh) for membrane docking, preventing its localization. Consistent with the loss of membrane localization and the polarity defect seen in dsh1 and fz mutants, this result indicates that membrane association is necessary for PCP signaling.
Figure 4.
Loss of Dishevelled (Dsh) membrane localization or asymmetry blocks polarity signaling. In all panels, proximal is left, distal is right. Dominant negative planar cell polarity phenotypes result from expression of Dsh(ΔbPDZ) (a) or Dsh(ΔDEP+) (b) in the patched expression domain. (c) Anterior (internal control) region of a 30-h after-puparium-formation wing in which patched-GAL4 drives expression of Dsh(ΔbPDZ), showing wild-type Dsh::green fluorescent protein (GFP) localization. (d) In the patched expression domain of the same wing, Dsh::GFP membrane association is lost. (e) Dsh::GFP pattern in the patched expression domain of a wing expressing Dsh(ΔDEP+). Note that the enrichment of Dsh::GFP at proximal–distal boundaries is diminished, and the pattern is more nearly symmetric. (f) Dsh::GFP at the anterior–posterior compartment boundary of a wing overexpressing Naked cuticle (Nkd) in the posterior, engrailed domain. High magnification of a portion of the anterior, wild-type domain (g) and the posterior domain overexpressing Nkd (h). Note the wild-type, zigzag pattern in the anterior, and the nearly complete loss of asymmetry in the posterior. In the posterior, Dsh is no longer enriched at the proximal–distal boundaries (compare darker arrowheads) and no longer suppressed at the anterior–posterior boundaries (compare lighter arrowheads).
In contrast, expression of Dsh(ΔDEP+), a (weaker) dominant negative construct lacking the DEP domain, also blocks polarity signaling (Fig. 4b; Axelrod et al. 1998), but in this case, Dsh::GFP (and presumably endogenous Dsh) retains its ability to localize to the membrane (Fig 4e). In these wings, the pattern of membrane-localized Dsh no longer displays the vertically oriented zigzags seen in the wild type. This result indicates that membrane association of Dsh, although necessary, is not sufficient for PCP signaling, and that the orientation of asymmetry is important for PCP signaling.
Finally, I took advantage of the observation that the Naked cuticle (Nkd) protein is known to bind the Dsh PDZ domain (Rousset et al. 2001). Nkd is thought not to play a role in PCP signaling. However, Nkd overexpression produces a PCP phenotype by binding Dsh and interfering with its ability to function in the PCP pathway (Rousset et al. 2001). Dsh localizes to the membrane throughout wings overexpressing Nkd in the posterior compartment (Fig. 4f). However, the transition from symmetric to asymmetric Dsh localization is abolished posteriorly (compare Fig. 4g,h). Whereas in the anterior, wild-type portion of the wing (Fig. 4f,g) Dsh accumulation is enriched along the proximal–distal boundaries and diminished at the anterior–posterior boundaries, in the posterior region where Nkd is overexpressed, Dsh accumulation is essentially symmetric around the cell periphery (Fig. 4f,h). Therefore, by binding Dsh, Nkd interferes with Dsh function, resulting in a loss of Dsh subcellular asymmetry, and producing an adult PCP phenotype. Furthermore, because specifically interfering with the ability of Dsh to signal blocks the acquisition of asymmetry, Dsh asymmetry does not simply result from passively colocalizing with asymmetrically distributed Fz (Strutt 2001). Rather, one can infer that Dsh function is required for generation of asymmetry, indicating that a feedback loop contributes to asymmetry.
Dsh membrane localization is associated with phosphorylation
Dsh may translocate to the membrane from an existing pool, or may be stabilized at the membrane, increasing the total cellular Dsh content. Furthermore, Dsh is a phosphoprotein (Yanagawa et al. 1995), and its phosphorylation state is potentially regulated during PCP signaling. Western blot analysis was therefore used to examine Dsh protein levels and phosphorylation state in pupal discs during PCP signaling. No significant difference in total Dsh levels was observed in wild type, fzR52, or dsh1 wings, indicating that membrane association represents a shift in Dsh localization from the cytoplasmic to the membrane compartment (Fig. 3i). However, more than half of the Dsh protein in wild type is in a hyperphosphorylated form, whereas very little of this form exists in fzR52 or dsh1 mutants. The PCP signal therefore results in phosphorylation of Dsh, and phosphorylation correlates with membrane localization, suggesting it is either required for, or is a response to, localization. This result is consistent with studies in Xenopus showing that XDsh phosphorylation and membrane association correlate with activity in convergent extension, a process homologous to PCP signaling, but not axis duplication, a β-catenin mediated process (Rothbacher et al. 2000; Tada and Smith 2000; Wallingford et al. 2000).
Fz signaling specificity in PCP and Wg signaling
Although both Fz and DFz2 transduce the Wg signal, only Fz can serve as a receptor for PCP signaling. Analysis of chimeras points to structural differences distal to the ligand binding domains as responsible for this difference (Boutros et al. 2000; Rulifson et al. 2000). However, the question of how Fz specifically transduces two distinct signals, both of which require Dsh function, still remains. Our previous work, using the frog animal cap assay, showed that Fz but not DFz2 could recruit Dsh to the membrane, suggesting that this difference may account for the unique ability of Fz to function in PCP signaling (Axelrod et al. 1998). However, others did not confirm this observation (Boutros et al. 2000). Here, this issue is addressed directly. During late third instar, Wg signals through both Fz and DFz2 to establish the proneural clusters that give rise to bristles near the D/V boundary of the wing (Phillips and Whittle 1993). However, no accumulation of Dsh is observed at membranes near the D/V boundary of third-instar wing discs (Fig. 1b). Furthermore, Dsh is not observed at membranes in embryos, nor in wing discs throughout third instar. During early pupal stages, when Dsh shows the earliest Fz-dependent membrane localization, no difference is observed between cells close to Wg expressing cells and those at greater distances (not shown). Recruitment of Dsh to the membrane is therefore a specific response to the Fz/PCP signal, and does not result from the Wg signaling activity of either Fz or DFz2.
Implications
The results presented here provide new insights into several key features of PCP signaling. Dsh localization is an early molecular marker of the proximal–distal polarity vector, and its unipolar redistribution to the distal end of the cell precedes and directs prehair assembly to the distal vertex. Furthermore, Dsh localization reflects the activation of Fz, in effect acting as a biosensor. The early, essentially symmetric pattern of Dsh membrane localization indicates that Fz signaling begins with undetectable asymmetry. The subsequent, gradual evolution of Dsh localization ultimately produces a pattern displaying unipolar asymmetry. Indeed, Fz has recently been shown to adopt distal localization similar to that of Dsh (Strutt 2001). Acquisition of the Dsh pattern could simply reflect the passive association of Dsh with Fz, which has been proposed to adopt this localization through a mechanism such as receptor clustering (Strutt 2001). However, Dsh is not a passive player in this process, because not only is its localization to the membrane required, but its ability to productively transduce signal is also necessary to generate asymmetry. This implies that a feedback mechanism is required to generate the steep intracellular gradient of Dsh localization seen by 30 h apf. The mutual requirement for Fmi, Dsh, and Fz for proper localization suggests that these three (and perhaps other) components function together in this process. Finally, because interfering with the generation of asymmetry by Dsh(ΔDEP+) expression or Nkd overexpression blocks polarity signaling, asymmetric cortical localization of Dsh, Fz, and Fmi (and perhaps other components) must determine the location of prehair assembly.
Cytosolic regulator of adenylyl cyclase (CRAC), a signal transducer in chemotaxing Dictyostelium cells (Parent et al. 1998), and the PH-domain protein AKT in chemotaxing neutrophils (Servant et al. 2000), both use feedback amplification to produce a steep intracellular gradient in response to a shallow gradient of extracellular signal. I propose that a shallow extracellular gradient, perhaps of a Wnt protein, initiates a slightly asymmetric PCP signal. This slight asymmetry is too subtle to detect by using the Dsh localization assay. Feedback then amplifies the asymmetry, resulting in the unipolar localization of Dsh and Fz, thereby determining the subcellular location for prehair assembly.
Materials and methods
Construction of GFP-tagged Dsh or Dsh1 transgenes
A 6.7-kb genomic fragment carrying dsh or dsh1 was modified by insertion of DNA-encoding-enhanced GFP at the 3′ end of the _dsh_-coding region (details available on request). Transgenic lines with insertions on the second and third chromosomes were created by standard methods.
Detection of Dsh::GFP
White prepupae were collected and aged at 25°C. At the appropriate time, heads were removed and the remainder subjected to fixation, either in PBS containing 4% formaldehyde and 0.2% tween-20 at 20°C for 20–30 min, or in 100% methanol at −20°C for 30 min. Wings were then dissected, and mounted in Vectashield (Vector Laboratories) for confocal microscopy, or immunostained for β-gal or Fmi (Usui et al. 1999) before mounting. Larval and early (2-h apf) pupal wing discs were treated similarly, except that formaldehyde fixation was done for 10 min.
Fmi mutant wings
Fmi mutant wings expressing Dsh::GFP were of the genotype fmiE45 GAL4_–_1407 / fmiE59; dsh::GFPIII / UAS-fmi. GAL4_–_1407; UAS-fmi drives fmi expression in the CNS, rescuing lethality, but not in the imaginal discs, thereby allowing development of mutant wing tissue (Usui et al. 1999). Wings in these animals are likely to be null for fmi, because no detectable expression of UAS–GFP can be detected from the neuron-specific GAL4_–_1407 driver.
Clonal analysis
Dsh::GFPII was recombined onto a second chromosome carrying FRTG13. Flies of the genotype hs-FLP / +; FRTG13, dsh::GFPII / FRTG13, arm-lacZ were heat shocked at 37°C for 2 h in late third instar to induce clones. Pupal wings were prepared as described earlier, and stained for βgal. Clones lacking Dsh::GFP expressed two copies of βgal.
Western blot
Wings, legs, and a small portion of the body wall of 30-h pupae were dissected and ground in reducing SDS gel loading buffer containing protease inhibitors, boiled for 5 min, and pelleted, and the supernatant was subjected to electrophoresis and blotting. The blot was probed with a rabbit anti-Dsh antibody (Nusse).
Other genotypes
Other genotypes were as follows:
y w dsh1; dsh1::GFPII / +
dsh::GFPII / +; dpp-GAL4 UAS-fz / +
dsh::GFPII / UAS-lacZ; dpp-GAL4 UAS-fz / +
dsh::GFPII / +; fzR52 / fzR52 or fzJ22 / fzJ22
dsh::GFPII / +; mwh3 / mwh1
ptc-GAL4 / UAS-Dsh(Δ_bPDZ); dsh::GFPIII_
ptc-GAL4 / UAS-Dsh(Δ_DEP_+); dsh::GFPIII
en-GAL4 / UAS-nkd 3–2; dsh::GFPIII
Acknowledgments
I thank members of my lab, as well as S. Eaton, C. Logan, H. McNeill, E. Rulifson, J. Shulman, and K. Wharton for discussions and technical help. Special thanks to T. Uemura for reagents and for openly discussing data before publication, and to D. Ma for help with Western blotting. This work was supported in part by DRS-16 of the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation, and by grants from the HHMI and NIH (R01GM59823–01).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.890501.
References
- Adler PN, Charlton J, Jones KH, Liu J. The cold-sensitive period for frizzled in the development of wing hair polarity ends prior to the start of hair morphogenesis. Mech Dev. 1994a;46:101–107. doi: 10.1016/0925-4773(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Adler PN, Charlton J, Park WJ. The Drosophila tissue polarity gene inturned functions prior to wing hair morphogenesis in the regulation of hair polarity and number. Genetics. 1994b;137:829–836. doi: 10.1093/genetics/137.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–949. doi: 10.1016/s0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the Planar Cell Polarity and Wingless signaling pathways. Genes & Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KM. frizzled and frizzled 2 play a partially redundant role in wingless signaling and have similar requirements to wingless in neurogenesis. Cell. 1998;95:1027–1036. doi: 10.1016/s0092-8674(00)81726-2. [DOI] [PubMed] [Google Scholar]
- Boutros M, Mihaly J, Bouwmeester T, Mlodzik M. Signaling specificity by Frizzled receptors in Drosophila. Science. 2000;288:1825–1828. doi: 10.1126/science.288.5472.1825. [DOI] [PubMed] [Google Scholar]
- Chae J, Kim MJ, Goo JH, Collier S, Gubb D, Charlton J, Adler PN, Park WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–5429. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- Jones KH, Liu J, Adler PN. Molecular analysis of EMS-induced frizzled mutations in Drosophila melanogaster. Genetics. 1996;142:205–215. doi: 10.1093/genetics/142.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell JR, Carthew RW. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes & Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol. 1999;146:427–437. doi: 10.1083/jcb.146.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer J, Klingensmith J, Perrimon N, Nusse R. dishevelled and armadillo act in the wingless signalling pathway in Drosophila. Nature. 1994;367:80–83. doi: 10.1038/367080a0. [DOI] [PubMed] [Google Scholar]
- Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Mahowald AP. Multiple functions of segment polarity genes in Drosophila. Dev Biol. 1987;119:587–600. doi: 10.1016/0012-1606(87)90061-3. [DOI] [PubMed] [Google Scholar]
- Phillips RG, Whittle JRS. wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing dsic development. Development. 1993;118:427–438. doi: 10.1242/dev.118.2.427. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset R, Mack JA, Wharton KA, Jr, Axelrod JD, Cadigan K, Fish MP, Nusse R, Scott MP. naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes & Dev. 2001;15:658–671. doi: 10.1101/gad.869201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Wu CH, Nusse R. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol Cell. 2000;6:117–126. [PubMed] [Google Scholar]
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, Perrimon N, Axelrod JD. Frizzled signaling and the developmental control of cell polarity. Trends in Genetics. 1998;14:452–458. doi: 10.1016/s0168-9525(98)01584-4. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Wilder EL, Perrimon N. Components of wingless signalling in Drosophila. Nature. 1994;367:76–80. doi: 10.1038/367076a0. [DOI] [PubMed] [Google Scholar]
- Steitz SA, Tsang M, Sussman DJ. Wnt-mediated relocalization of dishevelled proteins. In Vitro Cell Dev Biol Anim. 1996;32:441–445. doi: 10.1007/BF02723007. [DOI] [PubMed] [Google Scholar]
- Strutt DI. Asymmetric localization of Frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: Regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Thiesen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Torres MA, Nelson WJ. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J Cell Biol. 2000;149:1433–1442. doi: 10.1083/jcb.149.7.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, Shi DL. The C-terminal cytoplasmic lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/β-catenin signalling. EMBO J. 2000;19:4944–4954. doi: 10.1093/emboj/19.18.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing hairs. J Cell Biol. 1993;123:209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes & Dev. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J, Miller JR, Brown JD, Lai C-J, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]