PI-103 and Sorafenib Inhibit Hepatocellular Carcinoma Cell Proliferation by Blocking Ras/Raf/MAPK and PI3K/AKT/mTOR Pathways (original) (raw)
. Author manuscript; available in PMC: 2011 Jul 22.
Published in final edited form as: Anticancer Res. 2010 Dec;30(12):4951–4958.
Abstract
Background
Aberrant Ras/Raf/MAPK and PI3K/AKT/mTOR signaling pathways are found in hepatocellular carcinoma (HCC). This study reports how sorafenib (a multi-kinase inhibitor) and PI-103 (a dual PI3K/mTOR inhibitor) alone and in combination inhibit the proliferation of the HCC cell line, Huh7.
Materials and Methods
Huh7 proliferation was assayed by 3H-thymidine incorporation and by MTT assay. Western blot was used to detect phosphorylation of the key enzymes in the Ras/Raf and PI3K pathways.
Results
Sorafenib and PI-103, as single agents, inhibited Huh7 proliferation and epidermal growth factor (EGF)-stimulated Huh7 proliferation in a dose-dependent fashion; the combination of sorafenib and PI-103 produced synergistic effects. EGF increased phosphorylation of MEK and ERK, key Ras/Raf downstream signaling proteins; this activation was inhibited by sorafenib. However, sorafenib, as a single agent, increased AKT (Ser473) and mTOR phosphorylation. EGF-stimulated activation of PI3K/AKT/mTOR pathway components was inhibited by PI-103. PI-103 is a potent inhibitor of AKT (Ser473) phosphorylation; in contrast, rapamycin stimulated AKT(Ser473) phosphorylation. It was found that PI-103, as a single agent, stimulated MEK and ERK phosphorylation. However, the combination of sorafenib and PI-103 caused inhibition of all the tested kinases in the Ras/Raf and PI3K pathways.
Conclusion
The combination of sorafenib and PI-103 can significantly inhibit EGF-stimulated Huh7 proliferation by blocking both Ras/Raf/MAPK and PI3K/AKT/mTOR pathways.
Keywords: Epidermal growth factor, PI-103, rapamycin, mTOR complex 1, mTOR complex 2, negative feedback loop
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and represents the seventh most common cause of cancer-related deaths worldwide (1). The incidence of HCC is increasing in the USA. From the late 1970s to the late 1990s, HCC increased from 1.4 to 3.0 cases per 100,000 persons per year, and it is estimated that the incidence will continue to increase over the next two decades, primarily due to an increased incidence of hepatitis C (1;2).
Recent publications indicate that HCC cell activation by different factors is known to increase Ras/Raf/MAPK and PI3K/AKT/mTOR signaling. The Ras/Raf/MAPK pathway is activated in the majority of advanced stage of HCC as a result of increased signaling induced from upstream growth factors such as epidermal growth factor (EGF), hepatocyte growth factor (HGF) or insulin-like growth factor, and also because of inactivation of tumor suppressor genes (3). The PI3K/AKT/mTOR signaling pathway plays a pivotal role in HCC and is activated in 30–50% of HCC cases. The ribosomal protein S6 (RPS6), a target of p70S6K which is downstream of mTOR signaling, is aberrantly activated in 50% of HCC cases (4). The etiology of HCC tumorigenesis and recurrence is currently poorly understood. There is an urgent need to find an effective regimen to treat HCC and to prevent tumor recurrence.
The novel anticancer agent sorafenib is a multi-kinase inhibitor of more than a dozen kinases at nanomolar potency, including serine/threonine kinases c-Raf and B-Raf, the receptor tyrosine kinases VEGFR2, VEGFR3, platelet derived growth factor receptor (PDGFR), FLT3, Ret and c-kit (5). Sorafenib has been shown to inhibit tumor cell proliferation by blocking the Ras/Raf/MAPK pathway and to inhibit angiogenesis by blocking VEGFR and PDGFR signaling. A recent clinical trial describing the treatment of advanced HCC using sorafenib demonstrated promising results (6). However, sorafenib does not directly inhibit the PI3K/AKT/mTOR pathway which plays an important role in HCC proliferation. It has been previously demonstrated that sorafenib can even increase phosphorylation of mTOR targets (S6K and 4EBP1) (7). To overcome this problem, sirolimus has been used in combination with sorafenib to target the PI3K/AKT/mTOR pathway. PI-103, a dual PI3K/mTOR inhibitor, has been used in pre-clinical studies and has demonstrated promising inhibitory results for various cancers (8;9).
The aim of this study was to evaluate the anti-tumor effect of sorafenib and PI-103, as single agents and in combination, on HCC proliferation and to determine their effect on Ras/Raf/MAPK and PI3K/AKT/mTOR signaling.
Materials and Methods
Cell culture
The human HCC cell line, Huh7, was cultured in DMEM (Cat# 12100-046) medium (Invitrogen, Carlsbad, CA, USA) +10% heat inactivated fetal bovine serum (FBS) in a 37°C incubator with 5% CO2 in the air.
Chemicals and antibodies
Sorafenib p-Toluenesulfonate salt (sorafenib tosylate, purity >99%) was obtained from LC Laboratories, Woburn, MA, USA), PI-103 (purity>98%) from Cayman Chemicals (Ann Arbor, MI, USA), methyl-3H-thymidine (2Ci/mM) from MP Biomedicals (Costa mesa, CA, USA) and human EGF from Insight Genomics (Falls Church, VA, USA). Primary antibodies against AKT, phospho-AKT (Ser473), mTOR, phosphor-mTOR (Ser2448), ERK1/2, phosphor-ERK1/2(Thr 202/204), MEK1/2, phosphor-MEK1/2 (Ser 217/221), p70-S6K, phospho-p70-S6K (Thr389) and secondary goat anti-rabbit- HRP conjugated antibody were all obtained from Cell Signaling Technology (Danvers, MA, USA). Positive and negative controls for phosphor-MEK1/2 and phosphor-ERK1/2, protein markers and LumiGlo reagent A/B were also obtained from Cell Signaling Technology. Protease and phosphatase inhibitor cocktail and Restore-Plus Stripping Buffer for Western blot were obtained from Pierce (Rockford, IL, USA). Thiazolyl blue tetrazolium bromide (MTT), GW5074, LY294002, U0126, mouse anti-β-actin antibody, rabbit anti-mouse antibody conjugated with HRP and all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
3H-thymidine incorporation assay
Huh7 cells were plated in 96 well plates at 1,000 cells/well in 0.2 ml DMEM +10% FBS and treated with various concentrations of the sorafenib and PI-103, as single agents or in combination, and cultured for 72 h. Cells were pulsed with methyl-3H-thymidine (from MP, 2 Ci/mM) for 4h at 1 μCi/well. After aspiration with a fine gel loading tip, the wells were washed once with 0.35 ml of serum-free DMEM, and the cells were fixed in 0.3 ml of 10% Trichloroacetic Acid (TCA) for 12 min. After completely aspirating the TCA, the cells were lysed in 100 μl of 0.2M NaOH/0.2% SDS for 40 min. The cell lysates were transferred to scintillation vials; the wells were washed with 0.2 ml of scintillation cocktail (RPI, Mount Prospect, IL, USA) and the mixture was transferred to the scintillation vials. After addition of 3 ml of the scintillation mixture into each vial, the vials were mixed by hand shaking. 3H-thymidine incorporation was measured by scintillation counting in a Packard Scintillation Analyzer (Covina, USA) (model TRI-CARB 2100TR) using a previously published technique with modification in sample volume (10).
MTT assay
Huh7 cells were plated in 96 well plates at 5,000 cells/well (n=12) in 100 μl of DMEM + 10% FBS and cultured for 24 h. The cells were then cultured in 1% FBS or in 10% FBS in DMEM for 24 h. EGF, sorafenib or PI-103 was then added to the cells and cultured for another 48 h. Carrier DMSO was added in the zero controls (<0.1% final concentration). Ten μl of MTT (5mg/ml) were added to the wells and incubated for 4 h. After aspiration, 100 μl of 20% SDS/50% N,N-Dimethylformamide (DMF) was added to each well and incubated for 2 h at 37°C to solubilize the bio-reduced colored MTT-formazan and to lyse the cells. The optical density was read at 570 nm in a microplate reader. This method is according to instructions from the manufacturer (Insight Genomics, Falls Church, VA, USA) with minor-modification in cell-lysis time from overnight to 4h at 37°C..
Western blot analysis
Huh7 cells were cultured in DMEM + 10% FBS in 100×20 mm tissue culture dishes until ~70% confluence. The cells were treated with sorafenib (4 μM), PI-103(2 μM), rapamycin (20 nM), GW5074 (10 μM), LY294002 (10 μM) or U0126 (10 μM) for 1 h, then treated with EGF for 15 min. Carrier DMSO was added in the zero controls (<0.1% final concentration). After washing three times in cold PBS, the cells were lysed in 1 ml of radio-immunoprecipitation assay (RIPA) buffer (25 mM Tris, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) with addition of 1× protease and phosphatase inhibitor cocktail (Pierce, Rockford, IL) plus 2 μg/ml of pepstatin A (Sigma-Aldrich, St. Louis, MO, USA) on ice for 5 min. The cell lysates were collected in microcentrifuge tubes and centrifuged for 15 min at 15,000 rpm and 4°C. The supernatants were transferred to clean microcentrifuge tubes and kept at−80°C until use. Sample protein concentrations were determined by Coomassie blue protein assay reagent (Pierce, Rockford, I, USAL). Protein (60 μg in each sample) was mixed with equal volume of 2× loading buffer, heated at 100°C for 3 min and then cooled on ice for 3 min before loading to the gel. The protein was separated on 7.5% SDS-polyacrylamide gel and then transferred onto a nitrocellulose membrane. The membrane was blocked with 5% fat-free milk. The primary antibody (1:2000 dilution) incubation was performed overnight at 4°C and the secondary antibody incubation (1:2000 dilution) was performed for 1 h at room temperature. The protein bands were detected by a chemiluminescence staining with LumiGlo reagent A/B (Cell Signaling Technology, Danvers, MA, USA). The membrane was exposed to Kodak scientific imaging film (VWR, Batavia, I, USAL). β-actin was used as an internal control to normalize for loading equality.
Statistical analysis
All analyses were performed using the software SPSS version 16. Data are presented as mean ± standard error of the mean. One-way ANOVA followed by Tukey correction was used to compare means. The level of statistical significance was set at p<0.05.
Results
Sorafenib and PI-103 as single agents or in combination inhibited Huh7 proliferation in a dose-dependent fashion
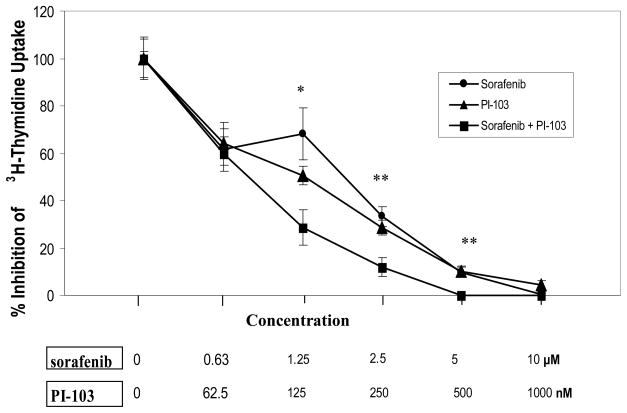
Sorafenib and PI-103, as single agents, inhibited Huh7 3H-thymidine incorporation in a dose dependent fashion. Combination of sorafenib and PI-103 caused synergistic inhibition of Huh7 3H-thymidine incorporation. This effect was noted to be significantly different from each single agent treatment at sorafenib concentrations of 1.25, 2.5 and 5 μM and PI-103 concentrations of 125, 250 and 500 nM (p<0.05, n=4) (Figure 1). The experiments were performed twice with similar results.
Figure 1. Effects of Sorafenib and PI-103 on inhibition of 3H-thymidine incorporation in Huh7 cells.
The Huh7 cells were plated to 96 well plates at 1000 cells/well in 0.2 ml DMEM +10% FBS with quadruplicate repeats (n=4). The cells were treated with various concentrations of the drugs (as indicated) and cultured for 72h. Carrier DMSO (<0.1% final concentration) was added to the zero controls. The cells were pulsed with methyl-3H-thymidine (specificity 2 Ci/mM) for 4h at 1 μCi/well. 3H-thymidine incorporation was measured by scintillation counting in a Packard Scintillation Analyzer (model TRI-CARB 2100TR). (*: drug combination vs mono-drug, p<0.05; **: drug combination vs mono-drug, p<0.01).
EGF-stimulated Huh7 proliferation in DMEM +1% FBS or in DMEM +10% FBS was synergistically and significantly inhibited by sorafenib and PI-103
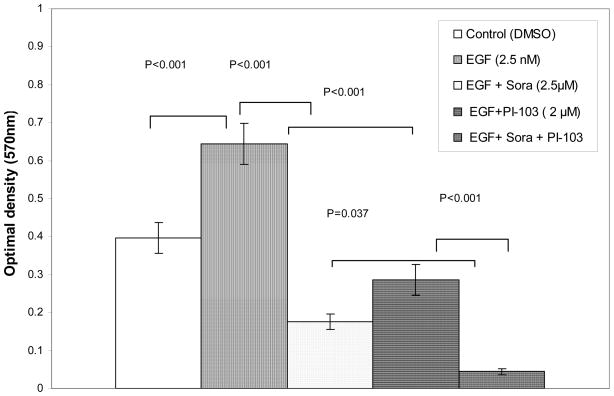
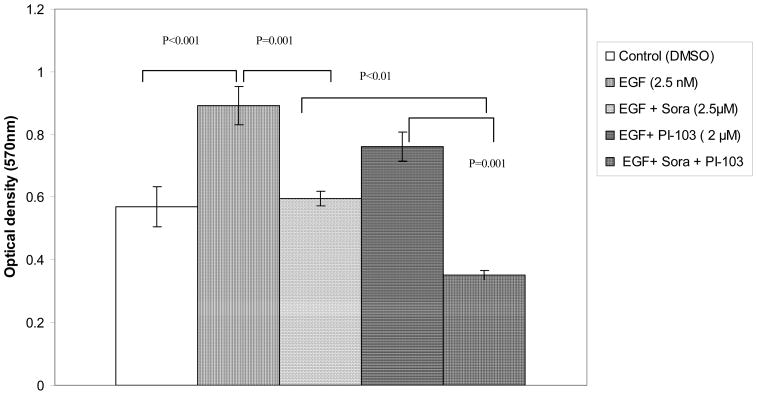
Since 3H-thymidine incorporation detects DNA synthesis in a short time period, the MTT assay was used to determine if sorafenib and PI-103 can inhibit Huh7 proliferation over a longer period of treatment. At first, an EGF concentration titration was performed to determine the optimum stimulating concentration of EGF on Huh7 proliferation cultured in DMEM +1% or 10% FBS. It was found that EGF (2.5 nM) is able to optimally stimulate Huh7 proliferation in both culture conditions. When the cells were cultured in 1% FBS, EGF (2.5 nM) stimulated Huh7 proliferation 63% as compared to control (_p_=0.001; n=12). The EGF-stimulated Huh7 proliferation was inhibited 73% (p<0.001; n=12) by sorafenib (at 2.5 μM). PI-103 (2 μM) inhibited EGF-stimulated Huh7 proliferation 56% (p<0.001; n=12). Combination of the two drugs synergistically inhibited EGF-stimulated Huh7 proliferation by 93% (p<0.001; n=12). The effect of the drug combination was significantly different from the inhibitory effect of sorafenib (_p_=0.037) or PI-103 (p<0.001). When the cells were culture in 10% FBS, EGF (2.5 nM) stimulated Huh7 proliferation 36% (p<0.001; n=12). The EGF-stimulated Huh7 proliferation was inhibited 33% by sorafenib (at 2.5 μM) (_p_=0.001; n=12). Although PI-103 alone (2 μM) did not inhibit EGF-stimulated Huh7 proliferation significantly in 10% FBS; the combination of sorafenib and PI-103 synergistically inhibited EGF-stimulated Huh7 proliferation 61% (p<0.001; n=12). The effect of combination of the two drugs was significantly different from the inhibitory effect of sorafenib (_p_=0.01) or PI-103 (_p_=0.001). (Figures 2A, B). The experiments were repeated with similar results.
Figure 2. Sorafenib and PI-103 inhibited EGF stimulated Huh7 proliferation.
(A) Cells cultured in 1% FBS starvation condition. Huh7 cells were plated in 96 well plate at 5,000 cells/well (n=12) in 100 μl of DMEM+10%FBS and cultured for 24 h. The cells were starved for 24 h in DMEM +1% FBS. Then the cells were cultured in 1% FBS in DMEM for 48h with DMSO (0.017%, control), EGF and inhibitors at the indicated concentration. MTT assay was performed as indicated in the Materials and Methods. (B) Cells cultured in 10% FBS. Huh7 cells were plated in 96 well plate at 5,000 cells/well (n=12) in 100 μl of DMEM+10% FBS and cultured for 24h. EGF and inhibitors were added for another 48 h. MTT assay was performed as indicated in the Materials and Methods.
The combination of sorafenib and PI-103 inhibited multiple key enzymes in the Ras/Raf/MAPK and PI3K/AKT/mTOR signaling pathways
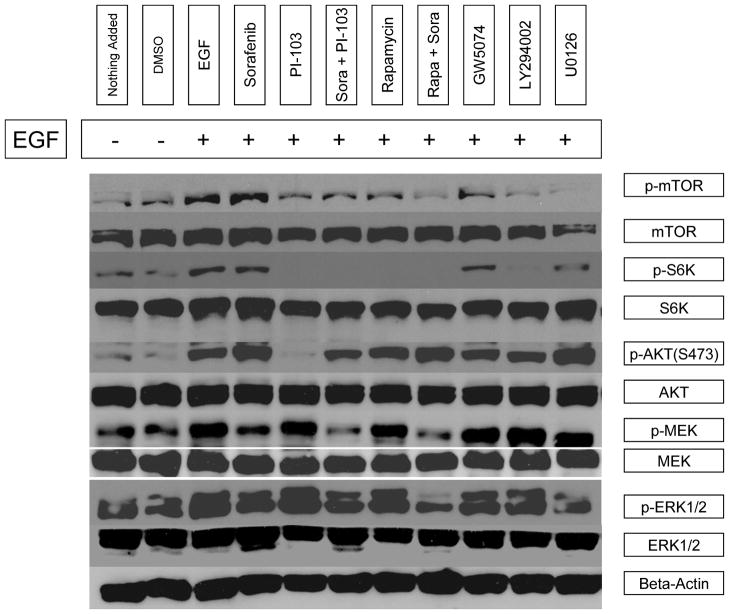
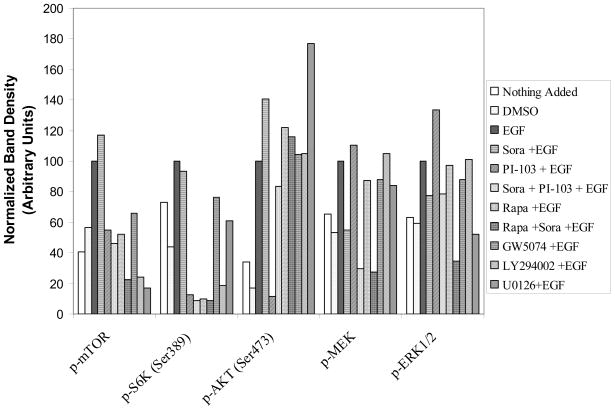
In comparison with DMSO control, EGF stimulated mTOR(Ser2448), S6K(Thr389), AKT(Ser473), MEK1/2(Ser217/221) and ERK1/2(Thr202/204) phosphorylation by 43%, 56%, 83%, 47% and 41%, respectively. Sorafenib inhibited EGF-stimulated MEK1/2 and ERK1/2 phosphorylation by 45% and 22%, respectively. However, sorafenib, as a single agent, only slightly inhibited S6K phosphorylation (7%) but did not inhibit EGF-stimulated mTOR, and AKT phosphorylation in the PI3K/AKT/mTOR signaling pathway. Interestingly, it was found that sorafenib stimulated mTOR and AKT phosphorylation by 17% and 41%, respectively. PI-103, as a single agent, inhibited EGF stimulated mTOR(Ser2448), S6K(Thr389) and AKT(Ser473) phosphorylation by 45%, 87% and 88%, respectively, thus indicating PI-103 inhibited both mTORC1 and mTORC2 activity. Rapamycin, as a single agent, inhibited mTOR(Ser2448), and S6K(Thr389) phosphorylation 48% and 90%, but rapamycin did not inhibit EGF-stimulated AKT(Ser473) phosphorylation. Rapamycin increased mTORC2 substrate AKT(Ser473) phosphorylation by 22%. PI-103, as a single agent, did not inhibit EGF-stimulated Ras/Raf/MAPK pathway and was found to increase MEK1/2(Ser217/221) and ERK1/2(Thr202/204) phosphorylation by 11% and 33%. The combination of sorafenib and PI-103 strongly inhibited both Ras/Raf/MAPK and PI3K/AKT/mTOR signaling pathways. The EGF-stimulated mTOR(Ser2448), S6K(Thr389), AKT(Ser473), MEK1/2(Ser217/221) and ERK1/2(Thr202/204) phosphorylation was inhibited by 54%, 91%, 17%, 70% and 21%, respectively (Figure 3A, B). These findings indicate that combination treatment with sorafenib and PI-103 has a significant advantage over single agent therapy at specific concentrations by preventing increased activity of non-targeted pathways.
Figure 3.
Sorafenib and PI-103 differentially inhibited or activated phosphorylation of several key enzymes in the Ras/Raf/MAPK and PI3K/Akt/mTOR pathways. (A) Western blot for p-mTOR (Ser2448), p-S6K (Thr389), p-AKT (Ser473), p-MEK1/2(Ser217/221) and p-ERK1/2(Thr202/204) level after different treatments. Also shown are endogenous mTOR, S6K, AKT, MEK1/2 and ERK1/2. Positive and negative controls for p-MEK and for p-ERK were ordered from Cell Signaling Technology (Danvers, MA) and included in the experiments but are not shown. Phosphorylated kinase band densities were assessed by Scion Image software and normalized by β-actin. (B) A plot generated based on β-actin. inormalized phosphorylated kinase band densities in Figure 4A.
Discussion
The 3H-thymidine incorporation and MTT assay experiments performed in this study indicated that sorafenib and PI-103, as single agents, are able to attenuate HCC cell proliferation. Sorafenib and PI-103, in combination, resulted in synergistic inhibition of HCC cell proliferation when cells were cultured in 1% or 10% FBS. Using 1% FBS allows the cells to reach a quiescent state after 24 h of starvation, so that EGF stimulation will be more prominent. At the same time, this condition mimics the limited nutrition from poor blood supply in tumor cells. The synergistic inhibition by the combination treatment indicates that the Ras/Raf/MAPK and PI3K/AKT/mTOR pathways play an important role in HCC cell proliferation. This was confirmed by Western blot experiments showing inhibition of key enzymes in these two pathways. It was found that EGF stimulates MEK and ERK phosphorylation in the Ras/Raf/MAPK pathway and enhanced mTOR, AKT and S6K phosphorylation in the PI3K/AKT/mTOR pathway, in agreement with previously published studies (10;11). PI-103 mono-therapy strongly inhibited EGF stimulated mTOR, S6K and AKT (Ser473) phosphorylation. In contrast, rapamycin only inhibited mTOR and S6K phosphorylation, but stimulated AKT (Ser473) phosphorylation.
S6K phosphorylation is a marker of mTOR complex 1 (mTORC1) activity, while AKT(Ser473) phosphorylation is a marker of mTOR complex 2 (mTORC2) activity (12). A different inhibition pattern was demonstrated between PI-103 and rapamycin. PI-103 inhibited both mTORC1 and mTORC2; however, rapamycin only inhibited mTORC1. Not surprisingly, PI-103 mono-therapy stimulated MEK and ERK phosphorylation. This may be explained by the fact that inhibition of mTORC1 can eliminate the S6K mediated negative feedback inhibitory loop, causing activation of Ras/Raf/MAPK pathway (13). Sorafenib mono-therapy inhibited EGF stimulated MEK and ERK phosphorylation, but interestingly it stimulated mTOR and AKT (Ser473) phosphorylation and had almost no effect on EGF stimulated S6K phosphorylation. It is hypothesized that sorafenib may induce other signals, such as HGF/HGFR, that further stimulates the EGF stimulated PI3K/AKT/mTOR pathway. Indeed, sorafenib stimulated HGF secretion in HCC cells and promoted c-Met, S6K and 4EBP1 phosphorylation (7). Increased HCC activation and mobility by HGF was reported to be mediated through PI3K signaling(14). Sorafenib, Rapamycin, MEK inhibitor U0126, Raf inhibitor GW5074 and PI3K inhibitor LY294002 in mono-therapy all enhanced EGF stimulated AKT (Ser473) phosphorylation; PI-103 was the only drug that caused near complete inhibition of EGF stimulated AKT (Ser473) phosphorylation. Since AKT plays an important role in tumor cell survival, AKT may be a target in HCC therapy.
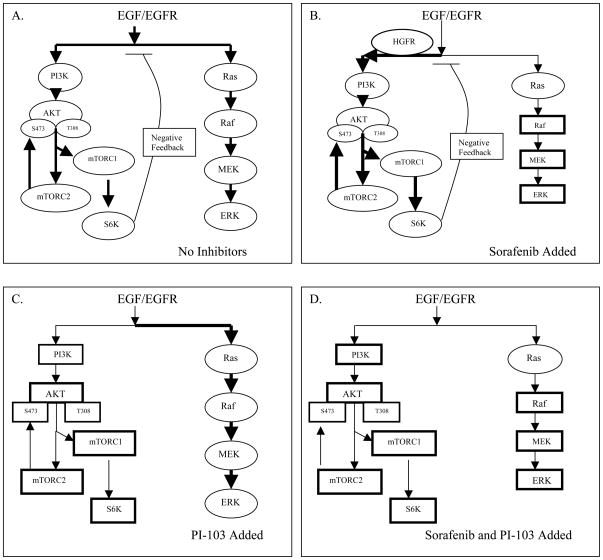
Sorafenib or PI-103 mono-therapy induced differential inhibition and activation of the key kinases in the two pathways, which underscores the importance of using sorafenib and PI-103 in combination. Combination treatment inhibited multiple kinases in the two pathways and caused synergistic inhibition of cell proliferation. Figure 4 summarizes the possible mechanisms of sorafenib and PI-103 on inhibition of EGF stimulated Ras/Raf/MAPK and PI3K/AKT/mTOR pathways.
Figure 4.
Possible mechanisms that sorafenib and PI-103 differentially inhibits and activates key kinases in the Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. (A) In the absence of added inhibitors, EGF/EGFR signaling activates Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Activation of S6K in the PI3K/AKT/mTOR pathway may induce feedback inhibition on Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. (B) When sorafenib is added, sorafenib directly or indirectly inhibits Raf, MEK and ERK (shown by rectangles). Sorafenib may activate HGF/HGFR signaling via activation of PI3K, thus further activating AKT, mTOC1 and mTORC2 and S6K based on the activated levels after EGF stimulation (shown by ovals). Activation of S6K still causes feedback inhibition on both pathways. (C) Addition of PI-103 directly inhibits PI3K, mTORC1 and mTORC2. PI-103 indirectly inhibits AKT and S6K in the PI3K/AKT/mTOR pathway (shown by rectangles). Inhibition of S6K eliminates the feedback inhibition effect of S6K, thus increasing the signal towards the Ras/Raf/MAPK pathway and further stimulated the Ras, Raf, MEK and ERK phosphorylation based on the activated levels after EGF stimulation (shown by ovals). (D) Combination of sorafenib and PI-103 blocks EGF stimulated Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Therefore the combination of sorafenib and PI-103 may have an advantage over mono-drug treatment in HCC therapy.
Recent studies have found that the Ras/Raf/MAPK and PI3K/AKT/mTOR are two activated pathways in HCC cells (11). Several attempts have been made to target these pathways as therapy. Villanueva et al. used a rapamycin analogue, everolimus, and an EGF/VEGF inhibitor, AEE788, to block these two pathways in the Huh7 HCC cell line, which resulted in the inhibition of mTOR signaling in vitro and decreased tumor progression and increased survival in xenograft model. The combination of everolimus and AEE788 enhanced tumor suppression as compared to everolimus mono-therapy (15). Newell et al. used sorafenib and rapamycin to target mTOR and Ras/Raf/MAPK signaling and decreased proliferation and induced apoptosis in HCC cell lines. In a xenograft mouse model, the combination of rapamycin and sorafenib enhanced tumor necrosis and ulceration when compared with sorafenib alone (10). The use of sorafenib in advanced HCC has become a common practice. Llovet et al.(6) demonstrated that this multi-kinase inhibitor has a significant impact on tumor progression and patient survival.
The use of mTOR inhibitors such as rapamycin to treat HCC is still a matter of debate and faces many challenges. A recent publication indicated that prolonged treatment with rapamycin is able to inhibit both mTORC1 and mTORC2 in several cancer cell lines, however, no inhibition of mTORC2 in HCC cell line was reported (16). Rapamycin disrupts the assembly of mTORC1 but has no effect on mTORC2 conformation. Because of this, more mTOR units are available to bind rictor and mSin1 to generate more mTORC2. mTORC2 phosphorylates AKT at Ser473 and promotes tumor cell survival by inducing resistance to contemporary mTOR inhibitors(17;18). Inhibition of mTORC1 by rapamycin can eliminate S6K mediated negative feedback inhibitory loop with subsequent activation of upstream PI3K and Ras/Raf/MAPK pathways (13;19). An agent that is able to inhibit both mTORC1 and mTORC2, and also block upstream of mTOR, will result in a stronger inhibition of the PI3K/AKT/mTOR pathway. The novel drug PI-103 is a chemically synthesized small molecule that exhibits potent dual inhibition of PI3K and mTOR (mTORC1 and mTORC2). It is highly bio-available and with low toxicity to normal cells (8). PI-103 has been used in preclinical studies to inhibit several cancer types, including glioma, leukemia, and drug resistant malignant peripheral nerve sheath tumors (18;20). PI-103 has not been used in HCC preclinical or clinical studies to date. Based on these observations, this in vitro study used a novel combination of sorafenib and PI-103 on the inhibition of HCC cell line Huh7. Future investigations will be focused on in vivo xenograft model to translate these in vitro results.
In conclusion sorafenib and PI-103 are potent anti-HCC drugs; their application in combination has significant advantages compared with mono-drug therapy in the inhibition of HCC pivotal pathways Ras/Raf/MAPK and PI3K/AKT/mTOR.
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2005;128:1752–1764. doi: 10.1053/j.gastro.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today (Barc ) 2005;41:773–784. doi: 10.1358/dot.2005.41.12.937959. [DOI] [PubMed] [Google Scholar]
- 4.Minguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol. 2009;25:186–194. doi: 10.1097/MOG.0b013e32832962a1. [DOI] [PubMed] [Google Scholar]
- 5.Chaparro M, Gonzalez ML, Trapero-Marugan M, Medina J, Moreno-Otero R. Review article: pharmacological therapy for hepatocellular carcinoma with sorafenib and other oral agents. Aliment Pharmacol Ther. 2008;28:1269–1277. doi: 10.1111/j.1365-2036.2008.03857.x. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Huynh H, Ngo VC, Koong HN, Poon D, Choo SP, Thng CH, Chow P, Ong HS, Chung A, Soo KC. Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Cell Mol Med. 2009;13:2673–2683. doi: 10.1111/j.1582-4934.2009.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kojima K, Shimanuki M, Shikami M, Samudio IJ, Ruvolo V, Corn P, Hanaoka N, Konopleva M, Andreeff M, Nakakuma H. The dual PI3 kinase/mTOR inhibitor PI-103 prevents p53 induction by Mdm2 inhibition but enhances p53-mediated mitochondrial apoptosis in p53 wild-type AML. Leukemia. 2008;22:1728–1736. doi: 10.1038/leu.2008.158. [DOI] [PubMed] [Google Scholar]
- 10.Newell P, Toffanin S, Villanueva A, Chiang DY, Minguez B, Cabellos L, Savic R, Hoshida Y, Lim KH, Melgar-Lesmes P, Yea S, Peix J, Deniz K, Fiel MI, Thung S, Alsinet C, Tovar V, Mazzaferro V, Bruix J, Roayaie S, Schwartz M, Friedman SL, Llovet JM. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol. 2009;51:725–733. doi: 10.1016/j.jhep.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu K, Shi C, Toral-Barza L, Lucas J, Shor B, Kim JE, Zhang WG, Mahoney R, Gaydos C, Tardio L, Kim SK, Conant R, Curran K, Kaplan J, Verheijen J, yral-Kaloustian S, Mansour TS, Abraham RT, Zask A, Gibbons JJ. Beyond rapalog therapy: preclinical pharmacology and antitumor activity of WYE-125132, an ATP-competitive and specific inhibitor of mTORC1 and mTORC2. Cancer Res. 2010;70:621–631. doi: 10.1158/0008-5472.CAN-09-2340. [DOI] [PubMed] [Google Scholar]
- 13.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi K, Fujimoto J, Ueki T, Kishimoto K, Hashimoto-Tamaoki T, Furuyama J, Itoh T, Sasaki Y, Okamoto E. Hepatocyte growth factor promotes migration of human hepatocellular carcinoma via phosphatidylinositol 3-kinase. Clin Exp Metastasis. 1999;17:507–514. doi: 10.1023/a:1006685218766. [DOI] [PubMed] [Google Scholar]
- 15.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, Battiston C, Van LS, Fiel MI, Di FA, Hoshida Y, Yea S, Toffanin S, Ramos A, Martignetti JA, Mazzaferro V, Bruix J, Waxman S, Schwartz M, Meyerson M, Friedman SL, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–83. 1983. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 18.Zou CY, Smith KD, Zhu QS, Liu J, McCutcheon IE. Dual targeting of AKT and mammalian target of rapamycin: A potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2009;8:1157–1168. doi: 10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]
- 19.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 20.Fan QW, Cheng CK, Nicolaides TP, Hackett CS, Knight ZA, Shokat KM, Weiss WA. A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67:7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]