The biology of fear- and anxiety-related behaviors (original) (raw)
Show available content in
Abstract
Anxiety is a psychological, physiological, and behavioral state induced in animals and humans by a threat to well-being or survival, either actual or potential. It is characterized by increased arousal, expectancy, autonomic and neuroendocrine activation, and specific behavior patterns. The function of these changes is to facilitate coping with an adverse or unexpected situation. Pathological anxiety interferes with the ability to cope successfully with life challenges. Vulnerability to psychopathology appears to be a consequence of predisposing factors (or traits), which result from numerous gene-environment interactions during development (particularly during the perinatal period) and experience (life events), in this review, the biology of fear and anxiety will be examined from systemic (brain-behavior relationships, neuronal circuitry, and functional neuroanatomy) and cellular/molecular (neurotransmitters, hormones, and other biochemical factors) points of view, with particular reference to animal models. These models have been instrumental in establishing the biological correlates of fear and anxiety, although the recent development of noninvasive investigation methods in humans, such as the various neuroimaging techniques, certainly opens new avenues of research in this field. Our current knowledge of the biological bases of fear and anxiety is already impressive, and further progress toward models or theories integrating contributions from the medical, biological, and psychological sciences can be expected.
Keywords: anxiety, fear, emotions, animial models, neurobiology, behavior
In a book published in 1878 (Physiologie des passions), Charles Letourneau, who was contemporary with the French neuroanatomist Paul Broca, defined emotions as “passions of a short duration” and described a number of physiological signs and behavioral responses associated with strong emotions.1 Emotions are “intimately linked with organic life,” he said, and either result in an “abnormal excitation of the nervous network,” which induces changes in heart rate and secretions, or interrupt “the normal relationship between the peripheral nervous system and the brain.” Cerebral activity is focused on the source of the emotion; voluntary muscles may become paralyzed and sensory perceptions may be altered, including the feeling of physical pain. This first phase of the emotional response is followed by a reactive phase, where muscles come back into action, but the attention still remains highly focused on the emotional situation. With the knowledge of brain physiology and anatomy that was available at the end of the 19th century, hypotheses on the mechanisms possibly involved in emotions were of course limited. However, Letourneau assumed that “the strong cerebral excitation” that accompanies emotions probably only concerned “certain groups of conscious cells” in the brain and “must necessitate a considerable increase of blood flow in the cell regions involved.”1 He also mentioned that the intensity, the expression, and the pathological consequences of emotions were directly linked to “temperaments” (which he defined within the four classic Hippocratic categories).
It is amazing to see how Letourneau's views on emotions, more than a century ago, were in many ways premonitory. The fact that emotions are “intimately linked with organic life,” his precise description of the sequence of the physiological and behavioral reactions that accompany a strong emotion, such as fear, the idea that emotions involve specific areas of the brain, and the theory that activation of these areas is associated with an increased blood flow have all been largely confirmed by modern neuroscience. The suggestion that temperament or personality traits influence the “affective style” and vulnerability to psychopathology is also an important aspect of our modern approach to anxiety and mood disorders.2
For a long time, emotions were considered to be unique to human beings, and were studied mainly from a philosophical perspective.3 Evolutionary theories and progress in brain and behavioral research, physiology, and psychology have progressively introduced the study of emotions into the field of biology, and understanding the mechanisms, functions, and evolutionary significance of emotional processes is becoming a major goal of modem neuroscience.
Three fundamental aspects of emotions
The modem era of emotion research probably started when it became obvious that emotions are not just “feelings” or mental states, but are accompanied by physiological and behavioral changes that are an integral part of them. This has progressively led to today's view of emotions being experienced or expressed at three different, but closely interrelated levels: the mental or psychological level, the (neuro)physiological level, and the behavioral level. These three complementary aspects are present in even the most basic emotions, such as fear.
A detailed account of the many “theories of emotion” is beyond the scope of this review. However, a brief historical survey of the more biologically oriented ones may help to set some important conceptual issues.3-8
One of the main questions addressed by earlier scientific theories of emotions was whether physiological changes precede the emotional experience, or if they are only a consequence of it. For James (1884) and Lange (1885), “[...] the bodily changes follow directly the perception of the existing fact, and [...] our feelings of the same changes as they occur IS the emotion.” In other words, according to the James-Lange theory of emotions, stimuli reaching the cerebral cortex induce visceral changes, which are then perceived as emotion. Cannon and Bard (1915-1932) criticized this theory and proposed that the neurophysiological aspects of emotions are subcortical and involve the thalamus.9 Stimuli from the environment activate the thalamus, which relays information to the cortex and viscera, and back again to the cortex to generate the “emotional state.” Watson, the father of behaviorism, was also very critical of what he called the “introverted viewpoint” of James' theory. He considered that there were only three types of unlearned emotional responses, which he called “fear,” “rage,” and “love” for convenience, although he wanted to “[...] strip them out of all their old connotations.”10 These three emotional responses can be elicited by three sets of specific stimuli. Thus, a sudden noise or loss of physical support can induce an innate fear reaction, and restraint of bodily movements triggers rage. He also mentioned the fact that these emotional responses can be conditioned and that, although these reactions are usually accompanied by specific behaviors, “[...] visceral and glandular factors predominate.” Papez's (1937) theory of emotions also had a physiological basis. For him, connections between the cerebral hemispheres and the hypothalamus, and between the cerebral hemispheres and the dorsal thalamus mediate emotions. He held the view that emotion implies behavior (expression) and feeling (experience, subjective aspects). Expression depends on the hypothalamus, and experience on the cortex. Although the “circuit of Papez” is still presented as “the emotional brain” in some handbooks, it is clear that many details of his original theory are now outdated. More recently, Schachter (1975) emphasized the importance of cognitive processes: bodily states are interpreted in a cognitive context and are modulated by experience. He also showed that the visceral response appears to be a necessary, although not sufficient, condition for the occurrence of emotion.
The view that there is a limited set of emotions (eg, fear, anger, etc) with specific neurophysiological and neuroanatomical substrates that can be considered as “basic” and serve as the primitive building blocks from which the other, more complex emotions are built, was challenged as late as 1990.11 However, Ekman has convincingly argued that there is now enough evidence of universals in expression and in physiology to suggest a biological basis for these elementary emotions.12 Panksepp added to these arguments by stating that “genetically dictated brain systems that mediate affective-emotional processes do exist, even though there are bound to be semantic ambiguities in how we speak about these systems.”13
The biology of fear and anxiety
Fear versus anxiety: is there a difference?
The main function of fear and anxiety is to act as a signal of danger, threat, or motivational conflict, and to trigger appropriate adaptive responses. For some authors, fear and anxiety are undistinguishable, whereas others believe that they are distinct phenomena.
Ethologists define fear as a motivational state aroused by specific stimuli that give rise to defensive behavior or escape.14 Animals may learn to fear situations in which they have previously been exposed to pain or stress, and subsequently show avoidance behavior when they reencounter that situation. Young animals may show an innate fear reaction to sudden noise or disturbances in the environment, but rapidly become habituated to them. When they are used to a familiar environment, then a fear of novelty may develop. Ethologists have also made the important observation that fear is often mixed up with other aspects of motivation. Thus, conflict between fear and approach behavior may results in displacement activities (eg, self-grooming in rats). Such displacement activities may be the behavioral expression of an anxious state, but anxiety is a concept that is apparently not used by ethologists, perhaps because their definition of fear does in fact include all the more biological aspects of anxiety.
Many authors, however, have argued that differences in their etiologies, response patterns, time courses, and intensities seem to justify a clear distinction between anxiety and fear.15 Although both are alerting signals, they appear to prepare the body for different actions. Anxiety is a generalized response to an unknown threat or internal conflict, whereas fear is focused on known external danger.15 It has been suggested that “[...] anxiety can only be understood by taking into account some of its cognitive aspects, particularly because a basic aspect of anxiety appears to be uncertainty. Also, it is reasonable to conclude that anxiety can be distinguished from fear in that the object of fear is 'real' or 'external' or 'known' or 'objective.' The origins of anxiety are unclear or uncertain [...].”3 Other authors pointed out that “[...] situations lacking in clear indications of situational contingencies or likely outcomes are associated with considerable stress. The uncertainty regarding these situations highlights a lack of control that contributes to feelings of anxiety and makes coping more difficult.”15 Barlow has described anxiety as “[...] a unique and coherent cognitive-affective structure within our defensive and motivational system [...]. At the heart of this structure is a sense of uncontrollability focused largely on possible future threats, danger, or other upcoming potentially negative events, in contrast to fear, where the danger is present and imminent.”16
The fact that anxiety and fear are probably distinct emotional states docs not exclude some overlap in underlying brain and behavioral mechanisms. In fact, anxiety may just be a more elaborate form of fear, which provides the individual with an increased capacity to adapt and plan for the future.16 If this is the case, we can expect that part of the fear-mediating mechanisms elaborated during evolution to protect the individual from an immediate danger have been somehow “recycled” to develop the sophisticated systems required to protect us from more distant or virtual threats.
Defense and coping strategies
Fear or anxiety result in the expression of a range of adaptive or defensive behaviors, which are aimed at escaping from the source of danger or motivational conflict. These behaviors depend on the context and the repertoire of the species. Active coping strategies are used when escape from threat is possible, and the autonomic changes associated with these active strategies are mediated predominantly by sympathetic activation (hypertension, tachycardia). This is the fight-or-flight response originally described by Cannon.17 Passive coping strategies, such as immobilization or freezing, are usually elicited when threat is inescapable, and are usually characterized by autonomic inhibition (hypotension, bradychardia), and a more pronounced increase in the neuroendocrine response (activation of the hypothalamopituitary-adrenal axis and increased glucocorticoid secretion). This type of passive response was originally described by Engel and Schmale as a conservation-withdrawal strategy.18 The concept of alternative (active/passive) strategies itself owes much to the work of Henry and coworkers. 19 Specific brain circuits appear to mediate distinct coping reactions to different types of stressors.20,21
According to Panksepp, flight and other active coping behaviors are unconditional responses to proximate threat, whereas passive coping strategies, such as freezing, are conditioned responses to distal stimuli predictive of danger. These two strategies have distinct and successive roles, and are modulated by the (cognitive) apprehension of the environment and probability of success, eg, whether or not there is a route of escape. Thus, when an animal faces a predator, freezing is preferentially activated when the source of known danger is still far away. When danger gets closer, and the stimulus passes through some critical “psychometric” distance, it becomes a true unconditional stimulus and a flight pattern is activated.22
Defensive behaviors have been studied in a large number of species,23 and it has recently been shown that human defensive behaviors to threat scenarios arc not unlike those seen in nonhuman mammals.24 The importance of risk assessment in making a proper decision about the best strategy to be used in a particular context has been emphasized.25
It should be underlined, however, that the choice between an active or passive defense strategy does not entirely depend on contextual clues. Individual differences in coping styles do exist and may also influence this choice. In a given situation, some individuals may react actively (“proactive” style), whereas other individuals may react in a more passive way (“reactive” style). These coping styles are characterized by consistent behavioral and neuroendocrine patterns, and may explain individual differences in vulnerability to stress-induced diseases.26 Differences in coping styles have also been found between various strains of mice,27 or between genetically selected rat lines,28 which suggests that they have a genetic basis.
The capacity to cope successfully with life challenges, whether innate or acquired, is probably a primary determinant of resistance to stress-induced diseases.29,30
Normal versus pathological anxiety
Although anxiety is a natural adaptive reaction, it can become pathological and interfere with the ability to cope successfully with various challenges and/or stressful events, and even alter body condition (eg, formation of gastric ulcers).
In 1926, following a major flooding disaster in Leningrad, Pavlov reported a state of “chronic inhibition” and learning impairment in the dogs that had been successfully trained for conditioned responses in his laboratory and had directly experienced the flood.31 This observation (which may be one of the first laboratory-based accounts of the symptoms of posttraumatic stress disorder) and other experiments were the basis for his later studies on “experimental neuroses” in dogs. Pavlov discovered large differences in dogs' individual susceptibility to psychopathology, and attributed these differences to “nervous types.” He described four types analogous to the four temperaments of Hippocrates, which, according to him, resulted from the combination of three factors: the “strength” of the nervous system (its degree of resistance to excitation or inhibition), the equilibrium between excitation and inhibition processes, and the capacity to shift from inhibition to excitation and vice versa.32
Although Pavlov's typology is outdated, it is now recognized that increased vulnerability to anxiety and its disorders is associated with particular traits or endophenotypes, ie, traits that may be intermediate in the chain of causality from genes to disease.33 These traits may be innate or acquired during development or through experience.
Barlow has defined three interacting sets of vulnerability factors for the development of human anxiety disorders in humans: (i) a generalized biological vulnerability, mainly of genetic origin; (ii) a generalized psychological vulnerability, resulting in particular from early life experiences; and (iii) a specific psychological vulnerability, focused on particular events or circumstances.16 The latter set is probably implicated in the development of specific anxiety disorders (as opposed to generalized anxiety disorders), ie, social phobia, obsessive-compulsive and panic disorders, and specific phobias.
Increased anxiety in animal models, as a trait, can be attributed to at least two sets of factors: (i) a genetic predisposition, essentially linked to the expression of genes that are involved in the various neurochemical mechanisms underlying fear and anxiety; and (ii) the influence of environmental factors. These environmental factors can interact with the expression of the relevant genes during early development and determine the functional properties of the neural and biochemical systems involved in coping with stressful events. They can also modulate the learning processes that occur at a later stage, when the individual is confronted with various life events, and determine the capacity to cope successfully with aversive or threatening situations in adulthood.
These predisposing factors, either innate or acquired, determine individual “affective styles”2,34 or coping strategies,26 which are thought to play an important role in vulnerability to psychopathology.
Animal models
Some of the neurobiological mechanisms underlying anxiety may already be present in very simple organisms, such as the snail Aplysia, which can show forms of learning akin to anticipatory and chronic anxiety.35 However, most animal models of anxiety are based on the use of mammalian species, particularly rats and mice.36-42 These models fall into two broad categories. In the first one, animals are confronted with situations that generate an anxious state (state anxiety models). This state of anxiety can be either conditioned (eg, conditioned fear, avoidance, and punishment-induced conflict tests) or unconditioned (eg, aversive and ethological conflict tests). In the second category, the models are concerned with trait or “pathological” anxiety: genetic manipulations (transgenic or “knockout” animals) or selective breeding creates lines of rats or mice that permanently express an increased or decreased level of anxiety.
Functional neuroanatomy
As already suspected by Letourneau and others, emotional. experience and the associated behavioral responses are likely to activate specific circuits in the brain. The search for the neuroanatomical substrates of fear and anxiety has been a successful field of research over the last decades.
For a long time, it was assumed that emotions, including fear and anxiety, were almost exclusively generated or processed in a “primitive” part of the brain, ie, the limbic system (“the emotional brain”). The view that emotions and cognitions are separate functions of the brain and must therefore have different underlying neuroanatomical substrates is probably responsible for this simplification. As pointed out by LeDoux in a recent review,43 modern research with the most advanced neuro-imaging technologies still uses this dichotomic approach to higher brain functions as a post hoc explanation: “When a so-called emotional task is used, and a limbic area is activated, the activation is explained by reference to the fact that limbic areas mediate emotions. And when a limbic area is activated in a cognitive task, it is often assumed that there must have been some emotional undertone to the task.” However, neuroanatomical and behavioral data obtained during the last decades clearly indicate that this dichotomy between cognitive and emotional processes is obsolete.
The locus ceruleus and arousal
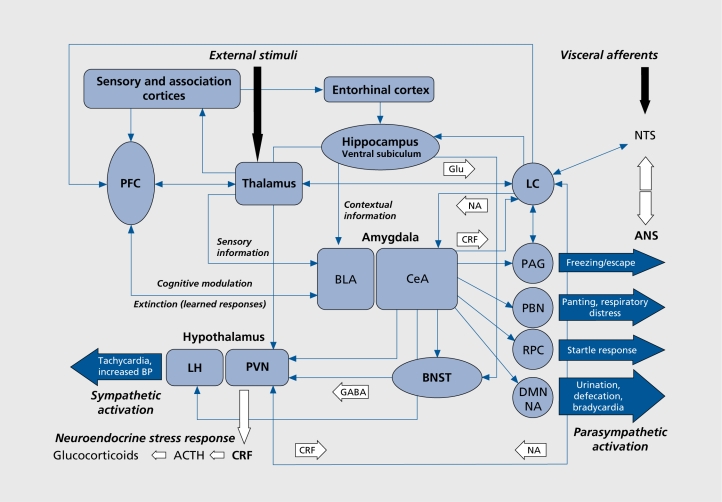
Autonomic activation and increased arousal are among the earlier psychophysiological responses observed in a state of fear or anxiety. Since the immediate consequences of autonomic activation (eg, tachycardia) are perhaps the most readily perceived when experiencing a state of fear or anxiety, it has been proposed that the ascending noradrenergic system originating from the locus ceruleus (LC) is the core around which feelings of anxiety are organized.44 The LC contains a large proportion of the noradrenaline (NA) cell bodies found in the brain and it is a key brain stem region involved in arousal (Figure 1). It is highly responsive to alerting/stressful stimuli. In rats, cats, and monkeys, increased LC neuronal firing rate is associated with alertness, selective attention to meaningful and/or novel stimuli, and vigilance. The meaning, as well as the intensity of stimuli, seems to be an important factor in LC response. In cats, confrontation with a novel, but non-threatening stimulus, such as a mouse, does not cause a specific increase in LC firing, whereas confrontation with a threatening stimulus (eg, a dog) causes a marked increase in LC firing. Thus, novelty by itself is not sufficient to activate the LC/NA system, but stimuli that signal reward, as those that signal danger, may activate the system.45 Recent data suggest that a phasic mode of LC activity may promote focused or selective attention, whereas a tonic mode may produce a state of high behavioral flexibility or scanning attentiveness.46 Some LC neurons project to the paraventricular nucleus (PVN) in the hypothalamus and activate the hypothalamopituitary-adrenocortical (HPA) axis, triggering or facilitating the stress response associated with increased anxiety (Figure 1). However, although 6-hydroxydopamine lesions of the LC in rats affect the HPA axis response to acute stress, they do not appear to substantially affect its response to chronic stress.47 Noradrenergic LC neurons also project to the amygdala (mainly to the central nucleus of the amygdala [CeA]), the prefrontal cortex (PFC), the bed nucleus of the stria terminalis (BNST), the hippocampus, the periaqueductal gray (PAG), the hypothalamus, the thalamus, and the nucleus tractus solitarius (NTS), which arc all areas involved in the fear/anxiety response (Figure 1). The LC is in turn innervated by areas such as the amygdala (which processes fear-related stimuli) and other areas receiving visceral stimuli relayed by the NTS. The LC is therefore in a key position to integrate both external sensory and internal visceral stimuli and influence stress- and fear-related neuroanatomical structures, including cortical areas.48
Figure 1. A schematic view of major brain circuits involved in fear and anxiety. External auditory, visual, olfactory, or somatosensory stimuli are relayed by the thalamus to the amygdala and cortex. The basolateral complex (BLA) of the amygdala is the input side of the system, which also receives contextual information from the hippocampal formation (entorhinal cortex, hippocampus, and ventral subiculum). After intra-amygdala processing of the emotional stimuli, the central nucleus of the amygdala (CeA), on the output side, activates the locus ceruleus (LC) and central and peripheral noradrenaline systems (via corticotropin-releasing factor [CRF] neurons), and the hypothalamus (paraventricular nucleus [PVN] and lateral hypothalamus [LH]). The bed nucleus of the stria terminalis (BNST, part of the “extended amygdala”) is also a control center for the neuroendocrine system by integrating information originating from both the hippocampus and the amygdala. In addition, the CeA directly activates various midbrain regions or nuclei responsible for different aspects of the fear/anxiety response: freezing or escape (periaqueductal gray [PAG]), increased respiratory rate (parabrachial nucleus [PBN]), startle (caudal reticulopontine nucleus of the reticular formation [RPC]), and the dorsal motor nucleus of the vagus (DMN) in the medulla, which (together with the lateral hypothalamus) is responsible for the increase in heart rate and blood pressure associated with emotional events. The prefrontal cortex (PFC) processes more elaborate (“cognitive”) information; it modulates the physiological, neuroendocrine, and behavioral responses (via the amygdala), and it is also involved in the extinction of fear- and anxiety-related conditional responses. ACTH, adrenocorticotropic hormone; ANS, autonomous nervous system; BP, blood pressure; GABA, β-aminobutyric acid; Glu, glutamate; NA, noradrenaline (neurotransmitter) or nucleus ambiguus (structure); NTS, nucleus tractus solitarius.
The septohippocampal system and behavioral inhibition
The inhibition of ongoing behaviors is the first behavioral manifestation of an anxious or fearful state. In the 1970s, Gray suggested that vulnerability to anxiety is associated with individual differences in the activity of a septohippocampal behavioral inhibition system (BIS). According to Gray, this is one of the three major emotional systems, which also include the behavioral approach system (BAS) and the fight/flight system (F/FLS). 49,50 The primary function of the BIS is to compare actual with expected stimuli. If there is a discrepancy between the actual and expected stimuli (ie, “novelty” or “uncertainty”), or if the predicted stimuli are aversive, the BIS is activated, arousal and attention to novel environmental stimuli is increased, and ongoing behaviors arc inhibited. Thus, according to Gray, anticipatory anxiety reflects a central state mediated by BIS activation, which is elicited by threats of punishment or failure, and by novelty or uncertainty.51
The central role of behavioral inhibition in generating an anxious state has also been pointed out by Laborit.52 Anxiety is associated with the “alarm reaction,” as defined in Selye's original description of the stress response (or general adaptation syndrome).53 According to Laborit, anxiety appears when one realizes that a proper adaptive action is not possible, ie, that there is loss of control over the situation, and it depends on the activation of the HPA axis.
Panksepp has argued that the activities of the ascending NA systems and the descending BIS are not causally related to the affective experience of fear and anxiety.22 They may be correlated, supportive, or permissive systems for establishing brain states that participate in the many brain readjustments accompanying fear. These systems certainly participate in the genesis of fear and anxiety behaviors: the NA system is involved in the initial alarm reaction, whereas freezing promoted by septohippocampal inhibition may help regulate the intensity and duration of fear. However, according to Panksepp, the amygdala-central gray axis plays an essential role in creating the emotional state associated with fear and anxiety.22
The amygdala-hypothalamus-central gray axis and fear
In all mammalian species, there are three distinct sites in the brain where electrical stimulation will provoke a full fear response: the lateral and central zones of the amygdala, the anterior and medial hypothalamus, and specific areas of the PAG. A circuit coursing from the lateral and central nuclei of the amygdala, throughout the ventral-anterior and medial hypothalamic areas, down to the mesencephalic PAG, may constitute the executive system for fear, since freezing, as well as flight behavior and the autonomic indices of fear (eg, increased heart rate and eliminative behavior) can be evoked along the whole trajectory of this system.41
In rats, stepwise increases in the electrical stimulation of the dorsolateral periaqueductal gray (d1PAG) produce alertness, then freezing and finally escape, replicating the sequence of natural defensive reactions when exposed to threat. Recent data suggest that d1PAG stimulation produces freezing independently of any contextual fear conditioning, whereas stimulation of the ventral periaqueductal gray (vPAG) appears to be critical to the expression of conditioned fear.54 Because electrical or pharmacological stimulation of PAG produces a range of fear-related responses similar to those seen in a panic attack, this area be could be directly implicated in panic disorder.55,56
The amygdala and fear conditioning
The elegant studies carried out by LeDoux, based on a simple fear conditioning paradigm in rats, have emphasized the primary role of the amygdala in controlling emotional behaviors.43,57-59 His approach is along the lines of earlier learning/behavioral theories, eg, those of Pavlov and Watson,3 which emphasize the role of conditioning processes in behavioral development. After a few pairings of a threatening stimulus (eg, electric shocks, the unconditioned stimulus [US]) with a formerly neutral cue (eg, a tone or visual signal, the conditioned stimulus [CS]), animals will experience a state of conditioned fear when only the cue is present. Conditioned fear provides a critical survival-related function in the face of threat by activating a range of protective (or defensive) behaviors. The neuroanatomical and neurochemical foundations of conditioned fear,60 based mainly on the behavioral models of freezing and fear-potentiated startle in rats61 have been worked out in detail. In LeDoux's model, the amygdala and thalamic pathways are responsible for the primary appraisal of threat by allowing a rapid, automatic analysis of potentially dangerous stimuli. Additional brain structures, including the hippocampus and cortical pathways, provide more information on the situational context and relevant stimulus characteristics (Figure 1). Thus, the amygdala plays a central role by integrating rapid, direct thalamic inputs, eg, visual information, with more detailed information, eg, cortical integration of sensory information, originating from longer and slower neuronal pathways.43 Activation of the amygdala by threatening stimuli then influences cognitive processes, perception, selective attention, and explicit memory.
The cognitive representation of fear may preferentially involve the left amygdala, as shown by recent functional magnetic resonance imaging (fMRI) studies.62 Interestingly, a sex difference in amygdala activation during the perception of facial affect has recently been reported.63 Amygdala activation (measured by fMRI) differed for men and women depending on the valence of the expression: happy faces produced greater right than left amygdala activation for males, but not for females. Both sexes showed greater left amygdala activation for fearful faces. These data suggest that the left amygdala may be more involved in the representation of negative affect.
The role of the various amygdala nuclei in fear conditioning is now well established, notably by lesion studies. 43,59,60,64 In rats, the central and medial nuclei of the amygdala are important in mediating conditioned aversive states, but conditioned freezing may be mediated independently.65 Thus, different types of fear-conditioned behavior may be mediated by separate nuclei within the amygdala.66
The amygdala plays a pivotal role in coordinating the behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in rats. In a fear-conditioning paradigm, pretraining amygdala lesions blocked freezing behavior, ultrasonic vocalizations, adrenocortical activation, and dopaminergic metabolic activation in the medial prefrontal cortex (mPFC). Posttraining lesions blocked mPFC dopamine, serotonin (5-hydroxytryptaminc [5-HT]), and NA activation and stress-induced freezing and defecation, and greatly attenuated adrenocortical activation.67
The amygdala and positive reinforcement and attention
The role of the amygdala is not limited to fear-conditioning and the processing of aversive stimuli. Studies in rats using food-motivated associative learning indicate that the basolateral amygdala may be involved in the acquisition and representation of positive reinforcement values (possibly through its connections with the ventral striatal dopamine systems and the orbitofrontal cortex).68 Therefore, the amygdala is probably a key structure for the integration of behavior in conflicting situations, when both potentially rewarding and aversive stimuli are present. Recent studies indicate that the human amygdala can also process both positively and negatively valenced stimuli.69
Recent studies also indicate that the CeA may contribute to attentional function in conditioning, by way of its influence on basal forebrain cholinergic systems and on the dorsolateral striatum.68
The amygdala and social behavior and phobia
The amygdala may play an important role in regulating social behavior. Thus, in adult macaque monkeys, selective bilateral lesions of the amygdala result in a lack of fear response to inanimate objects and a “socially uninhibited” pattern of behavior.70 The amygdala may function as a protective “brake” during evaluation of a potential threat, and it has been suggested that social anxiety may involve a dysregulation or hyperactivity of the amygdala evaluative process.70 Studies in rats also suggest that the basolateral nucleus of the amygdala may play a crucial role in the consolidation of information that leads to the formation of a specific phobia.71
The extended amygdala (BNST) and anxiety
Although the amygdala is clearly involved in conditioned fear, its role in anxiety is less evident, because it is often difficult to specify the stimuli that triggers anxiety.72,73
Thus, lesions of the rat amygdala that suppressed fearelicited startle or freezing behavior did not affect measures of anxiety in the elevated plus-maze and shock-probe-burying tests, two classic tests of anxiety for rodents.74 Moreover, diazepam was effective in these tests, even in amygdala-lesioned rats, suggesting that the anxiolytic effects of benzodiazepines are not necessarily mediated by the amygdala.75 Recent studies in primates also suggest that the amygdala is involved in mediating some acute unconditioned fear responses in rhesus monkeys, but that it is unlikely to be a key structure regarding the dispositional behavioral and physiological characteristics of the anxious temperament.76
The BNST is considered to be part of the extended amygdala.77 It appears to be a center for the integration of information originating from the amygdala and the hippocampus (Figure 1), and is clearly involved in the modulation of the neuroendocrine stress response.78,79
Activation of the BNST, notably by corticotropin-releasing factor (CRF), may be more specific for anxiety than fear. Studies in rats with the startle reflex suggest that explicit cues such as light, tone, or touch activate the amygdala, which then activates hypothalamic and brainstem target areas involved in the expression of fear, whereas less specific (or more complex) stimuli of longer duration, such as exposure to a threatening environment or intraventricular administration of CRF, may preferentially involve the BNST.73
The PFC and the control of emotional responses
The primary roles of the PFC appear to be the analysis of complex stimuli or situations and the control of emotional responses.
In a revised version of his original BIS model, Gray postulated that the PFC may modulate septohippocampal activity, and that lesions to this area would impair the processing of vital information for the subicular comparator, and subsequently affect behavioral inhibition and anticipatory anxiety.51 He also suggested that the role of cortical structures in anxiety was probably more prominent in primates, based on the increased anatomical relationship between the septohippocampal system and the prefrontal and cingulate cortices observed in monkeys. Recent studies in humans and primates have largely confirmed Gray's hypothesis, and it is now clear that the various subdivisions of the human PFC (dorsolateral, ventromedial, and orbital sectors) have specific roles in representing affect in the absence of immediate rewards or punishments and in controlling emotional responses.80,81 There appear to be important functional differences between the left and right sides within each of these sectors. Earlier studies on patients with unilateral brain lesions have already emphasized the role of cerebral lateralization in emotional information processing.82 More recently, brain electrical activity measures and positron emission tomography (PET) studies have indicated that negative affect and anxiety are associated with increased activation of the right PFC; moreover, individual differences in baseline levels of asymmetric activation in the PFC may be associated with individual differences in affective styles and vulnerability to mood and anxiety disorders.81
There is also increasing evidence that the PFC plays an important role in controlling anxiety and the associated stress response in rats, and that cerebral laterality is an important feature of the PFC system. Thus, in a recent study right, but not left, lesions of the ventral medial PFC were shown to have anxiolytic effects, and were also more effective in suppressing the neuroendocrine and autonomic stress response.83
Neurochemical correlates
A large number of neurotransmitters, peptides, hormones, and other neuromodulators have been implicated in fear and anxiety. We shall only discuss a few representative examples.
The noradrenergic system
Several preclinical studies have shown that stress and anxiety cause a marked increase in NA release in several rat brain regions, including the hypothalamus, the amygdala, and the LC.84
In agreement with these data, yohimbine, an α2-adrenergic receptor antagonist that increases NA release in the brain, has been shown to have anxiogenic effects in rats.84 However, pharmacological experiments involving the administration of various α2A-receptor agonists or antagonists in several animal models of anxiety are inconsistent, perhaps due to their interaction with other monoaminergic receptors.85 In a recent study, local administration into the LC region of an antisense oligodeoxynucleotide (AS-ODN) corresponding to the α2A-receptor mRNA was shown to have an anxiolytic effect,85 but another study has also shown that genetic knockout of the α2A-receptor in mice resulted in a more anxious phenotype than that of the corresponding C57BL/6 wild type.86
The role of the various NA receptor subtypes in mediating NA action on fear- and anxiety-related behaviors is therefore not settled. The precise location of the receptor subtypes within the complex circuitry mediating fear and anxiety responses is probably critical.
The serotonergic system
Data on the role of 5-HT in anxiety are conflicting: there is no agreement whether 5-HT enhances or, conversely, decreases anxiety. Thus, a 5-HT2C agonist such as _m_-chlorophenylpiperazine (mCPP) has anxiogenic effects in humans and may induce panic attacks, obsessions, and other neuropsychiatrie symptoms, whereas selective 5-HT reuptake inhibitors (SSRIs) and 5-HT1A or 5-HT3 receptor-selective drugs can have antianxiety effects in certain anxiety disorders and animal models.87
On the basis of data obtained from animal models, Graeff et al have proposed a “dual 5-HT fear hypothesis” postulating that 5-HT may enhance conditioned fear in the amygdala, while inhibiting innate fear in the dorsal PAG.88 The ascending 5-HT pathway originating from the dorsal raphe nucleus (DRN) and innervating the amygdala and frontal cortex facilitates conditioned fear, while the DRN-periventricular pathway innervating the periventricular and PAG matter inhibits inborn fight/flight reactions to impending danger, pain, or asphyxia.89 The same authors have also proposed that the pathway connecting the median raphe nucleus (MRN) to the hippocampus may promote resistance to chronic, unavoidable stress by facilitating hippocampal 5-HT1A transmission.89
These results demonstrate that it is not possible to conclude about an “anxiogenic” or “anxiolytic” role for 5-HT (or, for that matter, of any other neurotransmitter, peptide, or hormone) without considering its site of action in the brain and/or the receptor subtype implicated.
Indirect evidence that the anxiolytic action of 5-HT is mediated by the 5-HT1A receptor has been obtained by three independent groups who have reported an “anxious” phenotype in 5-HT1A receptor knockout mice compared with corresponding wild-type mice, using three different genetic backgrounds.90 Depending on this background, the null mutation may be associated with changes in GABAergic transmission.91 More recently, it has been shown that 5-HT1A receptor knockouts display an “anxious-like” phenotype not only at the behavioral, but also at the autonomic response level.92 This seems to provide a strong argument in favor of an important role of 5-HT1A receptor gene expression for anxiety-related behaviors. In contrast, 5-HT1B receptor knockout mice were found to be more aggressive, more reactive, and less anxious than their wild-type counterparts, suggesting that this receptor may also modulate 5-HT action on defense mechanisms.93 Serotonin transporter (5-HTT) knockout mice (5-HTT-/-) have also been produced, and shown to display elevated anxiety in various behavioral tests, and an increased stress response (adenocorticotropic hormone [ACTH] secretion) following a mild stress, which was also observed to a lesser degree in the 5-HTT+/- heterozygotes.94
The GABAergic system
γ-Aminobutyric acid (GABA) is the most abundant inhibitory neurotransmitter in the brain. The GABAA-benzodiazepine receptor is an important target for several anxiolytic drugs and may therefore play an important role in anxiety-related disorders.95 Several GABAA receptor subtypes have been described.96,97
The diazepam-sensitive α2-GABAA subtype appears to be specifically involved in anxiolysis.96 This subtype is largely expressed in the hippocampus, the amygdala, and the striatum.98 Two mouse lines were generated with a knockin point mutation on the α2 or α3 subunit, which rendered them insensitive to diazepam. The anxiolytic action of diazepam was suppressed in mice with the α2(H101R) point mutation, but not in those with the α3(H126R) point mutation.99
Heterozygous γ2-knockout mice (γ2+/-) have been generated (the homozygous mutation is not viable).98 These mice show enhanced reactivity to natural aversive stimuli, increased passive avoidance responses, and a deficit in ambiguous cue discrimination.100 They have been proposed as a model for trait anxiety characterized by harm avoidance behavior and explicit memory bias for threat cues (enhanced sensitivity to negative associations).
In contrast to the anxiolytic action of benzodiazepinelike compounds, inverse agonists of the GABA/benzodiazepine receptor such as the β-carbolines are well known to be anxiogenic. Recently, intrahippocampal injections of a novel inverse agonist (RY024) have been shown to produce a fear response (freezing) and to interfere with fear-conditioning in rats.101
The neurosteroids
The neurosteroids are a novel, interesting class of neuromodulators synthesized in the brain directly from cholesterol.102 They appear to act essentially via an allosteric modulation of the GABAA receptor, although other receptors may also be involved.102,103 As early as 1987, Majewska suggested that neurosteroids could play an important role in mood regulation.104 Several studies have shown that positive allosteric modulators (which potentiate GABA action), such as progesterone and allopregnanolone, have anxiolytic effects in various animal models.103 Neurosteroid synthesis is regulated by a peripheral benzodiazepine receptor (PBR) located on the outer mitochondrial membrane,105 and part of the anxiolytic effects of benzodiazepine could in fact involve increased neurosteroid synthesis. Compounds with a selective affinity for the PBR, such as FGIN-1-27, have shown an anxiolytic action in rats.106 Neurosteroids are currently attracting a lot of interest because of their potential role as natural, endogenous anxiolytics.
Hormones of the HPA axis
Hormones of the HPA axis, such as Cortisol, or corticosterone (in rodents), ACTH, and CRF are usually increased in a state of fear and anxiety. They also appear to modulate the response to threatening events.
Corticotropin-releasing factor
Intracerebral administration of CRF has been shown to elicit anxious-like behavior in rats.107 More recent pre-clinical studies suggest that CRF and its receptors play a pivotal, integrative role in the stress response and anxiety-related behaviors.108,109 There are two major CRF systems in the brain: the neuroendocrine system in the PVN, and another system with CRF cells located in the amygdala (CeA) and BNST, which would be more directly related to the physiological and behavioral responses associated with fear and anxiety. Whereas glucocorticoids restrain CRF production in the PVN (the neuroendocrine negative feedback loop), they appear to increase CRF expression in the amygdala and BNST, thus promoting fear- and anxiety-related behavior.110 CRF neurons originating from the amygdala project onto the LC (Figure 1) and contribute to increased arousal in fear and anxiety states.111 In a rat model, a full postsynaptic CRF agonist, CRF(1-41), increased arousal at low dosage and had an anxiogenic action at higher doses.112 This suggests that progressively increasing levels of CRF in the brain may ensure the transition from the initial state of increased arousal to the anxious state of expectancy in stressful situations.
Transgenic mice overexpressing CRF show a behavioral and neuroendocrine profile consistent with an increased level of stress and anxiety, including elevated plasma ACTH and corticosterone levels, and generally exhibit the same behavioral changes as those observed in mice following exogenous CRF administration.113-115 Recent data indicate a desensitization of postsynaptic, but not presynaptic 5-HT1A receptors in mice overproducing CRF.116 Another line of transgenic mice ovcrcxprcssing CRF (CRH-OE(2122)) has shown a reduced startle reactivity, habituation, and prepulse inhibition.117
Deletion of the CRF gene (CRF-KO mice) results in chronic glucocorticoid insufficiency, and this may cause severe developmental problems.114,118 Despite an impaired stress-induced activation of the HPA axis, the behavioral stress responses do not appear to be markedly affected in CRF-deficient mice, suggesting that other CRF-like molecules may be implicated in the behavioral effects mediated by CRF receptors.114,118-120 CRF-KO mice also display normal startle- and fear-conditioned responses.120
CRF receptors and CRF binding protein
Deletion of the genes coding for CRF receptors 1 (CRF-R1) or 2 (CRF-R2) have more profound behavioral effects.114,115,121-124 _CRF-R1_-deficient mice display decreased anxiety and an impaired stress response,125 whereas deletion of the CRF-R2 gene has the reverse effect in males (but not in females): anxiety is increased in Crhr2-/-.126 These data suggest that CRF-R1 mediates the anxiogenic effects of CRF, whereas CRF-R2 may be involved in anxiolysis. Recently, mice deficient in both CRF-R1 and CRF-R2 receptors have been generated.127 These double mutants display altered anxiety-related behavior and an impaired HPA axis response to stress. Interestingly, the effects on anxiety are again sex-dependent: females show a decreased anxiety similar to that observed in Crhr1-/- mutants, whereas the genotype has no effect on male anxiety-related behaviors. These studies have also demonstrated a novel role of the mother's genotype on the development of pup anxiety: pups born to a heterozygous or mutant mother display significantly more anxiety, regardless of that pup's genotype.127
The CRF binding protein (CRF-BP) may play an important modulatory role in CRF action.128 Interesting data consistent with a modulatory action of CRF-BP have recently been obtained with transgenic and knockout models: transgenic males overexpressing CRF-BP tend to show less anxiety, whereas the behavior of CRF-BP-deficient mice was consistent with increased anxiety.129
Corticosteroids
Corticosteroids effects on anxiety-related behaviors may be mediated by both genomic and nongenomic mechanisms (control of neuronal excitability). Hippocampal corticosteroid receptors play an important role in the termination of the acute stress response.130 Studies with a model of posttraumatic stress disorder in rats suggest an alteration of the mineralocorticoid receptor (MR) vs glucocorticoid receptor (GR) balance, as measured by the expression of mRNA levels in the hippocampus, during the recovery phase following acute stress: the MR/GR ratio was decreased, but only in animals with an enhanced fast feedback.131 Recent data also suggest that, at low circulating levels, corticosteroids exert a permissive action (via MRs) on acute freezing behavior and other acute fear-related behaviors. At higher levels, corticosteroids enhance acquisition, conditioning, and consolidation of an inescapable stressful experience, as well as processes underlying fear potentiation, via GR-dependent mechanisms.132 Mice with targeted mutation of the MR and GR receptors display altered anxiety-related behaviors.133
Other peptides, neurotransmitters, and hormones
Several peptides, such as cholecystokinin (CCK), neuropeptide Y (NPY), tachykinins (substance P, neurokinins A and B), and natriuretic peptides (atrial natriuretic peptide or C-type natriuretic peptide) may play important roles in fear- and anxiety -related behaviors.134 CCK may be particularly relevant for panic disorders,135,136 and may influence cognitive processes.137
Excitatory amino acids (EAA), such as glutamate, are also important. In rats, microinjections of EAA into the dorsolateral PAG induce a flight reaction. Part of the effects mediated by _N_-methyl-D-aspartate (NMDA) receptors may involve nitric oxide (NO). Nitric oxide synthase (NOS) inhibitors injected in the dorsolateral PAG have been shown to have anxiolytic effects, and psychological stress (restraint) induced an increased expression of neuronal NOS in the same area and in other areas related to defense mechanisms, suggesting that NO may participate in these defensive responses.138 We have also shown that anticipatory anxiety can lead to a decreased secretion of luteinizing hormone (LH) and testosterone in young, healthy male subjects.139
Genetic and environmental factors
Individual differences in sensitivity to threat or stress, and particular coping or affective styles appear to be critical predisposing factors for anxiety-related disorders. Genetic and environmental factors have been implicated, and how these factors interact during development is one of the major questions addressed by recent clinical and fundamental research.
Genetic determinants
A genetic basis for anxiety-related behaviors is now clearly established, notably through several family, twin, and adoption studies.
In mice, targeted gene mutations have shown that modifying the expression of particular genes can have a profound effect on anxiety-related behavioral phenotypes.39,140 Some examples were mentioned in the preceding section.
Natural variations in trait anxiety, or emotionality, in inbred rat and mouse strains are being extensively studied.27,39,141-146 Some of these strains show differences in sensitivity to anxiolytic agents such as diazepam.147,148 Crossbreeding of inbred rodents strains has shown the quantitative nature of many anxiety-related traits.149,150
The quantitative trait locus (QTL) method is based on a comparison between the allelic frequency of DNA markers and quantitative behavioral traits.146,150 It has been used to assess gene effects on fear, emotionality, and anxiety-related behaviors in mice from various genetic backgrounds.140,151 Loci on mouse chromosomes 1, 4, and 15 were found to operate in four tests of anxiety, whereas loci on chromosomes 7, 12, 14, 18, and X influenced only a subset of behavioral measures.152 A QTL influencing anxiety has also been found recently on rat chromosome 5.153
Selective breeding of mice and rats has also been used to create lines that show extreme behavioral characteristics within the range of the normal population.140 Various selection criteria can be used, which may not be directly related to anxiety. Thus, rat lines initially selected for their good versus poor performance in two-way, active avoidance were subsequently shown to differ in trait anxiety, or emotionality. For instance, the Roman high- (RHA/Verh) and low- (RLA/Verh) avoidance rat lines display clear differences in emotionality and anxiety-related behaviors.28,154 The more anxious (RLA/Verh) rats display increased neuroendocrine and autonomic reactivity to mild stressors.28,155,156 Differences in vasopressin, oxytocin, and CRF action at the level of the amygdala,156,157 dopaminergic and GABAergic neurotransmission,158 basal vasopressin mRNA expression in the hypothalamic PVN,159 and 5-HTT levels in the frontal cortex and hippocampus160 have been reported. We have shown an increased capacity (enzymatic activities) for the production of progesterone-derived, anxiolytic neurosteroids in the frontal cortex and BNST of RHA/Verh rats, which may explain in part the differences in emotional reactivity of these two lines.28 These two rat lines also differ in their respective coping styles and response to novelty,154,155 and this model may therefore prove useful for studying the interaction between anxiety and defense mechanisms.
Recently, two Wistar rat lines have been selected and bred for high anxiety-related behavior (HAB) or low anxiety-related behavior (LAB) on the elevated plusmaze, a classical test for anxiety in rodents.149 The neuroendocrine, physiological, and behavioral characteristics of these two lines are being extensively studied, and show some similarities, but also differences, as compared to the Roman rat lines.161-167 Further comparison between lines such as the RHA/RLA and HAB/LAB rats, which have been selected on different behavioral criteria (avoidance versus anxiety in the elevated plus-maze test), but show a similar, anxiety-related behavioral phenotype, may be extremely fruitful to delineate brain mechanisms underlying specific aspects of anxiety disorders.
Environmental influences
The role of environmental influences in the etiology of anxiety is also well established.15 Early adverse experience is a major developmental risk factor for psychopathology.168-170
Prenatal stress in animal models has been shown to permanently alter brain morphology, anxiety-related behavior, coping, and regulation of the HPA axis in adulthood.171 Naturally occurring variations in maternal care can also alter the regulation of genes controlling the behavioral and neuroendocrine responses to stress, as well as hippocampal synaptic development. These effects are responsible for stable, individual differences in stress reactivity, as well as the maternal behavior of female offspring.172 They could constitute the basis of a nongenetic mechanism for the transmission of individual differences in stress reactivity and coping styles across generations. In 1958, Levine reported that rats handled for the first 21 days of life exhibit reduced fearfulness compared with nonhandled controls. Since then, several studies have shown the beneficial effects of neonatal handling and a progressive habituation to stress on adults' stress responses and anxiety-related behaviors. Neonatal handling can even reverse the behavioral abnormalities induced by prenatal stress.173 These effects appear to be mediated essentially by the CRF/HPA axis system,174,175 although the serotonergic and catecholaminergic systems could be also involved.176,177 A study has shown that neonatal handling increases the expression of the peripheral benzodiazepine receptor (PBR), which has been implicated in the synthesis of endogenous, natural anxiolytic agents such as the neurosteroids, in rat adrenals, kidney, and gonads.178 It is likely that increased adrenal production of naturally anxiolytic compounds such as allopregnanolone contributed to the decrease in anxiety reported in this study.
Sex differences in the effects of neonatal handling have been recently reported: neonatal handling may provide males with a greater capacity to actively face chronic stressors.179 Recent data indicate that neonatal handling can also affect memory processes involved in contextual fear conditioning.180
In the Roman rat lines, neonatal handling has been shown to alter the behavioral phenotype of the more anxious RLA/Verh rats so that, in adulthood, they behave in the same way as their nonhandled, hypoemotional RHA/Verh counterparts. Females were found to be more sensitive than males to the positive influences of early stimulation.181 The effects of neonatal handling on RLA/Verh rats were not limited to behavioral stress responses and coping behaviors, but were accompanied by a concomitant decrease in stress-induced ACTH, corticosterone, and prolactin release, indicating that the neurochemical substrates underlying these responses were also permanently affected by early experience.182,183
This and other examples indicate that the developmental processes that determine individual sensitivity to stressors, or emotionality, and coping behaviors involve complex interactions between genetic and environmental factors, and that anxiety-related phenotypes cannot be predicted on the sole basis of a genetic predisposition or early adverse experience.
Conclusions
The biological bases of fear and anxiety are now recognized, and the major brain structures and neuronal circuits involved in emotional information processing and behavior are delineated. Emotional and cognitive processes cannot be dissociated, even when considering such a basic emotion as fear. The cognitive apprehension of events and situations is critically involved in emotional experiences and also influences coping strategies or defense mechanisms. This is reflected in the important role now attributed to the PFC in controlling emotional behavior in humans and animals.
Molecular biology techniques, such as those used to create transgenic and knockout mice, have been successful in exploring the role of various neurotransmitters, peptides, hormones, and their receptors in mediating the appraisal of stressful stimuli, information processing through the various neuronal circuits, and the physiological responses and behaviors associated with fear and anxiety.
It is now clear that individual differences in affective or coping styles, which are also observed in nonhuman species, are directly associated with vulnerability to psychopathology. Studying these individual differences, including sex-related differences, in humans and in animal models will give interesting clues about the brain mechanisms of emotional behavior.
Finally, the study of genetic predisposition and environmental influences, particularly during early development, in determining vulnerability traits and anxietyprone endophenotypes is certainly becoming one of the major, and perhaps most promising, domains of contemporary research with respect to our understanding of the etiology of anxiety and mood disorders.
Selected abbreviations and acronyms
ACTH
adenocorticotropic hormone
BIS
behavioral inhibition system
BNST
bed nucleus of the stria terminalis
CeA
central nucleus of the amygdala
CRF
corticotropin-releasing factor
GABA
γ-aminobutyric acid
HPA
hypothalamo-pituitary-adrenocortical (axis)
5-HT
5-hydroxytryptamine (serotonin)
5-HTT
serotonin transporter
LC
locus ceruleus
NA
noradrenaline
NTS
nucleus tractus solitarius
PAG
periaqueductal gray
PBR
peripheral benzodiazepine receptor
PFC
prefrontal cortex
PVN
paraventricular nucleus
The author would like to express his gratitude to the Swiss National Science Foundation for supporting work on the Roman rat lines in his laboratory (grant 32-51187-97).
REFERENCES
- 1.Letourneau C. Physiologie des passions. 2nd ed. Paris, France: C. Reinwald &Cie; 1878 [Google Scholar]
- 2.Davidson JR. Affective style, mood and anxiety disorders. An affective neuroscience approach. In: Davidson JR, ed. Anxiety Depression and Emotions. Oxford, UK: Oxford University Press; 2000:88–108. [Google Scholar]
- 3.Strongman KT. The Psychology of Emotion. Theories of Emotion in Perspective. Chichester, UK: John Wiley & sons; 1996 [Google Scholar]
- 4.Ekman P., Davidson RJ (eds). The Nature of Emotion. Oxford, UK: Oxford University Press; 1994 [Google Scholar]
- 5.Panksepp J. Affective Neuroscience. New York, NY: Oxford University Press; 1998 [Google Scholar]
- 6.Borod JC (ed). The Neuropsychology of Emotion. Oxford, UK: Oxford University Press; 2000 [Google Scholar]
- 7.Davidson JR (ed). Anxiety Depression, and Emotion. Oxford, UK: Oxford University Press; 2000 [Google Scholar]
- 8.Lewis M., Haviland-Jones JM (eds). Handbook of Emotions. New York, NY: The Guilford Press; 2000 [Google Scholar]
- 9.Cannon WB. The James-Lange theory of emotions: a critical examination and an alternative theory. By Walter B. Cannon, 1927. Am J Psychol. 1987;100:567–586. [PubMed] [Google Scholar]
- 10.Watson JB. Behaviorism. 7th ed. New York, NY: WW Norton & Company; 1970 [Google Scholar]
- 11.Ortony A., Turner TJ. What's basic about basic emotions? Psychol Rev. 1990;97:315–331. doi: 10.1037/0033-295x.97.3.315. [DOI] [PubMed] [Google Scholar]
- 12.Ekman P. Are there basic emotions? Psychol Rev. 1992;99:550–553. doi: 10.1037/0033-295x.99.3.550. [DOI] [PubMed] [Google Scholar]
- 13.Panksepp J. A critical role for “affective neuroscience” in resolving what is basic about basic emotions. Psychol Rev. 1992;99:554–560. doi: 10.1037/0033-295X.99.3.554. [DOI] [PubMed] [Google Scholar]
- 14.McFarland D. The Oxford Companion to Animal Behaviour. Oxford, UK: Oxford University Press; 1987 [Google Scholar]
- 15.Craig KJ., Brown KJ., Baum A. Environmental factors in the etiology of anxiety. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology: the Fourth Generation of Progress. New York, NY: Raven Press; 1995:1325–1339. [Google Scholar]
- 16.Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- 17.Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage. New York, NY: Appleton; 1915 [Google Scholar]
- 18.Engel GL., Schmale AH. Conservation withdrawal: a primary regulatory process for organic homeostasis. In: Physiology, Emotions and Psychosomatic Illness. New York, NY: Elsevier; 1972:57–95. doi: 10.1002/9780470719916.ch5. [DOI] [PubMed] [Google Scholar]
- 19.Henry JP., Stephens PM. Health and the Social Environment: a Sociobiological Approach to Medicine. Berlin, Germany: Springer; 1997 [Google Scholar]
- 20.Keay KA., Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 21.Bandler R., Price JL., Keay KA. Brain mediation of active and passive emotional coping. In: Mayer EA, Sapers CB, eds. Progress in Brain Research. Vol 122. Amsterdam, The Netherlands: Elsevier Science BV; 2000:333–349. doi: 10.1016/s0079-6123(08)62149-4. [DOI] [PubMed] [Google Scholar]
- 22.Panksepp J. The psychoneurology of fear: evolutionary perspectives and the role of animal models in understanding human anxiety. In: Burrows GD, Roth M, Noyes Jr R, eds. Handbook of Anxiety. Volume 3. The Neurobiology of Anxiety. Amsterdam, The Netherlands: Elsevier Science BV; 1990:3–58. [Google Scholar]
- 23.Bakshi VP., Shelton SE., Kalin NH. Neurobiological correlates of defensive behaviors. In: Mayer EA, Sapers CB, eds. Progress in Brain Research. Vol 122. Amsterdam, The Netherlands: Elsevier Science BV; 2000:105–115. doi: 10.1016/s0079-6123(08)62133-0. [DOI] [PubMed] [Google Scholar]
- 24.Blanchard DC., Hynd AL., Minke KA., Monemoto T., Blanchard RJ. Human defensive behaviors to threat scenarios show parallels to fear- and anxiety-related defense patterns of non-human mammals. Neurosci Biobehav Rev. 2001;25:761–770. doi: 10.1016/s0149-7634(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 25.Kavaliers M., Choleris E. Antipredator responses and defensive behavior: ecological and ethological approaches for the neurosciences. Neurosci Biobehav Rev. 2001;25:577–586. doi: 10.1016/s0149-7634(01)00042-2. [DOI] [PubMed] [Google Scholar]
- 26.Koolhaas JM., Korte SM., De Boer SF., et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 27.Parmigiani S., Palanza P., Rodgers J., Ferrari PF. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci Biobehav Rev. 1999;23:957–970. doi: 10.1016/s0149-7634(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 28.Steimer T., Driscoll P., Schulz P. Brain metabolism of progesterone, coping behaviour and emotional reactivity in male rats from two psychogenetically selected lines. J Neuroendocrinol. 1997;9:169–175. doi: 10.1046/j.1365-2826.1997.t01-1-00571.x. [DOI] [PubMed] [Google Scholar]
- 29.Perrez M., Reichert M. Stress, Coping, and Health. Seattle, Wash: Hogrefe & Huber Publishers; 1992 [Google Scholar]
- 30.Van Egeren L. Stress and coping and behavioral organization. Psychosom Med. 2000;62:451–460. doi: 10.1097/00006842-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Pavlov I. Oeuvres choisies. Moscow, Russia: Editions en langues étrangères; 1954:250–251. [Google Scholar]
- 32.Cosnier J. Les névroses expérimentales. Paris, France: Editions du Seuil; 1966 [Google Scholar]
- 33.Gottesman II. Schizophrenia Genesis: The Origins of Madness. New York, NY: WH Freeman and Co; 1991 [Google Scholar]
- 34.Goldsmith HH., Lemery KS. Linking temperamental fearfulness and anxiety symptoms: a behavior-genetic perspective. Biol Psychiatry. 2000;48:1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- 35.Kandel ER. From metapsychology to molecular biology: explorations into the nature of anxiety. Am J Psychiatry. 1983;140:1277–1293. doi: 10.1176/ajp.140.10.1277. [DOI] [PubMed] [Google Scholar]
- 36.Lister RG. Ethologically based animal models of anxiety disorders. Pharmacol Ther. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- 37.Shekhar A., McCann UD., Meaney MJ., et al. Summary of a National Institute of Mental Health workshop: developing animal models of anxiety disorders. Psychopharmacology (Berl). 2001;157:327–339. doi: 10.1007/s002130100859. [DOI] [PubMed] [Google Scholar]
- 38.Belzung C., Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 39.Tarantino ML., Bucan M. Dissection of behavior and psychiatric disorders using the mouse as a model. Hum Mol Genet. 2000;9:953–965. doi: 10.1093/hmg/9.6.953. [DOI] [PubMed] [Google Scholar]
- 40.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 2002;855:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 41.Panksepp J. The sources of fear and anxiety in the brain. In: Panksepp J, ed. Affective Neuroscience. New York, NY: Oxford University Press; 1998:206–222. [Google Scholar]
- 42.Coplan JD., Rosenblum LA., Gorman JM. Primate models of anxiety. Longitudinal perspectives. Psychiatr Clin North Am. 1995;18:727–743. [PubMed] [Google Scholar]
- 43.LeDoux J. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 44.Jr Redmond DE., Huang YH. Current concepts. II. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 1979;25:2149–2162. doi: 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- 45.Southwick SM., Bremner JD., Rasmusson A., Morgan CA III., Arnsten A., Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 46.Aston-Jones G., Rajkowski J., Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 47.Ziegler DR., Cass WA., Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan GM., Coplan JD., Kent JM., Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- 49.Gray JA. The structure of the emotions and the limbic system. In: Physiology, Emotions and Psychosomatic Illness. Amsterdam, The Netherlands: Elsevier; 1972:87–129. [Google Scholar]
- 50.Gray JA. Three fundamental emotion systems. In: Ekman P, Davidson JR, eds. The Nature of Emotion. New York, NY: Oxford University Press; 1994:243–247. [Google Scholar]
- 51.Gray JA. The Neuropsychology of Anxiety. An Enquiry into the Functions of the Septo-hippocampal System. Oxford, UK: Clarendon Press; 1987 [Google Scholar]
- 52.Laborit H. Inhibition of action: interdisciplinary approach to its mechanisms and pathophysiology. In: Traue HC, Pennebaker JW, eds. Emotion, Inhibition and Health. Seattle, Wash: Hogrefe & Huber Publishers; 1993:57–79. [Google Scholar]
- 53.Selye H. The Stress of Life. 2nd revised paperback ed. New York, NY: McGraw Hill; 1984 [Google Scholar]
- 54.Vianna DML., Landeira-Fernadez J., Brandão ML. Dorsolateral and ventral regions of the periaqueductal gray matter are involved in distinct types of fear. Neurosci Biobehav Rev. 2001;25:711–719. doi: 10.1016/s0149-7634(01)00052-5. [DOI] [PubMed] [Google Scholar]
- 55.Coplan JD., Lydiard RB. Brain circuits in panic disorder. Biol Psychiatry. 1998;44:1264–1276. doi: 10.1016/s0006-3223(98)00300-x. [DOI] [PubMed] [Google Scholar]
- 56.Goddard AW., Charney DS. Toward an integrated neurobiology of panic disorder. J Clin Psychiatry. 1997;58(suppl 2):4–11. [PubMed] [Google Scholar]
- 57.LeDoux J. Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- 58.LeDoux J. The Emotional Brain. New York, NY: Simon & Schuster; 1998 [Google Scholar]
- 59.LeDoux J. The amygdala and emotion: a view through fear. In: Aggleton JP, ed. The Amygdala. Oxford, UK: Oxford University Press; 2000:289–310. [Google Scholar]
- 60.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 61.Fendt M., Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 62.Phelps EA., O'Connor KJ., Gatenby JC., Gore JC., Grillon C., Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2002;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 63.Killgore WDS., Yurgelun-Todd DA. Sex differences in amygdala activation during the perception of facial affect. Neuroreport. 2001;12:2543–2547. doi: 10.1097/00001756-200108080-00050. [DOI] [PubMed] [Google Scholar]
- 64.Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, ed. The Amygdala. Oxford, UK: Oxford University Press; 2000:213–287. [Google Scholar]
- 65.Holahan MR., White NM. Conditioned memory modulation, freezing, and avoidance as measures of amygdala-mediated conditioned fear. Neurobiol Learn Mem. 2002;77:250–275. doi: 10.1006/nlme.2001.4012. [DOI] [PubMed] [Google Scholar]
- 66.Killcross S., Robbins TW., Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within the amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 67.Goldstein LE., Rasmusson AM., Bunney BS., Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holland PC., Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 69.Garavan H., Pendergrass JC., Ross TJ., Stein EA., Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12:2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- 70.Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 71.File S., Gonzalez LE., Gallants R. Role of the basolateral nucleus of the amygdala in the formation of a phobia. Neuropsychopharmacology. 1998;19:397–405. doi: 10.1016/S0893-133X(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 72.Davis M., Walker DL., Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann N Y Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- 73.Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- 74.Treit D., Pesold C., Rotzinger S. Dissociating the anti-fear effects of septal and amygdaloid lesions using two pharmacologically validated models of rat anxiety. Behav Neurosci. 1993;105:770–785. doi: 10.1037//0735-7044.107.5.770. [DOI] [PubMed] [Google Scholar]
- 75.Treit D., Pesold C., Rotzinger S. Noninteractive effects of diazepam and amygdaloid lesions in two animal models of anxiety. Behav Neurosci. 1993;107:1099–1105. doi: 10.1037//0735-7044.107.6.1099. [DOI] [PubMed] [Google Scholar]
- 76.Kalin NH., Shelton SE., Davidson RJ., Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aggleton JP. The Amygdala. A Functional Analysis. 2nd ed. Oxford, UK: Oxford University Press; 2000 [Google Scholar]
- 78.Herman JP., Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 79.Lopez JF., Akil H., Watson SJ. Neural circuits mediating stress. Biol Psychiatry. 1999;46:1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- 80.Davidson RJ., Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 81.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 82.Gainotti G., Caltagirone C. Emotions and the Dual Brain. Berlin, Germany: Springer-Verlag; 1989 [Google Scholar]
- 83.Sullivan RM., Gratton A. Behavioral effects of excitotoxic lesions of ventral medial prefrontal cortex in the rat are hemisphere-dependent. Brain Res. 2002;927:69–79. doi: 10.1016/s0006-8993(01)03328-5. [DOI] [PubMed] [Google Scholar]
- 84.Tanaka M., Yoshida M., Emoto H., Ishii H. Noradrenaline systems in the hypothalamus, amygdala and locus coeruleus are involved in the provocation of anxiety: basic studies. Eur J Pharmacol. 2000;405:397–406. doi: 10.1016/s0014-2999(00)00569-0. [DOI] [PubMed] [Google Scholar]
- 85.Shishkina GT., Kalinina TS., Sournina NY., Saharov DG., Kobzev VF., Dygalo NN. Effects of antisense oligodeoxynucleotide to the alpha2A-adrenoceptors on the plasma corticosterone level and on elevated plus-maze behavior in rats. Psychoneuroendocrinology. 2002;27:593–601. doi: 10.1016/s0306-4530(01)00095-6. [DOI] [PubMed] [Google Scholar]
- 86.Schramm NL., McDonald MP., Limbird LE. The <x2A-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci. 2001;21:4875–4882. doi: 10.1523/JNEUROSCI.21-13-04875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bagdy G. Serotonin, anxiety, and stress hormones. Focus on 5-HT receptor subtypes, species and gender differences. Ann N Y Acad Sci. 1998;851:357–363. doi: 10.1111/j.1749-6632.1998.tb09009.x. [DOI] [PubMed] [Google Scholar]
- 88.Graeff FG., Viana MB., Mora PO. Dual role of 5-HT in defense and anxiety. Neurosci Biobehav Rev. 1997;21:791–799. doi: 10.1016/s0149-7634(96)00059-0. [DOI] [PubMed] [Google Scholar]
- 89.Graeff FG., Guimaraes FS., De Andrade TG., Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 90.Gingrich JA., Hen R. Dissecting the role of the serotonin system in neuropsychiatrie disorders using knockout mice. Psychopharmacology (Berl). 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- 91.Olivier B., Pattij T., Wood SJ., Oosting R., Sarnyai Z., Toth M. The 5-HT(1A) receptor knockout mouse and anxiety. Behav Pharmacol. 2001;12:439–450. doi: 10.1097/00008877-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 92.Pattij T., Groenink L., Hijzen TH., et al. Autonomic changes associated with enhanced anxiety in 5-HT1A receptor knockout mice. Neuropsychopharmacology. 2002;27:380. doi: 10.1016/S0893-133X(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 93.Zhuang X., Gross C., Santarelli L., Compan V., Trillat AC., Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology. 1999;21(2, suppl):52S–60S. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 94.Murphy DL., Li Q., Engel S., et al. Genetic perspectives on the serotonin transporter. Brain Res Bull. 2001;56:487–494. doi: 10.1016/s0361-9230(01)00622-0. [DOI] [PubMed] [Google Scholar]
- 95.Nutt DJ., Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry. 2001;179:390–396. doi: 10.1192/bjp.179.5.390. [DOI] [PubMed] [Google Scholar]
- 96.Möhler H., Crestani F., Rudolph U. GABAA-receptor subtypes: a new pharmacology. Curr Opin Pharmacol. 2002;1:22–25. doi: 10.1016/s1471-4892(01)00008-x. [DOI] [PubMed] [Google Scholar]
- 97.Möhler H., Fritschy JM., Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 98.Rudolph U., Crestani F., Möhler H. GABAA receptors subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- 99.Löw K., Crestani F., Keist R., et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 100.Crestani F., Lorez M., Baer K., et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 101.Bailey DJ., Tezlaff JE., Cook JM., He X., Helmstetter FJ. Effects of hippocampal injections of a novel ligand selective for the α5β2γ2 subunits of the GABA/benzodiazepine receptor on Pavlovian conditioning. Neurobiol Learn Mem. 2002;78:1–10. doi: 10.1006/nlme.2001.4050. [DOI] [PubMed] [Google Scholar]
- 102.Compagnone NA., Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2002;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 103.Rupprecht R., di Michele F., Hermann B., et al. Neuroactive steroids: molecular mechanisms of action and implications for neuropsychopharmacology. Brain Res Brain Res Rev. 2001;37:59–67. doi: 10.1016/s0165-0173(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 104.Majewska MD. Steroids and brain activity. Essential dialogue between body and mind. Biochem Pharmacol. 1987;36:3781–3788. doi: 10.1016/0006-2952(87)90437-0. [DOI] [PubMed] [Google Scholar]
- 105.Costa E., Cheney DL., Grayson DR., et al. Pharmacology of neurosteroid biosynthesis. Role of the mitochondrial DBI receptor (MDR) complex. Ann N Y Acad Sci. 1994;746:223–242. doi: 10.1111/j.1749-6632.1994.tb39240.x. [DOI] [PubMed] [Google Scholar]
- 106.Costa E., Auta J., Guidotti A., Korneyev A., Romeo E. The pharmacology of neurosteroidogenesis. J Steroid Biochem Mol Biol. 1994;49:385–389. doi: 10.1016/0960-0760(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 107.Dunn AJ., Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 108.Arborelius L., Owens MJ., Plotsky PM., Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 109.Smagin GN., Heinrichs SC., Dunn AJ. The role of CRH in behavioral responses to stress. Peptides. 2001;22:713–724. doi: 10.1016/s0196-9781(01)00384-9. [DOI] [PubMed] [Google Scholar]
- 110.Schulkin J., Gold PW., McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 111.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 112.Heinrichs SC., Joppa M. Dissociation of arousal-like from anxiogeniclike actions of brain corticotropin-releasing factor receptor ligands in rats. Behav Brain Res. 2001;122:43–50. doi: 10.1016/s0166-4328(01)00174-7. [DOI] [PubMed] [Google Scholar]
- 113.Stenzel-Poore MP., Heinrichs SC., Rivest S., Koob GF., Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bakshi VP., Kalin NH. Corticotropin-releasing hormone and animal models of anxiety: gene-environment interactions. Biol Psychiatry. 2000;48:1175–1198. doi: 10.1016/s0006-3223(00)01082-9. [DOI] [PubMed] [Google Scholar]
- 115.Coste SC., Murray SE., Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–741. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- 116.van Gaalen MM., Reul JHM., Gesing A., Stenzel-Poore M., Holsboer F., Steckler T. Mice overexpressing CRH show reduced responsiveness in plasma corticosterone after a 5-HT1A receptor challenge. Genes Brain Behav. 2002;1:174–177. doi: 10.1034/j.1601-183x.2002.10305.x. [DOI] [PubMed] [Google Scholar]
- 117.Dirks A., Groenink L., Schipholt Ml., et al. Reduced startle reactivity and plasticity in transgenic mice overexpressing corticotropin-releasing hormone. Biol Psychiatry. 2002;51:583–590. doi: 10.1016/s0006-3223(01)01323-3. [DOI] [PubMed] [Google Scholar]
- 118.Muglia LJ., Jacobson L., Weninger SC., Karalis KP., Jeong K., Majzoub JA. The physiology of corticotropin-releasing hormone deficiency in mice. Peptides. 2001;22:725–731. doi: 10.1016/s0196-9781(01)00385-0. [DOI] [PubMed] [Google Scholar]
- 119.Dunn AJ., Swiergiel AH. Behavioral responses to stress are intact in CRF-deficient mice. Brain Res. 1999;845:14–20. doi: 10.1016/s0006-8993(99)01912-5. [DOI] [PubMed] [Google Scholar]
- 120.Weninger SC., Dunn AJ., Muglia LJ., et al. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci USA. 1999;96:8283–8288. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sleekler T., Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 122.Holmes A. Targeted gene mutation approaches to the study of anxiety-like behavior in mice. Neurosci Biobehav Rev. 2001;25:261–273. doi: 10.1016/s0149-7634(01)00012-4. [DOI] [PubMed] [Google Scholar]
- 123.Takahashi LK. Role of CRF1 and CRF2 receptors in fear and anxiety. Neurosci Biobehav Rev. 2002;25:627–636. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- 124.Reul JM., Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- 125.Smith GW., Aubry JM., Dellu F., et al. Corticotropin-releasing factor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 126.Kishimoto T., Radulovic J., Radulovic M., et al. Deletion of Crhr2 reveals an anxiolytic role for corticotropin-releasing hormone recptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 127.Bale TL., Picetti R., Contarino A., Koob GF., Vale WW., Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kemp CF., Woods RJ., Lowry PJ. The corticotropin-releasing factor-binding protein: an act of several parts. Peptides. 1998;19:1119–1128. doi: 10.1016/s0196-9781(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 129.Seasholtz AF., Burrows HL., Karolyi IJ., Camper SA. Mouse models of altered CRH-binding protein expression. Peptides. 2001;22:743–751. doi: 10.1016/s0196-9781(01)00387-4. [DOI] [PubMed] [Google Scholar]
- 130.Sapolsky RM., Krey LC., McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liberzon I., López JF., Flagel SB., Vázquez DM., Young EA. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol. 1999;11:11–17. doi: 10.1046/j.1365-2826.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 132.Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25:117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 133.Gass P., Reichardt HM., Strekalova T., Henn F., Tronche F. Mice with targeted mutations of glucocorticoid and mineralocorticoid receptors: models for depression and anxiety? Physiol Behav. 2001;73:811–825. doi: 10.1016/s0031-9384(01)00518-2. [DOI] [PubMed] [Google Scholar]
- 134.Griebel G. Is there a future for neuropeptide receptor ligands in the treatment of anxiety disorders? Pharmacol Ther. 1999;82:1–61. doi: 10.1016/s0163-7258(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 135.van Megen HJ., Westenberg HG., den Boer JA., Kahn RS. Cholecystokinin in anxiety. Eur Neuropsychopharmacol. 1996;6:263–280. doi: 10.1016/s0924-977x(96)00038-7. [DOI] [PubMed] [Google Scholar]
- 136.Rehfeld JF. Cholecystokinin and panic disorder - three unsettled questions. Regul Pept. 2000;93:79–83. doi: 10.1016/s0167-0115(00)00179-8. [DOI] [PubMed] [Google Scholar]
- 137.Dauge V., Lena I. CCK in anxiety and cognitive processes. Neurosci Biobehav Rev. 1998;22:815–825. doi: 10.1016/s0149-7634(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 138.De Oliveira RMW., Del Bel EA., Guimarães FS. Effects of excitatory amino acids and nitric oxide on flight behavior elicited from the periaqueductal gray. Neurosci Biobehav Rev. 2001;25:679–685. doi: 10.1016/s0149-7634(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 139.Schulz P., Walker JP., Peyrin L., Soulier V., Curtin F., Steimer T. Lower sex hormones in men during anticipatory stress. Neuroreport. 1996;7:3101–3104. doi: 10.1097/00001756-199611250-00061. [DOI] [PubMed] [Google Scholar]
- 140.Clement Y., Calatayud F., Belzung C. Genetic basis of anxiety-like behaviour: a critical review. Brain Res Bull. 2002;57:57–71. doi: 10.1016/s0361-9230(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 141.Rogers DC., Jones DNC., Nelson PR., et al. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 142.van Gaalen MM., Steckler T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav Brain Res. 2000;115:95–106. doi: 10.1016/s0166-4328(00)00240-0. [DOI] [PubMed] [Google Scholar]
- 143.Avgustinovich DF., Lipina TV., Bondar NP., Alekseyenko OV., Kudryavtseva NN. Features of genetically defined anxiety in mice. Behav Genet. 2000;30:101–109. doi: 10.1023/a:1001999020138. [DOI] [PubMed] [Google Scholar]
- 144.Bert B., Fink H., Huston JP., Voits M. Fisher 344 and Wistar rats differ in anxiety and habituation but not in water maze performance. Neurobiol Learn Mem. 2002;78:11–22. doi: 10.1006/nlme.2001.4040. [DOI] [PubMed] [Google Scholar]
- 145.Rex A., Sondern U., Voigt JP., Franck S., Fink H. Strain differences in fearmotivated behavior of rats. Pharmacol Biochem Behav. 1996;54:107–111. doi: 10.1016/0091-3057(95)02128-0. [DOI] [PubMed] [Google Scholar]
- 146.Wehner JM., Radcliffe RA., Bowers BJ. Quantitative genetics and mouse behavior. Annu Rev Neurosci. 2001;24:845–867. doi: 10.1146/annurev.neuro.24.1.845. [DOI] [PubMed] [Google Scholar]
- 147.Griebel G., Belzung C., Perrault G., Sanger DJ. Differences in anxietyrelated behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl). 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- 148.Garrett KM., Niekrasz I., Haque D., Parker KM., Seale TW. Genotypic differences between C57BL/6 and A inbred mice in anxiolytic and sedative actions of diazepam. Behav Genet. 1998;28:125–136. doi: 10.1023/a:1021424108213. [DOI] [PubMed] [Google Scholar]
- 149.Wigger A., Loerscher P., Weissenbacher P., Holsboer F., Landgraf R. Crossfostering and cross-breeding of HAB and LAB rats: a genetic rat model of anxiety. Behav Genet. 2001;31:371–382. doi: 10.1023/a:1012222402346. [DOI] [PubMed] [Google Scholar]
- 150.Plomin R., DeFries JC., McClearn GE., McGuffin P. Behavioral Genetics. 4th ed. New York, NY: Worth Publishers; 2000 [Google Scholar]
- 151.Wehner JM., Radcliffe RA., Rosman ST., et al. Quantitative trait locus analysis of contextual fear conditioning in mice. Nat Genet. 1997;17:331–334. doi: 10.1038/ng1197-331. [DOI] [PubMed] [Google Scholar]
- 152.Turri MG., Datta SR., DeFries J., Henderson ND., Flint J. QTL analysis identifies multiple behavioral dimensions in ethological tests of anxiety in laboratory mice. Curr Biol. 2001;11:725–734. doi: 10.1016/s0960-9822(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 153.Fernandez-Teruel A., Escorihuela RM., Gray JA., et al. A quantitative trait locus influencing anxiety in the laboratory rat. Genome Res. 2002;12:618–626. doi: 10.1101/gr.203402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Escorihuela RM., Fernandez-Teruel A., Gil L., Aguilar R., Tobeña A., Driscoll P. Inbred Roman high- and low-avoidance rats: differences in anxiety, novelty-seeking, and shuttlebox behaviors. Physiol Behav. 1999;67:19–26. doi: 10.1016/s0031-9384(99)00064-5. [DOI] [PubMed] [Google Scholar]
- 155.Steimer T., la Fleur S., Schulz PE. Neuroendocrine correlates of emotional reactivity and coping in male rats from the Roman high (RHA/Verh) and low (RLA/Verh)-avoidance lines. Behav Genet. 1997;27:503–511. doi: 10.1023/a:1021448713665. [DOI] [PubMed] [Google Scholar]
- 156.Roozendaal B., Wiersma A., Driscoll P., Koolhaas JM., Bohus B. Vasopressinergic modulation of stress responses in the central amygdala of the Roman high-avoidance and low-avoidance rat. Brain Res. 1992;596:35–40. doi: 10.1016/0006-8993(92)91529-n. [DOI] [PubMed] [Google Scholar]
- 157.Wiersma A., Knoellema S., Konsman JP., Bohus B., Koolhaas JM. Corticotropin-releasing hormone modulation of a conditioned stress response in the central amygdala of Roman high (RHA/Verh)-avoidance and low (RLA/Verh)-avoidance rats. Behav Genet. 1997;27:547–555. doi: 10.1023/a:1021457015482. [DOI] [PubMed] [Google Scholar]
- 158.Corda MG., Lecca D., Piras G., Di Chiara G., Giorgi O. Biochemical parameters of dopaminergic and GABAergic neurotransmission in the CNS of Roman high-avoidance and Roman low-avoidance rats. Behav Genet. 1997;27:527–536. doi: 10.1023/a:1021452814574. [DOI] [PubMed] [Google Scholar]
- 159.Aubry JM., Bartanusz V., Driscoll P., Schulz P., Steimer T., Kiss JZ. Corticotropin-releasing factor and vasopressin mRNA levels in Roman highand low-avoidance rats: response to open field exposure. Neuroendocrinology. 1995;61:89–97. doi: 10.1159/000126829. [DOI] [PubMed] [Google Scholar]
- 160.Charnay Y., Steimer T., Hugenin C., Driscoll P. [3H]Paroxetine binding sites: brain regional differences between two psychogenetically selected lines of rats. Neurosci Res Commun. 1995;16:29–35. [Google Scholar]
- 161.Liebsch G., Linthorst ACE., Neumann ID., Reul J., Holsboer FLR. Behavioral, physiological, and neuroendocrine stress responses and differential sensitivity to diazepam in two Wistar rat lines selectively bred for high- and low-anxiety-related behavior. Neuropsychopharmacology. 1998;19:381–396. doi: 10.1016/S0893-133X(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 162.Liebsch G., Montkowski A., Holsboer F., Landgraf R. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res. 1998;94:301–310. doi: 10.1016/s0166-4328(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 163.Landgraf R., Wigger A., Holsboer F., Neumann ID. Hyper-reactive hypothalamo-pituitary-adrenocortical axis in rats bred for high anxiety-related behaviour. J Neuroendocrinal. 1999;11:405–407. doi: 10.1046/j.1365-2826.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- 164.Henniger MSH., Ohl F., Hölter SM., et al. Unconditioned anxiety and social behavior in two rat lines selectively bred for high and low anxietyrelated behaviour. Behav Brain Res. 2000;111:153–163. doi: 10.1016/s0166-4328(00)00151-0. [DOI] [PubMed] [Google Scholar]
- 165.Hermann B., Landgraf R., Keck ME., et al. Pharmacological characterisation of cortical y-aminobutyric acid type A (GABAA) receptor in two Wistar rat lines selectively bred for high and low anxiety-related behaviour. World J Biol Psychiatry. 2000;1:137–143. doi: 10.3109/15622970009150581. [DOI] [PubMed] [Google Scholar]
- 166.Keck ME., Wigger A., Welt T., et al. Vasopressin mediates the response of the combined dexamethasone/CRH test in hyper-anxious rats: implications for the pathogenesis of affective disorders. Neuropsychopharmacology. 2002;26:94–105. doi: 10.1016/S0893-133X(01)00351-7. [DOI] [PubMed] [Google Scholar]
- 167.Henniger MSH., Spanagel R., Wigger A., Landgraf R., Hölter SM. Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacology. 2002;26:729–736. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 168.Sanchez MM., Ladd CO., Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 169.Heim C., Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 170.Heim C., Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 171.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 172.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 173.Wakshlak A., Weinstock M. Neonatal handling reverses behavioral abnormalities induced in rats by prenatal stress. Physiol Behav. 1990;48:289–292. doi: 10.1016/0031-9384(90)90315-u. [DOI] [PubMed] [Google Scholar]
- 174.Meaney MJ., Mitchell JB., Aitken DH., et al. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 1991;16:85–103. doi: 10.1016/0306-4530(91)90072-2. [DOI] [PubMed] [Google Scholar]
- 175.Plotsky PM., Meaney MJ. Early, post-natal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 176.Smythe JW., Rowe WB., Meaney MJ. Neonatal handling alters serotonin (5-HT) turnover and 5-HT2 receptor binding in selected brain regions: relationship to the handling effect on glucocorticoid receptor expression. Brain Res Dev Brain Res. 1994;80:183–189. doi: 10.1016/0165-3806(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 177.Tejedor-Real P., Costela C., Gibert-Rahola J. Neonatal handling reduces emotional reactivity and susceptibility to learned helplessness. Involvement of catecholaminergic systems. Life Sci. 1998;62:37–50. doi: 10.1016/s0024-3205(97)01036-9. [DOI] [PubMed] [Google Scholar]
- 178.Weizman R., Lehman J., Leschiner S., et al. Long-lasting effect of earlyhandling on the peripheral benzodiazepine receptor. Pharmacol Biochem Behav. 1999;64:725–729. doi: 10.1016/s0091-3057(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 179.Papaioannou A., Gerozissis K., Prokopiou A., Solaris S., Stylianopoulou F. Sex differences in the effects of neonatal handling on the animal's response to stress and the vulnerability for depressive behaviour. Behav Brain Res. 2002;129:131–139. doi: 10.1016/s0166-4328(01)00334-5. [DOI] [PubMed] [Google Scholar]
- 180.Beane ML., Cole MA., Spencer RL., Rudy JW. Neonatal handling enhances contextual fear conditioning and alter corticosterone stress responses in young rats. Horm Behav. 2002;41:33–40. doi: 10.1006/hbeh.2001.1725. [DOI] [PubMed] [Google Scholar]
- 181.Fernandez-Teruel A., Escorihuela RM., Driscoll P., Tobena A., Bättig K. Infantile (handling) stimulation and behavior in young Roman high- and low-avoidance rats. Physiol Behav. 1991;50:563–565. doi: 10.1016/0031-9384(91)90546-z. [DOI] [PubMed] [Google Scholar]
- 182.Fernandez-Teruel A., Escorihuela RM., Castellano B., Gonzàlez B., Tobena A. Neonatal handling and environmental enrichment effects on emotionality, novelty/reward seeking, and age-related cognitive and hippocampal impairments: focus on the Roman rat lines. Behav Genet. 1997;6:513–526. doi: 10.1023/a:1021400830503. [DOI] [PubMed] [Google Scholar]
- 183.Steimer T., Escorihuela RM., Fernandez-Teruel A., Driscoll P. Long-term behavioural and neuroendocrine changes in Roman high-(RHA/Verh) and low-(RLA/Verh) avoidance rats following neonatal handling. Int J Dev Neurosci. 1998;16:165–174. doi: 10.1016/s0736-5748(98)00032-x. [DOI] [PubMed] [Google Scholar]