Moderating the portion size of a protein-rich meal improves anabolic efficiency in young and elderly (original) (raw)
. Author manuscript; available in PMC: 2011 Oct 20.
Published in final edited form as: J Am Diet Assoc. 2009 Sep 1;109(9):1582–1586. doi: 10.1016/j.jada.2009.06.369
Abstract
Ingestion of sufficient dietary protein is a fundamental prerequisite for muscle protein synthesis and maintenance of muscle mass and function. The elderly are often at increased risk for protein-energy malnutrition, sarcopenia and a diminished quality of life. This study sought to compare changes in muscle protein synthesis and anabolic efficiency in response to a single moderate (113 g; 220 kcal; 30 g protein) or large serving (340 g; 660 kcal; 90 g protein) of 90% lean beef. Venous blood and vastus lateralis muscle biopsy samples were obtained during a primed, constant infusion (0.08 μmol/kg/min) of L-[ring-13C6] phenylalanine in healthy young (n=17; 34±3yrs) and elderly (n=17; 68±2yrs) individuals. Mixed muscle fractional synthesis rate (FSR) was calculated during a 3 h post-absorptive period and for 5 h following meal ingestion. Data were analysed using a two-way repeated measures ANOVA with Tukey's pair-wise comparisons. A 113 g serving of lean beef increased muscle protein synthesis by approximately 50% in both young and older volunteers. Despite a 3-fold increase in protein and energy content, there was no further increase in protein synthesis following ingestion of 340 g of lean beef in either age group. Ingestion of more than 30 g of protein in a single meal does not further enhance the stimulation of muscle protein synthesis in young and elderly.
Keywords: nutrition, stable isotopes, sarcopenia, diet, beef
Introduction
Ingestion of sufficient dietary protein is a fundamental prerequisite for muscle protein synthesis and maintenance of lean muscle mass and function. Elderly are at increased risk of protein-energy malnutrition (5, 8, 11) and sarcopenic muscle loss (2, 9). While it is clear that strategies to prevent and treat sarcopenia cannot exclusively target a single issue, recent commentary and research has suggested that moderately increasing dietary protein intake above the recommended dietary allowance of 0.8 g protein kg-1 d-1 may enhance muscle protein anabolism and offer additional benefits associated with increased satiety, thermogenesis and energy expenditure (1, 16, 25, 26, 29).
We recently demonstrated that a single moderate-size serving of a protein-rich food (113g lean beef) acutely increased muscle protein synthesis above fasting (baseline) values by 50% in both young and elderly individuals (20). A 113 g serving of 90% lean beef (220 kcal) contains approximately 30g of protein, 10 g of essential amino acids (EAA) and represents 50% of the Recommended Dietary Allowance for a 75 kg individual. While the results of this earlier study were particularly encouraging for older individuals, a number of questions remained unanswered. Cuthbertson et al. (6) noted that ingestion of 2.5, 5 or 10 g of rapidly digested free-form essential amino acids (EAA) increased myofibrillar protein synthesis in a dose-dependent manner. However, a larger 20-40 g serving of EAAs failed to elicit an additional stimulatory effect. In a practical sense, these data are consistent with the contention that a protein source containing approximately 10 g of essential amino acids provides a maximal acute protein synthetic effect. However, in the context of a more realistic meal-like setting, we do not know if a similar dose-response relationship exists in response to ingestion of a more slowly digested, high-quality intact protein such as lean beef (14, 15).
Compared to a moderately-sized protein meal (113 g lean beef; 30 g protein; 10 g EAA, 220 kcal), this study sought to determine if a 3-fold larger protein- and energy-rich meal (340 g lean beef; 90 g protein; 30 g EAA; 660 kcal), representative of the exaggerated portion size available in many restaurants, can be justified by an increased ability to acutely increase muscle protein synthesis in healthy young and elderly individuals.
Methods
Subjects and Experimental Design
Participants were recruited through The Sealy Center on Aging Volunteer Registry at The University of Texas Medical Branch (UTMB) and through newspaper advertisements and flyers. This study was approved by the Institutional Review Board at UTMB. An independent, internal monitoring board oversaw study procedures, data collection and analysis.
Medical screening included a medical history and physical, blood count, plasma electrolytes, blood glucose concentration, and liver and renal function tests. Eligible participants were free of any recent injury, the presence of a metabolically unstable medical condition, low hematocrit or hemoglobin, vascular disease, hypertension, or cardiac abnormality. All participants were physically active and independent but not athletically trained.
Seventeen young (8 male/9 female, 35±3 yr, 1.71±0.03 m, 79.2±7 kg; mean ±SD) and 17 elderly (10 male/7 female, 68±2 yrs, 1.70±0.04 m, 77.5±8 kg; mean ±SD). Apart from age, there were no between-group differences in demographic variables. Volunteers were randomly assigned to participate in one of four separate groups: young - 113 g beef (5 male, female), young - 340 g beef (3 male, 4 female), elderly - 113 g beef (5 male, 5 female), and elderly - 340 g beef (5 male, 2 female). There was no evidence of a gender effect (10, 21).
For seventy-two hours prior to admission, participants were asked to maintain their normal diet and avoided strenuous activity. Participants stayed overnight in the General Clinical Research Center and were studied the following morning after an overnight fast. Subjects remained largely physically inactive (i.e., rested in bed) for the duration of the study. On the morning of the study at approximately 0530, an 18-gauge polyethylene catheter (Insyte-W; Becton Dickinson, Sandy, UT) was inserted into a forearm vein for blood sampling. A second 18-gauge polyethylene catheter was inserted into a forearm vein of the contralateral limb for stable isotope tracer infusion. Background blood samples were drawn for the analysis of phenylalanine enrichments and concentrations, insulin (serum separator tubes; BD Vacutainer SST, Franklin Lakes, NJ) and glucose concentrations (CapiJect tubes; Terumo Medical Corp., Elkton, MD). A primed (2 μmol/kg), constant infusion (0.08 μmol·kg-1min-1) of L- [ring-13C6] phenylalanine (Cambridge Isotope Laboratories, Andover, MA) was started and maintained for 11 hours.
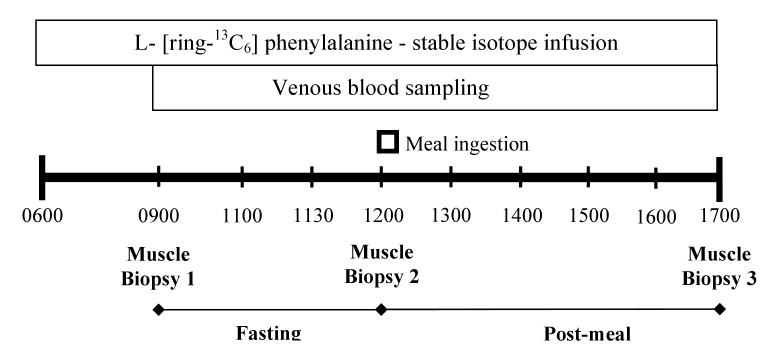
During the post-absorptive period (0900-1200), venous blood samples were obtained hourly. Following ingestion of the lean beef meal (113 or 340 g), venous blood samples were obtained every twenty minutes for the duration of the study (5 hours), (see Figure 1). Muscle biopsies, approximately 100 mg, were taken at three time points under local anesthesia (2% lidocane) from the lateral portion of the vastus lateralis of the leg using a 5 mm Bergstrom biopsy needle as previously described (3). The biopsy site was approximately 10 cm to 15 cm above the knee.
Figure 1.
Data were collected during an 11hr stable isotope infusion protocol. Muscle biopsy and venous blood samples were obtained to calculate post absorptive and postprandial mixed muscle protein synthesis.
The 90% lean ground beef patties (113 g; 220 kcal; 30 g protein, 11 g fat per patty) were prepared and supplied by Texas Tech University. Patties were pre-cooked, individually vacuumed sealed and frozen prior to delivery to UTMB. The patties were gently warmed in a microwave oven and provided to the participant without condiments immediately following the second biopsy. Participants in the higher protein group consumed 3 beef patties. All volunteers were able to consume the meal within 10-15 minutes.
Analytical Methods
All analytical methods have been described in detail previously (20, 27, 28). Briefly, plasma phenylalanine was extracted by cation exchange chromatography (Dowex AG 50W-8X, 100-220 mesh H+ form; Bio-Rad Laboratories, Richmond, CA) and dried under vacuum (Savant Instruments, Farmingdale, NY). Phenylalanine enrichments and concentrations were determined with _tert_-butyldimethylsilyl derivative using gas chromatography-mass spectrometry (6890 Plus GC, Agilent Technologies, Palo Alto, CA) with electron impact ionization. Ions 234, 238, 240, 336, 342, and 346 were monitored (17, 30).
Mixed muscle intracellular phenylalanine enrichments and concentrations were calculated with a _tert_-butyldimethylsilyl derivative. Mixed muscle protein-bound L-[ring-13C6] phenylalanine enrichments were determined using gas chromatography-mass spectrometry via the standard curve approach as previously described (28).
Calculations
Mixed muscle protein fractional synthesis rate (FSR) was calculated by measuring the direct incorporation of L- [ring-13C6] phenylalanine into protein, via the precursor-product model:
FSR=[(Ep2−Ep1)/(Em•t•CF)]•60•100
where E p1 and E p2 are the enrichments of bound L- [ring-13C6] phenylalanine in two sequential biopsies, t is the time interval between two biopsies, and _E_m is the mean L-[ring-13C6] phenylalanine enrichment in the muscle intracellular pool.
In order to account for the decreased plasma L- [ring-13C6] phenylalanine enrichment and isotopic non-steady state during the post-meal period (a source of non-labeled phenylalanine) a correction factor (CF) was employed (20).
where _E_v(AUC) is the actual venous enrichment area under the curve between sequential biopsies (i.e., biopsy 2 and 3) (Figure 1) and _E_v(m2,m3) is the average venous enrichment at each biopsy time point. This correction is based on the assumption that the transient post-meal decrease in plasma phenylalanine enrichment reflects the decrease in the muscle intracellular phenylalanine enrichment.
Statistical Analysis
Changes in muscle protein synthesis were analyzed using a two-way repeated measures analysis of variance with within (time) and between (age) group factors. Secondary analyses were performed using pair-wise multiple comparison procedures with Tukey correction. Data are presented as means ± standard error of the means (SEM). Statistical analysis was performed using SigmaStat for Windows (version 3.5; Systat Software, Inc., San Jose, CA). Statistical significance for all analyses was accepted at α = 0.05.
Results and Discussion
Fasting plasma phenylalanine enrichments (tracer/tracee ratio) were similar in the moderate (young: 0.112±0.003; elderly: 0.113±0.002) and high protein groups (young: 0.101±0.008; elderly: 0.113±0.002), (p>0.05). Following meal ingestion there was an expected dilution of the labeled plasma phenylalanine pool. Mean post-prandial enrichment values in the moderate protein group (113 g beef) were 0.105±0.002 (young) and 0.105±0.004 (elderly), (p>0.05), while enrichment values in the high protein group (340 g beef) were 0.090±0.008 (young) and 0.092±0.009 (elderly), (p>0.05). As described, a correction factor was applied to account for the transient post-prandial decrease in the precursor enrichment and subsequent underestimation of mixed muscle fractional synthesis rate (20).
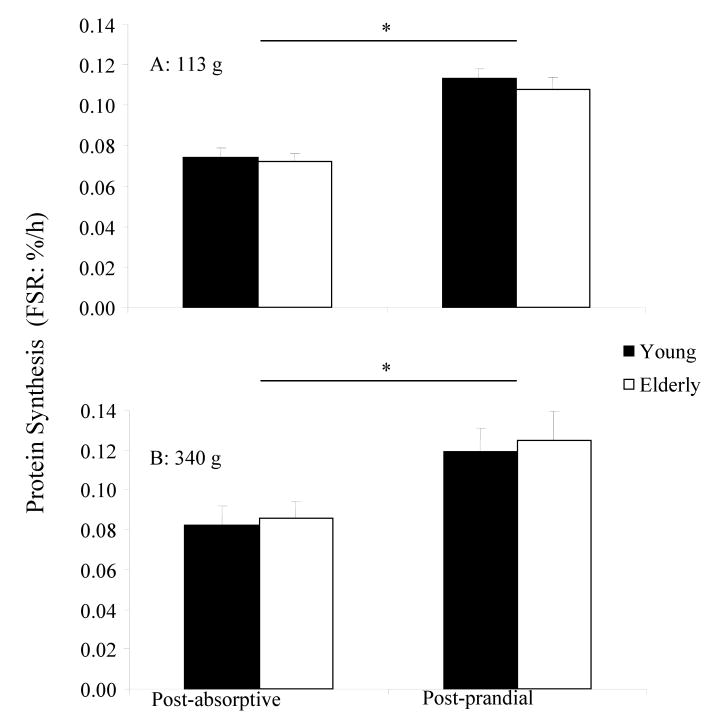
Protein synthesis following ingestion of 113 g and 340 g of lean beef are presented in Figure 2. Post-absorptive mixed muscle FSR values were similar in all groups and did not differ with age. Ingestion of 340 g of lean beef increased mixed muscle FSR increased by ∼46 % (p = 0.008) in both the young and the elderly. This was consistent with the 50% increase following ingestion of 113 g of lean beef (20). Dose- and age-specific differences were too small to be considered physiologically relevant, particularly if considered in the context of the myriad of additional factors that would influence protein synthesis in a real world setting.
Figure 2.
Mean (± SEM) mixed muscle fractional synthesis rate before and after ingestions of 113 g (A), 12 or 340 g (B) of 90% lean ground beef in young and the elderly. * Significant increase from fasting (young and elderly) following 113g and 340g of lean beef.
There is little debate that the ingestion of high quality protein is of paramount importance in the maintenance of muscle mass and function in the elderly. To this end, our findings are consistent with previous work demonstrating an improved protein synthetic response to intact protein source such as whey protein, milk and beef (4, 7, 14, 20). However, in circumstances where the total ingested protein content is low (i.e., EAA content less than ∼7 g), (12) or when glucose and amino acids are co-ingested (23) the protein synthetic response of elders may be blunted compared to their younger counterparts. These findings may have considerable practical significance should they reflect the response to the smaller, mixed nutrient meals commonly consumed by many older adults.
While a blunted protein-anabolic response to a small, mixed nutrient meal may, over-time, contribute to the development of sarcopenia (23), there is no age-related discrepancy in muscle protein synthesis following ingestion of a higher total amino acid load (6, 15, 22, 24). In the current study, participants consumed approximately 30 g or 90 g of high-quality protein in a single serving. The key finding was that no further protein synthetic advantage was elicited by the larger meal when compared to the response to a more moderate 30g protein serving (20). In terms of stimulating muscle growth, it therefore appears likely that under resting/non-exercising conditions, consumption of more than 30 g of protein in a single meal is not justified. Indeed, it may well be the case that a slightly smaller meal would produce a similar protein synthetic response.
The data presented in this study represent a practical extension of previous proof of concept research that has largely focused on amino acid or whey protein supplementation (12-15). Nevertheless, there are several limitations that could influence our results. Perhaps the most obvious is the fact that a single menu item, such as a serving of lean beef, is seldom eaten alone. As noted, there are some data suggesting elders may have a less robust protein synthetic response to the combined ingestion of protein and carbohydrate than their younger counterparts (23). This has yet to be explored in the context of an actual mixed-nutrient meal, but warrants further investigation. Further, there is the potential of an added protein synthetic response if protein were to be consumed in combination with physical activity (18, 19).
In summary, a large 340 g serving of lean beef, increases mixed muscle protein synthesis by approximately 50% in both the young and the elderly. However, a moderate size portion (113 g) represents an equally effective and more energetically efficient means of stimulating muscle protein synthesis than the 3-fold larger serving. We suggest that instead of a single, large protein-rich meal, ingestion of multiple moderate-sized servings of high quality protein-rich foods over the course of a day may represent an effective means of optimizing the potential for muscle growth while permitting greater control over total energy and nutrient intake.
Acknowledgments
The authors would like to thank David Chinkes, Tara Cocke, Christopher Danesi and Scott Schutzler for their assistance in data collection and analysis. DPJ and RRW contributed to the original experimental design. TBS, MSM, and DPJ were responsible for data acquisition and data analysis. TBS drafted the manuscript under the supervision of DPJ and MSM. All authors contributed to the interpretation of the results and take responsibility for the work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
T. Brock Symons, Division of Rehabilitation Sciences. The University of Texas Medical Branch*.
Melinda Sheffield-Moore, Email: melmoore@utmb.edu, Department of Internal Medicine. 301 University Blvd. The University of Texas Medical Branch. Galveston, Tx. 77555-1060. Tel: 409-772-8707; Fax: 409-772-8709.
Robert R. Wolfe, Department of Surgery. The University of Texas Medical Branch*.
Douglas Paddon-Jones, Email: djpaddon@utmb.edu, Departments of Physical Therapy and Internal Medicine. 301 University Blvd. The University of Texas Medical Branch. Galveston, Tx. 77555-1144. Tel.: 409-772-3073; Fax: 409-747-1613.
References
- 1.Astrup A. The satiating power of protein - a key to obesity prevention? Am J Clin Nutr. 2005;82:1–2. doi: 10.1093/ajcn.82.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 3.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 4.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–380. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 7.Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballevre O, Beaufrere B. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol. 2003;549:635–644. doi: 10.1113/jphysiol.2002.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 9.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 10.Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007;292:E77–83. doi: 10.1152/ajpendo.00173.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 12.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 13.Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJ, van Loon LJ. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006;84:623–632. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 14.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 16.Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr. 2008;87:1558S–1561S. doi: 10.1093/ajcn/87.5.1558S. [DOI] [PubMed] [Google Scholar]
- 17.Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metab. 1997;46:943–948. doi: 10.1016/s0026-0495(97)90084-6. [DOI] [PubMed] [Google Scholar]
- 18.Phillips SM, Hartman JW, Wilkinson SB. Dietary protein to support anabolism with resistance exercise in young men. J Am Coll Nutr. 2005;24:134S–139S. doi: 10.1080/07315724.2005.10719454. [DOI] [PubMed] [Google Scholar]
- 19.Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287:E513–522. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- 20.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 21.Tipton KD. Gender differences in protein metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:493–498. doi: 10.1097/00075197-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 25.Westerterp-Plantenga MS. The significance of protein in food intake and body weight regulation. Curr Opin Clin Nutr Metab Care. 2003;6:635–638. doi: 10.1097/00075197-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Westerterp-Plantenga MS, Lejeune MP, Nijs I, van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord. 2004;28:57–64. doi: 10.1038/sj.ijo.0802461. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine:Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. Protein synthesis and breakdown; pp. 377–417. [Google Scholar]
- 28.Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss; 1992. [Google Scholar]
- 29.Wolfe RR, Miller SL. The recommended dietary allowance of protein: a misunderstood concept. Jama. 2008;299:2891–2893. doi: 10.1001/jama.299.24.2891. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Chinkes DL, Sakurai Y, Wolfe RR. An isotopic method for measurement of muscle protein fractional breakdown rate in vivo. Am J Physiol (Endocrinol Metab) 1996;270:E759–E767. doi: 10.1152/ajpendo.1996.270.5.E759. [DOI] [PubMed] [Google Scholar]