Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition (original) (raw)
Abstract
There are currently few treatment options for pulmonary fibrosis. Innovations may come from a better understanding of the cellular origin of the characteristic fibrotic lesions. We have analyzed normal and fibrotic mouse and human lungs by confocal microscopy to define stromal cell populations with respect to several commonly used markers. In both species, we observed unexpected heterogeneity of stromal cells. These include numerous cells with molecular and morphological characteristics of pericytes, implicated as a source of myofibroblasts in other fibrotic tissues. We used mouse genetic tools to follow the fates of specific cell types in the bleomcyin-induced model of pulmonary fibrosis. Using inducible transgenic alleles to lineage trace pericyte-like cells in the alveolar interstitium, we show that this population proliferates in fibrotic regions. However, neither these cells nor their descendants express high levels of the myofibroblast marker alpha smooth muscle actin (Acta2, aSMA). We then used a Surfactant protein C-CreERT2 knock-in allele to follow the fate of Type II alveolar cells (AEC2) in vivo. We find no evidence at the cellular or molecular level for epithelial to mesenchymal transition of labeled cells into myofibroblasts. Rather, bleomycin accelerates the previously reported conversion of AEC2 into AEC1 cells. Similarly, epithelial cells labeled with our Scgb1a1-CreER allele do not give rise to fibroblasts but generate both AEC2 and AEC1 cells in response to bleomycin-induced lung injury. Taken together, our results show a previously unappreciated heterogeneity of cell types proliferating in fibrotic lesions and exclude pericytes and two epithelial cell populations as the origin of myofibroblasts.
Fibrosis, the replacement of normal tissue with ECM, is a common pathological response of organs to injury, inflammation, or stress. It imposes a major clinical burden when organ function is compromised (1, 2). In the lung, characteristic features of fibrosis include the focal accumulation of cells with fibroblast-like morphology and excessive production of ECM. Idiopathic pulmonary fibrosis (IPF) is a fatal lung disease, and the characteristic histology, including honeycombing or bronchiolization of the alveoli, is termed usual interstitial pneumonia (UIP) (3, 4). These changes disrupt tissue architecture and in the absence of effective repair, lead to a progressive loss of lung function. Several injurious stimuli have been associated with pulmonary fibrosis. Examples are autoimmune responses, environmental exposures, and mutations in genes highly expressed by lung epithelium, including those mutations causing endoplasmic reticulum stress (5). However, the etiology of IPF, the most common fibrotic lung disease in adults, is, by definition, not well-understood, and effective therapies are limited. Lung transplant, with the lowest 5-y survival of any solid organ, is the only therapy that can prolong survival in end-stage IPF (6).
At least three cell populations have been proposed as the source of the fibroblast-like cells that secrete ECM proteins in the context of pulmonary fibrosis. The most parsimonious model is that resident stromal cells are activated in response to local or systemic stimuli to proliferate and change their gene expression, including the up-regulation of α-smooth muscle actin (Acta2 and aSMA) and secretion of ECM (7). A second proposed source of fibrotic cells is circulating bone marrow-derived fibrocytes (8, 9). Finally, it has been suggested that epithelial cells of the lung, including type 2 alveolar epithelial cells (AEC2), undergo epithelial to mesenchymal transition (EMT) to give rise to fibroblasts or cells with fibroblast-like morphology (10–16). In some cases, these EMTs have been estimated to contribute 50% of the fibroblasts in fibrotic lesions (11, 15). Much of the human data to support each of these hypotheses is based on retrospective immunohistochemical analysis of fixed samples and the in vitro manipulation of primary cells from fibrotic lungs or cell lines. The analysis of these experiments is also complicated by the fact that the in vivo lineage relationships of stromal cells, even in the healthy lung, are poorly understood.
An in vivo model for IPF is bleomycin-induced pulmonary fibrosis in mice (17). In this model, administration of the antineoplastic drug bleomycin, either intratracheally or systemically, leads to the development of patchy fibrotic lesions followed by slow repair. This is in marked contrast to the progressive pathological remodeling that is characteristic of pulmonary fibrosis in humans. Several in vivo lineage tracing experiments carried out in the context of bleomycin-induced pulmonary fibrosis in mice have found evidence to support the EMT of AEC2 cells (11, 13, 15). However, these studies used a limited number of reporter alleles and markers for different stromal cell types and did not combine immunohistochemistry with confocal analysis of large areas of the lung.
Here, we use a combination of techniques to investigate the contributions of several cell types to pulmonary fibrosis. We use confocal analysis of normal and fibrotic human and mouse lungs with a wide range of immunohistochemical markers for stromal cell types, including pericytes. This cell type has been implicated as a source of myofibroblasts in other fibrotic processes (18, 19) but has not been studied in relation to pulmonary fibrosis. We perform in vivo genetic lineage tracing with inducible CreER alleles and fluorescent reporter alleles compatible with confocal analysis. Specifically, we follow the fates of cells expressing pericyte markers (herein, pericyte-like cells) within the alveolar interstitium as well as AEC2 cells and Scgb1a1-positive cells of the airways and alveoli. Our data suggest that resident stromal populations proliferate within fibrotic foci. However, lineage-labeled pericyte-like cells do not express high levels of the myofibroblast marker aSMA. We show that AEC2 and Scgb1a1-positive cells are epithelial progenitor populations that give rise to alveolar lineages, including AEC1, in response to bleomycin-induced lung injury. However, our data suggest that these cells are not a major source of myofibroblasts through EMT. These conclusions should help to focus future efforts at identifying the origin of the matrix producing fibroblasts and developing molecular therapies for fibrotic lung disease.
Results
Heterogeneity of Stromal Cell Types in a Mouse Model of Pulmonary Fibrosis.
There is surprisingly little information about the molecular identity, localization, and abundance of the different stromal cell types described by classic electron microscopic studies in the adult rodent and human lung. These cell types include fibroblasts, myofibroblasts, lipocytes, vascular and airway smooth muscle, pericytes, and lymphatics (20–23). Moreover, there is no consensus of the expression by these cells of markers commonly used to analyze pathologic changes in the fibrotic lung (aSMA, vimentin, S100a4, desmin, and _N_-cadherin) (11–16, 24). To begin to address this problem, we used immunofluorescence and confocal microscopy to localize stromal markers in the lungs of control mice or mice 14–21 d after the intratracheal administration of bleomycin (Fig. 1 and Fig. S1). Importantly, we examined multiple optical sections through the thickness of the tissue section for the unambiguous identification of cell boundaries.
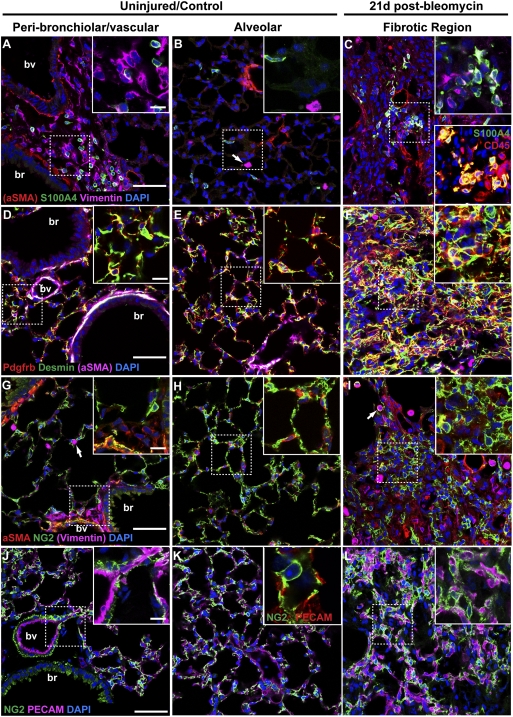
Fig. 1.
Heterogeneity of stromal cells in normal and fibrotic mouse lungs. Sections of normal mouse lungs (A, B, D, E, G, H, J, and K) and fibrotic lungs collected 21 d after the intratracheal administration of 1.25 U/kg bleomycin (C, F, I, and L) were stained with antibodies against stromal cell markers. Single images from representative confocal stacks are shown. In some cases, one marker (noted in parentheses) is omitted from high-magnification Insets for clarity. (A–C) aSMA (red), S100a4 (green), and vimentin (purple). Arrows in B, G, and I mark vimentin-positive macrophage identified by shape and location within the alveolar space. (C) Note increase in S100a4-positive cells in fibrotic regions. Some of these cells coexpress vimentin (Inset Upper, white) and CD45, a marker of hematopoietic cells (Inset Lower, yellow). (Note that this image is from a different fibrotic lung.) (D –F) Pdgfrb (red), desmin (green), and aSMA (purple). Note coexpression of desmin and aSMA (white) in smooth muscle cells around blood vessels and bronchioles. Desmin is also expressed in Pdgfrb-positive pericytes (D –F Insets, yellow) and Pdgfrb-negative fibroblasts (D and E Insets, green) in the alveoli. Desmin-positive cells, with and without coexpression of Pdgfrb, are abundant within fibrotic foci. (G–I) aSMA (red), NG2 (Cspg4; green), and vimentin (purple). NG2-positive cells coexpress aSMA (yellow) around blood vessels, but the abundant NG2-positive pericyte-like cells within the alveolar interstitium do not express aSMA. Fibrotic areas contain large numbers of NG2-positive cells, most of which do not express aSMA (yellow). (J–L) NG2 (green) and PECAM (CD31; purple). Pericyte-like cells are in close association with pulmonary microvasculature, even in areas of fibrosis. Dashed boxed region of each image is shown at higher magnification in Insets. J Inset shows NG2 (green) and PECAM (red) at high magnification for clarity. br, bronchiole; bv, blood vessel. (Scale bars: A, D, G, and J, 50 μm; Insets, 20 μm.)
As previously described for human and rat lungs, aSMA marks perivascular and peribronchiolar smooth muscle as well as cells present discontinuously around the walls of the smallest venules and fibroblasts at the alveolar entrance ring (20) (Fig. 1 A, B, D, E, G, and H and Fig. S1 A and D). Importantly and consistent with previous studies, we did not detect aSMA in abundant interstitial cells, including the alveolar myofibroblasts or contractile interstitial cells that are known from anatomical studies to reside in the uninjured stroma (Fig. 1 B, E, and H and Fig. S1 A and D) (20).
The intermediate filament protein vimentin marks stromal cells with a fibroblastic morphology around airways and vasculature (Fig. 1 A and G and Fig. S1_A_). Consistent with a previous report (25), some alveolar macrophages also express vimentin (Fig. 1 B and G). Desmin, another intermediate filament protein associated with contractile cells throughout the body, is present in aSMA-positive vascular and bronchiolar smooth muscle and numerous aSMA-negative interstitial cells (Fig. 1 D and E). Some of these interstitial cells also express Pdgfrb and are likely pericytes (see below) (26).
S100a4 (also known as fibroblast-specific protein 1) has been used as a marker of fibroblasts in the lung (11, 15, 24). We observed stellate S100a4-positive cells located near vasculature and airways, and other S100a4-positive cells were elongated and located within the interstitium (Fig. 1 A and B and Fig. S1_A_). S100a4 did not colocalize with aSMA in either population. Some S100a4-positive cells coexpressed vimentin and/or CD45 (Fig. 1 A and C and Fig. S1_A_).
Finally, we examined lungs for the expression of markers of pericytes, a heterogeneous cell type that is, by definition, intimately associated with the vasculature. In the pre- and postcapillary vessels of the lung, we found cells that express aSMA, Pdgfrb, and NG2 (nerve/glial antigen 2, Cspg4), validated markers of pericytes in other tissues (26, 27) (Fig. 1 G and J and Fig. S1_D_). With respect to the capillaries of the lung, the existence of pericytes has been a matter of debate. There is certainly good evidence in the literature for the existence of cells with ultrastructural similarities to pericytes, including partial localization within the capillary basement membrane and numerous processes that contact endothelium, on the microvasculature of the lung (23). Indeed, quantitative ultrastructural studies suggest that ∼90% of capillaries in bovine lungs contact pericytes that cover ∼25% of the abluminal capillary surface area (28, 29). We observed abundant NG2-positive, Pdgfrb-positive, and desmin-positive (but aSMA-negative) cells in the interstitium intimately associated with PECAM (CD31) positive pulmonary capillaries (Fig. 1 E, H, and K and Fig. S1_D_). The facts that these cells express three accepted markers of pericytes, are intimately associated with lung microvasculature, and are morphologically similar to pericytes in other tissues suggest that they are indeed pericytes. However, lacking ultrastructural immunolocalization data, we refer to them here as pericyte-like cells.
Next, we analyzed multiple fibrotic regions by immunohistochemistry and confocal microscopy 14 and 21 d after bleomycin (Fig. 1 C, F, I, and L and Fig. S1 B, C, E, and F). This analysis revealed several important features. First, there is increased expression of all stromal markers in the fibrotic regions. These markers include the markers for pericytes described above (NG2, desmin, and Pdgfrb) that, to our knowledge, have not been described previously in relation to pulmonary fibrosis (Fig. 1 F, I, and L and Fig. S1 E and F). Second, the abundance of aSMA-positive cells was higher at 14 than 21 d (Fig. S1). This finding, consistent with previous reports from rodent and human fibrotic lungs (30, 31), suggests that the expression of aSMA in myofibroblasts is dynamic over the course of fibrosis, with higher levels early in progression. Lastly, there is an increase in the number of cells expressing CD45 after bleomycin (Fig. 1_C_). The simplest explanation is that these are macrophages, dendritic cells, and other immune cells, subsets of which have been reported to express vimentin and S100a4 (25, 32, 33). However, at least one report suggests that fibroblasts coexpress CD45 and S100A4 (34).
Heterogeneity of Stromal Cell Types in Human Pulmonary Fibrosis.
Next, we performed immunofluorescent staining and confocal microscopic analysis of the markers described above on normal and fibrotic human lung tissue. We obtained samples from normal lungs taken at the time of transplantation (n = 2) and patients undergoing diagnostic open lung biopsy for interstitial lung disease (n = 8). All eight biopsy samples showed a UIP histologic pattern. Four patients were diagnosed with IPF, and the remaining four patients had some suggestion of underlying connective tissue disease (Table S1). In normal lungs, as in the mouse, aSMA-positive cells were usually associated with blood vessels, and some of these coexpressed NG2. However, there were not abundant aSMA-positive cells within the interstitium (Fig. S2_A_). Also, like the mouse, we observed a population of NG2-positive (aSMA-negative) interstitial cells in the normal human lung that we call pericyte-like cells based on morphology, marker expression, and close association with pulmonary microvasculature (Fig. S2_A_). In fibrotic lungs, we detected abundant cells expressing either NG2 or aSMA, suggesting that these populations had expanded. However, most cells (except those cells associated with the vasculature as in control lungs) did not express both markers (Fig. S2_B_). Quantitative colocalization analysis with Imaris software confirmed that some cells coexpress aSMA and NG2 in fibrotic lungs, and most of these cells seem to be associated with vasculature as in normal lungs (Fig. S2 C and C′). Cells expressing aSMA or NG2 were not prominent within fibroblast foci characteristic of IPF (Fig. S2 B and B′, arrows). Together, these data suggest that a similarly heterogeneous population of stromal cells contributes to pulmonary fibrosis in humans and the mouse model of bleomycin-induced pulmonary fibrosis.
Proliferation of Resident Stromal Populations in the Context of Pulmonary Fibrosis.
To test the hypothesis that pericyte-like cells are a source of myofibroblasts in the context of pulmonary fibrosis, we crossed a previously described NG2-CreER BAC transgenic mouse line (35) to a ROSA-farnesylated GFP (fGFP) reporter strain. Double heterozygous mice were given four doses of tamoxifen to label NG2-positive pericyte-like cells of the alveolar interstitium with membrane-associated GFP for lineage tracing studies. This regimen induced label in 14 ± 4.9% of NG2-positive pericyte-like cells when sections were scored 3–6 d after the final dose of tamoxifen (mean ± SEM, n = 3 mice). In control animals, labeled cells remain as single cells or small clones after 2 or 4 wk (Fig. 2 A and D–F). In contrast, in fibrotic regions 14 or 21 d after intratracheal bleomycin, NG2-positive cells constitute a large proportion of the cells in fibrotic regions, and lineage-labeled pericyte-like cells are frequently found in multicellular clusters within the lesions (Fig. 2 B, C, and G–I). These cells continue to express NG2 and Pdgfrb, but most do not express high levels of aSMA. To determine if these cells proliferate in response to bleomycin-induced lung injury, we gave NG2-CreER;ROSA-fGFP mice intratracheal bleomycin or saline control. Fourteen days later, mice were given i.p. BrdU, and their lungs were collected for histology 3 h later. In control lungs, only 0.51 ± 0.32% of lineage-labeled cells incorporated BrdU (mean ± SEM, n = 3 mice). The proportion of proliferating lineage-labeled cells increased nearly fivefold to 2.46 ± 0.15% in fibrotic regions after bleomycin (Fig. 2_C_) (mean ± SEM, n = 4 mice). These data show that pericyte-like interstitial cells proliferate in the context of bleomycin-induced pulmonary fibrosis.
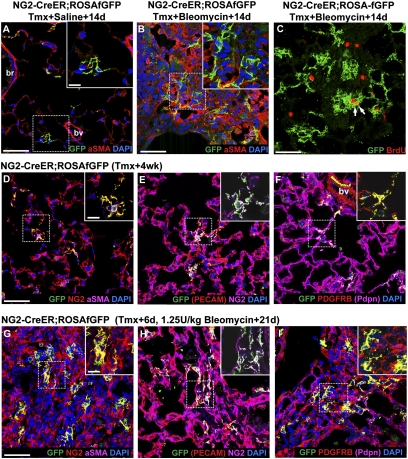
Fig. 2.
NG2-CreER labels pericyte-like interstitial cells that proliferate in pulmonary fibrosis. (A and B) NG2-CreER;ROSA-fGFP double heterozygous mice were given four doses of tamoxifen followed 4 d later with intratracheal (A) saline or (B) 1.25 U/kg bleomycin; 14 d later, sections were stained with antibodies against GFP and aSMA (red). Note that the lineage label does not colocalize with aSMA in normal or fibrotic lungs. (C) NG2-CreER;ROSA-fGFP double heterozygous mice were given tamoxifen and bleomycin as above. BrdU was given intraperitoneally 3 h before animals were killed 14 d later. Sections were stained with antibodies against GFP (green, lineage label) and BrdU (red, proliferative cells). The percentage of proliferating lineage-labeled pericyte-like cells (arrows) increases from 0.51% in controls (n = 3 mice) to 2.46% in fibrotic regions after bleomycin (n = 4 mice). (D –I) NG2-CreER;ROSA-fGFP double heterozygous mice were given four doses of tamoxifen followed 4 d later with intratracheal (D –F) saline or (G–I) 1.25 U/kg bleomycin; 21 d later, sections were stained with antibodies against GFP (green, lineage label), (D and G) NG2 (red) and aSMA (purple), (E and H) PECAM (red) and NG2 (purple), or (F and I) Pdgfrb (red) and Pdpn (purple). (D –F) Lineage-labeled pericyte-like cells (D, yellow) are intimately associated with the pulmonary microvasculature and express (E) NG2 and (F) Pdgfrb. (G–I) In fibrotic regions following bleomycin, the lineage-labeled pericyte-like population (yellow) expands and continues to express (G and H) NG2 and (I) Pdgfrb. The dashed boxed region of each image is shown at higher magnification in Insets. In some cases, one marker (noted in parentheses) is omitted from high-magnification Insets for clarity. br, bronchiole; bv, blood vessel. (Scale bars: A, B, C, D, and G, 50 μm; Insets A, D, and G, 20 μm.)
During the course of ongoing experiments, we determined that a transgenic FoxJ1-CreER allele developed in our laboratory (36) is also expressed in NG2-positive pericyte-like cells of the lung. We, therefore, injected adult FoxJ1-CreER;ROSA-fGFP mice with tamoxifen to induce recombination in these cells (as well as multiciliated cells of airways). This Cre driver labels pericyte-like interstitial cells more efficiently than NG2-CreER, but we do not know whether this finding reflects a transgene insertion artifact or expression of FoxJ1. Consistent with our results using the NG2-CreER allele, the pericyte-like population labeled with FoxJ1-CreER proliferates in response to bleomycin. These cells, which constitute a large proportion of cells within fibrotic regions of the lung, continue to express NG2, but most do not express high levels of aSMA (Fig. S3).
BrdU-positive proliferative cells were observed in fibrotic regions that were not lineage labeled with NG2-CreER (or Foxj1-CreER). This finding suggests that other stromal populations also proliferate in response to injury by bleomycin. A Pdgfra-H2B:GFP knock-in allele [Pdgfratm11(EGFP)Sor] (37) reports endogenous Pdgfra gene expression in perivascular and peribronchiolar cells. These cells coexpress Pdgfrb (and variably, aSMA) but are negative for NG2 and PECAM (Fig. S4 A and C). Pdgfra-H2B:GFP–positive cells are also widespread in the alveoli, and these cells do not express aSMA, Pdgfrb, or NG2 (Fig. S4_B_). These interstitial cells might correspond to a subset of alveolar fibroblasts, myofibroblasts, or contractile interstitial cells (20). We administered BrdU to adult Pdgfra-H2B:GFP mice 6 d after intratracheal bleomycin or saline to test whether these cells proliferate during the development of pulmonary fibrosis. We did not observe any BrdU-positive Pdgfra-H2B:GFP+ cells in saline controls (n = 1 mouse, 3,522 cells scored). In contrast, 3.15 ± 1.3% Pdgfra-H2B:GFP+ cells were BrdU-positive 6 d after bleomycin (mean ± SEM, n = 3 mice). These proliferative cells were usually near vessels and airways (Fig. S4_D_).
AEC2 Cells Are Not a Major Source of Fibroblasts Through the EMT.
According to one current hypothesis, up to 50% of the stromal cells associated with pulmonary fibrosis are derived from alveolar epithelial cells that undergo EMT (10, 11, 13, 16). To test this hypothesis, we generated an Sftpc-CreERT2 knock-in allele to lineage trace AEC2 cells in vivo in the context of bleomycin-induced pulmonary fibrosis. We injected adult Sftpc-CreERT2;ROSA-Tomato double heterozygous mice with four doses of tamoxifen to induce recombination at the ROSA locus and heritable expression of a td-Tomato variant of red fluorescent protein (RFP) in 84.2 ± 4% of Sftpc-positive AEC2 cells (Fig. 3_A_). Four to ten days later, 1.25 U/kg bleomycin were delivered intratracheally to induce pulmonary fibrosis. Control mice received tamoxifen followed by intratracheal saline. In control animals 5 d after injection with saline, 0.5 ± 0.5% of lineage-labeled cells were scored as AEC1 based on morphology and the expression of Aqp5 and Pdpn (Fig. 3_B_) (mean ± SD, n = 3 mice for each data point). This value rose modestly to 2.9 ± 2.5% at 10 d and remained relatively constant at 1.2 ± 0.2% after 3 wk. These data suggest that at least a subset of AEC2 is a progenitor population capable of giving rise to AEC1 under steady-state conditions. In contrast, the proportion of lineage-labeled AEC1 derived from Sftpc-positive cells was much greater in fibrotic regions after bleomycin compared with relatively unaffected areas and control animals. After 5, 10, and 21 d, the proportions of lineage-labeled AEC1 cells were 5.6 ± 4.6%, 34.4 ± 14.4%, and 41.1 ± 4.4%, respectively (Fig. 3_B_). Sixty days after bleomycin, fibrotic regions had been repaired to nearly normal histology, and large areas of lineage-labeled AEC1 were obvious (Fig. 3 C and D). Fewer lineage-labeled AEC1 cells were observed after the same period in saline controls (Fig. 3_E_). Consistent with a recent report (38), the proportion of lineage-labeled Sftpc-positive cells in fibrotic regions was decreased compared with histologically normal regions and the lungs of saline-treated control mice (Fig. S5). Together, these data suggest that the kinetics of AEC2 to AEC1 conversion are enhanced in the context of pulmonary fibrosis. Moreover, Sftpc-positive AEC2 cells can be derived from an Sftpc-negative progenitor population or at least, a population that is not efficiently labeled by Sftpc-CreER.
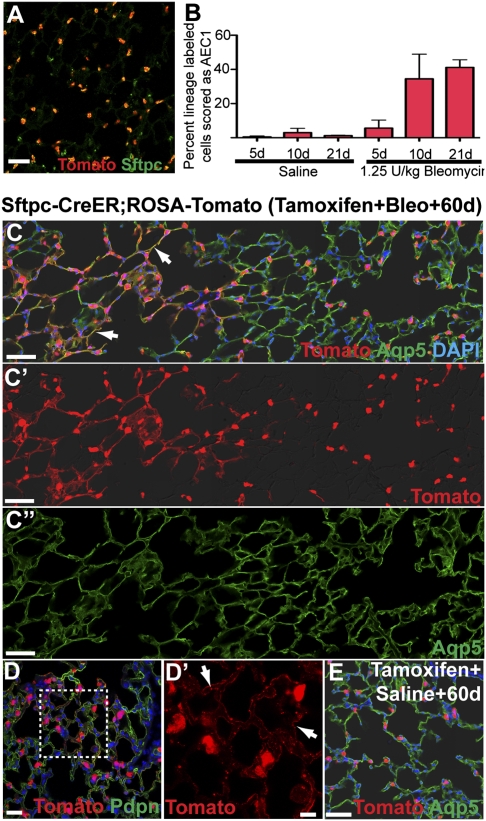
Fig. 3.
AEC2 cells give rise to AEC1 under steady-state conditions and in response to bleomycin. (A) Adult Sftpc-CreERT2;ROSA-Tomato mice were given four doses of tamoxifen to induce the heritable expression of the RFP variant tdTomato in AEC2 cells. This regimen labels ∼84.2 ± 4% of Sftpc+ AEC2 when sections are analyzed 4 d after the final dose of tamoxifen (n = 3 mice). (B) The proportion of lineage-labeled cells scored as AEC1 based on morphology and expression of Aqp5 or Pdpn was determined 5, 10, and 21 d after the intratracheal administration of 1.25 U/kg bleomycin or saline control. Data shown are means ± SD and n = 3 mice for each group. (C and D) Sixty days after bleomycin, the proportion of lineage-labeled Aqp5- and Pdpn-positive AEC1 (arrows) was increased compared with saline-treated controls (E) or relatively unaffected regions of bleomycin-treated lungs (C, right side). In C′, C″, and D′, DAPI and red and/or green channels are omitted for clarity. (Scale bars: A–E, 50 μm; D′, 20 μm.)
To assess the proliferative response of AEC2 cells to bleomycin, we gave Sftpc-CreER;ROSA-Tomato double heterozygous mice a regimen of tamoxifen and intratracheal bleomycin or saline as above followed 10 d later by a 3-h pulse of BrdU. In control lungs, 0.33 ± 0.05% of lineage-labeled cells were positive for BrdU incorporation, and this value increased about 10-fold to 3.6 ± 1.5% in mice exposed to bleomycin (mean ± SEM, n = 3 mice for each treatment).
Next, we used immunofluorescent staining of the Tomato lineage tag and markers of the stromal populations to determine if lineage-labeled AEC2 cells generate mesenchymal cells in bleomycin-induced pulmonary fibrosis. Again, multiple optical sections through the thickness of the tissue sections were captured by confocal microscopy for the unambiguous identification of cell boundaries. We did not detect colocalization of Tomato with aSMA, S100a4, vimentin, NG2, or Pdgfrb in fibrotic regions, either 10–14 d postbleomycin, when aSMA-positive myofibroblastasts are abundant (Fig. S6), or 21 d postbleomycin (Fig. 4 A–D). These findings were confirmed by the use of Imaris software to quantify the colocalization of the Tomato lineage tag with different markers in 3D through stacks of confocal images (Fig. S7). Together, these data strongly suggest that AEC2 cells are not major contributors to the fibrotic response by EMT.
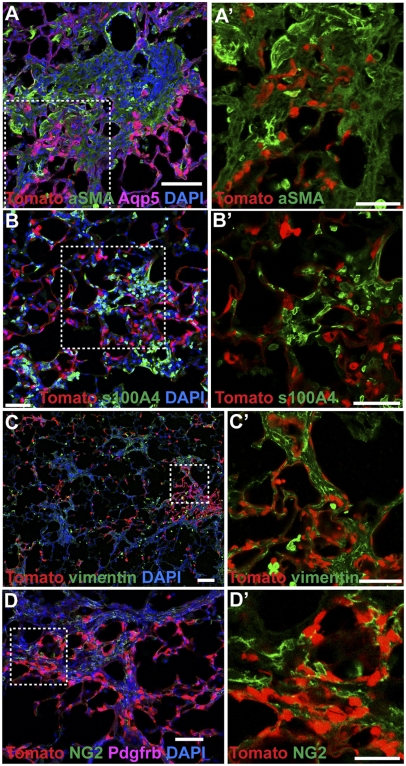
Fig. 4.
AEC2 cells do not directly contribute to pulmonary fibrosis by EMT. Sftpc-CreERT2;ROSA-Tomato double heterozygous mice were given four doses of tamoxifen followed 4–10 d later with 1.25 U/kg intratracheal bleomycin. After 21 d, we imaged endogenous Tomato lineage label and immunofluorescence against (A) aSMA (green) and Aqp5 (purple), (B) S100a4 (green), (C) vimentin (green), and (D) NG2 (green) and Pdgfrb (purple). Note that some Aqp5 AEC1 cells are lineage-labeled (A) but that there is no colocalization of lineage label with stromal markers. Red and green channels from boxed regions from A–D are shown at higher magnification in A′–D′. (Scale bars: A, A′, B, B′, C′, and D, 50 μm; C, 100 μm; D′, 25 μm.)
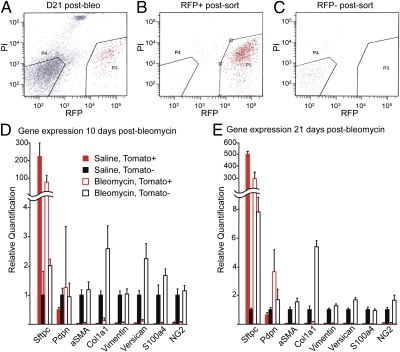
To further characterize the response of AEC2 to bleomycin at the molecular level, we performed quantitative PCR (qPCR) using template cDNA synthesized from sorted Tomato-positive cells (Sftpc-positive AEC2 cells and their daughters) and Tomato-negative cells (stromal and unlabeled epithelial cells) 10 and 21 d after intratracheal bleomycin (Fig. 5). As expected, the expression of aSMA, Col1a1, vimentin, versican, S100a4, and NG2 was confined to the Tomato-negative, predominantly stromal cell population (Fig. 5 D and E), and the transcripts for some of these genes were more abundant in this population after bleomycin. These data are consistent with the proliferation of resident stromal cells and their increased production of ECM. The expression of Sftpc decreased in the Tomato-positive population 14 and 21 d after bleomycin compared with saline controls. The expression of Pdpn was increased in the Tomato-positive population 21 d postbleomycin compared with saline-treated controls (Fig. 5 D and E). These data are consistent with the proportion of AEC2 cells in the labeled population being diluted, because they generate AEC1. The expression of Sftpc was modestly increased in the Tomato-negative population from bleomycin-treated lungs compared with the same population in controls, supporting our hypothesis that AEC2 can be derived from an Sftpc-negative (or Sftpc-CreER-unlabeled) progenitor in response to bleomycin-induced lung injury. Together, these findings support conclusions from our in vivo lineage analysis that AEC2 cells are progenitors for AEC1 but do not give rise to stromal cells through EMT.
Fig. 5.
Gene expression in freshly sorted cells from normal and fibrotic lungs. Adult Sftpc-CreERT2;ROSA-Tomato mice were given four doses of tamoxifen followed 4–10 d later by intratracheal saline or 1.25 U/kg bleomycin. (A) After 10 or 21 d, lungs were dissociated and sorted into Tomato-positive (P3; lineage labeled) and -negative (P4; unlabeled) populations by FACS. Resorting of (B) positive and (C) negative populations shows purity. Expression of Sftpc (AEC2), Pdpn (AEC1), aSMA, Col1a1, versican, vimentin, S100a4, and NG2 relative to GAPDH and Tbp was measured by qPCR in Tomato-positive (red bars) and -negative (black bars) populations (D) 10 or (E) 21 d after treatment with saline (solid bars) or bleomycin (open bars). The expression level of each gene in Tomato-negative cells from saline-treated lungs was set equal to one for comparison. Combined data from three independent experiments (each data point performed in triplicate) are shown, and error bars indicate 95% confidence interval. Note that Sftpc expression is highest in the Tomato-positive populations. The level of Pdpn is increased in the Tomato-positive population 21 d after bleomycin, consistent with the derivation of AEC1 from lineage-labeled AEC2 progenitors. Sftpc is modestly increased in the Tomato-negative population after bleomycin, presumably because AEC2 can derive from an Sftpc-negative unlabeled progenitor under these conditions. The expression of aSMA, Col1a1, versican, vimentin, S100a4, and NG2 is restricted to the Tomato-negative, predominantly stromal populations in normal and fibrotic lungs.
Behavior of Scgb1a1-Positive Cells in Response to Bleomycin.
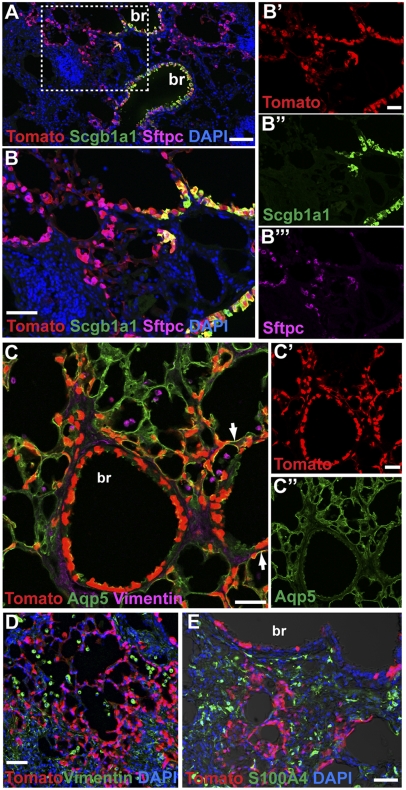
Because we found no evidence that Sftpc lineage-labeled cells undergo EMT in bleomycin-treated lungs, we asked whether the Scgb1a1-positive population was perhaps undergoing EMT instead. It had been reported that many cells in the terminal bronchioles proliferate within 2 d in response to bleomycin (39), and bronchiolization is a feature of fibrosis in the human lung. To address this possibility, we used our previously described Scgb1a1-CreER knock-in allele that is normally expressed in bronchiolar Clara cells, including the few Scgb1a1;Sftpc dual positive cells in the bronchioalveolar duct junction [putative bronchioalveolar stem cells (BASCs)] (40) and a population of dual positive AEC2 cells in the alveoli (41). We gave adult Scgb1a1-CreER;ROSA-Tomato mice either one dose of tamoxifen (to label predominantly bronchiolar Clara cells as well as some BASCs and a few dual positive AEC2s in the alveoli) or four doses of tamoxifen (to label Clara cells, putative BASCs, and more dual positive AEC2) (41). This process was followed 4–10 d later by the intratracheal administration of bleomycin. With both doses of tamoxifen, we found a striking increase in the number of lineage-labeled AEC2 and AEC1 cells in fibrotic regions 21 d postbleomycin (Fig. 6 A–C). This finding is in marked contrast to the results previously reported after injury induced by hyperoxia and naphthalene (41). Our present data do not show whether the lineage-labeled alveolar cells in fibrotic lungs are derived from bronchiolar Clara cells, putative BASCs, or alveolar Scgb1a1+;Sftpc+ cells. Importantly, with respect to the cell of origin of fibrosis, we did not detect colocalization of the Scgb1a1 lineage tag with markers of any stromal cell type, again strongly suggesting that epithelial cells are not a source of fibroblasts through EMT (Fig. 6 D and E).
Fig. 6.
Scgb1a1-positive epithelial cells give rise to AEC2 and AEC1 cells, but not stromal cells, in pulmonary fibrosis. Scgb1a1-CreER;ROSA-Tomato double heterozygous mice were given one or four doses of tamoxifen followed 4–10 d later with 2.5 U/kg intratracheal bleomycin. After 21 d, sections were stained with antibodies against RFP (red, Tomato lineage label) and (A and B) Scgb1a1 (green) and Sftpc (purple), (C) Aqp5 (green) and vimentin (purple), (D) vimentin (green), or (E) S100A4 (green). (A and B) Note that bronchiolar Scgb1a1-positive cells and alveolar Scgb1a1-negative cells are lineage labeled (red), suggesting that they are derived from an Scgb1a1-positive progenitor. Many of the lineage-labeled alveolar cells express (A and B) the AEC2 marker Sftpc or (C) AEC1 marker Aqp5 (yellow, arrows). In contrast, there is no colocalization of the lineage label with the stromal markers (C and D) vimentin or (E) S100a4. Boxed region in A shown at higher magnification in B. Single channels from B and C are shown independently in B′–B″′ and C′–C″′ for clarity. br, bronchiole. (Scale bars: A, 100 μm; B, B′, C, C′, D, and E, 50 μm.)
Discussion
The studies described here address two important topics in lung biology: epithelial cell lineage relationships in the distal lung and the cellular origins of pulmonary fibrosis. They argue against a major direct contribution of epithelial cells to fibroblast foci through the process of EMT. Rather, they support models in which resident stromal cells, of commonly underestimated heterogeneity, expand under fibrogenic conditions.
With respect to cell lineage relationships in the distal lung, our data clearly show that Sftpc-positive AEC2 are an epithelial progenitor population that generates AEC1 under steady-state conditions and more rapidly in response to bleomycin-induced pulmonary fibrosis. However, we cannot yet conclude that AEC2 cells function as stem cells that self-renew and generate AEC1 over the long term; experiments addressing this question are underway. However, our data do suggest that an Sftpc-negative or low progenitor pool generates AEC2 cells in fibrotic regions. We do not know whether these cells correspond to the integrin α6β4-positive progenitor population recently proposed by others (38). In addition, we do not yet know the origin of the Scgb1a1 lineage-labeled cells that give rise to large numbers of AEC2 and AEC1 cells in the alveoli after bleomycin treatment. The Scgb1a1-positive progenitors may initially reside in the terminal bronchioles and/or the alveoli and may possibly correspond to the proposed integrin α6β4-positive progenitors. Given that bronchiolization is a characteristic feature of human fibrosis, it is critical to identify the signals up-regulated by bleomycin (but not apparently by hyperoxia) that induce the proliferation of Scgb1a1-positive cells and their differentiation into alveolar lineages and their relationship to pathways, including Tgfb and Pdgf, with established roles in pulmonary fibrosis (reviewed in refs. 42 and 43).
The definition of EMT is evolving from the direct conversion of epithelial cells to mesenchymal fibroblasts to a less rigorous one that includes modest changes in epithelial morphology, motility, and gene expression. The loosest definition might cover the differentiation of AEC2 to AEC1 cells. Regardless, our data show that epithelial cells do not generate myofibroblasts in the context of pulmonary fibrosis. This conclusion contradicts previous studies (13, 15, 16). We provide the following possible explanations for this discrepancy. First, several studies have relied on the expression of β-gal as a lineage label (13, 15). The colorimetric X-gal reaction precludes confocal microscopy for the colocalization of lineage label with cell type-specific markers. Without confocal resolution, closely apposed cells in the distal lung might give the appearance of colocalization. Here, we have addressed this issue by using the heritable expression of the RFP variant td-Tomato or fGFP for lineage tags that are compatible with confocal analysis. In addition, visualization of Tomato does not require antibody staining on frozen tissue sections, and fGFP is membrane-associated for the unambiguous identification of cell borders. Another potential cause for our conflicting data is the method by which pulmonary fibrosis was induced. Differences have been reported between the responses to a single dose of bleomycin and the repeated instillation of bleomycin (11, 17). In at least one study that reported evidence of EMT, adenoviral TGF-β was used to induce pulmonary fibrosis (13). Another difference between this work and some previous studies is that we have used an Sftpc-CreERT2 knock-in allele to induce recombination specifically in cells that express Sftpc in the adult lung. Other studies have used a fragment of the human Sftpc promoter to drive recombination in embryonic or adult lung epithelial cells (11, 13, 15). Lastly, it is common to analyze marker expression in cultured primary cells and even cell lines to support claims of EMT (12–14). However, in vitro culture may alter gene expression. Here, we performed qPCR on cDNA made from freshly isolated lineage-labeled epithelial cells from fibrotic lungs. Our data show that these epithelial cells do not up-regulate the expression of stromal cell markers in response to bleomycin in vivo.
Pericytes have recently been implicated as the origin of fibroblasts that secrete ECM in the context of renal fibrosis and scar tissue after spinal cord injury (18, 19, 33). The contribution of pericytes to pulmonary fibrosis has not been investigated, even though they were implicated as a source of myofibroblasts more than 20 y ago and EM suggests that pericytes in fibrotic lungs are no longer confined by the capillary basement membrane (44, 45). Our data show that pericyte-like interstitial cells proliferate in response to bleomycin. It should be noted that our data likely grossly underestimate the proportion of proliferative cells that are pericyte-like cells, because the NG2-CreER is relatively inefficient at driving recombination in this population. It has been reported that pericytes express aSMA, at least transiently, in both renal fibrosis and spinal cord scar tissue (18, 19, 33). However, in the present study, most NG2-positive (or FoxJ1-positive) cells and their lineage-labeled daughters do not express high levels of aSMA in fibrotic lungs 14 or 21 d postbleomycin. This finding might reflect differences between contractile fibroblasts and pericytes of different organs (26, 27, 46) or tissue-specific variation in fibrotic responses. Importantly, there is no universal molecular definition of a pericyte; another pericyte or pericyte-like interstitial population that expresses neither NG2 nor FoxJ1, if it exists, represents a potential source of aSMA-positive myofibroblasts. Additional investigations are required to identify the molecular signals that trigger pericyte proliferation and the manner by which this population affects other cell types in the context of interstitial lung disease.
We hope that our work will emphasize the importance of understanding the many stromal cell types in the distal lung and their lineage relationships under healthy and pathological conditions. Recent studies, both in vivo and in vitro, have relied on the expression of a limited repertoire of markers at a single time in experimentally induced pulmonary fibrosis for the identification of fibrogenic myofibroblasts. This oversimplification has the potential to yield ambiguous data, because the expression of any one marker, including aSMA, is likely to be dynamic within the myofibroblast and other stromal lineages (20, 47). In the future, it will be important to carry out more genetic lineage tracing experiments of stromal cell populations, like those experiments reported here with NG2-CreER, to identify the source of aSMA-positive myofibroblasts in pulmonary fibrosis and precisely characterize changes in cellular behaviors over time. Although our qPCR data show that epithelial cells are not a major source of ECM in the fibrotic lung, the relative contributions of various stromal populations to the accumulation of ECM have not been investigated. Another important question that remains to be addressed is the relevance of findings with bleomycin-induced fibrosis in the mouse to the etiology of different fibrosing lung diseases in humans. Finally, as with the epithelial lineages, it is critical to identify the signaling pathways that regulate the proliferation and differentiation of stromal cells, including those pathways that produce fibrotic matrix. Such comprehensive insight has the potential to facilitate the development of therapies for fibrotic lung diseases.
Materials and Methods
Mice.
All experiments were approved by the Duke Institutional Animal Care and Use Committee. To generate Sftpctm1(cre/ERT)Blh (Sftpc-CreERT2) mice, the coding sequence and 3′ UTR of Sftpc were retrieved from a BAC by recombineering into a vector upstream of a diphtheria toxin cassette for negative selection. An IRES-CreERT2 cassette and a PGKneo cassette flanked with FRT sites were recombined into the 3′ UTR. The construct was electroporated into 129S6/SvEvTac ES cells, and cells from three correctly targeted clones were injected into C57BL/6 blastocysts. Mice heterozygous for Sftpc-CreER were bred to ROSA-FLP line 129S4-Gt(ROSA)26Sortm2(FLP*)Sor/J mice to remove the neocassette. ROSA-td-Tomato mice were a gift from Fan Wang (Durham, NC). The generation of this strain was identical to the ROSA-fGFP strain except the coding sequence for td-Tomato was inserted in place of fGFP (41). Scgb1a1-CreER (41), NG2-CreER (35), Pdgfra-H2B:GFP [Pdfgratm11(EGFP)Sor] (37), and ROSA-fGFP (41) mice have been described previously. The NG2-CreER strain was provided by Akiko Nishiyama (Storrs, CT) and rederived by the Duke Rodent Genetic Core.
Bleomycin Administration.
Adult (≥6 wk) mice were anesthetized with 100 mg/kg bodyweight ketamine + 5 mg/kg xylazine, and a single dose of bleomycin (1.25 U/kg − 5 U/kg body weight in 0.9% sterile saline) was injected into the trachea using a 28-gauge needle through a small incision.
Mouse Tissue Preparation.
After anesthesia, lungs were perfused with 5 mL cold PBS through the right ventricle. Lungs and trachea were removed and inflated to 20 cm H2O pressure with 4% paraformaldehyde. Lobes were fixed for 3 h in 4% PFA and washed with PBS overnight. Tissue for cryosectioning was cryoprotected in 30% sucrose and embedded in OCT. Tissue for paraffin sectioning was dehydrated, cleared in xylenes, and embedded in paraffin.
Immunohistochemistry of Mouse Tissue.
Cryosections (12 μm) and paraffin sections (7 μm) were stained by standard protocols. Rabbit anti-Sftpc (catalog #ab3786, 1:500; Millipore), goat antisecretoglobin 1a1 (Scgb1a1; 1:10,000; provided by Barry Stripp, Durham, NC), goat antivimentin (catalog #SC-7557, 1:50; Santa Cruz Biotechnology), mouse antismooth muscle actin (catalog #A2547, 1:250; Sigma-Aldrich), rabbit anti-S100A4 (1:250; provided by Eric Neilson, Nashville, TN), rabbit anti-S100A4 (catalog #ab27957, 1:250; Abcam), rat anti-PDGFRb (catalog #14–1402, 1:200; eBioscience), rabbit anti-NG2 (catalog #ab5320, 1:200; Millipore), rabbit anti-Aquaporin 5 (catalog #ab78486, 1:200; Abcam), rat anti-CD45 (catalog #553076, 1:100; BD Biosciences), rabbit antidesmin (catalog #RB-9014-P01:200; Thermo-Fisher), and rat anti-PECAM (CD31; catalog #5502741:200; BD Biosciences) were used without antigen retrieval on cryosections. Hamster anti-T1α (clone 8.1.1, 1:1,000; DSHB) was used without antigen retrieval on paraffin sections. Rabbit anti-RFP (catalog #600–4013791:250; Rockland) was used with antigen retrieval on paraffin sections (boiling for 5 min in 100 mM sodium citrate butter, pH 6, followed by heating for 10 additional min). Rabbit anti-Sftpc (see above) was used with antigen retrieval on paraffin sections (0.05% trypsin digestion at room temperature for 4 min). Alexa-Fluor–coupled secondary antibodies (Invitrogen) were used at 1:500. Z stacks of optical sections were captured on a Leica Sp2 laser-scanning confocal microscope or Zeiss LSM 710 laser-scanning confocal microscope. Unless otherwise noted, sections from at least three lungs were analyzed for each data point. Imaris colocalization (×64 7.1.1; Imaris) was used to quantitatively assess colocalization of stromal markers (in the green channel) with the Tomato lineage tag (in the red channel) on 3D images (i.e., z series) acquired on the Zeiss 710 confocal microscope. Data are reported as percentages of red volume above the threshold (percent red voxels) that is colocalized with green above the threshold.
BrdU.
BrdU (10 μL/g body weight; #RPN201, Cell Proliferation Reagent; GE Healthcare) was injected i.p. 3 h before sacrifice. After antigen retrieval (10 mM sodium citrate, pH 6, in a 2100 Retriever followed by 2M HCl at 37° for 20 min and 0.05% Trypsin in PBS at room temperature for 5 min), slides were stained with rat anti-BrdU (catalog #OBT0030, 1:500; Accurate Chemical and Scientific) and chicken anti-GFP (catalog #GFP-1020, 1:500; Aves Labs).
Lung Dissociation and FACS.
Animals were killed by Euthasol and perfused with 5 mL cold PBS through the right ventricle. Lungs were inflated with protease solution [1–2 mL; Collagenase Type I (catalog #17100–017, 450 U/mL; Gibco), Elastase (catalog #LS002279, 4 U/mL; Worthington Biochemical Corporation), Dispase (catalog #354235, 5 U/mL; BD Biosciences) and DNaseI (catalog #10104159001, 0.33 U/mL; Roche) in DMEM/F12], cut into small pieces (<2 mm2), and incubated in 1–2 mL protease solution for 25 min at 37 °C with frequent agitation. DMEM/F12 + 10% FBS was added, and tissue was disrupted by pipetting, washed with DMEM/F12, and incubated for 20 min at 37 °C in 2 mL 0.1% Trypsin-EDTA + 0.325 mg DNaseI with intermittent agitation. An equal volume of DMEM-F12 + 10% FBS was added, and tissue was dissociated by pipetting. After filtering through a 100-μm strainer, cells were washed with 5 mL DMEM/F12, incubated at room temperature for 1.5 min in 2 mL red blood cell lysis buffer (catalog #00–4333-57; eBioscience), filtered through a 40-μm strainer, centrifuged, and resuspended in DMEM + 2% BSA. Sorting was performed on FACS Vantage SE, and data was analyzed with FACS Diva (BD Biosciences).
RNA Isolation and cDNA Synthesis.
RNA was isolated using a Qiagen RNeasy Micro Kit. RNA (138 ng) from each population of three biological replicates was used to synthesize cDNA using iScript cDNA Synthesis Kit (Bio-Rad).
qPCR.
Gene expression levels were quantified by qRT-PCR on the StepOnePlus Real-Time PCR System (Applied Biosystems). Samples were run in duplicate, and 18-μL reactions were pipetted from a master mix including 2 μL sample cDNA, 20 μL 2× iQ SYBR Green Supermix (Bio-Rad), 10 μL water, and 8 μL gene-specific primers (200 nM each). Primer efficiency was determined by running standard curve reactions, and all pairs were >90% efficient across a >1,000-fold range. PCR cycling parameters are 95 °C for 10 min (one cycle); 95 °C for 15 s, 59 °C for 30 s, 72 °C for 30 s (40 cycles); and a melting curve to confirm primer specificity. Threshold cycle values (Ct) for triplicate samples were averaged and normalized to GAPDH and Tbp (ΔCt), and these values across samples were compared (ΔΔCt) to quantify relative expression. Primers are listed in Table S2. Data are the combined results of three cohorts of animals (pooled, sorted cells from the lungs of one to three mice) for each treatment at each time (10 or 21 d).
Human Samples.
All experiments were performed with Institutional Review Board approval. Samples were inflated and fixed in 4% PFA at 4 °C for 3 h followed by washing in PBS. Cryosections (12 μm) were stained by standard protocols. RaNG2/D2 rabbit anti-NG2 (1:50; provided by Bill Stallcup, La Jolla, CA) was used without antigen retrieval.
Supplementary Material
Supporting Information
Acknowledgments
We thank Scott Randell and Lynn Sakai for critically reading the manuscript and Steve Munger for assistance with qPCR. The rabbit anti-human NG2 antibody was a gift from Bill Stallcup, and the rabbit anti-S100a4 antibody was a gift from Eric Neilson. J.R.R. and C.E.B. were supported by Grant T32HL007538, and J.R.R. was supported by Grant HL102920. This work was supported by the Richard T. Schutt Medical Research Fund and in part, Richard T. Schutt Medical Research Fund Grant HL071303 (to B.L.M.H.).
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 20867.
References
- 1.Mehal WZ, Iredale J, Friedman SL. Scraping fibrosis: Expressway to the core of fibrosis. Nat Med. 2011;17:552–553. doi: 10.1038/nm0511-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011 doi: 10.1016/S0140-6736(11)60052-4. 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 4.Plantier L, et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax. 2011;66:651–657. doi: 10.1136/thx.2010.151555. [DOI] [PubMed] [Google Scholar]
- 5.Kottmann RM, Hogan CM, Phipps RP, Sime PJ. Determinants of initiation and progression of idiopathic pulmonary fibrosis. Respirology. 2009;14:917–933. doi: 10.1111/j.1440-1843.2009.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie JD, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-fifth official adult lung and heart/lung transplantation report—2008. J Heart Lung Transplant. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Vyalov SL, Gabbiani G, Kapanci Y. Rat alveolar myofibroblasts acquire alpha-smooth muscle actin expression during bleomycin-induced pulmonary fibrosis. Am J Pathol. 1993;143:1754–1765. [PMC free article] [PubMed] [Google Scholar]
- 8.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corvol H, Flamein F, Epaud R, Clement A, Guillot L. Lung alveolar epithelium and interstitial lung disease. Int J Biochem Cell Biol. 2009;41:1643–1651. doi: 10.1016/j.biocel.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Degryse AL, et al. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L442–L452. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felton VM, Borok Z, Willis BC. N-acetylcysteine inhibits alveolar epithelial-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L805–L812. doi: 10.1152/ajplung.00009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KK, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanjore H, et al. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem. 2011;286:30972–30980. doi: 10.1074/jbc.M110.181164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanjore H, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degryse AL, Lawson WE. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am J Med Sci. 2011;341:444–449. doi: 10.1097/MAJ.0b013e31821aa000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Göritz C, et al. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys BD, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapanci Y, Ribaux C, Chaponnier C, Gabbiani G. Cytoskeletal features of alveolar myofibroblasts and pericytes in normal human and rat lung. J Histochem Cytochem. 1992;40:1955–1963. doi: 10.1177/40.12.1333502. [DOI] [PubMed] [Google Scholar]
- 21.Rehan VK, et al. Evidence for the presence of lipofibroblasts in human lung. Exp Lung Res. 2006;32:379–393. doi: 10.1080/01902140600880257. [DOI] [PubMed] [Google Scholar]
- 22.Sirianni FE, Chu FS, Walker DC. Human alveolar wall fibroblasts directly link epithelial type 2 cells to capillary endothelium. Am J Respir Crit Care Med. 2003;168:1532–1537. doi: 10.1164/rccm.200303-371OC. [DOI] [PubMed] [Google Scholar]
- 23.Weibel ER. On pericytes, particularly their existence on lung capillaries. Microvasc Res. 1974;8:218–235. doi: 10.1016/0026-2862(74)90096-x. [DOI] [PubMed] [Google Scholar]
- 24.Lawson WE, et al. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:899–907. doi: 10.1164/rccm.200311-1535OC. [DOI] [PubMed] [Google Scholar]
- 25.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 26.Armulik A, Genové G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 28.Epling GP. Electron microscopic observations of pericytes of small blood vessels in the lungs and hearts of normal cattle and swine. Anat Rec. 1966;155:513–530. [Google Scholar]
- 29.Sims DE, Westfall JA. Analysis of relationships between pericytes and gas exchange capillaries in neonatal and mature bovine lungs. Microvasc Res. 1983;25:333–342. doi: 10.1016/0026-2862(83)90023-7. [DOI] [PubMed] [Google Scholar]
- 30.Kapanci Y, Desmouliere A, Pache JC, Redard M, Gabbiani G. Cytoskeletal protein modulation in pulmonary alveolar myofibroblasts during idiopathic pulmonary fibrosis. Possible role of transforming growth factor beta and tumor necrosis factor alpha. Am J Respir Crit Care Med. 1995;152:2163–2169. doi: 10.1164/ajrccm.152.6.8520791. [DOI] [PubMed] [Google Scholar]
- 31.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 32.Boomershine CS, et al. Autoimmune pancreatitis results from loss of TGFbeta signalling in S100A4-positive dendritic cells. Gut. 2009;58:1267–1274. doi: 10.1136/gut.2008.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue T, Plieth D, Venkov CD, Xu C, Neilson EG. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 2005;67:2488–2493. doi: 10.1111/j.1523-1755.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhu X, et al. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: Contribution of the ciliated lineage. Proc Natl Acad Sci USA. 2007;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol. 2003;23:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman HA, et al. Integrin &alpha6&beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawamoto M, Fukuda Y. Cell proliferation during the process of bleomycin-induced pulmonary fibrosis in rats. Acta Pathol Jpn. 1990;40:227–238. doi: 10.1111/j.1440-1827.1990.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 41.Rawlins EL, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 44.Adler KB, Callahan LM, Evans JN. Cellular alterations in the alveolar wall in bleomycin-induced pulmonary fibrosis in rats. An ultrastructural morphometric study. Am Rev Respir Dis. 1986;133:1043–1048. doi: 10.1164/arrd.1986.133.6.1043. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell J, et al. Alpha-smooth muscle actin in parenchymal cells of bleomycin-injured rat lung. Lab Invest. 1989;60:643–650. [PubMed] [Google Scholar]
- 46.Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 47.Schürch W, Seemayer TA, Gabbiani G. The myofibroblast: A quarter century after its discovery. Am J Surg Pathol. 1998;22:141–147. doi: 10.1097/00000478-199802000-00001. [DOI] [PubMed] [Google Scholar]
Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):20867–20868.
Author Summary
Idiopathic pulmonary fibrosis (IPF) is a debilitating disease in which the delicate gas exchange region of the lung is gradually replaced by accumulations of ECM and myofibroblasts. These cells are commonly thought to produce components of the ECM, including collagen. Conflicting ideas about the cellular origin of the fibrotic lesions may slow progress to the development of effective therapies for IPF. To address this problem, we combined the power of cell lineage tracing in mice with a model of pulmonary fibrosis induced by bleomycin, an anticancer agent that often results in pulmonary fibrosis as a side effect. We provide evidence that the behaviors of pericytes, a cell type associated with blood vessels, and epithelial cells are modulated in response to bleomycin-induced lung injury. However, neither cell type is a major source of myofibroblasts. These findings should help to focus future efforts at developing therapies for the treatment of IPF.
One factor complicating the study of IPF is that surprisingly little is known about the identities, behaviors, and lineage relationships of the different cell types that make up the gas exchange region of the lung where the disease occurs. This region has a complex 3D structure in which a vast network of capillaries and nonepithelial stromal cells surrounds the millions of thin-walled, air-filled epithelial sacs known as alveoli (Fig. P1; ref. 1). To fully understand the process of fibrosis, we focused on high-resolution microscopy of intact normal and fibrotic lung tissue rather than study of cells grown in culture.
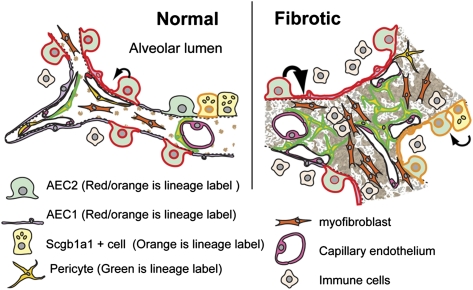
Fig. P1.
Schematic depiction of alveoli in a normal mouse lung (Left) and remodeling in a region of bleomycin-induced fibrosis (Right). In the normal lung, lineage-labeled AEC2 gives rise to AEC1 (black arrow), supporting the classical view of AEC2 cells as alveolar epithelial progenitor. The rate of this conversion is enhanced in response to bleomycin injury, but lineage-labeled epithelial cells do not give rise to fibroblasts through epithelial to mesenchymal transition. After bleomycin-induced lung injury, Scgb1a1+ lineage-labeled cells also proliferate and generate AEC2 and AEC1 cells. In fibrotic lungs, lineage-labeled pericytes proliferate but are not a major source of aSMA-positive myofibroblasts.
We began by staining tissue sections with antibodies against a panel of commonly used markers for stromal cells and viewing them by confocal microscopy to characterize these cell populations before bleomycin treatment and at different times after it. The markers included α-smooth muscle actin (aSMA and Acta2), which is generally considered a hallmark of myofibroblasts. We also examined other markers including S100a4 (Fsp1), desmin, vimentin, Pdgfra, Pdgfrb, and the surface glycoprotein Ng2 (Cspg4). Some of these markers mark pericytes, a population of cells implicated as a source of myofibroblasts in other fibrotic processes (2, 3). These studies revealed a surprising diversity of cells in the fibrotic lesions, with aSMA-positive cells showing the greatest abundance relatively early after the administration of bleomycin. A survey of fresh biopsy samples from patients with IPF suggested that a similar diversity exists in human fibrotic lesions.
With this foundation in place, we used genetic lineage tracing in the mouse to determine the origin of the fibroblasts within the bleomycin-induced lesions. First, we tested the hypothesis that pericytes are a source of myofibroblasts. We used two different mouse strains carrying transgenes, Ng2-CreER and FoxJ1-CreER, to induce the expression of a fluorescent protein that is heritable and specifically expressed in pericyte-like cells within the alveolar wall. This florescent marker allowed us to follow the fate of this cell type in response to bleomycin. We found that these cells proliferated and expanded within fibroblast foci. Surprisingly, most of the lineage-labeled cells did not express high levels of aSMA.
It has been suggested that 30–50% of the myofibroblasts in the bleomycin model originate from epithelial cells, including type 2 alveolar epithelial cells (AEC2), through a process known as epithelial to mesenchymal transition. According to this model, epithelial cells lose their polarity and assume a fibroblast phenotype, including the expression of stromal markers and secretion of ECM. We addressed this popular hypothesis by generating a knock-in allele to induce the heritable expression of a fluorescent lineage tag in AEC2 cells. In the distal lung, these cells are a source of surfactant proteins, which are critical for proper lung function, and they may also act as a population of stem cells capable of differentiating into type 1 alveolar epithelial cells (AEC1s) (4). The lineage tag also enabled us to purify AEC2s and their descendants to confirm the morphological findings by examining the expression of different marker genes. Using confocal microscopy and our panel of stromal markers, we saw no evidence that lineage-labeled AEC2 gave rise to any kind of stromal cell. Rather, they differentiate into AEC1 cells, supporting classical models for the lineage relationship of these two cell types. Significantly, the slow conversion of AEC2 to AEC1 cells seen in control lungs is greatly enhanced in response to bleomycin-induced lung injury. Currently, we do not understand the mechanism underlying this change in behavior, but additional analysis of gene expression in AEC2 cells will address this question. We also used our Secretoglobin1a1-CreER knock-in allele to follow the response of epithelial cells of the airways (bronchioles) and a subset of alveolar epithelial cells to bleomycin. Unlike our previous findings with other injury models (5), we found striking changes in the proliferation and differentiation of these cells after bleomycin injury. However, again, we found no evidence that this labeled population generates fibroblasts.
In conclusion, our studies address two important topics in lung biology: the lineage relationships of epithelial cells in the distal gas exchange region of the lung and the cellular origins of pulmonary fibrosis. A deeper understanding of epithelial progenitors will help to identify therapies to reverse the cell loss that occurs in a number of lung diseases and not just fibrosis. For fibrosis, defining the relative contributions of each of the different stromal cell types, including pericytes, to the fibrotic process will be important in future studies. In particular, we need to understand whether contributions are direct (through synthesis of ECM) or indirect (through production of growth factors and cytokines). This knowledge has the potential to lead to novel therapeutic strategies for the treatment of pulmonary fibrosis.
Footnotes
The authors declare no conflict of interest.
This is a Contributed submission.
See full research article on page E1475 of www.pnas.org.
References
- 1.Morrisey EE, Hogan BL. Preparing for the first breath: Genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Göritz C, et al. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys BD, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aso Y, Yoneda K, Kikkawa Y. Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Lab Invest. 1976;35:558–568. [PubMed] [Google Scholar]
- 5.Rawlins EL, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary Materials
Supporting Information