Liberal or Restrictive Transfusion in High-Risk Patients after Hip Surgery (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 29.
Published in final edited form as: N Engl J Med. 2011 Dec 14;365(26):2453–2462. doi: 10.1056/NEJMoa1012452
Abstract
BACKGROUND
The hemoglobin threshold at which postoperative red-cell transfusion is warranted is controversial. We conducted a randomized trial to determine whether a higher threshold for blood transfusion would improve recovery in patients who had undergone surgery for hip fracture.
METHODS
We enrolled 2016 patients who were 50 years of age or older, who had either a history of or risk factors for cardiovascular disease, and whose hemoglobin level was below 10 g per deciliter after hip-fracture surgery. We randomly assigned patients to a liberal transfusion strategy (a hemoglobin threshold of 10 g per deciliter) or a restrictive transfusion strategy (symptoms of anemia or at physician discretion for a hemoglobin level of <8 g per deciliter). The primary outcome was death or an inability to walk across a room without human assistance on 60-day follow-up.
RESULTS
A median of 2 units of red cells were transfused in the liberal-strategy group and none in the restrictive-strategy group. The rates of the primary outcome were 35.2% in the liberal-strategy group and 34.7% in the restrictive-strategy group (odds ratio in the liberal-strategy group, 1.01; 95% confidence interval [CI], 0.84 to 1.22), for an absolute risk difference of 0.5 percentage points (95% CI, −3.7 to 4.7). The rates of in-hospital acute coronary syndrome or death were 4.3% and 5.2%, respectively (absolute risk difference, −0.9%; 99% CI, −3.3 to 1.6), and rates of death on 60-day follow-up were 7.6% and 6.6%, respectively (absolute risk difference, 1.0%; 99% CI, −1.9 to 4.0). The rates of other complications were similar in the two groups.
CONCLUSIONS
A liberal transfusion strategy, as compared with a restrictive strategy, did not reduce rates of death or inability to walk independently on 60-day follow-up or reduce in-hospital morbidity in elderly patients at high cardiovascular risk. (Funded by the National Heart, Lung, and Blood Institute; FOCUS ClinicalTrials.gov number, NCT00071032.)
In the United States, more than 17 million red-cell units are collected annually, and 15 million units are transfused.1 Blood transfusions are frequently given to surgical patients and to the elderly.2,3 Yet, the indications for postoperative transfusion have not been adequately evaluated and remain controversial. Most clinical trials have been small.4 One adequately powered trial involving adults in intensive care units showed a nonsignificant decrease in 30-day mortality with a restrictive transfusion strategy, as compared with a liberal strategy (18.7% vs. 23.3%).5 However, the effect of a restrictive approach on functional recovery or risk of myocardial infarction in patients with cardiac disease has not been studied.4 We performed the Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) to test the hypothesis that a higher threshold for blood transfusion (a hemoglobin level of 10 g per deciliter) would improve functional recovery and reduce morbidity and mortality, as compared with a more restrictive transfusion strategy (a hemoglobin level of <8 g per deciliter or symptoms).
METHODS
PATIENTS
From July 19, 2004, through February 28, 2009, we enrolled patients at 47 clinical sites in the United States and Canada. Telephone follow-up ended on May 4, 2009. Patients 50 years of age or older who were undergoing primary surgical repair of a hip fracture and who had clinical evidence of or risk factors for cardiovascular disease were eligible if they had a hemoglobin level of less than 10 g per deciliter within 3 days after surgery. According to the original protocol, only patients with cardiovascular disease (a history of ischemic heart disease, electrocardiographic evidence of previous myocardial infarction, a history or presence of congestive heart failure or peripheral vascular disease, or a history of stroke or transient ischemic attack) were eligible. In December 2005, eligibility criteria were expanded to enhance recruitment by including patients with any of the following cardiovascular risk factors: a history of or treatment for hypertension, diabetes mellitus, or hypercholesterolemia; a cholesterol level of 200 mg or more per deciliter or a low-density lipoprotein cholesterol level of 130 mg or more per deciliter; current tobacco use; or a creatinine level of more than 2.0 mg per deciliter.6
We excluded patients if they were unable to walk without human assistance before hip fracture, declined blood transfusions, had multiple trauma (defined as having had or planning to undergo surgery for non–hip-related traumatic injury), had a pathologic hip fracture associated with cancer, had a history of clinically recognized acute myocardial infarction within 30 days before randomization, had previously participated in the trial with a contralateral hip fracture, had symptoms associated with anemia (e.g., ischemic chest pain), or were actively bleeding at the time of potential randomization.
The institutional review board or ethics committee at all 47 participating clinical sites approved the protocol (available with the full text of this article at NEJM.org). An independent data and safety monitoring board also approved the protocol and monitored the trial. Written informed consent was obtained from patients or their designated representatives. Methods were reported in detail previously.6
TREATMENT ASSIGNMENT AND FOLLOW-UP
We randomly assigned patients to the liberal-strategy group or the restrictive-strategy group using an automated telephone randomization system. Staff members at the data coordinating center prepared randomization schedules for each site using randomly ordered block sizes of two, four, six, or eight. After randomization, clinical-site staff members, clinicians, and patients were aware of study-group assignments.
Patients in the liberal-strategy group received 1 unit of packed red cells and additional blood as needed to maintain a hemoglobin level of 10 g or more per deciliter. An assessment of the hemoglobin level after transfusion was required, and an additional unit of blood was transfused if the patient’s hemoglobin level was below 10 g per deciliter.
Patients in the restrictive-strategy group were permitted to receive transfusions if symptoms or signs of anemia developed or at the discretion of their physicians if the hemoglobin level fell below 8 g per deciliter. Symptoms or signs that were considered indications for transfusion were chest pain that was deemed to be cardiac in origin, congestive heart failure, and unexplained tachycardia or hypotension unresponsive to fluid replacement. Blood was administered 1 unit at a time, and the presence of symptoms or signs was reassessed. Patients with clinically diagnosed dementia received transfusions when the hemoglobin level fell below 8 g per deciliter because they might not be able to report their symptoms.
Hemoglobin levels were measured during hospitalization on days 1, 2, 4, and 7 after randomization. Additional hemoglobin determinations were made as clinically indicated. The assigned transfusion strategy was to be followed until discharge or up to 30 days, whichever came first. Transfusion was permitted at any time without measuring a hemoglobin level if the patient was bleeding and emergency transfusion was considered necessary by the treating physician.
Nurses at the clinical coordinating center who were not involved with study implementation and were unaware of study-group assignments telephoned patients or proxies at or close to 30 days and 60 days after randomization to ascertain outcomes after hospital discharge. They spoke directly to patients who were accessible by telephone or to proxies if patients were cognitively impaired or could not talk on the telephone.
PRIMARY OUTCOME
The primary outcome was death or an inability to walk 10 ft (or across a room) without human assistance at the 60-day follow-up. We hypothesized that an increased hemoglobin level would allow patients to participate more actively in rehabilitation and therefore increase the proportion who were walking independently 60 days after randomization.
SECONDARY OUTCOMES
Secondary outcomes included a combined outcome of in-hospital myocardial infarction, unstable angina, or death for any reason; each of these outcomes was assessed individually.
Electrocardiography was performed before surgery, before randomization, and on day 4 after randomization (or at the time of discharge if before day 4). Blood (plasma or serum) specimens were collected for measurement of the cardiac troponin I level before surgery, before randomization, and on days 1 and 4 after randomization or before discharge (if before day 4). Electrocardiograms and results of testing of cardiac biomarkers that were performed in hospitals for clinical indications were also collected. Samples were analyzed at the core laboratory of the Minneapolis Medical Research Foundation of Hennepin County Medical Center for troponin I (Access 2 Immunoassay System, Beckman Coulter) with the use of a threshold of 0.06 µg per liter (1.5 times the 99th percentile [0.04 µg per liter] for healthy patients). We used the Universal Definition of Myocardial Infarction criteria7,8 to define myocardial infarction and unstable angina on the basis of review of clinical status, central interpretation of electrocardiograms at Saint Louis University, and results of core laboratory and clinical cardiac biomarkers (see the Supplementary Appendix, available at NEJM.org). Study investigators who classified cardiovascular outcomes and those who did follow-up telephone assessments were unaware of study-group assignments.
Other secondary outcomes that were determined on telephone follow-up at or close to 30 days and 60 days after randomization included current residence, survival, functional measures (lower-extremity physical and instrumental activities of daily living), and fatigue. These outcomes were ascertained with the use of methods described previously.6
TERTIARY STUDY OUTCOMES
We evaluated in-hospital morbidity up to 30 days after randomization, including pneumonia, wound infection, thromboembolism, stroke or transient ischemic attack, and clinically recognized myocardial infarction.6 We prespecified two composite outcomes: death, myocardial infarction, or pneumonia; and death, myocardial infarction, pneumonia, thromboembolism, or stroke.
VITAL STATUS AND WALKING CONFIRMATION
We validated the vital status of patients in the United States by searching the online Social Security Database. When discrepancies were identified between telephone reports and this database, we verified deaths using hospital records or published obituaries. We validated the vital status of Canadian patients by searching hospital medical records, vital-status records, and outpatient medical records. We validated vital status in 95.9% of patients (99.0% in the United States and 91.2% in Canada). Of 1934 vital-status confirmations, we found 7 discrepancies (0.4%) between telephone reports and vital-status records; in these cases, we used vital-status records. We assessed the reliability of the self-report of walking status in a subgroup of 814 patients for whom we had both self-report and proxy report and found high reliability (kappa = 0.90) between these reports.9
ADHERENCE DEFINITIONS
We defined major protocol violations as a lack of receipt of a transfusion or hospital discharge with a hemoglobin level of less than 10 g per deciliter in the liberal-strategy group and as the receipt of transfusion with a hemoglobin level of 8 g per deciliter or more in the absence of symptoms in the restrictive-strategy group.
STATISTICAL ANALYSIS
According to the original study design, we determined that a sample size of 2600 patients would provide a power of 90% and an experiment-wise alpha level of 0.05 allowing for interim analyses (four were performed by the data and safety monitoring board) and a level of 0.048 for the final comparison to detect an absolute between-group difference of 7 percentage points in the primary outcome (odds ratio, 0.75). In September 2007, the data and safety monitoring board approved a reduction of recruitment goal to 2000 patients. This change resulted in an absolute change of approximately 1 percentage point in the between-group difference in the primary outcome that could be excluded with a power of 90%.
We used the Mantel–Haenszel method10 to conduct the primary analysis, taking into account different clinical sites. We prespecified tests for interaction of the primary outcome11,12 with sex, age, race, and cardiovascular-disease status (known cardiovascular disease vs. risk factors only) without adjustment of the alpha level. Tests for interaction and differences in outcomes are presented without adjustment for clinical site. The primary outcome analysis is presented as a Mantel–Haenszel odds ratio with 95% confidence intervals. For secondary and tertiary analyses, we used standard methods for the comparison of proportions and means without adjustment for clinical site, using an alpha level of 0.01 (with 99% confidence intervals). Analyses were performed with the use of SAS software, version 9.2.
RESULTS
STUDY POPULATION
We screened 14,438 patients and randomly assigned 2016 to either the liberal-strategy group (1007 patients) or the restrictive-strategy group (1009) (see the Supplementary Appendix). There were 14 withdrawals, 2 losses to follow-up, and 1 incomplete follow-up ascertainment; follow-up for the primary analysis was obtained in 99.2% of the patients. Of the 1999 patients included in the primary analysis, we directly interviewed 1075 (53.8%) and obtained data on 923 (46.2%) by proxy; the source of information was missing for 1 patient.
The mean age of the study population was 81.6 years (range, 51 to 103), and cardiovascular disease was present in 62.9%. Baseline characteristics were similar in the two study groups (Table 1).
Table 1.
Baseline Characteristics of the Patients.*
| Variable | Liberal Strategy(N = 1007) | Restrictive Strategy(N = 1009) |
|---|---|---|
| Age — yr | 81.8±8.8 | 81.5±9.0 |
| Male sex — no. (%) | 250 (24.8) | 239 (23.7) |
| Race — no. (%)† | ||
| White | 944 (93.7) | 947 (93.9) |
| Black | 40 (4.0) | 42 (4.2) |
| Asian | 14 (1.4) | 13 (1.3) |
| Other | 9 (0.9) | 7 (0.7) |
| Residence in the United States — no. (%) | 609 (60.5) | 613 (60.8) |
| Cardiovascular disease — no. (%) | ||
| Any | 637 (63.3) | 631 (62.5) |
| Coronary artery disease | 402 (39.9) | 403 (39.9) |
| Congestive heart failure | 184 (18.3) | 167 (16.6) |
| Cerebrovascular disease | 249 (24.7) | 224 (22.2) |
| Peripheral vascular disease | 117 (11.6) | 102 (10.1) |
| Cardiovascular risk factors — no./total no. (%) | ||
| Hypertension | 824/1003 (82.2) | 821/1005 (81.7) |
| Diabetes mellitus | 252/1003 (25.1) | 256/1005 (25.5) |
| Hypercholesterolemia | 347/1002 (34.6) | 360/1001 (36.0) |
| Tobacco use | 116/1003 (11.6) | 113/1004 (11.3) |
| Creatinine >2.0 mg/dl | 83/1001 (8.3) | 86/1003 (8.6) |
| Chronic lung disease | 189/1003 (18.8) | 188/1007 (18.7) |
| History of dementia or confusion | 309/1004 (30.8) | 325/1008 (32.2) |
| History of cancer | 181/1003 (18.0) | 189/1008 (18.8) |
| Type of hip fracture — no./total no. (%) | ||
| Femoral neck | 432/1004 (43.0) | 422/1008 (41.9) |
| Intertrochanteric | 512/1004 (51.0) | 522/1008 (51.8) |
| Subtrochanteric | 88/1004 (8.8) | 95/1008 (9.4) |
| Reverse oblique | 13/1004 (1.3) | 8/1008 (0.8) |
| Type of anesthesia — no./total no. (%) | ||
| General | 543/1005 (54.0) | 566/1008 (56.2) |
| Spinal | 457/1005 (45.5) | 434/1008 (43.1) |
| Other | 5/1005 (0.5) | 8/1008 (0.8) |
| American Society of Anesthesiology risk score‡ | 3.0±0.6 | 2.9±0.6 |
| Residence — no./total no. (%) | ||
| Home or retirement home | 892/1005 (88.8) | 886/1008 (87.9) |
| Nursing home | 104/1005 (10.3) | 110/1008 (10.9) |
| Other | 9/1005 (0.9) | 12/1008 (1.2) |
HEMOGLOBIN LEVELS AND TRANSFUSION
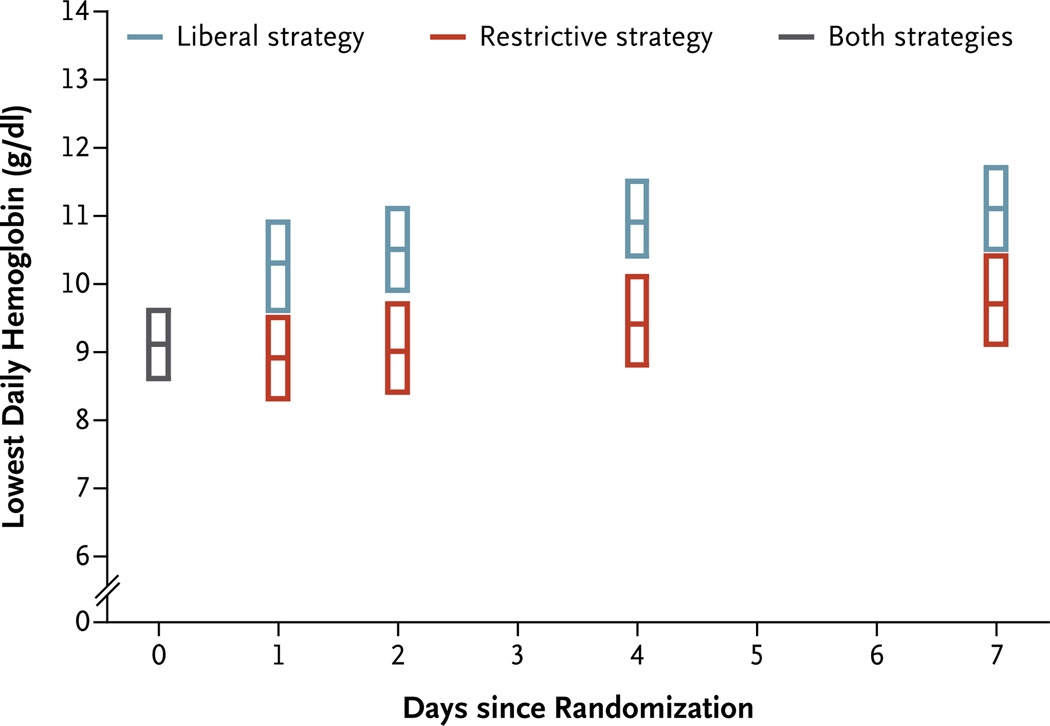
The average hemoglobin level before transfusion was 1.3 g per deciliter higher in the liberal-strategy group than in the restrictive-strategy group (P<0.001) (Table 2). The median number of units transfused was 2.0 (interquartile range, 1 to 2) in the liberal-strategy group and 0 (interquartile range, 0 to 1) in the restrictive-strategy group; 59.0% of patients in the restrictive-strategy group did not receive a transfusion after randomization. Figure 1 shows the average daily lowest hemoglobin levels in the two groups.
Table 2.
Hemoglobin Levels and Transfusions.*
| Variable | Liberal Strategy(N = 1007) | Restrictive Strategy(N = 1009) | P Value |
|---|---|---|---|
| Hemoglobin level — g/dl | |||
| Before surgery | 11.3±1.5 | 11.3±1.5 | 0.70 |
| During eligibility screening | 9.0±0.8 | 9.0±0.8 | 0.98 |
| Before transfusion | 9.2±0.5 | 7.9±0.6 | <0.001 |
| Estimated blood loss during surgery — ml† | 209±179 | 232±257 | 0.03 |
| Transfusions before randomization | |||
| 0 units — no./total no. (%) | 754/1006 (75.0) | 720/1008 (71.4) | |
| ≥1 unit — no./total no. (%) | 252/1006 (25.0) | 288/1008 (28.6) | 0.07 |
| Total no. of units | 452 | 531 | |
| Transfusions after randomization | |||
| 0 units — no./total no. (%) | 33/1003 (3.3) | 594/1007 (59.0) | |
| 1 unit — no./total no. (%) | 420/1003 (41.9) | 246/1007 (24.4) | |
| 2 units — no./total no. (%) | 346/1003 (34.5) | 127/1007 (12.6) | |
| 3 units — no./total no. (%) | 132/1003 (13.2) | 24/1007 (2.4) | |
| ≥4 units — no./total no. (%) | 72/1003 (7.2) | 16/1007 (1.6) | <0.001 |
| Total no. of units | 1866 | 652 | |
| Storage of units transfused after randomization — days‡ | 22.0±9.5 | 22.1±9.9 | 0.83 |
| Leukoreduced units transfused after randomization — %§ | 90.2 | 88.6 | 0.25 |
| Major protocol violation — no./total no. (%)¶ | 91/1006 (9.0) | 56/1007 (5.6) | 0.003 |
| Transfusion because of symptoms — no./total no. (%)‖ | |||
| Rapid bleeding | 5/1006 (0.5) | 14/1007 (1.4) | 0.04 |
| Chest pain | 4/1006 (0.4) | 9/1007 (0.9) | 0.17 |
| Congestive heart failure | 1/1006 (0.1) | 10/1007 (1.0) | 0.007 |
| Tachycardia or hypotension | 43/1006 (4.3) | 123/1007 (12.2) | <0.001 |
Figure 1. Lowest Daily Hemoglobin Levels.
Shown are the lowest daily hemoglobin levels among patients in the liberal-strategy group versus those in the restrictive-strategy group. Data for the two groups are pooled on the day of randomization and are presented for days 1, 2, 4, and 7, when hemoglobin levels were required to be measured while patients remained in the hospital. The center line within each box represents the median, and the extremes the interquartile range.
Violations in the transfusion protocol occurred in 9.0% of patients in the liberal-strategy group and in 5.6% of those in the restrictive-strategy group. Symptoms leading to transfusion are listed in Table 2.
OUTCOMES
The rates of death or an inability to walk without human assistance at 60-day follow-up were similar in the liberal-strategy group and the restrictive-strategy group (35.2% vs. 34.7%, P = 0.90) (Table 3). The odds ratio for the primary outcome associated with the liberal strategy versus the restrictive strategy was 1.01 (95% confidence interval [CI], 0.84 to 1.22), for an absolute risk difference of 0.5 percentage points (95% CI, −3.7 to 4.7). There was a significant interaction according to patients’ sex (P = 0.03), with an odds ratio associated with the liberal strategy of 1.45 (95% CI, 1.00 to 2.10) for men versus 0.91 (95% CI, 0.74 to 1.13) for women. Interactions according to age, race, and cardiovascular-disease status were not significant (see the Supplementary Appendix).
Table 3.
Outcomes at 30 Days and 60 Days.*
| Variable | 30-Day Period | 60-Day Period | ||||||
|---|---|---|---|---|---|---|---|---|
| Liberal Strategy(N = 1007) | Restrictive Strategy(N = 1009) | Odds Ratio(99% CI) | Absolute RiskDifference(99% CI) | Liberal Strategy(N = 1007) | Restrictive Strategy(N = 1009) | Odds Ratio(95% CI) | Absolute RiskDifference(95% CI) | |
| no./total no.(%) | percentage points | no./total no. (%) | percentage points | |||||
| Death or inability to walk independently | 459/995 (46.1) | 481/1000 (48.1) | 0.92 (0.73 to 1.16) | −2.0 (−7.7 to 3.8) | 351/998 (35.2) | 347/1001 (34.7) | 1.01 (0.84 to 1.22) | 0.5 (−3.7 to 4.7) |
| Inability to walk independently | 407/995 (40.9) | 438/1000 (43.8) | 275/998 (27.6) | 281/1001 (28.1) | ||||
| Death | 52/995 (5.2) | 43/1000 (4.3) | 1.23 (0.71 to 2.12) | 0.9 (−1.5 to 3.4) | 76/998 (7.6) | 66/1001 (6.6) | 1.17 (0.75 to 1.83)† | 1.0 (−1.9 to 4.0)† |
| P Value | P Value | |||||||
| Residence | 0.17 | 0.34 | ||||||
| Home or retirement home | 457/994 (46.0) | 425/999 (42.5) | 617/996 (61.9) | 603/1001 (60.2) | ||||
| Nursing home | 135/994 (13.6) | 161/999 (16.1) | 137/996 (13.8) | 161/1001 (16.1) | ||||
| Other | 402/994 (40.4) | 413/999 (41.3) | 242/996 (24.3) | 237/1001 (23.7) | ||||
| score | score | |||||||
| Function and symptom scales | ||||||||
| Lower-extremity physical ADL‡ | 7.3±4.0 | 7.4±3.9 | 0.72 | 5.1±4.2 | 5.1±4.3 | 0.85 | ||
| Instrumental ADL§ | 3.9±0.5 | 3.9±0.4 | 0.10 | 3.7±0.8 | 3.7±0.9 | 0.94 | ||
| FACIT-Fatigue scale¶ | 38.7±7.7 | 38.6±7.6 | 0.84 | 41.8±7.3 | 42.3±7.4 | 0.26 |
There were no significant between-group differences in the rates of death on 30-day follow-up (5.2% in the liberal-strategy group vs. 4.3% in the restrictive-strategy group), for an absolute risk difference of 0.9 percentage points (99% CI, −1.5 to 3.4), and on 60-day follow-up (7.6% in the liberal-strategy group vs. 6.6% in the restrictive-strategy group), for an absolute risk difference of 1.0 percentage point (99% CI, −1.9 to 4.0) (Table 3). The between-group differences were also not significant in the rates of in-hospital acute myocardial infarction, unstable angina, or death (4.3% in the liberal-strategy group vs. 5.2% in the restrictive-strategy group), for an absolute risk difference of −0.9 percentage points (99% CI, −3.3 to 1.6). The frequencies of in-hospital clinical events and serious adverse events did not differ significantly between groups (Table 4). Also similar in the two groups were the length of hospital stay, scores for lower-extremity physical activities of daily living, instrumental activities of daily living, and fatigue, as well as rates of residing at home at 30-day and 60-day follow-up (Table 3).
Table 4.
Hospital Outcomes.*
| Variable | Liberal Strategy(N = 1007) | Restrictive Strategy(N = 1009) | Odds Ratio(99% CI) | Absolute RiskDifference(99% CI) |
|---|---|---|---|---|
| number/total number (percent) | percentage points | |||
| Myocardial infarction, unstable angina, or in-hospital death† | 43/1005 (4.3) | 52/1008 (5.2) | 0.82 (0.48 to 1.42) | −0.9 (−3.3 to 1.6) |
| Myocardial infarction† | 23/1005 (2.3) | 38/1008 (3.8) | 0.60 (0.30 to 1.19) | −1.5 (−3.5 to 0.5) |
| Unstable angina† | 2/1005 (0.2) | 3/1008 (0.3) | 0.67 (0.06 to 7.03) | −0.1 (−0.7 to 0.5) |
| In-hospital death | 20/1005 (2.0) | 14/1008 (1.4) | 1.44 (0.58 to 3.56) | 0.6 (−0.9 to 2.1) |
| Isolated troponin elevation‡ | 62/1005 (6.2) | 59/1008 (5.9) | 1.06 (0.65 to 1.71) | 0.3 (−2.4 to 3.1) |
| Physician diagnosis of congestive heart failure | 27/1005 (2.7) | 35/1007 (3.5) | 0.77 (0.39 to 1.50) | −0.8 (−2.8 to 1.2) |
| Stroke or transient ischemic attack | ||||
| On CT or MRI | 5/1005 (0.5) | 1/1007 (0.1) | 5.03 (0.30 to 84.73) | 0.4 (−0.2 to 1.0) |
| On physician diagnosis or CT or MRI | 8/1005 (0.8) | 3/1007 (0.3) | 2.69 (0.47 to 15.42) | 0.5 (−0.3 to 1.3) |
| Chest radiograph with new or progressive infiltrate | 60/1005 (6.0) | 48/1007 (4.8) | 1.27 (0.76 to 2.12) | 1.2 (−1.4 to 3.8) |
| New-onset purulent sputum | 9/1005 (0.9) | 3/1007 (0.3) | 3.02 (0.54 to 16.91) | 0.6 (−0.3 to 1.5) |
| Wound infection | 14/1005 (1.4) | 8/1007 (0.8) | 1.76 (0.56 to 5.56) | 0.6 (−0.6 to 1.8) |
| Deep-vein thrombosis or pulmonary embolism | 12/1005 (1.2) | 8/1007 (0.8) | 1.51 (0.46 to 4.92) | 0.4 (−0.7 to 1.5) |
| Death, myocardial infarction, pneumonia | 89/1005 (8.9) | 90/1007 (8.9) | 0.99 (0.66, 1.48) | −0.1 (−3.4 to 3.2) |
| Death, myocardial infarction, pneumonia, thromboembolism, or stroke | 103/1005 (10.2) | 94/1007 (9.3) | 1.11 (0.75 to 1.63) | 0.9 (−2.5 to 4.3) |
| Returned to operating room | 15/1005 (1.5) | 18/1007 (1.8) | 0.83 (0.34 to 2.06) | −0.3 (−1.8 to 1.2) |
| Transfer to intensive care unit | 30/1005 (3.0) | 29/1007 (2.9) | 1.04 (0.53 to 2.05) | 0.1 (−1.8 to 2.0) |
| days | P Value | |||
| Time from randomization to discharge§ | ||||
| United States | 3.67±3.38 | 3.97±3.89 | 0.15 | |
| Canada | 12.03±9.31 | 12.70±9.48 | 0.32 |
DISCUSSION
We performed a randomized clinical trial involving 2016 patients undergoing surgery for hip fracture and found no evidence that maintaining the hemoglobin level above 10 g per deciliter was superior to transfusion for symptoms or maintaining a hemoglobin level of less than 8 g per deciliter with respect to the primary outcome (a composite of death or an inability to walk across the room without human assistance) and to several clinically relevant secondary outcomes, including cardiovascular event rates and other functional measures. We enrolled a high-risk group of patients with a mean age of more than 81 years for whom untreated anemia would probably be more harmful than in a healthier or younger population undergoing most surgical procedures.
An ability to walk across the room at 60 days was selected as a main component of the primary outcome because such a measure is recognized to be an important functional outcome after hip fracture and is likely to be affected by factors that transfusion might influence (e.g., aerobic capacity and muscle strength). We hypothesized, in particular, that a higher hemoglobin level might facilitate more active participation in rehabilitation, leading to more successful recovery of ambulation.
We achieved a clinically important difference in the use of packed red cells and a good separation in hemoglobin levels in the two transfusion groups (Fig. 1). Patients in the restrictive-strategy group received 65% fewer units of blood than those in the liberal-strategy group; more than half the patients in the restrictive-strategy group did not receive any blood transfusion. Widespread implementation of this restrictive approach to transfusion in similar patients would greatly reduce blood use.
We found an interaction between the transfusion strategy and sex in the liberal-strategy group, suggesting a higher rate of death or an inability to walk without human assistance at 60-day follow-up in men but not in women. This difference was not anticipated and could have been due to chance.
We obtained primary-outcome information (including data regarding deaths) for more than 99% of patients and validated vital status. However, we did not perform follow-up examinations, and our telephone ascertainment of functional outcomes was subject to possible miscommunication, poorly informed proxy respondents, and recording errors. Although we did not validate patients’ ability to walk, in cases in which both patients and their proxies answered the question about walking ability, we found strong agreement between the two reports. Scores for physical activities of daily living, instrumental activities of daily living, and fatigue were not validated and were not useful for analysis for 45 to 60% of patients. We revised eligibility criteria in the course of the trial to include lower-risk patients who had cardiovascular risk factors but no history of cardiovascular disease, and there was no important treatment interaction with cardiovascular-disease status.
Our study had excellent statistical power for determining the primary outcome of death or inability to walk. On the basis of the 95% confidence interval, the restrictive transfusion policy plausibly resulted in at most a 3.7% increase in the risk of death or inability to walk without human assistance, a composite outcome that occurred in about 35% of patients. We had less statistical power for in-hospital outcomes; our data are compatible with an absolute change in the composite outcome of in-hospital acute myocardial infarction, unstable angina, or death, ranging from an increase of 3.3 percentage points to a decrease of 1.6 percentage points for the restrictive transfusion strategy.
Our results are consistent with most of the findings of the Transfusion Requirements in Critical Care (TRICC) trial, in which outcomes did not differ significantly between a transfusion threshold of 7 g per deciliter and a threshold of 10 g per deciliter among patients in intensive care units.5,13 However, in contrast to that report, we did not find increased rates of myocardial infarction or congestive heart failure in the liberal-strategy group. Furthermore, we did not confirm findings from observational studies of markedly higher mortality in patients who received transfusion than in patients who did not.14 A randomized clinical trial allows us to evaluate transfusion while avoiding selection bias.15
In summary, we found that a liberal transfusion strategy, as compared with a restrictive strategy, did not result in reduced rates of death or an inability to walk on 60-day follow-up or in significant reductions in rates of in-hospital complications in this population at increased cardiovascular risk. Our findings suggest that it is reasonable to withhold transfusion in patients who have undergone surgery in the absence of symptoms of anemia or a decline in the hemoglobin level below 8 g per deciliter, even in elderly patients with underlying cardiovascular disease or risk factors.
Supplementary Material
Supplement1
Acknowledgments
Supported in part by grants from the National Heart, Lung, and Blood Institute (U01 HL073958 and U01 HL074815).
Dr. Carson reports receiving grant support to his institution from Amgen; Dr. Lewis, receiving a salary from the Orthopaedic Associates of Hartford, receiving a stipend for serving as president of the Hartford County Medical Association, and providing expert testimony representing the American Academy of Orthopaedic Surgery on the Medicare Evidence Development and Coverage Advisory Committee; Dr. Apple, serving as a scientific advisory board member for Abbott Laboratories, Alere, Beckman Coulter, Ortho Clinical Diagnostics, and Instrumentation Laboratories, receiving consulting fees from Abbott Diagnostics, Ortho Clinical Diagnostics, and Instrumentation Laboratories, receiving grant support to his institution from Abbott Diagnostics, Siemens, Ortho Clinical Diagnostics, Roche Diagnostics, BioRad, Response Biomedical, Radiometer, and BRAHMS, and receiving lecture fees and travel expenses from Abbott Diagnostics and Alere; Dr. Magazine, serving as a board member for Amgen, Novartis, and GlaxoSmithKline and receiving consulting fees from Eli Lily, Sanofi-Aventis, and Amgen, grant support to his institution from Novartis, Merck, and Eli Lilly, and lecture fees from Novartis.
APPENDIX
The authors’ affiliations are as follows: the Division of General Internal Medicine, Department of Medicine, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, New Brunswick (J.L.C., H.N., K.D.); the Department of Epidemiology and Public Health, University of Maryland School of Medicine (M.L.T., J.M.); and Johns Hopkins Bayview Medical Center (K.J.Z.) — both in Baltimore; the Division of Orthopaedic Surgery, University of Western Ontario, London (D.W.S.); the Department of Physical Therapy and Surgery and the Division of Orthopaedic Surgery (L.B.), University of Alberta, Edmonton; the Division of Orthopedic Surgery (K.H.) and Department of Medicine (D.R.C.), University of Calgary, Calgary, AB; and the Department of Orthopedic Surgery, QEII Health Sciences Centre, Halifax, NS (G.D.) — all in Canada; the Department of Medicine, Saint Louis University School of Medicine, St. Louis (B.R.C.); the Department of Epidemiology, University of Medicine and Dentistry of New Jersey–School of Public Health, Piscataway (G.G.R.); the Transfusion Medicine and Cellular Therapeutics Branch, Division of Blood Diseases and Resources, National Heart, Lung, and Blood Institute, Bethesda (G.N.); and the Cooperative Studies Program Coordinating Center, Veterans Affairs Medical Center, Perry Point (R.A.H.) — both in Maryland; the Department of Orthopedic Surgery, New York–Presbyterian Hospital at Columbia University, New York (W.M.); Hartford Hospital, Hartford, CT (C.L.); and Minneapolis Medical Research Foundation of Hennepin County Medical Center and University of Minnesota School of Medicine, Minneapolis (F.S.A.).
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Washington, DC: Department of Health and Human Services, Office of the Assistant Secretary for Health; 2011. Report of the Department of Health and Human Services: the 2009 national blood collection and utilization survey report. [Google Scholar]
- 2.Anderson SA, Menis M, O’Connell K, Burwen DR. Blood use by inpatient elderly population in the United States. Transfusion. 2007;47:582–592. doi: 10.1111/j.1537-2995.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 3.Cobain TJ, Vamvakas EC, Wells A, Titlestad K. A survey of the demographics of blood use. Transfus Med. 2007;17:1–15. doi: 10.1111/j.1365-3148.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 4.Carless PA, Henry DA, Carson JL, Hebert PP, McClelland B, Ker K. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2010;10 doi: 10.1002/14651858.CD002042.pub2. CD002042. [DOI] [PubMed] [Google Scholar]
- 5.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [Erratum, N Engl J Med 1999;340:1056.] [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Terrin ML, Magaziner J, et al. Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) Transfusion. 2006;46:2192–2206. doi: 10.1111/j.1537-2995.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- 7.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined — a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [Erratum, J Am Coll Cardiol 2001; 37:973.] [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Fleiss JL. Statistical methods for rates and proportions. New York: John Wiley; 1981. [Google Scholar]
- 10.Mantel N, Haenszel W. Statistical aspects of the analysis of data for retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 11.Breslow NE, Day NE. The analysis of case-control studies. Vol. 1. Lyon, France: International Agency for Research on Cancer; 1980. (IARC scientific publications no. 32.) [Google Scholar]
- 12.Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley; 1989. [Google Scholar]
- 13.Hébert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;29:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [Erratum, Crit Care Med 2008;36:3134.] [DOI] [PubMed] [Google Scholar]
- 15.MacMahon S, Collins R. Reliable assessment of the effects of treatment on mortality and major morbidity, II: observational studies. Lancet. 2001;357:455–462. doi: 10.1016/S0140-6736(00)04017-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1