Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review (original) (raw)
Abstract
The Olive tree (Olea europaea L.), a native of the Mediterranean basin and parts of Asia, is now widely cultivated in many other parts of the world for production of olive oil and table olives. Olive is a rich source of valuable nutrients and bioactives of medicinal and therapeutic interest. Olive fruit contains appreciable concentration, 1–3% of fresh pulp weight, of hydrophilic (phenolic acids, phenolic alchohols, flavonoids and secoiridoids) and lipophilic (cresols) phenolic compounds that are known to possess multiple biological activities such as antioxidant, anticarcinogenic, antiinflammatory, antimicrobial, antihypertensive, antidyslipidemic, cardiotonic, laxative, and antiplatelet. Other important compounds present in olive fruit are pectin, organic acids, and pigments. Virgin olive oil (VOO), extracted mechanically from the fruit, is also very popular for its nutritive and health-promoting potential, especially against cardiovascular disorders due to the presence of high levels of monounsaturates and other valuable minor components such as phenolics, phytosterols, tocopherols, carotenoids, chlorophyll and squalene. The cultivar, area of production, harvest time, and the processing techniques employed are some of the factors shown to influence the composition of olive fruit and olive oil. This review focuses comprehensively on the nutrients and high-value bioactives profile as well as medicinal and functional aspects of different parts of olives and its byproducts. Various factors affecting the composition of this food commodity of medicinal value are also discussed.
Keywords: Mediterranean diet, high-value components, bioactives, phytochemicals, virgin olive oil, medicinal uses, therapeutic potential
1. Introduction
The Olive (Olea europaea L.) is a small tree, which belongs to the family Oleaceae and is native to tropical and warm temperate regions of the world. The tree, famous for its fruit, also called the olive, is commercially important in the Mediterranean region as a prime source of olive oil [1]. The tree is typically distributed in the the coastal areas of the eastern Mediterranean Basin, the adjoining coastal areas of southeastern Europe, western Asia and northern Africa as well as northern Iran at the south end of the Caspian Sea. Although olive is now cultivated in several parts of the world, the Medetarrianen region still serves as the major production area accounting for about 98% of the world’s olive cultivation [2].
According to estimates, the cultivation of olive tree dates back more than 7000 years. Archaelogical evidence indicates that olives were grown commercially in Crete as far back as 3000 BC, by the Minoan civilization [3]. Ancient Greek literature reveals uses of olive oil for body health [3]. In the context of religious importance, olive tree and its fruit (olives) are narrated over several times in the Bible, both in the New and Old Testaments [2] as well as in the Quran [2,3]. Olive is praised as a blessed fruit in Chapter 24 Al-Nur (Quran 24:35).
The olive tree has a long history of medicinal and nutritional values. Over the centuries, extracts from olive leaf have been used for promoting health and preservation. For instance, ancient Egyptians used the leaves to mummify Pharaohs. Similarly, they have been valued as a famous folk remedy to treat fever and some tropical diseases such as malaria [4]. Economically, the fruit of olive is an important commodity as it yields nutritious edible oil with potential medicinal functions [5]. Olives are rarely used in their natural form due to severe bitterness; nevertheless, they are consumed in either one of the two forms, namely oil or table olives. Oleuropein is the bitterness-causing chemical component that must be eliminated from olivesto make them palatable [2,4].
Due to rising awareness about the beneficial effects of optimal nutrition and functional foods among todays’s health conscious cosmopolitan societies, the worldwide consumption of olives and olive products has increased significantly, especially in high-income countries such as the United States, Europe, Japan, Canada and Australia, resulting the rapid development of olive-based products [2,6]. The traditional “Mediterranean diet”, in which olive oil is the main dietary fat, is considered to be one of the healthiest because of its strong association with the reduced incidence of cardiovascular diseases and certain cancers [2,4,7,8]. The health benefits of olive oil are mainly ascribed to the presence of high content of monounsaturated fatty acid (MUFAs) and functional bioactives including tocopherols, carotenoids, phospholipids and phenolics, with multiple biological activities [9,10]. Such components also contribute to the unique flavour and taste of olive oil. As with other crops, the composition of olive and olive oil components varies in relation to various factors, namely cultivar, ripeness and harvesting regime, agroclimatic conditions as well as the processing techniques employed [10].
The main objective of the present review is to compile a comprehensive report, covering nutritional, medicinal and functional aspects of different parts of the olive, thus providing detailed information about the valuable nutrients and high-value bioactives of this multipurpose tree. The technological advancements to be applicable for the potential recovery of valuable components from olive processing wastes have also been discussed.
2. Distribution, Cultivation and Production of Table Olives and Olive Oil
The task of identifying and classifying different olive varieties is quite challenging [1]. There are around 2500 known varieties of olives, of which 250 are classified as commercial cultivars by the International Olive Oil Council as described in Table 1 [11]. These commercial cultivars are used for the production of either olive oil or table olives or both. The specific use of a given cultivar is determined by its oil content and size. Olive varieties with oil content less than 12% such as Ascolano, Calamata and Manzanillo are almost exclusively used for table olive production, while those withhigher oil yield (ca. 20%) such as Hojiblanca, Verdial, Picual, Gemlik, Nychati Kalamonand Arauco are usually preferred for the purpose of olive oil production [2]. The larger fruits (more than 4 g) are mostly favored for table olive consumption. In the case of table olive production, besides the fruit size, several other physical properties of fruits cultivars such as shape, flesh-to-pit ratio, colour and texture are of key importance (Table 2) but some other factors, namely harvesting procedure, type of cultivation (irrigated or non-irrigated system), and ripening cycle areregarded less important [12].
Table 1.
List of some common olive cultivars and their origins in the world [12].
| Origin | Cultivar |
|---|---|
| Greece | Adramitini, Amigdalolia, Amphissis, Chalkidikis (Chondrolia), Daphnoelia Doppia, Frantoio, Gordal, Koroneiki, Karidolia, Lianolia, Patrini, Chondrolia (aka Throumbolia), Tsounati, Valanolia |
| Italy | Biancolilla, Bosana, Canino, Casaliva, Cellina di Nardo, Coratina Dolce Agogio, Dritta, Moraiolo, Rosciola, Pisciottana, Grignan, Ottobratica |
| Spain | Alfafara, Arbequina, Bical, Blanqueta, Empeltre, Farga, Gordal, Lechin, Hojiblanca, Manzanilla de Jaén, Morrut Palomar, Picual, Sevillenca Verdiell, Vilallonga |
| France | Aglandau, Amellau, Cayon, Germaine, Picholine, Lucques, Sabine, Salonenque Picholine, Zinzala |
| Portugal | Cobrancosa, Galega |
| Croatia | Oblica and Leccino |
| Tunisia | Chemlali, Chetoui, Gerboui, Meski, Oueslati |
Table 2.
Some important properties of popular cultivars, their utilization and origin [12].
| Cultivar | %Oil yield | Ratio flesh to pit | Olive size (Fruits/kg) | Purpose | Shape of the fruits | Origin |
|---|---|---|---|---|---|---|
| Hojiblanca | 23–29 | 4.9 and 6.6:1 | 230–700 | Oil extraction and table olives | Regular | Spain |
| Verdial | 22–30 | 6:1 | 220–800 | Oil extraction and table olives | Ellipsoidal | Spain |
| Picual | 23–27 | 3.8 and 6.1:1 | 270–470 | Oil extraction and table olives | Prominent tip at the button end | Spain |
| Domat | 22 | - | 180–190 | Green table olive | Regular | Turkey |
| Gemlik | 27 | 6:1 | 270–280 | Black table olives | Pronounced tip at the end | Turkey |
| Memecik | 22 | 6:1 | 205–215 | Oil extraction | Pronounced tip at the end | Turkey |
| Memeli | 25 | 7 : 1 | 200–210 | Green-type table olives | Small tip at the end | Turkey |
| Conservolea | 22–25 | 8:1 | 180–200 | Table olive | Round to oval | Greece |
| Nychati Kalamon | 25 | 8:1 | 220–240 | Black table olive | Cylindro-conical, Curved | Greece |
| Chalkidiki | 19 | - | 120–140 | Green table olive, oil extraction | Pronounced tip at the end | Greece |
| Sevillano | 14 | 7.3:1 | 70–80 | Table olive | - | Spain |
| Ascolano | 19 | 8.2:1 | 110–120 | Table olive | - | Italy |
| Ascolana | 17 | - | 100–180 | Table olive | Spherica | Italy |
| Barouni | 17 | 6.8:1 | 130–140 | Green & black - ripe olives | - | Tunisia |
| Picholine marocaine | 17 | 5:1 | 300–500 | Table olive | - | Morocco |
| Arauco | 22–24 | 7:1 | 125–300 | Table olive and oil extraction | Pronounced tip | Argentina |
| Galega vulga | - | 4:1 | 430 | Black table olive | - | Portugal |
| Oblitza | 22 | 6.5:1 | 200 | Table olive | Apple and heart shape | Yugoslavia |
| Ladoelia | 18–21 | 4.6:1 | 330 | Table olive | - | Cyprus |
Naturally, olive fruits have a bitter flavor; hence they are typically subjected to fermentation or cured with lye or brine to make them more palatable. There are three important internationally used practices for preparation of table olives [13].
- Spanish-style (pickled) green olives in brine
- Californian-style (pickled) black olives in brine
- Greek-style natural black olives in brine
In the Spanish and Californian-style method, the bitterness of the olive is removed by using food grade sodium hydroxide treatment that caused several changes in some compounds of the fruit; however triglycerides composition remains unaffected by this treatment. After the preliminary brine treatment, the fruits are rinsed to remove alkali and then fermented in brine for several months. In the case of Greek-style, the fruits are placed directly in the brine to remove oleuropein completely or partially. In the Spanish-style, a lactic fermentation is principally used for green olive brine, while for the Greek-style; natural black olives in brine are fermented mostly using yeasts. In the Californian-style, black olives do not necessarily require fermentation at all. They are treated directly with lye and oxidized, then washed, placed in brine, and packed in cans with heat-sterilization [12].
Olives are known as one of the widely cultivated fruit crops worldover [14]. Presently, approximately more than 750 million olive trees are cultivated worldwide. About 98% of the total surface area, 99% of productive trees and 99% of total olive production belong to the countries around the Mediterranean basin and in the Middle-East. According to an estimate of Food and Agriculture Organization (FAO), in 2009, 9.9 million hectares (ha) were planted with olive trees. Spain, with a total cultivated area of 2,500,000 ha, is the biggest producer, followed by Italy (1,159,000 ha) and Greece (765,000 ha) [15]. Each olive tree produces an average of 15 to 50 kg of olives; depending on the nature of olive and environmental conditions [11]. World olive oil production in 2008–2009 was 2.9 million tones, of which Spain, contributing over 40%, was the top producer. After Spain and Italy, Greece holds third position in world olive oil production, producing approximately 332,000 tons of olive oil annually, of which 82% is extra-virgin olive oil. About half of the annual Greek olive oil produced is exported, but only some 5% of this reflects the origin of the bottled product [15]. Based on the data from the FAO, ten main olive producing countries are located in the Mediterranean region, which produce 95% of the world’s olives as shown in Table 3.
Table 3.
Major olive production countries in 2009 [15].
| Country | Production (tonnes) | Cultivated area (hectares) | Yield (quintal/hectar) |
|---|---|---|---|
| World | 18,241,809 | 9,922,836 | 18.383 |
| Spain | 6,204,700 | 2,500,000 | 24.818 |
| Italy | 3,600,500 | 1,159,000 | 31.065 |
| Greece* | 2,444,230 | 765,000 | 31.4 |
| Turkey | 1,290,654 | 727,513 | 17.740 |
| Syria | 885,942 | 635,691 | 13.936 |
| Morocco | 770,000 | 550,000 | 14.000 |
| Tunisia | 750,000 | 2,300,000 | 3.260 |
| Egypt | 500,000 | 110,000 | 45.454 |
| Algeria | 475,182 | 288,442 | 16.474 |
| Portugal | 362,600 | 380,700 | 9.524 |
| Libya | 180,000 | Na | Na |
| Argentina | 160,000 | 52,000 | 30.769 |
Virgin olive oil (VOO) is obtained solely through physical means by mechanical or direct pressing of the olives under mild thermal conditions that do not lead to alterations in the oil composition. Virgin olive oil is not subjected to any treatment except washing, decantation, centrifugation and filtration [16]. In this regard, the oils produced by solvent extraction or re-esterification processes, and those blended or mixed with other vegetable oils are excluded from the category of VOO.
World olive oil production in 2009 was 2.9 million tones, of which Spain supplied 1/3 of the world’s olive oil followed by Italy (1/4), and Greece (1/5). Greece has the maximum olive oil consumption per capita worldwide (around 23.7 liters/person/year), followed by Spain and Italy with around 13.62 liters and 12.35 liters, respectively (Table 4). On the commercial product value basis, olive oil contributes 15 percent share of the world oil trade [17]. The price of olive oil is usually 2–5 fold higher than that of other vegetable oils depending on the origin, category/type of the oil, and the harvesting period of the olive fruits [17].
Table 4.
World wide major production and consumption of olive oil [15].
| Country | Production in tons (2009)a | Production % (2009) | Consumption (2005)b | Consumption per person annually (liters/kg)c |
|---|---|---|---|---|
| World | 2,907,985 | 100% | 100% | 0.43 |
| Spain | 1,199,200 | 41.2% | 20% | 13.62 |
| Italy | 587,700 | 20.2% | 30% | 12.35 |
| Greece | 332,600 | 11.4% | 9% | 23.7 |
| Syria | 168,163 | 5.8% | 3% | 7.0 |
| Tunisia | 150,000 | 5.2% | 2% | 11.1 |
| Turkey | 143,600 | 4.9% | 2% | 1.2 |
| Morocco | 95,300 | 3.3% | 2% | 1.8 |
| Portugal | 53,300 | 1.8% | 2% | 7.1 |
| France | 6,300 | 0.2% | 4% | 1.34 |
| United States | 2,700 | 0.1% | 8% | 0.56 |
| Others | 169,122 | 5.8% | 18% | 1.18 |
3. Composition of High-Value Nutrients and Functional Bioactives in Different Parts of Olive
Different parts of the olive are valued for their nutrients and functional food components and health-promoting bioactives.
3.1. Olive Fruit
The olive fruit is an oval-shaped drupe and possesses a typical size of 2–3 cm (width and length) and pulp per stone ratios of 3.0–6.5. The olive fruit is essentially made up of 3 parts, epicarp or skin, mesocarp or pulp and endocarp or stone. The epicarp (skin) is covered with wax; during the growth phase the skin colour turns from light green to purple and brown or black. The mesocarp, with a soft, pulpy flesh, accounts for 84–90% (of the total fruit mass) while the hard endocarp (stone) containing the seed or kernel may differ from 13 to 30% of fruit weight. The seed contains 2–4 g oil /100 g. Olive fruit weight may range from 2–12 g, although some varieties may weigh as much as 20 g [18,19].
The growth and ripening of olive fruit is a long process, which takes about 5 months in usual climatic conditions. However, in cold climatic conditions, growth is slower. Olive fruit’s average composition includes water (50%), protein (1.6%), oil (22%), carbohydrate (19.1%), cellulose (5.8%), inorganic substances (1.5%) and phenolic compounds (1–3%). Other important compounds present in olive fruit are pectin, organic acids, and pigments [1]. Organic acids show metabolic activity and are intermediate products resulting from formation and degradation of other compounds [20].
The distribution and structure of the chemical constituents of olive fruit is complex and dependent on parameters including variety, cultivation practices, geographical origin, and the level of maturation. The olive phenols impart antimicrobial properties to different parts of the plant and are also responsible for the extent of browning in the fruit. These phenolic components also contribute towards the sensory and aromatic characteristics of the olive as well as impart pharmaceutical and physiological benefits [1,9,21].
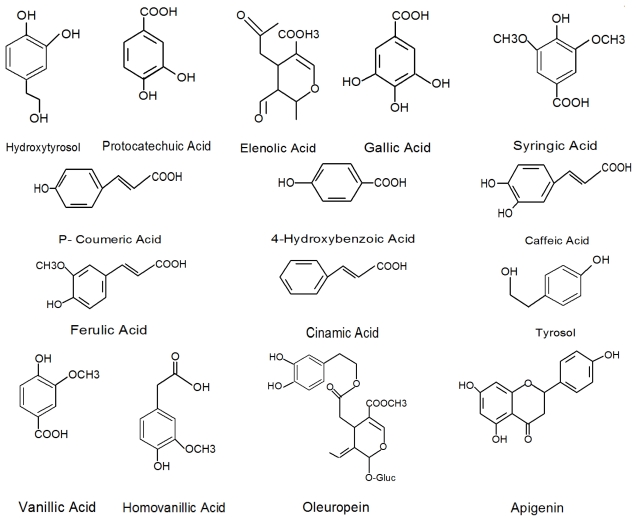
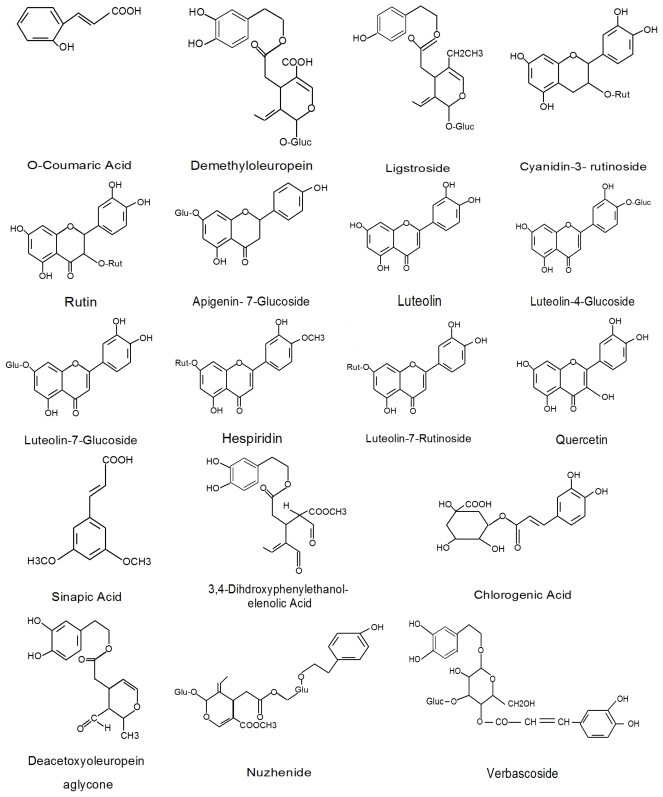
Both lipophilic and hydrophilic phenolics are distributed in olive fruit. The main lipophilic phenols are cresols while the major hydrophilic phenols include phenolic acids, phenolic alchohols, flavonoids and secoiridoids; they are present in almost all parts of the plant but their nature and concentration varies greatly between the tissues [1,9]. Phenolic acids are named as secondary aromatic plant metabolites that are commonly distributed throughout the plant kingdom [22,23]. Phenolic acids with basic skeleton of C6–C1 (hydroxybenzoic acid) such as vanillic acid, syringic acid, gallic acid; C6–C3 (hydroxycinnamic acid) such as caffeic acid, ferulic acid and sinapic acid, and flavonoids with the chemical structure of C6–C3–C6 such as cyanidin have been investigated in olive fruit [2,4,12,24,25]. The main hydroxycinnamic acid derivative reported in olive fruit is verbascoside [26]; its chemical structure was identified by Andary et al. [27] and confirmed by Servili et al. [26], as shown in Figure 1.
Figure 1.
Chemical structure of important bioactives in olive/olive oil [2,28].
The main phenolic alcohols of olives include oleuropein β-(3,4-dihydroxyphenylethanol) or hydroxytyrosol and _p_-hydroxyphenylethanol (tyrosol) [2,29]. Flavonoid compounds in olive are mainly comprised of flavonol glycosides such as luteolin 7-_O_-glucoside, rutin, apigenin 7-_O_-glucoside, anthocyanins, cyanidin 3-_O_-glucoside and cyanidin 3-_O_-rutinoside [30,31]. Hydroxytyrosol and tyrosol are present at the highest contents of 76.73 and 19.48 mg/100 g olives, respectively, in comparison to the rest of the phenolic compounds [24]. During ripening of olives, oleuropein is completely degraded, and is almost undetectable when the fruit darkens, but hydroxytyrosol, tyrosol, and verbascoside increase [4]. The presence of such medicinally important bioactive compounds in olive fruit supports the functional foods potential of the major products, table olives and virgin olive oil, produced from this species [1,2,10,12,24,25]. Besides, two important pentacyclic triterpenes, namely oleanolic and maslinic acids with potential antiproliferative activity, have been reported from olive fruit skin [32].
Oleuropein is the major secoiroidoids constituent of unripe olive fruit. The concentration of this compound decreases with maturation, while demethyloleuropein and the dialdehydic form of elenolic acid linked to β-(3,4-dihydroxyphenyl) ethanol), (3,4-DHPEA or hydroxytyrosol) increases [33]. Ragazzi et al. [34] was the first to separate and characterize demethyloleuropein in the ripe olives. Another polyphenol named ligstroside (deacetoxy-ligstroside aglycon), which contributes to the pungent odor of extra virgin olive oil, was identified in the olive fruit [35] (Figure 1). Different parts of olive fruit including seed, peel, and especially the pulp also contain oleuropein, demethyloleuropein and verbascoside, but nuzhenide is only detected in the seed [26] (Figure 1). The bitterness of the olive fruit is mainly attributed to the occurrence of oleuropein and it has to be removed in table olive processing [30]. The alkali (NaOH) treatment hydrolyses oleuropein into β-(3,4-dihydroxyphenyl) ethanol), oleoside 11-methylester and oleside making the olive palatable [21]. The detailed description of the main classes of olive phenolics is given in Table 5.
Table 5.
Main classes of phenolic compounds in olive fruit.
| Phenolic compounds | Reference |
|---|---|
| FlavonolsQuercetin-3-rutinoside, Luteolin-7-glucoside, Luteolin-5-glucoside, Apigenin-7-glucoside | [2,36] |
| Phenolic acidsChlorogenic acid, Caffeic acid, _p_-Hydroxybenzoic acid, Protocatechuic acid, Vanilic acid, Syringic acid, _p_-Coumaric acid, _o_-Coumaric acid, Ferulic acid, Sinapic acid, Benzoic acid, Cinnamic acid, Gallic acid | [2,12,36,37] |
| Phenolic alcohols(3,4-Dihydroxyphenyl) ethanol (3,4-DHPEA), (_p_-Hydroxyphenyl) ethanol (_p_-HPEA) | [2,31,38] |
| SecoiridoidsOleuropein, Demethyloteuropein, Ligstroside, Nuzhenide | [26,34,35,38,39] |
| Hydroxycinnamic acid derivativesVerbascoside | [26,27] |
Oxidative stress is considered as one of the major factors causing different diseases such as cancer, aging, inflammation and atherosclerosis [40]. It is now widely accepted that the risk of oxidative damage can be reduced by the high intake of plant-based antioxidants. In this regard, olive polyphenols, being free radical scavengers, contribute positively towards skin health by preventing the oxidative damage linked with the formation of wrinkles and other such disorders such as skin dryness and hyperproliferation. In a clinical study, it has been shown that olive intake increases polyphenols and total antioxidant potential (TAP) in plasma, thus indicating that olive polyphenols have good bioavailability, which is in accordance with their antioxidant efficacy [24]. Olive extracts contain 73.25% maslinic and 25.75% oleanolic acids, which potentially have cancer chemopreventive activity [41]. The unripe olive fruit extract has been shown to possess the calcium channel blocking activity, considered to be responsible for its effectiveness in cardiovascular disorders like hypertension [42]. Similarly, olive fruit extract has been shown to contain a combination of laxative and antidiarroeal activities mediated through the presence of cholinergic and calcium channel blocking constituents, respectively [43,44].
3.2. Olive Oil
Olive oil is widely used for food preparations (as salad oil, cooking oil, in frying and pasta sauces), in cosmetics and the pharmaceutical industry [45]. In the olive fruits, oil is mainly concentrated in the pericarp (96–98%). The formation and accumulation of oil in the drupe, a rich reservoir of many classes of lipids, is possibly the reason why the oil has a unique flavour and fragrance. The olive flesh components are transformed to the oil, which mainly consists of two components, namely saponifiables and unsaponifiables. The former, comprising triacylglycerols (TAG), partial glycerides, esters of fatty acids or free fatty acids and phosphatides, represent nearly 98% of the oil chemical composition, while the later, consisting of mainly minor components such as tocopherols, phytosterols, coloring pigments and phenolics, contribute around 1–2% of the oil composition [25]. The oil triglycerides are mainly represented by monounsaturates (oleic acid), alongwith small amount of saturates and considerable quantity of polyunsaturates (mainly of linoleic acid) [46].
Several public-health based studies have revealed that the traditional “Mediterranean diet”, which includes VOO as one of the most important food ingredients, is strongly linked with the reduced prevelance of cardiovascular diseases and certain cancers [47,48]. The nutritional value and health functions of VOO are ascribed to the presence of large amount of monounsaturated fatty acids (MUFAs) such as oleic acid and valuable minor components including aliphatic and triterpenic alcohols, sterols (mainly β-sitosterol), hydrocarbons (squalene), volatile compounds, tocopherols (chiefly α-tocopherol), pigments such as chlorophylls, carotenoids (β-carotene and lutein) and antioxidants [49].
In 2004, the Food and Drug Administration (FDA) of the USA allowed a claim on olive oil labels concerning “the benefits on the risk of coronary heart disease of eating about two tablespoons (23 g) of olive oil daily, due to the MUFAs in olive oil” [50]. Oleic acid (C18:1), the principal fatty acid in oilive oil, is claimed to increase the plasma high density lipoprotein (HDL) cholesterol and apoprotein A1 and decrease the low density lipoprotein (LDL) cholesterol and apoprotein B [51]. For this reason, this fatty acid (i.e., olieic acid) can prevent cardiovascular diseases that are the major cause of mortality in industrialized countries [52]. If these beneficial effects of olive oil can be attributed only to MUFAs contents, any type of high oleic acid oils such as rapeseed oil, or any MUFAs-rich food would have shown the same health benefits; this indicates that olive oil’s effects are due to more than MUFAs contents. It has been suggested that the phenolic profile of olive oil is likely to have far greater benefits on blood lipids and oxidative damage than those shown by MUFAs [10]. Based on this evidence, olive oil can be categorized as a functional food that besides having a high level of oleic acid, contains other medicinally important minor components with multiple biological activities [53].
3.2.1. Fatty Acid Composition of Olive Oil
Fatty acids present in olive oil include palmitic (C16:0), palmitoleic (C16:1), stearic (C18:0), oleic (C18:1), linoleic (C18:2), and linolenic (C18:3). Myristic (C14:0), margaric (C17:0) and gadoleic (C20:1) acids are found in trace amount (Table 6). Also traces of 11-_cis_-vaccenic and eicosenoic acids have been detected using C-13 Nuclear Magnetic Resonance (13C-NMR) spectroscopic approach [18].
Table 6.
Fatty acid composition (%) of olive oil from selected cultivars.
| Fatty Acid | IOOC (a) | Arbequina (b) | Abosasana (b) | Koroneiki (b) | Frantoio (c) | Leccino (d) | Busa (d) |
|---|---|---|---|---|---|---|---|
| Myristic acid | < 0.05 | ND | ND | ND | ND | ND | ND |
| Palmitic acid | 7.5–20.0 | 17.57 | 17.78 | 11.65 | 10.9 | 13.7 | 12.07 |
| Palmitoleic acid | 0.3–3.5 | 2.41 | 2.12 | 1.07 | 0.89 | 1.32 | 1.02 |
| Heptadecanoic acid | < 0.3 | ND | ND | ND | 0.07 | ND | ND |
| Stearic acid | 0.5–5.0 | 1.88 | 2.07 | 2.15 | 1.53 | 1.9 | 1.97 |
| Oleic acid | 55.0–83.0 | 58.82 | 64.79 | 75.53 | 78.3 | 75.69 | 74.54 |
| Linoleic acid | 3.5–21.0 | 12.93 | 12.09 | 8.56 | 6.79 | 5.65 | 8.36 |
| Linolenic acid | < 1.0 | 0.63 | 0.54 | 0.26 | 0.49 | 0.161 | 0.66 |
| Arachidic acid | < 0.6 | 0.40 | 0.33 | 0.42 | 0.33 | 0.3 | 0.33 |
| Gadoleic acid (eicosenoic) | < 0.4 | ND | ND | ND | 0.27 | ND | ND |
| Behenic acid | < 0.2 | ND | ND | ND | 0.18 | ND | ND |
| Lignoceric acid | < 0.2 | ND | ND | ND | ND | ND | ND |
Almost all the cultivars of olive oil have C16:0, C18:0, C18:1 and C18:2 as the main components; C16:1, C18:3, and C20:0 are present in small amounts, while C22:0, C20:1, and C24:0 are at levels often less than 0.2%. The principal component is always the oleic acid, contributing about 55–75% of the total fatty acids. Some parameters such as the area of production, the latitude, the climate, the variety, and the stage of maturity of the fruit greatly affect the fatty acid composition of olive oil. For example, varieties of olive oil from Greece, Italy, and Spain are low in linoleic and palmitic acids but they have a high percentage of oleic acid, while the Tunisian olive oil is high in linoleic and palmitic acids and low in oleic acid [57].
Polyunsaturated fatty acids (PUFAs) with 18 carbon (C18) atoms such as linoleic (18:2 ω-6), and α-linolenic (18:3 ω-3) are known as essential fatty acids (EFAs) in human nutrition. These fatty acids, although regarded as an indispensable component for cell structure and development and function, cannot be synthesized by the human body. The intake of PUFA is necessary through diet, and should account for only 6–8% of calories from fat [25]. At the same time, the consumption of saturated fatty acids should be moderate (approximately the same amount as polyunsaturates, with a ratio of 1:1). Saturated fatty acids increase plasma cholesterol level and acts as “promoters” of certain cancer development (e.g., colon, breast, and perhaps uterus and prostate). Nutritionists recommend a balanced lipid intake corresponding to a total amount of fats equal to 25 to 30% of total calories with a ratio in fatty acids as follows: 1-Saturates (6–8%), 2-Monounsaturates (12–14%), 3- Polyunsaturates as a ω-6 (6–7%), and 4-Polyunsaturates as a ω-3 (0.5–1.5%) [25].
The two series “ω-6 and ω-3″ are, however, in contrast with each other in many aspects. Therefore, it seems important that they be present in a correct ratio in the diet, because an excess of linoleic acid can prevent the endogenous synthesis of the long chains of α-linolenic acid (eicosapentaenoic acid and docosahexaenoic acid) with consequent damage to the body. The World Health Organization recommends a ratio of 5:1 to 10:1 for ω-6 to ω-3. The ratio between the ω-6 and the ω-3 series is very important, especially during growth, because the long-chain ω-3 series are fundamental for brain and retina development. Other important functions associated include anti-cancer, antiplatelet aggregation, anti-inflammatory, and protection against dryness of the skin. The given recommended ratio is found in olive oil, whereas the same cannot be established for other vegetable oils, with the exception of linseed and soy oils [25].
It is widely accepted that the oils with higher levels of MUFAs and lower in saturated fatty acids (SFAs) are superior due to the proven beneficial effect of MUFAs on serum cholesterol levels. Table 7 shows fatty acid groups of olive oil in comparison with other edible oils. The relatively high content of MUFAs and less SFAs and considerable EFAs impart olive oil a high nutritional status, while extra virgin olive oil, extracted directly from olive fruit through mechanical means, has appreciable antioxidant activity and medicinal benefits due to the presence of an array of high-value minor components such as phenolics [9,10,37,48].
Table 7.
Fatty acid groups of olive oil in comparison to some other edible oils [25].
| Saturates (%) | Monounsaturates (%) | ω-6 (%) | ω-3(%) | |
|---|---|---|---|---|
| Butter | 45–55 | 35–55 | 1.5–2.5 | 0.5 |
| Lard | 40–46 | 42–44 | 6–8 | 0.5–0.9 |
| Olive oil | 8–14 | 65–83 | 6–15 | 0.2–1.5 |
| Peanut oil | 17–21 | 40–70 | 13–28 | - |
| Maize oil | 12–28 | 32–35 | 40–62 | 0.1–0.5 |
| Soyabean oil | 10–18 | 18–30 | 35–52 | 6.5–9 |
| Sunflower oil | 5–13 | 21–35 | 56–66 | - |
3.2.2. Olive Oil Phenolics
Plant phenolic compounds are well known secondary metabolites. These can be synthesized naturally by plants in response to stress conditions such as infection, wounding, and UV radiation [58]. There are at least 30 phenolic compounds detected in olive oil belonging to the hydrophilic group [48]. The phenolic composition of olive and olive oil is very complex and the average concentration of these compounds depends on several factors including maturation stage, part of the fruit, variety, season, packaging, storage, climatologic conditions and the degree of technology used in its production [59]. If measured colorimetrically as total phenols in the methanolic extract of oil, their content may range between 40 and 900 mg/kg [60]. Biophenol compounds have potential antioxidants power and play an important role in the chemical, organoleptic and nutritional properties of the virgin olive oil (VOO) and the table olives. The main classes of phenols in virgin olive oil are phenolic acids, phenolic alcohols, hydroxy-isocromans, flavonoids, secoiridoids and lignans. The phenolic acids were the first group of phenolic compounds found in VOO; these compounds together with phenyl-alcohols, hydroxy-isochromans and flavonoids [61], are present in small amounts in VOO [62], while secoiridoids and lignans are the most prevalent phenolic compounds of oil.
Several phenolic acids, such as gallic acid, protocatechuic, _p_-hydroxybenzoic, vanillic acid, caffeic acid, syringic, _p_- and _o_-coumaric acid, ferulic acid, and cinnamic acid have also been determined and quantified in VOO (Figure 1) [37]. The contents of phenolic acids is often lower than 1 mg per kg of olive oil [63]. Secoiridoids are derivatives of oleuropein, demethyloleuropein and ligstroside. The most abundant secoiridoids of VOO are the dialdehydic form of decarboxymethyl elenolic acid linked to hydroxytyrosol (3,4-dihydroxyphenyl-ethanol) or _p_-hydroxyphenyl-ethanol (3,4-DHPEA or _p_-HPEA) (3,4-DHPEA-EDA or _p_-HPEA-EDA) and an isomer of the oleuropein aglycon 3,4-dihydroxyphenylethanol linked to elenolic acid (3,4-DHPEA-EA). Oleuropein and ligstroside aglycon and their dialdehydic forms were also detected as minor hydrophilic phenols of VOO [64,65]. The 3,4-dihydroxyphenyl-ethanol (3,4-DHPEA) and _p_-hydroxyphenyl-ethanol (_p_-HPEA) are the main phenolic alcohols of VOO; their concentration is usually low in fresh oils but increases during oil storage [66] due to the hydrolysis of VOO secoiridoids such as 3,4-DHPEA-EDA 3,4-dihydroxyphenyl-ethanol linked to dialdehydic form of elenolic acid, _p_-HPEA-EDA (_p_-hydroxyphenylethanol linked to dialdehydic form of elenolic acid) and 3,4-DHPEA-EA (3,4-dihydroxyphenyl-ethanol linked to elenolic acid) into hydroxytyrosol (3,4-dihydroxyphenylethanol) (3,4-DHPEA) and tyrosol (_p_-Hydroxyphenylethanol) (_p_-HPEA) [67]. Flavonoids such as luteolin and apigenin were also reported as phenolic components of VOO [68].
Lignans are another group of phenols found in VOO [64]; in fact, they have been recently isolated and (+)-1-acetoxypinoresinol and (+)-1-pinoresinol were characterized as the most concentrated lignans in VOO. Brenes et al. [69] have confirmed the occurrence of these compounds in Spanish VOO. The same author reported that the lignans concentrations discriminated the oils produced from Picual to the others virgin olive oils extracted from Hojiblanca, Coricabra and Arbequina varieties [69]. Hydroxy-isochromans is a new class of phenolic compounds of extra-virgin olive oil and the presence of 1-phenyl-6,7-dihydroxy-isochroman and 1-(39-methoxy-49-hydroxy) phenyl-6, 7-dihydroxy-isochroman has been shown in several samples [70].
Overall, a large number of phenolic compounds have been isolated from VOO, which can be further classified into Phenolic acids and derivatives, such as vanillic acid, syringic acid, _p_-coumaric acid**,** _O_-coumaric acid**,** gallic acid, caffeic acid, protocatechuic acid, _p_-hydroxybenzoic acid, ferulic acid, cinnamic acid, 4-(acetoxyethyl)-1,2-dihydroxybenzene, benzoic acid, hydroxy-isocromans [12,37,62,70], Secoiridoids, such as dialdehydic form of decarboxymethyl elenolic acid linked to 3,4-Dihydroxyphenyl-ethanol (3,4-DHPEA) (3,4-DHPEA-EDA) dialdehydic form of decarboxymethyl etenolic linked to _p_-hydroxyphenyl-ethanol _p_-HPEA (_p_-HPEA-EDA) oleuropein aglycon 3,4-dihydroxyphenyl-ethanol linked to elenolic acid (3,4-DHPEA-EA) ligstroside aglycon, oleuropein, _p_-HPEA-derivative, dialdehydic form of oleuropein aglycon, dialdehydic form of ligstroside aglycon [64,71,72], Lignans, (+)-1-acetoxypinoresinol, (+)-pinoresinol [64], Flavones, such as apigenin, luteolin [68] and Phenolic alcohols such as, 3,4-dihydroxyphenyl-ethanol (3,4-DHPEA), _p_-hydroxyphenyl-ethanol (_p_-HPEA) and 3,4-dihdroxyphenyl-ethanol-glucoside [73].
3.2.2.1. Biological Activities and Potential Health Benefits of Olive Oil Biophenols
Reactive oxygen species (ROS), formed as a result of oxidative stress, are known to be responsible for the development of some diseases targeting lipids, proteins and deoxyribonucleic acid (DNA) in living organisms. Diseases attributed to ROS include, for example, aging, arteriosclerosis, cancer and neurodegenerative diseases such as Parkinson’s [74].
Mostly the therapeutic potential of VOO is attributed to its antioxidant compounds. In animal systems, olive oil phenolics showed their antioxidant activities in vivo [75], and delayed the progression of atherosclerosis [76]. In fact, olive oil phenolic compounds have good bioavailability in humans, even from small doses (22 g per day) [77], which is lower than those reported in the Mediterranean diet (30–50 g per day). The two main phenolic compounds in olive oil, tyrosol and hydroxytyrosol, are absorbed dose-dependently from olive oil [75,77]; for this reason, they can function as useful indicator of olive oil consumption, and monitoring of the compliance in clinical studies [53]. Tyrosol and hydroxytyrosol are present in plasma and urine in their glucuronide conjugated forms (around 98%), suggesting an extensive first pass intestinal/hepatic metabolism of the ingested primary forms [78,79].
Several investigations both in men and women pointed out that replacing SFAs by MUFAs in the diet can lead to a decline in blood pressure [80,81]. Olive oil was more effective in decreasing blood pressure in hypertensive patientsthan PUFAs-high diets [80]. The effect of two similar MUFAs-rich diets (olive oil and high-oleic sunflower oil) in hypertensive women patients have been studied by Ruız-Gutierrez et al. [82], who observed that the diet rich in olive oil induced a significant reduction of blood pressure, which was also confirmed by Fito et al. [83]. The beneficial effects of olives and its oil and phenolic compounds on blood pressure could be considered through their protective effect on the vascular endothelial function [53] and presence of calcium antagonist constituents. Furthermore, an olive oil rich diet can reduce the risk of breast cancer [84–86]. Likewise, antitumor effects of olive have been reported for different organs of the body such as the pancreas [87], oral cavity [88], oesophagus [89], colon-rectum [90], prostate, [91], and lung [92]. In animal studies, the protective effect of olive oil against the UV rays on the skin has also been shown [93].
In addition to the antioxidant potential, the biological activities of olive oil phenolics on enzymes have been tested in a variety of cellular models (e.g., platelets, leukocytes, and macrophages) relevant to human pathology. Most olive oil phenolics are amphiphilic and possess the ability to modulate enzymes such as cyclo- and lipoxygenases, NADPH oxidase, and nitric oxide synthase, which are involved in key functions of those cells. Hydroxytyrosol was found to considerably inhibit chemically-induced in vitro platelet aggregation, the accumulation of the pro-aggregant agent thromboxane in human serum, the production of the pro-inflammatory molecules and leukotrienes by activated human leukocytes, and to inhibit arachidonate lipoxygenase activity [94–97].
The protective effects of olive against the chronic and degenerative diseases are attributed to the biophenol components, particularly, hydroxytyrosol rather than to the unsaturated fatty acids content of the olive oil. These protective effects of oil can be attributed to reduction of oxidized LDL [98–100]. Other potential mechanisms include inhibition of platelet aggregation by hydroxytyrosol [95], the anti-atherogenic activity [101], inhibition ofthe changes of DNA bases caused by peroxinitrites [102] and the reduction of free radical production in the faecal matrix [64], and increase in the ratio between the reduced and oxidized forms of glutathione [103]. Moreover, a protective effect against the inflammation has been shown in the animal model [104,105]. Also, several of the beneficial aspects of the olive against cardiovascular diseases exist via its vasodilatory, anti-platelet aggregation, anti-inflammatory, antioxidant and antimicrobial properties and are associated with its oleuropein component [4,48]. Oleuropein, vanillic and _p_-coumaric acids can also inhibit the growth of some bacteria, such as Escherichia coli, Klebsiella peneumoniae and Bacilluscereus in vitro [106], and in the presence of 6 mg/mL oleuropein, production of aflatoxin can be decreased greatly [107]. In vivo studies to confirm whether oleuropein possesses antimicrobial activities in the human body are still under study [4]. The multiple biological activities and potential health benefits of olive oil/olives biophenolsare are presented in Table 8.
Table 8.
Biological activities and potential health benefits relating to olives/olive oil phenolics.
| Biological Activity | Potential Clinical Target | References |
|---|---|---|
| Antioxidant activity | Cardiovascular and degenerative diseases | [101,104,108–125] |
| Anti-inflammatory activity | Inhibition of pro-inflammatory enzymes | [94–96,126,127] |
| Antimicrobial activity | Infectious diseases | [124,128–131] |
| Anti-atherogenic activity | Coronary heart diseases, stroke | [25,95,101,119] |
| Anti tumor activity | Various cancers | [84–86,95,96,132–135] |
| Anti platelet aggregation | Coronary heart diseases, stroke | [95,136,137] |
| Anti-hypertensive activity | Hypertension | [40,83,98,119,138,139] |
| Increased vitamin A and β-carotene activity | Antiaging/skin protection | [25] |
| Increased immune activity | Infectious diseases; various cancers | [25] |
| Anti-allergic activity | [25] | |
| Reduction in the levels of plasma cholesterol and oxidized LDL | Coronary heart diseases | [25,98–100] |
3.2.3. Volatile and Aromatic Compounds
Olive oil, compared to other vegetable oils, is distinguished by a characteristic aroma and flavour. These sensory characteristics, together with high nutritional value are the main features that have resulted in the increase of virgin olive oil consumption in recent years [11]. Analysis of volatile components can be used as an indicator to check the quality of olive oil [140], in terms ofdetection of adulterants [141], and magnitude ofpossible off-flavours produced [142], or to determine the variety of olive. Cultivar, geographic region, fruit maturity, processing methods and parameters influence the volatile composition of olive oil [143].
Approximately 280 compounds have been identified in the volatile fraction of virgin olive oils [57]. The distinctive aroma of virgin olive oil is attributed to a wide array of compounds of different chemical classes, such as aldehydes, alcohols, esters, hydrocarbons, ketones, furans and, probably, others unidentified yet (Table 9) [144–146]. Several chemical factors such as volatility, hydrophobic character, type and the position of functional groups seem to be more related to the odour intensity of a volatile compound than its concentration. Volatile compounds found in virgin olive oil are mainly produced in plant organs by the oxidation of fatty acids through intracellular biogenic pathways [145,147]. Some of these volatile compounds are present in the intact tissue of the fruit and others are formed during disruption of cell structure during virgin olive oil production due to the enzymatic reactions in the presence of oxygen. It is generally agreed that endogenous plant enzymes, through the lipoxygenase pathway, are responsible for the positive aroma perceptions in olive oil, whereas chemical oxidation and exogenous enzymes, usually from the microbial activity, are associated with sensory defects [145,147,148]. Moreover, the presence of minor volatile compounds may provide useful quality markers and lead to better understanding of the formation or degradation of the major volatile compounds [8,145].
Table 9.
Volatile compounds in olives/olive oil and their characteristics [145,149].
| Attribute/Aroma | Correlated compounds |
|---|---|
| Green | methyl acetate, 1,3-hexadien-5-yne,4-methyl pentan-2-one, 2-methyl-1-propanol, (Z)-3-hexenal, hexyl acetate, 3-hexenyl acetate, (Z)-2-penten-1-ol, (E)-2-hexen-1-ol, (Z)-3-hexen-1-ol |
| Sweet | ethyl furan, ethyl propanoate, 1-penten-3-one, butyl acetate, hexanal, Ethyl butanoate |
| bitter and pungent | ethyl benzene, (E)-2-hexenal, (Z)-2-hexenal, 6-methyl-5-hepten-2-one, quinine, caffeine, alkaloids. tridecene,1-penten-3-one, 1-penten-3-one |
| Undesirable | 1-penten-3-ol, 3-methyl butanol, 2-octanone, 1-hexanol, acetic acid |
| Fruity | 2-butanone, 3-methyl butanal, 2-methyl butyl propanoate, ethenyl benzene, 2-nonanone |
| Musty-humid | 2-heptanone and 2-nonanonetrans |
| Metallic | 1-penten-3-one |
| Rancid | unsaturated aldehydes |
3.2.4. Phytostrols
Plant sterols, also called phytosterols, include a major proportion of the unsaponifiables in vegetable oils. They are biosynthetically derived from squalene and form a group of triterpenes [150]. Total phytosterols content in olive oil varies between 1000 and 2300 ppm [16,151], and the amount of desmethylsterol components such as cholesterol, brassicasterol, stigmasterol, campesterol, delta-7-stigmastenol and apparent β-sitosterol in olive oil (% total sterols) are < 0.5 < 0.1 < campesterol < 4.0 < 0.5 and ≥ 93.0%, respectively [11].
The amount of sterols in the refined olive oil is considerably reduced as the refining process causes significant losses of sterols, which may be as high as 25% [152]. Phytosterols are structurally similar to cholesterol but with some modifications (Figure 2). These modifications involve the side chain and include the addition of a double bond and/or methyl or ethyl group [153]. In crude olive oil, the most common phytosterols are sitosterol (ca. 90%) and stigmasterol [154]. Sterol composition and content of olive oil are affected by cultivar, crop year, degree of fruit ripeness, storage time of fruits before oil extraction and the method of oil extraction [57].
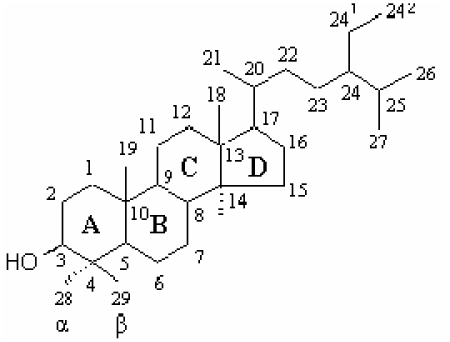
Figure 2.
Basic structure of a sterol with standard carbon numbering according to the IUPAC [155].
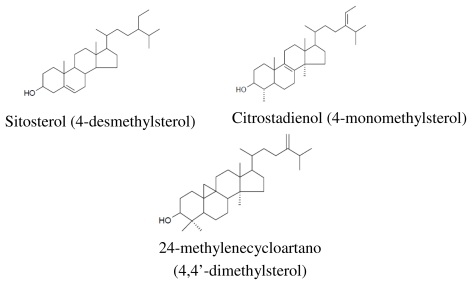
Phytosterols can be classified into three classes, namely 4-desmethylsterols (without methyl group), 4-monomethylsterols (one methyl group), 4, 4′-dimethylsterols (triterpene alcohols; two methyl groups) [156] based on the presence or absence of methyl groups at the C4 position in the A rings (Figure 2). 4-desmethylsterols include all of the common phytosterols with a 28- or 29-carbon skeleton, but also cholesterol with a 27-carbon skeleton [157] (Figure 2). It is classified into Δ5-sterols, Δ7-sterols and Δ5,7-sterols [157,158].
Methylsterols (4-monomethyl- and 4, 4′-dimethylsterols) are synthesised at an early stage in the biosynthetic pathway and are precursors of 4-desmethylsterols [159]. 4-monomethylsterol includes Citrostadienol, Obtusifoliol, Cycloeucalenol and Gramisterol. 4, 4′-dimethylsterol, can be categorised into 24-Methylenecycloartanol, Cycloartenol α-Amyrin and β-Amyrin. The effective dosage for phytosterols to decrease LDL-cholesterolin blood is as high as 8% to 15% is 1.5 to 3 g per day. The main mechanism of this action is the interference with the solubilisation of the cholesterol in the intestinal micelles so decreasing the absorption [160]. Also, they have some anti-cancer effects in colon, breast and prostate [161], possess anti-inflammatory properties [160] and act as immune system modulators [162]. There is no evidence of any side-effect and mutagenic activity of phytosterols in the in vitro studies [163] or subchronic toxicity studies in animals [164]. The only limitation is that they can interfere with the absorption of carotenoids, but this can be compensated in the diet by adding these compounds in appropriate amounts [160].
The amount and phytosterol classes of seed, pulp and whole olive fruit oil is different. Seed oil has a higher concentration of total 4-desmethylsterols (2.3-fold higher), sitosterol, campesterol, chlerosterol, Δ5–24-stigmastadienol, Δ7-stigmastenol and Δ7-avenasterol compared to other parts oils. Usually, pulp and whole olive fruit oil have the same amounts of 4-desmethylsterols. In this regard, the amount of 4, 4′-dimethylsterols and cycloartenol, 24-methylenecycloartanol in seed oil is low but β-amyrin, butyrospermol content compared with other extracted oils is high. In general, pulp and whole olive fruit oil have almost the same concentration of 4, 4′-dimethylsterols [165].
3.2.5. Tocopherols
Tocopherols are considered as the most important lipid soluable natural antioxidants, which prevent lipid peroxidation by scavenging radicals in membranes and lipoprotein particles [166]. Four different types of tocopherol, namely α-, β-, γ- and δ-tocopherol have been reported in olive oil. The amount of main component, α-tocopherol, varies from a few ppm up to 300 ppm [167]. The significant concentration of α-tocopherol in VOO supports itsideal E/PUFAs ratio. The ratio E/PUFAs can be described as the milligrams of vitamin E per gram of polyunstaurated fatty acids. This ratio which should never be less than 0.5, is rarely found in seed oils, however in VOO it is in the range of 1.5 to 2.0 [25]. The concentration of β-, γ- and δ-tocopherols are presented from traces to 25 ppm [168–169]. However, the tocopherols contents seem to be reduced during ripening fruits, refining and hydrogenation process [57].
These compounds are known to contribute to the antioxidant capacity of olive oil [108], as well as enhance oil stability during frying by protecting it from thermo-oxidative degradation [49]. Also tocopherols in virgin olive oils act not only as lipid radical scavengers, but also prevent the photoxidation by reacting with singlet oxygen by physical quenching or by chemical reactions [170]. Therefore, they increase oxidation stability of oils during storage due to preventingfrom the light [171]. Moreover, α-tocopherol defends the body against free radical attacks [172–174], and prevents skin disorders, cancer and arteriosclerosis [175–177]. However, the nature of this contribution isnot yet fully understood. Some researchers have demonstrated a synergistic relationship between the antioxidant actions of some phenolics and tocopherols [178].
3.2.6. Colouring Pigments
Olive oil, like other vegetable oils, contains considerable amount of pigments such as chlorophylls and carotenoids. Chlorophylls are encountered as pheophytin. Pheophytin α concentration in olive oil ranges from 3.3 to 40 ppm, while pheophytin b and chlorophyll b are present in trace amounts andchlorophyll a has not been detected [179]. The main carotenoids present in olive oil are β-carotene (0.3–4.4 ppm) and lutein (trace-1.4 ppm) [180]; for instance, the concentration of carotenoids in Spanish olive oils is 3.1–9.2 mg/kg [181].
Chlorophyll, a photosensitizer, may initiate oil oxidation in olive oil when exposed to light by converting ground state triplet oxygen (3O2) to highly reactive excited state singlet oxygen (1O2*) [182]. It is interesting to note that the photoxidation reaction (light-induced oxidation) proceeds about 1000 to 1500 times faster than the common triplet oxygen oxidation [183]. β-carotene quenches singlet oxygen [183], so enhances oxidative stability against light-induced oxidation (photo oxidation). It has also been suggested that the pigment absorbs light which would otherwise excite sensitizers, therefore reducing initiation reactions [184]. The effect of β-carotene during oxidation in the dark, where reactions are not initiated by pigment sensitization, depends on the conditions under which the reactions occur. Whether β-carotene is an antioxidant or a prooxidant and how effective it is, depends on its own concentration along with oxygen [185], as well as the chemical environment where the reaction occurs [186]. Lutein has an antioxidant effect and works in combination with lycopene, as a highly active agent against skin aging and cancer risk. An adequate intake of carotenoids derived from vegetable sources including that of VOO can act as a decisive skin protector factor [25].
3.2.7. Squalene
Squalene is a polyunsaturated triterpene comprising of six isoprene units and acts as a biochemical precursor of cholesterol and other steroids. It is widely produced by both plants and animals and iswidespread in nature, especially among olives, shark liver oil, wheat germ, and rice bran [187]. Thus, in addition to being synthesized within cells, it is consumed as an integral part of the human diet. Squalene content in olive oil is especially high, up to 0.7% (7 mg/g), compared to other oils and human dietary fats [188]. Only rice bran oil contained significant quantities (332 mg/100 g) [189] when compared with a large number of other seasoning oils.
Also, squalene is a major component (around 40%) of the oil unsaponifiable fraction, the material left after saponification with an alkaline hydroxide and extracted with a solvent (e.g., diethyl ether) [1]. All plants and animals including humans are capable of producing squalene. In humans, squalene is synthesized in the liver and the skin, transported in the blood by very low density lipoproteins (VLDL) and LDL, and secreted in large quantities by the sebaceous glands [190,191].
Other hydrocarbons have also been found in VOO, such as 6, 10-dimethyl-1-undecene, various sesquitterpenes, the series of _n_-alkanes from C14 to C35, _n_-heptadecene and _n_-9-alkenes [183]. Squalene has been considered to be an important component in the diet of Mediterranean people due to its chemopreventative potential against cancer. Levels of squalene (a sterol precursor) in the body achieved by including olive oil in the diet (around 40 g per day, a common value for people in Mediterranean countries) may have a considerable inhibitory effect on cancer development [192].
Special attention has been directed to squalene, present in a notable concentration in virgin olive oil nonsaponifiable fraction, which makes it similar to the composition of sebum. Squalene is found in high amounts in sebum (around 12% of its composition) and acts as a potent scavenger of singlet oxygen, inhibiting the lipoperoxidation induced by ultraviolet (UV) radiations [193], thus having anti-neoplastic influence on the colon, breast, and prostate, it seems to have immune-stimulating properties and it can inhibit the development of various tumors [194]. Also it has major protective effect against skin cancer, probably by scavenging singlet oxygen generated by UV light [189]. The oral intake as well as the external use of olive oil has been shown to provide photoprotection to the skin [195]. Such photoprotective effects of VOO against skin might be mainly correlated to the presence of notable amounts of squalane, which exerts antioxidant properties at the cutaneous level against solar rays thus behaving as a biological filter of singlet oxygen [25,64]. The presence of considerable amounts of squalane along with α-tocopherol and carotenoids in VOO provides an interesting aspect and supports the topical use of this valuable oil as an ingredient in cosmetics and dermo-potective creasems [25].
Moreover, squalene may act as a sink for highly lipophilic xenobiotics, assisting in their elimination from the body [196], and is frequently used in the preparation of stable emulsions as either the main ingredient or secondary oil [197,198]. Squalene emulsions have been used for various applications, especially for the delivery of vaccines, drugs, and other medicinal substances [199]. Since squalene is well absorbed orally, it has been used to improve the oral delivery of therapeutic molecules. Today, there are claims that squalene can enhance the quality of life, if taken continually and orally [194].
3.2.8. Triterpene Dialcohols
There is another group of compounds present in the unsaponifiables fraction of olive oil called triterpene dialcohols, which are co-chromatographed with 4-desmethylsterols [57] and present in the range of 500–3000 mg/kg [200]. Erythrodiol and uvaol are the two main triterpene dialcohols present in olive oils [16]. Their concentration is mainly affected by cultivar. The amount of total erythrodiol ranges from 19–69 mg/kg [201], and concentration of free erythrodiol is usually lower than 50 mg/kg. One way to distinguish between virgin olive oil and solvent extracted olive oil is on the basis of the amount of erythrodiol and uvaol [202]. Aaccording to IOOC standard [16], the total amount of these two compounds is ≤4.5% of total sterols in VOO, while in olive–pomace oil (solvent extracted oil) this limit is ≥4.5%.
3.3. Olive Leaf
Historically, olive leaves have been widely used as a remedy for the treatment of fever and other diseases like malaria [203–205] in European and Mediterranean countries such as Greece, Spain, Italy, France, Turkey, Palestine, Morocco and Tunisia. As a dietary component, the leaves have been consumed in the form of an extract, a whole herbor powder [206]. Olive leaves contain many potentially bioactive compounds that may have antioxidant, anti-hypertensive, anti-inflammatory, hypoglycaemic and hypocholesterolemic properties [206].
Several reports demonstrated that olive leaves can decrease blood pressure, increase blood flow in the coronary arteries [138,207,208], decrease arrhythmia and prevent intestinal muscle spasms [209]. The leaves also possess antimicrobial properties against some microorganisms such as bacteria, fungi, and mycoplasma [119,124,136,210–213].
These potential health benefits of olive leaves are mostly related to low molecular weight polyphenols such as oleuropein (up to 60–90 mg/g dry leaves weight), hydroxytyrosol, tyrosol, tocopherol, elenolic acid derivatives, caffeic acid, _p-_coumaric acid and vanillic acid as well as flavonoids: luteolin, diosmetin, rutin, luteolin-7-glucoside, apigenin-7-glucoside, and diosmetin-7-glucoside [214–216]. Moreover, the combined phenolic compounds have significantly higher antimicrobial activity than those of the individual phenolics [124].
Due to these activities and valuable biophenol compounds, usage of whole olive leaf and olive leaf extract has increased rapidly in both the pharmaceutical and food industries as food additives and functional food materials [204,217]. The whole leaf extract is recommended to achieve health benefits due to the presence of additive and/or synergistic effects of their phytochemicals [209].
3.4. Olive by-Products
As a result of olive processing, a huge quantity of olive by-products are produced. Typically, an olive oil processing industry produces approximately 35 kg olive cake and 440 L olive mill waste water (OMW) per 100 kg of processed olives [105]. A major environmental problem in the Mediterranean countries is the disposal and/or treatment of the large quantities of OMW and olive cake produced during olive oil processing [218]. The high-polluting power of OMW is generally associated with the high biochemical oxygen demand, chemical oxygen demand, total solids, organic carbon and the slight acidic character [219]. With the current trends in functional foods, appropriate techniques should be sought for the isolation of valuable bioactives from the olive residues for potential uses.
3.4.1. Olive Cake
Although olive cake is an economical biomass present in large quantities, it causes some environmental problems for Mediterranean countries [218]. For this reason, olive cake is often consumed as fuel, fertilizer and animal feed [220]. Olive cake is considered as a rich source of phenolic compounds with a wide array of biological activities. In fact, three aspects of antioxidant attributes have been investigated in olive cakes; antioxidant capacity [221,222], anti-radical activities and radical scavenging activities [58,218,223]. Accoording to some studies, hydroxytyrosol [224], oleuropein [225], tyrosol [226], caffeic acid [218], p_-coumaric acid, vanillic acid [227], verbascoside, elenolic acid [225], catechol [228] and rutin [29] are the main phenolic compounds in olive cake. However, little work has been doneuntil now on therecovery of phenolic compounds from olive cake as a potential source of bioactives for potential uses in the pharmaceutical and nutraceutical industries [229]. Recently, Aludatt_et al. [230] optimized some parameters for extraction of phenolic compounds from olive cake and reported that the highest total phenolic compounds and antioxidant activity were achievedusing methanol at 70 °C for 12 h. Also the major free phenolic compounds in full-fat and defatted olive cake were protocatechuic acid, sinapic acid, syringic acid, caffeic acid, and rutin. Table 10 shows the bound phenolic compounds profiles of extracts from full-fat and de-fatted olive cake derived in sequential extractions [230].
Table 10.
Amount (%a) of bound phenolic compounds analyzed in full-fat and defatted olive cake extracts by using RP-HPLC [230].
| Bound phenolic compounds | Full-fat olive cakeb | Defatted olive cakeb |
|---|---|---|
| Gallic acid | ND | ND |
| Protocatechuic acid | 21.2 ± 0.24 | 13.8 ± 0.41 |
| Hydroxybenzoic acid | 5.8 ± 0.17 | 7.1 ± 0.17 |
| Vanillic acid | 4.9 ± 0.12 | 4.9 ± 0.43 |
| Caffeic acid | 13.7 ± 0.28 | 11.1 ± 0.25 |
| Syringic acid | 22.4 ± 0.38 | 22.7 ± 0.36 |
| Sinapic acid | 13.1 ± 0.29 | 16.6 ± 0.59 |
| Ferulic acid | 7.2 ± 0.08 | 7.9 ± 0.35 |
| _p_-Coumaric acid | ND | ND |
| Rutin | 11.7 ± 0.39 | 8.2 ± 0.24 |
| Hesperidin | ND | 4.3 ± 0.13 |
| Quercetin | ND | 3.4 ± 0.19 |
| Cinnamic acid | ND | ND |
There is small variation observed for the amounts of the phenolic compounds (extracted by using alkaline hydrolysis) between full-fat and defatted olive cake except for the hesperidin and quercetin, which are only detected in defatted olive cake. Efforts should be made to explore the potential uses of this olive biomass in pharmaceutical, nutraceutical, functional food products not only for value addition but also to decrease the effect of olive oil production on the environment.
3.4.2. Olive Oil Mill Waste Water
Olive oil mill waste water (OMW) constitutes exert a serious problem with severe negative impact on soil and water quality, and thus on agriculture, environment and health [231]. On the other hand, the amount ofbiophenol compounds in olive oil is 2% of the total phenolic content of the olive fruits, the remaining 98% being lost in olive mill waste [229]. OMW, as a rich source ofbiophenol compounds with multiple biological activities, and free radical-scavenging and metal-chelating properties, have been shown to be more effective antioxidants in vitro than vitamins E and C on a molar basis [232]. GC–MS analysis revealed that the free phenols could be recovered from OMW by a simple liquid–liquid extraction process. The phenolic extract is composed of hydroxytyrosol as the major compound (66.5%), while tyrosol, cafeic acid, _p_-coumaric acid, homovanillic acid, protocatechuic acid, 3, 4-hihydroxymandelic acid; vanillic acid and ferulic acid are among others, which means that OMW extract can be a natural source of useful substances [233]. Some of the important bioactivities associated with OMW polyphenols, include antioxidant effect on intestinal human epithelial cells [234], anti-inflammatory activity through inhibition of 5-lipoxygenase [96], antiviral [28], molluscicidal [235], antibacterial and antifungal [106], cardioprotective, anti-atherogenic [236] and anti-tumour [64] activities.
The anti-microbial activity of OMW has been studied by Gonzalez et al. [237] and was reviewed by Moreno et al. [238], who attributed antimicrobial and phytotoxic activities to minor biophenols and phenolic acids [28]. A non-polar extract of OMW did not show any activity on the bacteria, so its bioactivity was correlated with the hydrophilic components such as phenolic content of OMW [239], and its anti-bacterial activity was more marked on Gram-positive than on Gram-negetive bacteria. Perez and colleagues [239] found that Ethylacetate and _n-_propanol OMW extracts had the strongest effect on Bacillus meganterium. Capasso et al. [240] determined the antimicrobial activity of OMW and found that hydroxytyrosol had activity just against Pseudomonas savastanoi but 4-methylcatechol was the strongest antibacterial compound against Pseudomonas syringae. Other compounds identified in OMW with antimicrobial activity were oleuropein, hydroxyltyrosol, 4-hydroxybenzoic acid, vanillic acid and _p_-coumeric acid [4], and hydroxytyrosol being more effective than oleuropein [28]. It was also observed that OMW extract can be used as natural antioxidants instead of synthetic antioxidants to protect edible oils and food products from oxidation [241].
3.4.3. Olive Stone
The olive stone and seed are important by-products generated in the olive oil extraction and pitted table olive industries. The whole olive stone consists of the wood shell (stone) and the seed. In the olive oil industry, only the olive stone without seed can be recovered by filtration of solid waste. From the pitted table olive industry, the whole olive stone (stone and seed) is recovered by separation of the pulp. The main lignocellulosic components in olive stone are hemicellulose, cellulose and lignin with 21.45–27.64%, 29.79–34.35%, 20.63–25.11%, respectively [242]. Protein, fat, phenols, free sugars and phenolics are also present in considerable quantities (Table 11). The main use of this biomass is in combustion to produce electric energy or heat. Other uses such as production of activated carbon, applied for removal of unwanted colours and dyes [243], odours, tastes or contaminants such as arsenic [244] or aluminium [245], furfural production [246], and plastic filling [247], have also been cited. Besides, this bio-mass has been reported to be used as metal bio-sorbent [248], animal feed [249], and in resin formation [250].
Table 11.
Chemical composition of olive whole stones and olive seeds (as % dry weight) [242].
| Components | Whole stone (%, w/w) | Seed (%, w/w) |
|---|---|---|
| Ash content | 0.01–0.68 | 0.03–0.13 |
| Moisture content | 9.79 | 9.98 |
| Fat | 5.53 | 1.01 |
| Protein | 3.20 | 1.29 |
| Free suger | 0.48 | 0.36 |
| Phenolics | 0.1 | 0.5–1 |
Interestingly, the whole olive stone is a rich source of bioactive compounds. These potentially valuable compounds are nuzhenide-oleoside, nuzhenide, salidroside, which are detected only in the olive seed;verbascoside only appears in significant quantities in the seed and pulp [214], but tyrosol, hydroxytyrosol, oleuropein and diadehydic form of decarboxymethyl oleuropein (3,4 DHFEA-EDA) are found in different parts of olive including the pulp, leaves, seed and stone [248].
3.4.4. Olive Wood
In view of the large amounts of wood generated from olive tree pruning that is now mostly burnt, olive wood would also be a very interesting and plentiful source of antioxidants. The isolation and radical scavenging activity of the six main components from olive wood, such as, tyrosol, hydroxytyrosol, (+)-cycloolivil, ligustroside, oleuropein and 7-deoxyloganic acid have been reported [251]. Besides having antioxidative activities, it has been reported that oleuropein and (+)-cycloolivil possess anti-platelet aggregation properties, and both compounds inhibit protein tyrosine phosphorylation, which suggests that they may prevent thrombotic complications associated with platelet hyperaggregability [252]. Recently, antioxidants have been isolated and identified in the ethylacetate extract of olive wood. A new secoiridoid compound, oleuropein-3′-methylether, together with six known secoiridoids,7′S-hydroxyoleuropein, jaspolyanoside, ligustroside 3′-_O_-β-D-glucoside, jaspolyoside, isojaspolyoside A and oleuropein 3′-_O_-β-D-glucoside, were isolated and the structures of these compounds determined by spectroscopic methods [253].
4. Factors Affecting Chemical Composition of Olives
The chemical composition of olives may vary depending upon different factors including agronomical factors (e.g., olive cultivar) [31], the ripening stage of the fruit [30], agroclimatic conditions [6] and irrigation management [254,255]. Several studies have already been carried out to describe the differences found between the phenolic profiles of different olive cultivars [6] as well as their distribution throughout the ripening process [30,26,256].
4.1. Cultivars
The composition of olives varies with cultivar and environmental conditions. Olive cultivar also affects the absolute concentration of the specific hydrophilic phenols of VOO, while the phenolic profile remains almost the same, but olive variety and harvest time have a statistically significant influence on the amount of total phenols, ortho-diphenols, as well as intensity of bitterness, while stage of ripeness in olives demonstrated a more notable effect than the species itself [257]. Many studies have been published demsonstrating considerable variations in the amounts of total phenolics among different cultivars of olives (Table 12).
Table 12.
Effect of different cultivars on the phenolic compounds in olives.
| Cultivar | Total phenols (mg/kg) | Reference |
|---|---|---|
| Arbequina | 108.27 | [54] |
| Arbosana | 137.84 | [54] |
| Koroneiki | 236.48 | [54] |
| Picual | 400 | [258] |
| Arbequina | 334 | [258] |
| Hojiblanca | 355 | [258] |
| Ornicabra | 495 | [258] |
| Leccino | 130 | [56] |
| Bianchera | 305 | [56] |
| Busa | 125 | [56] |
| Arbequina | 243.8 | [259] |
| Picolimon | 159.9 | [259] |
| Morisca | 435.4 | [259] |
4.2. Fruits Maturity
Phenolic compounds change qualitatively and quantitatively during growing period [30,31,260]. There are two maturation stages in olive fruits corresponding to green and black fruits. In the green maturation stage, olive fruits reach their final dimensions and their colour is yellow green. After, the green coloring pigments (i.e., chlorophylls) in the skin are replaced by anthocyanins resulting the transition to a “spotted,” “purple” and “black” stage. At the stage between the yellow green and purple skin, the olives have the highest amounts of phenolic compounds, especially oleuoropein.
Much research has concentrated on olives phenolics and especially on oleuropein, which is known to be the most important individual phenolic component of olive pulp, reaching concentrations of up to 14% on a dry weight basis in young Picoline olives [30]. The amount of total phenolic compounds, especially oleuropein, decreases during fruit maturity [30,261] because during this stage esterase activity degrades oleuropein [30] and it can reach zero in some cultivars when olives are absolutely dark [21]. Reduction of oleuropein concentration is accompanied by the accummulation of two compounds, namely demethyloleuropein and elenolic acid glycoside [262].
Besides the maturation state of fruits, oleuropein concentration can be affected by the dimensions of the fruits. Generally, some species with small olive fruits have a high level of oleuropein from the initial up to the end of maturation but in another species, which produce large fruits, quantities of oleuropein is low during the maturation process
Moreover, species with high levels of oleuropein have minimum verbascoside [21], but its concentration during fruit maturationincreases steadily [259]. The amount of ligstroside in young olives is higher but it decreases during maturation. Also, some changes have been observed in the level of oleoside 11-methylester, tyrosol, hydroxytyrosol and their glucosides within fruit developing [21]. Cimato et al. [263] showed that with fruit ripening, hydrolysis of components with ‘higher molecular weight’ occurred, resulting in the formation of tyrosol and hydroxytyrosol. Thus, the concentration of tyrosol and hydroxytyrosol was also shown to increase with the harvesting period, which has been correlated with an evident reduction in four unidentified, but [2] resumably phenolic components.
Besides the stage of maturation, some parameters such as fly (Bactrocera olaea) attack have an effect on the phenolic compounds in oils extracted from olives [264]. Several other studies have discussed the effect of maturation on the quantity of phenolics in various olive cultivars [6,69,262,265–274].
4.3. Irrigation
Ecological factors as well as agronomic practices such as irrigation have an effect on the phenolic content of VOO. Irrigation is an essential parameter, even in fields where water is unrestricted, for achieving better production, productivity and characteristics of olive oil, because good quality olive oil cannot be obtained from olive fruit harvested under high levels of water stress [275,276]. Several authors have determined variation in the chemical composition and the sensory qualities of VOO obtained from olive trees under irrigated and rain-fed conditions [201,277]. Some showed that irrigation had a clear effect on the phenolic compounds as major substances were affected [254,255,278]. Indeed, it has been observed that the total phenol content in different olive oils and its oxidative stability decreased when the trees received alarge amount of water [279].
The effects of irrigation with fresh and saline water on olive oil quality has also been studied [258], and the results showed that irrigation with saline and fresh water-type did not have a significant effect on olive oil composition, but the amount of total phenols decreased. According to published data [280], fundamental quality parameters, such as free acidity, peroxide value, and fatty acids profile did not change in olive oil during irrigation with saline water, though polyphenols and vitamin E content increased with the saline treatments. A few studies have reported that irrigation with treated waste water can affect the olive oil composition and decrease the total phenols content [281]. However, this irrigation did not exert any notable effect on the free fatty acid and specific ultraviolet absorbance K232 and K270 of olive oil [282].
4.4. Technological Aspects in Olive Oil Extraction
VOO is produced by mechanical and physical processes [283]. These processes involve collection, leaves removal, washing, olives crushing, malaxition the olive paste, centrifugation with or without adding water which is named “three-phase” or “two-phase” respectively, storage, filtration and bottling. The presence of hydrophilic phenols in VOO depends on different endogenous enzymes of olive fruits and extraction conditions. During the oil mechanical extraction process, crushing and malaxation play an important role [284,285]. Several modifications such as hydrolysis of glycerides by lipases, hydrolysis of glycosides and oligosaccharides by β-glucosidases, oxidation of phenolic compounds by phenoloxidases, and polymerization of free phenols can appear during these processes [2].
4.4.1. Crushing
This operation is designed to tear the fruit cells to release the droplets of oil from the inner cavity (vacuole). Crushing is an important part of extracting VOO, because it affects the physical and chemical properties of oil. Before crushing the fruits, the oil is protected inside the cell, but after crushing it contacts other constituents of the cell including enzymes, that affect the quality of oil. In the earlier days, the crushing process was carried out with a system of heavy wheels made of stones. Nowadays when the continuous extraction systems came into use the metal crusher-hammer or toothed-disc are used to grind the olives. Using the hammer increases the oil extraction yield because the intercellular structure is destroyed by using the stone mill and consequently oil droplets may be retained inside the cells, while the hammer cut the cells without destroying the intercellular structure [286]. The metal crusher may increase the yield of extraction from olives, but because of high speed it may create more emulsion than a stone crusher, therefore, the produced paste must stay longer in malaxation process. The concentration of phenols present in VOO depends on the way the olive paste is prepared [287]. Using a hammer crusher instead of a stone crusher increases the amount of total phenols components and ultimately the stronger antioxidant power in the oil, in terms of the concentration of polyphenolic compounds (Table 13). This can be ascribed to the higher temperature which is caused by the speed of the hammer crusher as well as solubilisation phenomena by which more phenolic compounds pass into the oil [286,287]. Also, when olives processed by these two crushing methods were analyzed by scanning electronic microscopy, the micrographs showed evidence that olives treated by hammer crushing system were better cut than those treated by stone mill because olive cell layers were broken and damaged by the later technique [286].
Table 13.
Concentration of polyphenolic compounds recovered in the olive oils produced by using different crushing methods [286,288].
| Compounds | Stone (ppm) | Hammer (ppm) |
|---|---|---|
| Gallic acid | 1.60 ± 0.20 | 1.32 ± 0.14 |
| Tyrosol | 2.99 ± 0.17 | 3.00 ± 0.54 |
| Vanilic acid | 1.83 ± 0.12 | 1.27 ± 0.12 |
| _p_-Coumaric acid | 2.25 ± 0.16 | 1.97 ± 0.42 |
| Ferulic acid | 1.95 ± 0.12 | 1.62 ± 0.22 |
| Luteolin | 4.66 ± 0.25 | 4.20 ± 0.31 |
| _trans_-Cinnamic acid | 0.12 ± 0.01 | 0.11 ± 0.01 |
| Apigenin | 1.64 ± 0.17 | 1.61 ± 0.14 |
4.4.2. Malaxation
Malaxation process (also called beating or kneading) is essential for increasing extraction yields. It is designed to enhance the effect of crushing and to make the paste uniform. The majority of oil in olive fruit is located in the vacuoles of mesocarp cells. During the malaxation step the small droplets of the oil, by means of slow and continuous kneading of the paste produced by metallic crusher, merge into large drops that can be easily separated by the separating apparatus [147]. This process also breaks the produced emulsion and helps to increase the oil extraction yield. The bioactives composition in olive oil can be significantly improved by various factors including malaxation temperature, time [289], and the use of microorganisms [290], or enzymes [291,292].
Addition of commercial enzyme preparations such as pectolytic, hemicellulolytic, and cellulolytic during the olive oil malaxation process resulted in degrading the cell wall of the fruit and reducing the complex of hydrophilic phenols with polysaccharides, increasing the concentration of free phenols in the olive paste and their consequent release into the oil and wastewaters through processing [293].
Oleuropein and demethyloleuropein and ligstroside hydrolyze within the crushing and malaxing step due to endogenous glycosidases and thus change to dialdehydic form of decarboxymethyloleuropein aglycone and aldehydic form of oleuropein aglycone [294].
4.4.3. Decantation
After the malaxation process, the oil must be separated from the paste and vegetation water by using a decantation process. Decanters are of three types, namely: (1) oldest three-phases decanter (50–100 L of added water per 100 kg of olive paste); (2) three-phases decanter that works using less water (10–30 L of added water per 100 kg of olive paste); (3) two-phases decanter that operates without adding any water. In this section, pressure and centrifugation as an extraction system play an important role in recovering the amounts of phenolic compounds. Actually, in three phase system in order to reduce the viscosity of pastes and to separate oil easily from the solid phase sufficient water needs to be added before centrifugation [73].
Disadvantages of this process include higher amounts of waste water (1.25 to 1.75 times more water than press extraction), loss of valuable components (e.g., natural antioxidants, phenolics) in the water phase, and problems of disposal of the oil mill waste water. Therefore, pressure system that does not need to add water to the olive paste offers a large amount of phenolic compounds in comparison to those obtained by the centrifugation system [73]. Two phase centrifugation systems have evolved within the last 10 years to need less water for the separation of oil. As shown in Table 14, higher contents of phenolic compounds are obtained in the oils produced by the new two phases’ decanter as compared to the traditional three-phase decanter [295,296]. Thus, the oil produced by a two-phase system has stronger antioxidant activity (2-fold greater) and higher resistance to oxidation than that obtained by a three-phase system due to the higher amount of hydroxytyrosol as an orthodiphenol compound [297–299].
Table 14.
Phenolic composition (ppm) of Coratina virgin olive oils with two phase and three phase centrifugation [295].
| Phenolic composition | Two phases (ppm) | Three phases (ppm) |
|---|---|---|
| (3,4-DHPEA) Hydroxytyrosol | 0.87 ± 0.02 | 0.58 ± 0.08 |
| (_p_-HPEA) Tyrosol | 3.74 ± 0.07 | 2.34 ± 0.08 |
| Vanillic acid | 0.41 ± 0.01 | 0.19 ± 0.01 |
| Caffeic acid | 0.16 ± 0.01 | 0.12 ± 0.02 |
| (3,4-DHPEA-EDA) 3,4-dihydroxyphenyl-ethanol linked to elenolic acid | 522.2 ± 13.5 | 427.2 ± 13.8 |
| (_p_-HPEA-EDA _p_-hydroxyphenylethanol linked to dialdehydic form of elenolic acid | 78.16 ± 0.52 | 67.26 ± 2.55 |
| _p_-HPEA-ester | 38.41 ± 0.10 | 35.62 ± 1.11 |
| (3,4-DHPEA-EA)3,4-dihydroxyphenyl-ethanol linked to elenolic acid | 351.71 ± 11.0 | 244.9 ± 13.6 |
| Total polyphenols | 673 ± 4 | 585 ± 7 |
4.4.4. Filtration
Filtration is the final step in olive oil processing and can be carried out with various materials or filter aids in combination with filtration hardware to improve filtration performance [300]. This process is also used for removing humidity and in this case cotton or paper filters can be used. Filtration also can affect the phenolic compounds. Using a cotton filter for removing humidity, hydroxytyrosol content in extra virgin olive oil was decreased [276,300]. However, filtration with cotton or paper plus anhydrous sodium sulphate led to an apparent increase in the phenolic content. Sometimes olive oil has a lot of suspended solids and in this case the diatomaceous earth is used for filtration purposes. Nowadays, organic materials such as cellulose fibrous materials and starch are used as filter aids. The solid residue waste after filtration with diatomaceous earth and organic filter aids may offer another source of phenolic compounds. Filtration by an organic filter aid is preferred over diatomaceous earth due to the high performance in the filtration process onlaboratory scale [300]. Thus, filtration especially dehydration can assist to improve the shelf-life and nutritive quality of olive oil in some cultivars such as Arbequina and Colombaia [301].
Different classes of the hydrophilic phenolics such as phenolic acids, phenolic alcohols, secoiridoids, lignans and flavones can be retained in the filter aids. Phenolic acids including, vanillin, vanillic, ferulic and _p_-coumaric acids have been identified in different filter aids. Similarly, phenolic alcohols, tyrosol and hydroxytyrosol in addition to secoiridoids have been found in almost all filter aids. The most important secoiridoids retained in filter aids include oleuropein aglycon, 10-hydroxy-oleuropein aglycon, decarboximethylated derivates of oleuropein aglyco, oxidation products of dialdehydic form of decarboxymethyl oleuropein aglycone and ligstroside aglycon. Lignans, pinoresinol, hydroxy-pinoresinol, and acetoxypinoresinol have also been detected. All the filters retained different flavones such as apigenin and luteolin, someunknown compounds have also been investigatedin the filter aids [300].
5. Potential for Recovery of Valuable Olive Natural Constituents
A more recent approach for obtaining olive mill waste (OMW) has involved the use of processing technologies to fractionate potential high-value components from olive agrowastes and residues. The recovered compounds may be broadly classified into insoluble, water-soluble and lipid solubles. One of the most important environmental problems in the Mediterranean countries, such as Spain, Italy and Greece, is the treatment and disposal of OMW. The main organic contents of OMW are sugars, nitrogenous compounds, volatile acids, polyalcohols, pectins, fats and polyphenols. During the past years, olive oil processing industries have used a continuous centrifugation system with a two-phase decanter, which separates VOO by recycling the vegetation water of the processed olives. This technology considerably decreases the volume of plant effluents and the disposal problems [238].
However, little information has been reported on the recovery of phenolic compounds from olive cake and OMW as a potential source of bioactive compounds for the pharmaceutical and nutraceutical industries [229]. In this regard some trials have been carried out by using physico-chemical or biological treatments to decrease the potential pollution load and obtain antioxidant compounds [233,302–305]. However, there are many limitations for the industrial-scale usage of the methods so far proposed. The major problems for the recovery of such valuable components are correlated to the complexity of the proposed processes, requiring water pre-treatment, or the huge costs for the purchase and the maintenance of the instruments.
One of the methods applied for recovering hydrophilic phenols from fresh OVW (olive vegetation water) is three consecutive membrane-filtration steps with decreasing cut-off values (microfiltration, ultra filtration and reverse osmosis) by a previous enzymatic treatment. Its products include crude phenolic concentrate permeate without any phenolic compounds and large amount of organic fraction that could also be re-used in the VOO-extraction process. Between membrane processes, nano-filtration is considered as an effective procedure for the removal of these compounds from waste water [306].
Another way to extract phenolic compounds is super critical fluid extraction (SFE) with carbon dioxide, which produces extracts with high antioxidant quality from spices and agricultural by-products but its phenolic yield is not appropriate. Typically, ethanolic extraction provides high yield with stronger antioxidant activity than butylated hydroxytolune (BHT), ascorbyl palmitate and vitamin E. Also, analysis by HPLC shows that hydroxytyrosol is a major phenolic compound in it [307]. Consequently, the OMW can be explored as an economical and renewable source of phenolic antioxidants. Phenolic extracts from OMW can be used as a natural alternative for commercial synthetic antioxidants or for other food and medicinal uses.
6. Conclusions
Consumption of olives and/or olive oil is recognized as a key factor supporting the beneficial effects of the “Mediterranean diet”. Olive oil, having been used as a nutritious food, drug, and as cosmetics for centuries by the Mediterranean people, has been a subject of much scientific interest in the last few decades, confirming its multiferous biological, therapeutic and functional food applications. Currently, due to continuing scientific evidence supported with numerious epidemiological and clinical experimental studies, the recognition of olive oil as a source of food and medicine is much acknowledged. The most important activities in olive oil are antioxidant, anti-microbial, anti-inflammatory and anti-cancer as evident from a variety of studies. Olive oil is resistant to oxidation and it has a special bitter and pungent taste. Principally, these biological activities and individual taste are due to the presence of unique bio-active compounds in the olives, namely phenolics (e.g., oleuropein, hydroxytyrosol, verbascoside and derivatives), tocopherols and carotenoids, amongst others. Several factors, such as agronomical conditions, climate, and level of ripening, olive cultivar and type of production process have the main effects on the profile and activities of bio-active compounds in olives and olive oil. During olive oil processing, in addition to the olive oil itself, olive cake and oil mill waste water are produced, which are considered to be good sources of phenolic compounds with multiple epidemiological and therapeutic activities, thus highlighting the potential of such olive by- products for the isolation of high-value bioactives for pharmaceutical, nutraceutical and food industries.
7. Future Prospects
Nowadays, the significance of functional foods is growing rapidly in food science, where inquires on the bioactivity, bioavailability and toxicology of phytochemicals and their stability and interactions with other food ingredients need to be carefully studied under in vitro and in vivo conditions [308]. Indeed, olive oil by-products are a good source of phytochemicals and natural antioxidants, which could be exploited for their health promoting properties. However, in this regard, significant efforts should be rendered to isolating, purifying and recovering optimum amounts of valuable compounds, in high class of purity followed by studying their detailed and standardized biological and pharmaceutical attributes. Efforts should also be focused on the isolation and structural elucidation of novel olive phenolic lipids and other bioactives using state-of-the-art chromatographic and spectroscopic tools. If the recovery and development of novel products from the olive oil by-products is well achieved, it can help to solve the environmental problems in “Mediterranean countries” and also promote application of olive oil in the food, pharmaceutical and cosmetic industries.
Industrial processes, which could minimise the loss of bioactive compounds, need to be developed. The standardization of olives and olive oil dietary intakes, based on the known chemical composition, will help to provide sound clinical basis for assessment of potential anti-atherosclerotic, anti-hypertensive, anticancer, anti-platelet aggregation and immune modulatory functionalities of olive bioactives and thus development of olive-based functional foods and nutraceuticals.
Figure 3.
Chemical structural of some important phytosterols in olive oil [18].
References
- 1.Boskou D. History and characteristics of the olive tree. In: Boskou D., editor. Olive Oil Chemistry and Technology. Am. Oil Chem. Soc. Press; Champaign, IL, USA: 1996. [Google Scholar]
- 2.Ryan D., Robards K. Phenolic compounds in olives. Analyst. 1998;123:31R–44R. [Google Scholar]
- 3.Gooch E. Ten plus one things you may not know about olive. Epikouria Magazine. Fall-Spring. 2005. [accessed on 5 November 2011]. Available online: http://www.epikouria.com/issue1/10+1-things-olives.php.
- 4.Soler-Rivas C., Epsin J.C., Wichers H.J. Oleuropein and related compounds. J. Sci. Food Agric. 2000;80:1013–1023. [Google Scholar]
- 5.Ribarova F., Zanev R., Shishkov S., Rizov N. α-Tocopherol, fatty acids and their correlations in Bulgarian foodstuffs. J. Food Compos. Anal. 2003;16:659–667. [Google Scholar]
- 6.Vinha A.F., Ferreres F., Silva B.M., Valentão P., Gonçalves A., Pereira J.A., Oliveira M.B., Seabra R.M., Andrade P.B. Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influences of cultivar and geographical origin. Food Chem. 2005;89:561–568. [Google Scholar]
- 7.Knoops K.T., de Groot L.C., Kromhout D. Mediteranean diet, lifestyle factors, and 10-year mortality in elderly European men and women. J. Am. Med. Assoc. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 8.Trichopoulou A., Costacou T., Bamia C., Trichopoulos D. Adherence to a mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 9.Covas M.I., Nyyssonen K., Poulsen H.E. The effect of polyphenols in olive oil on heart disease risk factors. Ann. Int. Med. 2006;145:333–431. doi: 10.7326/0003-4819-145-5-200609050-00006. [DOI] [PubMed] [Google Scholar]
- 10.Covas M.I. Bioactive effects of olive oil phenolic compounds in humans: Reduction of heart disease factors and oxidative damage. Inflammopharmacology. 2008;16:216–218. doi: 10.1007/s10787-008-8019-6. [DOI] [PubMed] [Google Scholar]
- 11.IOOC Home Page (International Olive Oil Council Activities: World Olive Oil Figures: World Olive Oil Consumption) [accessed on 25 November 2011]. Available online: www.internationaloliveoil.org.
- 12.Fernández A.G., Díez M.J.F., Adams M.R. Table Olives: Production and Processing. Chapman & Hall; London, UK: 1997. p. 478. [Google Scholar]
- 13.Fernandez Diez M.J. Olives. In: Rehm H.J., Reed J., editors. Biotechnology. Vol. 5. Verlag Chemie; Weinheim, Germany: 1983. pp. 379–397. [Google Scholar]
- 14.FAO Home Page. [accessed on 18 May 2009]. Available online: www.fao.org.
- 15.FAOSTAT Crops Processed Data for Olive Oil. FAO; Rome, Italy: 2009. [accessed on 5 October 2011]. Available online: http://faostat.fao.org/site/636/DesktopDefault.aspx?PageID=636#ancor. [Google Scholar]
- 16.Trade Standard Applying to Olive Oils and Olive-Pomace Oils. Internnationa Olive Council; Madrid, Spain: 2011. [accessed on 20 December 2011]. COI/ T.15/NC no. 3/Rev. 6. Available online: http://www.internationaloliveoil.org. [Google Scholar]
- 17.Luchetti F. Introduction. In: Harwood J.A., Aparicio R., editors. Handbook of Olive Oil: Analysis and Properties. Aspen Publishers, Inc; Gaithersburg, MD, USA: 2000. pp. 1–16. [Google Scholar]
- 18.Boskou D., Blekas G., Tsimidou M. Olive oil composition. In: Boskou D., editor. Olive Oil: Chemistry and Technology. Am. Oil Chem. Soc. Press; Champaign, IL, USA: 2006. pp. 1–33. [Google Scholar]
- 19.Niaounakis M., Halvadakis C.P. Waste Management Series. 2nd ed. Vol. 5. Elsevier; Amsterdam, the Netherlands: 2006. Characterization of Olive Processing Waste; pp. 23–64. Chapter 2. [Google Scholar]
- 20.Cunha S., Ferreira Isabel M.P.L.V.O., Fernandes J.O., Faria M.A., Beatriz M., Oliveira P.P. Determination of lactic, acetic, succinic and citric acids in table olive by HPLC/UV. J. Liq. Chromatogr. Relat. Technol. 2001;24:1029–1038. [Google Scholar]
- 21.Bianchi G. Lipids and phenols in table olives. Eur. J. Lipid Sci. Technol. 2003;105:229–242. [Google Scholar]
- 22.Andreasen M.F., Christensen L.P., Meyer A.S., Hansen Å. Content of phenolic acids and ferulic acid dehydrodimers in 17 rye (Secale cereale L.) varieties. J. Agric. Food Chem. 2000;48:2837–2842. doi: 10.1021/jf991266w. [DOI] [PubMed] [Google Scholar]
- 23.Prim N., Pastor F.I.J., Diaz P. Biochemical studies on cloned Bacillus sp. BP-7 phenolic acid decarboxylase PadA. Appl. Microbiol. Biotechnol. 2003;63:51–56. doi: 10.1007/s00253-003-1371-y. [DOI] [PubMed] [Google Scholar]
- 24.Kountouri A.M., Mylona A., Kaliora A.C., Andrikopoulos N.K. Bioavailability of the phenolic compounds of the fruits (drupes) of Olea europaea (olives): Impact on plasma antioxidant status in humans. Phytomedicine. 2007;14:659–667. doi: 10.1016/j.phymed.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Viola P., Viola M. Virgin olive oil as a fundamental nutritional component and skin protector. Clin. Dermatol. 2009;27:159–165. doi: 10.1016/j.clindermatol.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Servili M., Baldioli M., Selvaggini R., Macchioni A., Montedor G. Phenolic compounds of olive fruit: One- and two-dimensional nuclear magnetic resonance characterization of nüzhenide and its distribution in the constitutive parts of fruit. J. Agric. Food Chem. 1999;47:12–18. doi: 10.1021/jf9806210. [DOI] [PubMed] [Google Scholar]
- 27.Andary C., Wylde R., Laffite C., Privat G., Winternitz F. Structure of verbascoside and orobancoside, caffeic acid, suger esters from orobanche rapum-genistae. Phytochemistry. 1982;21:1123–1127. [Google Scholar]
- 28.Obied H.K., Allen M.S., Bedgood D.R., Prenzler P.D., Robards K., Stockmann R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005;53:823–837. doi: 10.1021/jf048569x. [DOI] [PubMed] [Google Scholar]
- 29.Romero C., Brenes M., Garcia P., Garrido A. Hydroxytyrosol 4-a-Dglucoside, an important phenolic compound in olive fruits and derived products. J. Agric. Food Chem. 2002;50:3835–3839. doi: 10.1021/jf011485t. [DOI] [PubMed] [Google Scholar]
- 30.Amiot M.J., Fleuriet A., Macheix J. Importance and evolution of phenolic compounds in olive during growth and maturation. J. Agric. Food Chem. 1986;34:823–826. [Google Scholar]
- 31.Romani A., Mulinacci N., Pinelli P., Vincieri F.F., Cimato A. Polyphenolic content in five tuscany cultivars of Olea europaea L. J. Agric. Food Chem. 1999;47:964–967. doi: 10.1021/jf980264t. [DOI] [PubMed] [Google Scholar]
- 32.Juan M., Planas J., Ruiz-Gutierrez V., Daniel H., Wenzel U. Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br. J. Nutr. 2008;100:36–43. doi: 10.1017/S0007114508882979. [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Bolaños J., Rodríguez G., Rodríguez R., Guillén R., Jiménez A. Extraction of interesting organic compounds from olive oil waste. Grasas Aceites. 2006;57:95–106. [Google Scholar]
- 34.Ragazzi E., Veronese G., Guitto A. The demethyloleuropein, a new glucoside extracted from ripe olives. Ann. Chim. 1973;63:13–20. [Google Scholar]
- 35.Kubo I., Matsumoto A. Molluscicides from olives Olea europaea and their efficient isolation by counter current chromatography. J. Agric. Food Chem. 1984;32:687–688. [Google Scholar]
- 36.Vasquez R.A., Costante E.G., Duran R.M. Components fenolicos de la aceituna. Polifenoles de la pulpa. Grasas Aceites. 1974;25:269–279. [Google Scholar]
- 37.Mannino S., Cosio M.S., Bertuccioli M. High performance liquid chromatography of phenolic compounds in virgin olive oil using amperometryc detector. Ital. J. Food Sci. 1993;4:363–370. [Google Scholar]
- 38.Panizzi L., Scarpati M.L., Oriente G. Chemical structure of Oleuropein, bitter glucoside of olive with hypotensive activity. Gazz. Chim. Ital. 1960;90:1449–1458. [Google Scholar]
- 39.Bourquelot E., Vintilesco J.C.R. Sur l oleuropein, nouveau principle de nature glucosidique reter de lolivier (Olea europaea L.) Compt. Rend. Hebd. Acad. Sci. 1908;147:533–535. [Google Scholar]
- 40.Huang C.L., Sumpio B.E. Olive oil, the Mediterranean diet, and cardiovascular health. J. Am. Coll. Surg. 2008;207:407–416. doi: 10.1016/j.jamcollsurg.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Juan M.E., Wenzel U., Ruiz-Gutierrez V., Daniel H., Planas J.M. Olive fruit extracts inhibit proliferation and induce apoptosis in HT-29 human colon cancer cells. J. Nutr. 2006;136:2553–2557. doi: 10.1093/jn/136.10.2553. [DOI] [PubMed] [Google Scholar]
- 42.Gilani A.H., Khan A.U., Shah A.J., Connor J., Jabeen Q. Blood pressure lowering effect of olives is mediated through calcium channel blockade. Int. J. Food Sci. Nutr. 2005;56:613–620. doi: 10.1080/09637480500539420. [DOI] [PubMed] [Google Scholar]
- 43.Gilani A.H., Khan A.U., Shah A.J. Calcium antagonist and cholinomimetic activities explain the medicinal uses of olives in gut disorders. Nutr. Res. 2006;26:277–283. [Google Scholar]
- 44.Gilani A.H., Khan A.U. Medicinal value of novel combination of cholinergic and calcium antagonist constituents in olive. In: Preedy V.R., Watson R.R., editors. Olives and Olive Oil in Health and Disease Prevention. Academic Press Elsevier; Amsterdam, the Netherlands: 2009. pp. 835–843. [Google Scholar]
- 45.Gavriilidou V., Boskou D. Chemical interesterification of olive oil-tristearin blends for margarines. Int. J. Food Sci. Technol. 1991;26:451–456. [Google Scholar]
- 46.Aparicio R., Aparicio-Ruíz R. Authentication of vegetable oils by chromatographic techniques. J. Chromatogr. A. 2000;881:93–104. doi: 10.1016/s0021-9673(00)00355-1. [DOI] [PubMed] [Google Scholar]
- 47.Artajo L.S., Romero M.P., Morelloa J.R., Motilva M.J. Enrichment of refined olive oil with phenolic compounds; evaluation of their antioxidant activity and their effect on the bitter index. J. Agric. Food Chem. 2006;54:6079–6088. doi: 10.1021/jf060874q. [DOI] [PubMed] [Google Scholar]
- 48.Tuck K.L., Hayball P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002;13:636–644. doi: 10.1016/s0955-2863(02)00229-2. [DOI] [PubMed] [Google Scholar]
- 49.Kochhar S.P. The composition of frying oils. In: Rossel J.B., editor. Frying Improving Quality. Woodhead Publishing Ltd; Cambridge, UK: 2001. pp. 87–114. [Google Scholar]
- 50.Health Claim Petition Docket No. 2003Q-0559. FDA; Rome, Italy: 2004. Monounsaturated fatty acids from olive oil and coronary heart disease. [Google Scholar]
- 51.Grundy S.M. Comparison of monounsaturated fatty acids and carbohydrates for lowering plasma cholesterol. N. Engl. J. Med. 1986;314:745–748. doi: 10.1056/NEJM198603203141204. [DOI] [PubMed] [Google Scholar]
- 52.Ranalli A., Angerosa F. Integral centrifuges for olive oil extraction. The qualitative characteristics of products. J. Am. Oil Chem. Soc. 1996;73:417–422. [Google Scholar]
- 53.Covas M.I. Olive oil and the cardiovascular system-Review. Pharm. Res. 2007;55:175–186. doi: 10.1016/j.phrs.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Allalout A., Krichene D., Methenni K., Taamalli A., Oueslati I., Daoud D. Characterization of virgin olive oil from super intensive Spanish and Greek varieties grown in northern Tunisia. Sci. Hort. 2009;120:77–83. [Google Scholar]
- 55.Paz Aguilera M., Beltran G., Ortega D., Fernandez A., Jimenez A., Uceda M. Characterisation of virgin olive oil of Italian olive cultivars: ‘Frantoio’ and ‘Leccino’, grown in Andalusia. Food Chem. 2005;89:387–391. [Google Scholar]
- 56.Skevin D., Rade D., Strucelj D., Mokrovãak Z., Nederal S., Benãiç D. The influence of variety and harvest time on the bitterness and phenolic compounds of olive oil. Eur. J. Lipid Sci. Technol. 2003;105:536–541. [Google Scholar]
- 57.Boskou D. Polar phenolic compounds. In: Boskou D., editor. Olive Oil: Chemistry and Technology. Am. Oil Chem. Soc. Press; Champaign, IL, USA: 2006. pp. 73–92. [Google Scholar]
- 58.Naczk M., Shahidib F. Extraction and analysis of phenolics in food. J. Chromatogr. A. 2004;1054:95–111. [PubMed] [Google Scholar]
- 59.Boskou D., Blekas G., Tsimidou M. Phenolic compounds in olive oil and olives. Curr. Top. Nutraceutical Res. 2005;3:125–136. [Google Scholar]
- 60.Baldioli M., Servili M., Perretti G.G.F. Montedoro antioxidant activity of tocopherols and phenolic compounds of virgin olive oil. J. Am. Oil Chem. Soc. 1996;73:1583–1593. [Google Scholar]
- 61.Bianco A., Coccioli F., Guiso M., Marra C. The occurrence in olive oil of a new class of phenolic compounds: Hydroxy-isochromans. Food Chem. 2001;77:405–411. [Google Scholar]
- 62.Montedoro G.F. Phenolic constituents of virgin olive oils. I. Identification of some phenolic acids and their antioxidant capacity. Sci. Technol. Alimenti. 1972;3:177–186. [Google Scholar]
- 63.Bendini A., Cerretani L., Carrasco-Pancorbo A., Gómez-Caravaca A.M., Segura-Carretero A., Fernández-Gutiérrez A. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods—An overview of the last decade. Molecules. 2007;12:1679–1719. doi: 10.3390/12081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Owen R.W., Mier W., Giacosa A., Hull W.E., Spiegelhalder B., Bartsch H. Phenolic compounds and sequalene in olive oils: the concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignans and sequalene. Food Chem. Toxicol. 2000;38:647–659. doi: 10.1016/s0278-6915(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 65.Rovellini P., Cortesi N. Liquid chromatography-mass spectrometry in the study of oleuropein and ligstroside aglycons in virgin olive oils: Aldehydic, dialdehydic forms and their oxidized products. Riv. Ital. Sostanze Grasse. 2002;79:1–14. [Google Scholar]
- 66.Montedoro G.F., Servili M. Tradizone ed Innovazioni Tecnologiche nell Estrazione degli Olii Extravergini Di Oliva. Proceedings of International Congress “Olive Oil Quality”; Firenze, Italy. 1–3 December, 1992; pp. 97–108. [Google Scholar]
- 67.Brenes M., García A., García P., Garrido A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001;49:5609–5614. doi: 10.1021/jf0107860. [DOI] [PubMed] [Google Scholar]
- 68.Rovellini P., Cortesi N., Fedeli E. Analysis of flavonoids from olea europaea by HPLC-UV and HPLC-electrospray-MS. Riv. Ital. Sostanze Grasse. 1997;74:273–279. [Google Scholar]
- 69.Brenes M., García A., Rios J.J., García P., Garrido A. Use of 1-acetoxypinoresinol to authenticate Picual olive oils. Int. J. Food Sci. Technol. 2002;37:615–625. [Google Scholar]
- 70.Bianco A., Buiarelli F., Cartoni G., Coccioli F., Muzzalupo I., Polidori A., Uccella N. Anlaysis by HPLC-MS/MS of biophenolic components in olives and oils. Anal. Lett. 2001;34:1033–1051. [Google Scholar]
- 71.Angerosa F., Alessandro N.D., Corana F., Mellerio G. Characterisation of phenolic and secoiridoid aglycons present in virgin olive oil by gas chromatography-chemical ionization mass spectrometry. J. Agric. Food Chem. 1995;43:1802–1807. [Google Scholar]
- 72.Perri E., Raffaelli A., Sindona G. Quantitation of oleuropein in virgin olive oil by ionspray mass spectrometry-selected reaction monitoring. J. Agric. Food Chem. 1999;47:4156–4160. doi: 10.1021/jf981161d. [DOI] [PubMed] [Google Scholar]
- 73.Montedoro G., Servili M., Baldioli M., Miniati E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992;40:1571–1576. [Google Scholar]
- 74.Jenner P., Olanow C.W. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology. 1996;47:161S–170S. doi: 10.1212/wnl.47.6_suppl_3.161s. [DOI] [PubMed] [Google Scholar]
- 75.Visioli F., Galli C., Bornet F., Mattei A., Patelli R., Galli G., Caruso D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000;486:159–160. doi: 10.1016/s0014-5793(00)01216-3. [DOI] [PubMed] [Google Scholar]
- 76.Aviram M. Interaction of oxidized low density lipoprotein with macrophages in atherosclerosis, and the antiatherogenicity of antioxidants. Eur. J. Clin. Chem. Clin. Biochem. 1996;34:599–608. [PubMed] [Google Scholar]
- 77.Marrugat J., Covas M.-I., Fitó M., Schröder H., Miró-Casas E., Gimeno E., López-Sabater M., Torre R., Farré M. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation.arandomized controlled trial. Eur. J. Nutr. 2004;43:140–147. doi: 10.1007/s00394-004-0452-8. [DOI] [PubMed] [Google Scholar]
- 78.Caruso D., Visioli F., Patelli R., Galli C., Galli G. Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism. 2001;50:1426–1428. doi: 10.1053/meta.2001.28073. [DOI] [PubMed] [Google Scholar]
- 79.Miro-Casas E., Covas M.-I., Farre M., Fito M., Ortuño J., Weinbrenner T., Roset P., Torre R.D.L. Hydroxytyrosol disposition in humans. Clin. Chem. 2003;49:945–952. doi: 10.1373/49.6.945. [DOI] [PubMed] [Google Scholar]
- 80.Mensink R.P., Janssen M.C., Katan M.B. Effect on blood pressure of two diets differing in total fat but not in saturated and polyunsaturated fatty acids in healthy volunteers. Am. J. Clin. Nutr. 1988;47:976–980. doi: 10.1093/ajcn/47.6.976. [DOI] [PubMed] [Google Scholar]
- 81.Rasmussen O., Thomsen C., Hansen K.W., Vesterlund M., Winther E., Hermansen K. Effects on blood pressure, glucose, and lipid levels of a high-monounsaturated fat diet compared with a high-carbohydrate diet in NIDDM subjects. Diabetes Care. 1993;16:1565–1571. doi: 10.2337/diacare.16.12.1565. [DOI] [PubMed] [Google Scholar]
- 82.Ruız-Gutierrez V., Muriana F.J., Guerrero A., Cert A.M., Villar J. Plasma lipids, erythrocyte membrane lipids and blood pressure of hypertensive women after ingestion of dietary oleic acid from two different sources. J. Hypertens. 1996;14:1483–1490. doi: 10.1097/00004872-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 83.Fitó M., Cladellas M., Torre R.D.L., Martí J., Alcántara M., Pujadas-Bastardes M., Marrugat J., Bruguera J., López-Sabater M.C., Vila J. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomised, crossover, controlled, clinical trial. Atherosclerosis. 2005;181:149–158. doi: 10.1016/j.atherosclerosis.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 84.Martin-Moreno J.M., Willet W.C., Gorgoio L., Banegas J.R., Rodriguez-Artalejo F., Fernandez-Rodriguez J.C., Maisonneuve P., Boyle P. Dietary fat, olive oil intake and breast cancer risk. Int. J. Cancer. 1994;58:774–780. doi: 10.1002/ijc.2910580604. [DOI] [PubMed] [Google Scholar]
- 85.Trichopoulou A., Katsouyanni K., Stuver S., Tzala L., Gnardellis C., Rimm E., Trichopoulo D. Consumption of olive oil and specific food groups in relation to breast cancer risk in Greece. J. Natl. Cancer Inst. 1995;87:110–116. doi: 10.1093/jnci/87.2.110. [DOI] [PubMed] [Google Scholar]
- 86.Vecchia C.L., Negri E., Franceschi S., Favero A., Nanni O., Filiberti R., Conti E., Montella M., Veronesi A., Ferraroni M. Hormone replacement treatment and breast cancer risk: A cooperative Italian study. Br. J. Cancer. 1995;72:244–248. doi: 10.1038/bjc.1995.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soler M., Chatenaud L., Vecchia C.L., Franceschi S., Negri S. Diet, alcohol, coffee and pancreatic cancer: Final results from an Italian study. Eur. J. Cancer Prev. 1998;7:455–460. doi: 10.1097/00008469-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 88.Franceschi S., Favero A., Conti E., Salamini R., Volpe R., Negri E., Barman L., Vecchia C.L. Food groups, oils and butter, and cancer of the oral cavity and pharynx. Br. J. Cancer. 1999;80:614–620. doi: 10.1038/sj.bjc.6690400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bosetti C., Trichopoulou A., Franceschi S., Negri E., Vecchia C.L. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive. Tract. Cancer Epidemiol. Biomarkers Prev. 2003;12:1091–1094. [PubMed] [Google Scholar]
- 90.Stoneham M., Goldacre M., Seagroatt V., Gill L., Epidemiol J. Olive oil, diet and colorectal cancer: An ecological study and a hypothesis. J. Epidemiol. Community Health. 2000;54:756–760. doi: 10.1136/jech.54.10.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hodge E., English D.R., McCredie M.R.E., Severi G., Boyle P., Hopper J.L., Giles G.G. Foods, nutrients and prostate cancer. Cancer Causes Control. 2004;15:11–20. doi: 10.1023/B:CACO.0000016568.25127.10. [DOI] [PubMed] [Google Scholar]
- 92.Fortes C., Forestiere F., Farchi S., Mallone S., Trequattrini T., Anatra F., Schmid G., Peducci C.A. the protective effect of the mediterranean diet on lung cancer. Nutr. Cancer. 2003;46:30–37. doi: 10.1207/S15327914NC4601_04. [DOI] [PubMed] [Google Scholar]
- 93.Ichihashi M., Ueda M., Budiyanto A., Bito T., Oka M., Fukunaga M., Tsuru K., Horikawa T. UV-Induced skin Damage. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 94.Kohyama N., Nagata T., Fujimoto S. Inhibition of arachidonate lipoxygenase activities by 2-(3,4-dihydroxyphenyl) ethanol, a phenolic compound from olives. Biosci. Biotechnol. Biochem. 1997;61:347–350. doi: 10.1271/bbb.61.347. [DOI] [PubMed] [Google Scholar]
- 95.Petroni A., Blasevich M., Salami M., Papini N., Montedoro G.F., Galli C. Inhibition of platelet aggregation and eicosanoid production by phenolic components of olive oil. Thromb. Res. 1995;78:151–160. doi: 10.1016/0049-3848(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 96.Puerta R.D.L, Gutierrez V.R., Hoult J.R. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999;57:445–449. doi: 10.1016/s0006-2952(98)00320-7. [DOI] [PubMed] [Google Scholar]
- 97.Turner R., Etienne N., Alonso M.G. Antioxidant and anti-atherogenic activities of olive oil phenolics. Int. J. Vitam. Nutr. Res. 2005;75:61–70. doi: 10.1024/0300-9831.75.1.61. [DOI] [PubMed] [Google Scholar]
- 98.Estruch R., Martinez-Gonzalez M.A., Corella D., Salas-Salvado J., Ruiz-Gutierrez V., Covas M.I. Effects of a Mediterranean- style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 99.Visioli F., Bellomo G., Montedoro G.F., Galli C. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis. 1995;117:25. doi: 10.1016/0021-9150(95)05546-9. [DOI] [PubMed] [Google Scholar]
- 100.Ruano J., Lopez-Miranda J., Fuentes F., Moreno J.A., Bellido C., Perez-Martinez P., Lozano A., Gómez P., Jiménez Y., Jiménez F.P. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J. Am. Coll. Cardiol. 2005;46:1864–1868. doi: 10.1016/j.jacc.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 101.Carluccio M.A., Siculella L., Ancora M.A. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003;23:622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 102.Dejana M., Trauma O.I., Bianchi M.P., Spencer J.P.E., Harparkash K., Halliwell B., Haeschbach R., Banni S., Dessi M.A., Corongiu F. Inhibition of peroxynitrite dependent DNA base modification and tyrosine nitration by the extra virgin olive oil-derived antioxidant hydroxytyrosol. Free Radic. Biol. Med. 1999;26:762–769. doi: 10.1016/s0891-5849(98)00231-7. [DOI] [PubMed] [Google Scholar]
- 103.Machowetz A., Poulsen H.E., Gruendel S., Weimann A., Fitó M., Marrugat J., Torre R.D.L., Salonen J.T., Nyyssönen K., Mursu J., et al. Effect of olive oils on biomarkers of oxidative DNA stress in Northern and Southern Europeans. FASEB J. 2007;21:45–52. doi: 10.1096/fj.06-6328com. [DOI] [PubMed] [Google Scholar]
- 104.Perona J.S., Cabello-Moruno R., Ruiz-Gutierrez V. The role of virgin olive oil components in the modulation of endothelial function. J. Nutr. Biochem. 2006;17:429–445. doi: 10.1016/j.jnutbio.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 105.Martinez-Dominguez E., de la Puerta R., Ruiz-Gutierrez V. Protective effects upon experimental inflammation models of a polyphenol-supplemented virgin olive oil diet. Inflamm. Res. 2001;50:102–106. doi: 10.1007/s000110050731. [DOI] [PubMed] [Google Scholar]
- 106.Aziz N.H., Farag S.E., Mousa L.A.A., Abo-Zaid M.A. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 1998;93:43–54. [PubMed] [Google Scholar]
- 107.Gourama H., Bullerman L.B. Effects of oleuropein on growth and aflatoxin production by Aspergillus parasiticus. Lebensm. Wiss. Technol. 1987;20:226–228. [Google Scholar]
- 108.Deiana M., Rosa A., Cao C.F., Pirisi F.M., Bandino G., Dessi A. Novel approach to study oxidative stability of extra virgin olive oils: Importance of α-tocopherol concentration. J. Agric. Food Chem. 2002;50:4342–4346. doi: 10.1021/jf020033t. [DOI] [PubMed] [Google Scholar]
- 109.Lavelli V. Comparison of the antioxidant activities of extra virgin olive oils. J. Agric. Food Chem. 2002;50:7704–7708. doi: 10.1021/jf020749o. [DOI] [PubMed] [Google Scholar]
- 110.Leenen R., Roodenburg A.J., Vissers M.N. Supplementation of plasma with olive oil phenols and extracts: Influence on LDL oxidation. J. Agric. Food Chem. 2002;50:1290–1297. doi: 10.1021/jf010968u. [DOI] [PubMed] [Google Scholar]
- 111.Carluccio M.A., Siculella L., Ancora M.A. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003;23:622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 112.Servili M., Esposto S., Fabiani R., Urbani S., Taticchi A., Mariucci F., Selvaggini R., Montedoro G.F. Phenolic compounds in olive oil: Antioxidant, health and sensory activities according to their chemical structure. Inflammopharmacology. 2009;17:76–84. doi: 10.1007/s10787-008-8014-y. [DOI] [PubMed] [Google Scholar]
- 113.Visioli F., Galli C. Olive oil phenols and their potential effects on human health. J. Agric. Food Chem. 1998;46:4292–4296. [Google Scholar]
- 114.Fitó M., Covas M.I., Lamuela-Raventós R.M., Vila J., Torrents J., Torre C.D.L., Marrugat J. Protective effect of olive oil and its phenolic compounds against low density lipoprotein oxidation. Lipids. 2000;35:633–638. doi: 10.1007/s11745-000-0567-1. [DOI] [PubMed] [Google Scholar]
- 115.Pellegrini N., Visioli F., Buratti S., Brighenti F. Direct analysis of total antioxidant activity of olive oil and studies on the influence of heating. J. Agric. Food Chem. 2001;49:2532–2538. doi: 10.1021/jf001418j. [DOI] [PubMed] [Google Scholar]
- 116.Manna C., D’Angelo S., Migliardi V., Loffredi E., Mazzoni O., Morrica P., Galletti P., Zappia V. Protective effect of the phenolic fraction from virgin olive oils against oxidative stress in human cells. J. Agric. Food Chem. 2002;50:6521–6526. doi: 10.1021/jf020565+. [DOI] [PubMed] [Google Scholar]
- 117.Stupans I., Kirlich A., Tuck K.L., Hayball P.J. Comparison of radical scavenging effect, inhibition of microsomal oxygen free radical generation, and serum lipoprotein oxidation of several natural antioxidants. J. Agric. Food Chem. 2002;50:2464–2469. doi: 10.1021/jf0112320. [DOI] [PubMed] [Google Scholar]
- 118.Gorinstein S., Martin-Belloso O., Katrich E., Lojek A., Èíž M., Gligelmo-Miguel N., Haruenkit R., Park Y.-S., Jung S.-T., Trakhtenberg S. Comparison of the contents of the main biochemical compounds and the antioxidant activity of some Spanish olive oils as determined by four different radical scavenging tests. J. Nutr. Biochem. 2003;14:154–159. doi: 10.1016/s0955-2863(02)00278-4. [DOI] [PubMed] [Google Scholar]
- 119.Somova L.I., Shode F.O., Ramnanan P., Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 2003;84:299–305. doi: 10.1016/s0378-8741(02)00332-x. [DOI] [PubMed] [Google Scholar]
- 120.Masella R., Varì R., D’Archivio M., Benedetto R.D., Matarrese P., Malorni W., Scazzocchio B., Giovannini C. Extra virgin olive oil biophenols inhibit cell-mediated oxidation of LDL by increasing the mRNA transcription of glutathione-related enzymes. J. Nutr. 2004;134:785–791. doi: 10.1093/jn/134.4.785. [DOI] [PubMed] [Google Scholar]
- 121.Bouaziz M., Grayer R.J., Simmonds M.S.J., Damak M., Sayadi S. Identification and antioxidant potential of flavonoids and low molecular weight phenols in olive cultivar chemlali growing in Tunisia. J. Agric. Food Chem. 2005;53:236–241. doi: 10.1021/jf048859d. [DOI] [PubMed] [Google Scholar]
- 122.Škergeta M., Kotnika P., Hadolinb M., Hrašb A.R., Simonièa M., Kneza Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. [Google Scholar]
- 123.Dabbou S., Issaoui M., Servili M., Taticchi A., Sifi S., Montedoro G.F., Hammami M. Characterisation of virgin olive oils from European olive cultivars introduced in Tunisia. Eur. J. Lipid Sci. Technol. 2009;111:392–401. [Google Scholar]
- 124.Lee O., Lee B. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010;101:3751–3754. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 125.Hashimoto T., Ibi M., Matsuno K., Nakashima S., Tanigawa T., Yoshikawa T., Yabe-Nishimura C. An endogenous metabolite of dopamine, 3,4-dihydroxyphenylethanol, acts as a unique cytoprotective agent against oxidative stress-induced injury. Free Radic. Biol. Med. 2004;36:555–564. doi: 10.1016/j.freeradbiomed.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 126.Trichopoulou A., Lagiou P. Healthy traditional Mediterranean diet: An expression of culture, history and lifestyle. Nutr. Rev. 1997;55:383–389. doi: 10.1111/j.1753-4887.1997.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 127.Tapiero H., Ba G.N., Couvreur P., Tew K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002;56:215–222. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 128.Tassou C.C., Nychas G.J. Inhibition of Staphylococous aureus by olive phenolics in broth and in a model food system. J. Food Protect. 1994;57:120–124. doi: 10.4315/0362-028X-57.2.120. [DOI] [PubMed] [Google Scholar]
- 129.Bisignano G., Tomaino A., Lo Cascio R.C.G., Uccella N., Saija A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999;51:971–974. doi: 10.1211/0022357991773258. [DOI] [PubMed] [Google Scholar]
- 130.Tranter H.S., Tassou S.C., Nychas G.J. The effect of the olive phenolic compound, oleuropein, on growth and enterotoxin B production by staphylococcus aureus. J. Appl. Bacteriol. 1993;74:253–259. doi: 10.1111/j.1365-2672.1993.tb03023.x. [DOI] [PubMed] [Google Scholar]
- 131.Sudjana A.N., Orazio C.D., Ryan V., Rasool N., Ng J., Islam N., Rileyae T.V., Hammer K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents. 2009;33:461–463. doi: 10.1016/j.ijantimicag.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 132.Gerber M. Olive oil and cancer. In: Hill M.J., Giacosa A., Caygill C.P.G., editors. Epidemiology of Diet and Cancer. Ellis Horwood; Chichester, UK: 1994. pp. 263–275. [Google Scholar]
- 133.Simopoulos A.P. The Mediterranean diets: What is so special about the diet of Greece? J. Nutr. 2001;131:3065S–3073S. doi: 10.1093/jn/131.11.3065S. [DOI] [PubMed] [Google Scholar]
- 134.Giovannini C., Scazzocchio B., Mattarrese P., Vari R., Archivio M.D., Benedetto R.D., Casciani S., Dessic M.R., Struface E., Malorni W., et al. Apoptosis induced by oxidized lipids is associated with upregulation of p66Shc in intestinal Caco-2 cells: Protective effects of phenolic compounds. J. Nutr. Biochem. 2008;19:118–128. doi: 10.1016/j.jnutbio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 135.García-Villalba R., Carrasco-Pancorbo A., Oliveras-Ferraros C., Vázquez-Martín A., Menéndez J.A., Segura-Carretero A., Fernández-Gutiérre A. Characterization and quantification of phenolic compounds of extra-virgin olive oils with anticancer properties by a rapid and resolutive LC-ESI-TOF MS method. J. Pharmaceut. Biomed. Anal. 2010;51:416–429. doi: 10.1016/j.jpba.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 136.Andrikopoulos N.K., Antonopoulou S., Kaliora A.C. Oleuropein inhibits LDL oxidation induced by cooking oil frying by-products and platelet aggregation induced by platelet-activating factor. LWT-Food Sci. Technol. 2002;35:479–484. [Google Scholar]
- 137.Driss F., Duranthon V., Viard V. Biological activity of olive tree polyphenolic compounds. OCL-Ol. Corps Gras Lipides. 1996;3:448–451. [Google Scholar]
- 138.Khayyal M.T., El-Ghazaly M.A., Abdallah D.M., Nassar N.N., Okpanyi S.N., Kreuter M.H. Blood pressure lowering effect of an olive leaf extract (Olea europaea) in L-NAME induced hypertension in rats. Arzneimittelforschung. 2002;52:797–802. doi: 10.1055/s-0031-1299970. [DOI] [PubMed] [Google Scholar]
- 139.Binukumar B., Mathew A. Dietary fat and risk of breast cancer. World J. Surg. Oncol. 2005;3:45. doi: 10.1186/1477-7819-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Angerosa F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci.Technol. 2002;104:639–660. [Google Scholar]
- 141.Lorenzo I.M., Pavon J.L.P., Laespada M.E.F., Pinto C.G., Cordero B.M. Detection of adulterants in olive oil by headspace-mass spectrometry. J. Chromatogr. A. 2002;945:221–230. doi: 10.1016/s0021-9673(01)01502-3. [DOI] [PubMed] [Google Scholar]
- 142.Morales M.T., Rios J.J., Aparicio R. Changes in the volatile composition of virgin olive oil during oxidation: Flavors and off-flavors. J. Agric. Food Chem. 1997;45:2666–2673. [Google Scholar]
- 143.Baccouri O., Bendini A., Cerretani L., Guerfel M., Baccouri B., Lercker G., Zarrouk M., Miled D.D.B. Comparative study on volatile compounds from Tunisian and Sicilian monovarietal virgin olive oils. Food Chem. 2008;111:322–328. doi: 10.1016/j.foodchem.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 144.Kiritsakis A.K., Nanos G.D., Polymenoupoulos Z., Thomai T., Sfakiotakis E.Y. Effect of fruit storage conditions on olive oil quality. J. Am. Oil Chem. Soc. 1998;75:721–724. [Google Scholar]
- 145.Kalua C.M., Allen M.S., Bedgood D.R., Bishop A.G., Prenzler P.D., Robards K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007;100:273–286. [Google Scholar]
- 146.Vichi S., Castellote A.I., Pizzale L., Conte L.S., Buxaderas S., Lopez-Tamames E. Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: Characterization of virgin olive oils from two distinct geographical areas of northern Italy. J. Agric. Food Chem. 2003;51:6572–6577. doi: 10.1021/jf030269c. [DOI] [PubMed] [Google Scholar]
- 147.Angerosa F., Basti C. Olive oil volatile compounds from the lipoxygenase pathway in relation to fruit ripeness. Ital. J. Food Sci. 2001;13:421–428. [Google Scholar]
- 148.Morales M.T., Alonso M.V., Rios J.J., Aparicio R. Virgin olive oil aroma: Relationship between volatile compounds and sensory attributes by chemometrics. J. Agric. Food Chem. 1995;43:2925–2931. [Google Scholar]
- 149.Morales M.T., Luna G., Aparicio R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005;91:293–301. [Google Scholar]
- 150.Goodwin T.W. Biosynthesis of sterols. In: Stumpf P.K., Conn E.E., editors. The Biochemistry of Plants. Lipids: Structure and Function. 4th ed. Academic Press; London, UK: 1980. pp. 485–507. [Google Scholar]
- 151.Benitez-Sánchez P.L., Camacho L.M., Aparicio R. A comprehensive study of hazelnut oil composition with comparisons to other vegetable oils, particularly olive oil. Eur. Food Res. Technol. 2003;218:13–19. [Google Scholar]
- 152.Morchio G., De Anreis R., Fedeli E. Investigations of total sterols content in the olive oil and their variation during refining process. Riv. Ital. Sostanze Grasse. 1987;64:185–192. [Google Scholar]
- 153.Sivakumar G., Bati C.B., Perri E., Uccella N.U. Gas chromatography screening of bioactive phytosterols from mono-cultivar olive oils. Food Chem. 2006;95:525–528. [Google Scholar]
- 154.Canabate-Díaz B., Carretero A.S., Fernández-Gutiérrez A., Belmonte Vega A., Garrido Frenich A., Martínez Vidal J.L., Duran Martos J. Separation and determination of sterols in olive oil by HPLC-MS. Food Chem. 2007;102:593–598. [Google Scholar]
- 155.Moss G.P. The nomenclature of steroids: Recommendations by the IUPAC-IUB joint commission on biochemical nomenclature. Eur. J. Biochem. 1989;186:429–458. [PubMed] [Google Scholar]
- 156.Akihisa T., Kokke W., Tamura T. Naturally occurring sterols and related compounds from plants. In: Patterson G.W., Nes W.D., editors. Physiology and Biochemistry of Sterols. American Oil Chemists’ Society; Champaign, IL, USA: 1991. pp. 172–228. [Google Scholar]
- 157.Moreau R.A., Whitakerb B.D., Hicksa K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 2002;41:457–500. doi: 10.1016/s0163-7827(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 158.Heupel R.C. Isolation and primary characterization of sterols. In: Nes D.W., Parish E.J., editors. Analysis of Sterols and Other Biologically Significant Steroids. Academic Press Inc.; San Diego, CA, USA: 1989. pp. 1–32. [Google Scholar]
- 159.Hartmann M.A. Plant sterols and the membrane environment. Trends Plant Sci. 1998;3:170–175. [Google Scholar]
- 160.Quillez J., Garcila-Lorda P., Salas-Salvadol J. Potential uses and benefits of phytosterols in diet: Present situation and future directions. Clin. Nutr. 2003;22:343–351. doi: 10.1016/s0261-5614(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 161.Awad A.B., Fink C.S. Phytosterols as anticancer dietary components: Evidence and mechanism of action. J. Nutr. 2000;130:2127–2130. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- 162.Wilt T.J., Mac Donald R., Ishani A. Beta-sitosterol for the treatment of benign prostatic hyperplasia: A systematic review. Br. J. Urol. Int. 1999;83:976–983. doi: 10.1046/j.1464-410x.1999.00026.x. [DOI] [PubMed] [Google Scholar]
- 163.Wolfreys A.M., Hepburn P.A. Safety evaluation of phytosterol esters. Part 7. Assessment of mutagenic activity of phytosterols, phytosterol esters and the cholesterol derivative, 4-cholesten-3-one. Food Chem. Toxicol. 2002;40:461–470. doi: 10.1016/s0278-6915(01)00099-0. [DOI] [PubMed] [Google Scholar]
- 164.Hepburn P.A., Horner S.A., Smith M. Safety evaluation of phytosterol esters. Part 2. Subchronic 90-day oral toxicity study on phytosterol esters a novel functional food. Food Chem. Toxicol. 1999;37:521–532. doi: 10.1016/s0278-6915(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 165.Ranalli A., Pollastri L., Contento S., di Loreto G., Iannucci E., Lucera L., Russi F. Sterol and alcohol components of seed, pulp and whole olive fruit oils. Their use to characterise olive fruit variety by multivariates. J. Sci. Food Agric. 2002;82:854–859. doi: 10.1021/jf011506j. [DOI] [PubMed] [Google Scholar]
- 166.Esterbauer H., Dieber-Rotheneder M., Striegl G., Waeg G. Role of vitamin E in preventing the oxidation of low-density lipoprotein. Am. J. Clin. Nutr. 1991;53:314S–321S. doi: 10.1093/ajcn/53.1.314S. [DOI] [PubMed] [Google Scholar]
- 167.Blekas G., Psomiadou E., Tsimidou M. On the importance of total polar phenols to monitor the stability of Greek virgin olive oil. Eur. J. Lipid Sci. Technol. 2002;104:340–346. [Google Scholar]
- 168.Dionisi F., Prodolliet J., Tagliaferri E. Assessment of olive oil adulteration by reversed-phase high-performance liquid chromatography/amperometric detection of tocopherols and tocoterienols. J. Am. Oil Chem. Soc. 1995;72:1505–1511. [Google Scholar]
- 169.Cunha S.C., Amaral J.S., Fernandes J.O., Oliveira M.B.P.P. Quantification of tocopherols and tocotrienols in Portuguese olive oils using HPLC with three different detection systems. J. Agric. Food Chem. 2006;54:3351–3356. doi: 10.1021/jf053102n. [DOI] [PubMed] [Google Scholar]
- 170.Kamal-Eldin A., Appelqvist L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 171.Yamauchi R., Matsushita S. Quenching effect of tocopherols on methyl linoleate photoxidation and their oxidation products. Agric. Biol. Chem. 1977;41:1425–1430. [Google Scholar]
- 172.Cheeseeman K.H., Slater T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 173.Kamal-Eldin A., Andersson R.A. Multivariate study of the correlation between tocopherol content and fatty acid composition in vegetable oils. J. Am. Oil Chem. Soc. 1997;74:375–380. [Google Scholar]
- 174.Doelman C.J. Antioxidant Therapy and Preventive Medicine. Vol. 9 Plenum Press; New York NY, USA: 1989. [Google Scholar]
- 175.Armstrong N., Paganga G., Brunev E., Miller N., Nanchahal K. Reference values for α-tocopherol and β-carotene in the Whitehall II study. Free Radic. Res. 1997;27:207–219. doi: 10.3109/10715769709097853. [DOI] [PubMed] [Google Scholar]
- 176.Caruso D., Berra B., Giovanini F., Cortesi N., Fedeli E., Galli G. Effect of virgin olive oil phenolic compounds on in vitro oxidation of human low density lipoproteins. Nutr. Metab. Cardiovasc. Dis. 1997;99:102–107. [PubMed] [Google Scholar]
- 177.Nicolaiew N., Lemort N., Adorni L., Berra B., Montorfano G., Rapelli S. Comparison between extra virgin olive oil and oleic acid rich sunflower oil: Effects on postprandial lipemia and LDL susceptibility to oxidation. Ann. Nutr. Metab. 1998;42:251–260. doi: 10.1159/000012741. [DOI] [PubMed] [Google Scholar]
- 178.Hudson B., Lewis J. Polyhydroxy flavonoid antioxidants for edible oils. Structural criteria for activity. Food Chem. 1983;10:47–55. [Google Scholar]
- 179.Psomiadou P., Tsimidou M. Simultaneous HPLC determination of tocopherols, carotenoids, and chlorophylls for monitoring their effect on virgin olive oil oxidation. J. Agric. Food Chem. 1998;46:5132–5138. [Google Scholar]
- 180.Lanzón A., Albi T., Cert A. The hydrocarbon fraction of virgin olive oil and changes resulting from refining. J. Am. Oil Chem. Soc. 1994;3:285–291. [Google Scholar]
- 181.Gandul-Rojas B., Minguez-Mosquera M.I. Chlorophyll and carotenoid composition in virgin olive oils from various Spanish olive varieties. J. Sci. Food Agric. 1996;72:31–39. [Google Scholar]
- 182.Kupper H., Dedic R., Svoboda A., Hala J., Kroneck P.M. Kinetics and efficency of excitation energy transfer from chlorophylls, their heavy metal-substituted derivatives, and pheophytins to singlet oxygen. Biochim. Biophys. Acta. 2002;1572:107–113. doi: 10.1016/s0304-4165(02)00284-2. [DOI] [PubMed] [Google Scholar]
- 183.Foote C.S., Denny R.W. Chemistry of singlet oxygen VII. quenching by β-carotene. J. Am. Chem. Soc. 1968;90:6233–6234. [Google Scholar]
- 184.Hansen E., Skibsted L.H. Light-induced oxidative changes in a model dairy spread. Wavelength dependence of quantum yields. J. Agric. Food Chem. 2000;48:3090–3094. doi: 10.1021/jf991232o. [DOI] [PubMed] [Google Scholar]
- 185.Burton G., Ingold K. β-Carotene: An unusual type of lipid antioxidant. J. Sci. Food Agric. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 186.Yanishlieva N.V., Aitzetmüller K., Raneva V. β-Carotene and lipid oxidation. Lipid/Fett. 1998;100:444–462. [Google Scholar]
- 187.Matyas G.R., Wassef N.M., Rao M., Alving C.R. Induction and detection of antibodies to squalene. J. Immunol. Methods. 2000;245:1–14. doi: 10.1016/s0022-1759(00)00268-4. [DOI] [PubMed] [Google Scholar]
- 188.Newmark H.L. Squalene, olive oil, and cancer risk: A review and hypothesis, Cancer Epidemiol. Biomark. Prev. 1997;6:1101–1103. [PubMed] [Google Scholar]
- 189.Owen R.W., Haubner R., Würtele G.W., Hull W.E., Spiegelhalder B., Bartsch H. Olives and olive oil in cancer prevention. Eur. J. Cancer Prev. 2004;13:319–326. doi: 10.1097/01.cej.0000130221.19480.7e. [DOI] [PubMed] [Google Scholar]
- 190.Koivisto P.V.I. Miettinen, Increased amount of cholesterol precursors in lipoproteins after ileal exclusion. Lipids. 1988;23:993–996. doi: 10.1007/BF02536349. [DOI] [PubMed] [Google Scholar]
- 191.Stewart M.E. Sebaceous gland lipids. Semin. Dermatol. 1992;11:100–105. [PubMed] [Google Scholar]
- 192.Newmark H.L. Squalene, olive oil, and cancer risk: Review and hypothesis. Ann. N. Y. Acad. J. Sci. Food Agric. 1999;889:193–203. doi: 10.1111/j.1749-6632.1999.tb08735.x. [DOI] [PubMed] [Google Scholar]
- 193.Dennis K., Shimamoto T. Production of malonyldialdehyde from squalene, a major skin surface lipid, during UV irradiation. Photochem. Photobiol. 1989;49:711–719. doi: 10.1111/j.1751-1097.1989.tb08445.x. [DOI] [PubMed] [Google Scholar]
- 194.Reddy L.H., Couvreur P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009;61:1412–1426. doi: 10.1016/j.addr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 195.Kelly G. Squalene and its potential clinical uses. Altern. Med. Rev. 1999;4:29–36. [PubMed] [Google Scholar]
- 196.Kalogeropoulos N., Chiou A., Gavala E., Christea M., Andrikopoulos K.N. Nutritional evaluation and bioactive microconstituents (carotenoids, tocopherols, sterols and squalene) of raw and roasted chicken fed on DHA-rich microalgae. Food Res. Int. 2010;43:2006–2013. [Google Scholar]
- 197.Whittenton J., Harendra S., Pitchumani R., Mohanty K., Vipulanandan C., Thevananther S. Evaluation of asymmetric liposomal nanoparticles for encapsulation of polynucleotides. Langmuir. 2008;24:8533–8540. doi: 10.1021/la801133j. [DOI] [PubMed] [Google Scholar]
- 198.Fox C.B., Anderson R.C., Dutill T.S., Goto Y., Reed S.G., Vedvick T.S. Monitoring the effects of component structure and source on formulation stability and adjuvant activity of oil-in-water emulsions. Colloids Surf. B. 2008;65:98–105. doi: 10.1016/j.colsurfb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 199.Kamimara H., Koga N., Oguri K., Yoshimura H. Enhanced elimination of theophylline, phenobarbital and strychnine from the bodies of rats and mice by squalene treatment. J. Pharmacobiodyn. 1992;15:215–221. doi: 10.1248/bpb1978.15.215. [DOI] [PubMed] [Google Scholar]
- 200.Kiritsakis Markakis. Advances in Food Research. Vol. 31. Elsevier Inc; New York, NY, USA: 1988. Olive oil: A review; pp. 453–482. [DOI] [PubMed] [Google Scholar]
- 201.Aparicio R., Luna G. Characterisation of monovarietal virgin olive oil. Eur. J. Lipid Sci. Technol. 2002;104:614–627. [Google Scholar]
- 202.Grob K., Lanfranchi M., Mariani C. Evaluation of olive oils through the fatty alcohols, the sterols and their esters by coupled LC-GC. J. Am. Oil Chem. Soc. 1990;67:626–634. [Google Scholar]
- 203.Gucci R., Lombardini L., Tattini M. Analysis of leaf water relations in leaves of two olive (Olea europaea) cultivars differing in tolerance to salinity. Tree Physiol. 1997;17:13–21. doi: 10.1093/treephys/17.1.13. [DOI] [PubMed] [Google Scholar]
- 204.Fernandez-Escobar R., Moreno R., Garcia-Creus M. Seasonal changes of mineral nutrients in olive leaves during the alternate-bearing cycle. Sci. Hort. -Amsterdam. 1999;82:25–45. [Google Scholar]
- 205.Ciafardini G., Zullo B.A. Microbiological activity in stored olive oil. Int. J. Food Microbiol. 2002;75:111–118. doi: 10.1016/s0168-1605(01)00739-5. [DOI] [PubMed] [Google Scholar]
- 206.karakaya S.E.S. Studies of olive tree leaf extract indicate seveal potential health benefits. Nutr. Rev. 2009;67:632–639. doi: 10.1111/j.1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- 207.Zarzuelo A. Vasodilator effect of olive leaf. Planta Med. 1991;57:417–419. doi: 10.1055/s-2006-960138. [DOI] [PubMed] [Google Scholar]
- 208.Samuelsson G. The blood pressure lowering factor in leaves of Olea europaea. Farmacevtisk Revy. 1951;15:229–239. [Google Scholar]
- 209.Pereira A.P., Ferreira I.C.F.R., Marcelino F., Valentão P., Andrade B.P., Seabra R., Estevinho L., Bento A., Pereira J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules. 2007;12:1153–1162. doi: 10.3390/12051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Benavente-Garcia O., Castillo J., Lorente J., Ortuno A., Del Rio J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000;68:457–462. [Google Scholar]
- 211.Furneri P.M., Marino A., Saija A., Uccella N., Bisignano G. In vitro antimycoplasmal activity of oleuropein. Int. J. Antimicrob. Agents. 2002;20:293–296. doi: 10.1016/s0924-8579(02)00181-4. [DOI] [PubMed] [Google Scholar]
- 212.Briante R., Febbraio F., Nucci R. Antioxidant properties of low molecular weight phenols present in the Mediterranean diet. J. Agric. Food Chem. 2003;51:6975–6981. doi: 10.1021/jf034471r. [DOI] [PubMed] [Google Scholar]
- 213.Skerget M., Kotnik P., Hadolin M., Hradolin A.R., Simoni M., Knez Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. [Google Scholar]
- 214.Ryan D., Prenzler P.D., Lavee S., Antolovich M., Robards K. Quantitative changes in phenolic content during physiological development of the olive (Olea europaea) cultivar Hardy’s Mammoth. J. Agric. Food Chem. 2003;51:2532–2538. doi: 10.1021/jf0261351. [DOI] [PubMed] [Google Scholar]
- 215.Bianco A., Uccella N. Biophenolic components of olives. Food Res. Int. 2000;33:475–485. [Google Scholar]
- 216.Tasioula-Margari M., Ologeri O. Isolation and characterization of virgin olive oil phenolic compounds by HPLC/UV and GC/MS. J. Food Sci. 2001;66:530–534. [Google Scholar]
- 217.Delgado-Pertinez M., Gomez-Cabrera A., Garrido A. Predicting the nutritive value of the olive leaf (Olea europaea): Digestibility and chemistry composition and in vitro studies. Anim. Feed Sci. Technol. 2000;87:187–201. [Google Scholar]
- 218.Lesage-Meessen L., Navarro D., Maunier S., Sigoillot J.C., Lorquin J., Delattre M. Simple phenolic content in olive oil residues as a function of extraction systems. Food Chem. 2001;75:501–507. [Google Scholar]
- 219.Sabbah I., Marsook T., Basheer S. The effect of pretreatment on anaerobic activity of olive mill wastewater using batch and continuous systems. Process Biochem. 2004;39:1947–1951. [Google Scholar]
- 220.Manios T. The composting potential of different organic solid wastes, experience from the island of Crete. Environ. Int. 2004;29:1079–1089. doi: 10.1016/S0160-4120(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 221.Ranalli A., Lucera L., Contento S. Antioxidizing potency of phenol compounds in olive oil mill wastewater. J. Agric. Food Chem. 2003;51:7636–7641. doi: 10.1021/jf034879o. [DOI] [PubMed] [Google Scholar]
- 222.Shahidi F., Naczk M. Phenolic in Food and Nutraceutical. CRC Press; Boca Raton, FL USA: 2004. [Google Scholar]
- 223.Amro B., Aburjai T., Al-Khalil S. Antioxidative and radical scavenging effects of olive cake extract. Fitoterapia. 2002;73:456–461. doi: 10.1016/s0367-326x(02)00173-9. [DOI] [PubMed] [Google Scholar]
- 224.Fernandez-Bolanos J., Rodriguez G., Rodriguez R., Heredia A., Guillen R., Jimenez A. Production in large quantities of highly purified hydroxytyrosol from liquid-solid waste of two phase olive oil processing or”Alperujo”. J. Agric. Food Chem. 2002;50:6804–6811. doi: 10.1021/jf011712r. [DOI] [PubMed] [Google Scholar]
- 225.Mulinacci N., Romani A., Galardi C., Pinelli P., Giaccherini C., Vincieri F.F. Polyphenolic content in olive oil wastewaters and related olive samples. J. Agric. Food Chem. 2001;49:3509–3514. doi: 10.1021/jf000972q. [DOI] [PubMed] [Google Scholar]
- 226.Allouche N., Fki I., Sayadi S. Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewaters. J. Agric. Food Chem. 2004;52:267–273. doi: 10.1021/jf034944u. [DOI] [PubMed] [Google Scholar]
- 227.Bianco A., Buiarelli F., Cartoni G., Coccioli F., Jasionowska R., Margherita P. Analysis by liquid chromatography-tandem mass spectrometry of biophenolic compounds in olives and vegetation waters, Part I. J. Sep. Sci. 2003;26:409–416. [Google Scholar]
- 228.Fiorentino A., Gentili A., Isidori M., Monaco P., Nardelli A., Parrella A.E., et al. Environmental effects caused by olive mill wastewaters: Toxicity comparison of low-molecular weight phenol components. J. Agric. Food Chem. 2003;51:1005–1009. doi: 10.1021/jf020887d. [DOI] [PubMed] [Google Scholar]
- 229.Rodis P.S., Karathanos V.T., Mantzavinou A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2002;50:596–601. doi: 10.1021/jf010864j. [DOI] [PubMed] [Google Scholar]
- 230.Aludatt M.H., Alli I., Ereifej K., Alhamad M., Al-Tawaha A.R., Rababah T. Optimisation, characterisation and quantification of phenolic compounds in olive cake. Food Chem. 2010;123:117–122. [Google Scholar]
- 231.Parades C., Cegarra J., Roig A., Sanchez-Monedero M.A., Bernal M.P., Brenes M. Characterization of olive mill wastewater (alpechin) audits sludge for agricultural purposes. Bioresour. Technol. 1999;67:111–115. [Google Scholar]
- 232.Rice-Evans C., Miller N.J., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 233.Khoufi S., Aloui F., Sayadi S. Extraction of antioxidants from olive mill wastewater and electro-coagulation of exhausted fraction to reduce its toxicity on anaerobic digestion. J. Hazard. Mater. 2008;151:531–539. doi: 10.1016/j.jhazmat.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 234.Manna C., Galletti P., Cucciolla V., Moltedo O., Leone A., Zappia V. The protective effect of the olive oil polyphenols (3,4-dyhydroxyphenyl)-ethanol counteracts reactive oxygen metabolite induced cytoxicity in caco-2-cells. J. Nutr. 1997;127:286–292. doi: 10.1093/jn/127.2.286. [DOI] [PubMed] [Google Scholar]
- 235.Kubo I., Hanke F.J. Chemically Mediated Interaction Between Plants and Other Organisims. Plenum; New York, NY, USA: 1985. Chemistry based resistence in plants. [Google Scholar]
- 236.Legar C.L., Kadiri-Hassani N., Descomps B. Decreased superoxide anion production in cultured human promonocyte cells (THP-l) due to polyphenol mixtures from olive oil processing wastewaters. J.Agric. Food Chem. 2000;48:5061–5067. doi: 10.1021/jf991349c. [DOI] [PubMed] [Google Scholar]
- 237.González M.D., Moreno E., Quevedo-Sarmiento J., Ramos-Cormenzana A. Studies on antibacterial activity of wastewaters from olive oil mills (alpechin): Inhibitory activity of phenolic and fatty acids. Chemosphere. 1990;20:423–432. [Google Scholar]
- 238.Moreno R., Benitez E., Melgar R., Polo A., Gomez M., Nogales R. Vermicomposting as an alternative for reusing by-products from the olive oil industry. Fresen. Environ. Bull. 2000;9:1–8. [Google Scholar]
- 239.Perez J., Delarubia T., Moreno J., Martinez J. phenolic content and antibacterial activity of olive oil wastewaters. Environ. Toxicol. Chem. 1992;11:489–495. [Google Scholar]
- 240.Capasso R., Evidente A, Schivo L., Orru G., Marcialis M.A., Cristinizo G. Antibacterial polyphenols from olive oil mill wastewaters. J. Appl. Bacteriol. 1995;79:393–398. doi: 10.1111/j.1365-2672.1995.tb03153.x. [DOI] [PubMed] [Google Scholar]
- 241.Fki I., Allouche N., Sayadi S. The use of polyphenolic extract, purified hydroxytyrosol and 3,4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: A potential alternative to synthetic antioxidants. Food Chem. 2005;93:197–204. [Google Scholar]
- 242.Heredia A., Guillén R., Fernández-Bolaños J., Rivas M. Olives stone as a source of fermentable sugars. Biomass. 1987;14:143–148. [Google Scholar]
- 243.Najar-Souissi S., Ouedereni A., Ratel A. Adsorption of dyes onto activated carbon prepared from olive stones. J. Environ. Sci. China. 2005;17:998–1003. [PubMed] [Google Scholar]
- 244.Budinova T., Petrov N., Razvigorova M., Parra J., Galiatsatou P. Removal of arsenic(III) from aqueous solution by activated carbons prepared from solvent extracted olive pulp and olive stones. Ind. Eng. Chem. Res. 2006;45:1896–1901. [Google Scholar]
- 245.Ghazy S.E., Samra S.E., May A.E.M., El-Morsy S.M. Removal of aluminium from some water samples by sorptive-flotation using powdered modified activated carbon as a sorbent and oleic acid as a surfactant. Anal. Sci. 2006;22:377–382. doi: 10.2116/analsci.22.377. [DOI] [PubMed] [Google Scholar]
- 246.Montane D., Salvado J., Torras C., Farriol X. High-temperature dilute acid hydrolysis of olive stone for furfural production. Biomass Bioenerg. 2001;22:295–304. [Google Scholar]
- 247.Siracusa G., La Rosa A.D., Siracusa V., Trovato M. Eco Compatible use of olive huso as filler in thermoplastic composites. J. Polym. Environ. 2001;9:157–161. [Google Scholar]
- 248.Rodríguez G., Lama A., Rodríguez R., Jiménez A., Guillén R., Pages J.F.B. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008;99:5261–5269. doi: 10.1016/j.biortech.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 249.Carraro L., Trocino A., Xiccato G. Dietary supplementation with olive stone meal in growing rabbits. Ital. J. Anim. Sci. 2005;4:88–90. [Google Scholar]
- 250.Tejeda-Ricardez J., Vaca-Garcia C., Borredon M.E. Design of a batch solvolytic liquefaction reactor for the vaporization of residues from the agricultural foodstuff. Chem. Eng. Res. 2003;81:1066–1070. [Google Scholar]
- 251.Pérez-Bonilla M., Salido S., van Beek T.A., Linares-Palomino P.J., Altarejos J., Nogueras M. Isolation and identification of radical scavengers in olive tree (Olea europaea) wood. J. Chromatogr. A. 2006;111:311–318. doi: 10.1016/j.chroma.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 252.Zbidi H., Salido S., Altarejos J., Pérez-Bonilla M., Bartegi A., Rosado J.A. Olive tree wood phenolic compounds with human platelet antiaggregant properties. Blood Cell Mol. Dis. 2009;42:279–285. doi: 10.1016/j.bcmd.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 253.Pérez-Bonilla M., Salido S., Teris A.V.B., Waard P.D., Linares-Palomino P.J., Sánchez A., Altarejos J. Isolation of antioxidative secoiridoids from olive wood (Olea europaea L.) guided by on-line HPLC-DAD-radical scavenging detection. Food Chem. 2011;124:36–41. [Google Scholar]
- 254.Patumi M., D’andria R., Marsilio V., Fontanazza G., Morelli G., Lanza B. Olive and olive oil quality after intensive monocone olive growing (Olea europaea L., cv. Kalmata) in different irrigation regimes. Food Chem. 2002;77:27–34. [Google Scholar]
- 255.Tovar M.J., Romero M.P., Alegre S., Girona J., Motilva M.J. Composition and organoleptic characteristics of oil from Arbequina olive (Olea europaea L.) trees under deficit irrigation. J. Sci. Food Agric. 2002;82:1755–1763. [Google Scholar]
- 256.Morelló J.R., Vuorela S., Romero M.P., Motilva M.J., Heinonen M. Antioxidant activity of olive pulp and olive oil phenolic compounds of the Arbequina cultivar. J. Agric. Food Chem. 2005;53:2002–2008. doi: 10.1021/jf048386a. [DOI] [PubMed] [Google Scholar]
- 257.Kevin D., Rade D., Trucelj D., Mokrovãak Î., Nederal S., Benãiç D. The influence of variety and harvest time on the bitterness and phenolic compounds of olive oil. Eur. J. Lipid Sci. Technol. 2003;105:536–541. [Google Scholar]
- 258.Garcia A., Brenes M., Garcia P., Romero C., Garrido A. Phenolic content of commercial olive oils. Eur. Food Res. Technol. 2003;216:520–525. [Google Scholar]
- 259.Gómez-Rico A., Fregapane G., Salvador M.D. Effect of cultivar and ripening on minor components in Spanish olive fruits and their corresponding virgin olive oils. Food Res. Int. 2008;41:433–440. [Google Scholar]
- 260.Esti M., Cinquanta L., La Notte E. Phenolic compounds in different olive varieties. J. Agric. Food Chem. 1998;46:32–35. doi: 10.1021/jf970391+. [DOI] [PubMed] [Google Scholar]
- 261.Gutiérrez F., Jímenez B., Ruíz A., Albi M.A. Effect of olive ripeness on the oxidative stability of virgin olive oil extracted from the varieties Picual and Hojiblanca and on the different components involved. J. Agric. Food Chem. 1999;47:121–127. doi: 10.1021/jf980684i. [DOI] [PubMed] [Google Scholar]
- 262.Ryan D., Robards K., Lavee S. Changes in phenolic content of olive during maturation. J. Food Sci. Technol. 1999;34:265–274. [Google Scholar]
- 263.Cimato A., Mattei A., Osti M. Variation of polyphenol composition with harvesting period. Acta Hortic. 1990;286:453–456. [Google Scholar]
- 264.Mraicha F., Ksantini M., Zouch O., Ayadi M., Sayadi S., Bouaziz M. Effect of olive fruit fly infestation on the quality of olive oil from Chemlali 3 cultivar during ripening. Food Res. Int. 2008;41:433–440. doi: 10.1016/j.fct.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 265.Amiot M.J., Fleuriet A., Macheix J.J. Accumulation of oleuropein derivatives during olive maturation. Phytochemistry. 1989;28:67–69. [Google Scholar]
- 266.Beltrán G., Sanchez S., Martinez L. Influence of fruit ripening process on the natural antioxidant content of Hojiblanca virgin olive oils. Food Chem. 2005;89:207–215. [Google Scholar]
- 267.Bouaziz M., Chamkha M., Sayadi S. Comparative study on phenolic content and antioxidant activity during maturation of the olive cultivar chemlali from Tunisia. J. Agric. Food Chem. 2004;52:5476–5481. doi: 10.1021/jf0497004. [DOI] [PubMed] [Google Scholar]
- 268.Garcia A., Brenes M., Romero C., Garcia P., Garrido A. Study of phenolic compounds in virgin olive oils of the Picual variety. Eur. Food Res. Technol. 2002;5:407–412. [Google Scholar]
- 269.Kotsifaki A.K.F., Stefanoudaki E. Effect of extraction system, stage of ripeness and kneading temperature on the sterol composition of virgin olive oil. J. Am. Oil Chem. Soc. 1999;76:1477–1481. [Google Scholar]
- 270.Morelloa J.R., Romero M.P., Motilva M.J. Effect of the maturation process of the olive fruit on the phenolic fraction of drupes and oils from arbequina, farga, and morrut cultivars. J. Agric. Food Chem. 2004;52:6002–6009. doi: 10.1021/jf035300p. [DOI] [PubMed] [Google Scholar]
- 271.Rotondi A., Bendni A., Cerretani L., Mari M., Lercker G., Toschi T.G. Effect of olive ripening degree on the oxidative stability and organoleptic properties of Cv. Nostrana di Brisighella extra virgin olive oil. Agric. Food Chem. 2004;52:3649–3654. doi: 10.1021/jf049845a. [DOI] [PubMed] [Google Scholar]
- 272.Rovellini P., Cortesi N. Determination of phenolic compounds in different cultivars during olive drupe ripening by liquid chromatography-mass spectrometry. Olive. 2003;95:32–38. [Google Scholar]
- 273.Salvador M., Aranda F., Fregapane G. Influence of fruit ripening on “Cornicabra” virgin olive oil quality. A study of four successive crop seasons. Food Chem. 2001;73:45–53. [Google Scholar]
- 274.Yousfi K., Cert R.M., Garcıa J.M. Changes in quality and phenolic compounds of virgin olive oils during objectively described fruit maturation. Eur. Food Res. Technol. 2006;223:117–124. [Google Scholar]
- 275.Moriana A., Orgaz F., Fereres E, Pastor M. Yield responses of a mature olive orchard to water deficits. J. Am. Soc. Hortic. Sci. 2003;128:425–431. [Google Scholar]
- 276.Gomez-Rico A., Desamparados Salvador M., Moriana A., Perez D., Olmedilla N., Ribas F., Fregapane G. Influence of different irrigation strategies in a traditional Cornicabra cv. olive orchard on virgin olive oil composition and quality. Food Chem. 2007;100:568–578. [Google Scholar]
- 277.Gómez-Rico A., Salvador M.D., La Greca M., Fregapane G. Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. Cv. Cornicabra) with regard to fruit ripening and irrigation management. J. Agric. Food Chem. 2006;54:7130–7136. doi: 10.1021/jf060798r. [DOI] [PubMed] [Google Scholar]
- 278.Artajo L.S., Romero M.P., Tovar M.J., Motilva M.J. Effect of irrigation applied to olive trees (Olea europaea L.) on phenolic compound transfer during olive oil extraction. Eur. J. Lipid Sci. Technol. 2006;108:19–27. [Google Scholar]
- 279.Berenguer M.J., Vossen P.M., Grattan R.S., Connell J.H., Polito V.S. Tree irrigation levels for optimum chemical and sensory properties of olive oil. HortScience. 2006;41:427–432. [Google Scholar]
- 280.Wiesman Z., Itzhak D., Dom N.B. Optimization of saline water level for sustainable Barnea olive and oil production in desert conditions. Sci. Hort. 2004;100:257–266. [Google Scholar]
- 281.Gharsallaoui M., Ben Amar F., Khabou W., Ayadi M. Valorisation des ressources en eau non conventionnelles au Sud Tunisien par la culture de l’olivier (Olea europaea L.). Meeting International: Gestion des Ressources et Application Biotechnologiques en Aridoculture et Culture Sahariennes: Perspectives pour la valorisation des potentialités du Sahara; Djerba, Tunisia. 25–28 December 2006; Djerba, Tunisia: Institue des Régions Arides; 2006. [Google Scholar]
- 282.Palese A.M., Celano G., Masi S., Xiloyannis C. Olivebioteq. Mazara del Vallo; Marsala, Italy: 2006. Treated wastewater for irrigation of olive trees: Effects on yield and oil quality; pp. 123–129. [Google Scholar]
- 283.European Community. Council Regulation (EC) No. 1513/2001 of 23 July 2001 Amending Regulations No. 136/66/EEC and (EC) No 1638/98 as regards the extension of the period of validity of the aid scheme and the quality strategy for olive oil. Off. J. Eur. Commun. 2001;L201:4–7. [Google Scholar]
- 284.Capella P., Fedeli E., Bonaga G., Lerker G. Manuale degli Oli e dei Grassi. Tecniche Nuove; Milano, Italy: 1997. [Google Scholar]
- 285.Servili M., Baldioli M., Montedoro G.F. Phenolic composition of virgin olive oil in relationship to some chemical and physical aspects of malaxation. Acta Hortic. 1994;356:331–336. [Google Scholar]
- 286.Veillet S., Tomao V., Bornard I., Ruiz K., Chemat F. Chemical changes in virgin olive oils as a function of crushing systems: Stone mill and hammer crusher. C.R. Chim. 2009;12:895–904. [Google Scholar]
- 287.Caponio F., Gomes T. Influence of olive crushing temperature on phenols in olive oils. Eur. Food Res. Technol. 2001;212:156–159. [Google Scholar]
- 288.Campeol E., Flamini G., Chericoni S., Catalano S., Cremonini R. Volatile compounds from three cultivars of Olea europaea from Italy. J. Agric. Food Chem. 2001;49:5409–5411. doi: 10.1021/jf010455n. [DOI] [PubMed] [Google Scholar]
- 289.Servili M., Selvaggini R., Taticchi A., Esposto S., Montedoro G.F. Volatile compounds and phenolic composition of virgin olive oil: Optimization of temperature and time of exposure of olive paste to air contact during the mechanical extraction process. J. Agric. Food Chem. 2003;51:7980–7988. doi: 10.1021/jf034804k. [DOI] [PubMed] [Google Scholar]
- 290.Kachouri F., Hamdi M. Use of Lactobacillus plantarum in olive oil process and improvement of phenolic compounds content. J. Food Eng. 2006;77:746–752. [Google Scholar]
- 291.Vierhuis E., Servili M., Baldioli M., Schols H.A., Voragen A.G.J., Montedoro G. Effect of enzyme treatment during mechanical extraction of olive oil on phenolic compounds and polysaccharides. J. Agric. Food Chem. 2001;49:1218–1223. doi: 10.1021/jf000578s. [DOI] [PubMed] [Google Scholar]
- 292.Faveri D.D., Aliakbariana B., Avogadroa M., Peregoa P., Converti A. Improvement of olive oil phenolics content by means of enzyme formulations: Effect of different enzyme activities and levels. Chem. Eng. J. 2008;41:149–156. [Google Scholar]
- 293.Aliakbarian B., Faveri D.D., Converti A., Perego P. Optimisation of olive oil extraction by means of enzyme processing aids using response surface methodology. Chem. Eng. J. 2008;42:34–40. [Google Scholar]
- 294.Morello J.R., Motilva M.J., Tovar M.J., Romero M.P. Changes in commercial virgin olive oil (cv. Arbequina) during storage, with special emphasis on the phenolic fraction. Food Chem. 2004;85:357–364. [Google Scholar]
- 295.Stefanoudaki E., Koutsaftakis A., Kotsifaki F., Angerosa F., DiGirolamo M. Quality characteristics of olive oils of dual- and three-phase decanters and laboratory mill. Acta Hortic. 1999;474:705–708. [Google Scholar]
- 296.Giovacchino L.D., Solinas M., Miccoli M. Effect of extraction systems on the quality of virgin olive oil. J. Am. Oil Chem. Soc. 1994;71:1189–1194. [Google Scholar]
- 297.Cert A., Alba J., León-Camacho M., Moreda W., Pérez-Camino M.C. Effects of talc addition and operating mode on the quality and oxidative stability of virgin olive oils obtained by centrifugation. J. Agric. Food Chem. 1996;44:3930–3934. [Google Scholar]
- 298.Galli C., Visioli F. Antioxidant and other properties of phenolics in olives/olive oil, typical compounds of the mediterranean diet. Lipids. 1999;34:S23–S26. doi: 10.1007/BF02562224. [DOI] [PubMed] [Google Scholar]
- 299.Manna C., Galletti P., Cucciolla V., Montedoro G., Zappia V. Olive oil hydroxytyrosol protects human erythrocytes against oxidative damages. J. Nutr. Biochem. 1999;10:159–165. doi: 10.1016/s0955-2863(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 300.Lozano-Sánchez J., Segura-Carretero A., Fernández-Gutiérrez A. Characterisation of the phenolic compounds retained in different organic and inorganic filter aids used for filtration of extra virgin olive oil. Food Chem. 2011;124:1146–1150. [Google Scholar]
- 301.Fregapane G., Lavelli V., León S., Kapuralin J., Salvador M.D. Effect of filtration on virgin olive oil stability during storage. Eur. J. Lipid Sci. Technol. 2006;108:134–142. [Google Scholar]
- 302.Agalias A., Magiatis P., Skaltsounis A., Mikros E., Tsarbopoulos A., Gikas E. A new process for the management of olive oil mill waste water and recovery of natural antioxidants. J. Agirc. Food Chem. 2007;55:2671–2676. doi: 10.1021/jf063091d. [DOI] [PubMed] [Google Scholar]
- 303.Gortzi O., Lalas S., Chatzilazarou A., Katsoyannos E., Papaconstandinou S., Dourtoglou E. Recovery of natural antioxidants from olive mill wastewater using Genapol-X080. J. Am. Oil Chem. Soc. 2008;85:133–140. [Google Scholar]
- 304.Paraskeva C.A., Papadakis V.G., Kanellopoulou D.G., Koutsoukos P.G., Angelopoulos K.C. Membrane filtration of olive mill wastewater and exploitation of its fractions. Water Environ. Res. 2007;79:421–429. doi: 10.2175/106143006x115345. [DOI] [PubMed] [Google Scholar]
- 305.Roig A., Cayuela M.L., Sanchez-Monedero M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006;26:960–969. doi: 10.1016/j.wasman.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 306.Arsuaga J.M., López-Muñoz M.J., Sotto A. Correlation between retention and adsorption of phenolic compounds in nanofiltration membranes. Desalination. 2010;250:829–832. [Google Scholar]
- 307.Lafka T.-I., Lazou A.E., Sinanoglou V.J., Lazos E.S. Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 2011;125:92–98. [Google Scholar]
- 308.Schieber A., Stintzing F.C., Carle R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001;12:401–413. [Google Scholar]