Biological, Clinical, and Psychosocial Correlates at the Interface of Cancer and Aging Research (original) (raw)
Abstract
In September 2010, the Cancer and Aging Research Group, in collaboration with the National Cancer Institute and the National Institute on Aging, conducted the first of three planned conferences to discuss research methodology to generate the highest quality research in older adults with cancer and then disseminate these findings among those working in the fields of cancer and aging. Conference speakers discussed the current level of research evidence in geriatric oncology, outlined the current knowledge gaps, and put forth principles for research designs and strategies that would address these gaps within the next 10 years. It was agreed that future oncology research trials that enroll older adults should include: 1) improved standardized geriatric assessment of older oncology patients, 2) substantially enhanced biological assessment of older oncology patients, 3) specific trials for the most vulnerable and/or those older than 75 years, and 4) research infrastructure that specifically targets older adults and substantially strengthened geriatrics and oncology research collaborations. This initial conference laid the foundation for the next two meetings, which will address the research designs and collaborations needed to enhance therapeutic and intervention trials in older adults with cancer.
Cancer is predominantly a disease of older adults; yet, there is a persisting misalignment between the age distribution of the general cancer population and the age distribution of the participants in clinical trials. The number of cancer patients older than 65 years is projected to increase substantially over the next 20 years (1). Although the majority of cancer patients are older than 65 years, only one-third of all participants in National Cancer Institute (NCI) trials fall into that age group (2). Thus, there is little evidence-based data from which to tailor appropriate treatments at a time when the number of older cancer patients is growing rapidly. The need to expand evidence-based research in older adults with cancer inspired the formation of the Cancer and Aging Research Group (CARG) (3), a coalition of investigators dedicated to linking geriatric oncology researchers, designing and implementing clinical trials in older adults and supporting the development of geriatric oncologists. In 2010, in collaboration with the NCI and the National Institute on Aging (NIA), CARG received a U13 grant (U13 AG038151) to conduct and disseminate findings from three conferences over 5 years on “Geriatric Oncology Research to Improve Clinical Care.” The first conference, “Clinical, Psychosocial, and Biological Correlates at the Interface of Aging and Cancer Research,” was held September 25–26, 2010, in Chicago, IL, in collaboration with the 2010 Cancer and Leukemia Group B (CALGB) committee meeting.
Conference Goals, Attendees, and Design
The conference goals, as stated in the U13 grant application, were to “identify the clinical, psychosocial, and biological factors necessary for high-quality research in older adults with cancer and to disseminate these recommendations to researchers across disciplines who work at the interface of cancer and aging research.” The oversight board for the conference grant includes senior-level representatives from the NIA, NCI, American Society of Clinical Oncology (ASCO), American Geriatrics Society (AGS), CALGB, International Society of Geriatric Oncology (SIOG), American Association for Cancer Research (AACR), and CARG (see Appendix for full list of attendees).
By design, the conference was limited to 50 recognized leaders in cancer and aging research. At the broadest level, this group included researchers trained in oncology and/or hematology (N = 24), geriatrics and/or aging (N = 15), or geriatric oncology (N = 15). Within these broader categories, there was specific research expertise in psychiatry, nursing, behavioral science, basic science, internal medicine, palliative medicine, and radiation oncology. There were also representatives from professional (eg, ASCO, AGS, SIOG, AACR) and government (eg, NCI, NIA) organizations. Included in this group were both senior-level (N = 40) and junior-level (N = 10) investigators, each of whom had an assigned participatory role in the conference.
The content of and speakers for the conference were selected by the U13 grant principal investigators, Dr Hurria, Dr Dale, and Dr Mohile, in collaboration with the oversight board. Speakers were asked to limit their presentations to 20 minutes, with an emphasis on identifying the knowledge gaps in geriatric oncology and proposing research methods and strategies to fill these gaps. Structured discussions followed the formal presentations. The full conference was audio recorded (audio recordings and accompanying slide shows are available on the CARG website at http://www.mycarg.org/home). Following the conference, the presentations were transcribed and reviewed by the principal investigators (A. Hurria, S. G. Mohile, W. Dale) to identify the main themes and the specific recommendations from this first conference. These recommendations were synthesized in collaboration with the advisory board and are summarized in this commentary.
Previous Research
What Is Known
Conference panelists with a wide spectrum of expertise summarized the current level of evidence in geriatric oncology research. Table 1 highlights a sample of clinical trials that focused on older patients with cancer. Several themes that emerged centered on study design and the level of evidence in geriatric oncology (Table 2). First, few clinical trials have been designed specifically for older adults with cancer. More commonly, evidence regarding the treatment of older adults emerges from studies that have enrolled patients of all ages. Notably, the number of older adults enrolled in standard clinical trials rarely reflects the proportion of older patients with the disease in the general population. Second, measures of functional or physiological age, which are routinely captured in geriatric assessment, are rarely included in study designs. Thus, it is difficult to know if the results of these studies can be extrapolated to the general geriatric oncology population.
Table 1.
A selection of clinical trials that included older patients with cancer*
| Cancer | First author, year (reference) | n | Age (y), other characteristics | Therapy | Functional assessment | Toxicity | Results |
|---|---|---|---|---|---|---|---|
| Breast | Hughes, 2004 (4) | 636 | ≥70 y, ER+, lumpectomy | Tamoxifen alone vs tamoxifen + radiation | None indicated | No statistically significant differences between treatments at 4 y of follow-up | Reduced recurrence in tamoxifen + radiation; no difference in survival or mastectomy |
| Breast | Muss, 2009 (5) | 600 | ≥65 y | Standard chemotherapy vs capecitabine | NCI PS 0–2 eligible | One or more grade 3 or 4 AE 60%–70% (standard chemotherapy) vs 34% (capecitabine) | Patients on capecitabine had twofold higher risk of recurrence or mortality |
| Lung | Hensing, 2003 (6) | 230; 67; 163 | Stage IIIB or IV NSCLC; ≥70 y; <70 y | Carboplatin and paclitaxel | KPS ≥ 70% | No difference in most common toxicities | No difference in survival or quality of life between ≥70 y and <70 y |
| Lung | Hesketh, 2007 (7) | 48; 89 | Stage IIIB or IV NSCLC; ≥80 y; <80 y | Vinorelbine and docetaxel or docetaxel | ECOG PS 0–2 eligible | Treatment well tolerated | Median survival: 7 mo for ≥80 y vs 11 mo for <80 y |
| Colorectal | D’Andre, 2005 (8) | 1748 | Age quartiles | 5-fluorouracil | None included | Patients older than 65 y had higher rates of severe toxicity | Response did not differ by age |
| Colorectal | Goldberg, 2006 (9) | 614; 3128 | ≥70 y; <70 y | Oxaliplatin plus fluorouracil or leucovorin (FOLFOX4) | ECOG PS 0–2 in two of four studies | Grade 3 or higher hematological toxicity and thrombocytopenia higher in older patients | All measures of benefits equal between groups |
| AML | Mayer, 1994 (10) | 346; 742 | ≥60 y; <60 y | Daunorubicin and cytarabine | None indicated | Only 29% of patients ≥60 y could tolerate high-dose cytarabine vs 62% of those <60 y | Complete remission after 4 y: <16% for patients ≥60 y vs >24% for patients <60 y |
| AML | Harousseau, 2009 (11) | 457; 110 | ≥70 y; ≥80 y | Tipifarnib vs best supportive care | ECOG PS 0–2 | 90% of patients treated with tipifarnib had one or more AE vs 75% of patients receiving best supportive care | 8% CR for tipifarnib, not statistically significantly different from best supportive care |
| Prostate | Italiano, 2009 (12) | 175 | ≥75 y | Castration-resistant cancer treated with docetaxel | ECOG PS, comorbidity count | 46% had grade 3 or 4 AE; toxicity risk correlated with poor PS | Median progression-free survival = 7.4 mo and OS = 15 mo |
| Prostate | Berthold, 2008 (13) | 1006 | Men with metastatic hormone-resistant prostate cancer | Docetaxel every 3 wk, weekly docetaxel, or mitoxantrone | KPS ≥ 70% | ND | Similar trends in survival between treatment arms were seen for men >65 y vs ≤65 y |
| Prostate | D’Amico, 2008 (14) | 206 | Localized, but unfavorable risk of prostate cancer (median age 72.5 y) | Radiation alone vs radiation + androgen suppression therapy | ECOG PS (0 or 1); ACE-27 for comorbidities | NA | Radiation + androgen suppression therapy improved survival only in men without moderate or severe comorbidity |
| Ovarian | McLean, 2010 (15) | 175; 34 | 65–79 y; ≥80 y | Neoadjuvant chemotherapy vs primary cytoreduction (chart review) | NA | No difference between ≥80 y and 65–79 y in frequency of surgery or chemotherapy complications | No difference in OS by treatment; median survival: 35 mo for 65–79 y vs 24 mo for ≥80 y |
Table 2.
Geriatric assessment domains, findings from the geriatrics and oncology literature, and remaining research gaps
| Comprehensive Geriatric Assessment (CGA) domain | Finding from geriatrics literature (reference) | Finding from oncology literature (reference) | Research gaps |
|---|---|---|---|
| Function | Low function predicts hospitalizations, institutionalization, and mortality (16) | Function does not necessarily correlate with comorbidity (17) | Which specific functional measures before treatment best predict toxicity to chemotherapy? |
| Pretreatment function predicts chemotherapy toxicity and survival (18) | How can we develop interventions to improve outcomes in patients with functional impairment? | ||
| A history of cancer is associated with lower function in older patients (19) | How do specific cancers and treatment regimens influence long-term functional outcomes? | ||
| Comorbidity | Higher comorbidity predicts hospitalizations, institutionalization, and mortality (20) | Comorbidity is associated with lower likelihood of undergoing diagnosis and treatment for cancer (21) | How do specific comorbidities and their interactions influence cancer treatment toxicity, cancer outcomes, and survivorship? |
| What is the number and severity of comorbidities among patients newly diagnosed with cancer compared with the general population of older adults? | |||
| How does the number and severity of comorbidities and their interactions change over the course of cancer therapy? | |||
| Pretreatment comorbidity is associated with mortality in cancer patients (22) | How do we estimate life expectancy in older patients with cancer and comorbidities, with or without cancer treatment? | ||
| How do we develop and successfully complete clinical trials for patients with comorbidities? | |||
| Polypharmacy | Greater numbers of medications leads to higher numbers of adverse events in community-dwelling and hospitalized older patients (23) | Cancer therapy adds to risks associated with adverse drug events in older patients (24) | How can we identify polypharmacy and measure the risk of adverse events due to drug interactions, including chemotherapy, in older cancer patients?What are the unique drug interactions in older cancer patients? |
| How do we tailor medication lists to best reduce risk of adverse drug reactions in older patients receiving treatment for cancer? | |||
| Cognition | Cognitive impairment and dementia are common and under-recognized in older patients, especially those aged ≥80 y (25) | Patients with dementia are less likely to undergo treatment for cancer and have worse survival (26) | How should we assess cognition and decision-making capacity for oncology clinical trials? |
| Cognitive impairment leads to adverse outcomes, including delirium, hospitalizations, and mortality (27) | Cancer treatment may affect cognition (28) | What are the side effects of cancer treatment in older patients with cognitive impairment? | |
| Psychological status | Depression affects 25% of medically ill older adults and is associated with poor health outcomes (29) | Depression is under-recognized in older patients with cancer (30) | How does depression or anxiety affect treatment choices and compliance in cancer patients? |
| Anxiety can affect treatment choices in older patients (31) | Are there interventions that could help patients and caregivers with depression or anxiety? | ||
| Nutrition | Weight loss is a marker of frailty in older persons (32) | Older patients with cancer have a higher prevalence of nutrition problems and weight loss than those without cancer (33) | How does nutritional status and weight loss affect prognosis in older patients?Are there supportive care interventions that could help mediate nutritional problems in older patients with cancer? |
| Social support | Lack of social support predicts institutionalization and mortality in community-dwelling older adults (34) | Lack of social support is an important barrier to enrollment of older adults onto clinical trials (35) | How does social support relate to treatment practice patterns in older adults with cancer?Can infrastructure be strengthened to address social support issues of older cancer patients? |
Given that the majority of cancers are diagnosed in adults aged 65 years or older, a rationale for formulating high-quality studies in older adults with cancer was discussed. Many studies simply show a difference in treatment patterns between older and younger cancer patients without identifying the reasons for these differences. Also, many studies demonstrate that older adults enrolled in cancer clinical trials derive similar treatment-related benefits as younger adults, but the older adults are at increased risk for treatment-related toxicities. Furthermore, few studies have measured specific age-associated characteristics that identify older adults who are at highest risk for toxic effects and poor outcomes. Finally, although it is possible to perform high-quality clinical trials of fit older adults in the cooperative group setting, few studies focus on enrolling vulnerable and/or frail older adults and those older than 75 years.
Research Gaps
Older patients who are enrolled in clinical trials typically have a high performance status, very few comorbidities, no functional losses, and well-preserved organ function, thereby limiting the generalizability of the findings to the majority of older adults who have cancer. More trials that specifically target older adults are needed to address the physiological differences between older and younger patients, differences that can affect both cancer biology and response to therapy. There is also a need to enroll more older patients into ongoing clinical trials for patients of all ages, as well as to develop tailored trials for vulnerable older adults with underlying deficits identified by geriatric assessment tools. To assess less fit patients and safely enroll them in such trials, we need to know how to build the necessary infrastructure to support them. Ways to fill these gaps were discussed, and resulting recommendations follow.
Addressing the Existing Gaps
Gap 1: Clinical Measures Most Relevant to Older Adults Are Rarely Incorporated Into Oncology Clinical Trials
We presume, largely on the basis of traditional measures of performance status (such as the Karnofsky score), that older patients who are typically enrolled in clinical trials are more “fit,” that is, not especially vulnerable to toxic side effects or poor outcomes. However, most oncology trial designs include few measures from geriatric assessment domains (eg, comorbidity, detailed functional status, cognition) (36). Most patients who are aged 65 years or older when diagnosed with cancer have multiple comorbidities, functional losses, geriatric syndromes, and/or physical disabilities (36). One study showed that these factors affect 74% of patients with breast cancer, 88% of patients with prostate cancer, and 86% of patients with colorectal cancer (37). An Italian study found that geriatric assessment provides prognostic information in addition to standard performance assessment in older patients with cancer (38). However, few studies measure these geriatric domains, despite the ability of such assessments to add important prognostic information (39).
Standardized geriatric assessment tools can help determine a patient's eligibility for cancer trials, choice of treatment, and underlying vulnerability to treatment-related toxicities. Incorporating such an assessment would improve the design of clinical trials and allow entry criteria to be individualized beyond the use of age or performance status cutoffs. Comprehensive standardized assessments would also allow investigators to analyze how more vulnerable patients respond to therapy, which may help to further refine the risk factors that are most important to consider when making treatment decisions for older cancer patients or stratifying such patients for clinical trial enrollment.
A variety of geriatric assessment measures have recently been assessed for their ability to predict toxic effects of chemotherapy. For example, investigators from the CARG group developed a risk stratification schema for chemotherapy toxicities. Factors found to predict the risk of chemotherapy toxicities included age, tumor and treatment characteristics (ie, cancer type, receipt of standard treatment dose, and use of polychemotherapy), specific laboratory values (ie, lower hemoglobin level, worse creatinine clearance), and geriatric assessment variables (ie, any hearing impairment, a fall in the previous 6 months, difficulty in walking one block, need for assistance with taking medications, and decreased social activities) (40). A scoring system based on these factors was developed to identify patients who are at low, intermediate, or high risk of chemotherapy toxicities. In addition, Extermann et al. (41) developed the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score, which predicts the likelihood of chemotherapy toxicity using measures such as albumin levels, blood pressure, the need for assistance with instrumental activities of daily living, reduced cognitive status, poor nutrition status, and abnormal performance status. However, additional research is needed to validate and generalize the use of these tools in older patients with different tumor types, receiving various treatment regimens, and in different care settings.
Filling Gap 1: Incorporation of Clinical Geriatric Assessment Into Oncology Research
Clinical trials that are designed to enroll substantial numbers of older adults with cancer should routinely include some form of validated geriatric assessment. Traditionally, a geriatric assessment includes (at least) measures of functional status, comorbidities, cognition, psychological state, social support, and nutritional status. Previous research has demonstrated that it is feasible to include a geriatric assessment in the cooperative group setting (42). However, the technology needed to capture these geriatric assessment data must be identified and evaluated for use outside academic medical centers. Close collaboration between oncologists and geriatric oncologists or geriatricians is necessary when designing clinical trials that involve older adults to ensure that geriatric principles and assessments are considered in oncology trial design. More research needs to be done regarding the utility of geriatric assessments in various tumor types and when using therapies with different toxic effects, both for toxicity prediction and for more appropriately measuring outcomes in older adults. Table 2 outlines questions that a geriatric assessment would help to answer.
Although mortality reduction is important for patients of all ages, for older patients, additional endpoints, such as the ability to live independently and with a high quality of life, may be equally (or more) important. Thus, the impact of cancer and its treatments on patients’ abilities to maintain function and independence should be more thoroughly investigated. Such data could be used to facilitate treatment discussions with older patients, as well as to inform decision making for patients who are faced with different treatment options. Findings from such studies would also allow researchers to incorporate age-appropriate health outcomes into studies of older adults and help guide care for older patients.
The relationship between cancer treatments and mental health and/or cognitive changes is an important concern for older patients, yet most existing data are based on small sample sizes. Larger studies are needed to assess the incidence of depression, anxiety, and stress in older patients with cancer, as well as risk factors that predict how treatment will affect these conditions and vice versa. Information about these psychological symptoms could be collected by using “patient-reported” methods such as in-home technology (such as smart phones) and web-based surveys. Validated measures assessing cognitive changes during and after treatment are another area ripe for additional research. Consideration should be given to implementing studies in parallel with standard phase III trials to measure the impact of treatment on physical function, cognition, and quality of life in older study participants.
Gap 2: Biological and Physiological Markers of Aging Are Inconsistently Incorporated in Oncology Research
Aging brings biological changes in tumor characteristics, as well as potential physiological changes in the “host organism” (ie, the patient). In both men and women, parameters such as body weight, grip strength, and aerobic capacity decline with age, leading to frailty, increased disease susceptibility, reduced healing, and other conditions of vulnerability (43). Aging is also marked by an increase in the circulating levels of several inflammatory factors, including the interleukins, C-reactive protein, and fibrinogen (44). For example, increased plasma levels of interleukin 6 were found to predict mobility deficits and worsened activities of daily living in people aged 71 years or older (45).
A biological link appears to exist between aging and cancer, and there are several age-associated molecular changes that contribute to carcinogenesis. In vitro and murine in vivo experiments suggest that a predisposition to cancer in older organisms could result from the combined effects of a high mutation load, poor epigenetic regulation, telomere dysfunction, and altered stromal milieu (46). One specific example of a biological link between cancer and aging is the theory of antagonistic pleiotropy, the hypothesis that evolutionarily selected biological functions benefit younger individuals at the expense of older ones (47). For example, cellular senescence is a defense mechanism against the unregulated cellular proliferation underlying carcinogenesis in younger individuals, whereas widespread senescence and the subsequent release of inflammatory factors possibly play a role in several diseases of older individuals, including cancer (48). Another possible biological link between cancer and aging is the proliferation of dysfunctional telomeres in both older individuals and those individuals with cancer (49). The reverse may also be true: it is possible that cancer and/or its treatments may lead to an acceleration of the aging process at the cellular level (50). Further research is needed to elucidate the causal relationships behind the identified associations.
Chemotherapy toxicity may also be influenced by age-related alterations in the pharmacokinetics and pharmacodynamics of cancer therapy (Table 1). For example, older adults with serum levels of creatinine in the “normal” range may in fact have a decreased glomerular filtration rate (51), which could influence chemotherapy toxicity. A small number of studies have examined changes in the toxicity of anticancer agents according to age. For example, a CALGB study of the chemotherapeutic agent paclitaxel assessed the pharmacokinetics and toxicities associated with the drug in three cohorts of older patients aged 55–65, 65–75, or older than 75 years and found that older patients cleared the drug more slowly than younger patients (52). However, no statistically significant association between age and clinically important adverse events, including hospitalization for toxicity, the need for intravenous antibiotics, or neutropenic fever, was detected.
Filling Gap 2: Consistent Incorporation of Biological and Physiological Markers of Aging in Oncology Clinical Trials
To assess whether tumor biology changes substantially with age, as has been observed for cytogenetic abnormalities in acute myeloid leukemia (53), a wide range of tumor samples from patients of all ages should be collected and analyzed. The connection between inflammatory factors, aging, and cancer also needs to be clarified, and further research into the association between chronic inflammation and cancer development is needed. In preclinical studies involving model organisms, increased use of animals that are older and more vulnerable to disease is encouraged to simulate the biological status of typical older cancer patients. Similarly, treatment parameters such as body composition for weight-based treatments, pharmacokinetics and pharmacodynamics of therapeutics, and chemotherapy dosing based on organ function are necessary to improve treatment selection and dosing choices for older adults.
Gap 3: Too Few Studies Focus on Frail Older Adults or Those Aged 75 Years or Older
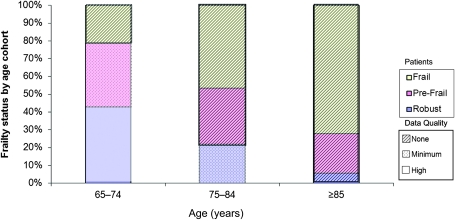
Although older age and frailty—a condition of increased vulnerability to disease and decline—are correlated (54), they are not identical. Some older adults are robust, not prone to develop diseases, and resistant to decline, whereas some older adults are most vulnerable to disease and decline. Unfortunately, there is a paucity of data on cancer treatments in either the frailest adults or those adults older than 75 years, primarily due to low enrollment of both groups in cancer clinical trials (Figure 1). A review of NCI-funded studies found that whereas 3% of cancer patients aged 30–64 years were represented in trials, only 1.3% of those aged 65–74 years and 0.5% of those older than 75 years were represented (2). One likely contributor to this age disparity in trial enrollment is the fact that frailer, older patients are rarely offered the opportunity to participate in trials. For example, half as many eligible women with breast cancer aged 65 years or older are offered a trial compared with women younger than 65 years (55,56). Reasons cited for not offering trial participation to older women include concerns about the number of comorbidities, perceived poor adherence to treatment, treatment toxicities, and straightforward exclusion by eligibility criteria. There is also often the erroneous perception that older patients are less accepting of trials, so they are not offered to these patients; however, when offered the opportunity, older patients are just as likely as younger patients to enroll in a clinical trial (55,56). Additionally, older patients are less likely to present for treatment at sites with access to clinical trials, such as NCI-designated cancer centers (57).
Figure 1.
Percentage of robust (0 of 5 deficits), pre-frail (1–2 of 5 deficits), and frail (3 or more deficits) older adults in three age groups and the quality of the evidence regarding appropriate cancer treatments by age. Based on data from Fried et al. (54).
Filling Gap 3: More Studies That Focus on Frailer Older Adults and/or Those Aged 75 Years or Older
There is a need to develop clinical trials and observational studies that actively enroll this older, more vulnerable population as well as criteria to guide the development of more appropriate trials for these individuals. For less fit older patients who are excluded from standard clinical trials because of frailty, performance status, or comorbidity restrictions, specific trials for an alternative dosing or schedule or enhanced supportive care measures should be designed and conducted. The efficacy and toxicity of treatment approaches in these patients should also be evaluated. Extra safety considerations can be built into the trial design to offset toxicity, address it promptly if it occurs, and maximize safety for this vulnerable population. Rather than focusing primarily on mortality, future studies should include equally important outcomes, such as the efficacy of reduced chemotherapy doses on cancer control as well as the short- and long-term impacts of therapy on functional abilities, cognition, symptom control, and other comorbidities. Inclusion of such outcomes could greatly improve the treatment of older and frailer cancer patients, especially given the paucity of data on the treatment of these patients, who are typically ineligible for standard clinical trials. Another option is to include a “registry” study to capture the decision making and outcomes of patients who do not qualify for a standard clinical trial.
Gap 4: Clinical Trial Infrastructure Incompatibility With Geriatric Needs
Research is needed to assess the barriers to clinical trial enrollment of older adults and to identify programs or trial designs to overcome these barriers. Assessments of needs that are built into the screening process to identify enrollment and treatment barriers—including lack of transportation, inability to get attention if sick, inability to complete daily activities, the need to serve as a caregiver for a dependent spouse, and financial barriers (eg, the cost of medications or for caregiver time)—are virtually never done. Oncology research staff rarely has specific training in these geriatrics issues. When caring for older cancer patients in the trial setting, additional time is not typically allotted to complete written informed consent documents, conduct geriatric assessments, or to manage potential toxicities. Additional infrastructure is rarely present to support participation in a trial for frail, older adults (ie, lowered examination tables, transportation assistance), or nearby housing if treatment or tests span several days.
Filling Gap 4: Incorporate Age-Associated Conditions of Older Adults Into Research Infrastructure
Better tailoring oncology care to meet the needs of older frail patients would almost surely increase enrollment of these patients in clinical trials. With the increased inclusion of older patients in medical research, facilities are more likely to make their clinical environments more convenient, such as providing wider lower examination tables and easier access to assistive devices. Sufficient time is also needed to complete geriatric assessments in clinical trials. Pilot studies are needed to quantify the impact of providing older adults with transportation to large centers on clinical trial enrollment and retention. In addition, developing and expanding community-based trials for older adults would eliminate the need for long distance travel to access clinical trials. Research nurses and data managers should receive specific training in the care of older adults. Data collection from remote locations can also be improved by incorporating advanced technology such as smart phones and tablet computers. Finally, home-monitoring programs that use visits by a nurse or social worker, telephone calls, or internet portals to support the well-being of older adults during cancer therapy are promising and must be explored further (58).
In addition, more consistent and earlier collaboration between oncology and geriatrics researchers is essential. A multidisciplinary team should closely collaborate to design, implement, and execute clinical trials for older adults. Integrating geriatricians and other providers who have geriatric training and experience into an older patient's treatment plan could potentially improve adherence and help minimize the impact of toxicities, particularly if the patient is frailer or has other conditions; measuring the size of that potential benefit alone constitutes a promising research topic. Targeted assistance based on particular comorbidities or other existing conditions is also strongly advised; this is a situation in which multidisciplinary collaboration between oncologists, geriatricians, and others would be enormously beneficial. The inclusion of other health-care providers, including clinical psychologists, social workers, physician extenders, and physical and occupational therapists, especially those with geriatric training, is critical in treating older patients. In short, both multi- and interdisciplinary teams that are led by oncologists and geriatricians are necessary to allow geriatric oncology research to progress.
Summary
Although the cancer community has made enormous strides in cancer treatment in general, several gaps in knowledge remain when it comes to research and treatment for older cancer patients. To address these gaps, future oncology research for older patients with cancer must include: 1) comprehensive clinical geriatric assessments, 2) improved biological assessments, 3) more trials specifically tailored for patients that are frail and older than 75 years, and 4) an enhanced research infrastructure that addresses the issues pertinent to older patients and strengthens collaborations between oncologists, geriatricians, and a multidisciplinary team (Box 1). As a result of this conference, the NCI is working to appoint members with expertise in geriatric oncology to its disease-specific steering committee task forces to address these knowledge gaps.
Box 1. Take-home messages for “Filling the Research Gaps” from the Cancer and Aging Research Group U13 Conference, 2010.
A. Need to consistently incorporate geriatric assessment into oncology research
- Trial design enrolling substantial numbers of older adults with cancer should routinely include validated geriatric assessment.
- Additional endpoints, such as maintenance of functional abilities and quality of survival, should be considered as important as mortality.
- More consistent precise measurement of mental health and/or cognitive changes is needed in trials of older cancer patients, especially those receiving chemotherapy.
B. Need to consistently incorporate biological and physiological markers of aging in oncology trials
- Tumor samples from patients across the age spectrum are needed to assess whether tumor biology changes with aging.
- Measurement of pharmacokinetics and pharmacodynamics of cancer therapeutics in older adults is necessary to improve treatment selection and dosing choices.
C. Need for more studies of vulnerable older adults and/or those aged 75 years or older
- There is a need to develop trials for older individuals with comorbidities, functional losses, cognitive impairment, and frailty.
- There is a need to recruit those older than 75 years to clinical trials for whom there are virtually no data.
D. Research infrastructure should incorporate specific age-associated support
- There is a need to better tailor oncology trials to the specific needs of older, more vulnerable patients.
- The efficacy of data collection from remote locations using advanced information technology needs to be studied.
- More consistent and earlier research collaboration between oncology and geriatrics researchers when designing trials is essential.
In upcoming U13 grant–supported conferences, we plan to highlight research designs and collaborations that will enhance therapeutic and intervention trials in older adults with cancer. By prioritizing the most important research in the field, we hope to focus our research efforts and substantially improve cancer care for older adults.
Appendix.
Attendees at the U13 Conference, September 25–26, 2010, Chicago, IL, in alphabetical order (affiliation at the time of the conference): Tim Ahles (Memorial Sloan-Kettering Cancer Center); Shabbir Alibhai (University Health Network in Toronto); Andrew Artz (The University of Chicago); William Barry (Duke University); Kathryn Bylow (Medical College of Wisconsin); Judith Campisi (Buck Institute, Lawrence Berkeley National Laboratory); Ben Clark (American Society of Clinical Oncology); Harvey Cohen (U13 Oversight Board member) (Duke University); William Dale (U13 Oversight Board member) (The University of Chicago); Basil Eldadah (U13 Oversight Board member) (National Institutes of Health – NIA); Linda Emanuel (Northwestern University Medical Center); Martine Extermann (U13 Oversight Board member) (H. Lee Moffitt Cancer Center); Betty Ferrell (U13 Oversight Board member) (City of Hope National Medical Center); Luigi Ferrucci (National Institute of Health – NIA); Gini Fleming (The University of Chicago); Ajeet Gajra (SUNY Upstate Medical University); Daniel Gardner (New York University); Richard Goldberg (North Carolina Cancer Hospital); John C. Grecula (Ohio State University); Cary Gross (Yale University School of Medicine); Abdo Haddad (Cleveland Clinic); Jimmie Holland (Memorial Sloan-Kettering Cancer Center); Arti Hurria (U13 Oversight Board member) (City of Hope National Medical Center); Danelle James (University of California San Diego); Gretchen G. Kimmick (Duke University); Heidi Klepin (Wake Forest University); Michelle Le Beau (The University of Chicago); Stuart M. Lichtman (Memorial Sloan-Kettering Cancer Center); Rogerio Lilenbaum (Mount Sinai Medical Center, Miami Beach); Ronald Maggiore (The University of Chicago); Jeanne Mandelblatt (Georgetown University); Supriya Mohile (U13 Oversight Board member) (University of Rochester); Vicki A. Morrison (University of Minnesota); Joanne Mortimer (City of Hope National Medical Center); Hyman Muss (U13 Oversight Board member) (University of North Carolina—Chapel Hill); Susan Nayfield (U13 Oversight Board member) (National Institute of Health—NIA; University of Florida); Cynthia Owusu (Case Western Reserve University); Blase Polite (The University of Chicago); Marilyn Raymond (American Society of Clinical Oncology); Donna Regenstreif (Gero-Concepts Inc); Ellen Ritchie (NewYork Presbyterian/Weill Cornell); Miriam Rodin (Saint Louis University); Saleha Sajid (The University of Chicago); Richard Schilsky (U13 Oversight Board member) (The University of Chicago); Kenneth Schmader (U13 Oversight Board member) (Duke University); Dale Shepard (Cleveland Clinic); Richard Stone (Dana-Farber Cancer Institute); William Tew (Memorial Sloan-Kettering Cancer Center); Edward Trimble (U13 Oversight Board member) (National Institutes of Health—NCI); Josie Van Londen (University of Pittsburgh); James Wallace (The University of Chicago); Tanya Wildes (Washington University School of Medicine).
Footnotes
We thank Robert Mitchum and Carol Pearce for assistance in preparing the commentary. The authors were solely responsible for the study design, literature review, interpretation of the data presented, writing of the commentary, and the decision to submit the commentary to the Journal of the National Cancer Institute for publication.
Funding
National Institutes of Health (National Institute on Aging/National Cancer Institute) (U13 AG038151), Cancer and Aging Research Group (CARG), Cancer and Leukemia Group B (CALGB) (7U10-CA31946-29), U13 Conference Attendees, University of Chicago Comprehensive Cancer Center, University of Rochester Cancer Center, City of Hope Cancer Center.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 3.Hurria A, Balducci L, Naeim A, et al. Mentoring junior faculty in geriatric oncology: report from the Cancer and Aging Research Group. J Clin Oncol. 2008;26(19):3125–3127. doi: 10.1200/JCO.2008.16.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 5.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hensing TA, Peterman AH, Schell MJ, Lee JH, Socinski MA. The impact of age on toxicity, response rate, quality of life, and survival in patients with advanced, stage IIIB or IV nonsmall cell lung carcinoma treated with carboplatin and paclitaxel. Cancer. 2003;98(4):779–788. doi: 10.1002/cncr.11548. [DOI] [PubMed] [Google Scholar]
- 7.Hesketh PJ, Lilenbaum RC, Chansky K, et al. Chemotherapy in patients > or = 80 with advanced non-small cell lung cancer: combined results from SWOG 0027 and LUN 6. J Thorac Oncol. 2007;2(6):494–498. doi: 10.1097/JTO.0b013e318060097e. [DOI] [PubMed] [Google Scholar]
- 8.D’Andre S, Sargent DJ, Cha SS, et al. 5-Fluorouracil-based chemotherapy for advanced colorectal cancer in elderly patients: a north central cancer treatment group study. Clin Colorectal Cancer. 2005;4(5):325–331. doi: 10.3816/ccc.2005.n.005. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24(25):4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 10.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331(14):896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 11.Harousseau JL, Martinelli G, Jedrzejczak WW, et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114(6):1166–1173. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]
- 12.Italiano A, Ortholan C, Oudard S, et al. Docetaxel-based chemotherapy in elderly patients (age 75 and older) with castration-resistant prostate cancer. Eur Urol. 2009;55(6):1368–1375. doi: 10.1016/j.eururo.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 13.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26(2):242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 14.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 15.McLean KA, Shah CA, Thompson SA, Gray HJ, Swensen RE, Goff BA. Ovarian cancer in the elderly: outcomes with neoadjuvant chemotherapy or primary cytoreduction. Gynecol Oncol. 2010;118(1):43–46. doi: 10.1016/j.ygyno.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 17.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 18.Hwang SS, Scott CB, Chang VT, Cogswell J, Srinivas S, Kasimis B. Prediction of survival for advanced cancer patients by recursive partitioning analysis: role of Karnofsky performance status, quality of life, and symptom distress. Cancer Invest. 2004;22(5):678–687. doi: 10.1081/cnv-200032911. [DOI] [PubMed] [Google Scholar]
- 19.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101(17):1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rochon PA, Katz JN, Morrow LA, et al. Comorbid illness is associated with survival and length of hospital stay in patients with chronic disability. A prospective comparison of three comorbidity indices. Med Care. 1996;34(11):1093–1101. doi: 10.1097/00005650-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Gross CP, McAvay GJ, Guo Z, Tinetti ME. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007;109(12):2410–2419. doi: 10.1002/cncr.22726. [DOI] [PubMed] [Google Scholar]
- 22.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120(2):104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Hajjar ER, Hanlon JT, Artz MB, et al. Adverse drug reaction risk factors in older outpatients. Am J Geriatr Pharmacother. 2003;1(2):82–89. doi: 10.1016/s1543-5946(03)90004-3. [DOI] [PubMed] [Google Scholar]
- 24.Maggiore RJ, Gross CP, Hurria A. Polypharmacy in older adults with cancer. Oncologist. 2010;15(5):507–522. doi: 10.1634/theoncologist.2009-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc. 2004;52(10):1681–1687. doi: 10.1111/j.1532-5415.2004.52461.x. [DOI] [PubMed] [Google Scholar]
- 27.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 28.Mitsiades N, Correa D, Gross CP, Hurria A, Slovin SF. Cognitive effects of hormonal therapy in older adults. Semin Oncol. 2008;35(6):569–581. doi: 10.1053/j.seminoncol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Lyness JM. Treatment of depressive conditions in later life: real-world light for dark (or dim) tunnels. JAMA. 2004;291(13):1626–1628. doi: 10.1001/jama.291.13.1626. [DOI] [PubMed] [Google Scholar]
- 30.Rao A, Cohen HJ. Symptom management in the elderly cancer patient: fatigue, pain, and depression. J Natl Cancer Inst Monogr. 2004;(32):150–157. doi: 10.1093/jncimonographs/lgh031. [DOI] [PubMed] [Google Scholar]
- 31.Dale W, Hemmerich J, Bylow K, Mohile S, Mullaney M, Stadler WM. Patient anxiety about prostate cancer independently predicts early initiation of androgen deprivation therapy for biochemical cancer recurrence in older men: a prospective cohort study. J Clin Oncol. 2009;27(10):1557–1563. doi: 10.1200/JCO.2008.18.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56(12):2211–116. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liv Wergeland S. Cancer in home care: unintended weight loss and ethical challenges. A cross-sectional study of older people at 11 sites in Europe. Arch Gerontol Geriatr. 2011;53(1):64–69. doi: 10.1016/j.archger.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Steinbach U. Social networks, institutionalization, and mortality among elderly people in the United States. J Gerontol. 1992;47(4):S183–S190. doi: 10.1093/geronj/47.4.s183. [DOI] [PubMed] [Google Scholar]
- 35.Basche M, Baron AE, Eckhardt SG, et al. Barriers to enrollment of elderly adults in early-phase cancer clinical trials. J Oncol Pract. 2008;4(4):162–168. doi: 10.1200/JOP.0842001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunello A, Sandri R, Extermann M. Multidimensional geriatric evaluation for older cancer patients as a clinical and research tool. Cancer Treat Rev. 2009;35(6):487–492. doi: 10.1016/j.ctrv.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006;24(15):2304–2310. doi: 10.1200/JCO.2005.03.1567. [DOI] [PubMed] [Google Scholar]
- 38.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20(2):494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 39.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25(14):1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 40.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Extermann M, Boler I, Reich R, et al. The Chemotherapy Risk Assessment Scale for High-Age patients (CRASH) score: design and validation. J Clin Oncol. 2010;28(15s) suppl Abstract 9000. [Google Scholar]
- 42.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in Cooperative Group Clinical Cancer Trials: CALGB 360401. J Clin Oncol. 2011;29(10):1290–1296. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alley DE, Koster A, Mackey D, et al. Hospitalization and change in body composition and strength in a population-based cohort of older persons. J Am Geriatr Soc. 2010;58(11):2085–2091. doi: 10.1111/j.1532-5415.2010.03144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 46.DePinho RA. The age of cancer. Nature. 2000;408(6809):248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 47.Campisi J. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Exp Gerontol. 2003;38(1–2):5–11. doi: 10.1016/s0531-5565(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 48.Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29(2):273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campisi J, Kim SH, Lim CS, Rubio M. Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol. 2001;36(10):1619–1637. doi: 10.1016/s0531-5565(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 50.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127(2):265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Giannelli SV, Patel KV, Windham BG, Pizzarelli F, Ferrucci L, Guralnik JM. Magnitude of underascertainment of impaired kidney function in older adults with normal serum creatinine. J Am Geriatr Soc. 2007;55(6):816–823. doi: 10.1111/j.1532-5415.2007.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lichtman SM, Hollis D, Miller AA, et al. Prospective evaluation of the relationship of patient age and paclitaxel clinical pharmacology: Cancer and Leukemia Group B (CALGB 9762) J Clin Oncol. 2006;24(12):1846–1851. doi: 10.1200/JCO.2005.03.9289. [DOI] [PubMed] [Google Scholar]
- 53.Klepin HD, Balducci L. Acute myelogenous leukemia in older adults. Oncologist. 2009;14(3):222–232. doi: 10.1634/theoncologist.2008-0224. [DOI] [PubMed] [Google Scholar]
- 54.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 55.Simon MS, Du W, Flaherty L, et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22(11):2046–2052. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21(12):2268–2275. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 57.Basche M, Barón AE, Eckhardt SG, et al. Barriers to enrollment of elderly adults in early-phase cancer clinical trials. J Oncol Pract. 2008;4(4):162–168. doi: 10.1200/JOP.0842001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Given B, Given CW. Cancer treatment in older adults: implications for psychosocial research. J Am Geriatr Soc. 2009;57(suppl 2):s283–s285. doi: 10.1111/j.1532-5415.2009.02513.x. [DOI] [PubMed] [Google Scholar]