DEG/ENaC ion channels involved in sensory transduction are modulated by cold temperature (original) (raw)
Abstract
Several DEG/ENaC cation channel subunits are expressed in the tongue and in cutaneous sensory neurons, where they are postulated to function as receptors for salt and sour taste and for touch. Because these tissues are exposed to large temperature variations, we examined how temperature affects DEG/ENaC channel function. We found that cold temperature markedly increased the constitutively active Na+ currents generated by epithelial Na+ channels (ENaC). Half-maximal stimulation occurred at 25°C. Cold temperature did not induce current from other DEG/ENaC family members (BNC1, ASIC, and DRASIC). However, when these channels were activated by acid, cold temperature potentiated the currents by slowing the rate of desensitization. Potentiation was abolished by a “Deg” mutation that alters channel gating. Temperature changes in the physiologic range had prominent effects on current in cells heterologously expressing acid-gated DEG/ENaC channels, as well as in dorsal root ganglion sensory neurons. The finding that cold temperature modulates DEG/ENaC channel function may provide a molecular explanation for the widely recognized ability of temperature to modify taste sensation and mechanosensation.
Temperature is known to modulate sensations such as taste and touch. For example, personal experience informs us that temperature is key to the taste of food and wine. The interplay between temperature and sensory perception may occur by many mechanisms, including the direct action of temperature on sensory receptors.
Recent studies have suggested that several members of the DEG/ENaC family of cation channels may function as sensory receptors (for reviews see refs. 1 and 2). In Caenorhabditis elegans, the DEG/ENaC channel subunits MEC-4 and MEC-10 are expressed in touch cell neurons, where they are thought to form part of the mechanoreceptor complex (3). In mammals, the DEG/ENaC channel BNC1 (4) [also called MDEG (5), BNaC1 (6), and ASIC2 (2)] is expressed in lanceolate nerve endings at the site of mechanotransduction (7). Targeted disruption of the BNC1 gene in mice reduced the sensitivity of low-threshold, rapidly adapting mechanoreceptors, indicating that BNC1 is required for normal touch sensation (7). The related channel subunits ASIC (8) [also called BNaC2 (6) and ASIC1 (2)] and DRASIC (9) [also called ASIC3 (2)] are also expressed in peripheral sensory neurons; because they are activated by acidic pH, it has been proposed that they play a role in nociception (2). The mammalian epithelial Na+ channel (ENaC) subunits may also function in touch sensation; β- and γENaC are expressed in Merkel cell/neurite complexes, in lamellated corpuscles of the rat foot pad, and in lanceolate nerve endings of the vibrissal follicle (10, 11). BNC1 and all three ENaC subunits (α, β, γ) are also expressed in the tongue. On the basis of their localization in the apical membrane of taste receptor cells, DEG/ENaC subunits may be receptors for salt taste (ENaC) (12–14) and sour taste (BNC1) (15). Consistent with these hypotheses, salt taste can be modulated in rats by the ENaC blocker amiloride or hormones that regulate ENaC activity (13, 14), and BNC1 is activated at proton concentrations that elicit sour taste (15).
Their location in the skin and tongue place these DEG/ENaC channels at sites where they are exposed to large fluctuations in environmental temperature. Importantly, even modest changes in temperature are well known to alter sensation. For example, it is widely recognized that temperature modifies taste sensation (16, 17). In addition, mechanosensation mediated by Merkel cells is potentiated by cold temperatures (18). The molecular bases for these phenomena are not known, but it is thought that temperature-dependent modulation of ion channel function may be responsible (19).
The potential role of DEG/ENaC channels in several aspects of sensation, coupled with their location at sites subjected to significant environmental temperature fluctuations, suggested that variations in temperature could have an important impact on their function. Therefore, we tested the hypothesis that temperature modulates the function of DEG/ENaC ion channels.
Materials and Methods
DNA Constructs.
cDNA constructs for human α-, β-, and γENaC (20, 21), human BNC1a (4), human ASIC, mouse DRASIC (22), and Helix aspersa FaNaCh (23) in pMT3 were generated as previously described. Ser520 in βENaC was mutated to lysine (βS520K), and Gly430 in BNC1 was mutated to valine (BNC1G430V) (24) by using the Quick-change kit (Stratagene), and sequenced in the University of Iowa DNA Core Facility.
Xenopus Oocyte Expression and Electrophysiology.
For measurement of whole-cell current, cDNAs encoding ENaC (α-, β-, and γENaC), BNC1, ASIC, or DRASIC (0.2–1.0 ng each) were injected into the nucleus of Xenopus oocytes. After incubation in modified Barth's solution at 18°C for 16–24 h, we measured whole cell Na+ currents (at −60 mV) by two-electrode voltage-clamp with the cells bathed in (in mM) 116 NaCl, 2 KCl, 0.4 CaCl2, 1 MgCl2, and 5 Hepes (pH 7.4). For experiments in which the pH was altered, Hepes was replaced with 2-(4-morpholino)ethanesulfonic acid (Mes). The bath temperature was changed by perfusion (60 ml/min, bath volume 0.3–0.4 ml) with solutions at 6°C to 43°C. The bath temperature was monitored with a thermistor probe. Changes in temperature did not alter amiloride-sensitive currents in uninjected oocytes, but did have modest effects on baseline membrane conductance. The effect of temperature on DEG/ENaC channel kinetics was reversible, and the sequence of temperature changes did not affect the results.
Total BNC1 current was calculated by integrating current amplitude for 35 s after pH alteration by using igor pro version 3.1.30 (WaveMetrics, Lake Oswego, OR). To obtain the desensitization time constant of DRASIC and FaNaCh, the desensitization phase of the transient current was fitted to a single exponential by usingigor pro. τ was determined from the equation
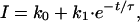
where I is the current at a given time (t). Activation energies (_E_a) were calculated from the τ at a given temperature T (in kelvin) by using an Arrhenius plot of ln(τ) versus 1/T. The slope of the line was then used to calculate the activation energy by the equation
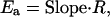
where R = 8.314 J mol−1 K−1. The _Q_10 over a 20–30°C range was then calculated from the equation
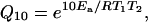
where _T_1 and_T_2 are the temperatures in kelvin (293 K and 303 K, respectively).
COS-7 Cell Expression and Electrophysiology.
COS-7 cells were transfected with cDNAs encoding human BNC1 (2 μg/ml) and green fluorescent protein (to identify transfected cells, 0.33 μg/ml pGreenlantern from GIBCO) by using Transfast reagent (Promega). Whole-cell currents (at −70 mV) in transfected COS-7 cells were recorded by patch-clamp with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). Pipettes were filled with (in mM) 100 KCl, 5 MgCl2, 10 EGTA, and 40 Hepes (pH 7.4 with KOH). The extracellular solution contained (in mM) 130 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, 10 Mes, and 5 glucose; pH was adjusted with tetramethylammonium hydroxide and the osmolarity was adjusted with tetramethylammonium chloride. Rapid extracellular solution changes were accomplished by using a computer-driven solenoid valve system (25), and the solution temperature was measured with a thermistor probe.
Dorsal Root Ganglia (DRG) Neuron Electrophysiology.
Mouse DRG neurons were cultured from C57BL/6 mice as described (7,25). Cells were incubated overnight at room temperature or at 37°C. Whole-cell currents (at −100 mV) in large to medium diameter neurons (25–40 μm) were recorded by patch-clamp with the Axopatch 200B amplifier. Pipettes were filled with (in mM) 120 KCl, 10 NaCl, 2 MgCl2, 5 EGTA, 10 Hepes, and 2 ATP, pH 7.2. The extracellular solution contained (in mM) 128 NaCl, 5 MgCl2, 1.8 CaCl2, 5.4 KCl, 5.55 glucose, and 20 Hepes, pH 7.4 or 6.0.
Results
Cold Temperature Modulates BNC1 Currents.
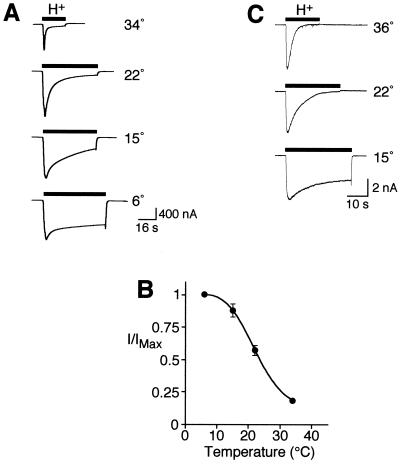
BNC1 generates Na+ currents that activate and rapidly close in the continued presence of acidic pH (desensitize) (24,26). We tested the effect of temperature on acid-evoked whole-cell Na+ currents. At pH 7.4, cold temperature did not induce current in Xenopus oocytes expressing BNC1 (not shown). However, currents elicited by acidic pH were potentiated by cold temperature (Fig. 1A). The predominant effect of low temperature was to slow desensitization. For example, at 6°C the channel desensitized much more slowly than at 34°C, and it failed to completely desensitize, resulting in a large sustained current. As a result, the integrated current was near maximal at 6°C, and decreased by 85% at 34°C (Fig. 1B). Temperature had a similar effect when BNC1 was expressed in_Xenopus_ oocytes (Fig. 1A) and mammalian cells (Fig. 1C).
Figure 1.
Cold-induced stimulation of BNC1 proton-gated current. (A) Representative recordings of human BNC1 proton-activated (pH 4) current in Xenopus oocytes at the indicated temperatures (−60 mV). (B) Plot of proton-activated current (relative to maximal current, mean ± SEM, n = 7 or 8) for BNC1. Current level was determined by integrating the current from 0 to 35 s after pH addition. (C) Representative recordings of BNC1 proton-activated (pH 5) currents in COS-7 cells measured by whole-cell patch clamp (−70 mV). Traces are representative of four cells.
Cold Stimulation of Other Proton-Gated Ion Channels.
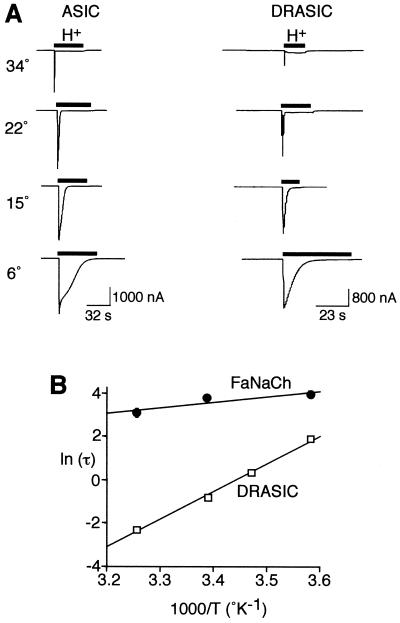
To determine whether temperature modulates the activity of other proton-gated DEG/ENaC channels, we tested ASIC and DRASIC. Acid-gated currents generated by ASIC and DRASIC also showed a marked temperature dependence (Fig. 2A); decreasing temperature potentiated the current in a manner similar to that observed with BNC1.
Figure 2.
Modulation of ASIC and DRASIC by temperature. (A) Representative recordings of human ASIC and DRASIC proton-activated current (pH 5 and 4, respectively) in Xenopus oocytes at the indicated temperatures (−60 mV). (B) Arrhenius plot of the temperature dependence of the τ of DRASIC desensitization after activation by pH 4 (mean ± SEM, n = 6) and FaNaCh desensitization after activation by addition of 10 μM FMRFamide (mean ± SEM, n = 9 or 10). τ of desensitization was determined by single-exponential fits of the desensitization phase of the transient current.
We investigated the effect of temperature on the kinetics of DRASIC after application of a pH 4 solution. The kinetics for desensitization were fit to a single exponential. To measure the temperature dependence, we used the Arrhenius plot of the desensitization time constant (τ) to determine activation energy (_E_a) of DRASIC desensitization (Fig.2B). We then calculated the_Q_10, a measurement of the temperature dependence over 10°C. Desensitization was highly temperature dependent; the _E_a was 109.3 ± 1.9 kJ mol−1, which corresponds to a_Q_10 of 4.4 (±0.12) between 20 and 30°C. This value was much greater than the_Q_10 of 1.3 for aqueous diffusion (27). BNC1 and ASIC showed similar responses to cold temperature, although their desensitization kinetics were more complex and did not fit a single exponential function.
To determine whether other ligand-gated DEG/ENaC channels were similarly affected, we investigated the temperature dependence of FaNaCh from the snail Helix aspersa. This ligand-gated DEG/ENaC channel activates and desensitizes in the continued presence of the peptide FMRFamide (28). In contrast to acid-gated channels, desensitization of FaNaCh showed little temperature dependence (_Q_10 = 1.3, Fig.2B).
Stimulation of ENaC by Cold Temperature.
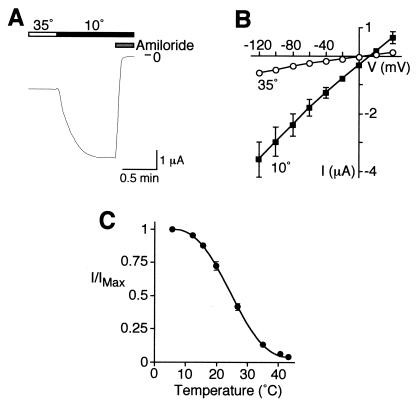
We asked whether a non-ligand-gated member of the DEG/ENaC family is also modulated by temperature. Mammalian ENaC subunits are also expressed in locations exposed to large fluctuations in temperature, including the tongue and peripheral sensory neurons. To determine whether temperature can alter ENaC function, we coexpressed the three subunits that form sodium channels in epithelia (α, β, and γ) and measured whole-cell Na+ current. Unlike the acid-gated channels, ENaC produces currents in the absence of a ligand (21, 29). Fig. 3A shows that decreasing the bath temperature from 35°C to 10°C produced a large and rapid increase in Na+ current. This current was completely inhibited by amiloride (Fig. 3A), a blocker of ENaC (30, 31). Stimulation by cold temperature was not dependent on voltage; a temperature of 10°C increased Na+ current at all voltages tested (−120 to +40 mV, Fig. 3B). Cooling the bath solution generated a graded response; current was maximal at 6°C, and half-maximal activation occurred at ≈25°C (Fig. 3C). Interestingly, cold temperature stimulated ENaC over the same range of temperatures that it stimulated BNC1.
Figure 3.
Cold-induced stimulation of ENaC. Expression of α-, β-, and γhENaC in Xenopus oocytes. Whole-cell Na+ current was measured by two-electrode voltage clamp (−60 mV). (A) Representative current recording for wild-type ENaC at the indicated temperatures. Amiloride was added to bathing solution, as indicated, at a final concentration of 10 μM. (B) Plot of amiloride-sensitive Na+ current at the indicated membrane potentials for cells at 35°C or 10°C (mean ± SEM,n = 4). (C) Plot of amiloride-sensitive Na+ current (relative to maximal current) at temperatures from 6°C to 44°C (mean ± SEM,n = 8) for ENaC.
DEG Mutations Abolish Cold Stimulation.
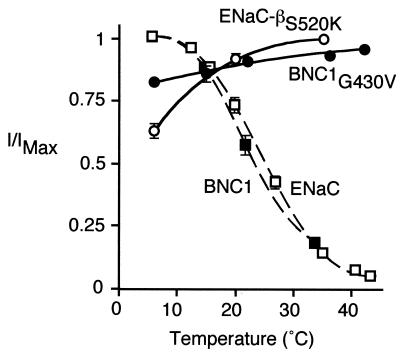
We asked whether the cold-induced stimulation of ENaC and BNC1 was disrupted by mutation of a residue that functions in the gating of DEG/ENaC channels. Earlier studies found that mutation of a conserved residue (the “Deg” residue) just preceding the second membrane-spanning segment activates DEG/ENaC channels by altering channel gating (32, 33). Equivalent mutations in some C. elegans DEG/ENaC channels (e.g., MEC-4, MEC-10) cause neurodegeneration (3). When ENaC contained a mutation at this position (βS520K), cold-induced stimulation was abolished (Fig. 4). Instead, cold temperature decreased Na+ current. The reduction can be at least partly explained by a decrease in ion conductivity as temperature decreases (34). In BNC1, mutation of the equivalent Deg residue (G430V) produces constitutively active Na+ channels (5, 24). Similar to mutant ENaC, the BNC1G430V current was not stimulated by cold temperature (Fig. 4). Thus, the cold-induced stimulation of ENaC and BNC1 requires normal channel gating.
Figure 4.
DEG mutations abolish cold-induced stimulation. Plot of amiloride-sensitive Na+ current (relative to maximal current) at temperatures from 6°C to 44°C in Xenopus oocytes expressing either βS520K with α- and γhENaC, or BNC1G430V (mean ± SEM, n = 8). Currents for wild-type ENaC and BNC1 (from Figs. 1 and 3) are included for comparison.
Temperature Modulation of Proton-Gated Currents in Sensory Neurons.
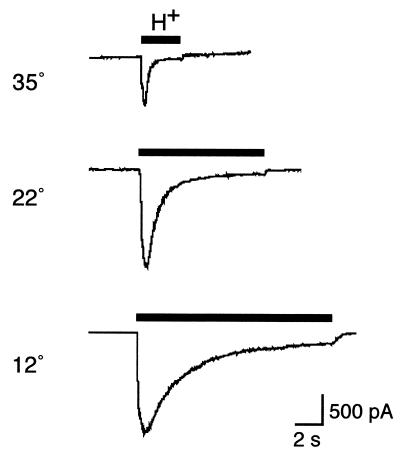
Sensory neurons of the DRG express proton-gated DEG/ENaC channels, including BNC1, ASIC, and DRASIC. We therefore tested the hypothesis that cold temperature modulates acid-evoked currents in cultured DRG neurons. At 37°C, protons (pH 6) generated a transient Na+ current (Fig.5). The current was potentiated by lowering the temperature to 22 and 12°C. Similar to the behavior of heterologously expressed DEG/ENaC channels, potentiation resulted from a slowing of channel desensitization (Fig. 5). When the neurons were returned to higher temperatures, the potentiation was reversed (not shown). Thus, cold temperature modulates proton-gated currents in sensory neurons, suggesting a potential role for this mechanism in the cold-induced modulation of sensory stimuli.
Figure 5.
Temperature modulation of proton-gated current in sensory neurons. Whole-cell patch-clamp recording (−100 mV) of proton-activated current (pH 6) in cultured DRG neurons at the indicated temperatures. The traces are representative of six cells.
Discussion
DEG/ENaC channels in sensory tissues are positioned at sites where they encounter significant temperature variations. Our findings that Na+ current was potentiated by reducing the temperature below 37°C, and that currents were half-maximally increased at 23–25°C, suggest that temperature variations in the physiologic range might alter the function of these channels.
The cold-induced increase in current could result from an increase in the number of channels in the membrane, the single-channel current, or the open-state probability. Cold temperature would not likely increase channel insertion into the membrane; low temperatures inhibit this process (35). Likewise as temperature falls, ionic activity is reduced and current flow through open channels decreases (27). Instead, the data suggest that cold temperature increased current by altering channel gating. Consistent with this conclusion, reducing the temperature progressively decreased the desensitization rate. The effect was highly temperature dependent, with a_Q_10 of 4.4 for DRASIC. Also supporting an effect of temperature on gating, mutation of a specific residue preceding the second membrane-spanning segment abolished the cold-induced stimulation of BNC1 and ENaC. This mutation activates DEG/ENaC channels by locking them in a high PO state (32, 33). Thus, the Deg mutation may prevent a further increase in PO as temperature falls. Additional work will be required to understand the mechanisms underlying the effect of cold temperature on desensitization and current.
One tissue where temperature could affect DEG/ENaC channel function is the tongue. In rats, ENaC and BNC1 are expressed at the apical membrane of taste cells, where they are hypothesized to mediate salt (13, 14) and sour (15) taste, respectively. However, the role of these channels in human taste is unclear. It has been proposed that as salt concentrations increase, cells expressing ENaC progressively depolarize, thereby activating neurons responsible for salt taste. Similarly, activation of BNC1 by acid is also thought to depolarize taste cells, generating the signal for sour taste. Importantly, temperature is well known to modify taste sensation (16, 17). Thus, we speculate that the cold-induced effects on ENaC and BNC1 might contribute to these phenomena.
The skin is another site where wide temperature swings could alter DEG/ENaC channel function. BNC1, ASIC, and DRASIC are expressed in sensory neurons of the DRG (7–9), and where tested, in specialized cutaneous nerve endings (7). In cutaneous fibers, these channels may play a role as mechanosensors (7). The ability of acid to activate current may provide a signature of DEG/ENaC channel mechanosensory function. β- and γENaC have also been localized in specialized cutaneous nerve endings (10, 11). Although they do not generate current when expressed without αENaC (21, 29), β- and γENaC may combine with other DEG/ENaC subunits to contribute to mechanosensation (10,11). Thus, our finding that cold temperature potentiated DEG/ENaC channel currents in heterologous cells, and in medium to large DRG neurons, suggests that cold temperature might modulate the sensory function of these channels. Interestingly, mechanosensation mediated by Merkel cell/neurite complexes is also potentiated by cold temperature (18). This mechanism may be responsible for the Weber phenomenon, in which cold objects feel heavier than warm objects (36). We speculate that DEG/ENaC channels may mediate this response.
The DEG/ENaC channels we studied are also expressed at sites where substantial temperature variations do not occur. For example, ENaC is expressed in kidney and intestinal epithelia (31), and the acid-gated channels are expressed in brain (5, 8) and in visceral sensory neurons (25, 37). Certainly, large temperature fluctuations do not occur at these sites. However, even relatively small changes in temperature, which occur with fever or hypothermia, might have biologically important effects on DEG/ENaC channel function. For example, specific neurons in the central nervous system (preoptic area and anterior hypothalamus) increase their firing rates in response to cooling (38). These neurons are thought to function in thermoregulation.
The ability of acid to activate current from BNC1, ASIC, and DRASIC has suggested that they may be involved in acid-evoked nociception (2). If that hypothesis is correct, one might expect increased nociceptor activity at reduced temperature, an eventuality that seems contrary to common observation. However in the case of nociception, as well as taste and mechanosensation, temperature could affect numerous other channels, transporters, and regulatory processes; the integration of all these processes may determine sensory stimulation. For example, temperature also affects another class of ion channel involved in sensation, the vanilloid receptors VR-1 and VRL-1 (39, 40). However, there are two important differences in the temperature responses. First, DEG/ENaC currents were enhanced by cold temperature, whereas the vanilloid receptors are stimulated by noxious heat. Second, the temperature stimulus directly activates the vanilloid receptors. In contrast, cold temperature alone did not stimulate BNC1, ASIC, or DRASIC; activation required protons.
Might DEG/ENaC channels be sensors for cold temperature? Our data do not answer this question, but they raise the possibility. Although cold temperature alone did not stimulate BNC1, ASIC, or DRASIC, it is possible that they detect cold temperature directly under some conditions. ENaC subunits are also candidates to sense cold temperature because they are open in the absence of an extracellular ligand. Interestingly, Cruz and Green (41) recently reported that temperature on its own could elicit taste. They found that exposing the tip of the tongue to cold temperature elicited salt and sour tastes in some subjects. This observation raises the intriguing possibility that ENaC might be involved in the response. Irrespective of whether these channels directly detect temperature, their modulation by cold temperature suggests that further understanding their function may yield new insights into the molecular mechanisms responsible for detecting changes in the environment.
Acknowledgments
We thank Diane Olson, Daniel Bucher, Jinghui Xie, and Jill Henss for excellent assistance. This work was supported by the National Heart, Lung, and Blood Institute (Grants HL-58812 and HL-03575) of the National Institutes of Health (P.M.S.) and by the Howard Hughes Medical Institute (M.J.W.). C.C.A. is an Associate and M.J.W. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
ENaC
epithelial Na+ channel
DRG
dorsal root ganglia
References
- 1.Mano I, Driscoll M. BioEssays. 1999;21:568–578. doi: 10.1002/(SICI)1521-1878(199907)21:7<568::AID-BIES5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann R, Lazdunski M. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 3.Tavernarakis N, Driscoll M. Annu Rev Physiol. 1997;59:659–689. doi: 10.1146/annurev.physiol.59.1.659. [DOI] [PubMed] [Google Scholar]
- 4.Price M P, Snyder P M, Welsh M J. J Biol Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. J Biol Chem. 1996;271:10433–6. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman B T, Corey D P. Proc Natl Acad Sci USA. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price M P, Lewin G R, McIlwrath S L, Cheng C, Xie J, Heppenstall P A, Stucky C L, Mannsfeldt A G, Brennan T J, Drummond H A, et al. Nature (London) 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. Nature (London) 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 9.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. J Biol Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 10.Drummond H A, Abboud F M, Welsh M J. Brain Res. 2000;884:1–12. doi: 10.1016/s0006-8993(00)02831-6. [DOI] [PubMed] [Google Scholar]
- 11.Fricke B, Lints R, Stewart G, Drummond H, Dodt G, Driscoll M, von During M. Cell Tissue Res. 2000;299:327–334. doi: 10.1007/s004419900153. [DOI] [PubMed] [Google Scholar]
- 12.Li X J, Blackshaw S, Snyder S H. Proc Natl Acad Sci USA. 1994;91:1814–1818. doi: 10.1073/pnas.91.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, Finger T E, Rossier B C, Kinnamon S C. J Comp Neurol. 1999;405:406–420. doi: 10.1002/(sici)1096-9861(19990315)405:3<406::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Boughter J D, Jr, Gilbertson T A. Neuron. 1999;22:213–215. doi: 10.1016/s0896-6273(00)81082-x. [DOI] [PubMed] [Google Scholar]
- 15.Ugawa S, Minami Y, Guo W, Saishin Y, Takatsuji K, Yamamoto T, Tohyama M, Shimada S. Nature (London) 1998;395:555–556. doi: 10.1038/26882. [DOI] [PubMed] [Google Scholar]
- 16.McBurney D H, Collings V B, Glanz L M. Physiol Behav. 1973;11:89–94. doi: 10.1016/0031-9384(73)90127-3. [DOI] [PubMed] [Google Scholar]
- 17.Green B G, Frankmann S P. Physiol Behav. 1988;43:515–519. doi: 10.1016/0031-9384(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 18.Iggo A, Muir A R. J Physiol. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adair R K. Proc Natl Acad Sci USA. 1999;96:11825–11829. doi: 10.1073/pnas.96.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald F J, Snyder P M, McCray P B, Jr, Welsh M J. Am J Physiol. 1994;266:L728–L734. doi: 10.1152/ajplung.1994.266.6.L728. [DOI] [PubMed] [Google Scholar]
- 21.McDonald F J, Price M P, Snyder P M, Welsh M J. Am J Physiol. 1995;268:C1157–C1163. doi: 10.1152/ajpcell.1995.268.5.C1157. [DOI] [PubMed] [Google Scholar]
- 22.Askwith C C, Cheng C, Ikuma M, Benson C, Price M P, Welsh M J. Neuron. 2000;26:133–141. doi: 10.1016/s0896-6273(00)81144-7. [DOI] [PubMed] [Google Scholar]
- 23.Snyder P M, Cheng C, Prince L S, Rogers J C, Welsh M J. J Biol Chem. 1998;273:681–684. doi: 10.1074/jbc.273.2.681. [DOI] [PubMed] [Google Scholar]
- 24.Adams C M, Snyder P M, Price M P, Welsh M J. J Biol Chem. 1998;273:30204–30207. doi: 10.1074/jbc.273.46.30204. [DOI] [PubMed] [Google Scholar]
- 25.Benson C J, Eckert S P, McCleskey E W. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 26.Lingueglia E, de Weille J R, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 27.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 28.Lingueglia E, Champigny G, Lazdunski M, Barbry P. Nature (London) 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- 29.Canessa C M, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J D, Rossier B C. Nature (London) 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 30.Benos D J, Awayda M S, Ismailov I I, Johnson J P. J Membr Biol. 1995;143:1–18. doi: 10.1007/BF00232519. [DOI] [PubMed] [Google Scholar]
- 31.Garty H, Palmer L G. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Anoveros J, Garcia J A, Liu J D, Corey D P. Neuron. 1998;20:1231–1241. doi: 10.1016/s0896-6273(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 33.Snyder P M, Bucher D B, Olson D R. J Gen Physiol. 2000;116:781–790. doi: 10.1085/jgp.116.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzoleni A P, Sisken B F, Kahler R L. Bioelectromagnetics. 1986;7:95–99. doi: 10.1002/bem.2250070111. [DOI] [PubMed] [Google Scholar]
- 35.Kuismanen E, Saraste J. Methods Cell Biol. 1989;32:257–274. doi: 10.1016/s0091-679x(08)61174-7. [DOI] [PubMed] [Google Scholar]
- 36.Spray D C. Annu Rev Physiol. 1986;48:625–638. doi: 10.1146/annurev.ph.48.030186.003205. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland S P, Benson C J, Adelman J P, McCleskey E W. Proc Natl Acad Sci USA. 2001;98:711–716. doi: 10.1073/pnas.011404498. . (First Published December 19, 2000; 10.1073/pnas.011404498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boulant J A, Dean J B. Annu Rev Physiol. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- 39.Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 40.Caterina M J, Rosen T A, Tominaga M, Brake A J, Julius D. Nature (London) 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 41.Cruz A, Green B G. Nature (London) 2000;403:889–892. doi: 10.1038/35002581. [DOI] [PubMed] [Google Scholar]