Determinants of vascular dementia in the Cardiovascular Health Cognition Study (original) (raw)
. Author manuscript; available in PMC: 2012 Jun 19.
Abstract
Objective
The authors evaluated 3,375 participants without dementia at the time of MRI in 1991 to 1994 over 5.7 years for incident dementia and type of dementia.
Methods
Incidence of and risk factors for vascular dementia (VaD) were measured using both pre-MRI and modified State of California Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC) post-MRI review and further classified Alzheimer disease (AD) by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDSADRDA) criteria.
Results
Approximately 44% (213) of 480 incident dementia cases were classified as possible or probable VaD by ADDTC. The incidence of VaD increased with age and was greater in blacks than whites. Risk factors for VaD included age, Modified Mini-Mental State Examination, high white matter grade, number of MRI infarcts, ventricular size, and history of stroke.
Conclusions
Vascular disease in the brain is prevalent among incident dementia cases. There is a substantial overlap between cases classified as Alzheimer disease by Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association and vascular dementia (VaD) by modified State of California Alzheimer’s Disease Diagnostic and Treatment Centers criteria. The substantial contribution of vascular disease would be missed without inclusion of MRI. Treatment of risk factors for VaD could have an important impact on incidence of dementia.
We evaluated risk factors for vascular dementia (VaD) in the Cardiovascular Health Cognition Study (CHCS)1,2 over 5.7 years among 3,608 participants who had MRI of the brain in 1991 to 1994.2 We also evaluated in detail the factors related to VaD, focusing specifically on the MRI measurements and subsequent incidence of vascular type of dementia post-MRI among 2,318 subjects and 480 subjects with incident dementia, excluding those with myocardial infarction (MI). We compared the incidence and risk factors for VaD before and after review of MRI. The pre-MRI classification is unbiased with regard to MRI variables, whereas post-MRI criteria for VaD include MRI measurements and therefore is biased when using those same MRI variables as measures of the risk of VaD. We used the National Institute of Neurological Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for Alzheimer disease (AD) so that we could evaluate risk factors for mixed dementia, i.e., VaD, by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC) criteria and AD by NINCDS-ADRDA criteria.3 Vascular disease in the brain in this study includes high white matter grade (WMG) and the presence of infarcts ≥3 mm with or without clinical stroke.4,5
In the Cardiovascular Health Study (CHS), measures of both clinical and subclinical cardiovascular disease (CVD) were positively correlated with probable ischemic pathology in the brain including high WMG and subcortical infarcts.6 Both a high WMG and the number of subcortical infarcts were independent predictors of the risk of clinical incident stroke.4,5 A recent report from the CHS has noted that carotid intimal medial thickness and stenosis of the left internal carotid artery were directly related to declines in cognitive function as measured by the Modified Mini-Mental State Examination (3MSE) among participants without a history of clinical stroke.7
In the CHCS, the presence of global ventricular atrophy, a high WMG, and one or more primarily subcortical infarcts on MRI were independent predictors of the risk of total dementia in multivariate models and of VaD with or without AD based on clinical classification before review of the MRI in the CHS.2
A companion paper compares the different criteria for VaD within the CHCS.
Methods
In 1998 to 1999, the CHCS was implemented for 3,608 of 5,888 participants who had MRI of the brain in 1991 to 1994.1 We evaluated the 3,608 participants using all available data to determine whether they were demented at the time of MRI and then for participants not demented, to determine the subsequent incidence of dementia over a 5.7-year follow-up.2 Of the 3,608 subjects included in this study, 778 (22%) died from the time of MRI to the end of study in 1998 to 1999 and are included in the analysis and at risk of dementia. There were 1,741 white and 357 black women, 1,282 white and 212 black men, and 16 subjects of other races in the CHCS. We excluded 227 participants with dementia (prevalent) at the time of MRI from the analysis (see table E-1 on the Neurology Web site at www.neurology.org). For participants classified as having possible dementia, we also obtained information from a proxy using the Dementia Questionnaire.1 For those participants who were still alive and coming to the clinic, a neuropsychological evaluation and a neurologic examination were included.1 Diagnosis of dementia was based on a progressive or static cognitive deficit of sufficient severity to affect the subject’s activities of daily living and a history of normal intellectual function before the onset of cognitive abnormalities.1 Participants were required to have impairments in at least two cognitive domains that did not necessarily include memory. A neurologist or psychiatrist with expertise in the diagnosis of dementia evaluated all potential dementia cases at each of the clinics. All the information was then sent to the Pittsburgh Center irrespective of diagnosis by the local neurologist or psychiatrist, i.e., classified as normal, MI, or dementia. An experienced neurologist (O.L.) reviewed each of the cases in the study and classified the cases as dementia, MI, and normal. A dementia adjudication committee that included neurologists and psychiatrists from each center in the study who had expertise in dementia diagnoses then reviewed records of participants classified as having dementia. The adjudications were first done without availability of MRI, (pre-MRI classification) and again after review of the MRI (post-MRI).1 The presence or absence of dementia and possibly the type of dementia were first determined. The pre-MRI classification of the type of dementia was based on the evaluation of the clinical information including all the neuropsychological and neuropsychiatric evaluations over time and history of clinical stroke but did not include the MRI evaluation. Information from the MRI was then used to classify the specific type of dementia.8–10
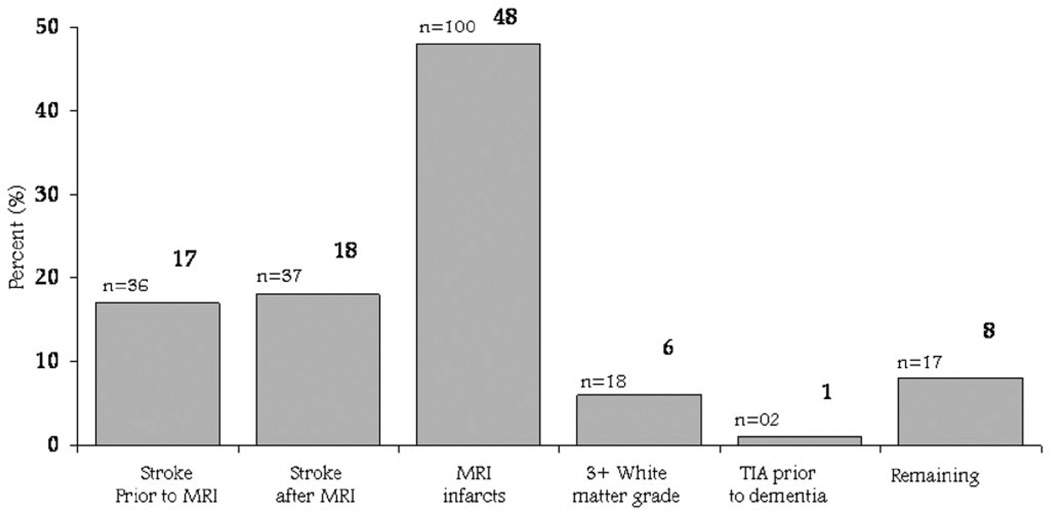
For analysis purposes in this article, we have classified VaD primarily by modified ADDTC criteria (ADDTC VaD) (figure 1).8 We have not included early-onset urinary incontinence not explained by urologic disease or gait disturbances not explained by peripheral causes, as neither of these two measurements has been included in the CHS database. A brief neurologic examination was included at the time of MRI in 1992 to 1994 including hemiplegia, ability to walk without assistance, and a timed walk. The prevalence of any abnormalities was very low (not shown) and was not included in the evaluation by the adjudication committee. The adjudication committee made the diagnosis and classification of ADDTC VaD after reviewing the MRI and clinical information such as history of stroke and changes in cognitive test scores over time. The MRI was, albeit, the major component of the classification but not the only component. We also included classification of VaD by an adjudication panel before review of MRI. AD was based on NINCDS-ADRDA criteria.9 Six cases could not be classified and are excluded from the analysis (see table E-1). MRI. Cranial MRI scans were performed between June 1991 and 1994.11 All images were sent to a central reading center for interpretation by neurologists trained in the CHS protocol. A cerebral infarct by MRI was defined as an area of abnormal signal in a vascular distribution that lacked mass effect. Infarcts of the cortical gray and deep nuclear regions had to be brighter on spin density and T2*-weighted images than normal gray matter. Infarcts in the white matter were similarly defined except that they had to be hypointense on T1-weighted images to distinguish them from diffuse white matter disease.12 An MRI infarct was considered a silent infarct if there was no self-report of TIA or stroke at base-line and no incident TIA or stroke before the MRI was performed as part of the study.12
Figure 1.
Criteria for vascular dementia by Alzheimer’s Disease Diagnostic and Treatment Centers criteria, mutually exclusive.
In this analysis, only infarcts of at least 3 mm based on the maximum of the anterior, posterior, and right-to-left or rostal-cortical dimensions were included in the analysis. We also evaluated the number of infarcts and their location. An infarct involved the cortex even if it spanned subcortical regions. Reproducibility studies have shown good reproducibility for lesions >3 mm but not for those <3 mm. Most infarcts were classified as MRI lacunar infarcts <20 mm in each measured dimension and subcortical. Thus, of the 923 infarcts identified in the total sample, 504 (55%) were single infarcts and 246 (27%) multiple infarcts. Of the infarcts on MRI, 791 were subcortical only, 72 were cortical, and 60 were both cortical and subcortical.12,13
Measurements of WMG had an interreader reliability within one grade of 92% and an intrareader reliability of approximately 94.5%. Most of the high WMG was periventricular rather than subcortical. Thus, of 1,115 subjects with ≥3 WMG intensity, in 828 (74%), periventricular was greater than subcortical WMG; in 205 (18%) participants, they were similar, and in 82 (7%), the subcortical was greater than the periventricular WMG.14
We identified measures of cerebral volume loss based on ventricular size recorded on a semiquantitative 0 to 9 scale. We have previously also shown high reproducibility of this measure, both within and between observers.14
Statistical methods
Analyses were done using SPSS for Windows (version 11.01).15 Incident dementia was defined as those cases in which year of dementia onset occurred after the MRI. Person-years of observation used to calculate rates in this study included time from the MRI until the midpoint of the year of onset of dementia for cases or until death/end of follow-up (June 1999) for noncases. Hazard ratios (HRs) for VaD were calculated on Cox proportional hazards regression, using the 213 cases that met the criteria by ADDTC and the 2,318 cases classified as normal through follow-up. The 577 cases with MI were excluded from most of the prospective analyses (see table E-1).
The classification of VaD using the MRI readings and subsequent use of the same MRI variables as a measure of risk of VaD are biased. They were used primarily for description of the characteristics of VaD over the 5.7-year follow-up. There is no independent measure of VaD. Results presented are consistent across four sites. Because of limited sample sizes, site-specific data are not presented.
Results
There were 480 (13.3%) cases of incident dementia over an approximate 5.7 years of follow-up among 3,375 participants without dementia at the time of MRI in 1991 to 1994. The pre-MRI review classified 330 (69%) cases as AD, 52 (11%) as VaD, 76 (16%) as both VaD and AD, and 22 (5%) as Parkinson disease and other dementia.
After reviewing the MRI, the adjudicators were then given access to the MRI to classify the specific types of dementia, including both the pre-MRI and MRI data. The comparison of ADDTC-diagnosed VaD and the pre-MRI classification of the type of dementia for incident cases is shown in table 1.
Table 1.
Comparison of modified ADDTC criteria for VaD and pre-MRI classification (clinical) incident cases
| Pre-MRI classification | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AD | Vasculardisease | Bothvasculardisease andAD | Other | Total | ||||||
| After measurement of MRI | n | % | n | % | n | % | n | % | n | % |
| Modified ADDTC criteria | ||||||||||
| Probable | 46 | 39 | 37 | 32 | 31 | 26 | 3 | 3 | 117 | 100 |
| Possible | 50 | 52 | 11 | 11 | 34 | 35 | 1 | 1 | 96 | 100 |
| Not vascular | 234 | 88 | 4 | 1 | 11 | 4 | 18 | 7 | 267 | 100 |
| Total | 330 | 69 | 52 | 11 | 76 | 16 | 22 | 5 | 480 | 100 |
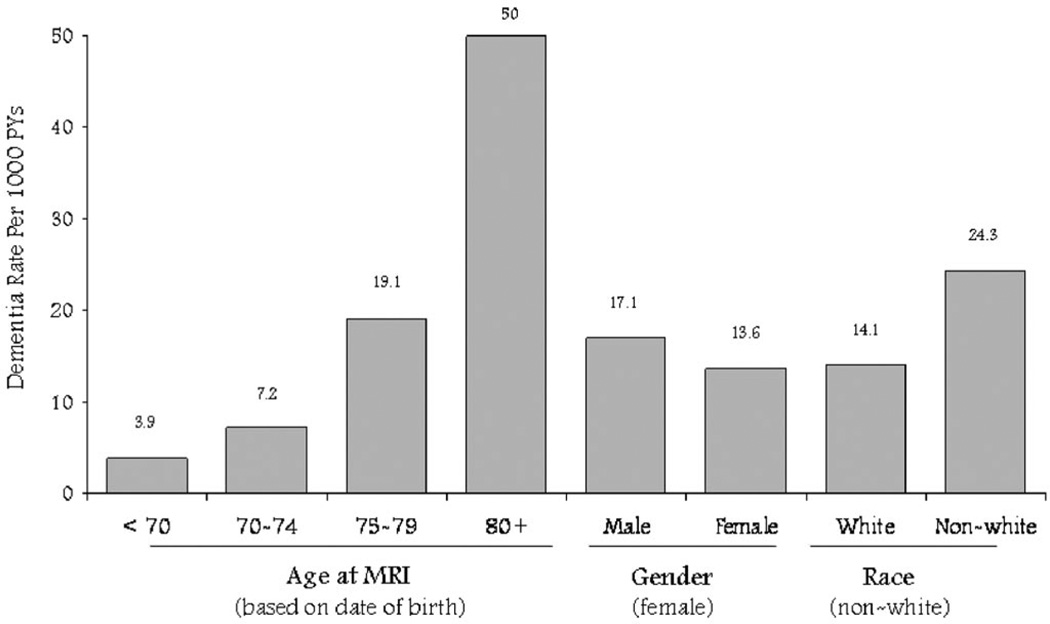
The incidence of VaD increased dramatically with age (figure 2). Rates were higher for blacks than for whites. There was not a significant sex difference.
Figure 2.
Incidence of vascular dementia (by Alzheimer’s Disease Diagnostic and Treatment Centers criteria) by age, sex, and race. PYs = person-years.
The relative risk of VaD (compared with subjects) in the presence of an incident stroke post-MRI was high: 4.5 (95% CI, 3.1 to 6.5; see table E-2). Among the 37 patients with VaD with an incident stroke post-MRI, 23 (62%) were diagnosed within the year of the stroke, 11 (30%) in the first year after the stroke, and three (8%) more than 1 year after. A history of coronary artery disease before the MRI was noted in 29% of patients classified as having VaD (HR: 1.4; 95% CI, 1.1 to 1.9; age, race, and sex adjusted; see table E-2). Similarly, 10% had a history of bypass surgery (HR: 2.6; 95% CI, 1.7 to 4.1). There was no association with a history of coronary angioplasty and risk of VaD (see table E-2).
Age-adjusted HRs for MRI variables associated with VaD were ventricular atrophy >5 + 2.1 (95% CI, 1.5 to 2.8); for WMG, 3 + 3.9 (95% CI, 2.9 to 5.2), and for one or more infarcts ≥3 mm on MRI, 4.8 (95% CI, 3.6 to 6.4). The incidence of VaD was linearly related to the number of infarcts identified on MRI, a higher WMG, and ventricular size (table 2).
Table 2.
Incidence of vascular dementia by baseline MRI findings, HRs, and 95% CIs adjusted for age, race, and sex: 213 vascular dementias and 2,318 normals
| Adjusted HRsand 95% CIs | |||
|---|---|---|---|
| VaD MRI characteristic | Rate, per 1,000person-years | HR | 95% CI |
| Number MRI infarcts ≥3 mm | |||
| 0 | 6.4 | 1.0 | 2.2–4.5 |
| 1 | 25.8 | 3.2 | 4.1–9.1 |
| 2 | 46.9 | 6.1 | 6.6–15.9 |
| 3 | 73.4 | 10.2 | 5.8–18.5 |
| 4 | 91.0 | 10.4 | 2.1–16.4 |
| 5 | 59.2 | 5.9 | 2.2–4.5 |
| White matter grade | |||
| 0 | 1.3 | 1.0 | |
| 1 | 4.3 | 2.7 | 0.4–20.2 |
| 2 | 10.5 | 5.2 | 0.7–38.0 |
| 3 | 24.3 | 12.3 | 1.7–89.0 |
| 4 | 47.9 | 19.0 | 2.6–138.9 |
| 5 | 52.2 | 19.0 | 2.5–143.0 |
| 6 | 65.7 | 21.1 | 2.7–163.0 |
| 7 | 62.6 | 20.2 | 2.4–166.5 |
| 8 | 66.7 | 34.0 | 3.1–376.9 |
| 9 | 0.0 | — | — |
| Ventricular grade | |||
| 1 | 2.9 | 1.0 | |
| 2 | 6.7 | 1.8 | 0.2–13.2 |
| 3 | 8.9 | 2.1 | 0.3–15.4 |
| 4 | 18.2 | 3.6 | 0.5–26.2 |
| 5 | 33.6 | 5.6 | 0.8–41.1 |
| 6 | 37.2 | 6.5 | 0.9–49.3 |
| 7 | 32.7 | 4.1 | 0.4–37.4 |
| 8 | 86.9 | 5.2 | 0.4–60.1 |
| 9 | 95.0 | 48.2 | 4.3–533.6 |
Among 177 patients with incident VaD with no clinical history of stroke before the MRI, there were 118 with the largest infarct on the MRI ≥3 mm, of which 11% were in the cortex, 6% were in the cerebellum, 71% were in the basal ganglion, 5% were classified as located in the deep cerebellum, and 7% were classified as in the deep white matter. The distribution for the 530 subjects with at least one infarct on MRI was similar, with the majority (73% or 395) in the basal ganglion.
The risk factors for VaD with or without AD by the NINCDS-ADRDA classification of AD after review of MRI (see table E-3). There were 61 subjects who were classified as having VaD by modified ADDTC and not AD by ADRDA criteria and 245 who were classified as having AD by ADRDA criteria and not VaD by modified ADDTC criteria. The patients who were classified as having VaD and not AD had a high percentage (56% [34 of 61]) of either prevalent stroke at the time of MRI or incident stroke as well as high WMG (p = 0.001), a greater number of MRI infarcts (p = 0.001), a history of hypertension (p = 0.003), and any subclinical CVD (p = 0.005) compared with those classified as having AD only (p = 0.001). Those (224 subjects) with AD without vascular components have a higher prevalence of ApoE4 (40%) compared with subjects with VaD (p = 0.001). There was also a higher percentage of women with AD but not VaD, 66% compared with 46% for VaD (p = 0.005) and 59% (p = 0.03) among normals.
We then compared risk factors for VaD only, VaD and AD, and AD only (table 3) by Cox models comparing subjects. Black race, high WMG, MRI infarcts, stroke before the MRI, hypertension, and history of angina pectoris were predictors of VaD. ApoE4 was an independent predictor of both AD with and without VaD. Diabetes was also a significant risk factor for AD alone and similarly related to AD with VaD. Risk factors for cases classified as VaD with AD were black race, high WMG, and the presence of infarcts on MRI. Female sex was a significant risk factor only for AD without a vascular component. Age, lower 3MSE score, and history of angina pectoris were significantly related to all three types of dementia (see table 3). Lower education was not a significant risk factor after including the 3MSE score in the Cox models.
Table 3.
Cox models comparing each of the three incident dementia groups defined by combinations of ADDTC VaD and NINCDSADRDA AD with normals
| ADDTC VaD possible/probable NINCDS-ADRDA Criteria for AD | NOT ADDTC VaDpossible/probableNINCDS-ADRDACriteria for AD | |||||
|---|---|---|---|---|---|---|
| Not present (n = 61) vsnormals (n = 2,318) | Probable/possible (n =151) vs normals (n =2,318) | Probable/possible (n =245) vs normals (n =2,318) | ||||
| Variables in the Model | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Age at MRI | 1.1 | 1.03–1.14 | 1.2 | 1.12–1.19 | 1.1 | 1.11–1.17 |
| Female | 0.7 | 0.39–1.24 | 1.2 | 0.84–1.76 | 1.6 | 1.17–2.13 |
| Race (nonwhite) | 2.5 | 1.16–5.41 | 1.9 | 1.06–3.27 | 1.3 | 0.84–2.06 |
| 3MSE at MRI | 0.9 | 0.87–0.94 | 0.9 | 0.9–0.94 | 0.9 | 0.89–0.92 |
| ApoE4 (yes) | 0.8 | 0.42–1.67 | 2.4 | 1.64–3.58 | 2.9 | 2.15–3.86 |
| White matter grade 3+ | 3.8 | 1.99–7.3 | 2.2 | 1.47–3.21 | 1.6 | 1.16–2.09 |
| Ventricular grade 5+ | 1.6 | 0.87–2.93 | 1.9 | 1.28–2.85 | 1.3 | 0.90–1.81 |
| Large infarcts (present) | 2.3 | 1.28–4.22 | 4.0 | 2.66–6.0 | 0.6 | 0.39–0.81 |
| Stroke before MRI | 2.4 | 1.23–4.85 | 1.5 | 0.88–2.59 | 0.4 | 0.14–1.43 |
| Education < grade 12 | 0.5 | 0.10–2.14 | 0.5 | 0.20–1.39 | 1.1 | 0.61–1.83 |
| Diabetes by ADA | 0.6 | 0.24–1.41 | 1.5 | 0.95–2.52 | 1.5 | 1.01–2.24 |
| Hypertension | 1.8 | 1.00–3.20 | 1.0 | 0.66–1.39 | 0.9 | 0.65–1.18 |
| MI before MRI | 1.1 | 0.50–2.41 | 0.9 | 0.48–1.61 | 0.8 | 0.45–1.40 |
| Angina before MRI | 2.1 | 1.05–4.01 | 1.4 | 0.90–2.21 | 1.6 | 1.08–2.31 |
The risk factors for VaD classified before or after review of MRI were similar. The pre-MRI review was less biased because MRI variables are part of post-MRI criteria for VaD (see table E-4).
Discussion
The estimated incidence of VaD increased dramatically by inclusion of measurement of MRI variables such as high WMG and infarcts. The definition of incident dementia included decline in cognitive function in at least two domains but not requiring memory loss as one of the domains.1
The high percentage, 35% (151 of 430), of incident dementia cases that are mixed, both AD and VaD, has important implications for both etiology and therapeutic research. Hypertension, diabetes, and markers of subclinical CVD are risk factors for dementia because they predict subclinical vascular disease in the brain.16 Many of these dementia cases would be classified as AD without available MRI, possibly accounting for the reported association of CVD and AD. The prevalence of ApoE4 is much higher for cases classified as AD by NINCDS-ADRDA criteria but not vascular by modified ADDTC criteria. The change in prevalence of ApoE4 among Alzheimer type dementia cases by age, race, sex, and other risk factors is likely a function of extent of vascular disease within the clinical AD classification, i.e., by ADRDA criteria.
It is possible that there are common determinants of both the high prevalence of brain vascular disease and neurofibrillary tangles and plaques of AD. A recent study reported that there is a positive association between atherosclerosis in the circle of Willis and AD compared with controls.17 Furthermore, there was also a strong association between both amyloid plaque score and neurofibrillary tangles and extent of stenosis in the circle of Willis.17 However, other recent pathology studies suggest no association of AD and vascular pathway.18 The vascular changes identified on the MRI might be correlated with components of small vessel vascular disease in the brain that were not measured by MRI, especially in the cerebral cortex.19 Small vessel disease may be causal to the pathogenesis of AD.20,21 Increased β-amyloid deposition may also have an adverse effect on cerebral arteries.22 There may be two unique diseases, one vascular associated with the small subcortical infarcts and higher WMG on MRI and one associated with the characteristic pathology of AD.23 Individuals may have predominantly one or the other type of dementia or, in many cases, a combination of both, given the high prevalence of vascular disease in the brain.24
The high prevalence of VaD or VaD and AD, i.e., mixed dementia, has important implications. There are very effective therapies to delay onset of VaD. If such therapies are effective in reducing VaD, then they could have a major impact on the overall dementia incidence in the community.25,26
Acknowledgments
Supported by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, and N01-HC-15103 from the National Heart, Lung, and Blood Institute, and grant AG15928 from the National Institute on Aging.
Contributor Information
L.H. Kuller, Department of Epidemiology, University of Pittsburgh, Pittsburgh, PA.
O.L. Lopez, Departments of Neurology and Psychiatry, University of Pittsburgh, Pittsburgh, PA.
W.J. Jagust, Department of Neurology, University of California-Davis, Sacramento, CA.
J.T. Becker, Departments of Neurology and Psychiatry, University of Pittsburgh, Pittsburgh, PA.
S.T. DeKosky, Departments of Neurology and Psychiatry, University of Pittsburgh, Pittsburgh, PA.
C. Lyketsos, Division of Geriatric Psychiatry and Neuropsychiatry, Department of Psychiatry, John Hopkins University, Baltimore, MD.
C. Kawas, Department of Neurology, University of California-Irvine, Irvine, CA.
J.C.S. Breitner, Division of Geriatric Psychiatry, Psychiatry and Behavioral Sciences, VA Puget Sound Health Care System.
A. Fitzpatrick, Department of Epidemiology, University of Washington, Seattle, WA.
C. Dulberg, Department of Biostatistics, University of Washington, Seattle, WA.
References
- 1.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 2.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 3.Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of VaD. Stroke. 2000;31:2952–2957. doi: 10.1161/01.str.31.12.2952. [DOI] [PubMed] [Google Scholar]
- 4.Bernick C, Kuller L, Dulberg C, et al. for the Cardiovascular Health Study. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 5.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ, Jr for the Cardiovascular Health Study Collaborative Research Group. White matter hyperintensity on cranial magnetic resonance imaging. A predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 6.Manolio T, Burke GL, O’Leary DH, et al. for the CHS Collaborative Research Group. Relationship of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:356–365. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 7.Johnston SC, O’Meara ES, Manolio TA, Lefkowitz D, O’Leary DH, Goldstein S, Carlson MC, Fried LP, Longstreth WT., Jr Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med. 2004;140:237–247. doi: 10.7326/0003-4819-140-4-200402170-00005. [DOI] [PubMed] [Google Scholar]
- 8.Chui HC, Victoroff JIB, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic VaD proposed by the state of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 9.McKhann G, Drachman DA, Folstein MF, Katzman R, Price DL, Stadlan E. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 10.Roman GC, Tatemichi TK, Erkinjuntti T, et al. VaD: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 11.Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate effects of cardiovascular disease on the brain: the Cardiovascular Health Study. Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 12.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3600 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 13.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 14.Yue NC, Arnold AM, Longstreth WT, Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the Cardiovascular Health Study. Radiology. 1997;2002:33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 15.Norusis MJ. SPSS for Windows, Advanced Statistics, Release 6.0. Chicago: SPSS, Inc.; 1993. [Google Scholar]
- 16.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a populationbased study: the Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 17.Roher AE, Esh C, Kokjohn TA, et al. Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–2062. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 19.Kovari E, Gold G, Herrmann FR, et al. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt W-P, Roesler A, Kretzschmar K, Ladwig K-H, Junker R, Berger K. Functional and cognitive consequences of silent stroke discovered using brain magnetic resonance imaging in an elderly population. J Am Geriatr Soc. 2004;52:1045–1050. doi: 10.1111/j.1532-5415.2004.52300.x. [DOI] [PubMed] [Google Scholar]
- 21.White L, Petrovitch H, Hardman J, Nelson J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. In: de la Torre JC, Kalaria R, Nakajima K, Nagata K, editors. Alzheimer’s disease. Vascular etiology and pathology. Ann NY Acad Sci. Vol. 977. 2002. pp. 9–23. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg SM, Gurol E, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35:2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- 23.Fox MC, Schott JM. Imaging cerebral atrophy: normal ageing to Alzheimer’s disease. Lancet. 2004;363:392–394. doi: 10.1016/S0140-6736(04)15441-X. [DOI] [PubMed] [Google Scholar]
- 24.Goulding JMR, Signorini DF, Chatterjee S, et al. Inverse relation between Braak stage and cerebrovascular pathology in Alzheimer predominant dementia. J Neurol Neurosurg Psychiatry. 1999;67:654–657. doi: 10.1136/jnnp.67.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke. 2004;35:2620–2622. doi: 10.1161/01.STR.0000143318.70292.47. [DOI] [PubMed] [Google Scholar]
- 26.Erkinjuntti T, Roman G, Gauthier S, Feldman H, Rockwood K. Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke. 2004;35:1010–1017. doi: 10.1161/01.STR.0000120731.88236.33. [DOI] [PubMed] [Google Scholar]