Convergent Evolution of Disease Resistance Gene Specificity in Two Flowering Plant Families (original) (raw)
Abstract
Plant disease resistance (R) genes that mediate recognition of the same pathogen determinant sometimes can be found in distantly related plant families. This observation implies that some R gene alleles may have been conserved throughout the diversification of land plants. To address this question, we have compared R genes from Glycine max (soybean), Rpg1-b, and Arabidopsis thaliana, RPM1, that mediate recognition of the same type III effector protein from Pseudomonas syringae, AvrB. RPM1 has been cloned previously, and here, we describe the isolation of Rpg1-b. Although RPM1 and Rpg1-b both belong to the coiled-coil nucleotide binding site (NBS) Leu-rich repeat (LRR) class of R genes, they share only limited sequence similarity outside the conserved domains characteristic of this class. Phylogenetic analyses of A. thaliana and legume NBS-LRR sequences demonstrate that Rpg1-b and RPM1 are not orthologous. We conclude that convergent evolution, rather than the conservation of an ancient specificity, is responsible for the generation of these AvrB-specific genes.
INTRODUCTION
Pathogen detection in plants often is mediated by genetically defined plant disease resistance (R) genes. The resistance these genes confer is highly specific and only is effective against pathogens expressing a corresponding avirulence (avr) gene. These observations are consistent with R genes encoding receptors that are able to detect, directly or indirectly, the products of the pathogen avr genes (Dangl and Jones, 2001). Consistent with this hypothesis, some R gene products have been shown to interact directly with their corresponding avr gene products (Scofield et al., 1996; Tang et al., 1996; Jia et al., 2000; Deslandes et al., 2003), whereas others interact with plant proteins modified in the presence of Avr proteins (Mackey et al., 2002, 2003; Axtell and Staskawicz, 2003). Pathogen avr genes often enhance disease symptoms on hosts that lack a matching R gene; thus, they likely encode virulence determinants that the plant has evolved the ability to detect (Collmer, 1998).
R gene–mediated resistance, at least in crop plants, often is overcome rapidly in the field, and it has been proposed that R genes and their corresponding avr genes are locked in a relentless cycle of coevolution. A given R gene allele will select for pathogen races that have lost, or modified, the matching avr gene. In turn, once R genes have been overcome, virulent pathogens will select for plants that evolve new R genes able to detect other pathogen determinants. This model would predict that defeated R genes would be rapidly lost from the population, assuming that there is a fitness cost to maintaining R genes of no use. Thus, R genes would be constantly replaced, and ancient functional alleles would be rare. However, at the Arabidopsis thaliana RPM1 and RPS5 loci, functional and nonfunctional alleles have coexisted for millions of years (Stahl et al., 1999; Tian et al., 2002). Stahl et al. propose that in natural populations, frequency-dependent selection can maintain a given R gene allele in the population even in the presence of pathogen strains lacking the corresponding avr gene (Stahl et al., 1999). This model predicts that the frequencies of the avr and R genes in their respective populations will cycle over time. Just how long a given R gene specificity could be maintained in this manner is not known.
R genes sharing the same specificity sometimes are found in distantly related plant species (Whalen et al., 1991; Dangl et al., 1992; Fillingham et al., 1992; Ronald et al., 1992; Innes et al., 1993; Simonich and Innes, 1995), raising the possibility that some R gene specificities have been maintained, perhaps because of balancing selection, in lineages leading to multiple plant species. The alternative explanation is that particular specificities may have evolved independently in different lineages as a result of convergent evolution. Resolving this question would provide important insights into the longevity of R gene specificities and thus provide clues to the dynamics of R gene evolution.
To address this question, we studied two functionally analogous R genes, RPM1 from A. thaliana and Rpg1-b from Glycine max (soybean). Both genes confer resistance to races of Pseudomonas syringae (the causative agent of bacterial blight) that express the avirulence gene avrB (Keen and Buzzel, 1991; Innes et al., 1993). RPM1 is unusual in that it mediates recognition of a second P. syringae avirulence gene, avrRpm1, which has no detectable sequence similarity to avrB (Debener et al., 1991; Bisgrove et al., 1994). Interestingly, recognition of AvrB and AvrRpm1 in G. max is mediated by two distinct but tightly linked genes (Ashfield et al., 1995).
RPM1 is a member of the largest class of plant R genes, which is characterized by a central nucleotide binding site (NBS) and C-terminal Leu-rich repeats (LRR) (Grant et al., 1995). NBS-LRR R genes can be subdivided according to the presence or absence of N-terminal homology to the Toll and Interleukin1 receptors (TIR) and non-TIR classes, respectively (Dangl and Jones, 2001). Many genes from the non-TIR class, including RPM1, contain a putative coiled-coil (CC) domain and are referred to as CC-NBS-LRR genes. Phylogenetic analysis suggests an ancient divergence of the TIR and non-TIR classes (Meyers et al., 1999). The non-TIR class can be further divided into four major subgroups, N1 through N4, which also are of ancient origin, found in both monocots and dicots (Cannon et al., 2002). RPM1 belongs to the N2 subgroup.
Here, we report the positional cloning of the G. max Rpg1-b gene. Although both RPM1 and Rpg1-b belong to the CC-NBS-LRR class of R genes, they share only limited sequence similarity, and phylogenetic analyses reveal a lack of orthology. Thus, R genes specific for avrB have evolved at least twice during the evolution of land plants.
RESULTS
Molecular Isolation of the G. max Rpg1-b Gene
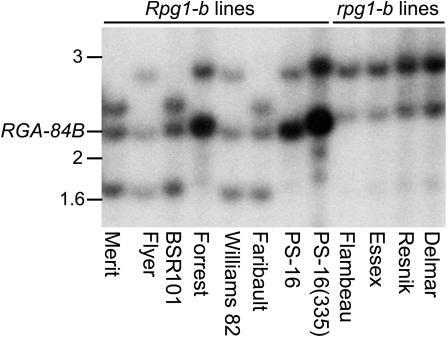
We previously have mapped Rpg1-b to a cluster of R genes that confer resistance to viral, bacterial, oomycete, and nematode pathogens (Ashfield et al., 1998). Fine mapping localized Rpg1-b to a genetic interval of <0.2 centimorgans, encompassed by two overlapping BAC clones (Ashfield et al., 2003). At least five CC-NBS-LRR sequences cosegregate with Rpg1-b (Ashfield et al., 2003). Figure 1 shows that one of these sequences (RGA-84B) gives a characteristic restriction fragment length polymorphism (RFLP) in all Rpg1-b lines examined. This RFLP is absent in lines that do not express _Rpg1-b_–mediated resistance, identifying RGA-84B as a likely Rpg1-b candidate gene. RGA-84B was cloned and sequenced from the _Rpg1-b_–expressing line PS-16 and from PS-16(335), an ethyl methanesulfonate (EMS)–induced mutant lacking Rpg1-b function. RGA-84B was found to encode an NBS-LRR sequence with a putative CC domain at the N terminus and 24 predicted LRRs (Figure 2). A single base difference, causing a Gly-to-Asp substitution (G1154D) in the last LRR (Figure 2B), was found between the two alleles, consistent with RGA-84B being Rpg1-b.
Figure 1.
The NBS-LRR Gene RGA-84B Displays a Characteristic RFLP in _Rpg1-b_–Expressing Cultivars.
Genomic DNA samples from a collection of G. max cultivars that express, or don't express, Rpg1-b specificity were digested with HindIII and analyzed by DNA gel blot hybridization with the RGA-84A probe (Ashfield et al., 2003). A band of ∼2.2 kb (representing the RGA-84B gene) was found only in the _Rpg1-b_–containing lines. Line PS-16(335) is an EMS mutant derived from line PS-16, which carries a mutation in the Rpg1-b gene. The positions of size markers are indicated at left in kilobases.
Figure 2.
Sequence of RGA-84B and Comparison with the A. thaliana RPM1 Protein.
(A) Alignment of the RPM1 and RGA-84B sequences from the first predicted Met to the end of the fifth LRR. Conserved motifs, as described by van der Biezen and Jones (1998b), are indicated. The region of RPM1 previously shown to interact with RIN4 is underlined (Mackey et al., 2002).
(B) Alignment of the LRRs of Rpg1-b. Residues conforming to the intracellular LRR consensus are shown in red. The Gly (G) residue that is mutated to an Asp in the PS-16(335) allele is boxed.
Primers that flank the RGA-84B gene in line PS-16 were used to amplify putative homologs from the G. max cultivars Flambeau and Bonminori, which lack Rpg1-b specificity. We reasoned that if RGA-84B encodes Rpg1-b, homologs in cultivars that lack AvrB specificity should contain polymorphisms not found in the PS-16 sequence and other functional alleles. These primers amplified only single products from line PS-16 and each of the two rpg1-b lines, indicating that they are locus specific in these cultivars. The RGA-84B homologs found in Flambeau and Bonminori differ from each other at only 11 amino acid positions (Figure 3). Significantly, comparison of these homologs to the RGA-84B sequence found in PS-16 revealed an identical large deletion (78 amino acids) within their LRRs, possibly accounting for their inability to mediate AvrB recognition. These homologs also contain multiple small insertions and deletions compared with the PS-16 sequence and many substitutions, mostly concentrated in the NBS domain. This nonrandom distribution of polymorphic residues suggests that gene conversion or unequal exchange may have substituted sequences from a related NBS-LRR gene. Consistent with this hypothesis, phylogenetic analysis revealed that the Flambeau and Bonminori NBS regions group with a subfamily of CC-NBS-LRR sequences different from PS-16 (Figure 4).
Figure 3.
Alignment of Rpg1-b Homologs from G. max Cultivars That Express, or Don't Express, avrB Specificity Indicates Substantial Divergence between Functional and Nonfunctional Homologs.
Cultivars PS-16, Williams 82, and Forrest all express the Rpg1-b phenotype; cultivars Flambeau and Bonminori do not. Line PS-16(335) was isolated from an EMS-mutagenized population derived from cultivar PS-16. The position of the mutation in the PS-16(335) allele is indicated with an asterisk. Sequences were aligned using ClustalX. Motifs conserved among NBS-LRR proteins are underlined. The rpg1b+ designation indicates a nonfunctional allele generated by chemical mutagenesis.
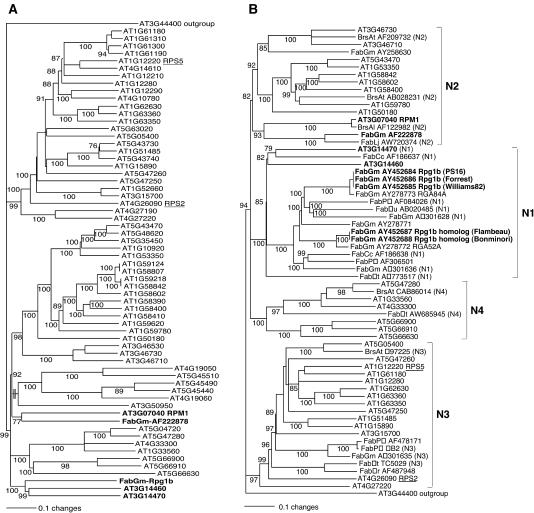
Figure 4.
Phylogenetic Analysis Indicates That RPM1 and Rpg1-b Are Not Orthologous.
(A) Maximum likelihood tree of Rpg1-b and the A. thaliana non-TIR-NBS-LRR sequences. The NBS domains from the entire set of A. thaliana non-TIR-NBS-LRR genes (http://niblrrs.ucdavis.edu) and from the G. max Rpg1-b protein were aligned using ClustalX, and a maximum likelihood tree was generated using MrBayes, a program for the Bayesian inference of phylogeny (see Methods). Numbers on branches indicate the probability that a given grouping is correct (only values >75 are shown). Sequence AF222878 is a G. max sequence included to allow comparison with the tree in (B). The tree was rooted using a TIR-NBS-LRR sequence.
(B) Maximum likelihood tree of A. thaliana and legume non-TIR-NBS-LRR sequences, including RPM1 and Rpg1-b. The region between the P-loop and GLPL domains from the indicated sequences was aligned, and a tree was generated as described in (A). Sequences marked with N1, N2, N3, or N4 have been previously assigned to one of the four non-TIR-NBS-LRR major subgroups (Cannon et al., 2002). Sequences with the AT prefix are A. thaliana sequences also represented in (A). All other sequences are identified by an abbreviation indicating the family (Fab, Fabaceae; Brs, Brassicaceae), the genus and species of origin (Al, A. lyrata; At, A. thaliana; Cc, Cajunas cajan; Gm, G. max; Lj, Lotus japonicus; Mr, Medicago ruthenica; Mt, M. truncatulata; Pv, Phaseolus vulgaris), and the GenBank accession number.
The RGA-84B alleles from two additional _Rpg1-b_–expressing cultivars, Forrest and Williams 82, also were sequenced. Consistent with its ability to mediate AvrB recognition, the Williams 82 allele differs from the PS-16 allele at only four amino acid positions, all of which are in the LRR domain positions (Figure 3). The PS-16 and Forrest alleles are identical.
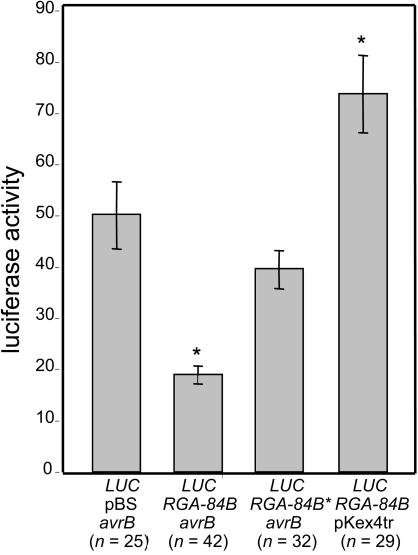
The identity of Rpg1-b was confirmed using a transient complementation assay (Mindrinos et al., 1994). This assay exploits the fact that the interaction between an R gene and its corresponding avr gene often leads to programmed cell death (the hypersensitive response [HR]) in the challenged plant cell (Dangl et al., 1996). This cell death can be detected by expressing a reporter gene (in this case, the luciferase-encoding LUC gene) in the challenged cells; those cells undergoing HR express less of the reporter. G. max leaves from a cultivar lacking the Rpg1-b gene were cobombarded with the LUC gene and avrB, both driven by a constitutive 35S promoter of Cauliflower mosaic virus. A relatively high level of luciferase activity was measurable in the bombarded tissue 2 d later, indicating that many of the transformed cells had remained viable (Figure 5). The addition of a genomic RGA-84B clone expressed under its own promoter resulted in a significantly lower level of luciferase activity, consistent with RGA-84B and avrB interacting to trigger hypersensitive cell death. Replacing the wild-type RGA-84B gene with a mutated allele containing the G1154D substitution restored luciferase activity to that observed with avrB/LUC treatment. To rule out toxicity of RGA-84B, we also cobombarded only RGA-84B and LUC. Interestingly, this bombardment yielded significantly higher luciferase activity than the avrB/LUC bombardment. This result suggests that avrB reduces G. max cell viability, even in the absence of Rpg1-b, perhaps reflecting the role of AvrB in enhancing virulence on susceptible hosts (Ashfield et al., 1995). Together, these data demonstrate that RGA-84B is Rpg1-b.
Figure 5.
NBS-LRR Gene RGA-84B Conditions an _avrB_-Dependent Reduction in Reporter Gene Expression.
Particle bombardment was used to transiently coexpress the LUC reporter gene with the indicated plasmids. RGA-84B* indicates RGA-84B containing the G-to-D substitution identified in the PS-16(335) mutant. pBS indicates pBluescript SK+, the empty vector for RGA-84B. pKex4tr is the empty vector control for the avrB plasmid. Leaves of G. max cv Flambeau (which does not express Rpg1-b) were used in all cases. Values represent the mean ± se of >25 samples, which were pooled from a minimum of three independent experiments. The asterisks indicate values that are significantly different (P < 0.05; Tukey multiple comparison test) from the avrB/Luc treatment.
We previously have shown that Rpg1-b and Rpg1-r (an R gene effective against P. syringae strains that express the effector gene avrRpm1) are tightly linked (Ashfield et al., 1995). The avrB and avrRpm1 specificities are encoded by a single gene, RPM1, in A. thaliana (Bisgrove et al., 1994). Analyses of recombinant inbred lines derived from cultivars Merit (Rpg1-b) and Flambeau (Rpg1-r) indicate that the Rpg1-b homolog from Flambeau (Figure 3) does not correspond to Rpg1-r; thus, Rpg1-r is likely encoded by a different paralog (data not shown).
Rpg1-b Shares Only Limited Sequence Similarity with RPM1, a Functionally Analogous R Gene from A. thaliana
The cloning of the G. max Rpg1-b gene allowed the comparison of two R genes sharing the same specificity from distantly related plant species. Alignment of the predicted RPM1 and Rpg1-b protein sequences revealed a relatively low level of amino acid sequence identity across the NBS region (∼34%) (Figure 2A). The LRR regions were so divergent that they could not be confidently aligned beyond the first five LRRs. In addition, the Rpg1-b LRR region is much longer than that of RPM1, containing 24 repeats versus 15 in RPM1 (Figure 2B) (Grant et al., 1995). Despite this low level of sequence identity, a search of the Arabidopsis genome protein database using the Basic Local Alignment Search Tool (BLAST) algorithm identified RPM1 as one of the six most similar proteins to Rpg1-b.
Rpg1-b and RPM1 Are Not Orthologous
To assess the evolutionary relationship between Rpg1-b and RPM1, we conducted a phylogenetic analysis using a Bayesian inference approach. The NBS region from Rpg1-b (residues 207 to 558) was aligned with the same region from the complete family of non-TIR-NBS-LRR genes present in the A. thaliana genome, and a phylogenetic tree was constructed (Figure 4A). Significantly, this analysis demonstrated that RPM1 is not the A. thaliana sequence most closely related to Rpg1-b. Rpg1-b is more closely related to two other A. thaliana CC-NBS-LRR sequences of unknown function (At3g14460 and At3g14470). A BLAST search with only the LRR region of Rpg1-b also identified these two genes as the most similar A. thaliana genes; thus, the entire Rpg1-b sequence is more similar to these two genes than it is to RPM1. At3g14460 and At3g14470 are adjacent genes on A. thaliana chromosome 3 and presumably are the result of a tandem duplication. Importantly, an earlier study has demonstrated that RPM1 and At3g14460 and At3g14470 (and by inference, Rpg1-b) are members of two different NBS-LRR clades of ancient origin (clades N2 and N1, respectively) (Cannon et al., 2002). Thus, RPM1 and Rpg1-b share only a very distant evolutionary relationship.
If the A. thaliana RPM1 and G. max Rpg1-b genes are not orthologs, then one would predict that there would be G. max NBS-LRR sequences more closely related to RPM1 than Rpg1-b is to RPM1. Several partial non-TIR-NBS-LRR sequences from G. max have been identified, allowing us to test this prediction. The region between the P-loop and the GLPL motif of RPM1 (residues 209 to 379) was aligned with these G. max sequences (including Rpg1-b), together with a representative selection of A. thaliana sequences from Figure 4A, and a tree was generated. Several non-G. max legume sequences used in previous phylogenetic studies were included to facilitate comparisons and to illustrate the distribution of sequences among the four previously defined ancient clades of non-TIR-NBS-LRR genes (Cannon et al., 2002). As expected, Rpg1-b was not the G. max sequence most closely related to RPM1 (Figure 4B). RPM1 is most closely related to a G. max sequence of unknown function, AF222878 (Figure 4B). Consistent with its relatively close relationship with RPM1, AF222878 is a member of clade N2 in the previously defined NBS-LRR phylogenetic tree (Cannon et al., 2002). This analysis also was conducted on a data set that included all of the A. thaliana NBS-LRR sequences shown in Figure 4A. The resulting tree strongly supported our conclusion that RPM1 and Rpg1-b share only a very distant ancestry (see supplemental data online).
The above data demonstrate that the G. max Rpg1-b and A. thaliana RPM1 genes are not orthologs. Given that the N1 and N2 clades diverged before the divergence of monocots and dicots (Cannon et al., 2002) and given the low level of sequence similarity between RPM1 and Rpg1-b, it is highly unlikely that the avrB specificities of RPM1 and Rpg1-b are derived from a common ancestral gene. For this to be the case, the lineage that gave rise to soybean and Arabidopsis families would have had to maintain two R genes with avrB specificity for tens of millions of years (i.e., before the split between monocots and dicots). Thus, we conclude that R genes with avrB specificity have evolved at least twice during the evolution of land plants.
The Type III Effector Protein AvrRpt2 Suppresses Rpg1-b–Mediated Recognition of AvrB
It has been shown previously in A. thaliana that the _RPM1_-mediated response to AvrB is dependent on an additional A. thaliana gene, RIN4 (Mackey et al., 2002). Furthermore, AvrB induces the phosphorylation of the A. thaliana RIN4 protein (Mackey et al., 2002). We attempted to use the antiserum raised against the A. thaliana RIN4 protein (Mackey et al., 2002) to look for AvrB-induced modifications of RIN4-like proteins in G. max. Although this antiserum detected multiple G. max proteins, we detected no AvrB-induced changes in migration of these proteins through SDS-PAGE (data not shown).
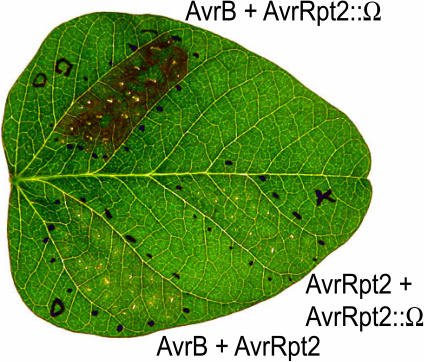
The A. thaliana RIN4 protein also appears to be a target of the P. syringae type III effector AvrRpt2, because RIN4 protein levels are dramatically reduced in the presence of AvrRpt2, and this reduction correlates with suppression of RPM1 function (Ritter and Dangl, 1996; Axtell and Staskawicz, 2003; Mackey et al., 2003). Significantly, we found that avrRpt2 also strongly suppresses the response to avrB in G. max plants expressing Rpg1-b (Figure 6). We attempted to correlate this observation with a loss of at least one of the G. max proteins detected by the A. thaliana RIN4 antiserum. In some experiments, a partial loss of a doublet of cross-reacting bands (∼34 and 36 kD) was observed on protein gel blots after inoculation of G. max leaves with P. syringae expressing avrRpt2 (data not shown). However, this loss was incomplete, and the observation was poorly reproducible; thus, firm conclusions regarding the identity of these cross-reacting bands and their relationship to Rpg1-b resistance cannot be drawn.
Figure 6.
The Type III Effector Protein AvrRpt2 Suppresses Rpg1-b–Mediated Recognition of AvrB.
Each leaf section was injected with a 1:1 mixture of P. syringae pv glycinea race 4 strains carrying the indicated avr genes. AvrRpt2::Ω is a nonfunctional allele generated by insertional disruption of the ORF. The black dots indicate the perimeter of the infiltrated region. The photograph was taken 24 h after inoculation.
The suppression of Rpg1-b function by avrRpt2 is intriguing because it suggests that despite their independent origins, RPM1 and Rpg1-b may use related mechanisms to detect avrB. Whether one common factor is a RIN-like protein remains to be shown.
DISCUSSION
Two models have been proposed to account for R gene specificity. The receptor-ligand model (Gabriel and Rolfe, 1990) proposes a direct interaction between the R gene product and its cognate Avr protein. However, no such interaction has been detected between RPM1 and AvrB (Mackey et al., 2002). A second model, the guard hypothesis (van der Biezen and Jones, 1998a), suggests that R proteins guard the targets of pathogen virulence factors (encoded by avr genes) and are activated by modifications to these targets. In the context of the guard model, the AvrB-induced phosphorylation of RIN4 in A. thaliana has been proposed to activate RPM1 (Mackey et al., 2002). Both AvrB and RPM1 have been shown to interact physically with RIN4 (Mackey et al., 2002). The latter interaction is mediated by the 176 N-terminal amino acids of RPM1. This part of the protein is poorly conserved with Rpg1-b (Figure 2A), suggesting either that Rpg1-b does not interact with a RIN4-like protein or if it does that it uses different contact points. It is quite possible that AvrB has multiple targets in the plant cell, and Rpg1-b could conceivably guard any one of these proteins, and not necessarily the one guarded by RPM1. Similarly, AvrRpt2 could be suppressing the Rpg1-b HR by targeting proteins other than, or in addition to, RIN4 in G. max.
The trench warfare model proposes that a range of alleles will be maintained at R gene loci through the mechanism of balancing selection (Stahl et al., 1999), but it is unknown whether specific alleles could have survived long enough to be present in different plant families. An RPM1 ortholog is present in another genus of the Brassicaceae family (B. napus), but this gene appears not to have retained avrB or avrRpm1 specificity (Grant et al., 1998). A functional ortholog of the Lycopersicon esculentum (tomato) Pto R gene, which is required for recognition of the avrPto gene from P. syringae, has been identified in a distantly related species of the Lycopersicon genus (Riely and Martin, 2001). Pto encodes a Ser/Thr kinase rather than an NBS-LRR protein. Significantly, however, _Pto_-mediated resistance is dependent on the NBS-LRR gene Prf. Furthermore, silencing of the ortholog of Prf in Nicotiana benthamiana blocks the HR induced by coexpression of the tomato Pto gene and avrPto (Chang et al., 2002). This observation implies that the specificity of Prf has been conserved during speciation within the Solanaceae family, suggesting that at least some NBS-LRR alleles may be maintained for long periods of evolutionary time. However, our data demonstrate that identical specificity among R genes does not necessarily indicate conserved alleles.
If R genes are not typically conserved beyond the family level, then one questions why the same specificity arises in multiple plant families. In the context of bacterial Avr protein recognition, it is plausible that avr genes with strong virulence effects, once they arise, are spread among host-specific pathogen strains by horizontal transfer. This would lead to selection for R genes with identical specificities in multiple plant families. A second explanation, which is not mutually exclusive, is that there are a limited number of targets in plant cells that can be exploited by pathogens. Independent selection of R genes that guard these targets in different plant families then would give the appearance of having the same specificity, even if the recognition mechanisms are distinct, and the selection pressures giving rise to these R genes unrelated. A corollary of this is that different Avr proteins that target the same host protein may be recognized by a single R protein. This appears to be the case for RPM1, which mediates recognition of both AvrB and AvrRpm1 (Bisgrove et al., 1994). In either scenario, the longevity of a given R gene allele will likely depend on the cost to the pathogen of modifying or deleting the matching avr gene and the geographical distribution of the host and pathogen. Although balancing selection has been proposed as a mechanism for maintaining specific R gene alleles over long evolutionary times (Stahl et al., 1999; Tian et al., 2002), conservation of an ancient specificity in different plant families remains to be shown.
METHODS
Isolation of the PS-16(335) rpg1-b Mutant G. max Line
Mutant PS-16(335) was isolated from a population of EMS-mutagenized M2 families derived from the line PS-16, which expresses Rpg1-b. This population was kindly supplied by B. Carroll (University of Queensland, Brisbane, Australia). In all, 344 M2 families were screened for susceptibility to P. syringae expressing avrB (Psg (avrB)) using a dip inoculation assay (Ashfield et al., 1998). Up to 15 individuals from each family were scored to enable the detection of recessive mutations. A single M2 family (line PS16-335) that contained individuals susceptible to Psg (avrB) was identified. Analysis with microsatellite markers confirmed that the mutant was not a seed contaminant. F2 individuals derived from crosses to the rpg1-b cultivars Flambeau and Vinton were all susceptible to Psg (avrB) indicating that the mutation was in Rpg1-b.
Hypersensitive Response Disease Assays
Plant and bacterial growth conditions, inoculum preparation, and hand inoculations were as described by Ashfield et al. (1995). The P. syringae pv glycinea race 4 strains expressing avrB and avrRpt2 used in this study are described by Innes et al. (1993) and Whalen et al. (1991), respectively. The control strain containing avrRpt2::Ω expresses a nonfunctional allele of avrRpt2 (Whalen et al., 1991). Two strains were mixed (1:1) for each inoculation such that each strain was represented in the inoculum at an OD600 of 0.1. Injections were made into leaves of G. max cv Merit (Rpg1-b). Inoculated leaves were photographed after 24 h using transmitted light.
Primer Sequences
Primers used for this work are as follows: RGA-50N7A-1, 5′-CCAAGCAGAATCAATCACTTGAAAC-3′; RGA-50N7A-3, 5′-CAAGAGGTACCCTCAGCAGAATC-3′; RGA-84D9-1, 5′-ATGGGTAAGACCACACTTGCT-3′; RGA-84D9-2, 5′-ACATAATCTTTGGGGAATAAGG-3′; E33-mut1, 5′-GAAATGGATTTGGGCAGATCCTCCTCTGGTAAGCATTG-3′; and E33-mut2, 5′-CAATGCTTACCAGAGGAGGATCTGCCCAAATCCATTTC-3′.
DNA Gel Blot Hybridizations
Plant genomic DNA was prepared using DNeasy spin columns (Qiagen, Valencia, CA) according to the manufacturer's instructions. Approximately 10-μg DNA samples were digested with HindIII, separated through 0.9% agarose, and capillary blotted onto Hybond N membranes (Amersham Biosciences, Piscataway, NJ) as described by Ashfield et al. (1998). The membrane was hybridized with an ∼700-bp fragment amplified from the RGA-84A NBS domain (Ashfield et al., 2003) with the primers RGA-84D9-1 and RGA-84D9-2 using a subclone of BAC IS_084_D09 as the template. Probe labeling and hybridization were as described by Ashfield et al. (1998). The G. max genotypes represented on the blot shown in Figure 1 are as follows (from left to right): Merit (Rpg1-b), Flyer (Rpg1-b), BSR101 (Rpg1-b), Forrest (Rpg1-b), Williams 82 (Rpg1-b), Faribault (Rpg1-b), PS-16 (Rpg1-b), PS-16(335) (rpg1-b+), Flambeau (rpg1-b), Essex (rpg1-b), Resnik (rpg1-b), and Delmar (rpg1-b). The _rpg1-b_+ designation indicates a nonfunctional allele generated by chemical mutagenesis.
Amplification and Sequencing of Rpg1-b Alleles
Rpg1-b was initially subcloned from BAC SIU_050_N07 (insert from cultivar Forrest) as a 4.5-kb EcoRI fragment (clone E3-3) in the pBluescript SK+ vector (Stratagene, La Jolla, CA), which then was sequenced using the ABI Prism BigDye terminator cycle sequencing ready reaction kit (Applied Biosystems, Foster City, CA) and an ABI3700 Sequencer. The sequence of the Williams 82 allele was determined from shotgun sequencing of the BAC IS_052_D01. Rpg1-b was amplified from other cultivars using the primers RGA-50N7A-1 and RGA-50N7A-3, which flank the Rpg1-b coding region. PCR amplifications used TaKaRa LA Taq (PanVera, Madison, WI). Sequences were assembled using the Sequencher 3.1.1 software (Gene Codes, Ann Arbor, MI), and alleles were aligned using ClustalX (Thompson et al., 1997).
Constructs Used in Transient Assays
RGA-84B was constructed from a 4.5-kb EcoRI fragment containing RGA-84B from cultivar Forrest cloned into the EcoRI site of the pBluescript SK+ vector. RGA-84B (G1154D) was generated by site-directed mutagenesis of RGA-84B, using a Quikchange kit (Stratagene) with the primers E33-mut1 and E33-mut2. The 35S:LUC construct was previously described by Chern et al. (1996), pKex4tr was described by Tao et al. (2000), and pKex4tr:AvrB was described by Leister et al. (1996).
Transient Expression Assays
Leaf bombardments were performed in a Biolistic PDS-1000/He particle delivery system using 1350-p.s.i. rupture disks (Bio-Rad, Hercules, CA). One-micrometer gold particles (Bio-Rad) were prepared according to the manufacturer's instructions. For each bombardment, 400 μg of gold particles were coated with 1 μg of 35S:LUC and different combinations of the following constructs: 0.68 μg of pKex4tr:AvrB, 1 μg of RGA-84B (or RGA-84B[G1154D]), 0.4 μg of pBluescript SK+ (Stratagene), and 0.53 μg of pKex4tr. Young expanding leaves (2 to 4 cm) from 2- to 4-week-old G. max cv Flambeau were screened by plastic disks with a 1.6-cm hole during bombardment to equalize transformed areas. Bombarded leaves were incubated for 48 h with their petioles submerged in 300 μL of water. Bombarded leaf disks were subsequently excised, ground in liquid N2, and resuspended in 240 μL of cell culture lysis reagent (Promega, Madison, WI). Luciferase assays were performed in a Turner Designs (Sunnyvale, CA) TD-20/20 single-tube luminometer using the Luciferase assay system (Promega) according to the manufacturer's instructions. Data from multiple experiments were pooled after analysis of variance, and average values for each treatment were subjected to a Tukey multiple comparison test to determine significant differences (P < 0.05).
Phylogenetic Analysis
The phylogenetic trees presented in Figure 4 were generated using a Bayesian inference method. Amino acid sequences were aligned using ClustalX (Thompson et al., 1997), and the aligned sequences were analyzed using MrBayes version 2.01 (Huelsenbeck and Ronquist, 2001). Each analysis shown in Figure 4 was run for >400,000 generations, and burn-in was achieved by 50,000 cycles. Trees were sampled every 100 generations. The Dayhoff amino acid substitution model was used for the analyses shown. Consensus trees were generated by PAUP* (Swofford, 2003) using the 50% majority rule. The numbers at the interior branches are the percent of the time that the clade occurs among the sampled trees (i.e., the posterior probability of that clade existing). A TIR-NBS-LRR class sequence was included in each data set as an outgroup.
The GenBank accession numbers for the G. max Rpg1-b alleles and homologs are as follows: Rpg1-b (from line PS16), AY452684; Rpg1-b (from cultivar Williams 82), AY452685; Rpg1-b (from cultivar Forrest), AY452686; Rpg1-b homolog (from cultivar Flambeau), AY452687; Rpg1-b homolog (from cultivar Bonminori), AY452688.
Sequence data from this article have been deposited with the EMBL/GenBank Data libraries under accession numbers AY452684–AY452688.
Supplementary Material
[Supplemental Data]
Acknowledgments
We thank J. Colbourne, J. Danzer, M. Kitayama, M. Henderson, and N. Kane for technical advice and laboratory assistance, B. Carroll for providing mutagenized G. max seed, and F. Katagiri for providing plasmids 35S:LUC, pKex4tr, and pKex4tr:AvrB. We also thank B. Meyers and R. Michelmore for providing a sequence alignment of the A. thaliana CC-NBS-LRR genes, and L. Rieseberg, J. Palmer, C. Lively, and C. Golstein for providing comments on the manuscript. L.E.O. was supported by a National Science Foundation predoctoral fellowship. This work was supported by Grant 99-35300-7693 from the USDA National Research Initiative Competitive Grants Program and Grant GM46451 from the National Institutes of Health to R.W.I. We dedicate this paper to the memory of Noel Keen, who first characterized the genetic interaction between avrB and Rpg1.
W⃞
On-line version contains Web-only data.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Roger W. Innes (rinnes@bio.indiana.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.016725.
References
- Ashfield, T., Bocian, A., Held, D., Henk, A.D., Marek, L.F., Danesh, D., Peñuela, S., Meksem, K., Lightfoot, D.A., Young, N.D., Shoemaker, R.C., and Innes, R.W. (2003). Genetic and physical localization of the soybean Rpg1-b disease resistance gene reveals a complex locus containing several tightly linked families of NBS-LRR genes. Mol. Plant Microbe Interact. 16**,** 817–826. [DOI] [PubMed] [Google Scholar]
- Ashfield, T., Danzer, J.R., Held, D., Clayton, K., Keim, P., Saghai Maroof, M.A., Webb, P.M., and Innes, R.W. (1998). Rpg1, a soybean gene effective against races of bacterial blight, maps to a cluster of previously identified disease resistance genes. Theor. Appl. Genet. 96**,** 1013–1021. [Google Scholar]
- Ashfield, T., Keen, N.T., Buzzell, R.I., and Innes, R.W. (1995). Soybean resistance genes specific for different Pseudomonas syringae avirulence genes are allelic, or closely linked, at the RPG1 locus. Genetics 141**,** 1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell, M.J., and Staskawicz, B.J. (2003). Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112**,** 369–377. [DOI] [PubMed] [Google Scholar]
- Bisgrove, S.R., Simonich, M.T., Smith, N.M., Sattler, A., and Innes, R.W. (1994). A disease resistance gene in Arabidopsis with specificity for two different pathogen avirulence genes. Plant Cell 6**,** 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, S.B., Zhu, H., Baumgarten, A.M., Spangler, R., May, G., Cook, D.R., and Young, N.D. (2002). Diversity, distribution, and ancient taxonomic relationships within the TIR and non-TIR NBS-LRR resistance gene subfamilies. J. Mol. Evol. 54**,** 548–562. [DOI] [PubMed] [Google Scholar]
- Chang, J.H., Tai, Y.S., Bernal, A.J., Lavelle, D.T., Staskawicz, B.J., and Michelmore, R.W. (2002). Functional analyses of the Pto resistance gene family in tomato and the identification of a minor resistance determinant in a susceptible haplotype. Mol. Plant Microbe Interact. 15**,** 281–291. [DOI] [PubMed] [Google Scholar]
- Chern, M.S., Bobb, A.J., and Bustos, M.M. (1996). The regulator of MAT2 (ROM2) protein binds to early maturation promoters and represses PvALF-activated transcription. Plant Cell 8**,** 305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer, A. (1998). Determinants of pathogenicity and avirulence in plant pathogenic bacteria. Curr. Opin. Plant Biol. 1**,** 329–335. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L., Dietrich, R.A., and Richberg, M.H. (1996). Death don't have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 8**,** 1793–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl, J.L., and Jones, J.D. (2001). Plant pathogens and integrated defence responses to infection. Nature 411**,** 826–833. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L., Ritter, C., Gibbon, M.J., Mur, L.A., Wood, J.R., Goss, S., Mansfield, J., Taylor, J.D., and Vivian, A. (1992). Functional homologs of the Arabidopsis RPM1 disease resistance gene in bean and pea. Plant Cell 4**,** 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener, T., Lehnackers, H., Arnold, M., and Dangl, J.L. (1991). Identification and molecular mapping of a single Arabidopsis thaliana locus determining resistance to a phytopathogenic Pseudomonas syringae isolate. Plant J. 1**,** 289–302. [DOI] [PubMed] [Google Scholar]
- Deslandes, L., Olivier, J., Peeters, N., Feng, D.X., Khounlotham, M., Boucher, C., Somssich, I., Genin, S., and Marco, Y. (2003). Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc. Natl. Acad. Sci. USA 100**,** 8024–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingham, A.J., Wood, J., Bevan, J.R., Crute, I.R., Mansfield, J.W., Taylor, J.D., and Vivian, A. (1992). Avirulence genes from Pseudomonas syringae pathovars phaseolicola and pisi confer specificity towards both host and non-host species. Physiol. Molec. Plant Pathol. 40**,** 1–15. [Google Scholar]
- Gabriel, D.W., and Rolfe, B.G. (1990). Working models of specific recognition in plant-microbe interactions. Annu. Rev. Phytopathol. 28**,** 365–391. [Google Scholar]
- Grant, M.R., Godiard, L., Straube, E., Ashfield, T., Lewald, J., Sattler, A., Innes, R.W., and Dangl, J.L. (1995). Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269**,** 843–846. [DOI] [PubMed] [Google Scholar]
- Grant, M.R., McDowell, J.M., Sharpe, A.G., de Torres Zabala, M., Lydiate, D.J., and Dangl, J.L. (1998). Independent deletions of a pathogen-resistance gene in Brassica and Arabidopsis. Proc. Natl. Acad. Sci. USA 95**,** 15843–15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck, J.P., and Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17**,** 754–755. [DOI] [PubMed] [Google Scholar]
- Innes, R.W., Bisgrove, S.R., Smith, N.M., Bent, A.F., Staskawicz, B.J., and Liu, Y.C. (1993). Identification of a disease resistance locus in Arabidopsis that is functionally homologous to the RPG1 locus of soybean. Plant J. 4**,** 813–820. [DOI] [PubMed] [Google Scholar]
- Jia, Y., McAdams, S.A., Bryan, G.T., Hershey, H.P., and Valent, B. (2000). Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19**,** 4004–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen, N.T., and Buzzel, R.I. (1991). New disease resistance genes in soybean against Pseudomonas syringae pv. glycinea: Evidence that one of them interacts with a bacterial elicitor. Theor. Appl. Genet. 81**,** 133–138. [DOI] [PubMed] [Google Scholar]
- Leister, R.T., Ausubel, F.M., and Katagiri, F. (1996). Molecular recognition of pathogen attack occurs inside of plant cells in plant disease resistance specified by the Arabidopsis genes RPS2 and RPM1. Proc. Natl. Acad. Sci. USA 93**,** 15497–15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey, D., Belkhadir, Y., Alonso, J.M., Ecker, J.R., and Dangl, J.L. (2003). Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112**,** 379–389. [DOI] [PubMed] [Google Scholar]
- Mackey, D., Holt, B.F., Wiig, A., and Dangl, J.L. (2002). RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for _RPM1_-mediated resistance in Arabidopsis. Cell 108**,** 743–754. [DOI] [PubMed] [Google Scholar]
- Meyers, B.C., Dickerman, A.W., Michelmore, R.W., Sivaramakrishnan, S., Sobral, B.W., and Young, N.D. (1999). Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding superfamily. Plant J. 20**,** 317–332. [DOI] [PubMed] [Google Scholar]
- Mindrinos, M., Katagiri, F., Yu, G.L., and Ausubel, F.M. (1994). The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78**,** 1089–1099. [DOI] [PubMed] [Google Scholar]
- Riely, B.K., and Martin, G.B. (2001). Ancient origin of pathogen recognition specificity conferred by the tomato disease resistance gene Pto. Proc. Natl. Acad. Sci. USA 98**,** 2059–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter, C., and Dangl, J.L. (1996). Interference between two specific pathogen recognition events mediated by distinct plant disease resistance genes. Plant Cell 8**,** 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald, P.C., Salmeron, J.M., Carland, F.M., and Staskawicz, B.J. (1992). The cloned avirulence gene avrPto induces disease resistance in tomato cultivars containing the Pto resistance gene. J. Bacteriol. 174**,** 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield, S.R., Tobias, C.M., Rathjen, J.P., Chang, J.H., Lavelle, D.T., Michelmore, R.W., and Staskawicz, B.J. (1996). Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274**,** 2063–2065. [DOI] [PubMed] [Google Scholar]
- Simonich, M.T., and Innes, R.W. (1995). A disease resistance gene in Arabidopsis with specificity for the avrPph3 gene of Pseudomonas syringae pv. phaseolicola. Mol. Plant Microbe Interact. 8**,** 637–640. [DOI] [PubMed] [Google Scholar]
- Stahl, E.A., Dwyer, G., Mauricio, R., Kreitman, M., and Bergelson, J. (1999). Dynamics of disease resistance polymorphism at the Rpm1 locus of Arabidopsis. Nature 400**,** 667–671. [DOI] [PubMed] [Google Scholar]
- Swofford, D.L. 2003. PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods), Version 4. (Sunderland, MA: Sinauer Associates).
- Tang, X., Frederick, R.D., Zhou, J., Halterman, D.A., Jia, Y., and Martin, G.B. (1996). Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 274**,** 2060–2063. [DOI] [PubMed] [Google Scholar]
- Tao, Y., Yuan, F., Leister, R.T., Ausubel, F.M., and Katagiri, F. (2000). Mutational analysis of the Arabidopsis nucleotide binding site-leucine-rich repeat resistance gene RPS2. Plant Cell 12**,** 2541–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F., and Higgins, D.G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25**,** 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, D., Araki, H., Stahl, E., Bergelson, J., and Kreitman, M. (2002). Signature of balancing selection in Arabidopsis. Proc. Natl. Acad. Sci. USA 99**,** 11525–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Biezen, E.A., and Jones, J.D.G. (1998. a). Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem. Sci. 23**,** 454–456. [DOI] [PubMed] [Google Scholar]
- van der Biezen, E.A., and Jones, J.D.G. (1998. b). The NB-ARC domain: A novel signalling motif shared by plant resistance gene products and regulators of cell death in animals. Curr. Biol. 8**,** R226–R227. [DOI] [PubMed] [Google Scholar]
- Whalen, M.C., Innes, R.W., Bent, A.F., and Staskawicz, B.J. (1991). Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 3**,** 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]