Minireview: The Neuroendocrinology of the Suprachiasmatic Nucleus as a Conductor of Body Time in Mammals (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 21.
Published in final edited form as: Endocrinology. 2007 Sep 27;148(12):5640–5647. doi: 10.1210/en.2007-1083
Abstract
Circadian rhythms in physiology and behavior are regulated by a master clock resident in the suprachiasmatic nucleus (SCN) of the hypothalamus, and dysfunctions in the circadian system can lead to serious health effects. This paper reviews the organization of the SCN as the brain clock, how it regulates gonadal hormone secretion, and how androgens modulate aspects of circadian behavior known to be regulated by the SCN. We show that androgen receptors are restricted to a core SCN region that receives photic input as well as afferents from arousal systems in the brain. We suggest that androgens modulate circadian behavior directly via actions on the SCN and that both androgens and estrogens modulate circadian rhythms through an indirect route, by affecting overall activity and arousal levels. Thus, this system has multiple levels of regulation; the SCN regulates circadian rhythms in gonadal hormone secretion, and hormones feed back to influence SCN functions.
For optimal function, organisms and cells must locate and operate in a defined temporal and spatial niche. The need for resource partitioning is obvious in the spatial domain and is also important in the temporal domain. The daily rising and setting of the sun provides a predictable environmental time cue to which organisms are sensitive. In mammals, a master circadian clock located in the suprachiasmatic nucleus (SCN) ensures that peripheral clocks throughout the brain and body function in the appropriate phase. Entrainment (synchronization to the environment) in mammalian systems involves the resetting of this master clock, which then communicates timing information to the rest of the body. In the absence of environmental cues, the internal clock runs at its endogenous, free-running period. This system permits the prediction of environmental changes while maintaining plastic responses to changes in the environment.
SCN as Brain Clock
Evidence that the SCN is the locus of a master circadian clock in mammals has been gathered over many years from many labs and entails methodologically distinct experimental paradigms with converging lines of evidence (1, 2). Briefly, lesions of the SCN result in a loss of all circadian rhythmicity in behavior and physiology, including hormone secretion. The SCN expresses rhythmic electrical and metabolic activity, even when placed in a dish in isolation from the rest of the brain (3, 4). After creation of a hypothalamic island containing the SCN, rhythmic electrical activity continues within the island (5), and hamsters bearing such hypothalamic islands continue to show rhythmic locomotor activity although the gonadal regression response to long-term constant darkness is lost (6). Transplantation of fetal hypothalamic tissue containing the SCN into the third ventricle of an SCN-lesioned animal restores circadian locomotor rhythms with a period reflecting that of the donor animal, but again, the gonadal regression response to constant darkness is lost (7, 8). Interestingly, rhythms in hormone secretion are not restored by SCN grafts that do restore rhythmic behavior (9). Taken together, the data strongly indicate that rhythmic hormone and locomotor activity are controlled, at least in part, by different SCN output mechanisms and that rhythmic hormone secretion requires neural efferents from the SCN (10).
Body-Wide Impact of Circadian Clocks
Because of the vast array of physiological systems regulated by the SCN, rhythmicity affects many seemingly unrelated processes. Circadian rhythms occur in virtually all organs and tissues, although the specific genes expressing rhythmicity differ among tissues (11). The immediate consequence is that experimenters must take time of day into account when performing studies on any tissue, and even on cell lines, because the circadian clock regulates key rate-limiting steps in cell physiology, as evidenced by gene-chip analyses (12). At a more personal level, we experience circadian effects of jet lag when we fly across multiple time zones and suffer consequences to health when required to work at night or perform shift work (13, 14). There is also suggestive evidence that rhythm abnormalities are associated with various metabolic syndromes and impact cell cycle progression, with consequences for cancer treatment (15). It is of interest to understand how a small hypothalamic nucleus can act so broadly.
Understanding the Brain Clock: From Black Box to Circuits
In early studies, the SCN was visualized as a “black box” with phase-setting inputs and efferents to local neural sites (16, 17). Today, its cellular elements and neural circuits are better understood in rodents. The paired bilateral SCN are composed of 8,000–10,000 neurons in each hemisphere. The SCN tissue as a whole oscillates on a circadian basis as can be shown by rhythmic glucose metabolism (18) or gene expression studies (19). In this view, coherent rhythmicity is seen at the level of the whole tissue and is an emergent property of all these individual oscillators working together in synchrony. Individual SCN cells have circadian rhythms in electrical activity, but the period and phase differ among cells (20, 21). This finding is consistent with the possibility that all SCN neurons are oscillators (reviewed in Refs. 20 and 22) and that weak coupling among oscillators might account for coherent tissue response (23). As discussed below, subsequent work indicates that rhythmicity of SCN oscillators is spatially and temporally organized and that coupling among neurons occurs, but this mechanism alone is not a sufficient explanation.
Heterogeneity of SCN Organization: Peptides and Clock Genes
In all mammalian species examined, the SCN is a heterogeneous structure, evident both in the intrinsic anatomy (16) and in the peptidergic and neurochemical content of SCN cells (24–30). Broadly speaking, the SCN is organized into two anatomically distinct components, termed shell (or dorsomedial) and core (or ventrolateral), with distinct functional properties (26, 27, 29). Peptidergic phenotypes of SCN cells have long been used to delineate these components. In most species, arginine vasopressin (AVP) cells populate the shell. The core is somewhat more variable among species and contains cells that are positive for vasoactive intestinal polypeptide (VIP) and gastrin-releasing peptide (GRP). In hamsters, a dense population of the calcium-binding protein calbindin-D28k (CalB) cells also defines the core, whereas in mice, CalB is scattered throughout the SCN (24, 31, 32). In addition, cholecystokinin, somatostatin, and substance P neurons are present in several species (24, 32–35). Given species differences, and the absence of strictly delineated areas (characteristic of other hypothalamic nuclei), broad generalizations about core and shell SCN regions can be ambiguous (36). The importance of spatial arrangements of the SCN neurons is nevertheless confirmed in the functional analyses.
We have characterized this SCN organization in both mice and hamsters (25, 37–40). The dorsomedial area, or shell SCN, is rhythmic on a circadian basis, measured by clock protein expression and electrical activity, and by current estimates, about 60% of SCN cells are rhythmic (41). The ventrolateral area, or core, has very low-amplitude or non-detectable rhythmicity using measures of clock protein or clock gene expression. Importantly, these comments on peptidergic and functional phenotypes refer to regions and not to individual cells. Data on individual cells nevertheless fit well with the generalizations about core and shell regions; CalB cells in the core of hamster SCN are not detectably electrically rhythmic (42).
Circuit Organization of the SCN
Early studies indicated that circadian rhythmicity was sustained as long as about 25% of any part of the nucleus survived ablation (43). This work, however, was done before the use of immunochemical markers to delineate SCN regions and before our knowledge of the importance of the SCN’s functional arrangements. Importantly and surprisingly, we now know that destruction of the core SCN region results in the loss of all measured circadian responses, including rhythmic melatonin and corticosterone secretion, body temperature, locomotor activity, and drinking (39, 44). This suggests that the core region is essential for the production of a rhythmic output SCN signal. Also in the core are cells that are first responders to photic input from the retina (31, 45, 46). This suggests that the core is an important site for input signals to the SCN. Such results beg the question of the relationship of core to shell with regard to SCN function and provoke a quest for the SCN circuits underlying entrainment and rhythmicity. How does information travel from the environment and from the body to reach the SCN? Within the SCN, how are incoming signals filtered and communicated to cellular oscillators, and how does information course from SCN neurons to target sites in the brain?
Spatial and Temporal Aspects of SCN Activation
Both under entrained and under free-running conditions, there is an order to cellular activation within the SCN. In its daily expression of rhythmicity, there is not a random but a systematic pattern of activation within the nucleus. In hamsters, the dorsomedial shell cells are the first to rhythmically express both Per1 and VP mRNA, with gene expression then spreading very slowly through the rest of the nucleus for the next 12 h before receding to baseline levels (47). Importantly, after a light pulse, mPer1 mRNA expression increases after 1 h in the core SCN and soon thereafter in the shell (48). This shell increase of mPer1 occurs earlier in animals exposed to a light pulse than in animals housed in constant darkness but given no light exposure, yet it still follows the same spatial and temporal expression pattern. The same conclusion has been drawn in similar studies of rat (49) and in mouse SCN slices in vitro (50, 51). The results suggest not only that the SCN is organized into light-responsive and rhythmic regions, but also that the rhythmic region of the SCN itself has an ordered arrangement in the activation of SCN oscillator cells.
Function of SCN Heterogeneity
Ever since the discovery of its complex neurochemical organization, the function of SCN heterogeneity has been a curiosity. If it is the case that the nucleus is made up of a uniform population of oscillators that produce a coherent rhythmic signal, what need is there for various cell types? On the other hand, anatomists will categorically state (and historical precedents confirm) that anatomical differences are associated with functional specializations. The problem and next question is: What are the functional specializations of the heterogeneous SCN neurons? And what is the significance of their spatial and temporal response properties?
At first, it seemed possible that heterogeneity might be directly related to SCN outputs. For instance, cells containing AVP might control one suite of rhythmic responses, whereas VIP cells might regulate some other aspect of rhythmicity. However, the connections of the SCN that control the daily release pattern of a number of hormones show that neuroendocrine target neurons are controlled by different subgroups that do not necessarily correspond to phenotypic differences among SCN neurons (52). Another hypothesis, that core and shell regions of the SCN send efferents to different brain target sites, was also not supported (53). A view that does have some experimental support is that various SCN peptidergic phenotypes are important for achieving synchronization with the environment under various circumstances that the animal encounters in its lifetime. These include seasonal changes, alterations in food availability, and variation of endogenous hormones, among others. In this aspect, we believe gonadal hormones appear to be significant players, and this topic is the focus of the rest of this review.
Gonadal Hormones: Clock Controlled and Clock Controller
It is well established that the SCN controls the daily timing of gonadal hormone secretion (54, 55). In males, one of the first reports of a diurnal pattern of testosterone secretion was published by Plant (56) in rhesus macaques, showing that there was a late-night peak in this gonadal hormone. This diurnal pattern is circadian in nature (57). Interestingly, in humans, the diurnal pattern of gonadotropin (e.g. LH and FSH) secretion is most robust during the peripubertal period, with both factors increasing during the night. This pattern is reduced in adulthood, although there still remains a robust pulse profile throughout the day. However, daily rhythms in LH and FSH are detected in juvenile and adult male mice. Thus, daily rhythms in blood levels of testosterone may be regulated downstream of the effects of LH and FSH (reviewed in Ref. 58).
In females, the duration of the estrous cycle is determined by the circadian timing system (59–61). For example, the period of the circadian cycle determines the period of the estrous cycle in Syrian hamsters (62). The induction of estrus occurs primarily through the precise timing of the LH surge on the early afternoon of proestrus, which may be mediated via direct SCN projections to GnRH neurons (63) or via a more indirect route through its projections to the anteroventral periventricular nucleus (64), another important neural center in the regulation of GnRH (65). Thus, the SCN controls the timing of rhythmicity in secretion of gonadal hormones through its action on neurosecretory cells.
Hormonal Influences on Timing
Daan (86) wrote in 1975, with prescience: “With the emergence of … new views concerning the mammalian circadian clock, the question of endocrine involvement … [is] … whether there is any feedback regulation from peripheral organs that might be involved in its homeostatic properties.”
Although hormone effects on circadian rhythmicity have been studied for over 30 yr (reviewed in Refs. 55 and 66), hormone effects on rhythmic responses have not been well integrated into the framework of SCN function(s). The past decade has seen tremendous interest in the ways in which hormones affect neural systems and behavior. The availability of new tools to investigate hormone actions directly on the brain clock or on circuits that are known to modulate circadian functioning have brought renewed interest to this topic. Although light is the most salient synchronizer of circadian rhythms, there are many other stimuli that can impact the circadian clock to cause phase resetting. These so-called non-photic effects include forced or voluntary wheel running (i.e. exercise), social cues, and metabolic cues (67–70). As will be discussed, such stimuli also impact peripheral hormone levels.
Ovarian Hormones and Circadian Behavior
The effects of ovarian hormones have been extensively studied. In female hamsters, estrogens modulate circadian locomotor rhythms by shortening the free-running period and advancing the phase angle of entrainment (62, 71). Ovariectomy also reduces the amount of daily activity in rats, hamsters, and mice (62, 71–73). Treatment with estradiol restores the activity levels to normal, with the medial pre-optic area implicated as a site of action (74). Estradiol also modulates overall locomotor activity levels in males (72, 73). Treatment with estradiol benzoate reduces the number of rhythm desynchronies in ovariectomized female hamsters housed in constant light, but no effect of either testosterone or estradiol benzoate is seen in males (75, 76). The effects of activity on circadian rhythmicity are well known (77), and it is likely that a component of the estrogenic effect on circadian timing is a result of indirect activity effects that can then modulate rhythmicity.
There are numerous sites of action of ovarian hormones on circadian timing, although the consensus is that most of these effects are not directly on the SCN. The SCN has a paucity of either estrogen receptor (ER_α_ or ER_β_) mRNA or protein (78–81). As a result, examination of the role of estrogens (and other gonadal hormones) in the circadian system have focused primarily on extra-SCN sites of action and on afferents to the SCN from ER-rich brain sites (e.g. preoptic area, corticomedial amygdala, bed nucleus of the stria terminalis, and arcuate nucleus) (82). Moreover, estradiol modulates the light-induced FOS response of serotonergic neurons of the dorsal raphe nucleus, apparently affecting the SCN through a projection via the median raphe (83). As such, estrogenic effects on circadian rhythms could be a result of indirect actions of estrogens on SCN afferents as well as through the effects of general activity on the circadian clock.
Testicular Hormones and Circadian Behavior
In male hamsters, either surgical or photoperiodic (short day-length induced testicular regression and decreased plasma testosterone) castration results in loss of cohesion of the daily running bout as well as loss of precision in onset of locomotor activity (84). These responses are restored by testosterone replacement. Circadian phase angle of entrainment and activity levels are modulated by testosterone in male Octodon degus (85), although no clear effects on period or precision were reported. In contrast to the rather subtle effects described in other species, the effects of castration and testosterone replacement are very robust in male mice (86). Castration results in lengthened period and loss of the onset bout of activity, whereas testosterone replacement restores these responses (86). Testosterone can be aromatized into estradiol and thus may have dual androgenic/estrogenic impacts on the system. We explored this possibility and found that both testosterone and the nonaromatizable metabolite dihydrotestosterone restored these measures (87), suggesting an androgenic effect. This is significant because precision and period are thought to be determined by the brain clock itself (88, 89), prompting a search for androgen receptor (AR) localization and mechanism of action.
Scattered AR immunoreactivity has been reported in the SCN of the ferret, baboon, rhesus, and human (90–93). Moreover, in humans, AR staining is sexually dimorphic within the SCN (93). In the mouse, we found a highly localized concentration of AR-containing cells restricted to the core SCN (Fig. 1), with few AR-positive cells in the shell. Investigation of AR distribution within the various peptidergic cell types shows that almost all SCN GRP cells and some VIP cells are positive for AR. Conversely, very few AVP cells in the shell contain the AR (87).
Fig. 1.
Photomicrographs depict double-label immunocytochemistry for AVP (green) and AR (red) from the rostral to caudal SCN in a male mouse. AVP delineates the shell region of the SCN, whereas AR is largely restricted to the core region. Scale bar, 150 _μ_m.
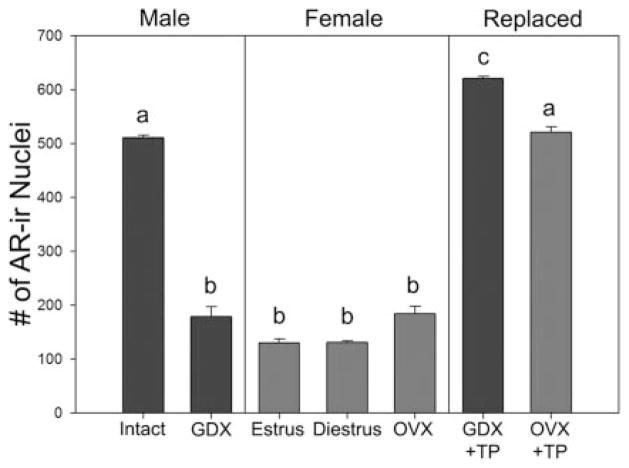
SCN AR expression is sexually dimorphic (Fig. 2); males have more AR-positive cells, and more than 2-fold the amount of AR protein, than females (116). Nevertheless, AR in female SCN is restricted to core cells. Castration of males reduces SCN AR to levels observed in intact females, whereas treatment with testosterone restores it (87). There are no differences in AR between estrus and diestrus, nor does ovariectomy affect SCN AR. However, treating ovariectomized females with testosterone propionate for 7 d augments SCN AR. After treatment with identical doses of testosterone propionate, males have higher AR expression than do females (116), suggesting sexually dimorphic development of this system. This is consistent with findings of sex differences in SCN morphology in human (94, 95) and rat (96–98) and in some aspects of circadian timing as well as the sensitivity of the circadian system to gonadal hormones (99–101). Sexually differentiated sleep patterns that appear at puberty and disappear in old age, presumably involving the circadian system, have also been reported (102).
Fig. 2.
Average number of AR-positive cell nuclei within the SCN of male and female mice under various hormonal conditions. AR levels are high in intact mice, but reduced after castration (GDX). In contrast, AR levels are low in females and not affected by either the estrous cycle (D, diestrus; E, estrus) or by ovariectomy (OVX). Replacing castrated males with a sc testosterone propionate (TP) capsule for 7d restores AR levels, whereas similar treatment of OVX females also results in increased levels, although not to the same levels as TP-replaced males. n = 4–6 animals per group. Columns sharing a common letter are not statistically different from each other, P < 0.01. Modified from Iwahana et al. (116).
Development of ARs in SCN
Gonadal hormones play an important role throughout development in the sculpting of neural circuits (103, 104). During postnatal development, AR expression in the SCN stays flat from d 7–14, rises at d 21, and reaches adult levels by d 28 (Fig. 3A). Comparing males and females, AR expression is similar in the sexes from postnatal d 7–21, but from d 21 through adulthood, males have significantly higher levels of AR than females (unpublished data from our lab and Fig. 3B). Compared with the sex difference in the medial preoptic area observed during development in the rat (which arises by postnatal d 14) (105), the sex difference in SCN AR becomes clear slightly later, by d 21. In mice, testosterone is almost undetectable at d 21 (Ref. 106 and data from our laboratory). That the SCN AR increase at d 21 antecedes the increase in plasma testosterone suggests a gonadal hormone-independent regulation of AR and that the sex difference observed in adulthood may occur during the pre- or perinatal period.
Fig. 3.
A, Photomicrographs depict AR staining in the mid-SCN of male mice at 7, 14, 21, 28, and 60 d of age. AVP is labeled in green, and AR nuclei are labeled in red. Scale bar, 100 _μ_m. B, AR staining in the mid-SCN of females (pink, ○) and males (blue, ●) during postnatal development. *, _P <_0.01; **, _P <_0.001. n = 3–4 animals per group.
What Is the Function of SCN AR?
The network properties of the SCN tissue suggest a function for AR cells in circadian timing. As discussed above, the core SCN can be considered a SCN input site. The ARs are localized to the core, and the retina, intergeniculate leaflet, and median raphe all project to the core region. Because the core is the first SCN compartment to respond to photic pulses from the environment, and core cells contain AR, it is interesting to think that androgens may alter SCN responses to light. We found that after castration, the male mouse SCN shows a blunted FOS expression in response to either an early-night (phase-delaying) or late-night (phase-advancing) light pulse. Moreover, replacing animals with the nonaromatizable testosterone metabolite dihydrotestosterone for 7d restores the FOS response to normal at both time points (87). This shows that androgens alter the responses of the SCN to external photic pulses. Thus, there is a complex interplay between signals from the external environment (namely, light) and changes in internal physiology (e.g. hormone levels) at the level of the SCN that can alter SCN function.
The Organization of the SCN: Universals and Specializations
A central theme in this review has been the anatomical and functional organization of the SCN and how this organization contributes to its functioning as a brain clock that regulates, and is in turn regulated by, gonadal hormones.
SCN universals
Some aspects of SCN organization appear to be universal, whereas others are specializations associated with species adaptations to their unique environments. First, in all species, SCN neurons are heterogeneous with respect to cellular morphology and peptidergic and neurotransmitter content. Second, the function of individual SCN cells is also heterogeneous, with some SCN cells being very robust self-sustained oscillators, whereas others are weak oscillators or not oscillators at all. Third, the SCN is organized into two regions, with a shell associated with oscillation and a core that receives afferent input. Both the shell and the core send outputs to local hypothalamic regions (53), and some SCN cells communicate with either the parasympathetic or sympathetic nervous system or, in some cases, both (107, 108, 117). Fourth, the phase of clock gene expression is similar in nocturnal and diurnal animals, indicating that common clock mechanisms underlie opposite daily activity profiles (109). Fifth, rhythmic gene and protein expression within the different SCN regions are not uniform but are orderly, with some SCN regions expressing these genes earlier in the day than others (47). Finally, changing photoperiod can alter this orderly phasing of different SCN regions (110–112), an example of the plasticity of the SCN network in response to changes in the external environment.
Species specializations: effects of androgens in rat, hamster, and mouse
As noted in the introductory section, there are species differences in the peptidergic content of the SCN core cells that receive photic and nonphotic input. This presumably reflects species differences in sensitivity to environmental and bodily input cues. It is possible that AR localization to the core aspect of the mouse SCN is a specialization. Castration of male rats or hamsters leads only to subtle changes in daily behavioral activity, with small changes to daily onset precision but no alteration of period (84). Additionally, SCN AR are sparse in these species (113, 114). Whether these reports actually reflect true species differences or whether they are merely a result of a lack of a detailed analysis of AR throughout the rostral-caudal extent of the SCN remains to be elucidated.
Conclusion: Hormones Act Directly and Indirectly on the SCN
This review has highlighted the two-way interactions between gonadal hormone and the circadian system. In broad strokes (Fig. 4), the SCN sits on top of a hierarchical system, acting to orchestrate the timing of the endocrine system and reproductive responses. In turn, hormones have many effects on circadian behavior. These include amount of activity, activity duration, onset precision, and free-running period. Of these influences, some are on the SCN directly and others may act through extra-SCN sites. Free-running period and precision are determined directly by the SCN (88, 115). We have shown that AR are present in the SCN, and these AR-bearing cells are obvious sites of action for direct effects on the SCN. In contrast, the effects of gonadal hormones on locomotor activity are likely due to extra-SCN sites of action. Activity itself can set the phase of SCN oscillators (reviewed in Ref. 70), and this represents an indirect route whereby hormones can influence circadian rhythmicity. Thus, hormones can regulate circadian rhythmicity through direct and indirect routes, closing a neuroendocrine loop regulated by the SCN.
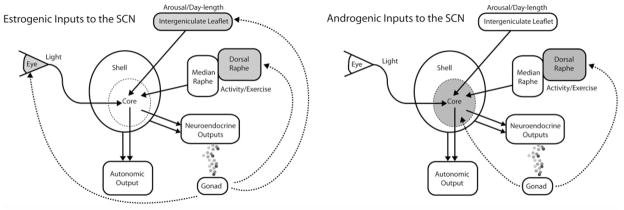
Fig. 4.
Schematic representation of differences between the site of action of estrogenic and androgenic hormones on the SCN and its inputs. Upper panel shows ER-rich nuclei (gray) that project to the SCN, including the retina, the intergeniculate leaflet, and the dorsal raphe nucleus, via the median raphe. Lower panel shows AR-rich areas in the core SCN and also the dorsal raphe. Testosterone can be aromatized into estradiol and thus may have dual androgenic/estrogenic impacts on the system. The SCN regulates circadian timing in physiology and behavior by sending outputs to autonomic and neuroendocrine systems. The SCN core receives direct input from the retina, the intergeniculate leaflet, and the median raphe nucleus, providing resetting information from photic cues and from activity/exercise.
Acknowledgments
This work was supported by a Natural Sciences and Engineering Research Council of Canada Predoctoral Fellowship (Canada) to I.N.K. and the National Institutes of Health (NS37919 and 023224) to R.S.
Abbreviations
AR
Androgen receptor
AVP
arginine vasopressin
CalB
calbindin-D28k
ER
estrogen receptor
GRP
gastrin-releasing peptide
SCN
suprachiasmatic nucleus
VIP
vasoactive intestinal polypeptide
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Based on talk given at the “From Molecular Clocks to Human Health” session at the Sixth International Congress of Neuroendocrinology, Pittsburgh, PA, June 2006.
References
- 1.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind’s clock. New York: Oxford University Press; 1991. [Google Scholar]
- 2.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 3.Gillette MU, Reppert SM. The hypothalamic suprachiasmatic nuclei: circadian patterns of vasopressin secretion and neuronal activity in vitro. Brain Res Bull. 1987;19:135–139. doi: 10.1016/0361-9230(87)90176-6. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki S, Kerbeshian MC, Hocker CG, Block GD, Menaker M. Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. J Neurosci. 1998;18:10709–10723. doi: 10.1523/JNEUROSCI.18-24-10709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakim H, DeBernardo AP, Silver R. Circadian locomotor rhythms, but not photoperiodic responses, survive surgical isolation of the SCN in hamsters. J Biol Rhythms. 1991;6:97–113. doi: 10.1177/074873049100600201. [DOI] [PubMed] [Google Scholar]
- 7.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 8.Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- 10.LeSauter J, Silver R. Output signals of the SCN. Chronobiol Int. 1998;15:535–550. doi: 10.3109/07420529808998706. [DOI] [PubMed] [Google Scholar]
- 11.Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417:329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- 12.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 13.Haus E, Smolensky M. Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control. 2006;17:489–500. doi: 10.1007/s10552-005-9015-4. [DOI] [PubMed] [Google Scholar]
- 14.Lack LC, Wright HR. Chronobiology of sleep in humans. Cell Mol Life Sci. 2007;64:1205–1215. doi: 10.1007/s00018-007-6531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levi F. Chronotherapeutics: the relevance of timing in cancer therapy. Cancer Causes Control. 2006;17:611–621. doi: 10.1007/s10552-005-9004-7. [DOI] [PubMed] [Google Scholar]
- 16.Van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- 17.Eskin A. Identification and physiology of circadian pacemakers. Introduction. Fed Proc. 1979;38:2570–2572. [PubMed] [Google Scholar]
- 18.Schwartz WJ, Gainer H. Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science. 1977;197:1089–1091. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- 19.Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 21.Herzog ED, Schwartz WJ. A neural clockwork for encoding circadian time. J Appl Physiol. 2002;92:401–408. doi: 10.1152/japplphysiol.00836.2001. [DOI] [PubMed] [Google Scholar]
- 22.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- 24.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 25.Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Moore RY, Silver R. Suprachiasmatic nucleus organization. Chronobiol Int. 1998;15:475–487. doi: 10.3109/07420529808998703. [DOI] [PubMed] [Google Scholar]
- 27.Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell Tissue Res. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- 28.van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- 29.Moore RY. Entrainment pathways and the functional organization of the circadian system. Prog Brain Res. 1996;111:103–119. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- 30.Morin LP, Blanchard J, Moore RY. Intergeniculate leaflet and suprachiasmatic nucleus organization and connections in the golden hamster. Vis Neurosci. 1992;8:219–230. doi: 10.1017/s095252380000287x. [DOI] [PubMed] [Google Scholar]
- 31.Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport. 1996;7:1224–1228. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- 32.Silver R, Sookhoo AI, LeSauter J, Stevens P, Jansen HT, Lehman MN. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in mouse SCN. Neuroreport. 1999;10:3165–3174. doi: 10.1097/00001756-199910190-00008. [DOI] [PubMed] [Google Scholar]
- 33.Card JP, Moore RY. The suprachiasmatic nucleus of the golden hamster: immunohistochemical analysis of cell and fiber distribution. Neuroscience. 1984;13:415–431. doi: 10.1016/0306-4522(84)90240-9. [DOI] [PubMed] [Google Scholar]
- 34.Castel M, Belenky M, Cohen S, Wagner S, Schwartz WJ. Light-induced c-Fos expression in the mouse suprachiasmatic nucleus: immunoelectron microscopy reveals co-localization in multiple cell types. Eur J Neurosci. 1997;9:1950–1960. doi: 10.1111/j.1460-9568.1997.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 35.Hartwich M, Kalsbeek A, Pevet P, Nurnberger F. Effects of illumination and enucleation on substance-P-immunoreactive structures in subcortical visual centers of golden hamster and Wistar rat. Cell Tissue Res. 1994;277:351–361. doi: 10.1007/BF00327783. [DOI] [PubMed] [Google Scholar]
- 36.Morin LP. SCN organization reconsidered. J Biol Rhythms. 2007;22:3–13. doi: 10.1177/0748730406296749. [DOI] [PubMed] [Google Scholar]
- 37.Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci. 2001;21:7742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karatsoreos IN, Yan L, LeSauter J, Silver R. Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J Neurosci. 2004;24:68–75. doi: 10.1523/JNEUROSCI.1666-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeSauter J, Silver R. Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J Neurosci. 1999;19:5574–5585. doi: 10.1523/JNEUROSCI.19-13-05574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeSauter J, Yan L, Vishnubhotla B, Quintero JE, Kuhlman SJ, McMahon DG, Silver R. A short half-life GFP mouse model for analysis of suprachiasmatic nucleus organization. Brain Res. 2003;964:279–287. doi: 10.1016/s0006-8993(02)04084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci USA. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jobst EE, Allen CN. Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. Eur J Neurosci. 2002;16:2469–2474. doi: 10.1046/j.1460-9568.2002.02309.x. [DOI] [PubMed] [Google Scholar]
- 43.Davis FC, Gorski RA. Development of hamster circadian rhythms: role of the maternal suprachiasmatic nucleus. J Comp Physiol A. 1988;162:601–610. doi: 10.1007/BF01342635. [DOI] [PubMed] [Google Scholar]
- 44.Kriegsfeld LJ, LeSauter J, Silver R. Targeted microlesions reveal novel organization of the hamster suprachiasmatic nucleus. J Neurosci. 2004;24:2449–2457. doi: 10.1523/JNEUROSCI.5323-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryant DN, LeSauter J, Silver R, Romero MT. Retinal innervation of calbindin-D28K cells in the hamster suprachiasmatic nucleus: ultrastructural characterization. J Biol Rhythms. 2000;15:103–111. doi: 10.1177/074873040001500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan L, Takekida S, Shigeyoshi Y, Okamura H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience. 1999;94:141–150. doi: 10.1016/s0306-4522(99)00223-7. [DOI] [PubMed] [Google Scholar]
- 47.Hamada T, Antle MC, Silver R. Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur J Neurosci. 2004;19:1741–1748. doi: 10.1111/j.1460-9568.2004.03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci. 2002;16:1531–1540. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan L, Okamura H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur J Neurosci. 2002;15:1153–1162. doi: 10.1046/j.1460-9568.2002.01955.x. [DOI] [PubMed] [Google Scholar]
- 50.Quintero JE, Kuhlman SJ, McMahon DG. The biological clock nucleus: a multiphasic oscillator network regulated by light. J Neurosci. 2003;23:8070–8076. doi: 10.1523/JNEUROSCI.23-22-08070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 52.Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 53.Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol. 2004;468:361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turek FW, Swann J, Earnest DJ. Role of the circadian system in reproductive phenomena. Recent Prog Horm Res. 1984;40:143–183. doi: 10.1016/b978-0-12-571140-1.50009-8. [DOI] [PubMed] [Google Scholar]
- 55.Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: timing is everything. Horm Behav. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plant TM. Time courses of concentrations of circulating gonadotropin, prolactin, testosterone, and cortisol in adult male rhesus monkeys (Macaca mulatta) throughout the 24 h light-dark cycle. Biol Reprod. 1981;25:244–252. doi: 10.1095/biolreprod25.2.244. [DOI] [PubMed] [Google Scholar]
- 57.Dubey AK, Puri CP, Puri V, Anand Kumar TC. Day and night levels of hormones in male rhesus monkeys kept under controlled or constant environmental light. Experientia. 1983;39:207–209. doi: 10.1007/BF01958904. [DOI] [PubMed] [Google Scholar]
- 58.Kriegsfeld LJ, LeSauter J, Hamada T, Pitts S, Silver R. Circadian rhythms in the endocrine system. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, brain and behavior. New York: Academic Press; 2002. [Google Scholar]
- 59.Alleva JJ, Waleski MV, Alleva FR. A biological clock controlling the estrous cycle of the hamster. Endocrinology. 1971;88:1368–1379. doi: 10.1210/endo-88-6-1368. [DOI] [PubMed] [Google Scholar]
- 60.Fitzgerald K, Zucker I. Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci USA. 1976;73:2923–2927. doi: 10.1073/pnas.73.8.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stetson MH, Anderson PJ. Circadian pacemaker times gonadotropin release in free-running female hamsters. Am J Physiol. 1980;238:R23–R27. doi: 10.1152/ajpregu.1980.238.1.R23. [DOI] [PubMed] [Google Scholar]
- 62.Morin LP, Fitzgerald KM, Rusak B, Zucker I. Circadian organization and neural mediation of hamster reproductive rhythms. Psychoneuroendocrinology. 1977;2:73–98. doi: 10.1016/0306-4530(77)90035-x. [DOI] [PubMed] [Google Scholar]
- 63.Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384:569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 64.Watson RE, Jr, Langub MC, Jr, Engle MG, Maley BE. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res. 1995;689:254–264. doi: 10.1016/0006-8993(95)00548-5. [DOI] [PubMed] [Google Scholar]
- 65.Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- 66.Hastings MH. Neuroendocrine rhythms. Pharmacol Ther. 1991;50:35–71. doi: 10.1016/0163-7258(91)90072-t. [DOI] [PubMed] [Google Scholar]
- 67.Davidson AJ, Menaker M. Birds of a feather clock together–sometimes: social synchronization of circadian rhythms. Curr Opin Neurobiol. 2003;13:765–769. doi: 10.1016/j.conb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 68.Davidson AJ, Tataroglu O, Menaker M. Circadian effects of timed meals (and other rewards) Methods Enzymol. 2005;393:509–523. doi: 10.1016/S0076-6879(05)93026-7. [DOI] [PubMed] [Google Scholar]
- 69.Mrosovsky N. Phase response curves for social entrainment. J Comp Physiol A. 1988;162:35–46. doi: 10.1007/BF01342701. [DOI] [PubMed] [Google Scholar]
- 70.Mrosovsky N, Reebs SG, Honrado GI, Salmon PA. Behavioural entrainment of circadian rhythms. Experientia. 1989;45:696–702. doi: 10.1007/BF01974561. [DOI] [PubMed] [Google Scholar]
- 71.Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- 72.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor α: specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 73.Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neurosci Biobehav Rev. 2004;28:55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 74.Fahrbach SE, Meisel RL, Pfaff DW. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol Behav. 1985;35:985–992. doi: 10.1016/0031-9384(85)90270-7. [DOI] [PubMed] [Google Scholar]
- 75.Morin LP. Effect of ovarian hormones on synchrony of hamster circadian rhythms. Physiol Behav. 1980;24:741–749. doi: 10.1016/0031-9384(80)90406-0. [DOI] [PubMed] [Google Scholar]
- 76.Morin LP, Cummings LA. Splitting of wheelrunning rhythms by castrated or steroid treated male and female hamsters. Physiol Behav. 1982;29:665–675. doi: 10.1016/0031-9384(82)90236-0. [DOI] [PubMed] [Google Scholar]
- 77.Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 78.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 79.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 80.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 81.Shughrue PJ, Komm B, Merchenthaler I. The distribution of estrogen receptor-β mRNA in the rat hypothalamus. Steroids. 1996;61:678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- 82.De La Iglesia HO, Blaustein JD, Bittman EL. Oestrogen receptor-α-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol. 1999;11:481–490. doi: 10.1046/j.1365-2826.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- 83.Abizaid A, Mezei G, Thanarajasingam G, Horvath TL. Estrogen enhances light-induced activation of dorsal raphe serotonergic neurons. Eur J Neurosci. 2005;21:1536–1546. doi: 10.1111/j.1460-9568.2005.03964.x. [DOI] [PubMed] [Google Scholar]
- 84.Morin LP, Cummings LA. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol Behav. 1981;26:825–838. doi: 10.1016/0031-9384(81)90106-2. [DOI] [PubMed] [Google Scholar]
- 85.Jechura TJ, Walsh JM, Lee TM. Testicular hormones modulate circadian rhythms of the diurnal rodent, Octodon degus. Horm Behav. 2000;38:243–249. doi: 10.1006/hbeh.2000.1624. [DOI] [PubMed] [Google Scholar]
- 86.Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus) Proc Natl Acad Sci USA. 1975;72:3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148:5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daan S, Oklejewicz M. The precision of circadian clocks: assessment and analysis in Syrian hamsters. Chronobiol Int. 2003;20:209–221. doi: 10.1081/cbi-120019309. [DOI] [PubMed] [Google Scholar]
- 89.Pittendrigh CS, Daan S. The stability and lability of spontaneous frequency. J Comp Physiol. 1976;106:223–252. [Google Scholar]
- 90.Kashon ML, Arbogast JA, Sisk CL. Distribution and hormonal regulation of androgen receptor immunoreactivity in the forebrain of the male European ferret. J Comp Neurol. 1996;376:567–586. doi: 10.1002/(SICI)1096-9861(19961223)376:4<567::AID-CNE6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 91.Wu SS, Nathanielsz PW, McDonald TJ. Immunocytochemical distribution of androgen receptors in the hypothalamus and pituitary of the fetal baboon in late gestation. Brain Res Dev Brain Res. 1995;84:278–281. doi: 10.1016/0165-3806(94)00184-2. [DOI] [PubMed] [Google Scholar]
- 92.Rees HD, Michael RP. Brain cells of the male rhesus monkey accumulate 3H-testosterone or its metabolites. J Comp Neurol. 1982;206:273–277. doi: 10.1002/cne.902060307. [DOI] [PubMed] [Google Scholar]
- 93.Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 94.Swaab DF. Development of the human hypothalamus. Neurochem Res. 1995;20:509–519. doi: 10.1007/BF01694533. [DOI] [PubMed] [Google Scholar]
- 95.Hofman MA, Zhou JN, Swaab DF. Suprachiasmatic nucleus of the human brain: an immunocytochemical and morphometric analysis. Anat Rec. 1996;244:552–562. doi: 10.1002/(SICI)1097-0185(199604)244:4<552::AID-AR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 96.Robinson SM, Fox TO, Dikkes P, Pearlstein RA. Sex differences in the shape of the sexually dimorphic nucleus of the preoptic area and suprachiasmatic nucleus of the rat: 3-D computer reconstructions and morphometrics. Brain Res. 1986;371:380–384. doi: 10.1016/0006-8993(86)90380-x. [DOI] [PubMed] [Google Scholar]
- 97.Guldner FH. Numbers of neurons and astroglial cells in the suprachiasmatic nucleus of male and female rats. Exp Brain Res. 1983;50:373–376. doi: 10.1007/BF00239203. [DOI] [PubMed] [Google Scholar]
- 98.Guldner FH. Sexual dimorphisms of axospine synapses and postsynaptic density material in the suprachiasmatic nucleus of the rat. Neurosci Lett. 1982;28:145–150. doi: 10.1016/0304-3940(82)90143-4. [DOI] [PubMed] [Google Scholar]
- 99.Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of the circadian system in the golden hamster. Am J Physiol. 1980;238:R97–R101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]
- 100.Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. Am J Physiol. 1983;244:R93–R105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- 101.Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241:R62–R66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- 102.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 103.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 104.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 105.Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- 106.Jean-Faucher C, Berger M, de Turckheim M, Veyssiere G, Jean C. Developmental patterns of plasma and testicular testosterone in mice from birth to adulthood. Acta Endocrinol (Copenh) 1978;89:780–788. doi: 10.1530/acta.0.0890780. [DOI] [PubMed] [Google Scholar]
- 107.Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- 108.Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- 109.Hastings MH, Reddy AB, Garabette M, King VM, Chahad-Ehlers S, O’Brien J, Maywood ES. Expression of clock gene products in the suprachiasmatic nucleus in relation to circadian behaviour. Novartis Found Symp. 2003;253:203–217. doi: 10.1002/0470090839.ch15. discussion 102–209, 218–222, 281–284. [DOI] [PubMed] [Google Scholar]
- 110.Inagaki N, Honma S, Ono D, Tanahashi Y, Honma K. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc Natl Acad Sci USA. 2007;104:7664–7669. doi: 10.1073/pnas.0607713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rohling J, Meijer JH, Vanderleest HT, Admiraal J. Phase differences between SCN neurons and their role in photoperiodic encoding: a simulation of ensemble patterns using recorded single unit electrical activity patterns. J Physiol Paris. 2007;100:261–270. doi: 10.1016/j.jphysparis.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 112.VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, Meijer JH. Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol. 2007;17:468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 113.Doherty PC, Sheridan PJ. Uptake and retention of androgen in neurons of the brain of the golden hamster. Brain Res. 1981;219:327–334. doi: 10.1016/0006-8993(81)90295-x. [DOI] [PubMed] [Google Scholar]
- 114.Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134:2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]
- 115.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents: pacemaker structure: a clock for all seasons. J Comp Physiol A. 1976;106:333–355. [Google Scholar]
- 116.Iwahana EI, Karatsoreos IN, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptor in mice. Horm Behav. doi: 10.1016/j.yhbeh.2007.11.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kalsbeek A, Kreier F, Fliers E, Sauerwein HP, Romijn JA, Buijs RM. Circadian control of metabolism by the suprachiasmatic nuclei. Endocrinology. 2007;148:5635–5639. doi: 10.1210/en.2007-0776. [DOI] [PubMed] [Google Scholar]