Cooperative elastic stresses, the hydrophobic effect, and lipid tilt in membrane remodeling (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 26.
Summary
One of the fundamental properties of biological membranes is the high lateral integrity provided by the lipid bilayer, the structural core and the foundation of their barrier function. This tensile strength is due to the intrinsic properties of amphiphilic lipid molecules, which spontaneously self-assemble into a stable bilayer structure due to the hydrophobic effect. In the highly dynamic life of cellular membrane systems, however, this integrity has to be regularly compromised. Membranes constantly change their topology, so specialized proteins have been developed to control membrane fusion and fission. The involvement of highly-curved membrane intermediates in such processes is now well established. Here we review the latest experimental and theoretical data on how such deformations can be conducted.
Introduction
The integrity of cellular membranes is provided by lipid bilayers, bimolecular films consisting of amphiphilic molecules held together due to the propensity of lipids tails to self-assemble into layers expelling water, known as hydrophobic effect [1,2]. The high energy penalty for formation of the oil-water interface makes pulling a single lipid out of the bilayer a hard work, estimated as at least 10 times higher than kBT, the characteristic energy of thermal fluctuations at physiological temperatures [3–5]. Thus, once assembled, the lipid bilayer remains stable, the lipid heads screening the oily tails from water. The headgroups also guard the membrane in the crowded intracellular environment – they repel the apposing membranes via electrostatic repulsion and at shorter, nanometer distances, via strong hydration repulsion provided by the “surface water”, the ordered water layer associated with the lipid headgroups [2,6]. Thus membranes tend to stay as closed continuous surfaces “covered” by two layers of tightly packed, fully hydrated headgroups. This structure is at the core of the “barrier function” of the cellular membranes separating the intracellular space into distinct compartment and guarding their hydrophilic contents. Importantly, it does not compromise the morphological plasticity of membrane systems, as lipid bilayers remain bendable. Indeed, due to their small thickness of lipid bilayers, their bending modulus k generally varies between 10 and 40 kBT [7] and the corresponding bending energy per lipid EI is small even in highly bent membrane vesicles (E = 8Π_k_/Nlipids ≤ kBT for 50nm vesicle). Nor did it prevent membrane remodeling, as cells evolved ways to change topological structure of their membrane systems. New closed membrane compartments are routinely produced (by the process of membrane fission) and existing compartments unified to exchange their contents (by the process of membrane fusion). Those processes, termed here topological remodeling, are the core elements of intracellular membrane transport, secretion and other morphological transformations of the cellular membrane systems [8,9]. However, the energy of these transformations is substantially higher than a kBT, as topological remodeling implies that the structural stability of the membrane barrier(s) will be transiently compromised. Thus such remodeling in cells is usually conducted by specialized proteo-lipid complexes designed as effective transducer of energy into the membrane deformations [8,10,11].
Cells take extreme care to separate their external and internal world milieu (the non-specific loss of this separation usually indicates a pathological process, such as apoptosis, characterized by leakage [12]). It follows that remodeling cannot be done by simple cut and reseal. Intuitively, membranes are to be squished together so that a tight contact is formed first and then the lipid bilayers are rearranged within the contact area to allow for fusion or fission, preferably without allowing any leakage (Figure 1), as proposed decades ago [13–15]. However, it was also realized that closely apposed lipid bilayers form a uniform lamellar phase, characterized by equilibrium separation between the lamella, determined by the balance between attractive Van Der Waals and repulsive forces [14,15]. This stable lamellar arrangement can be compromised by factors (such as Ca2+) altering hydration and/or the average configurational volumes of the lipid molecules (termed lipid intrinsic curvature), leading to bending of monolayers into non-bilayer lipid arrangements [14–17]. These phases are also formed by lipid species directly involved in regulation of membrane remodeling in cells [18,19]. At high densities, these components also impair the structural stability of a membrane [13], so if they act in biological remodeling their action must be confined. Thus, the hypothesis emerged that membrane remodeling in cells relies on the formation of localized non-bilayer intermediates, i.e. local and transient connections between apposed membranes [14,15,20,21]. Since then, tremendous efforts have been applied to detect and describe such connections, leading to establishment of the stalk paradigm [16,17].
Figure 1.
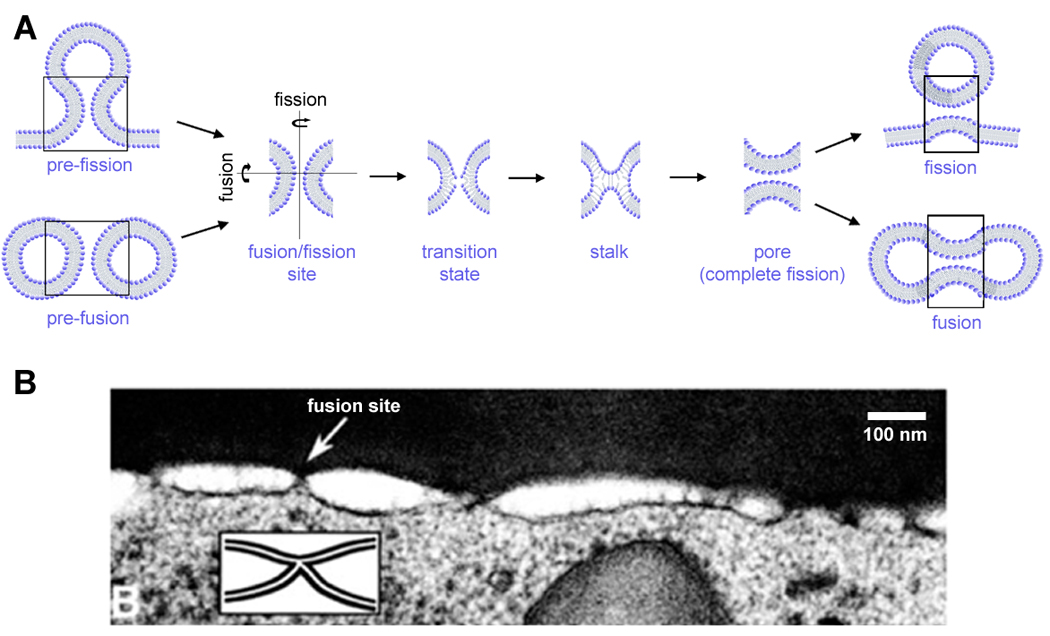
Membrane fusion and fission as localized membrane restructuring. A. A simplified representation of the pathway of membrane fusion and fission illustrating the common intermediates and the transition stage characterized by formation of a defect (“opening(s)”) in the contacting membrane monolayer(s). B. Electron microscopy resolves formation of membrane protrusions, induced by GPI-HA, leading to formation of contact points between fusion membranes.
Early studies considered the stalk, an hour-glass connection between two membranes, as a hypothetical intermediate, a stationary (although short-living) structure [13]. Direct experimental detection to verify the existence of the stalk in a biological process is still lacking. However, multiple indirect proofs have been accumulated from the experimental studies on lipid sensitivity of fusion, theoretical estimations and simulations (reviewed in [22,23]). These works have outlined the main geometrical features and energetics of the stalk intermediate. Importantly, this intermediate has been recovered in numerical simulations [23], confirming the ability of lipids to self-assemble in such structures under defined constraints. Furthermore, recent results indicate the involvement of similar structures in membrane fission [24].
The initial hypothesis of a stationary stalk intermediate, although being extremely successful in predicting the effects of lipids, cannot explain how the hydration repulsion is overcame and the lipid bilayer “opens up” to let the stalk form (Figure 1). It ascribes the main energy barrier of the fusion reaction to the elastic energy of the stalk. Satisfactory as an initial approach, it is being extensively developed in the recent literature to account for the whole pathway of the membrane remodeling (reviewed in [22]).
These studies reiterate the role of elastic (curvature and tensile) stresses at the initiation of membrane remodeling, as has been suggested earlier [15]. In application for protein-driven membrane shaping, these studies lead to refinement of the ways in which the protein machinery can affect the intrinsic pathway of membrane remodeling defined by minimization of the energy of lipid intermediates. We will discuss the newly proposed pathways of lipid rearrangement in the contact zone and corresponding structural features of the proteo-lipid machineries evolved to regulate the cooperative membrane deformations needed for topological remodeling.
The fusion site
Intracellular membrane fusion must proceed in a heterogeneous and dynamic membrane environment. This fact puts severe restrictions on the process: rearrangements need to be fast (often triggered) and localized (especially when small and tightly packed membrane compartments fuse, since there is simply no space for a large fusion machine). Indeed, electrophysiological measurements directly indicate the nanometer size range of the detectable metastable intermediates, the fusion and fission pores (Figure 1A, [24–26]). Other restrictions come from the lipid resistance to be deformed to fit into extremely curved stalk. Thus fusion (and for that matter fission) appears in distinct fusion sites well packed with the specialized proteins capable of providing sufficient energy for such deformations (e.g. SNARE proteins, influence hemagglutinin (HA) or dynamin). Those sites are transient and highly energized formations, often in a metastable state, which allows for fast triggering of membrane remodeling. Perhaps, the most illustrative are the dramatic changes in membrane geometry preceding fusion by HA, when multiple localized connections form as revealed by electron microscope imaging (Figure 1B, [27]). However, higher resolution images are still needed to resolve the structure of the fusion complexes at the relevant scale of nanometers.
Our knowledge about fusion sites has expanded dramatically during the last decade, revealing clear evidence of force transduction from proteins to lipids. Perhaps one of the central problems has been (and still remains) the role of lipids. Experimental data support their deep involvement, as summarized in several excellent reviews [17,22]. The generally accepted paradigm stipulates that proteins bring membranes to the state of “spontaneous fusion”, from which lipids gain the control on the process. Lipid elasticity defines the final outcome of the membranes’ interaction. The assumption of spontaneous fusion upon reaching membrane proximity (the proximity hypothesis) has been supported by both experimental data and simulations, demonstrating that pure lipid bilayers, being brought sufficiently close together, can indeed fuse under certain conditions [22,23]. In this framework, fusion and fission (at its final, most critical stage) can be described in terms of spontaneous (stochastic) transitions between arbitrary defined states (Figure 1), the transition probability being defined (at the simplest level) by the energy difference between the states.
However, in cellular systems there is a clear “protein sensitivity” of fusion, i.e. a clear difference in the kinetics of fusion of the same two membranes conducted by different fusion proteins, indicating that the simples forms of the proximity hypothesis would not be valid [28]. Furthermore, there are many indications in the literature that spontaneous fusion of pure lipid systems is highly parametrically sensitive. One needs to construct the fusion or fission sites accurately in order for the stalk energy to be within the reach. It involves tuning of the lipid composition, contact geometry and elastic stresses applied to the membranes [24,29–34]. The same requirements, establishing the tight membrane contact, adding non-bilayer lipids and/or tension, are often evident in simulations works [35–37]. However, some simulations as well as experimental works indicate that the stresses theoretically required to initiate membrane rearrangements might be high enough to induce leakage, a non-desirable companion of the remodeling [37,38]. Thus, the proteins governing formation of the fusion/fission sites must provide the equivalent of these special energetic and structural demands to apply stresses leading to membrane remodeling without substantial leakage of contents.
Lipid rearrangements on the way to hemi- fusion and fission: deformation fields and local disorganization of lipid monolayers
The idea that drastic localization of membrane merger would help to avoid extended structural destabilization of lipid bilayer and leakage, is routed several decades back [20]. It is based on a general physical assumption of point defects in lateral membrane organization (which can be either pre-existent, such as impurities, or specifically induced, e.g. by deforming the membranes, as shown in Figure 2A). Such defects can serve as natural nucleation points for the formation of trans-monolayer contacts [14,15]. The main reason is that these defects allows for “dodging” the repulsive forces between the two membranes, via local disordering of the hydration layers and thus triggering an hydrophobic attraction between the defects (Figure 2B). In a simple [15] theoretical framework, this idea corresponds to the semi-stalk hypothesis [13], assuming spontaneous formation of small hydrophobic “openings”, the places where the lipid heads are spread apart so that their tails become exposed (Figure 1, 2A, B). Such openings, if they meet, allow for the reconnection of contacting monolayers of the membranes, directly leading to hemifusion. However, formation of even extremely small semi-stalks requires prohibitively large energies, making the appearance of spontaneous openings in stable lipid lamellae impossible under physiological conditions unless some bulk factors, such as alcohol, are added to the membrane forming solution [39]. In simulations of membrane remodeling, this barrier is revealed as through the force required for pulling lipid tails out of a stable bilayer [3,35]. Thus membranes indeed need to find ways around this barrier.
Figure 2.
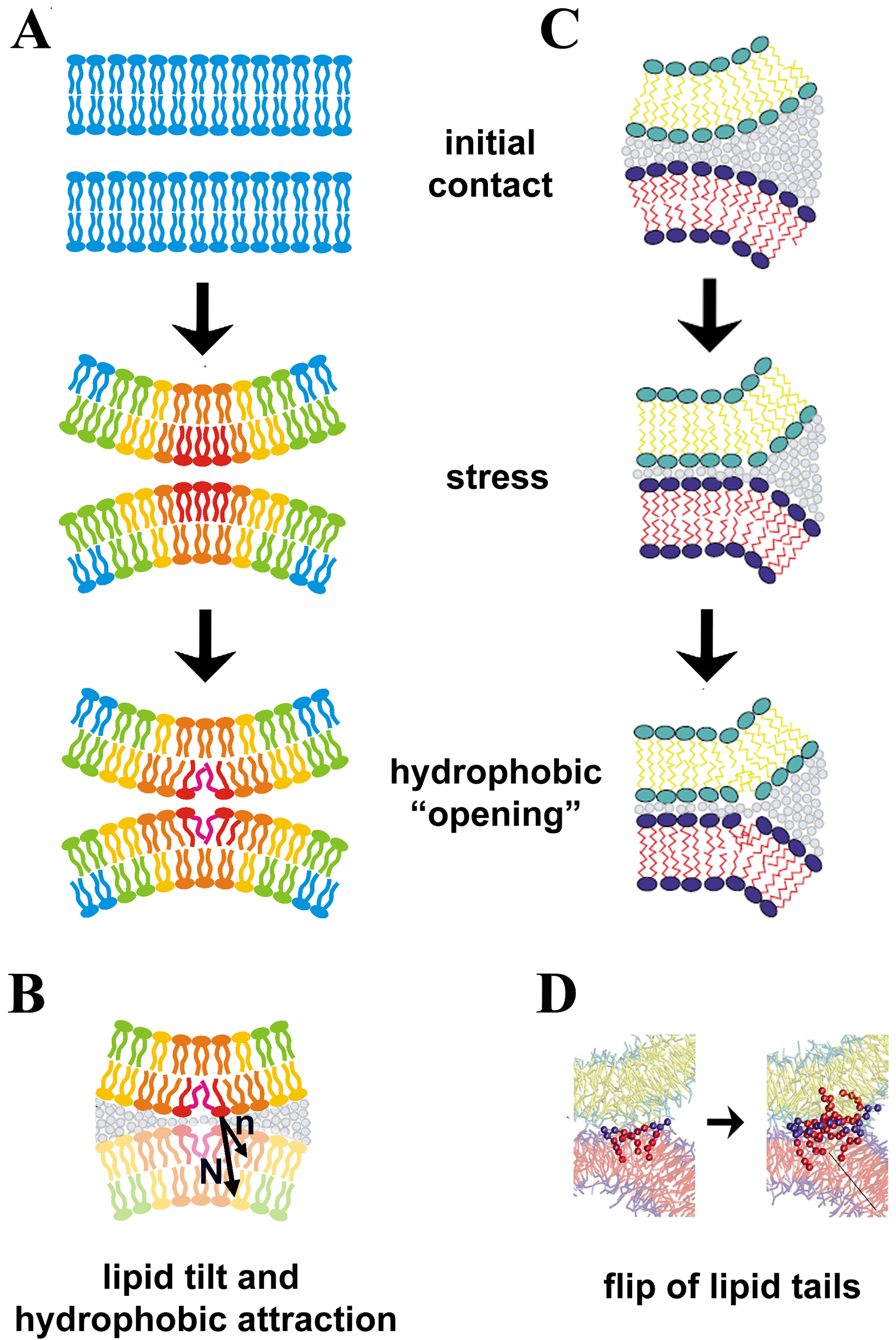
Membrane deformations in fusion and fission. A. Membrane bending with high curvature leads to non-uniform distribution of elastic stress. Note the higher stress (indicated in red) in the contacting monolayers, explaining why they "open up" first while the distal monolayers follow; such sequence ensure the non-leaky membrane remodeling along the hemi-fusion/fission pathway B. Formation of such “openings” can be associated with the tilt of the lipid molecules [24], the deviation of the mean direction (n) of the lipids close to the “opening” from the normal to the neutral surface of the monolayer (N). C. Strong membrane adhesion creates stresses in the perimeter of the adhesion zone leading to singularities in the monolayer continuity and lipid tilting (from [36]). D. Lipids in the perimeter can flip one of their hydrophobic tails into the opposite monolayer, leading to formation of molecular bridges between the contacting monolayers [35,36]
The first, relatively obvious, step was to assume that membrane remodeling is driven by substantial membrane stretching augmenting the probability of defect nucleation [15]. Furthermore, semi-stalks can form and expand cooperatively, helping one-another via disordering of the water in between them, the effect associated with the so called “hydrophobic attraction” of the oily interfaces in water [15,24,29]. Such attraction forces, yet to be measured directly for freely suspended lipid bilayers, act at sub-nanometer distances, so additional forces have to be applied to ensure the requisite close membrane apposition. The need of a close and localized membrane contact led to the concept of small membrane protrusions developing within the fusion sites [29,40]. Depending upon their geometry, such protrusions can support the spatial distribution of elastic stresses so that they maximize (“focus”) at the contact points between the two membranes, thus promoting their merger (Figure 2). This effect is related to the fact that at high membrane curvatures the elastic stresses in two membrane monolayers can not be minimized simultaneously [24,30], so that the stresses can be accumulated in the contacting monolayers, or in the inner monolayer of the fission neck (Figure 2A). This way the sequential rupture of two membrane monolayers can be induced, ensuring a non-leaky membrane remodeling via a hemi-fusion (or hemi-fission) path.
Different types of such protrusions have been considered. The early works considered interaction of membrane undulations, an approach that proved relatively effective for extensive membrane contacts [41]. These works indicate that the assistance of proteins is required for small trans-membrane contacts, typical for cellular membrane systems [29]. Indeed, some fusion proteins, such as influenza hemagglutinin, can induce formation of, membrane protrusions leading to localized membrane contacts in the fusion site, as resolved by electron microscopy (Figure 1, insert) [29]. This conception links curvature stress with the induction of the special kind of membrane deformation only recently considered with rigor for interaction of fluid lipid bilayers, the lipid tilt (Figure 2B, [29,30]). The tilt, or deviation of the mean orientation of the lipid molecule from the surface normal (Figure 2A), has been traditionally associated with gel lipid phases. For fluid-like membranes the energy of such deformation is high so that tilt generally appears in the situation of high elastic stress, such as formation of highly bent membrane intermediates. There, considering the tilt deformation allowed for more effective packing of the lipid tails [29,30], making the phenomenological description of membrane deformations more realistic, and consistent with the results of simulations [35,36]. It also helped to lower dramatically the energies associated with local membrane remodeling, so that now the tilt deformation is generally considered when the shape of highly curved membrane structures is to be calculated.
Recently, the tilting of lipids in the contact area between closely apposed lipid monolayers was directly linked to the induction of hydrophobic openings leading to the self-merger of the inner monolayer of a membrane nanotube (Figure 2B). Thus the curvature-tilt-hydrophobic opening link has been established [24]. We note, however, that, despite the seemingly similar intermediates involved in both processes, the initial deformation fields leading to the monolayer “opening” can be very different for membrane fission and fusion depending upon the imposed symmetries (for two possibilities see Figure 3 below).
Figure 3.
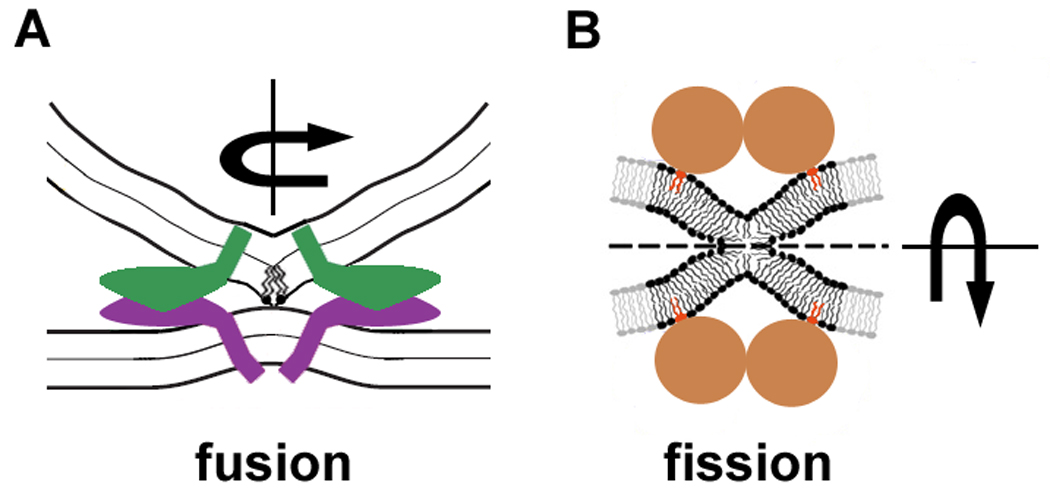
Protein action in membrane remodeling. Fusion (A) and fission (B) proteins encircle the sites of membrane remodeling, they induce membrane deformations characterized by high non-uniform curvature stresses and tilt deformations leading to localize rupture of contacting membrane monolayer(s) inside the fusion/fission site.
The nucleation of cooperative lipid tilting and the formation of packing defects can also be observed in the one-dimensional case, the highly-deformed perimeter of a zone of tight membrane adhesion (Figure 2C). Instabilities in such a perimeter have been detected by simulation techniques (coarse-grained [36], dissipative particle dynamics [35], reviewed by [23]). These works revealed a very interesting phenomenon, the induction of lipid flips between contacting monolayers, i.e. an ability of one of the lipid tails to jump from one monolayer to another forming a single molecular bridge (Figure 2D). Such mono-lipid bridges further self-assemble into structures corresponding to points defects, stalks and finally can expand to a pronounced hemi-fusion (Figure 2D [35]). Importantly, these lipid flips are initiated only when membranes are at very close proximity, corroborating the prediction of Helfrich-based theories and mean field calculations [29,34]. Figure 2B illustrates that close membrane adhesion induces tilt-like deformations over the perimeter of the adhesion zone, where the lipid flipping starts. The analysis of these simulations leads to the hypothesis of energy barriers on the way to forming a metastable stalk intermediate. The hydration repulsion is overcome not by a cooperative (synchronous) tilting of a group of lipids, but one by one, each lipid jumping across the kinetic barrier, so that 2 barriers precede stalk formation (first for a single lipid jump, second, for association of the flipped lipids into a stalk [35]). It remains to be shown experimentally whether lipids jump one by one or cooperatively (i.e. as a whole bunch whose size is defined by energy minimization). However, all of the theoretical approaches agree upon the fact that large elastic stresses and a very specific lipid deformation, tilt, are deeply involved in the regulation of membrane fusion.
The open problem remains the coupling of tilt and the formation of hydrophobic openings to the lipid composition. The fusion probability depends greatly on the spontaneous curvature, seen even in coarse-grained simulations using simplified models of lipid molecules [e.g. [37]]. So called non-bilayer lipid species destabilize the lipid bilayer (as at equilibrium form different, non-bilayer phases) and decrease the energy of curved monolayers of certain geometry. But how the spontaneous curvature affects the initial barrier(s) for membrane remodeling (including the barrier for the lipid flipping) remains unclear.
We add here that similar principles of the accumulation of stress and deformation can be applied for the next transition (from stalk/hemifusion to fusion pore [8,29]). Reversibility of stalk formation indicates that it is a metastable intermediate [37,42], and its development depends critically on the elastic stresses and membrane asymmetry. However, the molecular details of the direct stalk to pore formation (without formation of a macroscopic hemifusion diaphragm) are less studied and are out of the scope of this review.
In artificial model systems this particular deformation field is imposed through boundary conditions, indicating the role of protein insertion. Simply speaking, the point defect requires cooperative action, indicated by the nucleotide sensitivity of the cellular membrane fusion.
Ring-like protein arrangements and topological remodeling: focusing membrane stresses
Relevant structural information about protein complexes specialized in fusion and fission are scarce. Attempts to crystallize or crosslink proteins and complexes might lead to bulk structures that lose their physiological phenotype (e.g. long dynamin spirals [24,43]). But modeling based on biochemical methods, electrophysiology and, recently, simulations indicates that protein complexes arrange in space and in time to produce what is required: stresses correlated with bringing lipid monolayers into close proximity [24,29,44]. Figure 3 illustrates how small arrangements of membrane protein create membrane deformations leading to a local destabilization of monolayer integrity. The key goal of such complexes is the creation of membrane deformation with stress distribution leading to close membrane apposition and merger of contacting monolayers, as depicted in Figure 2. The localization is provided by ring-like assemblies of the proteins leading to the protrusion-type membrane deformations (Figure 3A) or formation of very narrow fission necks (Figure 3B).
In both cases the deformation is transferred from the place of direct proteo-lipid interaction to the contact point, illustrating the principle of action at a distance. This principle, directly corresponding to the needs of creation of stressed membrane contacts, is also related to the structural features of the proteins mediating membrane remodeling. They are generally quite bulky, possessing domains responsible for protein-protein and protein-nucleotide interactions, so they simply cannot be close to the contacting points as they would critically augment steric repulsion between membranes [45]. Several proteins are generally required to fulfill the energy demands for the creation of high membrane curvature (Figure 3). Thus proteins assemble a complex, which can be depicted in a simplified way as a ring-like assembly where proteins act from the boundary of the fusion or fission site (Figure 3). Their action is transmitted to membrane deformations via specialized insertion domains, creation of lipid clusters and curvature scaffolding [46]. They can also act from the outside of the fusion or fission site, via alteration of bulk mechanical stresses (such as lateral tension provided by protein coats far from the point of actual membrane rearrangement [8,47]). These actions assume a high level of integration in membrane remodeling, leading to the concept of specialized domains. There is increasing evidence for the involvement of proteo-lipid membrane domains in membrane trafficking and remodeling [9].
The energy required for membrane deformation comes from coil-coiled zipping, GTP hydrolysis and protein-lipid interactions providing a sufficient basis for the creation of high membrane curvature. The mechanisms of the cooperative mechano-chemical energy transduction are only beginning to be understood. In many cases, especially in fast triggered remodeling (such as membrane fusion leading to neurotransmitter release), the important requirement is to ensure that the conformational change(s) of the proteins are tightly coupled to the membrane remodeling. This is achieved via careful spatial organization of the fusion site, including the pre-accumulation of the curvature stress via auxiliary protein [48]. In minimalistic systems, involving only part of the fusion/fission machinery, the process becomes stochastic. However, cyclic assembly of the fission complexes is made possible by the constant influx of energy provided by a nucleotide. It is important to emphasize once again, however, that optimization of the fusion/fission machinery requires not only careful formation of the corresponding protein complexes, but also lipids: membrane remodeling can be severely inhibited by lipid species modifying the spontaneous curvature and elasticity of the membrane [8,24]. This way, topological membrane remodeling should be considered as a phenomenon where proteins and lipids cooperate to create highly localized critical membrane deformations leading to sequential rearrangements of lipid monolayers of the interacting membrane(s), the foundation of non-leaky fusion and fission of biological membranes.
Acknowledgements
This work was supported by the Intramural Program of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tanford C. The hydrophobic effect: Formation of micelles and biological membranes. John Wiley & Sons Inc; 1973. [Google Scholar]
- 2.Meyer EE, Rosenberg KJ, Israelachvili J. Recent progress in understanding hydrophobic interactions. Proc Natl Acad Sci USA. 2006;103:15739–15746. doi: 10.1073/pnas.0606422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrink SJ, Berger O, Tieleman P, Jahnig F. Adhesion forces of lipids in a phospholipid membrane studied by molecular dynamics simulations. Biophys J. 1998;74:931–943. doi: 10.1016/S0006-3495(98)74016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cevc G, Marsh D. Phospholipid Bilayers: Physical Principles and Models. New York: John Wiley & Sons Inc.; 1987. [Google Scholar]
- 5.Garcia-Manyes S, Sanz F. Nanomechanics of lipid bilayers by force spectroscopy with AFM: A perspective. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamem.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Rand RP, Parsegian VA. Hydration forces between phospholipid bilayers. Biochimica et Biophysica Acta - Reviews on Biomembranes. 1989;988:351–376. [Google Scholar]
- 7.Marsh D. Elastic curvature constants of lipid monolayers and bilayers. Chem Phys Lipids. 2006;144:146–159. doi: 10.1016/j.chemphyslip.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 9.Shnyrova AV, Frolov VA, Zimmerberg J. Domain-driven morphogenesis of cellular membranes. Curr Biol. 2009;19:R772–R780. doi: 10.1016/j.cub.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lentz BR, Malinin V, Haque ME, Evans K. Protein machines and lipid assemblies: current views of cell membrane fusion. Curr Opin Struct Biol. 2000;10:607–615. doi: 10.1016/s0959-440x(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 11.Basanez G. Membrane fusion: the process and its energy suppliers. Cell Mol Life Sci. 2000;59:1478–1490. doi: 10.1007/s00018-002-8523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 13.Chernomordik LV, Melikyan GB, Chizmadzhev YA. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987;906:309–352. doi: 10.1016/0304-4157(87)90016-5. [DOI] [PubMed] [Google Scholar]
- 14.Rand RP, Parsegian VA. Mimicry and mechanism in phospholipid models of membrane fusion. Annu Rev Physiol. 1986;48:201–212. doi: 10.1146/annurev.ph.48.030186.001221. [DOI] [PubMed] [Google Scholar]
- 15.Helm CA, Israelachvili JN, McGuiggan PM. Molecular mechanisms and forces involved in the adhesion and fusion of amphiphilic bilayers. Science. 1989;246:919–922. doi: 10.1126/science.2814514. [DOI] [PubMed] [Google Scholar]
- 16.Chernomordik LV, Zimmerberg J. Bending membranes to the task: structural intermediates in bilayer fusion. Curr Opin Struct Biol. 1995;5:541–547. doi: 10.1016/0959-440x(95)80041-7. [DOI] [PubMed] [Google Scholar]
- 17.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 18.Rigoni M, Caccin P, Gschmeissner S, Koster G, Postle AD, Rossetto O, Schiavo G, Montecucco C. Equivalent effects of snake PLA2 neurotoxins and lysophospholipid-fatty acid mixtures. Science. 2005;310:1678–1680. doi: 10.1126/science.1120640. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Huang HW. Observation of a membrane fusion intermediate structure. Science. 2002;297:1877–1879. doi: 10.1126/science.1074354. [DOI] [PubMed] [Google Scholar]
- 20.Hui SW, Stewart TP, Boni LT, Yeagle PL. Membrane fusion through point defects in bilayers. Science. 1981;212:921–923. doi: 10.1126/science.7233185. [DOI] [PubMed] [Google Scholar]
- 21.Kozlov MM, Markin VS. [Possible mechanism of membrane fusion] Biofizika. 1983;28:242–247. [PubMed] [Google Scholar]
- 22.Tamm LK, Crane J, Kiessling V. Membrane fusion: a structural perspective on the interplay of lipids and proteins. Curr Opin Struct Biol. 2003;13:453–466. doi: 10.1016/s0959-440x(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 23.Marrink SJ, de Vries AH, Tieleman DP. Lipids on the move: simulations of membrane pores, domains, stalks and curves. Biochim Biophys Acta. 2009;1788:149–168. doi: 10.1016/j.bbamem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Bashkirov PV, Akimov SA, Evseev AI, Schmid SL, Zimmerberg J, Frolov VA. GTPase cycle of dynamin is coupled to membrane squeeze and release, leading to spontaneous fission. Cell. 2008;135:1276–1286. doi: 10.1016/j.cell.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spruce AE, Iwata A, White JM, Aimers W. Patch clamp studies of single cell-fusion events mediated by a viral fusion protein. Nature. 1989;342:555–558. doi: 10.1038/342555a0. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald PE, Eliasson L, Rorsman P. Calcium increases endocytotic vesicle size and accelerates membrane fission in insulin-secreting INS-1 cells. J Cell Sci. 2005;118:5911–5920. doi: 10.1242/jcs.02685. [DOI] [PubMed] [Google Scholar]
- 27.Frolov VA, Cho MS, Bronk P, Reese TS, Zimmerberg J. Multiple local contact sites are induced by GPI-linked influenza hemagglutinin during hemifusion and flickering pore formation. Traffic. 2000;1:622–630. doi: 10.1034/j.1600-0854.2000.010806.x. [DOI] [PubMed] [Google Scholar]
- 28.Plonsky I, Kingsley DH, Rashtian A, Blank PS, Zimmerberg J. Initial size and dynamics of viral fusion pores are a function of the fusion protein mediating membrane fusion. Biol Cell. 2008;100:377–386. doi: 10.1042/BC20070040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuzmin PI, Zimmerberg J, Chizmadzhev YA, Cohen FS. A quantitative model for membrane fusion based on low-energy intermediates. Proc Natl Acad Sci U S A. 2001;98:7235–7240. doi: 10.1073/pnas.121191898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozlovsky Y, Kozlov MM. Stalk model of membrane fusion: solution of energy crisis. Biophys J. 2002;82:882–895. doi: 10.1016/S0006-3495(02)75450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozlovsky Y, Kozlov MM. Membrane fission: model for intermediate structures. Biophys J. 2003;85:85–96. doi: 10.1016/S0006-3495(03)74457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markin VS, Albanesi JP. Membrane fusion: stalk model revisited. Biophys J. 2002;82:693–712. doi: 10.1016/S0006-3495(02)75432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malinin VS, Lentz BR. Energetics of vesicle fusion intermediates: comparison of calculations with observed effects of osmotic and curvature stresses. Biophys J. 2004;86:2951–2964. doi: 10.1016/S0006-3495(04)74346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JY, Schick M. Calculation of free energy barriers to the fusion of small vesicles. Biophys J. 2008;94:1699–1706. doi: 10.1529/biophysj.107.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grafmuller A, Shillcock J, Lipowsky R. Pathway of membrane fusion with two tension-dependent energy barriers. Phys Rev Lett. 2007;98:218101. doi: 10.1103/PhysRevLett.98.218101. [DOI] [PubMed] [Google Scholar]
- 36.Stevens MJ, Hoh JH, Woolf TB. Insights into the molecular mechanism of membrane fusion from simulation: evidence for the association of splayed tails. Phys Rev Lett. 2003;91:188102. doi: 10.1103/PhysRevLett.91.188102. [DOI] [PubMed] [Google Scholar]
- 37.Muller M, Katsov K, Schick M. A new mechanism of model membrane fusion determined from Monte Carlo simulation. Biophys J. 2003;85:1611–1623. doi: 10.1016/S0006-3495(03)74592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frolov VA, Lizunov VA, Dunina-Barkovskaya AY, Samsonov AV, Zimmerberg J. Shape bistability of a membrane neck: a toggle switch to control vesicle content release. Proc Natl Acad Sci U S A. 2003;100:8698–8703. doi: 10.1073/pnas.1432962100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chanturiya A, Leikina E, Zimmerberg J, Chernomordik LV. Short-chain alcohols promote an early stage of membrane hemifusion. Biophys J. 1999;77:2035–2045. doi: 10.1016/S0006-3495(99)77044-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Efrat A, Chernomordik LV, Kozlov MM. Point-like protrusion as a prestalk intermediate in membrane fusion pathway. Biophys J. 2007;92:L61–L63. doi: 10.1529/biophysj.106.103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leikin SL, Kozlov MM, Chernomordik LV, Markin VS, Chizmadzhev YA. Membrane fusion: overcoming of the hydration barrier and local restructuring. J Theor Biol. 1987;129:411–425. doi: 10.1016/s0022-5193(87)80021-8. [DOI] [PubMed] [Google Scholar]
- 42.Katsov K, Muller M, Schick M. Field theoretic study of bilayer membrane fusion: II. Mechanism of a stalk-hole complex. Biophys J. 2006;90:915–926. doi: 10.1529/biophysj.105.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pucadyil TJ, Schmid SL. Real-time visualization of dynamin-catalyzed membrane fission and vesicle release. Cell. 2008;135:1263–1275. doi: 10.1016/j.cell.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu S, Guo H. Simulation Study of Protein-Mediated Vesicle Fusion. The Journal of Physical Chemistry B. 2008;113:589–591. doi: 10.1021/jp808776z. [DOI] [PubMed] [Google Scholar]
- 45.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 47.Roux A, Uyhazi K, Frost A, De Camilli P. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 48.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]