Cochlear mechanisms from a phylogenetic viewpoint (original) (raw)
Abstract
The hearing organ of the inner ear was the last of the paired sense organs of amniotes to undergo formative evolution. As a mechanical sensory organ, the inner-ear hearing organ's function depends highly on its physical structure. Comparative studies suggest that the hearing organ of the earliest amniote vertebrates was small and simple, but possessed hair cells with a cochlear amplifier mechanism, electrical frequency tuning, and incipient micromechanical tuning. The separation of the different groups of amniotes from the stem reptiles occurred relatively early, with the ancestors of the mammals branching off first, approximately 320 million years ago. The evolution of the hearing organ in the three major lines of the descendents of the stem reptiles (e.g., mammals, birds-crocodiles, and lizards-snakes) thus occurred independently over long periods of time. Dramatic and parallel improvements in the middle ear initiated papillar elongation in all lineages, accompanied by increased numbers of sensory cells with enhanced micromechanical tuning and group-specific hair-cell specializations that resulted in unique morphological configurations. This review aims not only to compare structure and function across classification boundaries (the comparative approach), but also to assess how and to what extent fundamental mechanisms were influenced by selection pressures in times past (the phylogenetic viewpoint).
The hearing organs of modern amniotes (reptiles, birds, and mammals) show an almost bewildering variety of morphologies. This variety provides the functional morphologist with an exciting natural experiment to assess the functional consequences of structural diversity in an organ whose function largely depends on just this structure. To do this presupposes an understanding of (i) the evolutionary history of the amniotes, (ii) the extent and nature of the morphological variation, and (iii) those mechanisms of hearing that are directly influenced by morphology. This review emphasizes cochlear mechanisms that may have been influenced by morphological changes during amniote phylogeny. As is now customary, the term cochlear will be used loosely for the mechanisms involved in the hearing organ of the cochlear duct (Scala media) of all amniotes.
The present discussion is based on certain assumptions, the most important being that the hearing organs of all amniotes are homologous (1). They have a common ancestry, share a common structure, and develop from the same genetic substrate, and their position in the organisms'Bauplan is the same. Thus the functional units of the hearing process—the hair cells and their innervating nerve fibers—are also homologous.
A second assumption, based on the comparative anatomy and physiology of putatively primitive amniote hearing organs, is that the archetypal auditory papilla was short (≈1 mm) with only a few hundred hair cells. It is assumed that these hair cells (i) had inherited electrical tuning from their vestibular-system ancestors, (ii) were innervated by both afferent and efferent nerve fibers, and (iii) contained an active process in their stereovillar bundles (2).
The final assumption is that evolution is a conservative process. Rather than developing functions or structures de novo, existing structures and processes tend to be modified, sometimes to accomplish new tasks. This conservatism also applies, of course, to subcellular molecular mechanisms. Using comparative studies of extant vertebrates, I will estimate how inner-ear mechanisms have been modified during phylogeny. To begin, it is appropriate to briefly introduce middle-ear evolution and the different hearing-organ morphologies of modern amniote lineages.
The Tympanic Middle Ear as the Initiator of Profound Change
The development of a tympanic middle ear in the late Paleozoic was crucial to the initiation of selection pressures for further phylogeny of the hearing organ. In early stem reptiles, there was little resembling what we would now refer to as a tympanic middle ear, i.e., an efficient impedance-matching device between air and water, with a tympanum moved by sound-pressure variations in air. Recent paleontological studies indicate that a tympanic ear was established in all lineages during the Triassic [250–220 million years (MY) ago, Fig. 1], 150 MY after the origin of the amniotes (3). This was 100 MY after mammal-like reptiles (synapsids) and 75 MY after the avian-crocodilian and lizard-snake lineages (referred to below as avian and lizard lineages) diverged from stem reptiles. We do not know which selective pressures led to the essentially simultaneous development of tympanic ears in all lineages. The new middle ears enabled the perception of higher frequencies, producing selection pressures for sensitivity to high-frequency sounds (>1 kHz).
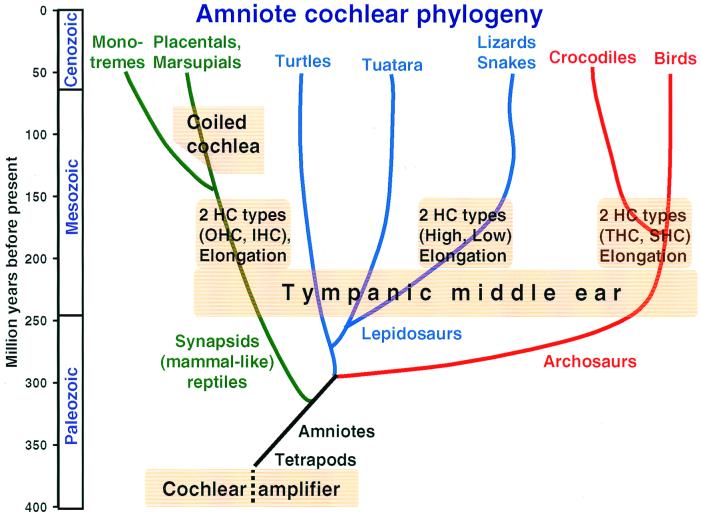
Figure 1.
Highly schematic representation of the amniote phylogenetic tree over 400 million years to illustrate the approximate time of origin of particular features of auditory systems. Amniotes arose from the earliest tetrapods early in the paleozoic and inherited from them a simple hearing organ with a cochlear amplifier in the stereovillar bundles. Apart from the lineages to the turtles and the Tuatara, that remained primitive in a number of respects, three main lineages to modern amniotes are distinguished here. Splitting off first were mammalian ancestors, which gave rise to both the egg-laying monotremes and the marsupial-placental line. Later, the archosaur line originated and led to the dominant land organisms of the mesozoic. Of these, only the crocodile-alligator and bird groups survived to modern times. The last group to split off was the lizards and snakes within the lepidosaurs. The tympanic middle ear originated independently in all groups during the Triassic, initiating the evolution of unique configurations of papillae, with all groups showing papillar elongation and hair-cell specializations. In mammals IHC and OHC, in birds THC and SHC populations developed. In lizards, the populations segregated along frequency lines (low- and high-frequency populations). Because the hair-cell populations in the monotreme and marsupial-placental mammal groups are so similar, they almost certainly arose before these lineages diverged. The same applies to the birds and Crocodilia. In lizards, there are great family-specific variations, suggesting that these hair-cell populations arose soon after the Triassic. Because monotremes do not have a coiled cochlea, coiling almost certainly developed in the marsupial-placental lineage. [Modified after ref. 5 and used with permission of Wiley-VCH Press (Copyright 2000, Wiley).]
This transformation had very profound consequences for the auditory papilla, for which the following sequence of evolutionary developments is likely. The refinement of micromechanical tuning extended the frequency range and made papillar elongation an advantage, enlarging the frequency space constants (Fig. 1). The increase in the number of sensory cells permitted the specialization of two hair-cell populations. In lizards, the two populations are at different locations along the tonotopic gradient, whereas in birds and mammals, they are separated across the papilla but present all along the tonotopic gradient. In birds and mammals, a new division of labor specialized one of the two populations for cochlear amplification. The independent evolution of larger and more specialized auditory organs also led to size increases and specialization of the neural auditory pathway. Their parallel evolution may explain the difficulties of establishing homologies between brain nuclei in different groups (e.g., ref.4).
The tympanic ear of mammals evolved differently to that of other groups. In the mammalian lineage, the jaw joint shifted from the primary to the more superficial secondary joint. The quadrate (malleus) and articular (incus), in a quirk of phylogeny, were integrated into the chain of three ossicles. This initially achieved the same result as the single-ossicle system of the avian and lizard lineages. Once selection pressures for responses to higher frequencies had begun to work, however, the mammalian solution was, quite accidentally, better at transmitting high frequencies (Fig. 2) (5).
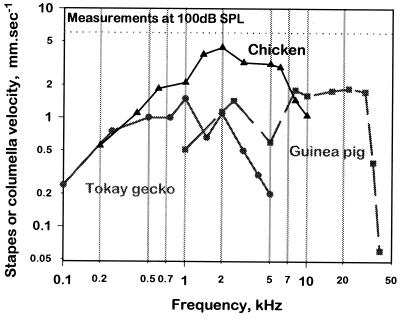
Figure 2.
Middle-ear sensitivity in representatives of three amniote groups, shown as the velocity of the center of the eardrum (the tip of the malleus or extracolumella) as a function of frequency at 100 dB sound pressure level (SPL). At this sound pressure, air particles have a velocity of 6 mm/sec (dashed line). The Tokay gecko (gray line, ●) is a sensitive lizard (6), the chicken (black line, ▴) represents birds (7), and the guinea pig (gray line, ■) represents mammals (8). The main difference observed is not in sensitivity, but in the upper frequency limits and the high-frequency flanks.
The Lineages of Modern Amniotes and Their Characteristic Hearing-Organ Morphologies
There are four basic types of amniote ear, in largely natural, i.e., phylogenetic, groups (9). I will refer to mammalian, avian (including Crocodilia), lizard (including snakes), and turtle (standing for a stem-reptile or unspecialized type) lineages. Mammalian, avian, and lizard papillae show unique constellations of features, suggesting that these features developed early in their respective phylogenies. Except for the turtles, all groups developed a sensitivity to high frequencies through unique morphological changes (2).
Turtles and Tuataras Probably Represent the Unspecialized State of the Ear.
Turtles (and the Tuatara lizard, Sphenodon) are regarded as primitive (9) and have the least specialized hearing organ (Fig.3) (10–12), most likely to resemble that of early stem reptiles. The hair cells are unspecialized, innervated by both afferent and efferent nerve fibers, and respond only to low frequencies (<1 kHz). The stereovillar bundles of almost all hair cells are uniformly oriented with weak morphological gradients that play only a minor role in determining hair-cell response frequencies. Instead, the ion-channel complement of hair-cell membranes create electrical resonances at preferred frequencies (e.g., ref. 13). The calibration of the individual cell is achieved by varying the number and kinetics of the channels (Fig.4D), with preferred frequencies from <100 Hz apically to about 600 Hz basally.
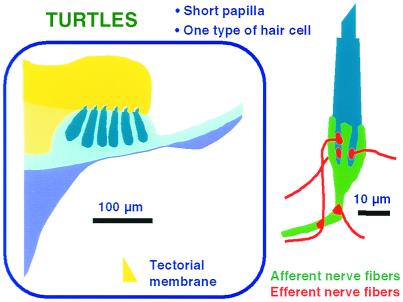
Figure 3.
A schematic summary of the structure of the auditory papilla in a primitive amniote, the red-eared turtle. Most of the hair cells are placed over the BM and covered by a thick TM (yellow). There is only one type of hair cell, which is innervated by both afferent and efferent fibers (Right).
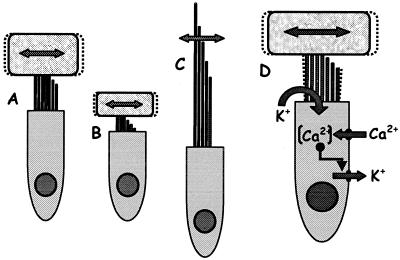
Figure 4.
(A_–_C) Basic hair-cell structures contributing to micromechanical frequency tuning. In most cases, a tectorial structure covers the hair-cell bundle. A hair cell with a taller stereovillar bundle and larger tectorial cover (A) responds best to much lower frequencies than a hair cell with a shorter bundle and less massive tectorial structure (B). Equivalent frequencies in hair cells without tectorial structures (C) result from much taller bundles. (D) Schematic of the ion channels involved in electrical tuning of hair cells. The flow of ions (mainly K+) through transduction channels depolarizes the cell and activates voltage-sensitive Ca2+ channels in the baso-lateral membrane, raising the Ca2+ concentration [Ca2+] in the cell and activating Ca2+-sensitive K+ channels. This leads to K+ outflow, hyperpolarization, and a new cycle. Channel number, kinetics, and the temperature determine the frequency of oscillation. In turtles, frequencies between 50 and 600 Hz have been measured, but in other amniotes, higher frequencies are reached (see text).
The basilar papilla was the first hair-cell organ to form over a moveable membrane (basilar membrane, BM). In turtles, as in lizards (see below), the BM shows no special frequency selectivity (14). In birds, there is some selectivity (15). In mammals, BM selectivity is the same as that of primary auditory afferents (e.g., ref. 16), but much is because of the motor activity of hair cells; the passive BM response is only crudely frequency selective. The primary focus in understanding the function of the inner ear needs to be on the hair cells themselves.
Lizards—A Playground of Evolution.
The morphology of lizard auditory epithelia varies widely, but tends to be consistent within each family (10, 12, 17). It varies in length from <100 μm to >2 mm and in the number of hair cells from <60 to >2,000 (Fig. 5). Hair cells may be covered by a continuous tectorial membrane (TM), by a TM that is divided into a chain of sallets, or have no TM at all (Fig. 5). The tonotopic organization (the arrangement of frequencies along the epithelium) may run from apex to base or from base to apex. Each lizard family shows particular combinations of features, and hearing-organ structure can even be species-specific.
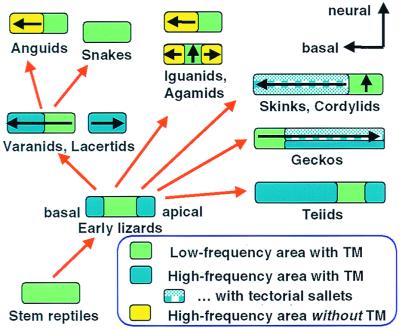
Figure 5.
Highly schematic representation of a possible evolutionary sequence of the papillae of modern lizard families. Where known, the direction of the tonotopicity, from low to high frequencies, is shown (arrow). From stem reptile papillae with uniform hair cells, a papilla arose with new, mirror-imaged, micromechanically tuned hair-cell areas at both ends, flanking the low-frequency (green) area. From this, the various papillar configurations of different lizard families and the snakes can be derived as shown, including the reversed tonotopic organization in geckos. Placing similarly formed papillae together does not necessary imply close systematic relationships. In different families, the TM over the high-frequency areas was either retained (uniformly blue areas), divided up into sallets (patterned blue areas), or lost (yellow areas) (see text; partly after ref. 10). [Modified after ref. 5, and used with permission of Wiley-VCH Press (Copyright 2000, Wiley).]
To reconstruct the phylogeny of the lizard hearing organ, it is necessary to recognize common features between lizard families. The first is that lizard papillae always show two hair-cell types (Fig.6) (10). One papillar area contains hair cells with a greater basal diameter, large numbers of, and larger, afferent nerve fibers and an efferent innervation. In most lizard papillae, hair-cell bundles in such areas all have the same (abneural) orientation. Functionally, these cells always respond to low frequencies (below about 1 kHz; ref. 17). This area is like the entire papilla of turtles, with weak morphological gradients and a similar upper limit (<1 kHz). Its frequency selectivity also may be largely determined by electrical tuning, but there is only indirect evidence for this (18).
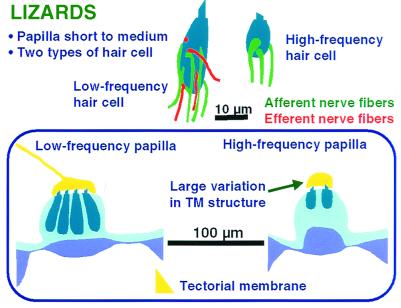
Figure 6.
A schematic summary of the structure of the auditory papilla of lizards, which always have a low- and a high-frequency area. Over the latter, the tectorial structure (yellow) is highly variable between and even within families and is missing in some groups (see Fig. 5). Low- and high-frequency hair cells differ both in their size and their innervation pattern; high-frequency hair cells (Upper Right) never receive an efferent innervation.
The second hair-cell type is characterized by its smaller size, by smaller and fewer afferents, and the complete lack of an efferent innervation (Fig. 6). Almost all such regions have groups of neurally and abneurally oriented hair-cell bundles (bidirectional orientation). These cells always respond to frequencies above about 1 kHz, with an upper limit of at least 4 kHz and are micromechanically tuned. The high-frequency areas show a large variation in the TM; some totally lack a TM. There are sometimes two high-frequency areas located apically and basally (Fig. 5) (10). If there is only one such area, it can be apical (e.g., geckos, in which case the tonotopic organization is reversed as compared with other amniotes, ref. 19) or basal (almost all others).
The evolution of the tympanic middle ear probably initiated the development of the high-frequency hair-cell areas of stem lizards; these are thus a synapomorphy (a derived common feature) of lizards. The two types of lizard hair cells are confined to separate frequency ranges and thus not functionally equivalent to the hair-cell populations of mammals or birds.
As in turtles, the lizard BM is not involved in frequency selectivity (20, 21), and the frequency selectivity of afferent nerve fibers is much higher than that of the BM (20). The primary stimulatory motion of hair cell bundles is transverse to the BM (e.g., ref. 22), and frequency selectivity depends on bundle and TM micromechanics (23).
Birds and Mammals—The Parallel Evolution of Specialized Hair-Cell Types.
Avian and mammalian auditory papillae are characteristically different and easily recognizable. Both have specialized hair-cell populations located across the width of the papilla, essentially at all frequency locations (24), and thus within a continuous tonotopic organization (25). Both groups have—independently—developed responses to high frequencies, in some birds up to 10 kHz, in some mammals even beyond 100 kHz (26, 27). Papillar elongation was generally much more extensive than the maximum of 2 mm found in lizards. Some owl papillae reach 11 mm and some whale papillae 105 mm. The coiled cochlea, which evolved after the divergence of the marsupial-placental line in the late Mesozoic (Fig. 1), was probably simply a mechanism for accommodating a long papilla.
Although the anatomical differentiation of different hair-cell types in birds is not as clear-cut as in mammals, important structural parallels exist between the tall and short hair cells (THCs and SHCs) of birds and the inner and outer hair cells (IHCs and OHCs) of mammals, respectively (Fig. 7) (24). THCs and IHCs are the less specialized hair cells and receive a strong afferent innervation. OHCs are innervated nonexclusively by relatively few afferent fibers (≈5%), and SHCs receive no afferent innervation at all (28).
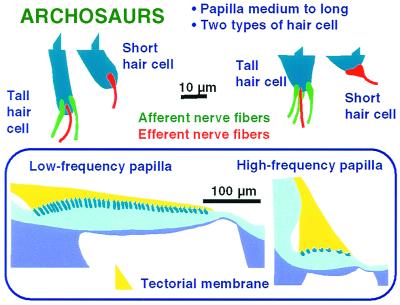
Figure 7.
A schematic summary of the structure of the auditory papilla of birds, which are between 2 and 11 mm in length and contain between 3,000 and 17,000 hair cells. The hair cells form a continuum, with the tallest cells at the apical end (Left, transverse section and typical hair-cell shape), where there are many hair cells across the papilla. Hair cells become shorter toward the base, where the number of hair cells across the papilla is much smaller (Right, transverse section and typical hair-cell shapes), and toward the abneural side. All hair cells are covered by a wedge-shaped TM (yellow). Short hair cells have no afferent innervation.
In mammals, the transition from IHC to OHC is sudden, and the populations are separated by specialized supporting cells. In birds, the transition is usually gradual (Fig. 7). Phylogenetically, the most interesting feature of avian and mammalian papillae that accompanied the development of high-frequency hearing is the setting aside of hair cells as effectors in a cochlear amplification process, as follows: IHCs and THCs retain the classical sensory function, passing information via afferents to the brain. OHCs and SHCs, in contrast, respond to low-level stimuli by creating motion that amplifies stimuli, that are passed on to IHCs or THCs (2). This occurred despite the fact that birds have retained electrical tuning of hair cells (25) and married it seamlessly to micromechanical tuning, whereas mammals appear to have abandoned the ancestral electrical tuning and to rely completely on micromechanics. Perhaps the latter was possible because of—or even necessary for—the development of a new cellular mechanism of amplification (see below).
A uniquely evolved feature of mammals is the intimate involvement of the BM in the response, such that its frequency selectivity is essentially identical to that of IHCs and afferent fibers (e.g., ref.16). The response is thus a feature of the entire organ, and no component is separable without reducing sensitivity and selectivity. This linking is not so well developed in birds (15, 29), perhaps because those hair cells that connect to most of the afferent fibers are not over the free BM, but over the limbus.
Frequency-Selectivity Mechanisms and Tonotopicity from a Phylogenetic Viewpoint
All vertebrate hearing organs, including those of fish and amphibians, consist of frequency-selective hair cells. Of the two mechanisms of frequency selectivity in hair cells (30), the phylogenetically older is electrical tuning of the hair-cell membrane, as described above for the turtle inner ear (Fig. 4). Strong evidence for such tuning is available for hair cells of the frog sacculus and the avian basilar papilla. At the higher temperatures of endotherms such as birds, electrical tuning should function to at least 4 kHz (31). Preferred intervals in the spontaneous activity of primary auditory neurons, a probable correlate of electrical tuning, have been reported from turtles, lizards, and birds (25). In the barn owl, Köppl (32) found preferred intervals in fibers of characteristic frequencies up to 5 kHz. Because both electrical tuning and phase locking work well at low frequencies, there was presumably little selective pressure for developing new selectivity mechanisms until the detection of high frequencies became important.
Responses to higher frequencies are best mediated by the second mechanism providing frequency selectivity, micromechanical tuning (Fig.4 A_–_C). Inner-ear structures are extremely small and have high resonance frequencies. The response frequency is determined by the mass and stiffness of their stereovillar bundles and the mass of any tectorial material covering the hair cells or of fluid coupled to the hair cells. In the later evolution of some groups, especially mammals, larger structures such as the BM were recruited into oscillating units. This resulted in significant changes in supporting-cell structure, influencing the mechanical properties of the entire organ. Thus, although micromechanical (at the hair-cell level) and macromechanical (involving larger structural units) tuning often are distinguished for discussion purposes, in the living organism they form a continuum. Phylogenetic variations on micromechanical tuning are discussed below.
The Factors Influencing Micromechanical Tuning Illustrated by Using Lizard Papillae.
Almost the entire variability in papillar structure between different lizard families is in the high-frequency, micromechanically tuned regions (6). Thus phylogenetically, high-frequency hearing arose near the time when the lizard families diverged. Beginning with small, micromechanically tuned areas at both ends of the low-frequency area, each family specialized these high-frequency areas differently. Despite this, the upper frequency limit is similar in all cases and lies between about 4 and 7 kHz (17).
The two most important determinants of frequency selectivity of micromechanicallytuned units in lizard papillae are (i) the length of the papilla (correlated with the number of hair cells), and (ii) the presence or absence of a tectorial structure and how that structure is shaped (Fig. 8). In a model of frequency tuning developed for the Tokay gecko (23), it was shown that tectorial structures increase sensitivity (the amplitude factor for free-standing hair bundles is only one-third of that for a tectorial sallet system) and frequency selectivity of hair cells (the amplitude maxima of hair-cell bundles without a TM are significantly less sharp than those with one; see also Fig. 8). The difference is largest when comparing free-standing hair cells with those covered by a chain of sallets. Under a continuous TM, the coupling between neighboring frequency regions is stronger, and in short papillae this would negatively influence frequency selectivity. The presence of essentially independent tectorial sallets in hair-cell areas in geckos, skinks, and cordylid lizards thus can be regarded as a method of micromechanical optimization in a small system covering a wide frequency range.
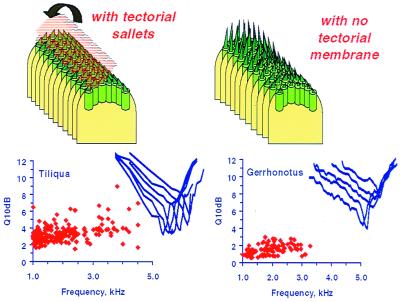
Figure 8.
The effect of TM coupling on the responses of lizard papillae. (Left) The salletal TM is shown lifted, to reveal hair-cell bundles. In the alligator lizard Gerrhonotus (Right), the high-frequency papillar area contains free-standing hair cells that lack a TM over their bundles. The requisite frequency range is attained by a strong gradient in bundle height along the papilla. Here, tuning sharpness of afferent fibers is poor (given as the Q10dB of tuning curves—the center frequency divided by the tuning bandwidth at 10 dB above the best sensitivity). (Inset) Representative tuning curves are shown. Where hair cells are attached to tectorial sallets (Left, represented by the Australian Bobtail lizard_Tiliqua)_, the coupling of neighboring hair cells leads to greater sensitivity and higher frequency selectivity, manifest as greater Q10dB values. In addition, the tuning curve flanks in Tiliqua are steeper (6).
Most lizards with small basilar papillae (e.g., iguanids, anguids, and agamids) have only free-standing hair cells in the high-frequency papillar area. Without a TM, such hair cells can respond at the lowest to about 1 kHz (Fig. 4C); even then, their bundles contain few stereovilli, and these are remarkably tall (>30 μm; Fig.4C) (6). Coupling the small number of hair cells by using tectorial material would allow only very few distinct frequency responses. Loss of the TM in very small papillae was thus probably a phylogenetic mechanism for deriving the maximum amount of information on frequencies. The cost involved was some loss of sensitivity and frequency selectivity (Fig. 8) (6).
Hair-cell systems with continuous TMs tend to be large, thus neighboring hair cells are tuned to similar frequencies. Any minor loss of frequency selectivity caused by TM coupling is more than matched by the resulting gain in sensitivity. The larger the papilla, the less detrimental to frequency selectivity is the coupling of hair cells and adding a TM is advantageous. All large lizard papillae and all avian and mammalian papillae have hair cells covered by a TM. In intermediate cases, a sallet-chain TM optimizes the various influences on sensitivity and selectivity.
Arranging Frequency Responses Systematically: The Phylogeny of Tonotopicity.
Next to frequency selectivity, a tonotopic organization (i.e., the systematic arrangement of frequencies along the epithelium) is a primordial feature of hearing organs of terrestrial vertebrates. Hearing epithelia always present frequency as their main organizational feature, presumably because of developmental processes creating gradients along certain axes. In amniotes, the base of the hearing epithelium (nearest the sacculus) is almost always tuned to the highest frequencies. After a systematic drop in frequencies along the epithelium, the lowest frequencies are found at the apex. As so often, some lizards are an exception.
The ancestral lizard hearing epithelium was probably small, with the primordial low-frequency hair-cell area in the center, and incipient high-frequency areas at both ends (Fig. 5) (17). From this archetype, each lizard family or family group evolved its own arrangement (Fig.5). In most iguanids, anguids, and agamids, the archetype was preserved with perhaps a reduction in size. The selection pressure for the preservation of redundant mirror-image hair-cell areas was presumably not very great, however, and some genera of these families lost the apical high-frequency area. The apical high-frequency area was also lost in most other lizard families, resulting in a tonotopic organization resembling that of birds and mammals (Fig. 5). That there is no universal law of tonotopic arrangements is shown by the geckos, which lost the basal high-frequency area of the archetype, and thus the tonotopic arrangement is the reverse of the amniote norm (Fig. 5). Other, more bizarre cases exist among lizards, in which a physical separation developed between one of the high-frequency areas and the rest of the papilla, resulting not only in two subpapillae, but in a diversification of the frequencies in the two high-frequency areas (Fig. 5). This is found in the monitor-lizard family (Varanidae), the Lacertidae, and some isolated iguanid lizards (6).
In birds, the gradients for both electrical and micromechanical tuning run parallel and in register. Whether they develop simultaneously in ontogeny is unknown: Perhaps one gradient develops first and influences the expression of genes responsible for the other gradient. In fact, there are many gradients, both along and across the avian papilla, resulting in hair cells that are all unique. Every avian hair cell has a phenotype differing slightly from that of its neighbors (33).
Although the avian pattern is rather consistent across species, the barn owl has a severely distorted tonotopic organization. In its auditory fovea, the octave vital for prey detection (4–8 kHz) occupies half of the papillar length (e.g., ref. 34). Similarly, some mammalian papillae show auditory foveae; in some species of bats, space constants of more than 50 mm/octave occur in frequency ranges used in detecting prey. These foveae are thought to have developed independently in different bat groups, because their anatomical and physiological characteristics differ (35). The immense amount of afferent information from foveal regions is processed by enlarged regions of brain nuclei. The phylogeny of such foveae and the complexity of lizard tonotopicities demonstrate the flexibility of the genetic substrate determining tonotopic arrangement, responding to selection pressures within a short evolutionary time.
Increasing Papillar Length During Evolution: Space Constants Do Matter.
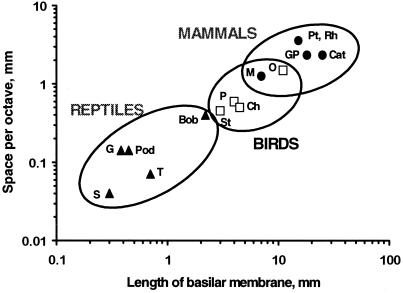
One of the most consistent findings across the amniotes is an increase in length of the auditory epithelia during phylogeny. The increase in length is proportionately greater than the increase in the upper frequency, especially in birds and mammals. Thus a mammal with a papillar length of 25 mm and an upper limit of 50 kHz has a hearing range of about 10 octaves and a frequency space constant of 2.5 mm/octave (an average for mammals; Fig.9). This compares to ≈3 octaves and a space constant of 0.3 mm in the putative ancestral form. In birds and in the high-frequency areas of large lizard papillae, the average space constant is <1 mm/octave. In small lizard papillae, it can be <0.1 mm. In some cases, only 20 columns of hair cells code for 2.5 octaves. But why are space constants important?
Figure 9.
The length of the BM and thus of the auditory organ correlates with the space constant for frequency (averaged along the papilla) in reptiles (▴), birds (□), and mammals (●). Because their hearing range did not increase in proportion to the length of the cochlea, avian and mammalian papillae gained much more space for the analysis of any given octave. The increase was greater in most mammals than in most birds, but the distributions overlap. The resulting increase in hair cells and nerve fibers per octave increased parallel processing. Lizards: G, alligator lizard, Gerrhonotus; T, turtle; Pod, European lizards of the genus Podarcis; S, the granite spiny lizard_Sceloporus_; Bob, Australian Bobtail lizard_Tiliqua._ Birds: St, starling, Sturnus; Ch, chicken, Gallus; P, pigeon, Columba; O, barn owl, Tyto. Mammals: M, mouse,Mus; GP, guinea pig, Cavia; Cat, house cat, Felis; Pt, Rh, two bat species,Pteronotus, the mustached bat, and_Rhinolophus_, the horseshoe bat. For refs see also refs.30, 34, and 36. [Modified after ref. 5, and used with permission of Wiley-VCH Press (Copyright 2000, Wiley).]
For electrically tuned hair cells, the frequency response of neighboring hair cells is, in principle, quite unimportant. The development of micromechanical tuning, however, placed a greater premium on space, because micromechanical influences from neighboring hair cells became critically important, as seen above. In the evolution of most amniote papillae, a continuous TM was kept, but the papilla was strongly elongated, increasing the number of hair cells and resulting in larger frequency space constants (Fig. 9). This maintained sensitivity and selectivity while simultaneously increasing parallel processing. Questions about space constants and those related to tonotopicity are thus interwoven.
Coding of Intensity from a Phylogenetic Viewpoint
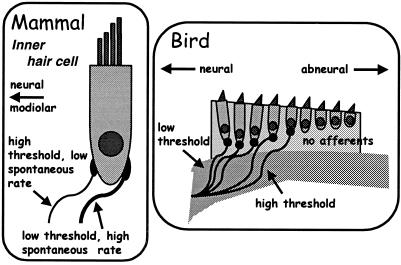
The sound-driven activity of auditory afferents also encodes information on the signal level. Because the natural world offers a huge range of intensities, we might expect phylogenetic adaptations to cope with it. Range fractionation is one solution, in which individual nerve fibers have different thresholds and code over different level ranges. A wide threshold range across primary auditory nerve fibers is the rule (e.g., refs. 6, 25, and 37). In mammals, auditory afferents can be divided into three groups (that are, however, part of a continuum). The most sensitive fibers contact the abneural edge of IHCs (Fig. 10) and have a small dynamic range. The two other types of fiber are less sensitive, have wider dynamic ranges, and contact the neural side of IHCs (38). Yates_et al._ (40) showed that these types of rate-intensity function reflect the existence of a cochlear amplifier driving the BM, with a strong compressive nonlinearity above a certain level. Creating a more effective coding of sound intensity also may have been a selection pressure in the development of amplification systems.
Figure 10.
Innervational patterns of primary afferents in mammals and birds that encode auditory sensitivity partly through the specialization of afferent fibers. In mammals (Left), the most sensitive fibers, which also have a higher spontaneous activity, are thicker and innervate the outer side of the IHC. Less sensitive fibers, which have lower spontaneous rates, innervate the modiolar side of the IHC (38). In the starling (Right), different sensitivities of afferent fibers contact different hair cells. The more sensitive fibers contact hair cells near the neural edge, less sensitive fibers hair cells near the middle of the papilla (e.g., ref. 39). Hair cells lying further abneurally have no afferent innervation (28). [Modified after ref. 5, and used with permission of Wiley-VCH Press (Copyright 2000, Wiley).]
In birds, primary afferent fibers often show a range of thresholds of >50 dB (6, 25). All three types of rate-intensity function described for mammals also exist, but in different proportions (40, 41). The particular pattern of rate-intensity types in birds suggests that the cochlear amplifier in birds acts locally rather than globally on the entire organ (40). There is evidence that the most sensitive afferents innervate hair cells near the neural edge of the papilla (Fig. 10) (25). In the emu, more than half the fibers code for a dynamic range greater than 50 dB (40).
The Phylogeny of the Cochlear Amplifier
Hair cells harbor a mechanism capable of increasing motion induced by low-level sounds (2, 42–44). Active motor mechanisms at the hair-cell level are responsible for the cochlear amplification at the organ level. In mammals, several phenomena correlate with the activity of a cochlear amplifier. These include the high sensitivity and frequency selectivity of hearing and their sensitivity to physiological insults, the existence of compressive nonlinearities in afferent fiber rate-level functions, and the existence and characteristics of otoacoustic emissions (OAE), especially spontaneous OAE (SOAE). SOAE are peaks in the sound-pressure spectra in the external meatus that are present in the absence of external stimuli and represent spontaneous hair-cell motion (45). All these phenomena also exist in nonmammals (2,44). SOAE are more common in some nonmammals and resemble mammalian SOAE to a remarkably high degree. There is thus no reason to doubt the existence of a cochlear amplifier mechanism in nonmammals, including amphibians. The most recently discovered cochlear mechanism may thus be phylogenetically one of the oldest.
Active motion in hair-cell bundles has been demonstrated in vestibular hair cells (e.g., refs. 46–49) and auditory hair cells of the turtle (50). In nonmammals, it appears that the force of the cochlear amplifier operates parallel to the surface of the papilla, and thus at the interface between the TM and the hair cells. In birds, this motion may be transmitted via the TM to THCs, producing maximal motion amplitudes at a point near the neural edge of the epithelium (29, 51). This is where the starling, for example, has its most sensitive hair cells (Fig. 10) (39). Such motions might be only very poorly reflected in BM motion. In mammals, there is evidence that the OHCs have an amplification motor located in the lateral cell membrane that causes cell contraction and elongation in a plane almost at right angles to the BM and thus the movement is reflected in BM motion (52).
The two putative active processes correlate with morphological differences between birds and mammals. In birds, there are more stereovilli per hair cell than in mammals (25), which might be expected if the amplifier were located in the bundles. In contrast, mammals have a thin BM and papillae that are full of specialized supporting cells. Mammals may have abandoned the active motion of hair-cell bundles, at least at high frequencies, but there is as yet no evidence for this. In mammals as in nonmammals, a bundle motor also would be an effective way of coupling cellular power into organ motion (53).
In sum, it can be said that the most important changes in cochlear mechanisms during phylogeny were initiated by changes in the middle ear. This led in most lineages to a predominance on micromechanical tuning, a profound elongation of the papilla, and the specialization of hair cells that went as far as to generate a division of labor in birds and mammals. Many of the most fundamental cochlear mechanisms have, however, changed little or not at all during amniote phylogeny. Plus cà change, plus cés la mème chose.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft within the SFB 204 “Gehör” and DFG-Grant MA 871/10–2.
Abbreviations
BM
basilar membrane
IHC
inner hair cell
OAE
otoacoustic emissions
OHC
outer hair cell
SHC
short hair cell
THC
tall hair cell
TM
tectorial membrane
Footnotes
This paper was presented at the National Academy of Sciences colloquium “Auditory Neuroscience: Development, Transduction, and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Nielsen C. Animal Evolution. Oxford: Oxford Univ. Press; 1995. [Google Scholar]
- 2.Manley G A, Köppl C. Curr Opin Neurobiol. 1998;8:468–474. doi: 10.1016/s0959-4388(98)80033-0. [DOI] [PubMed] [Google Scholar]
- 3.Clack J A. Brain Behav Evol. 1997;50:198–212. doi: 10.1159/000113334. [DOI] [PubMed] [Google Scholar]
- 4.Carr C. In: The Evolutionary Biology of Hearing. Fay R R, Popper A N, Webster D B, editors. New York: Springer; 1992. pp. 511–543. [Google Scholar]
- 5.Manley G A. In: Auditory Worlds: Sensory Analysis and Perception in Animals and Man. Manley G A, Fastl H, Kössl M, Oeckinghaus H, Klump G M, editors. Weinheim: Wiley; 2000. pp. 7–17. [Google Scholar]
- 6.Manley G A. Peripheral Hearing Mechanisms in Reptiles and Birds. Heidelberg: Springer; 1990. [Google Scholar]
- 7.Saunders J C. Hear Res. 1985;18:253–268. doi: 10.1016/0378-5955(85)90042-5. [DOI] [PubMed] [Google Scholar]
- 8.Manley G A, Johnstone B M. J Acoust Soc Am. 1974;56:571–576. doi: 10.1121/1.1903292. [DOI] [PubMed] [Google Scholar]
- 9.Carroll R L. Vertebrate Paleontology and Evolution. New York: Freeman; 1988. [Google Scholar]
- 10.Miller M R. In: The Evolutionary Biology of Hearing. Webster D B, Fay R R, Popper A N, editors. New York: Springer; 1992. pp. 463–487. [Google Scholar]
- 11.Sneary M G. J Comp Neurol. 1988;276:573–587. doi: 10.1002/cne.902760410. [DOI] [PubMed] [Google Scholar]
- 12.Wever E G. The Reptile Ear. Princeton, NJ: Princeton Univ. Press; 1978. [Google Scholar]
- 13.Fettiplace R, Fuchs P A. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill M P, Bearden A. Hear Res. 1995;84:125–138. doi: 10.1016/0378-5955(95)00018-y. [DOI] [PubMed] [Google Scholar]
- 15.Gummer A W, Smolders J W T, Klinke R. Hear Res. 1987;29:63–92. doi: 10.1016/0378-5955(87)90206-1. [DOI] [PubMed] [Google Scholar]
- 16.Sellick P M, Patuzzi R, Johnstone B M. J Acoust Soc Am. 1982;72:131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- 17.Köppl C, Manley G A. In: The Evolutionary Biology of Hearing. Fay R R, Popper A N, Webster D B, editors. New York: Springer; 1992. pp. 489–509. [Google Scholar]
- 18.Manley G A. In: Comparative Hearing: Birds and Reptiles, Springer Handbook of Auditory Research. Dooling R, Popper A N, Fay R R, editors. New York: Springer; 2000. , in press. [Google Scholar]
- 19.Manley G A, Köppl C, Sneary M. Hear Res. 1999;131:107–116. doi: 10.1016/s0378-5955(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 20.Manley G A, Köppl C, Yates G K. In: Mechanics of Hearing. Wilson J P, Kemp D, editors. New York: Plenum; 1989. pp. 143–150. [Google Scholar]
- 21.Peake W T, Ling A. J Acoust Soc Am. 1980;67:1736–1745. doi: 10.1121/1.384300. [DOI] [PubMed] [Google Scholar]
- 22.Holton T, Hudspeth A J. Science. 1983;222:508–510. doi: 10.1126/science.6623089. [DOI] [PubMed] [Google Scholar]
- 23.Authier S, Manley G A. Hear Res. 1995;82:1–13. doi: 10.1016/0378-5955(94)00138-g. [DOI] [PubMed] [Google Scholar]
- 24.Manley G A, Gleich O, Kaiser A, Brix J. J Comp Physiol A. 1989;164:289–296. [Google Scholar]
- 25.Gleich O, Manley G A. In: Comparative Hearing: Birds and Reptiles, Springer Handbook of Auditory Research. Dooling R, Popper A N, Fay R R, editors. New York: Springer; 2000. , in press. [Google Scholar]
- 26.Manley G A. Nature (London) 1971;230:506–509. doi: 10.1038/230506a0. [DOI] [PubMed] [Google Scholar]
- 27.Manley G A. Evolution. 1973;26:608–621. doi: 10.1111/j.1558-5646.1972.tb01968.x. [DOI] [PubMed] [Google Scholar]
- 28.Fischer F P. Scanning Microsc. 1994;8:351–364. [PubMed] [Google Scholar]
- 29.Manley G A. In: Advances in Hearing Research. Manley G A, Klump G M, Köppl C, Fastl H, Oeckinghaus H, editors. Singapore: World Scientific; 1995. pp. 219–229. [Google Scholar]
- 30.Manley G A. In: Auditory Frequency Selectivity. Moore B, Patterson R, editors. London: Plenum; 1986. pp. 63–72. [Google Scholar]
- 31.Wu Y-C, Art J J, Goodman M B, Fettiplace R. Prog Biophys Mol Biol. 1995;63:131–158. doi: 10.1016/0079-6107(95)00002-5. [DOI] [PubMed] [Google Scholar]
- 32.Köppl C. J Neurophysiol. 1997;77:364–377. doi: 10.1152/jn.1997.77.1.364. [DOI] [PubMed] [Google Scholar]
- 33.Köppl, C., Manley, G. A. & Konishi, M. (2000)Curr. Opin. Neurobiol., in press. [DOI] [PubMed]
- 34.Köppl C, Gleich O, Manley G A. J Comp Physiol. 1993;171:695–704. [Google Scholar]
- 35.Vater M. In: Auditory Worlds: Sensory Analysis and Perception in Animals and Man. Manley G A, Fastl H, Kössl M, Oeckinghaus H, Klump G M, editors. Weinheim: Wiley; 2000. pp. 61–69. [Google Scholar]
- 36.Köppl C, Manley G A. J Comp Physiol A. 1990;167:101–112. [Google Scholar]
- 37.Yates G K, Winter I M, Robertson D. Hear Res. 1990;45:203–220. doi: 10.1016/0378-5955(90)90121-5. [DOI] [PubMed] [Google Scholar]
- 38.Liberman C, Oliver M E. J Comp Neurol. 1984;223:163–176. doi: 10.1002/cne.902230203. [DOI] [PubMed] [Google Scholar]
- 39.Gleich O. Hear Res. 1989;37:255–268. doi: 10.1016/0378-5955(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 40.Yates G K, Manley G A, Köppl C. J Acoust Soc Am. 2000;107:2143–2154. doi: 10.1121/1.428496. [DOI] [PubMed] [Google Scholar]
- 41.Köppl C, Yates G K. J Neurosci. 1999;19:9674–9686. doi: 10.1523/JNEUROSCI.19-21-09674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis H. Hear Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- 43.Hudspeth A J. Curr Opin Neurobiol. 1997;7:480–486. doi: 10.1016/s0959-4388(97)80026-8. [DOI] [PubMed] [Google Scholar]
- 44.Manley G A. In: Recent Developments in Auditory Mechanics. Wada H, Takasaka T, Ohyama K, Ikeda K, Koike T, editors. Singapore: World Scientific; 2000. , in press. [Google Scholar]
- 45.Köppl C. In: Advances in Hearing Research. Manley G A, Klump G M, Köppl C, Fastl H, Oeckinghaus H, editors. Singapore: World Scientific; 1995. pp. 207–216. [Google Scholar]
- 46.Benser M E, Marquis R E, Hudspeth A J. J Neurosci. 1996;16:5629–5643. doi: 10.1523/JNEUROSCI.16-18-05629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denk W, Webb W W. Hear Res. 1992;60:89–102. doi: 10.1016/0378-5955(92)90062-r. [DOI] [PubMed] [Google Scholar]
- 48.Martin P, Hudspeth A J. Proc Natl Acad Sci USA. 1999;96:14206–14301. [Google Scholar]
- 49.Rüsch A, Thurm U. Hear Res. 1990;48:247–264. doi: 10.1016/0378-5955(90)90065-w. [DOI] [PubMed] [Google Scholar]
- 50.Crawford A C, Fettiplace R. J Physiol. 1985;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steele C R. In: Diversity in Auditory Mechanics, eds. Lewis E, Long G, Lyon R F, Narins P, Steele C R, Hecht-Poinar E, editors. Singapore: World Scientific; 1996. pp. 455–461. [Google Scholar]
- 52.Holley M C. In: The Cochlea. Dallos P, Popper A N, Fay R R, editors. New York: Springer; 1996. pp. 386–434. [Google Scholar]
- 53.Yates G K, Kirk D L. J Neurosci. 1998;18:1996–2003. doi: 10.1523/JNEUROSCI.18-06-01996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]