Activation of αvβ3-Vitronectin Binding Is a Multistage Process in which Increases in Bond Strength Are Dependent on Y747 and Y759 in the Cytoplasmic Domain of β3 (original) (raw)
Abstract
Integrin receptors serve as mechanical links between the cell and its structural environment. Using αvβ3 integrin expressed in K562 cells as a model system, the process by which the mechanical connection between αvβ3 and vitronectin develops was analyzed by measuring the resistance of these bonds to mechanical separation. Three distinct stages of activation, as defined by increases in the αvβ3-vitronectin_binding strength_, were defined by mutational, biochemical, and biomechanical analyses. Activation to the low binding strength stage 1 occurs through interaction with the vitronectin ligand and leads to the phosphorylation of Y747 in the β3 subunit. Stage 2 is characterized by a 4-fold increase in binding strength and is dependent on stage1 and the phosphorylation of Y747. Stage 3 is characterized by a further 2.5-fold increase in binding strength and is dependent on stage 2 events and the availability of Y759 for interaction with cellular proteins. The Y747F mutant blocked the transition from stage 1 to stage 2, and the Y759F blocked the transition from stage 2 to stage 3. The data suggest a model for tension-induced activation of αvβ3 integrin.
INTRODUCTION
The primary function of integrins is the mechanical connection of cells to extracellular matrix and to other cells by binding to specific ligands. This mechanical connection is essential for the integrity of tissues within the body. A consequence of these mechanical linkages is the modulation of cellular signaling circuits in many cell systems (Menko and Boettiger, 1987; Hynes, 1992; Schwartz_et al._, 1995). In addition to these “outside-in” signals, intracellular signals and cytoskeletal rearrangements can alter the adhesion function of integrins to their extracellular ligands (also called “inside-out” signaling; Ginsberg et al., 1992). αIIbβ3-mediated adhesion of platelets (Bennett et al., 1999) and LFA-1–mediated adhesion of lymphocytes (Kucik et al., 1996) appear to require a release of the integrins from a constrained nonbinding state to allow initial integrin-ligand binding. This reaction has been called activation and has been analyzed by the increase in the binding of the ligand-mimetic antibody, PAK-1, to αIIbβ3, and WOW-1 to αvβ3 (Hato et al., 1998; Pampori_et al._, 1999). The actual binding of αΙΙbβ3 or αvβ3 to ligand or to RGD-containing peptides, or the addition of Mn2+, induces a conformational change that increases the affinity for the binding of LIBS (ligand-induced binding site) antibodies to these β3 integrins (Frelinger et al., 1991). One consequence of this binding reaction can be the phosphorylation of the cytoplasmic domain of β3, which is necessary for subsequent steps in the adhesion process (Blystone et al., 1996). Binding of adhesion receptors to immobilized ligand is more complex to analyze but has distinct biological and biochemical consequences that are not revealed by the soluble ligand analyses (Boettiger et al., 1989;Schwartz, 1993; Garcia et al., 1999).
The attachment of cells to an immobile substrate stimulates cytoskeletal rearrangements and the assembly of actin filaments. This process also appears to affect integrin-mediated cell adhesion. Disruption of the actin cytoskeleton leads to reduction in cell adhesion, which seems to be due to a failure of the cytoskeletal-integrin linkages (Lotz et al., 1989;Garcia et al., 1998). The importance of the cytoskeletal connections to β3 integrin cytoplasmic domains has been reinforced by mutational analysis of the β3 cytoplasmic domain (Hughes et al., 1995; O'Toole et al., 1995;Schaffner-Reckinger et al., 1998). Of particular interest are transgenic knock-in experiments in which the tyrosines in the cytoplasmic domain of β3 were substituted with phenylalanines. This mutation did not affect the initial activation of αIIbβ3 but caused a rebleeding after wounding due to a failure in the clot structure (Law et al., 1999). Thus, the cytoplasmic domain of β3 integrin appears to play a critical role in the strength of the attachment of cells or platelets through the extracellular domain of β3 integrin to surface-bound ligands.
To analyze the more complex problem of integrin binding to surface-bound ligands, we developed a system that uses hydrodynamic shear forces to measure the strength of the integrin-ligand bonds. This analysis was combined with biochemical and genetic approaches to dissect the process of αvβ3 binding to immobilized vitronectin. The results support a model in which the cytoplasmic and extracellular domains collaborate to generate successive increases in the strength of both the αvβ3-cytoskeletal bonds and the αvβ3-vitronectin bonds. Both connections are necessary for stable adhesion of cells to a substrate.
METHODS AND PROCEDURES
Cells and Antibodies
The Kαvβ3, Kαvβ3(Y747F), and Kαvβ3(Y759F) cells were derived from K562 cells and the CSβ3, CSβ3(Y747F), and CSβ3(Y759F) were derived from CS-1 melanoma cells by transfection with both αv and β3 or β3 mutant integrins as previously described (Blystone_et al._, 1997). The K562-derived cells were adapted to growth as nonadherent cells in serum-free K562 medium (cytoSF4 from Kemp Biotechnologies, Fredrick, MD). AIIB2 (anti-human β1 integrin) monoclonal antibody was a gift from Caroline Damsky (UCSF). LM609 (anti-human β3 integrin) monoclonal antibody was a gift from David Cheresh (Scripps). Secondary antibodies for FACS analysis were obtained from Jackson Laboratories. Vitronectin was purified from human plasma by the method of Yatohga et al. (1988).
Vitronectin Adsorption
Bellco glass coverslips (Vineland, NJ) were washed in 95% ethanol overnight and stored in ethanol. Immediately before use, coverslips were washed two times with distilled water, once with PBS, and incubated for 30 min at room temperature with various concentrations of vitronectin dissolved in PBS. The coverslips were blocked with 1% heat-denatured BSA (56°C, 30 min) for 30 min and placed in complete PBS. For analysis of vitronectin adsorption kinetics, vitronectin was iodinated using the Bolton-Hunter reaction as described by the manufacturer (NEN, Arlington, MA). Iodinated vitronectin was adsorbed, washed, and blocked as described above, and the coverslips were counted in duplicate in a gamma counter.
Adhesion Analysis
The spinning disk device and the method have been described (Garcia et al., 1997, 1998). Briefly, cells were washed and resuspended in adhesion buffer (24 mM HEPES, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 2 mM glucose, pH 7.4). Cells were counted and diluted, and PMA (20 nM final concentration) was added as required and plated on 25-mm round, coated coverslips mounted on the spinning disk device in a total volume of 400 μl. After a 15-min incubation, the chamber of the spinning disk device was carefully filled with adhesion buffer, and the cells were spun for 5 min. Note that the K αvβ3 and mutant β3 cells do not exhibit significant spreading, and adhesion remains essentially constant from 15 to 60 min (data not shown). After spinning, the coverslips were fixed with formalin, permeabilized with Triton X-100, and stained with ethidium homodimer (Molecular Probes, Eugene, OR). The coverslips were analyzed by counting the number of cells in 61 10× microscope fields using an Optiphot fluorescent microscope (Nikon, Garden City, NY) with a Ludl XYZ stage and Ludl filter wheel/shutter (LUDL Electronics Products Ltd., Hawthorne, NY) driven by ScopePro V1.0 software and a Photometrics SenSys cooled CCD camera (Tucson, AZ). Data were analyzed by Image-Pro V3.0 software, and curve fits were made using SigmaPlot. For experiments in which the cells were cross-linked before spinning, cells were plated for 10 min, and then 4 mM sulfo-BSOCOES (Pierce, Rockford, IL) was added for 5 min before spinning.
Cross-linking and Extraction
Cells were plated in DPBS + 2 mM glucose for 15 min on dishes coated with 2 μg/ml vitronectin and then cross-linked with 1 mM sulfo-BSOCOES for 5 min. Excess cross-linker was quenched with 50 mM Tris-HCl (pH 7.2) for 10 min, and cells were extracted with 0.1% SDS, 350 μg/ml PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotonin in PBS to give an extracted fraction. Plates were washed three times with PBS and then incubated in 50 mM carbonate buffer (pH 11.6), 0.1% SDS on a rocker at 37°C for 2 h to reverse the cross-linking. The fraction containing the cross-linked integrins was concentrated using Microcon YM-30 (Millipore Corp., Bedford, MA). Both the extracted and the cross-linked fractions were analyzed by SDS-PAGE using nonreducing conditions, blotted on PVDF membranes (Millipore Corp.), blocked with 5% nonfat dry milk (Blotto) and probed with a rabbit polyclonal antibody to the cytoplasmic domain of β3, or with a polyclonal antibody for the cytoplasmic domain of αv (both from Chemicon Inc., Temecula, CA). The blots were developed with alkaline phosphatase-conjugated secondary antibodies (Jackson Laboratories) and ECF chemifluorescent reagent (Amersham Pharmacia Biotech, Piscataway, NJ) and quantitated using a Fuji LAS1000 system (Stamford, CT) and ScienceLab v2.5 software (Guilderland, NY).
Analysis of β3 Integrin Phosphorylation
Kαvβ3, Kαvβ3(Y747F), and Kαvβ3(Y759F) cells, with or without 20 nM PMA, were incubated for 2 hours in the presence of 75 μM sodium orthovanadate either in suspension or on vitronectin. The cells were extracted with PBS buffer containing 1% NP-40, sodium orthovanadate (2 mM), aprotonin (10 μg/ml), PMSF (350 μg/ml), and leupeptin (10 μg/ml). Lysates were precleared overnight with gelatin sepharose and immunoprecipitated with PM6/13 antibody (Chemicon) and goat anti-mouse IgG beads (ICN Pharmaceuticals, Costa Mesa, CA). Samples were separated on 8% SDS-PAGE, transferred to nitrocellulose, and blotted with 4G10 (UBI, Lake Placid, NY) for phosphotryrosine or rabbit polyclonal antibody to β3 cytoplasmic domain (AB1932; Chemicon). Blots were developed with ECL (Amersham Pharmacia Biotech) and quantitated using a Fuji LAS-1000 system and ScienceLab 2.5 software.
RESULTS
PMA Induces an Increase in the Strength of αVβ3-mediated Adhesion
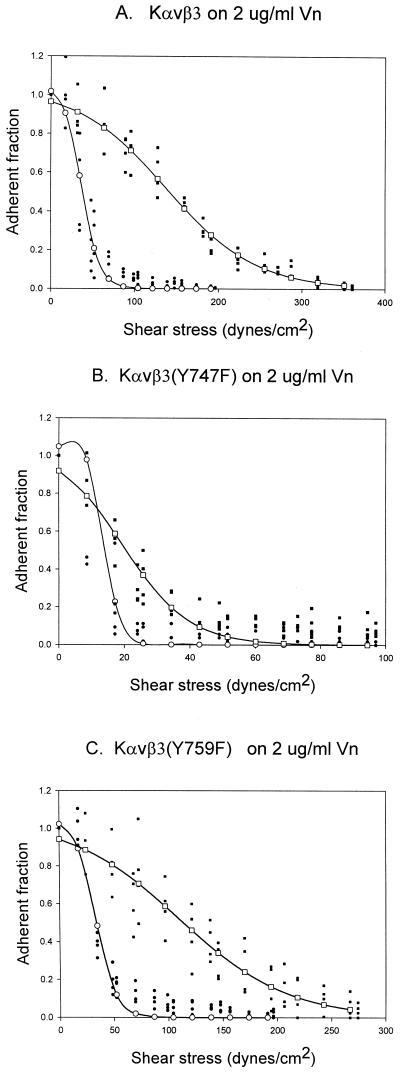
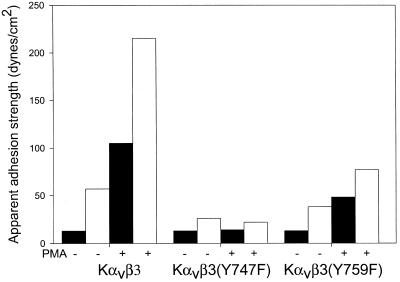
K562 cells stably transfected with αv and β3 or β3 mutants were used as a model system to understand the process of activating the ligand-binding function of αvβ3 integrin. Previous analysis has shown that treatment of these cells with either PMA or thrombin along with the availability of Y747 for phosphorylation was required for adhesion to vitronectin (Blystone et al., 1997). To analyze this process from the perspective of the strength of adhesion, we used the recently developed spinning disk assay (Garcia et al., 1998). This assay applies a calibrated linear hydrodynamic shear gradient to a cell population to determine the shear force necessary to detach the cells from their substrate. Figure1 shows that PMA induced a rightward displacement of the plots of the proportion of cells remaining as a function of applied hydrodynamic shear for Kαvβ3 and Kαvβ3(Y759F) cells (Figure2, A and C). In contrast, the Kαvβ3(Y747F) cells showed no significant displacement of the 50% adhesion point (note that this plot has been expanded relative to A and C to allow better visualization of the data details). Thus, PMA induced an increase in the αvβ3-vitronectin bond strength, and this increase required the presence of Y747. The broadening of the force-detachment profiles for the PMA-treated cells is caused by the effect of this treatment on the cytoskeleton because the cytoskeleton transmits the applied force to integrin receptors (Garcia_et al._, 1998). As expected, the adhesion strength measured in this assay could be reduced to the background levels observed for a BSA substrate by antibodies that block αvβ3 function but were unaffected by antibodies that block α5β1 function (data not shown). This data recapitulate previously published results using this model system (Blystone et al., 1997).
Figure 1.
Spinning disk comparison of Kαvβ3 (A), Kαvβ3(Y747F) (B), and Kαvβ3(Y759F) (C) cells, with and without PMA activation. Data from two different discs are plotted on the same axis. ●, control data points; ○, control curve fit; ▪, PMA data points; □, PMA curve fit. Lines represent sigmoid curve fits.
Figure 2.
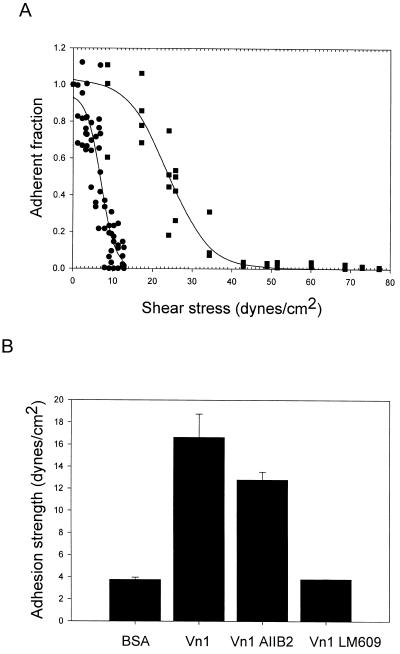
Stage 1 activation of αvβ3. (A) Adhesion of Kαvβ3(Y747F) cells to vitronectin were plated for 15 min on either BSA (●) or vitronectin-coated coverslips (coated at 2 μg/ml; ▪) and analyzed using the spinning disk. Solid lines represent the curve fits for each data set. The adhesion strength is defined as the force required to detach 50% of the cells. Adhesion strength for BSA = 6.4 ± 1.65 dynes/cm2 (means ± SD; n = 6). Adhesion strength for vitronectin = 23.9 ± 3.3 dynes/cm2 (n = 8). (B) Specificity αvβ3(Y747F)-mediated adhesion. Data show adhesion strength and SD derived from cell detachment profiles as shown in A: BSA cells plated on BSA only, Vn1 cells plated on vitronectin (1 μg/ml), AIIB2 cells preincubated with antibody to β1, and LM609 cells preincubated with antibody to β3.
To determine whether this effect of PMA required occupancy of αvβ3 by its vitronectin ligand, experiments were carried out using immobilized LM609 (a β3 integrin-specific, adhesion-inhibiting monoclonal antibody) as a surrogate ligand. The adhesion strength measured for Kαvβ3 cells to the LM609 ligand in the absence of PMA was 72 ± 12 dynes/cm2; addition of PMA had no significant effect on this adhesion strength. The failure of PMA to affect the LM609-mediated adhesion demonstrates that anchoring of αvβ3 to the substrate was not sufficient to induce the increase in the strength of the αvβ3-mediated adhesion.
Adhesion of the Initial αvβ3 Vitronectin Binding Complex
If the binding of αvβ3 to vitronectin was necessary for the increase in adhesion strength induced by PMA, this would require a specific interaction between αvβ3 and vitronectin in the absence of PMA. The most stringent system to look for this interaction is in the K αvβ3 (Y747F) cells, which cannot respond to PMA or other stimuli and which showed no significant adhesion to vitronectin using a standard adhesion assay (Blystone et al., 1997). Figure 2A shows the raw data and the sigmoid curve fit for the force required to detach Kαvβ3(Y747F) cells from BSA or vitronectin-coated substrates. The higher force required for detachment from the vitronectin substrate (rightward shift of the force/detachment profile) represents specific binding to vitronectin. Specific adhesion-blocking antibodies for β1 integrin (AIIB2) and β3 integrin (LM609) were used to confirm that αvβ3 was responsible for this specific adhesion to vitronectin (Figure 2B). Thus, by using this more sensitive method, it was possible to demonstrate that αVβ3(Y747F) expressed on K562 cells bound specifically to a vitronectin substrate but that the strength of this adhesion was weak. This level of adhesion strength is similar to that seen for cells pretreated with cytochalasin D to disrupt their actin microfilaments (Garcia et al., 1998).
Biochemical Demonstration of Multiple Binding States for αvβ3 Integrin to Vitronectin
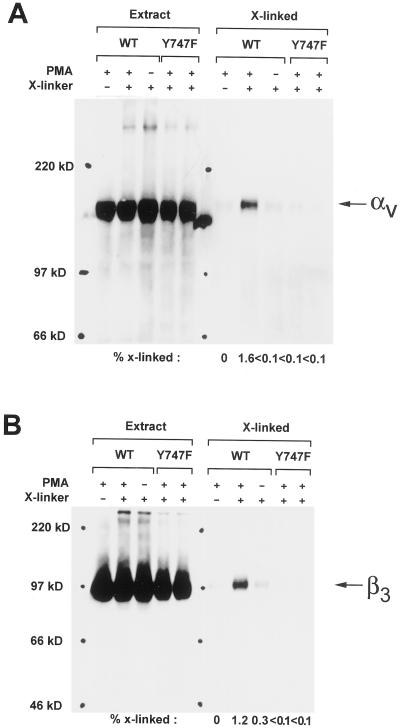
Conformational differences between the inactive or nonbinding state and an active state of αvβ3 have been demonstrated biochemically using antibody binding and fluorescence energy transfer experiments (Shattil et al., 1985; Frelinger_et al._, 1991). Here, it was necessary to distinguish between the weak binding state for vitronectin (which binds LIBS-1) and stronger binding state(s) induced by PMA stimulation of the Kαvβ3 cells. This required a new approach. To detect these differences, we used sulfo-BSOCOES, a cell impermeant, primary amine-specific, chemical cross-linker with a spacer arm length of 11.2A (Enomoto-Iwamoto et al., 1993; Garcia et al., 1998; Garcia and Boettiger, 1999). Figure3 shows that compared with the level of cross-linking for PMA-activated αvβ3, the level of cross-linkable αv was reduced ∼16-fold. Cross-linkable β3 was reduced ∼4-fold in the unstimulated Kαvβ3 and reduced ∼12-fold in the mutant Kαvβ3(Y747F) cells, which cannot be activated by external stimuli. The clear differences in cross-linking efficiency for the initial and the PMA-activated binding states show that PMA induced a change in physical interaction between αvβ3 integrin and vitronectin.
Figure 3.
Cross-linking of activated αvβ3: αv and β3 subunits. Kαvβ3 and Kαvβ3(Y747F) cells were plated on vitronectin (2 μg/ml) coated plates for 15 min and cross-linked with sulfo-BSOCOES for 5 min. Extract lanes show that αv and β3 levels were unaffected by either PMA or cross linking. The X-linked lanes represent the levels of cross-linked αv and β3 recovered after cleavage of the cross-linker. Treatment of Kαvβ3 cells with PMA increased the level of cross-linked αv and β3, demonstrating a change in the αvβ3-vitronectin binding conformation.
Kinetic and Genetic Analysis of αvβ3 Binding to Vitronectin: Three Distinct Ligand-bound States
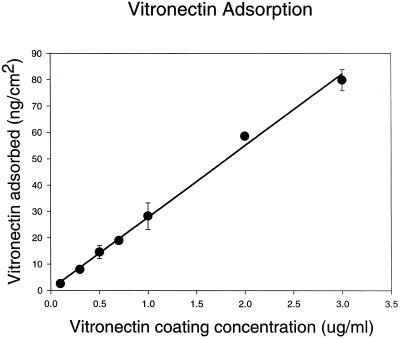
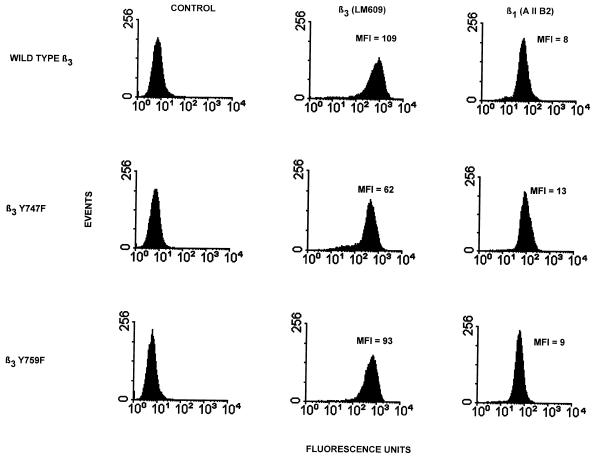
Relative binding strength constants for the αvβ3-vitronectin bonds, which are analogous to receptor-ligand dissociation constants, can be obtained from the spinning disk analysis (Garcia et al., 1998). These constants are derived from the initial slope of the plot of adhesion strength as a function of ligand density. For this analysis, it is necessary to determine ligand density, receptor density, and the kinetics of the interaction. Ligand density was determined by analysis of the adsorption kinetics for 125I-labeled vitronectin binding to glass using the same adsorption and BSA blocking protocol used for the adhesion studies. Figure4 shows that adsorption was a linear function of added vitronectin concentration up to 3 μg/ml. The relative receptor density for the cell lines used was determined using flow cytometry. Figure 5 shows the relative surface densities for both β3 and β1 integrin. The level of β1 measured is similar to that on the parental K562 cells (Garcia et al., 1998). The expression levels based on the mean fluorescence index were converted to surface density of receptors, assuming spherical cells.
Figure 4.
Adsorption of vitronectin to glass.125I labeled-vitronectin was adsorbed to glass using the same procedures used to coat glass coverslips for the spinning disk assays. Above 3 μg/ml coating concentration, the vitronectin saturated the surface, reaching a plateau value of 100 ng/cm2 (plateau data points are not shown). Error bars, SD; solid line is a linear regression.
Figure 5.
Comparison of surface expression of β1 and β3 integrin on transfected K562 cells. For FACS analysis the control is secondary antibody alone; LM609 was used to quantify β3 integrin, and AIIB2 was used to quantify β1 integrin. Mean fluorescence index (MFI) was calculated: [(geometric mean of the positive fluorescence) − (geometric mean of the control)]/(geometric mean of the control).
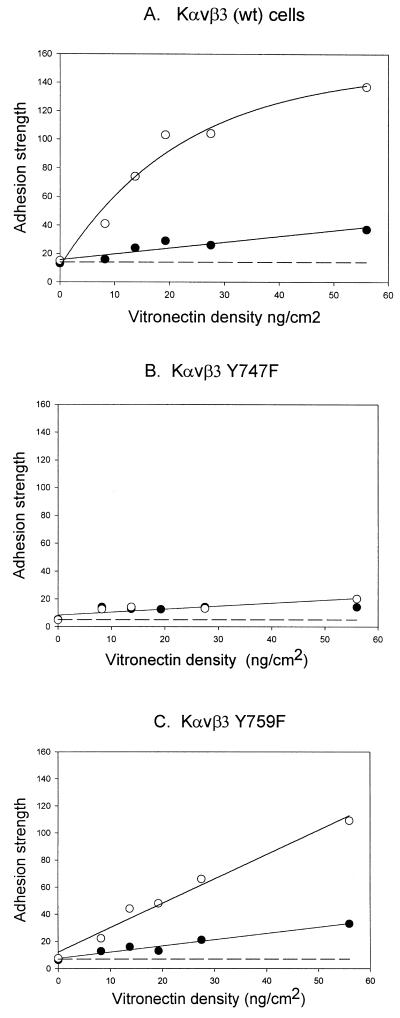
The binding kinetics for αvβ3 expressed on the cell surface to substrate-bound vitronectin was measured using the spinning disk method for each cell line in the presence and absence of PMA for a range of vitronectin surface densities. Mean adhesion strengths were calculated from individual experiments as shown in Figure 1 for each set of conditions. Figure6 shows the mean adhesion strength and SD for these measurements plotted as a function of the vitronectin surface density. For the PMA-treated Kαvβ3 cells, the adhesion strength was linear up to 20 ng/cm2 and then reached a plateau (Figure 6A). Each of the plots shows a linear increase in adhesion strength as a function of increasing vitronectin surface density (Figure 6, A–C). Thus, there is a direct relationship between the number of bonds, which would increase as a function of ligand density according to the laws of mass action, and the measured adhesion strength. The differences in slope include differences in cell surface expression of αvβ3 and differences in the strength of the interaction.
Figure 6.
Analysis of adhesion strength constants for different binding states of αvβ3. Kαvβ3 (A), Kαvβ3(Y747F) (B), and Kαvβ3(Y759F) (C) cells were analyzed using the spinning disk device on different vitronectin densities for 15 min of plating: control (●) or PMA-treated (○). The adhesion strength was determined from the sigmoid curve fits for each determination and plotted as a function of vitronectin density. Dashed line, the background adhesion, is not subtracted from the other plotted values. Figure shows one representative of four experiments.
The initial slopes of the plots in Figure 6 were combined with a normalized receptor density (Figure 5) to provide relative binding strength constants (Figure 7). Three distinct binding strengths were observed: a) initial binding shown for all cell lines in the absence of PMA-stimulation (stage 1 binding); b) a 4-fold increase in binding shown for the PMA-stimulated αvβ3(Y759F) mutant; and c) a 10-fold overall increase in binding shown by the PMA-stimulated WT αvβ3.
Figure 7.
The relative binding strength constants To calculate the relative binding strength, the expression levels of αvβ3 were normalized to the level of expression on the K αvβ3 cells based on the mean fluorescence index in Figure 5. The initial slope of the plots in Figure 6 were divided by the relative expression level of β3 integrin to give the relative binding strength constants. Background nonspecific adhesion has not been subtracted.
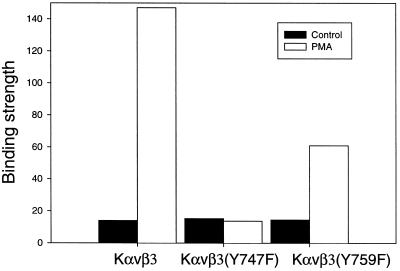
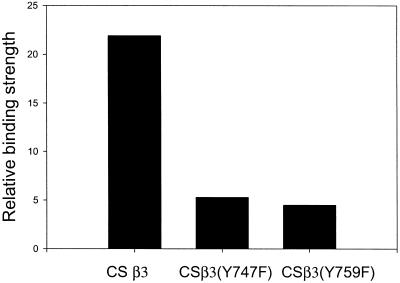
These data demonstrate that both Y747 and Y759 were required for different intracellular reactions necessary to achieve full activation and binding strength for the vitronectin-αvβ3 integrin bond in Kαvβ3 cells. To determine whether Y747 and Y759 were also required for αvβ3-mediated adhesion to vitronectin in other cell types, WT β3 and the β3 mutants Y747F and Y759F were transfected into CS-1 melanoma cells (Filardo et al., 1995;Blystone et al., 1997). In previous reports, differences in adhesion among these transfected cells were not detected. Figure8 shows that both the Y747F and the Y759F mutations reduced the binding strength of αvβ3 to vitronectin. These experiments were done without the addition of PMA because it is not required for the activation of αvβ3 in the CS-1 cells. Thus, Y747 and Y759 appear to play a general role in αvβ3-mediated adhesion.
Figure 8.
Adhesion Strength for αvβ3, αvβ3(Y747F), and αvβ3(Y759F) expressed in CS1 melanoma cells. The adhesion strength for each cell line was measured for vitronectin coating of 30 ng/cm2 in a 10-min incubation. The measured adhesion strengths were normalized for the level of β3 expression using the mean fluorescent index as measured by FACS.
The Increase in αvβ3-Vitronectin Binding Strength Is Mediated by the Connection of αvβ3 to the Cytoskeleton
The importance of the actin cytoskeleton in integrin-mediated cell adhesion was demonstrated by the reduction of adhesion in the presence of cytochalasin D (Lotz et al., 1989). In cell detachment assays, force is applied to the cell usually through centrifugation or hydrodynamic shear (e.g., washing). This force is transmitted to the cytoskeleton, which is in turn mechanically linked to active integrin, which is bound to ligand, which is bound to the substrate (plastic or glass). The application of force will break the weakest connection in this chain. In most assays used to measure integrin-mediated cell adhesion, the weak link is the bond between integrin and its ligand (Garcia et al., 1998). When the cells were treated with cytochalasin D to disassemble the actin cytoskeleton, the force required to detach cells was reduced because the force applied could not be transferred efficiently to integrin and the cells were broken from the surface, leaving the integrin behind (Garcia_et al._, 1998). Figure 3 demonstrates that active αvβ3 could be chemically cross-linked to vitronectin adsorbed to the substrate. This adds a covalent bond to the αvβ3-vitronectin linkage, which strengthens the mechanical connection; now the application of force will break the chain at a different point. An increase in the cross-linked adhesion strength after chemical cross-linking with a cell-impermeant cross-linker would demonstrate that originally the weak link was outside the cell and susceptible to cross-linking. For the experiments described here, the only noncovalent linkage that fits these criteria is the link between αvβ3 and vitronectin.
To control for contributions of cross-linking of other cell surface proteins, the strength of adhesion of K562 cells, which did not express αvβ3, was measured. The adhesion strength for the control K562 cells was indistinguishable from the cross-linked adhesion strength measured for cross-linked K562cells. Thus, the differences between the adhesion strength and cross-linked adhesion strength shown in Figure 9 were due to the cross-linking of αvβ3 to the vitronectin substrate. The observed increase in the presence of cross-linker in each case demonstrates that the weakest link was the αvβ3-vitronectin bond. Because the cross-linked adhesion strength is dependent on an intact actin cytoskeleton and on the linkage of integrin to that cytoskeleton, it provides a measure of the strength of the αvβ3-cytoskeletal linkage (Garcia et al., 1997). The αvβ3-cytoskeleton bond strength was about twice that for the αvβ3-vitronectin bond strength for each treatment and each mutant (Figure 9).
Figure 9.
Adhesion strength after αvβ3 cross-linking to vitronectin. The adhesion strength (▪) and the cross-linked adhesion strength (□) after chemical cross-linking of αvβ3 to substrate-bound vitronectin were determined using the spinning disk analysis to Kαvβ3, Kαvβ3(Y747F), and Kαvβ3(Y759F) cells seeded on vitronectin (60 ng/cm2) for 15 min in the absence or presence of PMA. The αvβ3-cytoskeletal apparent binding strengths were approximately twice the corresponding αvβ3-vitronectin binding strengths.
Phosphorylation of β3 Integrin Is Required for the β3-Cytoskeletal Linkages
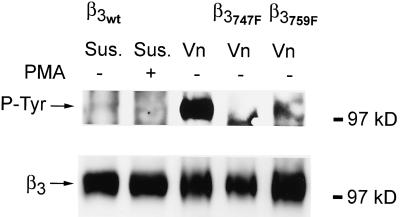
The differential effects on adhesion strength of the Y747F and Y759F mutants of β3 integrin suggest that the binding of proteins to the cytoplasmic domain of β3 integrin may be controlled by phosphorylation and dephosphorylation of β3 integrin and that these interactions mediate the conformational changes necessary to alter the binding strength of αvβ3 to vitronectin. To further investigate this model, the phosphorylation states of β3 integrin were analyzed. Cells were incubated either in suspension or plated on vitronectin for 2 hours and analyzed by immunoprecipitation and Western blot for β3 integrin and phosphotyrosine. Figure10 shows that no phosphorylated β3 integrin was detected in Kαvβ3 cells incubated in suspension either in the presence or absence of PMA, demonstrating a low level of background phosphorylation and the inability of PMA to induce phosphorylation of β3 integrin in suspended cells (see also Blystone et al., 1996). In contrast, plating the cells on vitronectin in the absence of PMA induced a high level of phosphorylation on β3 integrin. Plating Kαvβ3(Y747F) cells on vitronectin resulted in no phosphorylation of β3, whereas plating of Kαvβ3(Y759F) cells on vitronectin resulted in a reduced phosphorylation of β3 (14% of WT levels; Figure 10). As reported for activation of αvβ3 by binding of RGD peptides (Blystone et al., 1996), there was no phosphorylation of the available Y759 in the absence of phosphorylation of Y747 in the Y747F mutant. The reduced level of phosphorylation of β3 in the Y759F mutant relative to WT β3 suggests that Y759 is also a target for phosphorylation. The Y747F mutation blocks tyrosine phosphorylation and the increase in adhesion strength from stage 1 to stage 2, whereas the Y759F allows phosphorylation of Y747 but not Y759 and blocks the increase in adhesion strength from stage 2 to stage 3.
Figure 10.
Phosphorylation of β3 integrin. Kαvβ3, Kαvβ3(Y747F), and Kαvβ3(Y759F) cells were held in suspension in the presence or absence of PMA or plated for 2 h on vitronectin-coated plates. Blots were first probed for phosphotyrosine (top), stripped, and then reprobed for β3 integrin (bottom). Binding of cells to vitronectin-stimulated phosphorylation of β3 but PMA did not. No phosphorylation of β3 was seen for the Y747F mutant and 14% of WT levels of phosphorylation were observed for the Y759 mutant.
DISCUSSION
This report describes three basic findings: a) elucidation of the initial αvβ3 binding to vitronectin as a low-strength interaction, b) demonstration that the increase in adhesion strength involves an increase in the strength of the individual αvβ3-vitronectin bonds, and c) the increase in adhesion strength involves an increase in the strength of the αvβ3-actin cytoskeleton linkage. The models that have been developed to understand integrin–ligand interactions are not adequate to account for the data presented. Our working model is shown in Figure 11. The common usage of the terms affinity, avidity, and activation include operational definitions and theoretical models both of which require reevaluation to understand the data presented. Both affinity and avidity describe the behavior of ligand binding, which is driven by thermal diffusion. Affinity is the equilibrium binding constant for individual receptor–ligand bonds. Avidity was developed to account for the production of antibody–antigen molecular complexes, which were more stable than predicted on the basis of affinity measurements and was explained by the multivalency of the interactions. Integrin-mediated adhesion of cells to a substrate is indeed multivalent, which precludes the measurement of affinities in this configuration. Nevertheless, these bonds can be dissociated by the application of force and the amount of force required can be measured. The data presented here demonstrate that the increase in the strength of adhesion is caused by a change in the strength of individual integrin-ligand bonds. Thus, what is regulated is more similar to our concepts of affinity than avidity.
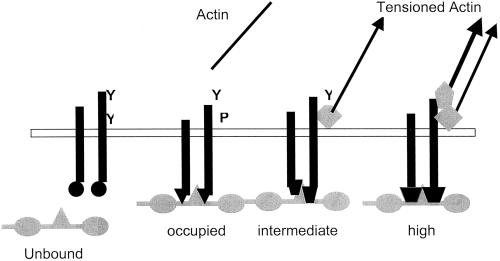
Figure 11.
Model for activation of αvβ3. Four states for αvβ3 integrin are given each with a different interaction with the vitronectin ligand from unbound to high-strength binding and with different linkages to the actin cytoskeleton. Y, available tyrosines; P, phosphorylated tyrosines. Diamonds and pentagons represent actin–integrin linking proteins, which may include talin and a-actinin. Actin arrows indicate the application tension by the actin cytoskeleton.
The meaning of activation depends on the assay that is applied. Most analyses of integrin activation depend on the use of CLIBS (cation- and ligand-induced binding site) antibodies as reporters to distinguish between the “inactive” (CLIBS not bound) and the “active” (CLIBS bound) states of the integrin (Shattil_et al._, 1985; O'Toole et al., 1994; Pampori_et al._, 1999). This approach includes the inherent assumption that there are only two states. In the Kαvβ3 cell system, measuring activation by CLIBS binding would identify the low-strength binding form as the “activated” form, whereas use of traditional adhesion assays would identify a later stage (Blystone et al., 1997). The measurement of binding parameters for the integrin-ligand interaction provides a less biased approach to this question and permits the identification of multiple activation states. As applied here, integrin activation includes all steps that increase the strength of integrin-ligand bonds.
The Initial αvβ3-Vitronectin Bond
Nonligated integrins on the surface of cells in suspension are generally in an “inactive” state. The conversion of these inactive integrins to forms that can bind ligand has been called inside-out signaling or affinity modulation (Ginsberg et al., 1992; Faull et al., 1993). In platelet and lymphocyte systems, these inactive forms of integrin are restrained from binding to ligand even when it is presented. Signaling through other receptor systems or treatment with low-dose cytochalasin D is required to relieve this inhibition and allow ligand binding (Kucik et al., 1996; Bennett et al., 1999). It was the identification of this agonist-induced removal of the inhibition to ligand binding that led to the concept of integrin activation (Shattil et al., 1985). However, this is not what happens during integrin binding in Kαvβ3 cells and most other cell types. As numerous adhesion assays have demonstrated, the presentation of substrate-bound ligand is sufficient to activate integrin-specific cell adhesion. Low-dose cytochalasin D, which activates some platelet and lymphocyte integrins, does not activate the integrins on Kαvβ3 cells (Kucik et al., 1996; Bennett et al., 1999; Boettiger et al., unpublished results). The binding of αvβ3 on Kαvβ3 cells to ligand is accompanied by a conformational change that is identified by the same LIBS 1 antibody that recognizes the activated state of αIIbβ3 on platelets (Frelinger_et al._, 1991; Blystone et al., 1997). The strong integrin-specific adhesion of fibroblasts and of Kαvβ3 cells requires metabolic energy, suggesting that intracellular signaling is required for full activation (Boettiger, unpublished results). What is the receptor that initiates this signaling process?
The Kαvβ3 cell system is unusual in that one can separate the initial binding reaction from the subsequent events, which are dependent on intracellular signaling because these signals require initiation through protein kinase C or thrombin receptors (Blystone et al., 1997). In this report, we identify a weak, but αvβ3-specific, adhesion to vitronectin that occurs in the absence of stimulation by PMA and occurs in β3 mutants, which are defective in the downstream activation of αvβ3 in these cells. In addition, this initial binding induces the phosphorylation of Y747 in the cytoplasmic domain of β3, demonstrating direct outside-in signaling, which is essential for subsequent activation of αvβ3 to provide strong cell adhesion. Thus, αvβ3 in this initial binding state has all the properties necessary to perform the function of the missing integrin activation receptor.
Signaling-Induced Ligand Binding States for αvβ3
After the initial ligand binding, there is an increase in the strength of the cell adhesion, and it is this increase that is usually measured using traditional adhesion assays (Blystone et al., 1997). Because the spinning disk analysis isolates the integrin-ligand bond strength from other parameters that may affect the strength of cell adhesion, it can be used to ask if this observed increase is due to changes in the properties of individual αvβ3-vitronectin bonds (Garcia et al., 1998). Our data identify two distinct levels of αvβ3-vitronectin bond strength based on the comparison of the WTβ3 with the Y759F mutant. In the Kαvβ3 cells, which are grown as suspension cells and have limited ability to spread, it is possible to account for all of the increased adhesion observed in these assays by these increases in the strength of the αvβ3-vitronectin bond. This is shown in the linearity of the relationship between the strength of adhesion and ligand density or number of receptor–ligand bonds. Thus, it is increases in the strength of individual bonds and not the clustering of integrins that is responsible for the increased strength of adhesion. The ability of integrins not only to assume “on” and “off” states but to regulate the strength of their “on” states is a fundamental property of these receptors.
The signals that regulate αvβ3-virtronectin bond strength appear to accomplish this through regulation of the binding of proteins to the cytoplasmic domain of β3. In the Y759F mutant, it is expected that the NPLY (744–747) domain is available for interacting with cytoplasmic proteins. Both talin and filamin bind to this region of β3 integrin and adhesion of αvβ3 to vitronectin can be abolished by either a N744A or Y747A mutation (Filardo et al., 1995;Ylanne et al., 1995). Either talin or filamin could provide a link between β3 and the actin cytoskeleton. In the wt β3, both the NPLY (744–747) and the NPIY (756–759) domains are available for binding. The approximately twofold increase in adhesion suggests that these binding events play approximately equal roles in contributing to the final binding strength.
In the Kαvβ3 cells, it appears that the phosphorylation of Y759 was dependent on the phosphorylation of Y747. This is consistent with the milder phenotype reported for Y759 mutants. Y759A had a mild effect on spreading of transfected CHO cells on fibrinogen but still formed normal adhesion plaques (Ylanne et al., 1995; Schaffner-Reckinger et al., 1998). The Y759F mutant had no distinctive phenotype in adhesion, spreading, or signaling assays (Blystone et al., 1997; Schaffner-Reckinger_et al._, 1998). However, this sequential reaction may not hold for all cells, because in the CS-1 cells either Y747F or Y759F reduced the adhesion strength by a similar amount.
Mechanisms of Activation of αvβ3 Integrin
Numerous data point to a role for the actin cytoskeleton in the activation of integrins (Lotz et al., 1989; Burridge and Chrzanowska-Wodnicka, 1996; Kucik et al., 1996; Zhong_et al._, 1997). It has been proposed that the role of the cytoskeleton is to control the distribution of integrins on the cell surface, either through restricting their movement (Kucik et al., 1996) or by clustering to form focal contacts (Burridge and Chrzanowska-Wodnicka, 1996). The clustering of integrins could contribute either directly to the increase in adhesion strength, if clusters produce stronger adhesion, or indirectly, if clustering controls the activation signaling process (Bazzoni and Hemler, 1998). If clustering were to contribute directly to adhesion strength, the relationship between the adhesion strength and the ligand density would be second order, and this difference would be apparent in the curve-fit of the data. Thus, it is unlikely that clustering has any direct contribution to adhesion strength in these cells. The issue of the contribution of clustering to the activation signals is not addressed by our experiments.
Alternatively, the cytoskeleton could exert tension on the cytoplasmic domain of integrin. There are two reports that demonstrate that mechanical strain increases the linkage of integrin to cytoskeleton. Choquet et al. (1997) placed fibronectin-coated beads on the surface of fibroblasts, which were then bound by integrin and transported centripetally by the actin cytoskeleton. Restraining the bead increased the force that the cytoskeleton could apply, implying that the integrin-actin link was increased by the mechanical strain. Riveline et al. (2001) demonstrated that prodding cells with a micropipet could substitute for the action of rho kinase and actin tensioning in the assembly of focal contacts. The data presented in this report show that using a combination of chemical cross linking and hydrodynamic shear demonstrates a parallel increase in the strength of αvβ3-vitronectin links and αvβ3-actin links. This extends the results of Choquet et al. and suggests that, like the integrin-actin link, the strength of the integrin-ligand link (integrin activation) is also a strain-activated process. The mutant analysis showed that the domains controlled by Y747 and Y759 contributed approximately equally to the overall strength of the αvβ3-vitronectin activation. This could be explained if they each formed the basis for a connection between integrin and the actin cytoskeleton. The need for actin tensioning by myosin would explain the sensitivity of activation to metabolic energy and also provide a means to expend energy inside the cell to effect a conformational change outside the cell. Thus, αvβ3 could be mechanically activated by the tensioning of the actin filaments that are linked to the β3 cytoplasmic domain. This strain could be used to alter the conformation of the ligand binding domain of αvβ3 and produce a stronger bond to its vitronectin ligand.
ACKNOWLEDGMENTS
We thank Richard Assoian (University of Pennsylvania) for critical reading of the manuscript, Caroline Damsky (UCSF) for the donation of AIIB2, and David Cheresh (Scripps) for the donation of LM609 monoclonal antibodies. This work was supported by grants RO1 CA49866 and RO1 CA16502 from the National Cancer Institute and grant RO1 GM 57388 from the Institute of General Medicine.
REFERENCES
- Bazzoni G, Hemler ME. Are changes in integrin affinity and conformation overemphasized? Trends Biochem Sci. 1998;23:30–34. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- Bennett JS, Zigmond S, Vilaire G, Cunningham ME, Bednar B. The platelet cytoskeleton regulates the affinity of the integrin alpha(IIb)beta(3) for fibrinogen. J Biol Chem. 1999;274:25301–25307. doi: 10.1074/jbc.274.36.25301. [DOI] [PubMed] [Google Scholar]
- Blystone SD, Lindberg FP, Williams MP, McHugh KP, Brown EJ. Inducible tyrosine phosphorylation of the beta3 integrin requires the alphaV integrin cytoplasmic tail. J Biol Chem. 1996;271:31458–31462. doi: 10.1074/jbc.271.49.31458. [DOI] [PubMed] [Google Scholar]
- Blystone SD, Williams MP, Slater SE, Brown EJ. Requirement of integrin beta3 tyrosine 747 for beta3 tyrosine phosphorylation and regulation of alphavbeta3 avidity. J Biol Chem. 1997;272:28757–28761. doi: 10.1074/jbc.272.45.28757. [DOI] [PubMed] [Google Scholar]
- Boettiger D, George-Weinstein M, Menko AS. Triggering terminal myogenic differentiation. In: Stockdale F, Kedes L, editors. UCLA Symposia on Molecular and Cellular Biology, New Series: Cellular and Molecular Biology of Muscle Development. New York: Alan R. Liss, Inc.; 1989. pp. 57–66. [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfield DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeletal linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Menko AS, Philp N, Boettiger D. Evaluation of integrin molecules involved in substrate adhesion. Cell Adhesion Commun. 1993;1:191–202. doi: 10.3109/15419069309097253. [DOI] [PubMed] [Google Scholar]
- Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Affinity modulation of integrin α5β 1: regulation of the functional response by soluble fibronectin. J Cell Biol. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Brooks PC, Deming SL, Damsky C, Cheresh DA. Requirement of the NPXY motif in the integrin beta 3 subunit cytoplasmic tail for melanoma cell migration in vitro and in vivo. J Cell Biol. 1995;130:441–450. doi: 10.1083/jcb.130.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger AL, Du XP, Plow EF, Ginsberg MH. Monoclonal antibodies to ligand-occupied conformers of integrin αIIbβ3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J Biol Chem. 1991;266:17106–17111. [PubMed] [Google Scholar]
- Garcia AJ, Boettiger D. Integrin-fibronectin interactions at the cell-material interface: initial integrin binding and signaling. Biomaterials. 1999;20:2427–2433. doi: 10.1016/s0142-9612(99)00170-2. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, Ducheyne P, Boettiger D. Quantification of cell adhesion using a spinning disc device and application to surface-reactive materials. Biomaterials. 1997;18:1091–1098. doi: 10.1016/s0142-9612(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, Huber F, Boettiger D. Force required to break α5β1 integrin-fibronectin bonds in intact adherent cells is sensitive to integrin activation state. J Biol Chem. 1998;273:10988–10993. doi: 10.1074/jbc.273.18.10988. [DOI] [PubMed] [Google Scholar]
- Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MH, Du X, Plow EF. Inside-out integrin signaling. Curr Opin Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Hato T, Pampori N, Shattil SJ. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin alphaIIb beta3. J Cell Biol. 1998;141:1685–1695. doi: 10.1083/jcb.141.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PE, O'Toole TE, Ylanne J, Shattil SJ, Ginsberg MH. The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J Biol Chem. 1995;270:12411–12417. doi: 10.1074/jbc.270.21.12411. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest. 1996;97:2139–2144. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D, DeGuzman FR, Heiser P, Ministri-Madrid K, Kileen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in αIIβ3 signaling in platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- Lotz MM, Burdsal CA, Erickson HP, McClay DR. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989;109:1795–1805. doi: 10.1083/jcb.109.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko AS, Boettiger D. Occupation of the extracellular matrix receptor integrin is a control point for myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- O'Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole TE, Ylanne J, Culley BM. Regulation of integrin affinity states through an NPXY motif in the beta subunit cytoplasmic domain. J Biol Chem. 1995;270:8553–8558. doi: 10.1074/jbc.270.15.8553. [DOI] [PubMed] [Google Scholar]
- Pampori N, Hato T, Stupack DG, Aidoudi S, Cheresh DA, Nemerow GR, Shattil SJ. Mechanisms and consequences of affinity modulation of integrin alpha(V)beta(3) detected with a novel patch-engineered monovalent ligand. J Biol Chem. 1999;274:21609–21616. doi: 10.1074/jbc.274.31.21609. [DOI] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban N, Kam Z, Geiger B, Bershadsky A. Focal contact as a mechanosenser: directional growth in response to local strain. Mol Biol Cell. 2001;10(suppl):341a. [Google Scholar]
- Schaffner-Reckinger E, Gouon V, Melchior C, Plancon S, Kieffer N. Distinct involvement of beta3 integrin cytoplasmic domain tyrosine residues 747 and 759 in integrin-mediated cytoskeletal assembly and phosphotyrosine signaling. J Biol Chem. 1998;273:12623–12632. doi: 10.1074/jbc.273.20.12623. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J Cell Biol. 1993;120:1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb-IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- Yatohga T, Masako I, Heiachiro K, Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988;13:292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- Ylanne J, Huuskonen J, O'Toole TE, Ginsberg MH, Virtanen I, Gahmberg CG. Mutation of the cytoplasmic domain of the integrin beta 3 subunit. Differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis. J Biol Chem. 1995;270:9550–9557. doi: 10.1074/jbc.270.16.9550. [DOI] [PubMed] [Google Scholar]
- Zhong C, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]