Estrogen Plus Progestin and Colorectal Cancer Incidence and Mortality (original) (raw)
Abstract
Purpose
During the intervention phase in the Women's Health Initiative (WHI) clinical trial, use of estrogen plus progestin reduced the colorectal cancer diagnosis rate, but the cancers were found at a substantially higher stage. To assess the clinical relevance of the findings, analyses of the influence of combined hormone therapy on colorectal cancer incidence and colorectal cancer mortality were conducted after extended follow-up.
Patients and Methods
The WHI study was a randomized, double-blind, placebo-controlled clinical trial involving 16,608 postmenopausal women with an intact uterus who were randomly assigned to daily 0.625 mg conjugated equine estrogen plus 2.5 mg medroxyprogesterone acetate (n = 8,506) or matching placebo (n = 8,102). Colorectal cancer diagnosis rates and colorectal cancer mortality were assessed.
Results
After a mean of 5.6 years (standard deviation [SD], 1.03 years) of intervention and 11.6 years (SD, 3.1 years) of total follow-up, fewer colorectal cancers were diagnosed in the combined hormone therapy group compared with the placebo group (diagnoses/year, 0.12% v 0.16%; hazard ratio [HR], 0.72; 95% CI, 0.56 to 0.94; P = .014). Bowel screening examinations were comparable between groups throughout. Cancers in the combined hormone therapy group more commonly had positive lymph nodes (50.5% v 28.6%; P < .001) and were at higher stage (regional or distant, 68.8% v 51.4%; P = .003). Although not statistically significant, there was a higher number of colorectal cancer deaths in the combined hormone therapy group (37 v 27 deaths; 0.04% v 0.03%; HR, 1.29; 95% CI, 0.78 to 2.11; P = .320).
Conclusion
The findings, suggestive of diagnostic delay, do not support a clinically meaningful benefit for combined hormone therapy on colorectal cancer.
INTRODUCTION
The Women's Health Initiative (WHI) randomized, placebo-controlled trial evaluating estrogen plus progestin identified more risks than benefits for the use of combined hormone therapy.1 However, during the intervention phase of the trial, there was a statistically significant 44% lower rate of colorectal cancer diagnoses in the estrogen plus progestin group,2 a finding in agreement with the preponderance of observational studies.3 Consequently, review articles,4,5 position statements,6–10 and executive summaries11 of professional societies commonly listed reduction of colorectal cancer risk as a benefit of estrogen plus progestin use.
Despite the general perception of colorectal cancer benefit for combined hormone therapy use, the WHI clinical trial findings raised several questions. The colorectal cancers in the combined hormone therapy group had more lymph node involvement and were diagnosed at a substantially higher stage.2 In addition, colorectal cancer deaths did not differ in the estrogen plus progestin and placebo groups in an early analysis based on the distribution of 44 deaths.12 Postintervention follow-up through a mean of 7.9 years found that a lower colorectal cancer diagnosis rate was no longer seen after discontinuation of hormones.13 Therefore, to assess whether combined hormone therapy is associated with meaningful influence on colorectal cancer, we report updated information on colorectal cancer diagnoses and colorectal mortality through a mean of 11.6 years of follow-up.
PATIENTS AND METHODS
The WHI trial of estrogen plus progestin randomly assigned 16,608 postmenopausal women to daily conjugated equine estrogens (0.625 mg/d) plus medroxyprogesterone acetate (2.5 mg/d; Prempro, Wyeth-Ayerst, Rouses Point, NY) or placebo at 40 US clinical centers between 1993 and 1998.1,14 Initially, participants were also randomly assigned to estrogen alone. When published results from the Heart and Estrogen/Progestin Replacement Study (HERS)15 indicated adherence was not feasible in women with a uterus, the protocol was changed to a 1:1 randomization excluding estrogen alone. The 331 women randomly assigned to estrogen alone were unblinded and reassigned to the estrogen plus progestin group.
The study was approved by institutional review boards at each institution, and all participants signed written informed consent. Study design and implementation have been described previously.1,14 Eligible women were between 50 and 79 years of age, postmenopausal, with life expectancy of ≥ 3 years. Women with prior hysterectomy, any prior breast cancer, or prior colorectal cancer within 10 years were ineligible. Hormone users were eligible after a 3-month washout. Random assignment was performed by the WHI Clinical Coordinating Center by using a computerized permuted block algorithm stratified by clinical center and age group and was implemented at local clinical centers by using a bar code dispensing procedure to ensure participant and staff blinding.
Colorectal cancer diagnoses were elicited semiannually by mail or by telephone questionnaires. Participant self-reports or next-of-kin (proxy) reports of colorectal cancer were verified by centrally trained physician adjudicators at the local clinical centers after medical record review.16 Final adjudication and coding were performed at the Clinical Coordinating Center by using the Surveillance, Epidemiology, and End Results (SEER) system.17 Cause of death was based on medical record review by physician adjudicators at the local clinical centers, with final adjudication at the Coordinating Center. Reviewers were blind to randomization allocation.
Colorectal screening was not protocol defined. At 6-month intervals, self-administered questionnaires or structured telephone interviews were used to collect information on the frequency of rectal examinations, fecal occult blood tests, sigmoidoscopy and colonoscopy (asked as one question), and barium enema examinations. Because the clinical centers did not provide comprehensive health care, work-ups related to colorectal cancer diagnosis were made largely by participants' local physicians.
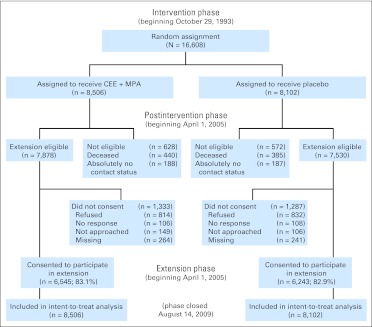
After net harm for estrogen plus progestin use was seen, participants were instructed to stop study medication on July 7, 2002. Follow-up continued according to the protocol through March 31, 2005, the original trial completion date. An extension phase began on April 1, 2005, which required reconsent for additional follow-up. Of 15,408 surviving participants, 12,788 or 83% reconsented. A CONSORT diagram detailing the flow of study participants was published previously18 and is provided in Figure 1.
Fig 1.
CONSORT diagram. CEE, conjugated equine estrogen; MPA, medroxyprogesterone acetate.
Our analyses included patients with invasive colorectal cancer and excluded two patients with squamous cell carcinomas and two with infiltrating ductal carcinomas. Prior reports included 115 cases of colorectal cancer reported after mean follow-up of 5.6 years (standard deviation [SD], 1.3 years)2 with an additional 74 cases reported after mean follow-up of 7.9 years (SD, 1.4 years).13 Previously, 44 deaths after colorectal cancer were reported.12 Now, with a mean follow-up of 11.6 years (SD, 3.1 years) through September 30, 2010, there are 263 colorectal cancers and 90 deaths following colorectal cancer diagnosis. In addition, we report, for the first time (to the best of our knowledge), on deaths after colorectal cancer measured from the date of diagnosis.
The sample size was based primarily on hypothesized coronary heart disease benefit. Colorectal cancer was a designated secondary end point. Results for invasive colorectal cancer incidence, deaths directly attributed to colorectal cancer (deaths from colorectal cancer) and deaths from all causes following colorectal cancer diagnosis (deaths after colorectal cancer) were assessed with time-to-event methods based on the intent-to-treat principle. The total number of events and the annualized percentages for these outcomes were reported. Analyses included all 16,608 randomly assigned participants.
Hazard ratio (HR) estimation for colorectal cancer diagnoses was based on Cox proportional hazards regression defined relative to the date of random assignment. Stratification was based on 10-year baseline age groups, colorectal cancer history, WHI Dietary Modification trial randomization (intervention, control, or nonparticipant), and calcium and vitamin D trial randomization (active, placebo, or nonparticipant). Nominal 95% CIs are presented for HRs, and all significance levels are two-sided. Thirteen interactions with baseline characteristics were tested. Less than one would be expected to be positive by chance alone. Kaplan-Meier plots were used to display rates of colorectal cancer over time. Cumulative incidence curves were computed and were nearly identical to the Kaplan-Meier estimates; hence, they are not presented. For colorectal cancer diagnosis rate analyses, women who did not reconsent to active follow-up after March 31, 2005, were censored at that time. The original consent permitted continued follow-up for vital status. Vital status information from the National Death Index (NDI) was included for all participants with all mortality information censored on September 30, 2010.
To examine the potential effect of censoring follow-up times for women who did not reconsent to follow-up after March 31, 2005, several secondary analyses were performed, including comparison of reconsent rates by random assignment and adjusting the HR analyses for reconsent status. Adherence was routinely measured by weighing or counting returned pills at the annual visits. Sensitivity analyses for colorectal cancer diagnosis and mortality rates by medication adherence were conducted by using inverse probability weighting analyses. Nonadherence (using < 80% of study pills or initiating nonprotocol hormone therapy) probabilities were estimated by logistic regression models that included baseline variables of age, ethnicity, education, body mass index, smoking, self-reported general health, night sweats, hot flashes, breast tenderness, and treatment assignment; at year 1, breast tenderness, night sweats, and hot flashes, and the inverse of these estimated probabilities were used as the weights in the Cox models for HR estimation.
To facilitate comparison with observational studies, we systematically reviewed the literature (PubMed) from 1970 to December 2011 and identified 10 cohort studies that examined estrogen plus progestin association with colorectal cancer risk. Relative risks across all studies were combined by using a random effects model. The relative risks used were from the multivariable adjusted estimates provided in the original studies.
The study sponsor provided input into the design and conduct of the trial and participated in the review of this report but not in its preparation. The corresponding authors had full access to all data and final responsibility for submitting the report for publication.
RESULTS
Baseline characteristics for the 16,608 initially randomly assigned participants have been published,1 and characteristics of participants in the two randomly assigned groups were comparable in the initial population and in the reconsenting population of 12,788 women with somewhat more women in the placebo group having a family history of colorectal cancer (Appendix Table A1, online only).
Outcome information was available on 16,560 (99.7%) of the 16,608 originally randomly assigned participants. Survival status was available for 12,430 (97.2%) of 12,788 participants who consented for the extension follow-up and through September 2010 for 3,121 (81.74%) of the remaining 3,820 participants. The mean follow-up was 11.6 years (SD, 3.1 years) with maximum follow-up of 16.5 years.
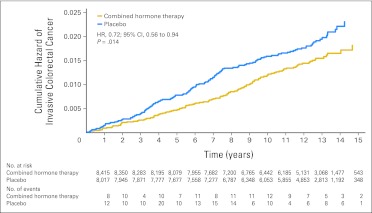
There were 118 women in the combined hormone therapy group with a diagnosis of invasive colorectal cancer compared with 145 in the placebo group (diagnoses/year, 0.12% v 0.16%; HR, 0.72; 95% CI, 0.56 to 0.94; P = .014; Table 1 and Fig 2). There were fewer colon cancers in the estrogen plus progestin group (103 v 129 cases; 0.10% v 0.14%; HR, 0.77; 95% CI, 0.59 to 1.01; P = .059). Only 37 rectal cancer cases were diagnosed with no difference between randomization groups (20 v 17 cases; 0.02% v 0.02%; HR, 1.16; 95% CI, 0.60 to 2.25; P = .65). The influence of estrogen plus progestin on the rate of colorectal cancer diagnosis was limited to the intervention period (HR, 0.75; 95% CI, 0.57 to 1.00) because no reduction was seen postintervention (HR, 0.96; 95% CI, 0.67 to 1.39; P = .83).
Table 1.
Invasive Colorectal Cancer Outcomes by Tumor Location and by Study Group
| Outcome | Combined Hormone Therapy Group* | Placebo Group | HR† | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| No. | % Per Year | No. | % Per Year | ||||
| Cancer incidence | |||||||
| Colorectal cancer (all) | 118 | 0.12 | 145 | 0.16 | 0.72 | 0.56 to 0.94 | .014 |
| Colon cancer‡ | 103 | 0.10 | 129 | 0.14 | 0.77 | 0.59 to 1.01 | .059 |
| Rectal cancer | 20 | 0.02 | 17 | 0.02 | 1.16 | 0.60 to 2.25 | .65 |
| Deaths from colorectal cancer§ | |||||||
| Colorectal cancer (all) | 37 | 0.04 | 27 | 0.03 | 1.29 | 0.78 to 2.11 | .32 |
| Colon cancer | 30 | 0.03 | 25 | 0.03 | 1.14 | 0.67 to 1.94 | .67 |
| Rectal cancer | 7 | 0.007 | 2 | 0.002 | 3.11 | 0.65 to 15.0 | .16 |
| Deaths after colorectal cancer¶ | |||||||
| Colorectal cancer (all) | 46 | 0.04 | 44 | 0.04 | 0.96 | 0.63 to 1.45 | .83 |
| Colon cancer | 40 | 0.04 | 41 | 0.04 | 0.89 | 0.57 to 1.38 | .61 |
| Rectal cancer | 10 | 0.01 | 3 | 0.003 | 3.14 | 0.86 to 11.4 | .08 |
Fig 2.
Colorectal cancer incidence by random allocation group. HR, hazard ratio.
Colorectal cancer incidence results were similar for analyses excluding 54 women with a remote colorectal cancer history (HR, 0.77; 95% CI, 0.60 to 0.98; P = .033) and for analyses adjusting for a family history of colorectal cancer (HR, 0.72; 95% CI, 0.56 to 0.94; P = .014).
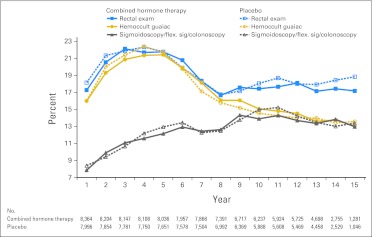
Forty-one percent of participants in each group reported having had colonoscopy or sigmoidoscopy before study entry. The frequency of bowel examinations during the study was similar in both randomly assigned groups over time (Appendix Fig A1, online only). During the study course, 67% of participants had at least one colonoscopy or sigmoidoscopy and 67% had at least one fecal occult blood test.
Vaginal bleeding was more common in the combined hormone therapy group (58% v 7%; P < .001). In the 96 women with vaginal bleeding before colorectal cancer was diagnosed, the mean number of positive lymph nodes (2.5 ± 4.3) was greater than in the 167 women with no such bleeding (1.2 ± 2.5 positive nodes; P = .014). The patients with colorectal cancer had similar histology, location, and grade in the two randomly assigned groups. However, patients with colorectal cancer in the hormone group were more likely to have positive lymph nodes (50.5% v 28.6%; P < .001) and were more likely to have been diagnosed with distant disease (19.3% v 6.5%; P = .003; Table 2).
Table 2.
Characteristics of Invasive Colorectal Cancer Cases, According to Treatment Group*
| Characteristic | Combined Hormone Therapy Group | Placebo Group | P† | ||||
|---|---|---|---|---|---|---|---|
| No. of Patients | % | Mean | SD | No. of Patients | % | Mean | SD |
| Invasive colorectal cancer | 118 | 1.4 | 145 | 1.8 | |||
| Tumor size, cm | (n = 85) | 4.3 | 2.3 | (n = 108) | 4.2 | 2.3 | .751 |
| ≤ 3.9 | 38 | 44.7 | 54 | 50.0 | .465 | ||
| 4.0-5.9 | 25 | 29.4 | 34 | 31.5 | |||
| ≥ 6.0 | 22 | 25.9 | 20 | 18.5 | |||
| No. of positive lymph nodes | (n = 90) | 2.3 | 4.1 | (n = 130) | 1.1 | 2.5 | .011 |
| None | 42 | 46.7 | 91 | 70.0 | .007 | ||
| 1 | 16 | 17.8 | 13 | 10.0 | |||
| 2-3 | 15 | 16.7 | 13 | 10.0 | |||
| ≥ 4 | 17 | 18.9 | 13 | 10.0 | |||
| Lymph node involvement | (n = 101) | (n = 133) | |||||
| No | 50 | 49.5 | 95 | 71.4 | < .001 | ||
| Yes | 51 | 50.5 | 38 | 28.6 | |||
| Stage of disease‡ | (n = 109) | (n = 138) | |||||
| Localized | 34 | 31.2 | 67 | 48.6 | .003 | ||
| Regional | 54 | 49.5 | 62 | 44.9 | |||
| Distant | 21 | 19.3 | 9 | 6.5 | |||
| Morphologic grade | (n = 100) | (n = 130) | |||||
| Well differentiated | 6 | 6.0 | 14 | 10.8 | .173 | ||
| Moderately differentiated | 68 | 68.0 | 91 | 70.0 | |||
| Poorly differentiated | 26 | 26.0 | 22 | 16.9 | |||
| Anaplastic | 0 | 0.0 | 3 | 2.3 | |||
| Location of cancer§ | (n = 109) | (n = 117) | |||||
| Proximal | 56 | 51.4 | 56 | 47.9 | .519 | ||
| Distal | 31 | 28.4 | 31 | 26.5 | |||
| Rectum | 22 | 20.2 | 30 | 25.6 | |||
| Histologic features | (n = 111) | (n = 141) | |||||
| Adenocarcinoma, not otherwise specified | 66 | 59.5 | 92 | 65.2 | .134 | ||
| Adenocarcinoma in adenomatous polyp | 7 | 6.3 | 15 | 10.6 | |||
| Adenocarcinoma in villous adenoma | 6 | 5.4 | 2 | 1.4 | |||
| Adenocarcinoma in tubulovillous adenoma | 13 | 11.7 | 16 | 11.3 | |||
| Mucin secreting | 4 | 3.6 | 5 | 3.5 | |||
| Signet ring cell | 0 | 0.0 | 2 | 1.4 | |||
| Medullary | 1 | 0.9 | 0 | 0.0 | |||
| Other | 14 | 12.6 | 9 | 6.4 |
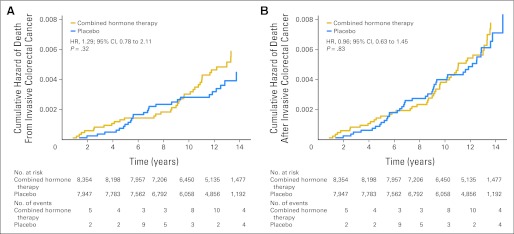
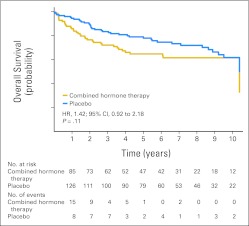
Forty-six women who were diagnosed with colorectal cancer in the estrogen plus progestin group died during follow-up compared with 44 in the placebo group (0.04% v 0.04%; HR, 0.96; 95% CI, 0.63 to 1.45; P = .83; Table 1). Of these, 37 deaths were directly attributed to colorectal cancer in the estrogen plus progestin group compared with 27 in the placebo group (0.04% v 0.03%; HR, 1.29; 95% CI, 0.78 to 2.11; P = .32; Table 1). Colorectal cancer mortality from random assignment date by study group is depicted in Figure 3. Thus, although there were 27 fewer colorectal cancers diagnosed in the combined hormone therapy group, there were 10 more deaths attributed to the disease. For this reason, an exploratory analysis examined the survival of women from the time of their colorectal cancer diagnosis by random assignment group. Survival after colorectal cancer diagnosis appeared to be greater in the placebo group compared with the combined hormone therapy group, although these differences were not statistically significant (HR, 1.42; 95% CI, 0.92 to 2.18; P = .11; Fig 4). The estimated 5-year survival rates were 0.65 (SE, 0.050) for combined hormone therapy and 0.78 (SE, 0.038) for the placebo group.
Fig 3.
(A) Risk of death from invasive colorectal cancer and (B) risk of death after invasive colorectal cancer from date of random allocation by randomly assigned group. HR, hazard ratio.
Fig 4.
Overall survival after colorectal cancer diagnosis by random allocation group. HR, hazard ratio.
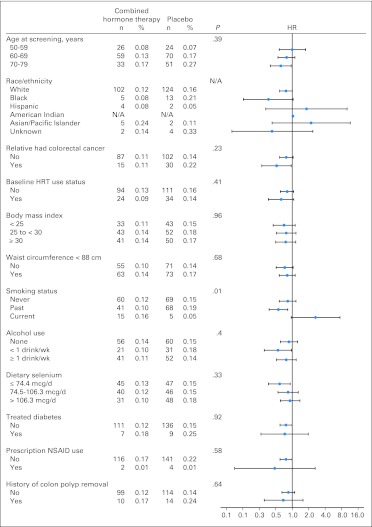
Of 13 subgroups examined, only smoking status had a nominally significant interaction with the risk of colorectal cancer diagnosis (Appendix Fig A2, online only). Current smokers in the combined hormone therapy group had an increased rate of colorectal cancer diagnosis (HR, 2.65; 95% CI, 0.96 to 7.37) compared with never smokers (HR, 0.83; 95% CI, 0.58 to 1.17; interaction P = .01), although the finding is based on only 20 cases among current smokers.
Reconsent status was similar for the combined hormone therapy (76.9%) and placebo groups (77.0%). Adjusting for reconsent status did not change the colorectal cancer incidence (HR, 0.72; 95% CI, 0.56 to 0.94; P = .014) or colorectal mortality results (HR, 1.31; 95% CI, 0.80 to 2.15; P = .29). Colorectal cancer incidence results were similar when the estimated probability of nonadherence to study medication was used as a weighting factor in the models (HR, 0.73; 95% CI, 0.51 to 1.04; P = .08).
In the meta-analyses of 10 cohort studies, estrogen plus progestin use was associated with a modest but statistically significant 14% lower colorectal cancer incidence (HR, 0.86; 95% CI, 0.76 to 0.97; P < .001; Table 3).
Table 3.
Meta-Analyses: Cohort Studies of Estrogen Plus Progestin Association With Colorectal Cancer Risk
| Study | Sample Size | No. of Patients | Outcome | Follow-Up (years) | RR | 95% CI |
|---|---|---|---|---|---|---|
| Risch and Howe19 | 32,973 | 464 | CRC | 15 | 1.07 | 0.58 to 1.98 |
| Persson et al20 | 22,597 | 233 | CRC | 13 | 0.60 | 0.38 to 0.95 |
| Pukkala et al21 | 94,505 | 83 | CRC | 3.2 | 0.85 | 0.64 to 1.12 |
| Tannen et al22 | 18,462 | N/A | CRC | 5.5 | 0.56 | 0.36 to 0.88 |
| Green et al23 | N/A | 383 | CRC | 7.4 | 0.83 | 0.73 to 0.94 |
| Johnson et al24 | 56,733 | 717 | CRC | 14 | 0.78 | 0.60 to 1.02 |
| Hildebrand et al25 | 67,412 | 776 | CRC | 13.2 | 0.93 | 0.70 to 1.23 |
| Henderson et al26 | 56,864 | 442 | Invasive colon cancer | 11 | 0.71 | 0.48 to 1.06 |
| Prentice et al12 | 32,084 | 175 | CRC | 5.5 | 1.15 | 0.74 to 1.79 |
| Tsilidis et al27 | 136,275 | 1,186 | CRC | 9 | 0.94 | 0.77 to 1.14 |
| Overall | 517,915 | 0.86 | 0.76 to 0.97 |
DISCUSSION
In the WHI randomized, placebo-controlled trial, estrogen plus progestin use was associated with a lower colorectal cancer diagnosis rate. However, the advanced stage of the cancers and the absence of lower colorectal cancer mortality in the hormone group raise concern regarding the clinical relevance of the findings. Because colorectal cancer is the third most common cancer in women in the United States,27,28 it is important to determine the influence of the still commonly used estrogen plus progestin therapy19 on the clinical course of this disease.
Although there were fewer colorectal cancers in the combined hormone therapy group, the cancers were diagnosed at a more advanced stage. Because screening bowel examinations and grade of cancers were similar across randomization groups, diagnostic delay represents a potential contributor to the difference in diagnosis rate. In any event, because colorectal cancer presents with localized disease in only approximately 40% of cases and has a 5-year risk of death of approximately 30% for all newly diagnosed cases,4 it is improbable that an intervention that reduced colorectal cancer incidence by 44% during active use would not have some favorable influence on colorectal mortality after 11.9 years of follow-up. In contrast, measured from random assignment, there is no suggestion of lower colorectal cancer mortality for women in the combined hormone therapy group.
Most colorectal cancers are not identified by bowel screening examinations, but patients commonly present with nonspecific findings, including abdominal pain and change in bowel habits that lead to diagnostic work-up.29,30 Because receiving an alternative diagnosis is associated with delay in the diagnosis of colorectal cancer,31 the association seen in the trial between antecedent vaginal bleeding and increased lymph node status suggests that attention to vaginal bleeding may have delayed assessment of the colorectal problem.
It is unknown whether implementing a prospective bowel screening program would identify the same number of colorectal cancers in women using estrogen plus progestin but find them at an earlier stage, which suggests clinical benefit or, alternatively, would find substantially more colorectal cancers earlier in combined hormone therapy users, which suggests diagnostic delay.
Early observational case-control studies, which uncommonly separated influence of estrogen alone from estrogen plus progestin use, associated menopausal hormone therapy use with lower colorectal cancer diagnosis rates.32 Several cohort studies have specifically evaluated the association between use of estrogen plus progestin and colorectal cancer.3,12,19–27 In both a meta-analysis of eight such studies27 and this meta-analysis incorporating 10 studies, a modest but statistically significant lower colorectal cancer incidence is associated with combined hormone therapy use. Such results agree with the current randomized clinical trial results regarding diagnosis rates but do not address the question of clinical relevance of the findings, since influence on colorectal mortality was not reported in the observational studies.
Four prior studies examined postmenopausal hormone therapy and survival measured from colorectal cancer diagnosis date and provided mixed results. In three studies,33–35 between 36% and 41% fewer cancer-related deaths were seen after colorectal cancer diagnosis in recent hormone users. In contrast, Newcomb et al36 found that neither estrogen alone nor estrogen plus progestin users had a lower colorectal cancer mortality compared with nonusers. Of the three positive observational studies, two did not adjust for screening33,34 and one adjusted for stage,35 potentially adjusting away an adverse effect of stage on combined hormone therapy use. In this randomized trial, median survival after the date of colorectal cancer diagnosis in the combined hormone therapy group was about 2 years shorter than that in the placebo group. In the WHI randomized clinical trial evaluating estrogen alone, survival in the hormone and placebo groups measured from diagnosis date was similar.37 Currently, there is no compelling explanation for divergent survival results after colorectal cancer seen between most observational studies and the randomized trials.
HERS evaluated the same estrogen plus progestin regimen used in the WHI trial in 2,763 postmenopausal women with or at risk for coronary disease. Fewer colorectal cancers were diagnosed during the intervention period in the hormone therapy group, but the difference was not statistically significant.38
Smoking status was the one subgroup with a significant interaction in which increased colorectal cancer incidence was seen with estrogen plus progestin use. An association between colorectal neoplasia and cigarette smoking has been described,39,40 particularly for rectal cancers.41 Our findings, taken together with the increased lung cancer mortality risk previously described in this trial,42 suggest that smokers who use estrogen plus progestin may be at increased risk for adverse cancer outcomes.
Study strengths include the randomized double-blind trial design, the large and diverse study population, serial assessment of bowel screening, long duration of follow-up, and central adjudication of cancers. Limitations include the study medication discontinuation rate, the limited number of colorectal cancer deaths, and absence of information on cancer therapy. However, therapy is fairly uniform by stage with surgery only for localized disease and one commonly used adjuvant chemotherapy regimen for node-positive disease.43
Despite concerns raised by prior colorectal cancer findings in this trial,2,12 position statements6–10 and executive summaries11 of professional societies continue to list reduction of colorectal cancer risk as a benefit of estrogen plus progestin use. Our results suggest that this assessment, with its potential for broad influence on clinical practice, should be re-evaluated.
In the WHI randomized trial, a lower rate of colorectal cancer diagnosis with estrogen plus progestin use was seen. However, the cancers were diagnosed at a more advanced stage, and no suggestion of reduced colorectal cancer mortality emerged with extended follow-up. These findings, in a cancer that can run a fatal course if there is a delay in diagnosis,4,27,28 do not support a clinically meaningful benefit for use of estrogen plus progestin in colorectal cancer. Future studies of estrogen plus progestin use and colorectal cancer should go beyond incidence analyses to address influence on tumor characteristics, stage, and colorectal cancer mortality.
Acknowledgment
We acknowledge the dedicated efforts of investigators and staff at the Women's Health Initiative (WHI) clinical centers, the WHI Clinical Coordinating Center, and the National Heart, Lung and Blood program office (listing available at https://cleo.whi.org/researchers/SitePages/Write%20a%20Paper.aspx). We also recognize the WHI participants for their extraordinary commitment to the WHI program.
Appendix
Table A1.
Baseline Characteristics of Reconsenting Participants (n = 12,784)
| Characteristic | Combined Hormone Therapy Groups(n = 6,542) | Mean ± SD | Placebo Group(n = 6,242) | Mean ± SD | P * |
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age at screening, years | (n = 6,542) | 62.8 ± 7 | (n = 6,242) | 63 ± 6.9 | .209 |
| 50-59 | 2,266 | 34.6 | 2,128 | 34.1 | .738 |
| 60-69 | 3,018 | 46.1 | 2,886 | 46.2 | |
| 70-79 | 1,258 | 19.2 | 1,228 | 19.7 | |
| Race/ethnicity | |||||
| White | 5,613 | 85.8 | 5,356 | 85.8 | .973 |
| Black | 406 | 6.2 | 401 | 6.4 | |
| Hispanic | 291 | 4.4 | 261 | 4.2 | |
| American Indian | 16 | 0.2 | 14 | 0.2 | |
| Asian/Pacific Islander | 132 | 2.0 | 128 | 2.1 | |
| Unknown | 84 | 1.3 | 82 | 1.3 | |
| Education | (n = 6,507) | (n = 6,198) | |||
| Primary school (≤ 8 years) | 94 | 1.4 | 90 | 1.5 | .265 |
| Some high school | 230 | 3.5 | 238 | 3.8 | |
| High school diploma or equivalent | 1,254 | 19.3 | 1,225 | 19.8 | |
| Some education after high school | 2,568 | 39.5 | 2,328 | 37.6 | |
| College or postgraduate degree | 2,361 | 36.3 | 2,317 | 37.4 | |
| Colon disease | (n = 5,906) | (n = 5,615) | |||
| First-degree relatives with colorectal cancer | |||||
| 0 | 5,153 | 87.3 | 4,768 | 84.9 | .008 |
| 1 | 673 | 11.4 | 751 | 13.4 | |
| ≥ 2 | 80 | 1.4 | 96 | 1.7 | |
| History of polyp removal | (n = 5,822) | (n = 5,752) | |||
| Yes | 411 | 7.1 | 411 | 7.1 | .857 |
| Ulcerative colitis or Crohn's disease | (n = 6,456) | (n = 6,154) | |||
| Yes | 60 | 0.9 | 43 | 0.7 | .150 |
| History of colorectal cancer | (n = 6,489) | (n = 6,193) | |||
| Yes | 20 | 0.3 | 23 | 0.4 | .541 |
| Diabetes | (n = 6,539) | (n = 6,241) | |||
| Current or past | 319 | 4.9 | 300 | 4.8 | .851 |
| Treatment (pills or shots) | 244 | 3.7 | 222 | 3.6 | .599 |
| Body mass index | (n = 6,513) | 28.5 ± 5.9 | (n = 6,201) | 28.4 ± 5.8 | .217 |
| < 25 | 1,996 | 30.6 | 1,948 | 31.4 | .196 |
| 25- < 30 | 2,278 | 35.0 | 2,215 | 35.7 | |
| ≥ 30 | 2,239 | 34.4 | 2,038 | 32.9 | |
| Waist circumference, cm | (n = 6,521) | 87.8 ± 13.7 | (n = 6,221) | 87.5 ± 13.6 | .223 |
| ≤ 88 | 3,520 | 54.0 | 3,438 | 55.3 | .145 |
| > 88 | 3,001 | 46.0 | 2,783 | 44.7 | |
| Hemoglobin, g/dL | (n = 6,538) | 13.6 ± 1.1 | (n = 6,240) | 13.6 ± 1.0 | .996 |
| Physical activity, MET/wk | (n = 5,920) | (n = 5,858) | |||
| None | 1,062 | 17.9 | 984 | 16.8 | .417 |
| < 5 | 1,323 | 22.3 | 1,313 | 22.4 | |
| 5-12 | 1,367 | 23.1 | 1,387 | 23.7 | |
| ≥ 12 | 2,168 | 36.6 | 2,174 | 37.1 | |
| Use of NSAIDs | (n = 6,542) | (n = 6,242) | |||
| Yes | 2,067 | 31.6 | 2,039 | 32.7 | .195 |
| Ibuprofen | 722 | 11.0 | 699 | 11.2 | .771 |
| Prescribed agent | 293 | 4.5 | 297 | 4.8 | .452 |
| Aspirin (≥ 80 mg/d) | 1,265 | 19.3 | 1,279 | 20.5 | .103 |
| Use of acetaminophen | |||||
| Yes | 647 | 9.9 | 626 | 10.0 | .793 |
| Daily dietary intake | (n = 6,339) | (n = 6,062) | |||
| Energy, kcal | 1,686 ± 656 | 1,672 ± 650 | .217 | ||
| Energy from fat, kcal | 64 ± 34 | 63 ± 34 | .151 | ||
| Fiber, g | 15.9 ± 6.7 | 16 ± 6.8 | .592 | ||
| Selenium, μg | 93 ± 39 | 93 ± 40 | .519 | ||
| Fruit servings | 1.8 ± 1.2 | 1.8 ± 1.2 | .928 | ||
| Vegetable servings | 2.2 ± 1.2 | 2.2 ± 1.2 | .561 | ||
| Red meat servings | (n = 6,522) | 0.8 ± 0.6 | (n = 6,225) | 0.7 ± 0.6 | .559 |
| Daily use of vitamins and supplements | (n = 6,541) | (n = 6,242) | |||
| Multivitamin | 3,129 | 47.8 | 3,027 | 48.5 | .457 |
| Calcium | 3,238 | 49.5 | 3,154 | 50.5 | .246 |
| Vitamin D | 2,863 | 43.8 | 2,745 | 44.0 | .814 |
| Selenium | 123 | 1.9 | 138 | 2.2 | .187 |
| Current alcohol use, drinks per week | (n = 6,493) | (n = 6,190) | |||
| None | 1,743 | 26.9 | 1,687 | 27.2 | .190 |
| < 1 | 2,238 | 34.4 | 2,040 | 32.9 | |
| ≥ 1 | 2,512 | 38.7 | 2,463 | 39.8 | |
| Smoking status | (n = 6,482) | (n = 6,167) | |||
| Never | 3,286 | 50.7 | 3,138 | 50.9 | .941 |
| Past | 2,596 | 40.0 | 2,452 | 39.8 | |
| Current | 600 | 9.3 | 577 | 9.4 | |
| Current or prior use of oral contraceptives | (n = 6,542) | (n = 6,242) | |||
| Yes | 2,959 | 45.2 | 2,789 | 44.7 | .532 |
| Prior use of hormones during menopause, years | (n = 6,241) | ||||
| None | 4,796 | 73.3 | 4,618 | 74.0 | .260 |
| < 5 | 1,207 | 18.5 | 1,137 | 18.2 | |
| 5-9 | 343 | 5.2 | 284 | 4.6 | |
| ≥ 9 | 196 | 3.0 | 202 | 3.2 | |
| Prior colonoscopy, sigmoidoscopy, or flexible sigmoidoscopy | (n = 6,242) | ||||
| Yes | 5,990 | 91.6 | 5,698 | 91.3 | .576 |
Fig A1.
Bowel examinations by treatment arm and year. flex. sig, flexible sigmoidoscopy
Fig A2.
Cumulative annualized incidence rates for invasive colorectal cancer by subgroups. Data are plotted as hazard ratios (HRs) with error bars showing 95% CIs. HRT, hormone replacement therapy; N/A, not applicable; NSAID, nonsteroidal anti-inflammatory drug.
Footnotes
Supported by Contracts No. N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221 from the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), US Department of Health and Human Services to the Women's Health Initiative program and by Grant No. NIH:NCI P30CA022453 from Cancer Center Support.
Presented as a poster presentation at the 103rd Annual Meeting of the American Association for Cancer Research, Chicago, IL, March 31-April 4, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Rowan T. Chlebowski, AstraZeneca (C), Novartis (C), Amgen (C), Pfizer (C) Stock Ownership: None Honoraria: Rowan T. Chlebowski, AstraZeneca, Novartis, Amgen, Pfizer; Ross L. Prentice, Wyeth Ayerst Research Funding: Rowan T. Chlebowski, Amgen Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Rowan T. Chlebowski, Jean Wactawski-Wende, Ross L. Prentice
Financial support: Jean Wactawski-Wende
Administrative support: Michael S. Simon, Rowan T. Chlebowski, Jean Wactawski-Wende
Provision of study materials or patients: Michael S. Simon, Rowan T. Chlebowski, Jean Wactawski-Wende
Collection and assembly of data: Michael S. Simon, Rowan T. Chlebowski, Karen C. Johnson
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA . 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med . 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 3.Lin KJ, Cheung WY, Lai JY, et al. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer . 2012;130:419–430. doi: 10.1002/ijc.26026. [DOI] [PubMed] [Google Scholar]
- 4.Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol . 2005;23:378–391. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 5.Solimando R, Bazzoli F, Ricciardiello L. Chemoprevention of colorectal cancer: A role for ursodeoxycholic acid, folate and hormone replacement treatment? Best Pract Res Clin Gastroenterol . 2011;25:555–568. doi: 10.1016/j.bpg.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 6.North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause . 2010;17:242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 7.Practice Committee of American Society for Reproductive Medicine. Estrogen and progestogen therapy in postmenopausal women. Fertil Steril . 2008;90:S88–S102. doi: 10.1016/j.fertnstert.2008.08.091. [DOI] [PubMed] [Google Scholar]
- 8.Pines A. Guidelines and recommendations on hormone therapy in the menopause. J Midlife Health . 2010;1:41–42. doi: 10.4103/0976-7800.66990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowring CE, Francis RM. National Osteoporosis Society's Position statement on hormone replacement therapy in the prevention and treatment of osteoporosis. Menopause Int . 2011;17:63–65. doi: 10.1258/mi.2011.011012. [DOI] [PubMed] [Google Scholar]
- 10.Sturdee DW, Pines A, Archer DF, et al. Updated IMS recommendations on postmenopausal hormone therapy and preventive strategies for midlife health. Climacteric . 2011;14:302–320. doi: 10.3109/13697137.2011.570590. [DOI] [PubMed] [Google Scholar]
- 11.Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: An Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(suppl 1):s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prentice RL, Pettinger M, Beresford SA, et al. Colorectal cancer in relation to postmenopausal estrogen and estrogen plus progestin in the Women's Health Initiative clinical trial and observational study. Cancer Epidemiol Biomarkers Prev . 2009;18:1531–1537. doi: 10.1158/1055-9965.EPI-08-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA . 2008;299:1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13(suppl 9):s5–s17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 15.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women: Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA . 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 16.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(suppl 9):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Surveillance, Epidemiology, and End Results: About SEER. 2011. http://www.seer.cancer.gov/
- 18.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA . 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Risch HA, Howe GR. Menopausal hormone use and colorectal cancer in Saskatchewan: A record linkage cohort study. Cancer Epidemiol Biomarkers Prev . 1995;4:21–28. [PubMed] [Google Scholar]
- 20.Persson I, Yuen J, Bergkvist L, et al. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy: Long-term follow-up of a Swedish cohort. Int J Cancer . 1996;67:327–332. doi: 10.1002/(SICI)1097-0215(19960729)67:3<327::AID-IJC4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Pukkala E, Tulenheimo-Silfvast A, Leminen A. Incidence of cancer among women using long versus monthly cycle hormonal replacement therapy, Finland 1994-1997. Cancer Causes Control . 2001;12:111–115. doi: 10.1023/a:1008934919159. [DOI] [PubMed] [Google Scholar]
- 22.Tannen RL, Weiner MG, Xie D, et al. Estrogen affects post-menopausal women differently than estrogen plus progestin replacement therapy. Hum Reprod . 2007;22:1769–1777. doi: 10.1093/humrep/dem031. [DOI] [PubMed] [Google Scholar]
- 23.Green J, Czanner G, Reeves G, et al. Menopausal hormone therapy and risk of gastrointestinal cancer: Nested case-control study within a prospective cohort, and meta-analysis. Int J Cancer . 2012;130:2387–2396. doi: 10.1002/ijc.26236. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JR, Lacey JV, Jr, Lazovich D, et al. Menopausal hormone therapy and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev . 2009;18:196–203. doi: 10.1158/1055-9965.EPI-08-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hildebrand JS, Jacobs EJ, Campbell PT, et al. Colorectal cancer incidence and postmenopausal hormone use by type, recency, and duration in cancer prevention study II. Cancer Epidemiol Biomarkers Prev . 2009;18:2835–2841. doi: 10.1158/1055-9965.EPI-09-0596. [DOI] [PubMed] [Google Scholar]
- 26.Delellis Henderson K, Duan L, Sullivan-Halley J, et al. Menopausal hormone therapy use and risk of invasive colon cancer: The California Teachers Study. Am J Epidemiol . 2010;171:415–425. doi: 10.1093/aje/kwp434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsilidis KK, Allen NE, Key TJ, et al. Menopausal hormone therapy and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011;128:1881–1889. doi: 10.1002/ijc.25504. [DOI] [PubMed] [Google Scholar]
- 28.American Cancer Society. Atlanta, GA: American Cancer Society; 2010. Cancer Facts and Figures 2010. [Google Scholar]
- 29.Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol . 1999;94:3039–3045. doi: 10.1111/j.1572-0241.1999.01454.x. [DOI] [PubMed] [Google Scholar]
- 30.Langenbach MR, Schmidt J, Neumann J, et al. Delay in treatment of colorectal cancer: Multifactorial problem. World J Surg . 2003;27:304–308. doi: 10.1007/s00268-002-6678-9. [DOI] [PubMed] [Google Scholar]
- 31.Siminoff LA, Rogers HL, Thomson MD, et al. Doctor, what's wrong with me? Factors that delay the diagnosis of colorectal cancer. Patient Educ Couns . 2011;84:352–358. doi: 10.1016/j.pec.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: A review and meta-analysis. Am J Med . 1999;106:574–582. doi: 10.1016/s0002-9343(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 33.Slattery ML, Anderson K, Samowitz W, et al. Hormone replacement therapy and improved survival among postmenopausal women diagnosed with colon cancer (USA) Cancer Causes Control . 1999;10:467–473. doi: 10.1023/a:1008974215622. [DOI] [PubMed] [Google Scholar]
- 34.Mandelson MT, Miglioretti D, Newcomb PA, et al. Hormone replacement therapy in relation to survival in women diagnosed with colon cancer. Cancer Causes Control . 2003;14:979–984. doi: 10.1023/b:caco.0000007970.04094.76. [DOI] [PubMed] [Google Scholar]
- 35.Chan JA, Meyerhardt JA, Chan AT, et al. Hormone replacement therapy and survival after colorectal cancer diagnosis. J Clin Oncol . 2006;24:5680–5686. doi: 10.1200/JCO.2006.08.0580. [DOI] [PubMed] [Google Scholar]
- 36.Newcomb PA, Chia VM, Hampton JM, et al. Hormone therapy in relation to survival from large bowel cancer. Cancer Causes Control . 2009;20:409–416. doi: 10.1007/s10552-008-9255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritenbaugh C, Stanford JL, Wu L, et al. Conjugated equine estrogens and colorectal cancer incidence and survival: The Women's Health Initiative randomized clinical trial. Cancer Epidemiol Biomarkers Prev . 2008;17:2609–2618. doi: 10.1158/1055-9965.EPI-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulley S, Furberg C, Barrett-Connor E, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study follow-up (HERS II) JAMA . 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 39.Giovannucci E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev . 2001;10:725–731. [PubMed] [Google Scholar]
- 40.Krishnan S, Wolf JL. Colorectal cancer screening and prevention in women. Womens Health (Lond Engl) . 2011;7:213–226. doi: 10.2217/whe.11.7. [DOI] [PubMed] [Google Scholar]
- 41.Paskett ED, Reeves KW, Rohan TE, et al. Association between cigarette smoking and colorectal cancer in the Women's Health Initiative. J Natl Cancer Inst . 2007;99:1729–1735. doi: 10.1093/jnci/djm176. [DOI] [PubMed] [Google Scholar]
- 42.Chlebowski RT. Menopausal hormone therapy, hormone receptor status, and lung cancer in women. Semin Oncol . 2009;36:566–571. doi: 10.1053/j.seminoncol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 43.National Cancer Institute. Colorectal cancer treatment (PDQ) http://www.cancer.gov/cancertopics/pdq/treatment/colon.