Embryonic Ductal Plate Cells Give Rise to Cholangiocytes, Periportal Hepatocytes and Adult Liver Progenitor Cells (original) (raw)
. Author manuscript; available in PMC: 2012 Nov 10.
Published in final edited form as: Gastroenterology. 2011 Jun 25;141(4):1432–1438.e4. doi: 10.1053/j.gastro.2011.06.049
Abstract
Background & Aims
Embryonic biliary precursor cells form a periportal sheet called the ductal plate, which is progressively remodeled to generate intrahepatic bile ducts. A limited number of ductal plate cells participate in duct formation; those not involved in duct development are believed to involute by apoptosis. Moreover, cells that express the SRY-related HMG box transcription factor 9 (SOX9), which include the embryonic ductal plate cells, were proposed to continuously supply the liver with hepatic cells. We investigated the role of the ductal plate in hepatic morphogenesis.
Methods
Apoptosis and proliferation were investigated by immunostaining of mouse and human fetal liver tissue. The post-natal progeny of SOX9-expressing ductal plate cells was analysed after genetic labeling, at the ductal plate stage, by Cre-mediated recombination of a ROSA26RYFP reporter allele. Inducible Cre expression was induced by SOX9 regulatory regions, inserted in a bacterial artificial chromosome. Livers were studied from mice under normal conditions and during diet-induced regeneration.
Results
Ductal plate cells did not undergo apoptosis and showed limited proliferation. They generated cholangiocytes lining interlobular bile ducts, bile ductules and canals of Hering, as well as periportal hepatocytes. Oval cells that appeared during regeneration also derived from the ductal plate. We did not find that liver homeostasis required a continuous supply of cells from SOX9-expressing progenitors.
Conclusions
The ductal plate gives rise to cholangiocytes lining the intrahepatic bile ducts, including its most proximal segments. It also generates periportal hepatocytes and adult hepatic progenitor cells.
Keywords: hepatic cell differentiation, liver development, liver regeneration
Introduction
Hepatocytes and cholangiocytes derive from common embryonic liver precursor cells, which are referred to as hepatoblasts. In developing liver, the segregation of the hepatocyte and cholangiocyte lineages is detectable by the expression of the transcription factor SRY-related HMG box transcription factor 9 (SOX9) in a subset of hepatoblasts which encompass the biliary precursor cells1. Hepatoblasts that remain SOX9-negative are located throughout the liver parenchyma and differentiate toward hepatocytes by gradual acquisition of hepatocyte-specific functions2, 3. After birth, periportal and pericentral hepatocytes progressively display distinct properties, a phenomenon called liver zonation4. In contrast, embryonic SOX9-positive biliary precursor cells line up around the periportal mesenchyme to constitute a near-complete and single-layered sleeve of cells which is termed “ductal plate” (reviewed in references5-7). Each ductal plate gives on average rise to two bile ducts per portal tract8, and only a minority of ductal plate cells participate in bile duct morphogenesis. It has been proposed that the ductal plate cells not involved in bile duct formation regress, and that such regression occurs by apoptosis9. In parallel, there is evidence that ductal plate cells have stem cell properties. Indeed, when they are isolated by immunoselection and maintained in culture, ductal plate cells display self-renewal capacity and an ability to differentiate toward hepatoblast-like cells. When transplanted in vivo, these cells engraft in liver and give rise to cells that have characteristics of mature epithelial liver cells10, 11. Recently, it was also proposed that SOX9-expressing cells in fetal and adult liver continuously generate hepatic cells under normal physiological conditions12.
These observations prompted us to revisit the fate of the ductal plate cells in vivo. Here we address this issue by evaluating apoptosis and proliferation of the ductal plate cells at several stages of development, and by using a genetic lineage tracing approach. Our data show that ductal plate cells give rise to periportal hepatocytes and to cholangiocytes lining the interlobular bile ducts, the intralobular ductules and the canals of Hering. Oval cells, which appear during diet-induced liver regeneration and are believed to derive from the intralobular ductules or canals of Hering harboring facultative stem cells (reviewed in reference13), also originate from the ductal plate.
Materials and Methods
Animals
Experiments were performed with approval of the University Animal Welfare Committee. SOX9-CreERT2 mice express the T2 variant of tamoxifen-inducible cyclization recombinase-estrogen receptor ligand binding domain (CreERT2) and were obtained by injection into fertilized oocytes of a bacterial artificial chromosome containing the cDNA of CreERT2 cloned in-frame into the SOX9-coding region14. ROSA26RYFP mice were as described15. Tamoxifen (Sigma, Bornem, Belgium) was dissolved in corn oil at a concentration of 30 mg/ml and injected intraperitoneally in pregnant mice at embryonic day (E) 15.5 at 100 mg/kg of body weight. For diet-induced liver regeneration, mice were fed a choline-deficient (MP Biomedicals, Irvine, CA, USA) ethionine supplemented (Sigma, Bornem, Belgium; 0.15% in water) diet (CDE), or a 3,5-diethoxycarbonyl-1,4-dihydro-collidine (DDC) diet (Sigma, Bornem, Belgium; 0.1% DDC in standard diet (Altronim, Lage, Germany)).
Human fetal liver specimens
Tissue samples were obtained from spontaneous or therapeutic abortion, in compliance with the French legislation, the 1975 Declaration of Helsinki, and the European Guidelines for the use of human tissues.
Immunofluorescence
Mouse liver preparation and immunofluorescence analysis were as described16 (Supplementary Table). Immunodetection of Cre was carried out with Tyramide Signal Amplification kit (Molecular Probes, Invitrogen, Merelbeke, Belgium). For multiple immunostaining with primary antibodies raised in the same species, staining-elution cycles were performed as described17. Pictures were taken with a Zeiss Cell Observer Spinning Disk confocal microscope or an Axiovert200 fluorescence microscope (Carl Zeiss, Zaventem, Belgium).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
TUNEL assay was performed with In Situ Cell Death detection kit, Fluoresceine (Roche Applied Science, Mannheim, Germany) according to manufacturer's instructions. Liver samples were dewaxed, rehydrated and treated by microwave irradiation in 0.1M citrate buffer, pH 6.0. TUNEL reaction with fluoresceine-coupled dUTP was performed prior to immunofluorescence detection of E-cadherin. DNAseI pretreatment served as positive control.
Results
Lack of apoptosis and low proliferation of ductal plate cells
To investigate the fate of ductal plate cells we first looked at apoptosis in developing mouse liver. The ductal plate was identified by staining for SOX9 or E-Cadherin. Co-stainings for activated Caspase 3 or TUNEL were performed from E15.5 to E18.5, namely from the initiation of ductal plate formation to the stage at which it is actively regressing. No apoptosis was detected in ductal plate cells at any stage, indicating that apoptosis is not the main mechanism of remodeling (Figure 1A-B). Similarly, analysis of a human fetal liver at 11 weeks of gestation did not reveal apoptosis in developing ducts. Human embryonic liver showed biliary structures at several stages of maturation, namely ductal plate cells with small lumina and adjacent to the parenchyma, as well as more mature ductal structures becoming incorporated in the mesenchyme (Figure 1A-B). Apoptosis was detected by activated caspase 3 and TUNEL stainings outside the ductal plate on the same sections of mouse and human liver, thereby providing positive controls (data not shown).
Figure 1.
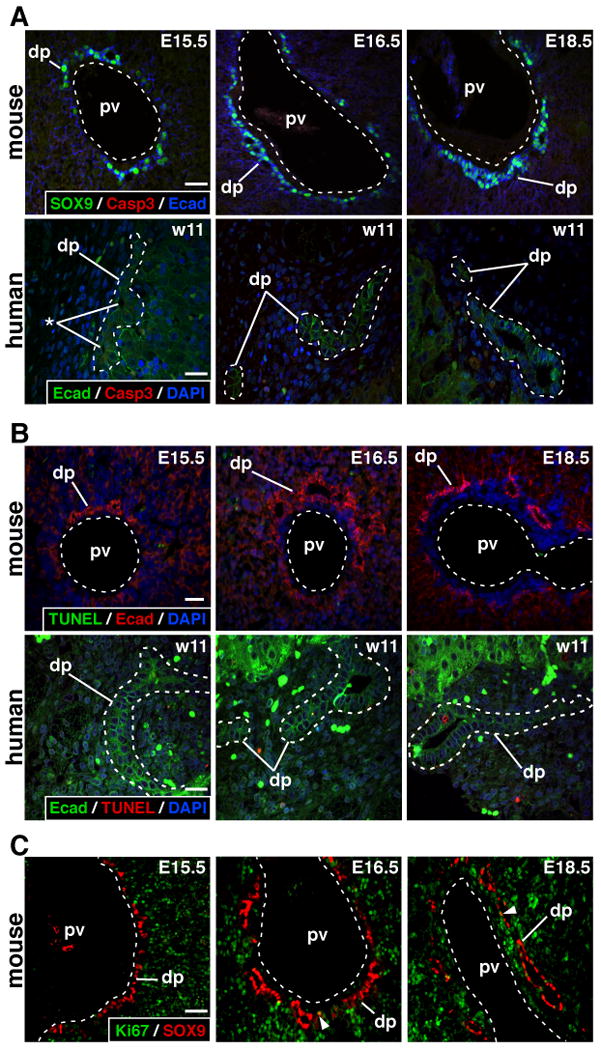
Lack of apoptosis and very low proliferation in ductal plate. (A-B) Mouse and human ductal plates were analyzed at the stages indicated. No evidence for apoptosis (activated caspase 3 and TUNEL) was found. (C) Only a few SOX9+ ductal plate cells were proliferating (Ki67; arrowhead). dp, ductal plate; pv, portal vein; *, lumen of developing duct; size bar, 20 μm.
Proliferation of SOX9-expressing ductal plate cells was also assessed in mouse liver (E15.5 to E18.5) using co-immunodetection of Ki67, which labels cells in the G1, S, G2 and M phases of proliferation. The data show that only a very low number of ductal plate cells was proliferating (arrowheads, Figure 1C), while proliferation was intense in the parenchyma.
A mouse model for genetic lineage tracing of ductal plate cells
Since the ductal plate cells were not undergoing apoptosis, we determined their fate using a genetic lineage tracing approach. To this end we first validated a mouse line that expresses inducible CreERT2 in SOX9-expressing cells14 (SOX9-CreERT2 mice). The cell-type specificity of CreERT2 expression was analyzed from E14.5 to E16.5 by immunofluorescent co-detection of CreERT2 and SOX9, and of CreERT2 and the hepatoblast marker HNF4. As shown in Figure 2, 18 h after tamoxifen injection, CreERT2 was exclusively found in nuclei of SOX9+ ductal plate cells, and never in HNF4+ hepatoblasts; 87.47% of SOX9-expressing cells showed nuclear CreERT2 (Supplementary Figure 1A). We concluded that CreERT2 expression in developing liver is specific to SOX9-expressing ductal plate cells.
Figure 2.
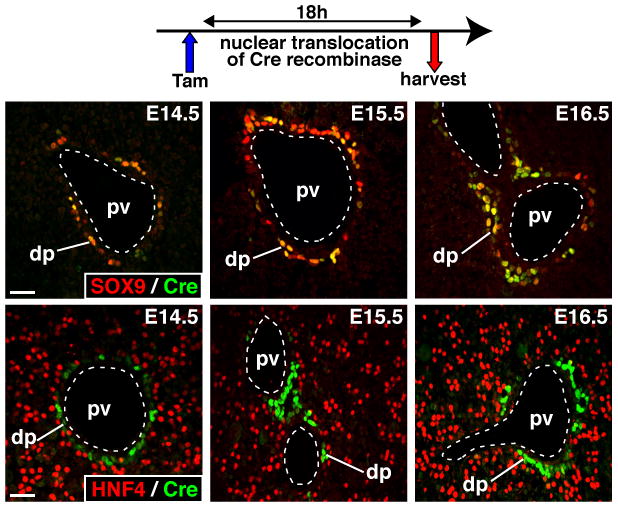
Expression of CreERT2 in developing liver is restricted to SOX9-expressing ductal plate cells. Pregnant females were injected with tamoxifen 18h prior to collection of the liver at the stages indicated. The livers were stained to detect CreERT2 and biliary (SOX9) or hepatoblast (HNF4) markers. dp, ductal plate; pv, portal vein; size bar, 20 μm.
The ductal plate gives rise to cholangiocytes and to periportal hepatocytes
To trace the fate of SOX9+ ductal plate cells we administered a single dose of tamoxifen to pregnant females from intercrosses between SOX9-CreERT2 mice and ROSA26RYFP mice. Tamoxifen transiently induces CreERT2 nuclear translocation in SOX9+ cells, leading to recombination of the ROSA26RYFP locus and to permanent expression of YFP in the cells' progeny. We also verified that CreERT2 remained inactive in the absence of tamoxifen (Supplementary Figure 1B). Pregnant mice were injected at E15.5, and the livers of SOX9-CreERT2;ROSA26RYFP offspring were collected after birth. Interlobular bile ducts expressed YFP in the postnatal period, confirming the presumed ductal plate origin of the bile ducts. These YFP+ bile ducts were identified by co-staining for YFP and SOX9, osteopontin (OPN) or cytokeratin 19 (CK19)1 (Figure 3A).
Figure 3.
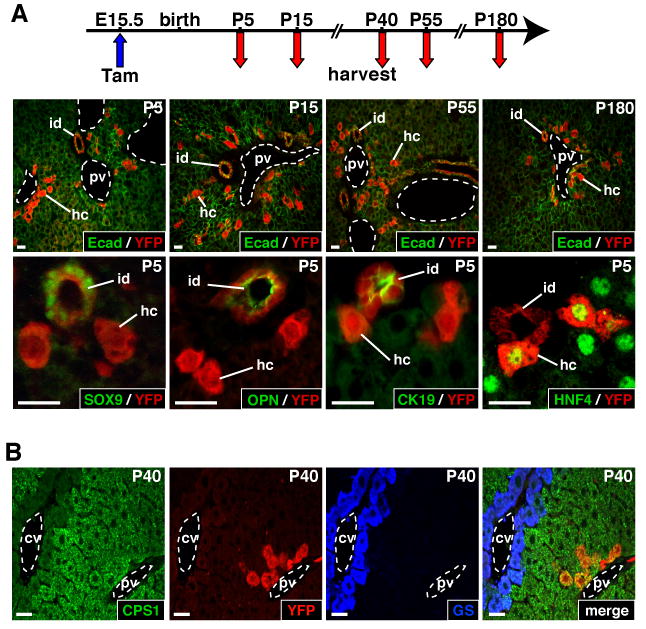
The ductal plate gives rise to cholangiocytes lining interlobular bile ducts and periportal hepatocytes. Pregnant females were injected with tamoxifen at E15.5, and the SOX9-CreERT2;ROSA26RYFP offspring were analyzed at the time points indicated. (A) The YFP+ progeny of the ductal plate consisted of interlobular ducts and of hepatocytes which persisted at least until 7 months of age. The biliary markers (SOX9, OPN, CK19) were expressed in the YFP+ ducts but not in YFP+ hepatocytes; the latter expressed the hepatocyte marker HNF4. (B) The YFP+ hepatocytes were restricted to the periportal zone and expressed periportal (CPS1) but not perivenous (GS) markers. cv, central vein; hc, hepatocyte; id, interlobular bile duct; pv, portal vein; size bar, 20 μm.
A number of hepatocytes also expressed YFP in the postnatal period. They displayed the typical polygonal shape of hepatocytes and expressed the hepatocyte marker HNF4, but not the biliary markers SOX9, OPN and CK19 (Figure 3A). These hepatocytes were located in the periportal area. Given that the hepatic lobule is zonated along its porto-central axis, we verified that the YFP-expressing hepatocytes displayed the expected enzymatic profile of periportal hepatocytes. This was the case, since they expressed carbamoyl-phosphate-synthetase1 (CPS1), but not glutamine synthetase (GS) (Figure 3B). These hepatocytes remained periportal and maintained a periportal enzymatic profile; also, they did not display differential proliferation as compared to YFP- hepatocytes (Supplementary Figure 2A).
Several YFP+ cells were located in the periportal mesenchyme and were distinct from hepatocytes and from cholangiocytes lining interlobular ducts. These cells had a flattened morphology and lined the biliary ductules and the canals of Hering (Figure 4), as defined according to the current nomenclature18. The ductules were delineated by small CK+ cholangiocytes and surrounded by laminin (Figure 4A). The canals of Hering (Figure 4B) were delineated on one side by hepatocytes and on the other side by CK+ cells; the latter had a basal pole lined by laminin, as shown earlier in rodents19. Expression of CEACAM, which stains the bile canaliculi at the apical pole of hepatocytes, further confirmed the identification of the canals of Hering.
Figure 4.
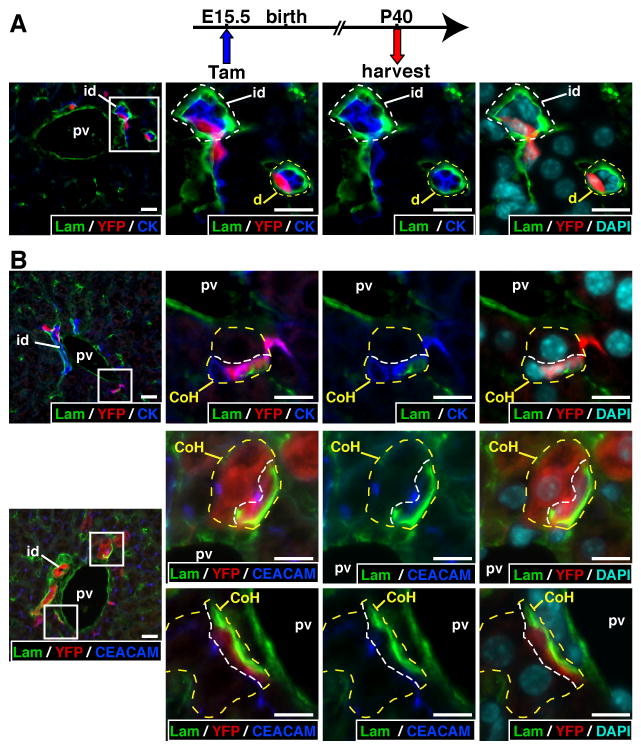
The YFP+ progeny of the ductal plate includes cholangiocytes lining the ductules and the canals of Hering. (A) Laminin (lam) fully surrounds ductules (yellow dotted line) and interlobular ducts (white dotted line). (B) Upper panels: Canals of Hering (yellow dotted line) were delineated by hepatocytes with a large nucleus and cholangiocytes expressing cytokeratin (pan-cytokeratin antibody); the white dotted line separates the cholangiocyte from the hepatocyte. Laminin is only found along the basal pole of the cholangiocytes. Middle and lower panels: canals of Hering are also identified by CEACAM staining which marks the bile canaliculi at the apical pole of hepatocytes. The middle pictures show a canal of Hering lined by a YFP+ hepatocyte and a YFP+ cholangiocyte. In A-B, the right three pictures correspond to magnified areas delineated in the left picture by a white box. CoH, canal of Hering; d, ductule; id, interlobular bile duct; pv, portal vein; size bar, 10 μm.
Finally, although the SOX9-expressing cell populations (cholangiocytes lining interlobular ducts, ductules and canals of Hering) contained YFP-labeled cells, we found no evidence that the liver becomes colonized by cells that express YFP and that derive from SOX9+YFP+ progenitors (Figure 3A). This was in contrast with the observations of Furuyama and coworkers12.
Thus, we concluded that SOX9+ ductal plate cells give rise to cholangiocytes lining the bile ducts including the most proximal biliary structures, as well as to periportal hepatocytes.
Ductal plate cells are the embryonic precursors of oval cells
When the liver is injured under conditions that prevent hepatocyte proliferation, the organ regenerates by proliferation and differentiation of oval cells20. These cells are transit-amplifying cells, considered to derive from facultative progenitor cells located most likely in the ductules or canals of Hering (reviewed in references13, 21, 22). Since biliary cells, including those lining the ductules and canals of Hering, originate from the ductal plate, we determined if the ductal plate is the embryonic origin of oval cells. To this end, tamoxifen was administered at E15.5 to SOX9-CreERT2;ROSA26RYFP embryos, and 4 weeks after birth the mice were fed a CDE diet until the livers were collected 21 days after the onset of the diet. Such diet is known to induce oval cell-dependent liver regeneration. Oval cells expressed the oval/biliary cell markers CK19, SOX9 and OPN23 as well as the oval cell-specific marker TROP224. A number of oval cells expressed YFP, indicating that their embryonic origin is the SOX9+ ductal plate (Figure 5). Not all oval cells expressed YFP, which is consistent with the fact that CreERT2 is not detectable in the nucleus of 100% of the ductal plate cells at E15.5, and with the efficiency or CreERT2 activity (Figure 2; Supplementary Figures 1A and 3). Therefore, we quantified the percentage of YFP+SOX9+ oval cells (ratio of YFP+SOX9+ oval cells over SOX9+ oval cells). The results showed that some periportal areas had close to 100% of SOX9+ oval cells that co-expressed YFP, suggesting that at least in these periportal areas nearly all oval cells derived from the ductal plate (Figure 5, Supplementary Figure 3).
Figure 5.
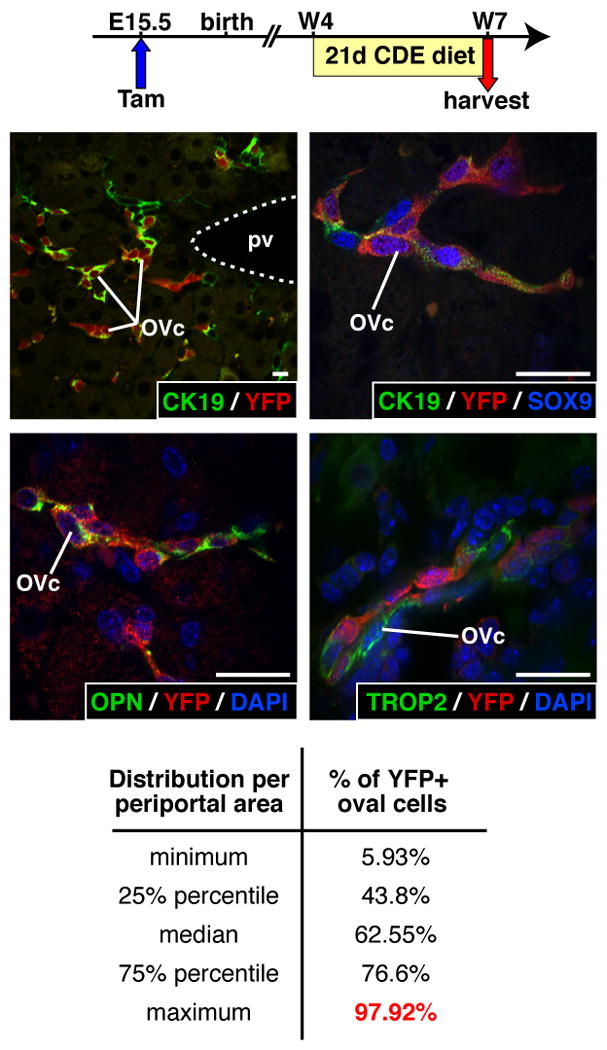
CDE diet-induced oval cells derive from the ductal plate. Pregnant females were injected with tamoxifen at E15.5, and 4 week-old SOX9-CreERT2;ROSA26RYFP offspring were fed a CDE diet. YFP+ oval cells express typical markers (CK19, SOX9, OPN, TROP2). The percentage of YFP+ oval cells [(YFP+SOX9+/SOX9+) × 100] per periportal area can reach close to 100%. 13,419 SOX9+ oval cells were counted over 146 periportal areas. OVc, oval cell; pv, portal vein; w, week after birth; size bar, 10 μm.
A regenerative response similar to an oval cell response is also induced in mice fed a DDC diet25. To further test if oval cells derive from the ductal plate, tamoxifen was administered at E15.5 to pregnant females, and their 4 week-old SOX9-CreERT2;ROSA26RYFP progeny was fed a DDC diet for 15 days, before analysis of the liver. Proliferation of atypical ductular structures was observed, as expected (Figure 6). The ductular structures expressed the oval/biliary cell markers SOX9, OPN and CK, and most ductular cells co-expressed YFP, indicating that they derive from the ductal plate. We concluded that the SOX9-expressing ductal plate cells give rise to at least a fraction of adult hepatic progenitor cells.
Figure 6.
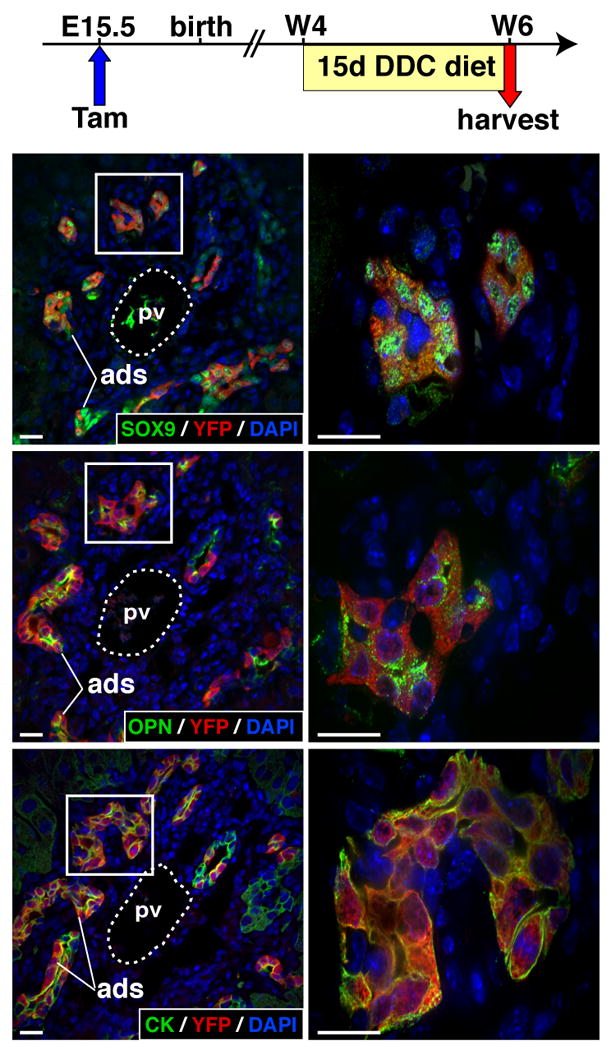
DDC diet-induced oval cells derive from the ductal plate. Pregnant females were injected with tamoxifen at E15.5, and 4 week-old SOX9-CreERT2;ROSA26RYFP offspring were fed a DDC diet for two weeks. Atypical ductular structures were induced. They co-express YFP and biliary/oval cells markers SOX9, OPN and CK (pan-cytokeratin antibody). The right pictures correspond to magnified areas delineated by a white box in the left panels. ads, atypical ductular struture; pv, portal vein; w, week after birth; size bar, 10 μm.
Discussion
During liver development the biliary precursor cells constitute the ductal plate, of which only a fraction of the cells is considered to participate in the formation of the biliary tree. It was proposed that, at least in humans, ductal plate remodeling occurs by proliferation and apoptosis9. Our analysis of mouse and human fetal livers did not reach the same conclusion, since no apoptosis and nearly no proliferation was found in the ductal plate. In addition, we show that the ductal plate differentiates toward cholangiocytes and periportal hepatocytes. The latter finding is in line with recent data12, which we extend here by demonstrating that the ductal plate gives rise to cholangiocytes lining the interlobular bile ducts, the ductules and the canals of Hering, as well as to periportal, but not pericentral, hepatocytes.
Several metabolic functions are exerted according to a porto-central gradient, but the steepness of each gradient differs, making it difficult to make a clear-cut distinction between hepatocyte sub-types. An exception is GS expression, which is strictly pericentral and non overlapping with CPS1. No YFP+ cell expressed GS, eliminating the possibility that ductal plate-derived hepatocytes contribute to the pericentral hepatocytes. CPS1 expression extends from the periportal zone to the midlobular zone. Co-labeling of YFP and CPS1 was always detected within the six to eight hepatocytes around the portal space, which corresponds to the periportal area called zone 14. Based on this anatomical location we propose that ductal plate-derived hepatocytes are periportal. However, considering the limitations of such anatomical interpretation, and considering that some section areas show a low number of cells between the portal space and the central vein, we cannot rule out that some mid-lobular hepatocytes derive from the ductal plate.
The discrepancy between earlier data9 and our observations on apoptosis and proliferation most likely results from technical reasons. Our data suggest that neither apoptosis nor proliferation are the most significant determinants of the fate of ductal plate cells. We propose that most remodeling of the ductal plate occurs by differentiation towards cholangiocytes and hepatocytes. However, we cannot determine if subpopulations of SOX9-expressing cells contribute to one and/or the other cell type.
Importantly, our data do not support a recent model of hepatic homeostasis. SOX9-expressing cells in adult liver line interlobular ducts, intralobular ductules and canals of Hering. Furuyama and coworkers12 proposed that SOX9-expressing cells are progenitors which continuously supply the liver with hepatic cells. In these experiments 8 week-old SOX9-IRES-CreERT2;ROSA26RYFP mice were injected with tamoxifen, and the progeny of SOX9-expressing cells were proposed to colonize the liver. In our experimental setting, SOX9-expressing cells in adult liver were YFP-labeled as a result of CreERT2-mediated recombination in the embryo; no ectopic expression of CreERT2 was detected after tamoxifen injection. We found no evidence that the progeny of YFP-labeled SOX9+ cells colonize the liver. Instead, YFP+ cells persisted as biliary cells or periportal hepatocytes. This was verified until 7 months of age (data not shown). A definitive reason explaining the discrepancy between our work and that of Furuyama cannot be provided. Still, a number of points need attention. The SOX9-CreERT2 mouse line used in our study expresses Cre recombinase from a bacterial artificial chromosome, thereby leaving the genomic SOX9 loci intact. In the work of Furuyama and coworkers, IRES-CreERT2 recombinase coding sequences were inserted in the 3′ untranslated region of the SOX9 locus. This raises the possibility that SOX9 expression levels may not be maintained at physiological levels, leading to a potential impact on the physiology of SOX9-expressing cells. We cannot eliminate the possibility that our work and that of Furuyama and coworkers trace the fate of distinct subpopulations of SOX9+ cells. Still, Furuyama and coworkers proposed that proliferation of SOX9+ cells contributes to the supply of hepatic cells. Our quantifications of proliferating SOX9+ cells showed progressive decrease of proliferation with age (Supplementary Figure 2B). The decreasing and low proliferation levels from birth to week 8 does not provide support for a mode of liver homeostasis that depends on SOX9+ cell proliferation. Finally, in our hands, injection of tamoxifen in 8 week-old mice (a single dose of 10 mg/kg body weight of tamoxifen) induces ectopic expression of SOX9 in hepatocytes (Supplementary Figure 2C). This raises the possibility that, in adult mice, tamoxifen induces ectopic CreERT2-mediated recombination of a ROSA reporter locus in hepatocytes. In that case, lineage tracing would not distinguish between the progeny of normal SOX9+ cells or of hepatocytes expressing tamoxifen-induced SOX9.
During CDE and DDC diet-induced liver regeneration, oval cells proliferate. There is mounting evidence that they derive from the ductules or canals of Hering (reviewed in references21, 22). Our data indicate that oval cells emerge from the progeny of the ductal plate. Oval cells are unlikely to derive from the ductal plate-derived hepatocytes, since the latter are injured during the diet (data not shown). The oval cells are also unlikely to derive from interlobular ducts, since there is no correlation between the periportal distribution of YFP+ oval cells and YFP+ interlobular duct cells (Supplementary Figure 3). Finally, there is controversial evidence that bone marrow cells may be a source of hepatic progenitor cells (reviewed in reference22), raising the possibility that YFP+ oval cells in our experiments derive from bone marrow cells which would express SOX9-CreERT2 at E15.5. This is unlikely: first, bone marrow precuror cells are mainly located in the parenchyma of fetal liver, and SOX9-CreERT2 expression in E15.5 fetal liver was exclusively found in the ductal plate, not in cells colonizing the parenchyma; second, bone marrow precursors are also found in the circulation from E12.5 to E17.5, but these cells are not distinct from the fetal liver hematopoietic cells, since they display the same stem cell surface markers; third, hematopoietic stem cells are not detectable in the limb bone until E17.526.
In conclusion, our work shows that the ductal plate in embryonic liver gives rise to cholangiocytes lining all segments of the intrahepatic bile ducts, as well as to periportal hepatocytes and hepatic progenitor cells. Our data bear significance to understand the mechanisms of biliary malformations and of liver regeneration.
Supplementary Material
01
02
03
04
05
Acknowledgments
The authors thank the lab members, M. Karamaga Rusingizwa, C. Sempoux and B. Sosa-Pineda for help and advice.
The work was supported by the Interuniversity Attraction Poles Program (Belgian Science Policy, to FPL and IAL), the D.G. Higher Education and Scientific Research of the French Community of Belgium, the Alphonse and Jean Forton Fund, and the Fund for Scientific Medical Research (Belgium). IAL and PJ are Research Associate of the F.R.S.-FNRS. MS was supported by NIH/NIDDK (R01-DK078803), JDRF (43-2009-791), and JLK by NIH/F32 CA136124.
Abbreviations
SOX9
SRY-related HMG box transcription factor 9
Cre
cyclization recombinase
CreER
cyclization recombinase-estrogen receptor ligand binding domain
E
embryonic day
CDE
choline-deficient ethionine supplemented
DDC
3,5-diethoxycarbonyl-1,4-dihydro-collidine
YFP
yellow fluorescent protein
TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
OPN
osteopontin
CK19
cytokeratin 19
P
postnatal day
CPS1
carbamoyl-phosphate-synthetase1
GS
glutamine synthetase
CEACAM
carcinoembryonic antigen-related cell adhesion molecule
Footnotes
Disclosures: The authors declare no conflict of interest.
Author contributions: RC: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript; RES, NVH, JBB, SC, AA, PR: acquisition of data, analysis and interpretation of data; JLK, MS characterization and provision of mouse strain; SL: provision and analysis of human fetal material; PJ, IAL: study concept and design; FPL: study concept, design and supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antoniou A, Raynaud P, Cordi S, et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–33. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jochheim A, Cieslak A, Hillemann T, et al. Multi-stage analysis of differential gene expression in BALB/C mouse liver development by high-density microarrays. Differentiation. 2003;71:62–72. doi: 10.1046/j.1432-0436.2003.700606.x. [DOI] [PubMed] [Google Scholar]
- 3.Petkov PM, Zavadil J, Goetz D, et al. Gene expression pattern in hepatic stem/progenitor cells during rat fetal development using complementary DNA microarrays. Hepatology. 2004;39:617–27. doi: 10.1002/hep.20088. [DOI] [PubMed] [Google Scholar]
- 4.Torre C, Perret C, Colnot S. Molecular determinants of liver zonation. Prog Mol Biol Transl Sci. 2010;97:127–50. doi: 10.1016/B978-0-12-385233-5.00005-2. [DOI] [PubMed] [Google Scholar]
- 5.Raynaud P, Carpentier R, Antoniou A, et al. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Biol. 2011;43:245–256. doi: 10.1016/j.biocel.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) 2008;291:628–35. doi: 10.1002/ar.20710. [DOI] [PubMed] [Google Scholar]
- 7.Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–89. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: a quantitative reference standard. Hepatology. 1998;28:323–31. doi: 10.1002/hep.510280206. [DOI] [PubMed] [Google Scholar]
- 9.Terada T, Nakanuma Y. Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am J Pathol. 1995;146:67–74. [PMC free article] [PubMed] [Google Scholar]
- 10.Schmelzer E, Zhang L, Bruce A, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–87. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Theise N, Chua M, et al. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology. 2008;48:1598–607. doi: 10.1002/hep.22516. [DOI] [PubMed] [Google Scholar]
- 12.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 13.Yanger K, Stanger BZ. Facultative stem cells in liver and pancreas: Fact and fancy. Dev Dyn. 2011;240:521–9. doi: 10.1002/dvdy.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopp JL, Dubois CL, Schaffer AE, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–65. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Eyll JM, Pierreux CE, Lemaigre FP, et al. Shh-dependent differentiation of intestinal tissue from embryonic pancreas by activin A. J Cell Sci. 2004;117:2077–86. doi: 10.1242/jcs.01067. [DOI] [PubMed] [Google Scholar]
- 17.Pirici D, Mogoanta L, Kumar-Singh S, et al. Antibody elution method for multiple immunohistochemistry on primary antibodies raised in the same species and of the same subtype. J Histochem Cytochem. 2009;57:567–75. doi: 10.1369/jhc.2009.953240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–45. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 19.Paku S, Dezso K, Kopper L, et al. Immunohistochemical analysis of cytokeratin 7 expression in resting and proliferating biliary structures of rat liver. Hepatology. 2005;42:863–70. doi: 10.1002/hep.20858. [DOI] [PubMed] [Google Scholar]
- 20.Shinozuka H, Lombardi B, Sell S, et al. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res. 1978;38:1092–8. [PubMed] [Google Scholar]
- 21.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–81. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalopoulos GK. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol. 2011;43:173–9. doi: 10.1016/j.biocel.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo A, Yoshida T, Yasukawa T, et al. Epiplakin1 is expressed in the cholangiocyte lineage cells in normal liver and adult progenitor cells in injured liver. Gene Expr Patterns. 2011;11:255–62. doi: 10.1016/j.gep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Okabe M, Tsukahara Y, Tanaka M, et al. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–60. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 25.Preisegger KH, Factor VM, Fuchsbichler A, et al. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab Invest. 1999;79:103–9. [PubMed] [Google Scholar]
- 26.Christensen JL, Wright DE, Wagers AJ, et al. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2:E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03
04
05