Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization (original) (raw)
. Author manuscript; available in PMC: 2012 Nov 18.
Abstract
The mammalian target of rapamycin (mTOR) is a serine-threonine kinase that controls global protein synthesis, in part, by modulating translation initiation, a rate-limiting step for many mRNAs. Previous studies implicate mTOR in regulating stimulant-induced sensitization and antidepressive-like behavior in rodents, as well as drug craving in abstinent heroin addicts. To determine if signaling downstream of mTOR is affected by repeated cocaine administration in reward-associated brain regions, and if inhibition of mTOR alters cocaine-induced behavioral plasticity, C57BL/6J mice received 4 intraperitoneal (IP) injections of 15 mg/kg cocaine and levels of phosphorylated P70S6 kinase and ribosomal S6 protein - two translational regulators directly downstream of mTOR - were analyzed by immunoblotting across several brain regions. Cocaine place-preference and locomotor sensitization were elicited by 4 pairings of cocaine with a distinct environment and the effects of mTOR inhibition were assessed by pretreating the mice with 10 mg/kg rapamycin, 1 hr prior to (a) each saline/cocaine conditioning session, (b) a post-conditioning test or (c) a test for locomotor sensitization conducted at 3 weeks withdrawal. While systemic pretreatment with 10 mg/kg rapamycin during conditioning failed to alter the development of a cocaine place-preference or locomotor sensitization, pretreatment prior to the post-conditioning test attenuated the expression of the place-preference. Additionally, rapamycin pretreatment prior to a cocaine challenge 3 weeks post-conditioning blocked the expression of the sensitized locomotor response. These ndings suggest a role for mTOR activity, and perhaps translational control, in the expression of cocaine-induced place preference and locomotor sensitization.
Keywords: cocaine, mTOR, P70S6K, rapamycin, S6, sensitization
Introduction
Repeated exposure to cocaine and other abused psychostimulants causes enduring neuroadaptations within interconnected dopamanergic, GABAergic and glutamatergic projections between the ventral tegmental area, nucleus accumbens (NAC), prefrontal cortex (PFC), dorsal striatum (DST), amygdala, and hippocampus (Hyman, Malenka and Nestler, 2006; Nestler, 2005). Among the basic molecular aspects of cocaine-induced neuroplasticity are changes in protein expression that can regulate important properties of neuron physiology such as transcriptional activation (e.g., Bannon, Kapatos and Albertson, 2005; Hemby, 2006; Rhodes and Crabbe, 2005). While many studies have focused on alterations in gene expression at the transcriptional level, relatively few have directly examined the proteomic profile of cocaine abuse or other factors that control levels of protein expression, such as the rate of mRNA translation or mRNA/protein stability and degradation (e.g., Heiman et al., 2008; Hemby, 2006).
A major regulator of protein synthesis and cell growth is the evolutionarily conserved kinase mammalian target of rapamycin (mTOR), which integrates environmental signals pertinent to cell survival, such as the presence of growth factors, nutrient availability, cellular energy levels, and hypoxic or genotoxic stress (Wullschleger, Loewith and Hall, 2006). Among the many processes downstream of mTOR, stimulation of the initiation of protein translation via phosphorylation of P70S6 kinase (P70S6K), S6 ribosomal protein (S6), and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) is one of the best understood. The macrolide drug rapamycin, a potent and specific inhibitor of mTOR, greatly attenuates the phosphorylation of P70S6K (T389) and S6 (S235/236), as well as mTOR-dependent translation, transcription, and ribosome biogenesis (Hay and Sonenberg, 2004; Wullschleger, Loewith and Hall, 2006). Based on the ability of mTOR to modulate translation and its presence at post-synaptic sites (Tang et al., 2002), various studies have investigated the potential involvement of mTOR in long-term memory formation and have shown that rapamycin inhibits long-term potentiation and memory formation in the hippocampus (Slipczuk et al., 2009; Tang et al., 2002). Moreover, rapamycin inhibits long-term depression between glutamate and dopamine neurons in the ventral tegmental area (Mameli et al., 2007), synapses that are notably remodeled by cocaine and other drugs of abuse (e.g., Saal et al., 2003; Ungless et al., 2001). While there are limited studies on the effects of rapamycin upon behavior, subchronic rapamycin treatment is reported to exert antidepressant-like activity in mice and rats (Cleary et al., 2008). Of direct relevance to addiction, rapamycin blocks the sensitization of a methamphetamine-induced conditioned place preference in rats (Narita et al., 2005) and significantly reduces cue-induced drug craving in abstinent human heroin addicts (Shi et al., 2009). Together, such data suggest that rapamycin may well serve as a potential pharmacotherapeutic for treating emotional and motivational dysfunction associated with addiction and that drug-induced aberrations in mTOR signaling may contribute to the addiction process.
As the potential effects of rapamycin upon cocaine-induced changes in behavior have not yet been investigated, the present study tested the hypothesis that mTOR mediates the induction and/or expression of cocaine-induced behavioral plasticity by assessing the effects of rapamycin pretreatment within conditioned place-preference and locomotor sensitization paradigms. To relate our behavioral observations to indices of mTOR signaling within addiction-relevant brain regions, we immunoblotted for two common measures of mTOR activation, phosphorylation of P70S6K and S6 (Hay and Sonenberg, 2004).
Materials and methods
Subjects
Adult male C57BL/6J mice (6–8 weeks of age; 22–30g; The Jackson Laboratory, Bar Harbor, ME) were acclimated to a temperature (25°C) and humidity (71%) controlled colony room for 7 days prior to experimentation. Animals were housed in groups of 3–5 per cage with food and water ad libitum and maintained on a 12-hr light/dark cycle with lights on at 8:00 A.M. Conditioning and injection procedures took place during the light cycle. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Santa Barbara and were consistent with the National Institute of Health (NIH) Guide for Care and Use of Laboratory Animals (NIH Publication NO. 80-23, revised 1996).
Drugs
For behavioral procedures and immunoblotting, animals received intraperitoneal (IP) injections of either 15 mg/kg cocaine HCl (a generous gift from NIDA, Bethesda, MD) dissolved in physiological saline or an equivalent volume of saline alone (10 ml/kg). Rapamycin (LC Laboratories, Woburn, MA) was dissolved completely in 100% DMSO and then diluted 10-fold in nanopure water. For behavioral procedures and immunoblotting, animals received 10 mg/kg rapamycin IP in 10% DMSO vehicle or an equivalent volume of 10% DMSO vehicle alone (30 ml/kg). Rapamycin dose, vehicle composition, and pretreatment time were chosen based on a previous report of antidepressant-like effects without changes in spontaneous locomotor activity using these parameters in mice (Cleary et al., 2008).
Cocaine-induced place-conditioning and locomotor activity
Procedures for cocaine place-conditioning and assessment of locomotor activity were similar to those described previously (Penzner et al., 2008; Szumlinski et al., 2007). Briefly, all experiments were conducted in a Plexiglas apparatus (46 cm long × 24 cm high × 22 cm wide) with a sound-attenuating lid and a removable center divider separating 2 compartments of equal size. Each side of the apparatus differed in wall pattern and floor texture. On conditioning sessions and the test for locomotor sensitization (see below), the 2 compartments were separated by solid divider, confining the animal to 1 compartment. On all other sessions, animals had free-access to both compartments through a divider with a door. All sessions were 15-min long, and two digital video cameras interfaced to a computer with ANYMaze software (Stoelting Company, Wood Dale, IL) simultaneously recorded the total distance traveled and time spent on each side of the apparatus for each mouse.
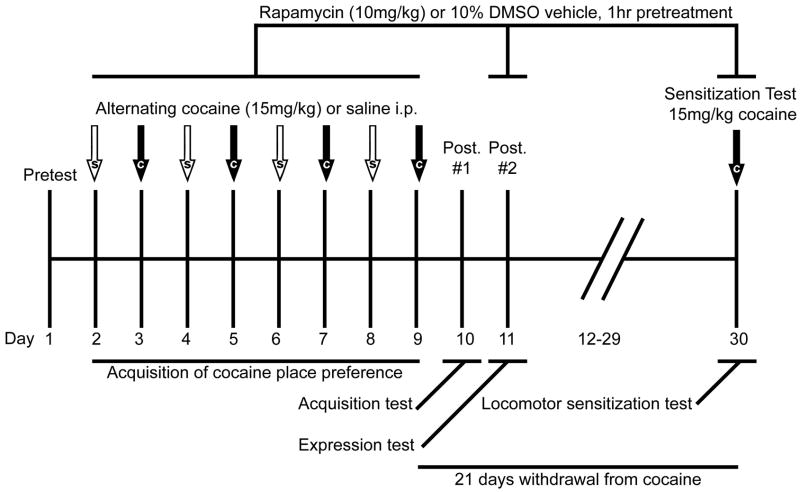
A schematic of the schedule for behavioral experiments is presented in Fig. 1a. Conditioning began with a preconditioning session (Pretest), in which experimentally-naïve animals had free-access to both compartments to establish initial compartment bias. This was followed by 8 once daily conditioning sessions, divided into 4 alternating pairings of distinct compartments with either 15 mg/kg cocaine or saline, using an unbiased design. During these daily conditioning sessions, animals were pretreated with either 10 mg/kg rapamycin (n=12) or vehicle (n=12) 1 h prior to placement in the appropriate compartment. To assay the effects of rapamycin pretreatment upon the acquisition of cocaine place-preference, a post-conditioning test (Posttest #1; Acquisition Test) was conducted in which the animals were completely drug-free. As rapamycin pretreatment during conditioning did not affect the acquisition of a cocaine place-preference (see Results), we next determined whether or not inhibition of mTOR signaling could block the expression of conditioned reward by pretreating our formerly rapamycin-naïve (i.e., vehicle pretreated) animals with 10 mg/kg rapamycin (n=6) or vehicle (n=6) 1 hr prior to a second post-conditioning test (Posttest #2; Expression Test). To increase the power of our statistical analysis of the data from this second post-conditioning test, a second cohort of mice was run through our place-conditioning procedures (without any pretreatment), subjected to a drug-free post-test, and then pretreated with either vehicle or 10 mg/kg rapamycin 1 hr prior to the second post-conditioning test (n=4 for vehicle; n=5 for rapamycin). Finally, as rapamycin pretreatment also did not alter the development of cocaine-induced locomotor sensitization across the 4 cocaine conditioning sessions, we conducted a test for the expression of locomotor sensitization 21 days after the last cocaine pairing (Sensitization Test). As repeated rapamycin exposure failed to alter cocaine-induced locomotion at any time during conditioning (Fig. 1b), mice tested for the expression of cocaine-induced locomotor sensitization were randomly assigned to be pretreated with either 10 mg/kg rapamycin or vehicle and then injected with 15 mg/kg cocaine, 1 hr later. The 10 mg/kg rapamycin dose and 1-hr pretreatment interval used in all of the experiments were chosen based on a previous report of antidepressive-like behavioral effects in mice and rats with no effects upon spontaneous locomotion (Cleary et al., 2008).
Figure 1.
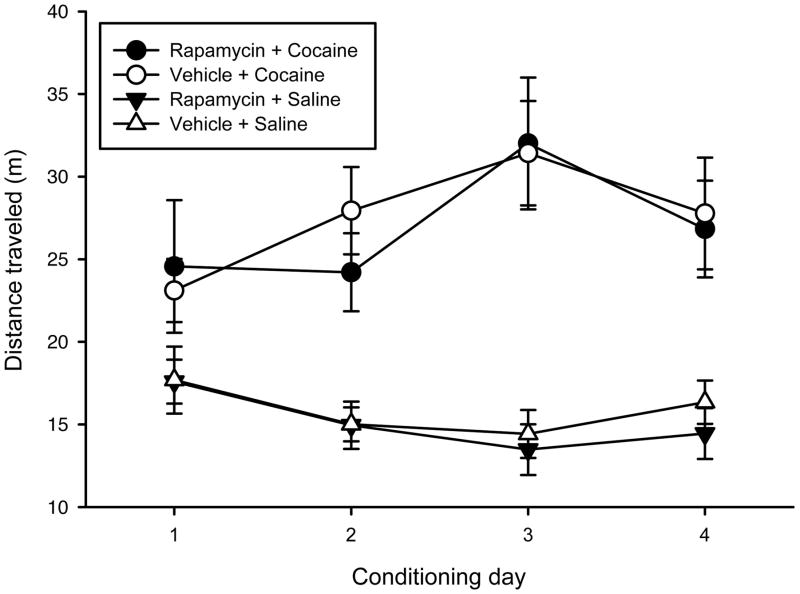
Rapamycin pretreatment during conditioning does not affect the acquisition of a cocaine-induced place preference or locomotor sensitization. (a) Schematic of experimental design. Mice received IP DMSO vehicle (n=12) or 10 mg/kg rapamycin (n=12) 1 hr prior to each cocaine/saline pairing, followed by a drug-free posttest (#1), a rapamycin-pretreated posttest (#2), a 21-day withdrawal period, and a test for the expression of cocaine-induced locomotor sensitization, following 1-hr rapamycin or vehicle pretreatment. (b) Rapamycin pretreatment during place-conditioning procedures had no effect on locomotor activity elicited by cocaine or by saline. (c) Rapamycin pretreatment during place-conditioning procedures also did not block a conditioned place-preference when animals were tested 24 hrs later in a drug-free state. Data are expressed as means ± SEM (error bars). *p<0.05 vs. saline-paired compartment (i.e., place-preference).
Treatment of mice for immunoblotting
To verify that repeated treatment with rapamycin effectively inhibited mTOR signaling in two brain regions associated with addiction, the prefrontal cortex (PFC) and dorsal striatum (STR), mice received 8 IP injections of 10mg/kg rapamycin or vehicle (n=8/group) once/day and were sacrificed 24 hr following the last injection (i.e., at a time corresponding to the Acquisition Test for place-preference; see Fig. 1a). To examine possible effects of cocaine on mTOR signaling, 3 groups of mice (n=12/group) received different treatment regimens of cocaine or saline: repeated cocaine (4X 15mg/kg), acute cocaine (3X saline; 1X 15mg/kg cocaine), or repeated saline (4X saline) with injections occurring every other day to mimic the treatment pattern of the conditioning paradigm. Mice were sacrificed 24 hr after the last injection.
Dissection and immunoblotting
Dissection of brain tissue was performed as described previously (Ary et al., 2007; Ary and Szumlinski, 2007; Shin et al., 2003). Mice were sacrificed by rapid decapitation and brains were coronally sectioned at the level of the PFC and again at the level of the NAC/STR in a chilled 0.5mm mouse brain matrix (Braintree Scientific, Braintree, MA). Over ice-cold glass, the PFC (incl. ventral prelimbic/dorsal infralimbic regions) and DST were dissected out with forceps, while the core and shell subregions of the NAC were removed separately with a cooled 18-G micropunch. Samples were immediately frozen on dry ice and stored at −80°C until homogenization. Tissue was homogenized in ice-cold RIPA lysis buffer with protease and phosphatase inhibitors (50mM Tris-HCl; pH 8.0, 150mM NaCl, 1mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, Complete Protease Inhibitor Cocktail Tablets (Roche, Indianapolis, IN), 1mM PMSF, 50mM NaF, 10mM β-glycerophosphate, 10mM sodium pyrophosphate, 1mM sodium orthovanadate). Lysates were cleared by centrifiguation at 4°C and total protein was quantified using the Pierce BCA Protein Assay Kit microplate procedure as per the manufacturer’s instructions (Pierce-Thermo Fisher Scientific, Rockford, IL). The total protein concentrations and were equalized across samples for each brain region and denatured in SDS-PAGE sample loading buffer. Immunoblotting was performed as described previously (Xu et al., 2010). Equal amounts of total protein (30–35ug) were loaded into each well of 10% acrylamide gels, separated by SDS-PAGE, and transferred to Immobilon 0.45μM PVDF membranes (Millipore, Billerica, MA) using a Semi Dry Electroblotting System (Thermo Scientific Owl Separation Systems, Rochester, NY). After drying, membranes were incubated in a 1:1 mixture of Rockland Blocking Buffer for Fluorescent Western Blotting (Rockland Immunochemicals Inc., Gilbertsville, PA) and PBST (PBS with 0.1% Tween 20) with one of the following primary antibodies overnight at 4°C: rabbit anti-Phospho-S6 ribosomal protein S235/236 (1:4000; Cell Signaling, Beverly, MA), rabbit anti-S6 ribosomal protein (1:2000; Cell Signaling), rabbit anti-Phospho-P70 S6 Kinase (1:2500; Millipore), rabbit anti-P70 S6 Kinase (1:5000; Millipore). Membranes were subsequently washed with PBST, incubated in goat anti-rabbit DyLight 680 secondary antibody (Thermo Scientific), dried, and imaged on an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). This imaging system was chosen as it provides quantitative fluorescence detection over a much wider linear dynamic range than chemiluminescence. Raw values for each band were measured, and first normalized to the average value of the vehicle control for each gel (DMSO vehicle for Fig. 2 blots, saline for Table 1 blots). Subsequently, the ratio of phospho-protein to total protein was obtained for each measurement, and the average of the vehicle ratios for each condition was set equal to 1 (100%).
Figure 2.
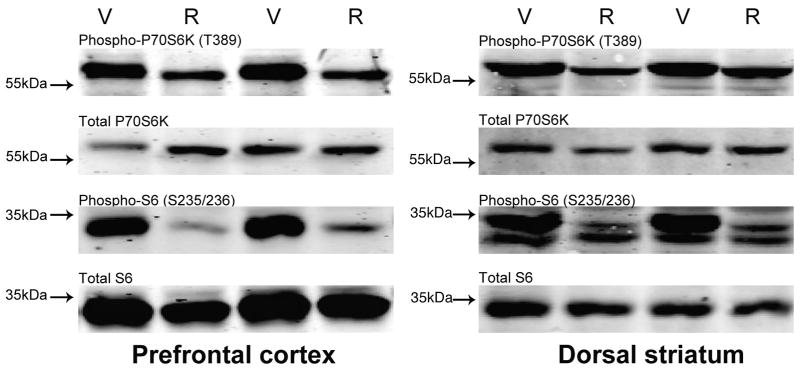
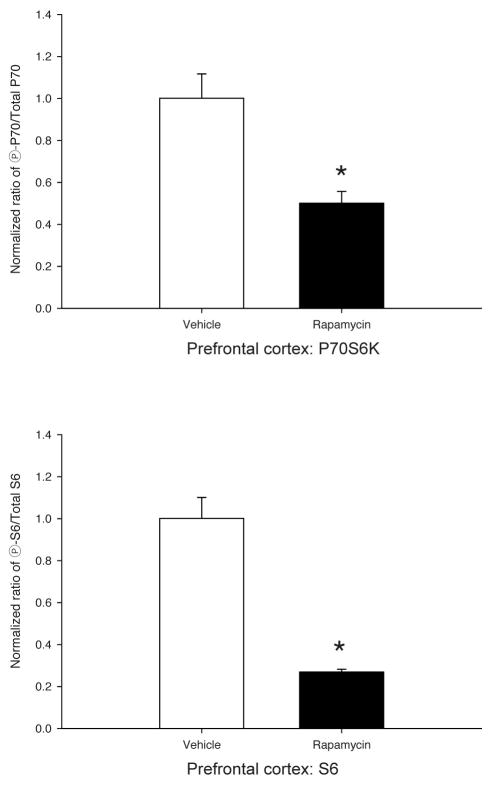
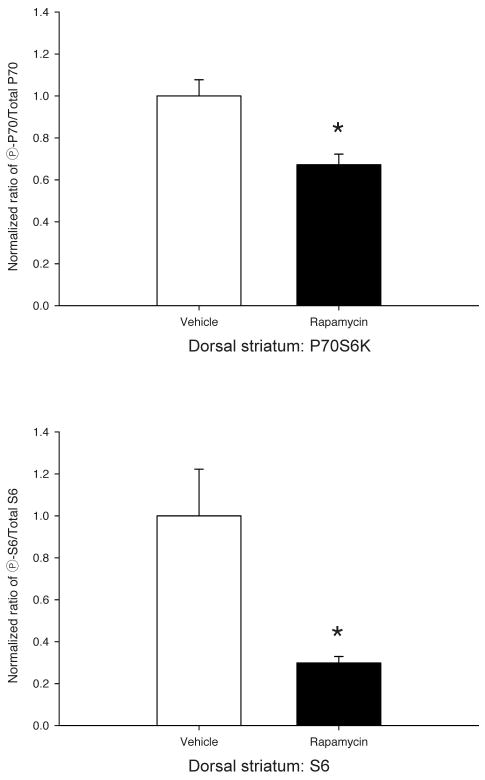
Rapamycin pretreatment reduces P70S6K and S6 phosphorylation in the prefrontal cortex and dorsal striatum. (a) Western blots from prefrontal cortex (left) and dorsal striatum (right) lysates of four representative mice (V= DMSO vehicle, R= 10mg/kg rapamycin). Once daily 10 mg/kg IP injections of rapamycin dissolved in 10% DMSO for 8 days was sufficient to significantly decrease phospho-P70S6K (T389) and phospho-S6 (S235/236) levels, relative to total levels of each protein at 24hrs after the final injection. Apparent molecular weight is denoted to the left of each series of blots. (b) Quantification of P70S6K (top) and S6 (bottom) western blots from prefrontal cortex lysates of vehicle- vs. rapamycin-treated mice obtained using Li-COR Odyssey Infrared Imaging System. (c) Similar quantification of P70S6K (top) and S6 (bottom) western blots from dorsal striatum of the same mice. Values in (b,c) bar graphs represent the means ± SEMs of 6–8/group and were normalized as described in “Materials and Methods.” *p<0.05 vs. Vehicle (t-tests).
Table 1.
Neither acute nor repeated cocaine treatment significantly altered the ratio of phospho-P70S6K or phospho-S6 levels to total levels of each protein (n=8–12/group, t-tests; p<0.05, mean ±SEM). Values are given as percent of saline control.
No effect of acute or repeated cocaine on phosphorylation of P70S6K or S6. Mice (n=8–12/group) received 4 injections of saline (saline), 3 injections of saline + 1 injection of 15 mg/kg cocaine (acute cocaine), or 4 injections of 15 mg/kg cocaine (repeated cocaine) and were sacrificed 24 hr after the last injection. Western blotting of PFC, NA core, and NA shell from these mice failed to yield significant differences in P70S6K or S6 phosporylation (all t-tests, p<0.05). All values were quantified and normalized as described in “Materials and Methods” and are represented as the mean ± SEM.
| Acute cocaine | Repeated cocaine | ||||||
|---|---|---|---|---|---|---|---|
| PFC | NA core | NA shell | PFC | NA core | NA shell | ||
| P-P70/Total P70 | Saline | 100±9.0 | 100±8.5 | 100±8.1 | 100±9.4 | 100±5.8 | 100±6.8 |
| Cocaine | 90±5.9 | 109±6.4 | 93±7.4 | 96±6.0 | 95±5.8 | 96±11.4 | |
| P-S6/Total S6 | Saline | 100±13.6 | 100±14.7 | 100±9.4 | 100±6.3 | 100±9.5 | 100±7.6 |
| Cocaine | 89±8.0 | 85±11.6 | 85±8.7 | 110±11.3 | 113±17.1 | 131±20.5 |
Statistical analysis
All statistical analyses were performed with SPSS Statistics 17 (IBM, Chicago, IL). For locomotor activity, mixed design analyses of variance (ANOVA), with the between-subjects factor of Pretreatment (rapamycin vs. saline) and repeated measures on the Day factor, were used to assess the effects of rapamycin pretreatment on locomotor activity during saline-conditioning, as well as during cocaine-conditioning and the test for the expression of cocaine-induced locomotor sensitization (Fig 3b). A mixed design ANOVA, with Pretreatment as the between-subjects factor and repeated measures on the Side factor (saline- or cocaine-paired) was used to assess the effects of rapamycin pretreatment upon the acquisition and expression of the place-preference. Two-way, independent-sample t-tests were used to compare quantified data from western blots. α=0.05 for all analyses.
Figure 3.
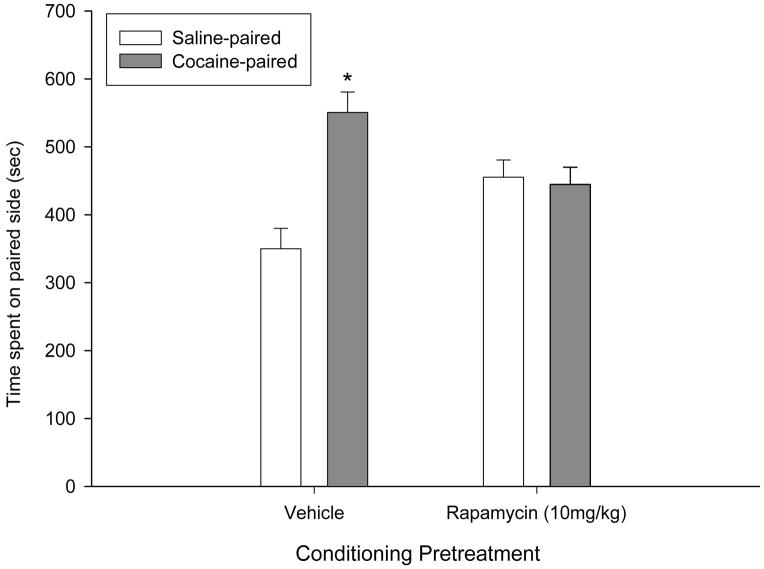
Rapamycin pretreatment attenuates cocaine-induced place preference and blocks long-term locomotor sensitization. (a) Compared to vehicle-pretreated animals (n=10), 10 mg/kg rapamycin pretreatment (n=11) 1 hr prior to a post-conditioning test attenuated the expression of a cocaine-induced place-preference. (b) Compared to vehicle-pretreated animals (n=11) rapamycin pretreatment (n=12) 1 hr prior to a 15 mg/kg cocaine challenge injection conducted at 3 weeks withdrawal blocked sensitization of the locomotor-activating effects of cocaine. Data are expressed as means ± SEMs. *p<0.05 vs. Vehicle; +p<0.05 vs. acute cocaine (sensitization).
Results
Rapamycin pretreatment does not affect the acquisition of cocaine place-preference or the development of locomotor sensitization
Mice received behavioral training and drug treatments according to the schedule depicted in Fig. 1a. As illustrated in Fig. 1b, cocaine-induced locomotor activity (measured by total distance traveled in meters) increased, or sensitized, over the 4 days of conditioning in both vehicle- and rapamycin-pretreated mice (n=12/group; Day effect: F3,66=6.55, p<0.001), but group differences were not observed regarding the amount of cocaine-induced locomotor activity nor the extent to which repeated cocaine treatment elicited sensitization (Pretreatment effect & Pretreatment X Day, n.s.). An analysis of saline-induced locomotor activity also failed to indicate a significant effect of rapamycin pretreatment upon the habituation of locomotor activity across the 4 saline sessions (Day effect: F3,66=7.47, p<0.001; Pretreatment effect & Pretreatment X Day, n.s.).
As illustrated in Fig. 1c, the repeated pairing of cocaine with a distinct environment elicited a robust place-preference when the animals were tested in a completely drug-free state (Side effect: F1,22=38.63, p<0.0001). However, prior rapamycin pretreatment during conditioning had absolutely no effect upon the magnitude of the place-preference exhibited on this test (Pretreatment X Side: n.s.). Thus, rapamycin pretreatment during cocaine-conditioning does not prevent the acquisition of a conditioned place-preference.
Repeated rapamycin decreases markers of mTOR activity in addiction-related brain regions
The negative behavioral data presented in Fig. 1 suggested that perhaps a tolerance might develop to the inhibitory effects of rapamycin upon mTOR signaling with its repeated administration across the 8 days of conditioning. Thus, to test this hypothesis, mice were randomly assigned to receive 8, once daily, IP injections of 10 mg/kg rapamycin or vehicle and were sacrificed 24 hr after the last injection. Immunoblotting was performed on PFC and STR lysates for rapamycin-sensitive phosphorylation sites on P70S6K and S6 ribosomal protein. In rapamycin-treated mice, both brain regions showed significantly reduced levels of phospho-P70S6K (T389) and phospho-S6 (S235/236) normalized to total levels of P70S6K and S6 respectively (Fig. 2a). Statistical analyses confirmed significant decreases from vehicle of 50±5.5% for phospho-P70S6K and 73±1.4% for phospho-S6 in the PFC of rapamycin treated mice [Fig. 2b; P70S6K, t(14)= 3.88, p<0.01; S6, t(7)= 7.18, p<0.001]. Similar reductions in the levels of phospho-P70S6K (33±5.2%) and phospho-S6 (70±3.1%) were observed in the STR [Fig 2c; P70S6K, t(12)= 3.68, p<0.01; S6, t(5)= 3.13, p=0.025]. For S6 in both PFC and STR, Levene’s test indicated unequal variances (PFC S6, F=13.8, p=0.002; STR S6, F=7.11, p=0.025), so degrees of freedom for these t-tests were adjusted accordingly. Thus, despite not affecting our behavioral outcomes (Fig. 1), the repeated rapamycin pretreatment regimen employed during conditioning was sufficient to inhibit significantly at least two downstream targets of mTOR in at least two brain regions relevant to addiction.
Rapamycin pretreatment attenuates the expression of cocaine place-preference
Since our rapamycin pretreatment was sufficient to inhibit mTOR targets in the brain (Fig. 2), but did not significantly affect the acquisition of a cocaine place-preference when the animals were tested in a drug-free state (Fig. 1c), we next tested the hypothesis that rapamycin pretreatment might alter the expression of a cocaine-conditioned place-preference by pretreating rapamycin-naïve animals with either vehicle (n=10) or 10 mg/kg rapamycin (n=11) 1 hr prior to a post-conditioning test. As illustrated in Fig. 3a, vehicle pretreatment prior to testing did not influence the expression of a conditioned place-preference, as mice spent significantly more time on the cocaine-paired side of the apparatus (Side effect: F1,19=5.98, p=0.024). In contrast, rapamycin pretreatment prior to testing completely blocked the expression of a place-preference, as mice spent equivalent amounts of time in both compartments (Pretreatment X Side: F1,19=7.38, p=0.014). Together, our place-preference data indicate that while repeated rapamycin pretreatment does not affect the development of a cocaine-conditioned place-preference, acute rapamycin pretreatment can block the expression of a place-preference in cocaine-conditioned animals.
Rapamycin pretreatment blocks the expression of cocaine locomotor sensitization after 3 weeks withdrawal
Based on our observation that a single injection of rapamycin 1 hr prior to behavioral testing blocked the expression, but not the acquisition, of a cocaine-induced place-preference, we next investigated whether or not rapamycin might also reduce the expression of enduring behavioral sensitization. For this, mice received rapamycin (n=12) or vehicle (n=11) pretreatment 1 hr prior to a challenge injection of 15mg/kg cocaine, administered 3 weeks following the last cocaine-conditioning injection. As illustrated in Fig. 3b, vehicle-pretreated mice exhibited significantly higher cocaine-induced locomotor activity on the Sensitization Test, compared to that expressed on day 1 of cocaine-conditioning (i.e., sensitization). In contrast, the level of cocaine-induced locomotor activity exhibited by mice pretreated with rapamycin prior to the Sensitization Test was similar to that expressed on day 1 of cocaine-conditioning, indicating no sensitization (Pretreatment X Day: F1,21=5.49, p=0.029). Together, our cocaine locomotor data indicates that while repeated rapamycin pretreatment does not prevent the development of cocaine-induced locomotor sensitization (Fig. 1b), acute pretreatment is sufficient to block the expression of sensitization in mice withdrawn from repeated cocaine treatment.
Neither acute nor repeated cocaine significantly alter levels of mTOR pathway activation in several addiction-related brain regions
As rapamycin was found to reduce the expression of two forms of cocaine-induced behavioral plasticity, we wondered whether or not rapamycin might exert this effect by inhibiting cocaine-induced activation of major mTOR pathway targets. To test the hypothesis that cocaine can activate mTOR pathway targets, mice were treated either acutely or repeatedly (4 injections) with 15 mg/kg cocaine. Control animals received 4 injections of saline. Lysates from PFC, as well as the core and shell subregions of the NAC were immunoblotted for levels of total and phospho- P70S6K and S6. Illustrated in Table 1, no significant changes in the phospho/total ratio of either mTOR target were observed in any of the selected brain regions (t-tests, all p’s n.s.). Although not without caveat, these data indicate that neither acute nor repeated cocaine induces mTOR activation at least within the PFC and NAC, and that the mechanism of rapamycin action to inhibit reward-associated behavior may not involve constitutively heightened mTOR activity.
Discussion
The present study advances our current knowledge of the role for mTOR pathway activation in mediating drug-induced behavioral plasticity by demonstrating that pretreatment with the mTOR inhibitor rapamycin (Fig. 2) inhibits the expression of a cocaine-conditioned place-preference, as well as the long-term expression of cocaine-induced locomotor sensitization (Fig. 3). Interestingly, rapamycin pretreatment during repeated cocaine exposure failed to alter significantly cocaine-induced locomotion behavior (Fig. 1b) and was insufficient to produce an enduring effect upon a cocaine-conditioned place-preference when animals were tested in a drug-free state (Fig. 1c). As neither acute nor repeated cocaine was found to alter the phosphorylation state of P70S6K and S6 within PFC or NAC (Table 1), the combination of our behavioral data suggest that rapamycin-mediated inhibition of cocaine-induced changes in conditioned reward and sensitized behavior does not likely involve a reversal of cocaine’s effects upon mTOR signaling within corticolimbic regions. Rather, rapamycin must interact with some other cocaine-induced neuroadaptation(s) independent of these 2 major downstream targets of mTOR to exert its apparent “anti-addictive” and “anti-sensitizing” effects.
Our observations that rapamycin blocks the expression of cocaine-conditioned reward and locomotor sensitization in mice are consistent with several lines of evidence that support a role for mTOR and the upstream regulator phosphoinositide kinase-3 (PI3K) in drug-induced neuroplasticity. Of direct relevance to the clinical condition of addiction, rapamycin (5 mg) was recently found to inhibit cue-induced heroin craving in abstinent human addicts (Shi et al., 2009). This finding in humans is consistent with data from an earlier study in rats demonstrating that an intra-NAC infusion of rapamycin (0.025 pmol) blocks the sensitization of a methamphetamine-induced place-preference elicited by prior methamphetamine experience (Narita et al., 2005), as well as data from alcohol studies conducted in mice indicating the effectiveness of an intra-NAC infusion of PI3K inhibitors for reducing the long-term expression of cocaine-induced locomotor sensitization (Izzo et al., 2002) and binge alcohol drinking in alcohol-experienced animals (Cozzoli et al., 2009). Interestingly, similar to our results for systemic rapamycin and cocaine, intra-NAC rapamycin pretreatment during methamphetamine-conditioning does not alter a place-preference when animals are tested in a drug-free state (Narita et al., 2005).
While it might be argued that the failure of rapamycin to block a stimulant-induced place-preference when tested 24 hr following the last rapamycin pretreatment could relate to pharmacokinetic factors, we show clearly that rapamycin-mediated inhibition of 2 major markers of mTOR pathway activation – the phosphorylation of P70S6K and S6 – persists in forebrain for at least 24 hrs following repeated rapamycin treatment (Fig. 2). The level of mTOR inhibition by repeated rapamycin ranged from 40–80%, depending upon the substrate and brain region examined (Fig. 2); while significantly lower than control animals, the possibility exists that this reduction of P70S6K and S6 phosphorylation is insufficient to produce a behavioral effect. In some support of this possibility, rapamycin administration 1 hr, but not 24 hrs, prior to testing was effective at reducing a cocaine-conditioned place-preference (Fig. 3a vs. Fig. 1c), suggesting that higher rapamycin levels or greater mTOR inhibition may be required in order to interfere with cocaine-induced changes in behavior. The 10 mg/kg rapamycin dose was selected for study as it produces maximal antidepressant-like effects in rats with no overt locomotor side-effects that could confound interpretation of our behavioral measures (Fig. 1b; Cleary et al., 2008). While a full dose-response study might assist in addressing this issue, the fact that no rapamycin pretreatment effect was observed upon cocaine-induced locomotion 1 hr following pretreatment during repeated cocaine exposure (Fig. 1), but an identical pretreatment blocked locomotor sensitization 3 weeks later (Fig. 3b), suggests factors other than rapamycin pharmacokinetics in the selective effect of pretreatment upon the expression of conditioned reward and locomotor sensitization.
Indeed, the neural substrates responsible for the acquisition/development of place-conditioning and locomotor sensitization are distinct from those mediating the expression of these two forms of drug-induced behavioral plasticity (Tzschentke, 2007; Vanderschuren and Kalivas, 2000). The fact that rapamycin pretreatment (either systemic or intra-NAC) appears to be behaviorally effective in addiction-related paradigms only when administered to animals with prior drug experience (Fig. 3; Narita et al., 2005) suggests some important interaction between rapamycin and the neuroplasticity resulting from this prior drug experience. One likely candidate in this regard could relate to drug-induced changes in PI3K/mTOR signaling within mesocorticolimbic circuits mediating the rewarding/reinforcing and psychomotor-activating properties of various drugs of abuse. Indeed, repeated (but not acute) methamphetamine treatment increases NAC phospho-P70S6K levels (Narita et al., 2005) and the expression (but not the acquisition) of cocaine-induced behavioral sensitization is associated with increased NAC PI3K activity (Izzo et al., 2002; Zhang et al., 2006). While the effects of binge alcohol drinking upon downstream indices of mTOR pathway activation have yet to determined, repeated bouts of binge alcohol drinking also up-regulates NAC PI3K activity and high basal PI3K activity within the NAC is observed in mice with a genetic propensity to consume high amounts of alcohol (Cozzoli et al., 2009; Goulding et al., 2010). Together, these reports support the involvement of mTOR in the neuroplasticity associated with drugs of abuse. This all being said, chronic dietary alcohol consumption (4% over 16-weeks) reduces phospho/total ratios of mTOR, P70S6K, and 4E-BP1 in cerebral cortex (Li and Ren, 2007), and we failed to detect any significant effect of either acute or repeated cocaine upon P70S6K activity in either subregion of the NAC or within PFC (Table 1). It is inherently difficult to compare results across studies employing drugs of abuse with different targets or mechanisms of action, routes of administration and treatment regimens. While the available data does not indicate a clear-cut link between the manifestation of addiction-related behaviors and common indices of corticoaccumbens mTOR activity, mTOR influences many downstream processes such as autophagy and neuron size/morphology (Sarbassov, Ali and Sabatini, 2005), and it is possible that P70S6K and S6 are not the behaviorally-relevant targets of mTOR inhibition. However, the ability of rapamycin to abolish behavioral sensitization when administered at 3 weeks withdrawal (Fig. 3b) and to reduce craving in heroin-abstinent individuals (Shi et al., 2009) supports a function for mTOR in maintaining the long-term neuroplasticity produced by repeated cocaine experience.
How precisely rapamycin regulates the expression of behavioral sensitization and conditioned place preference remains an important mechanistic question to be answered. One attractive rapamycin-sensitive mechanism reported to regulate neuroplasticity and memory consolidation of relevance to long-term drug-induced changes in behavior involves brain-derived neurotrophic factor (BDNF)-dependent alterations in the expression of AMPA receptor subunits. Rapamycin reduces the surface expression of GluR2/3 in primary cortical cultures (Wang, Barbaro and Baraban, 2006) and inhibits GluR2-dependent LTD in the VTA (Mameli et al., 2007). Moreover, rapamycin blocks BDNF-dependent Homer2 translation in dendrites (Mameli et al., 2007; Schratt et al., 2004), as well as the consolidation of fear-conditioned long-term memory and the associated GluR1 increase in the dorsal hippocampus (Slipczuk et al., 2009). GluR1 and GluR3 surface expression, as well as BDNF, are increased in the NAC after cocaine withdrawal and these changes contribute to the expression of behavioral sensitization, as well as drug-seeking, in the long-term (e.g., Bahi et al., 2008; Boudreau and Wolf, 2005; Conrad et al., 2008; Graham et al., 2007; Grimm et al., 2003; Lu et al., 2004). As intracerebroventricular infusion of the AMPA/kainate antagonist DNQX also blocks the expression of a cocaine-conditioned place-preference without affecting its induction (Cervo and Samanin, 1995), future studies in our laboratory will be examining the involvement of BDNF and AMPA receptors in mTOR-dependent regulation of cocaine-induced behavioral plasticity.
Acknowledgments
The authors would like to thank Dr. Haim Einat for technical advice. This work was supported by funding from NIDA to KKS and NARSAD to KKS and DM.
References
- Ary AW, Aguilar VR, Szumlinski KK, Kippin TE. Prenatal stress alters limbo-corticostriatal Homer protein expression. Synapse. 2007;61:938–941. doi: 10.1002/syn.20439. [DOI] [PubMed] [Google Scholar]
- Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Chandrasekar V, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Bannon M, Kapatos G, Albertson D. Gene expression profiling in the brains of human cocaine abusers. Addict Biol. 2005;10:119–126. doi: 10.1080/13556210412331308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Cleary C, Linde JA, Hiscock KM, Hadas I, Belmaker RH, Agam G, Flaisher-Grinberg S, Einat H. Antidepressive-like effects of rapamycin in animal models: Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res Bull. 2008;76:469–473. doi: 10.1016/j.brainresbull.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, Szumlinski KK. Accumbens Homer2-mediated signaling: A factor contributing to mouse strain differences in alcohol drinking? Genes Brain Behav. 2010 doi: 10.1111/j.1601-183X.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE. Chapter 9 Assessment of genome and proteome profiles in cocaine abuse. Progress in Brain Research. 2006:173–195. doi: 10.1016/S0079-6123(06)58009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Izzo E, Martin-Fardon R, Koob GF, Weiss F, Sanna PP. Neural plasticity and addiction: PI3-kinase and cocaine behavioral sensitization. Nat Neurosci. 2002;5:1263–1264. doi: 10.1038/nn977. [DOI] [PubMed] [Google Scholar]
- Li Q, Ren J. Chronic alcohol consumption alters mammalian target of rapamycin (mTOR), reduces ribosomal p70s6 kinase and p4E-BP1 levels in mouse cerebral cortex. Exp Neurol. 2007;204:840–844. doi: 10.1016/j.expneurol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Narita M, Akai H, Kita T, Nagumo Y, Sunagawa N, Hara C, Hasebe K, Nagase H, Suzuki T. Involvement of mitogen-stimulated p70-S6 kinase in the development of sensitization to the methamphetamine-induced rewarding effect in rats. Neuroscience. 2005;132:553–560. doi: 10.1016/j.neuroscience.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Penzner JH, Thompson DL, Arth C, Fowler JK, Ary AW, Szumlinski KK. Protracted ‘anti-addictive’ effects of adolescent phenylpropanolamine exposure in C57BL/6J mice. Addict Biol. 2008;13:310–325. doi: 10.1111/j.1369-1600.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Crabbe JC. Gene expression induced by drugs of abuse. Curr Opin Pharmacol. 2005;5:26–33. doi: 10.1016/j.coph.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Jun W, Zhao LY, Xue YX, Zhang XY, Kosten TR, Lu L. Effect of rapamycin on cue-induced drug craving in abstinent heroin addicts. Eur J Pharmacol. 2009;615:108–112. doi: 10.1016/j.ejphar.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003;162:293–303. doi: 10.1083/jcb.200210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Liu A, Penzner JH, Lominac KD. Protracted ‘pro-addictive’ phenotype produced in mice by pre-adolescent phenylpropanolamine. Neuropsychopharmacology. 2007;32:1760–1773. doi: 10.1038/sj.npp.1301306. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Wang Y, Barbaro MF, Baraban SC. A role for the mTOR pathway in surface expression of AMPA receptors. Neurosci Lett. 2006;401:35–39. doi: 10.1016/j.neulet.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xu Z, Xia B, Gong Q, Bailey J, Groves B, Radeke M, Wood SA, Szumlinski KK, Ma D. Identification of a deubiquitinating enzyme as a novel AGS3-interacting protein. PLoS One. 2010;5:e9725. doi: 10.1371/journal.pone.0009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mi J, Wetsel WC, Davidson C, Xiong X, Chen Q, Ellinwood EH, Lee TH. PI3 kinase is involved in cocaine behavioral sensitization and its reversal with brain area specificity. Biochem Biophys Res Commun. 2006;340:1144–1150. doi: 10.1016/j.bbrc.2005.12.114. [DOI] [PubMed] [Google Scholar]