Offense and defense: microbial membrane vesicles play both ways (original) (raw)
. Author manuscript; available in PMC: 2013 Nov 1.
Published in final edited form as: Res Microbiol. 2012 Oct 30;163(9-10):607–618. doi: 10.1016/j.resmic.2012.10.020
Abstract
Microbes have evolved over millennia to become adapted and specialized to the environments that they occupy. These environments may include water or soil, extreme environments such as hydrothermal vents, and can even include a host organism. To become adapted to these locations, microbes have evolved specific tools to mediate interactions with the environment. One such tool that prokaryotes have evolved includes the production of membrane vesicles (MVs). MVs are 10–300 nm spherical blebs derived from the outermost membrane and have known functions in protein secretion, immune activation and suppression, stress response, attachment, internalization and virulence. In this review, we consider the highly conserved role of membrane vesicles derived from Gram-negative, Gram-positive and archaeal species as a mechanism to facilitate intermicrobial and microbe-host interaction. We examine both the offensive and defensive capabilities of MVs in regard to the interaction of MVs with both host and microbial cells in their environment.
Keywords: OMV, MV, Bleb, Bacterial pathogenesis, Archaea, biofilm, Antibiotic resistance, Envelope stress response
1. Introduction
Microbial life has evolved over the millennia to survive and thrive in a wide variety of environments across the globe. Many diverse species live in a temperate environment, with some, like bacterial pathogens, specialized to colonize host organisms. Other organisms, like archaea, occupy the extreme environments of the world. Regardless of the location, microbes have needed to evolve tools to cope with and grow within a changing environment. One such tool that has evolved to facilitate microbe-microbe, microbe-host and microbe-environment interactions is the production of membrane vesicles (MVs). MVs are typically 10–300 nm spherical blebs that originate from the outermost cell membrane, although their size range can vary depending on the species. MVs are distinct from membranous blebs produced during cell lysis as they are produced as a regulated, selective secretion event (Ferrari et al., 2006; McBroom et al., 2006; Mug-Opstelten and Witholt, 1978; Zhou et al., 1998). The MV secretion mechanism functions to disseminate virulence factors including toxins and degradative enzymes into the extracellular milieu. Once released, MVs have been demonstrated to function offensively as a virulence factor delivery mechanism, as well as defensively, to aid in the colonization of a host and the survival of an organism in a hostile environment. This review will highlight the evolutionarily conserved nature of MVs derived from Gram-negative, Gram-positive and archaeal species and present how membrane vesicles facilitate microbial interactions with cells and factors in the environment.
2. Microbial membrane vesicle formation
2.1. Background and history
For nearly five decades, the study of MV formation by prokaryotes has focused on Gram-negative bacteria. Since the 1960’s, observations have been made regarding spherical membrane blebs present in electron micrograph images of Gram-negative bacteria (Birdsell and Cota-Robles, 1967; Knox et al., 1966). The content, composition and purpose of these structures were largely unknown. It was often assumed that the observed membranous structures were cell debris fragments caused by lysis. Since that time, the production and presence of MVs has been ubiquitously identified for a variety of Gram-negative species, including environmental, laboratory, as well as clinical and pathogenic isolates (Ellis and Kuehn, 2010; Kuehn and Kesty, 2005; Unal et al., 2011).
More recently, attention has turned to determining if MV production is an evolutionarily conserved process that also occurs for Gram-positive and archaeal species. Gram-positive bacteria and archaea notably lack a second lipid bilayer which was the site of MV production in Gram-negative bacteria. Despite the obvious physical differences in cell wall and membrane structure, spherical membrane blebs have been observed in the culture supernatant of Gram-positive bacteria and archaea, (Deatherage and Cookson, 2012; Ellen et al., 2010; Lee et al., 2009; Rivera et al., 2010; Soler et al., 2008).
Because the motif of secretion of the outermost membrane is evolutionarily conserved, MVs are expected to hold great importance for prokaryotic survival. By examining the mechanics of MV formation and regulation and the roles of MVs in intracellular interactions, we hope to gain insight into how MVs benefit the microbes. This review will serve to briefly highlight conserved aspects of MV production and focus more closely on their role as molecular tools to perform offensive and defensive tasks for a variety of prokaryotic microbes.
2.2. Gram-negative OMVs
MVs produced by Gram-negative bacteria are typically 10–300 nm spherical blebs derived from the outer membrane (OM) and therefore are termed “OMVs.” The Gram-negative bacterial OM is distinct from the inner, cytoplasmic membrane (IM) and is separated from the IM by the periplasmic space and a thin layer of peptidoglycan (PG). OM is composed of phospholipids and endotoxin (or lipopolysaccharide, LPS) as the lipid constituent of the inner and outer leaflets, respectively, in addition to integral OM proteins (OMPs) and lipid-anchored lipoproteins. As derivatives of the OM, OMVs contain these OM components as well as soluble content inside and attached to their outer surface of the vesicles. The contents of the vesicle lumen are derived from the periplasm between the IM and OM. Additionally, proteins and macromolecules have been found associated with LPS on the external surface of OMVs (Horstman and Kuehn, 2000; Schooling and Beveridge, 2006; Schooling et al., 2009). Much recent research has focused on OMV proteins that could play a role in bacterial pathogenesis, including toxins, degradative enzymes, and other virulence factors, and these will be discussed in depth later in this review.
Several studies have provided important evidence supporting the hypothesis that OMV production represents a pathway for the secretion of specific proteins. Proteomic analysis has revealed that the protein profiles of OMVs are distinct from those of the cellular subfractions (Choi et al., 2011; Lee et al., 2007; Lee et al., 2008). It was also demonstrated by McBroom et al., that particular lumenal cargo are enriched in OMVs as compared to the periplasm (McBroom and Kuehn, 2007). Also, particular LPS subtypes are enriched in OMVs as compared to the OM and these can participate in OMV cargo selection (Haurat et al., 2011; Kadurugamuwa and Beveridge, 1995; Kadurugamuwa et al., 1993). These data form the basis of the theory that OMVs are a secretion pathway, rather than the random product of the loss of bacterial integrity or lysis.
Elucidating the mechanism(s) for OMV formation has been the focus of more recent efforts. As past publications have discussed mechanisms of Gram-negative OMV formation in detail (Deatherage et al., 2009; Kuehn and Kesty, 2005; Kulp and Kuehn, 2010), we will only highlight the conserved mechanistic elements.
Since OMVs originate from the bacterial envelope, it was logical for initial investigations of OMV formation to focus on the contributions of envelope components (OM, IM, and PG). Membrane-anchored lipoproteins covalently and non-covalently link the PG to the two membrane bilayers, giving the cell rigidity. It is commonly hypothesized that in order for OMVs to form, a localized depletion of these linkages is necessary. Indeed, strains lacking the genes (e.g. tol-pal, lpp, or ompA) or residues critical for the non-covalent and covalent OM–PG linkages exhibited increased OMV production (Bernadac et al., 1998; Deatherage et al., 2009; Moon et al., 2012; Walburger et al., 2002). These data confirmed that the loss of PG linkages to the OM can lead to OMV shedding; however, whether regulated depletion or breaking of those links occurs in wild-type cells remains an unanswered question in the field.
In addition to the role of interactions between envelope components, the role of cell surface-linked sugars in OMV formation has been investigated. In the Gram-negative opportunistic pathogen Pseudomonas aeruginosa, two distinct forms of LPS are present in the OM (Kadurugamuwa and Beveridge, 1995; Kadurugamuwa et al., 1993). The O-specific antigen, or B-band LPS, is composed of many tandem sugar repeats that vary depending on the specific strain serotype and is highly charged. The common antigen, or A-band LPS, is composed of a limited number of rhamnose sugar repeats and is uncharged. Beveridge and coworkers observed that a majority, if not all, of the LPS in naturally-produced OMVs was B-band LPS (Kadurugamuwa and Beveridge, 1995; Kadurugamuwa et al., 1993). It was proposed that the charged nature of B-band LPS created repulsion forces in the membrane facilitating OM blebbing. In contrast, when they analyzed OMVs isolated from P. aeruginosa treated with the cell well perturbing agent, gentamicin, both A- and B-band LPS were present. This supported the model that native OMV budding sites are enriched in B-band LPS. Further support for the correlation of B-band LPS and OMV production was made by Sabra et al., when they observed increased OMV production with increased saturated oxygen levels, which increases B-band LPS levels (Sabra et al., 2003). Additionally, not only was a particular outer polysaccharide component of LPS found to be selected in OMVs, but the saccharide also appears to be important in cargo selection for Porphyromonas gingivalis (Haurat et al., 2011). Finally, McMahon et al., demonstrated that Serratia marcescens lacking polysaccharide-containing extracellular common antigen (ECA) resulted in an increase in OMV production (McMahon et al., 2012). The mechanism by which bacterial surface sugars serve to regulate OMV production and content remains unclear.
OMV production levels are modulated by altering levels of envelope proteins, as well as temperature and quorum sensing (QS) signals, suggesting that OMV formation is a regulated process (Bielig et al., 2011; Mashburn and Whiteley, 2005; McMahon et al., 2012, McBroom and Kuehn, 2007; Tashiro et al., 2009). The changes that occur in the envelope that could serve to affect OMV production in these situations are being investigated and could be different for different bacterial species.
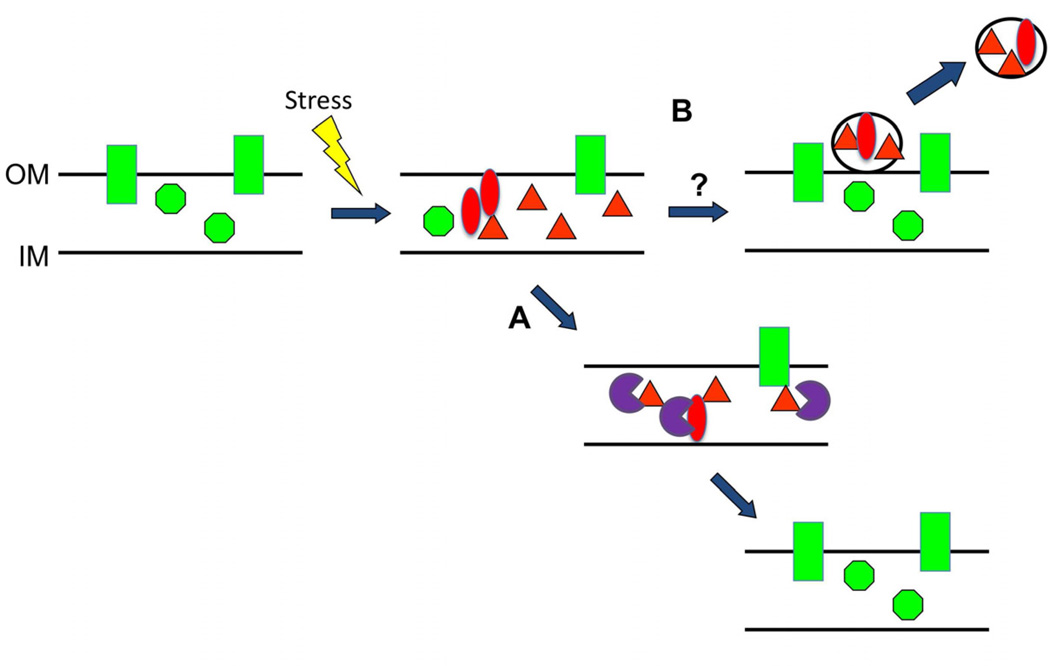
OMV production has been found to serve as an envelope stress response pathway for Gram-negative bacteria. OMV production was first linked to envelope stress when it was discovered that mutations in genes encoding σE envelope stress pathway components exhibited vesiculation phenotypes (McBroom et al., 2006). This pathway functions by sensing misfolded OMPs in the envelope, transmitting this signal to proteases which cause the release of σE into the cytosol, resulting in activation of the σE regulon (Hasselblatt et al., 2007, Kulp and Kuehn, 2011; Sohn et al., 2007; Walsh et al., 2003). When the pathway is altered by mutation, OMV production is increased, likely due to the accumulation of protein in the envelope (McBroom and Kuehn, 2007)(Fig 1). This pathway is also conserved in a divergent bacterial species, P. aeruginosa, where it appears to function similarly (Ramsey and Wozniak, 2005; Schurr et al., 1996; Wood and Ohman, 2009, 2006). Indeed, when the P. aeruginosa regulon mutant mucD was analyzed, it overproduced OMVs similar to the corresponding degP mutant in E. coli and S. typhmimurium, whereas modest levels of expression of the periplasmic chaperone/protease MucD or DegP resulted in decreased OMV production (McBroom and Kuehn, 2007; Tashiro et al., 2009). Further analysis of σE pathway and regulon mutants provided evidence that elevated σE levels are sufficient, but not necessary, for increasing OMV production in E. coli, since elevated levels of non- σE activating proteins also caused increased OMV production (McBroom and Kuehn, 2007). These data demonstrate a conserved link between envelope homeostasis and OMV formation for Gram-negative bacteria.
Fig. 1.
OMV-related mechanism for releasing envelope stress in Gram-negative bacteria. Upon exposure to a stressor, OMPs and periplasmic proteins (green shapes) can become misfolded and accumulate in the envelope (red shapes). (A) Misfolded envelope proteins can activate stress response pathways, inducing the recruitment of proteases and chaperones (purple shapes) that can remove or refold the stress products. (B) Through a yet to be elucidated mechanism, OMV production may also be induced as an alternative to, or as a supplement to, the chaperone/protease pathway, and these OMVs can remove concentrated misfolded envelope protein.
Gram-negative QS signals have also been found to influence OMV production and function. The Pseudomonas QS molecule PQS was discovered to significantly induce OMV production by P. aeruginosa, although it is not required (Mashburn and Whiteley, 2005; Tashiro et al., 2009). The upregulation of OMVs upon PQS exposure was explained by the generation of a repulsive force caused by the interaction between PQS and the bacterial LPS (Mashburn-Warren et al., 2009; Mashburn-Warren et al., 2008; Schertzer and Whiteley, 2012). OMV-mediated innate immune suppression in Vibrio cholerae was also linked to QS signals (Bielig et al., 2011). Although OMV production was unchanged in V. cholerae strains lacking the QS regulator HapR, OMVs lacking this QS regulator resulted in a decreased inflammatory response. This led the authors to speculate whether a mutation of HapR may provide the cell a means of immune evasion. The species-specific impact of QS on the regulation and function of OMVs, in combination with the variety of QS signals produced by Gram-negative bacteria, make this area of the field likely to be fruitful for future study.
Despite the identification of species- and environment-specific mechanisms and phenotypes of mutant strains, no unifying mechanism for OMV formation by Gram-negative bacteria has been identified to date. The presence of both conserved and species-specific contributing mechanisms highlights the complexity that is involved in the regulation and production of OMVs from highly diverse species.
2.3. Gram-positive MVs
Until recently, the study of MVs had largely been limited to Gram-negative bacteria without much inquiry into their production by Gram-positive organisms. Increased investigation of Bacillus anthracis, B. subtilis, B. cereus, Staphylococcus aureus and Mycobacterium ulcerans has resulted in the identification of MV production by Gram-positive species ranging in size from 20–150 nm (Dorward and Garon, 1990; Lee et al., 2009; Rivera et al., 2010). As dictated by the differences in the envelope structure of Gram-positive bacteria, these MVs are entirely distinct in composition from the OMVs of Gram-negative bacteria: Gram positive MVs originate from the cytoplasmic membrane and contain cytosolic proteins. Nevertheless, they appear to function in pathways similar to their OMV counterparts, including virulence, general metabolism, stress response and transport proteins (Lee et al., 2009).
Enrichment and selection of specific factors associated with Gram-positive MVs indicate that a regulatory mechanism for sorting MV cargo also exists in Gram-positive cells. It was noted that for B. anthracis, almost all of the protective antigen (PA), lethal factor (LF) and edema factor (EF) toxins were located in MV instead of free in the supernatant (Rivera et al., 2010). Further, lipid analysis revealed that the MVs derived from B. anthracis were enriched in the minor lipid components myristic and palmitic acids (Rivera et al., 2010). In addition, proteomics experiments indicated that the protein profiles of MVs were distinct from the composition of membrane and soluble subcellular compartments (Lee et al., 2009).
Possible mechanisms for the production of Gram-positive MVs have been proposed, although none have been directly tested or proven. Gram-positive MVs were observed to be produced by Thermoanerobacterium thermosulfurogenes (formerly Clostridium thermosulfurogenes EM1)(Antranikian, 1987). At that time, it was considered that MV production could be a consequence of α-amylase and pullulanase overproduction. However, it was also possible that overproduction of α-amylase and pullulanase could have resulted in cell wall degradation leading to membrane instability and hence the appearance of MV-like material in the supernatant (Antranikian, 1987). The N-acetylmuramoyl-L-alanine amidase of S. aureus MVs may be acting in a similar manner, but on the lipoteichoic acid of the Gram-positive membrane (Lee et al., 2009). This model was later disproven as the authors observed MV production from many Gram-positive species without a correlation between enzyme activity or cell wall instability (Specka et al., 1991).
To account for MV production independent of cell wall degradation, a role for the bacterial cytoskeleton was hypothesized (Mayer and Gottschalk, 2003). It has been demonstrated that the EF-Tu protein functions as an actin-like component of the bacterial cytoskeleton in addition to its role as an elongation factor (Beck et al., 1978). It was proposed that a lack of stability from the loss of properly localized EF-Tu in addition to the upregulated secretion of protein could account for MV formation (Mayer and Gottschalk, 2003). Increased production of secreted or membrane-bound enzymes would force an increase in membrane production to provide the secreted proteins a place in the membrane, resulting in excess membrane surface area and the formation of blebs.
2.4 Archaeal MVs
Archaea are prokaryotic life forms which have become well-adapted at surviving extreme environments such as high salt, low pH, as well as temperature extremes. If membrane secretion by MVs is a conserved stress response as it is hypothesized for Gram-negative OMVs, then it can be assumed that organisms living at the extremes of life would have all the more reason to rely on MV production. Indeed, now extracellular MVs have been observed in the thermophilic crenarchaeote Sulfolobus as well as in strains of Thermococcus (Ellen et al., 2009; Prangishvili et al., 2000; Soler et al., 2008).
Initially confused with virus particles in one report, the structural features of MVs derived from archaea resemble Gram-negative OMVs (Soler et al., 2008). MVs derived from Sulfolobus were spherical structures 90–230 nm in diameter, with MVs derived from Thermoccocus averaging a slightly smaller 50–100 nm diameter (Ellen et al., 2009; Ellen et al., 2010; Soler et al., 2008). A unique structural feature of MVs from Sulfolobus is their coat, which is composed of crystalline surface layer (S-layer) protein (Ellen et al., 2009; Ellen et al., 2010). The presence of S-layer protein confirms that these MVs bud from the cell membrane. Proteomic analysis of archaeal MVs has only been performed recently in strains of S. solfataricus, S. acidocaldarius and S. tokodaii (Ellen et al., 2009). The authors noted that the MVs from all three species contained ESCRT-III, Vps4, von Willebrand factor (vWA), thiosulfate sulfur transferase, flotillin, disulfide oxioreductase and S-layer proteins. It was also noted that the lipid content of MVs from Sulfolobus did not match that of the cell membrane. Lipid analysis determined that MVs were enriched in archaeal tetraether lipids and had reduced amounts of cyclopentane rings compared to the cell membrane. These data indicate that, like Gram-negative and Gram-positive bacteria, MV production by archaea represents a specific secretion event that is highly conserved.
A hypothetical mechanism for archaeal MV formation was based on the proteins identified in archaeal MVs. The proteins thought to be responsible for MV formation in Sulfolobus include ESCRT-III and Vps4. ESCRT-III and Vps4 have homologs in eukaryotes which function in multivesicular body formation (MVB), a process strikingly similar to membrane budding in archaea (Deatherage and Cookson, 2012). In eukaryotes, ESCRT proteins sort proteins into the endosomal transport pathway (Ellen et al., 2010). ESCRT-III interacts with other ESCRT complexes and this leads to inward vesicle budding to form MVBs inside of the endosomal lumen. The ATPase Vps4 releases ESCRT-III subunits from the endosomal membrane in eukaryotes. It is hypothesized that, like their eukaryotic homologs, ESCRT-III and Vps4 of Sulfolobus are working together to cause MV budding from the cell membrane.
3. Microbial MVs on the offensive
3.1. Pathogen-host interactions
Over the course of evolution, pathogenic bacteria have emerged by developing tools to colonize a host and cause infection and disease. Some of these tools serve to mitigate the stressful environment of a host organism and its defense mechanisms. MV production is one of these tools, as MVs can mediate bacterial attachment, biofilm formation, internalization and virulence factor transmission, as well as modulate the immune response.
The presence of MVs during the infectious process has been observed in human patient samples and tissue biopsies. OMVs were observed in the cerebrospinal fluid of an infant diagnosed with Neisseria meningitidis as well as from a case of fatal septic shock caused by N. meningitidis serogroup B as determined by electron microscopy (Namork and Brandtzaeg, 2002; Stephens et al., 1982). Several reports of clinical isolates of Helicobacter pylori show OMVs in contact with host epithelial cells (Fiocca et al., 1999; Keenan et al., 2000). Moraxella catarrhalis was reported to also induce OMV production when in contact with leukocytes (Tan et al., 2007), suggesting that cross-talk between the pathogen and host cells can elicit regulated bacterial vesicle production. Similarly, non-typeable H. influenzae (NTHI) released OMVs during extended tissue co-culture (Ren et al., 2012). Gram-positive, Staphylococcus aureus 06ST1048, was also observed budding from the bacterial cell surface and in the extracellular milieu of mouse lung tissue post infection demonstrating that Gram-positive MVs were present during the course of infection (Gurung et al., 2011).
The specific contribution to virulence of MVs in an infected host is unknown due to the lack of a strain or mutant that cannot produce MVs. However, investigators can speculate how OMVs might contribute to virulence, since the effect of purified OMVs from pathogens can be readily observed on cultured and primary host cells. Using in vitro assays, OMVs were often found to contact host epithelial or immune cells, a finding consistent with observations of clinical and biopsy studies (Fiocca et al., 1999; Keenan et al., 2000; Ren et al., 2012; Sharpe et al., 2011). Because receptor-binding adhesins are associated with the bacterial OM, it is logical that MVs would also contain adhesins. Indeed, specific host cell interaction was observed for OMVs from several bacterial species including E. coli, H. pylori, Shigella, Actinobacillus and Borrelia (Heczko et al., 2000; Horstman and Kuehn, 2002; Kadurugamuwa and Beveridge, 1998; Kesty et al., 2004; Meyer and Fives-Taylor, 1994; Shoberg and Thomas, 1993). OMV adherence has been studied in molecular detail for the heat-labile enterotoxin (LT) which is associated with OMVs produced by enterotoxigenic E. coli (ETEC). LT binds the surface of ETEC OMVs using an LPS binding pocket that is distinct from the host receptor GM1 binding site, and thus LT functions as an attachment bridge between the ETEC OMV the host cell. (Horstman and Kuehn, 2002; Horstman et al., 2004; Mudrak et al., 2009). Similarly, M. catarrhalis OMVs attach directly to host cells by the adhesion and virulence factor UspA1, since UspA1 interacts with the CEACAM-1 adhesion molecule on host cells (Schaar et al., 2011; Slevogt et al., 2008). It was also demonstrated that the adhesins BabA, SabA and VacA mediate H. pylori OMV attachment to epithelial cells (Olofsson et al., 2010). The OMV-associated aminopeptidase of P. aeruginosa has been demonstrated to facilitate binding to A549 lung epithelial cells in tissue culture (Bauman and Kuehn, 2009). However it is unknown whether the P. aeruginosa aminopeptidase mediates attachment directly, or whether the active enzyme cleaves surface antigens on the host cell surface uncovering other ligands for binding.
After attachment to host cells, OMVs can sometimes fuse with the host cell membrane or become internalized by the host cell. Evidence for fusion of OMVs into the host membrane was demonstrated in a few studies and represents a means for soluble, lumenal OMV cargo to be directly deposited into the target cell. Kadurugamuwa et al., demonstrated that OMVs derived from Shigella flexneri were able to entrap and deliver gentamicin into the eukaryotic host cytoplasm (Kadurugamuwa and Beveridge, 1998). OMV-mediated delivery of insoluble molecules into host cells has also been observed. LPS from Salmonella enterica serovar typhimurium was identified within the host cells vacuole compartments, as determined by antibodies directed against the O-antigen (Garcia-del Portillo et al., 1997). Also, OMVs from A. actinomycetemcomitans transferred a lipid tracking dye to the host cell plasma membrane (Demuth et al., 2003). Although evidence for direct OMV fusion is growing, it is unknown how the asymmetric, LPS/phospholipid architecture of the bacterial OMV membrane is able to integrate into a symmetric phospholipid-based eukaryotic membrane without the help of some type of fusion machinery.
Examples are abundant which demonstrate the internalization of intact OMVs. LT-containing OMVs from ETEC were shown to enter epithelial cells via binding to the GM1 ganglioside and lipid raft-mediated endocytosis (Kesty et al., 2004). NTHI OMVs also enter epithelial cells via a caveolae-mediated process (Sharpe et al., 2011). OmpA is the common OMP which interacts with the Ecrp receptor on brain microvascular endothelial cells, and has been linked to host cell entry resulting in neonatal meningitis. Host receptor tropism has been observed to facilitate OMV uptake by particular cell types. For example, N. meningitidis OMV uptake was facilitated by the bactericidal/permeability-increasing protein, but not by the LPS binding protein on dendritic cells (Schultz et al., 2007). Not only do OMVs become internalized inside host cells, but OMVs can facilitate bacterial invasion of host cells. Cytotoxic necrotizing factor 1 (CNF1) was recently shown to be associated with E. coli OMVs and secreted CNF1 leads to host cell invasion (Yu and Kim, 2012).
Finally, it should be considered that the destination of all OMVs may not be merely the outer surface or interior of the host cell, but it may be through the cell, such as the basolateral side of the epithelial barrier. Evidence for this came from studies of Treponema denticola OMVs which can disrupt a cell monolayer using their OMV-associated dentilysin activity, thereby breaking through the barrier of an intact epithelium (Chi et al., 2003).
One of the benefits of MV production to bacteria is their ability to function as a secretion mechanism for complex mixtures of soluble and insoluble factors. Due to the small size, complex composition and protective aspect of MVs, they are capable of facilitating the dissemination of a concentrated cohort of protease-protected virulence factors at a distance. The ability for MVs to act distal to the parent organism is important especially for the establishment of chronic infections and biofilms.
The transport of toxins by MVs is thought to be one of the primary purposes for MVs, but this emphasis may be simply because the toxic activity of MVs has been studied the most. In studies of S. aureus and B. anthracis MVs, these were found to be lethal to host cells (Gurung et al., 2011; Rivera et al., 2010). Even with antibodies directed against the anthrax toxins of B. anthracis, MVs were still lethal to macrophages due to an additional toxin, anthrolysin. For ETEC, 95% of the LT is associated with OMVs and LT is trafficked into host cells by OMV internalization and subsequent trafficking of the active A subunit through the endoplasmic reticulum, resulting in toxicity (Horstman and Kuehn, 2002; Kesty et al., 2004). Not only are OMVs a means for toxin delivery, but they modulate the host response to the toxin. Similar amounts of soluble and OMV-bound toxin stimulated different immune responses by different signaling pathways in polarized intestinal epithelial cells (Chutkan and Kuehn, 2011). Soluble and OMV context-dependent differences were also found for the adenylate cyclase toxin of Bordetella pertussis (Donato et al., 2012).
In some cases, the toxin seemingly relies on the MV compartment as a means to achieve proper folding or antigen presentation. The secreted E. coli toxin, ClyA, was found to require the reducing environment of OMV to oligomerize and become active (Wai et al., 2003). When oligomerized, ClyA forms observable pores in the OMVs. Interestingly, the periplasmic environment of the cell is an oxidizing environment, leaving ClyA inactive until it is secreted. In addition, B. anthracis packages all three components of the tripartite anthrax toxin (protective antigen, lethal factor and edema factor), and it is likely that co-localization in MVs greatly facilitates the interaction of these toxin components (Rivera et al., 2010).
Proteomic analysis of MVs has recently led to the identification of many other components of MVs, including other virulence factors. P. aeruginosa OMVs were previously reported to contain virulence determinants such as phospholipase C, proteases, alkaline phosphatase, an aminopeptidase, murein hydrolases and hemolysins (Kadurugamuwa and Beveridge, 1995; Li et al., 1998). With the advent of proteomics, the list of virulence factors for P. aeruginosa OMVs has increased to include EstA, LasA, IcmP, OprG and OprL (Bauman and Kuehn, 2006; Choi et al., 2011). EstA is an esterase which was reported to induce nitric oxide and cytokines in macrophages (Kang et al., 2009). LasA is a protease commonly associated with invasive P. aeruginosa and chronic cystic fibrosis (Storey et al., 1998). IcmP was demonstrated as a metalloprotease capable of cleaving fibrinogen (Fricke et al., 1999). OprG and OprL are both OMPs which aid in toxicity and generating an immune response, respectively (Gatypova et al., 2009; McPhee et al., 2009). _S. aureu_s MVs are now known to contain enzymes which function during the course of infection against the host (Lee et al., 2009). The alpha and gamma hemolysin were discovered in MVs, and these MVs produce pores in eukaryotic cells (Bhakdi and Tranum-Jensen, 1991; Choi et al., 2011).
A response from host cells is often mediated through contact, and thus it was not surprising that OMVs, which have been observed to contain both immunomodulatory determinants and adhesins, generate host cell responses. One of the primary constituents of OMVs is LPS. LPS is recognized by the TLR4 ligand on host cells and leads to an inflammatory response. Porins, another abundant component of OMVs, are also important stimulators of proinflammatory cytokines, as observed in H. pylori, Salmonella, Fusobacterium and Yersinia species (Galdiero et al., 1993; Galdiero et al., 1999; Takada et al., 1988, Tufano et al., 1994a; Tufano et al., 1994b). The host cell cytokine response to OMV challenge has been well-studied for many species including Klebsiella pneumoniae, S. typhimurium, P. aeruginosa, H. pylori, ETEC and N. meningitides, and commonly include upregulation of proinflammatory cytokines IL-8, IL-6 and IL-1β (Bauman and Kuehn, 2006; Chutkan and Kuehn, 2011; Durand et al., 2009; Ellis and Kuehn, 2010; Ellis et al., 2010; Hong et al., 2009; Lapinet et al., 2000; Lee et al., 2012). It is important to note that the cytokine profiles for OMV challenges do not directly mimic that for whole cells or purified antigens due to the distinct protein profiles of OMVs as well as synergistic effects of OMV components (Chutkan and Kuehn, 2011; Ellis et al., 2010).
3.2 Intermicrobial interactions
Establishing an infection in a host is not solely focused on the interaction between the microbe and the host. Interactions between competing commensal organisms and the invading pathogenic species are also important for the pathogen to survive and establish a niche in the host.
Just as MVs can disseminate virulence factors to host cells to facilitate virulence, likewise, MVs can facilitate aggressive microbial attacks that benefit the parent microbe. The predatory nature of OMVs against other bacteria was demonstrated by a report that OMVs derived from P. aeruginosa have the ability to kill other Gram-negative as well as Gram-positive bacterial species (Kadurugamuwa and Beveridge, 1999, 1996; Kadurugamuwa et al., 1998; Li et al., 1998). The mechanism of killing was shown to be by vesicle fusion or attachment leading to the delivery of murein hydrolases capable of degrading the murein sacculi of other, but not parental, species. A similar predatory nature of MVs has been identified from the archaea Sulfolobus islandicus (Prangishvili et al., 2000). S. islandicus produces a proteinaceous toxin referred to as sulfolobicin, which has the ability to kill specific species of Sulfolobus including S. solfataricus P1 and S. shibatae B12. The mechanism of killing was determined to be similar to that of other toxic bacteriocin-like proteins, although sulfolobicin was only found associated with MVs instead of being solubly secreted into the supernatant. The Gram-positive organism S. aureus produces MVs which also have an offensive function. The N-acteylmuramoyl-L-alanine amidase-containing MVs from S. aureus are capable of killing neighboring cells by degrading their peptidoglycan, resulting in membrane disruption (Lee et al., 2009).
The predatory activity of MVs may serve to clear out commensal flora so that the parent bacteria can colonize a particular host site; however, it should also be considered that MV-mediated lysis of other microbes in the immediate environment of a pathogen can be a source for nutrition and novel genetic material for the pathogen, as well as an indirect source of inflammation of the infected host (Rice and Bayles, 2008). However, these roles for MVs during host infection remain unconfirmed for the same reason that their role in virulence remains speculative: There is not yet a means to test the role of OMVs supplied in trans on the colonization or infection by a non-OMV producing mutant.
4. Microbial MVs as a defense mechanism
4.1. Introduction
The secretion of MVs as a mechanism for disseminating damaging virulence factors is a relatively obvious tool that can promote a bacterial infection; however, sometimes the best offense is a strong defense. Downregulating and diverting the host innate immune system are means by which MVs can allow pathogens time to establish an infection in a host. It has also been considered that non-pathogenic species also produce MVs, and so MV production may also deflect chemical and biological environmental insults and thereby benefit microbial survival.
4.2. Decoy activity
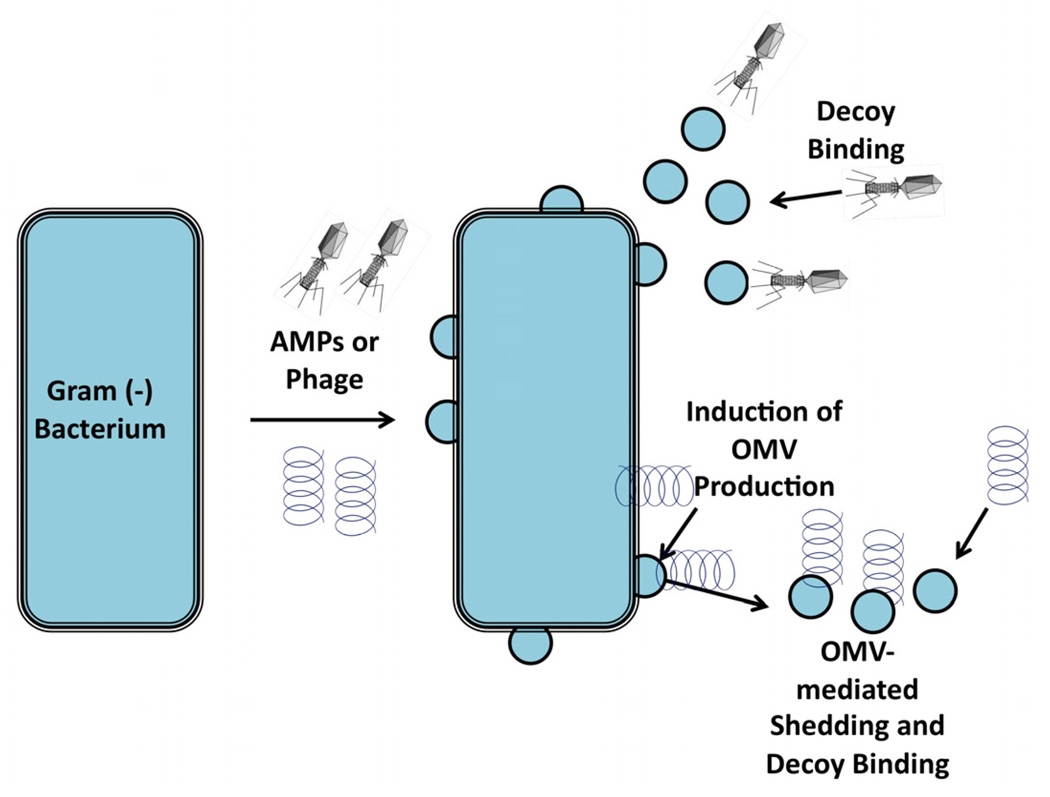
Cell wall-lysing compounds and membrane-disrupting cationic antimicrobial peptides (AMPs) are common man-made and natural mediators of microbial cell death. Not only do microbes encounter synthetic antibiotic compounds during clinical treatment, but they are also attacked by natural antimicrobials produced by bacteria and host epithelial and innate immune cells. MVs naturally mimic the outside of a microbe, and as such, released MVs can function as decoys, absorbing antimicrobial compounds (Fig 2). A recent report supports this decoy hypothesis by demonstrating that stressors directed at disrupting the cell wall were absorbed by the exogenous addition of OMVs, and OMV-hyperproducing mutants of E. coli survived treatment with AMPs better than wild type (Manning and Kuehn, 2011). These data demonstrate that producing large amounts of OMVs can convey a selective advantage and may provide insight into why clinical isolates often produce more MVs than laboratory adapted strains.
Fig. 2.
MVs as a defensive “decoy” mechanism. MVs secreted in response to cell-wall-directed challenge such as AMPs and bacteriophage can absorb these lethal antimicrobials. Bacteria can respond to cell wall perturbations by increasing MV production, thereby helping remove the stressor from the bacterial environment.
Non-pathogenic bacterial species are also subjected to MV-mediated protection against a completely different class of antimicrobials, bacteriophage. Bacteria are outnumbered by bacteriophage and thus are bound to encounter these potentially lethal viruses during their lifespan. As mimics of the microbial surface, OMVs theoretically should have a capacity to absorb free phage in the culture. Indeed, not only were _E coli_-specific T4 phage found to bind to E. coli OMVs, but this occurred in an irreversible manner, indicating that the phage has attached and injected its viral DNA into the OMV (Manning and Kuehn, 2011). Attachment to an OMV, instead of a bacterial cell, removes that virus from possible further infection and spread (Fig. 2). As with defense against cell wall-directed AMPs, strains which produced more OMVs displayed higher resistance when infected with bacteriophage.
4.3 Releasing internal stress
During the course of infection, bacteria face many other stressors that are not direct cell wall attacks to be mitigated by the production of decoys. Several of these more general stressors, including temperature changes and oxidative stress have also been linked to an increase in MV production. As discussed above, MV production is elevated in response to envelope stress. OMV production by E. coli increased upon the increase of protein misfolding stress caused by ethanol and elevated temperature (McBroom and Kuehn, 2007)(Fig. 1). Sabra et al., observed an increase in OMV production for P. aeruginosa treated with increasing oxygen saturation (Sabra et al., 2003). Further, we have observed an increase in OMV production upon addition of sublethal hydrogen peroxide (MacDonald and Kuehn, manuscript in preparation). Free radicals, like those from oxidative stressors, are known to cause DNA damage as well as possible direct oxidation of proteins and lipids. Induction of the SOS DNA damage response by ciprofloxacin was confirmed to increase OMV production, and the DNA damaging compound mitomycin C also resulted in an increase in OMV release (Maredia et al., 2012)(unpublished data). Despite all of these observations, the mechanism for how stress results in an increase in OMV production remains unknown.
The production of OMVs in response to environmental and chemical stressors that cause internal damage has been proposed to contribute to bacterial survival by enabling the elimination of potentially toxic damaged protein (McBroom and Kuehn, 2007; Tashiro et al., 2009) (Fig1). This idea was tested and mutants which hyperproduce OMVs did survive conditions generating misfolded envelope protein significantly better than strains with lower vesicle production phenotypes (McBroom and Kuehn, 2007). Current studies indicate that the inability to increase OMV production in response to stress results in accumulated misfolded proteins and leads to cell death (Schwechheimer and Kuehn, manuscript in preparation).
4.4. Suppression of host response
An uninhibited immune response can lead to septic shock and death of the host organism which is obviously not beneficial for the colonizing bacteria. As an apparent response to the potentially harmful but unavoidable consequence of a pathogen stimulating the immune system, certain bacterial strains have incorporated proteins into their MVs which facilitate immune suppression. Examples of MV production that mediate immune suppression or immune evasion can be found. The UspA1/A2 virulence factor found in M. catarrhalis OMVs was observed to bind and inactivate complement protein C3 in addition to its role in aiding attachment (Nordstrom et al., 2004; Nordstrom et al., 2005; Singh et al., 2010; Tan et al., 2007). Inactivation of complement causes the loss of one arm of the innate immune system. Like M. catarrhalis, Neisseria OMVs have been shown to remove bactericidal factors found in serum (Pettit and Judd, 1992). P. gingivalis causes CD14 degradation on O937 human macrophages by incorporating gingipain proteases in their OMVs (Duncan et al., 2004). Also, P. gingivalis OMVs induce the expression of ICAM-1 and E-selectin which function in inhibiting the synthesis of interferon γ MHC class II on vascular endothelial cells (Srisatjaluk et al., 1999; Srisatjaluk et al., 2002). S. aureus MVs contain the IgG-binding protein SbI which interacts with host IgG, as well as binding and inactivating complement C3 (Burman et al., 2008; Lee et al., 2009). Thus for certain pathogens, MV production may have evolved to benefit virulence by exerting immune suppressing effects.
4.5. Contribution to polymicrobial communities
An explosion of recent studies has highlighted the benefits of community living for microbes that can organize into biofilms. The components and importance of the extracellular matrix which facilitate biofilm formation have been elucidated, and MVs represent an important piece of that matrix (Beveridge et al., 1997; Mashburn-Warren and Whiteley, 2006; Schooling and Beveridge, 2006; Whitchurch et al., 2002; Yonezawa et al., 2009). Although the exact contribution of MVs to biofilm formation and maintenance remains unclear, it is thought that the charged nature of MVs facilitates interaction with extracellular DNA, that extracellular DNA acts as a scaffold for additional extracellular polysaccharides and other compounds and that these interactions result in a robust biofilm (Kulp and Kuehn, 2010; Schooling and Beveridge, 2006; Whitchurch et al., 2002). MV-associated extracellular DNA has also been observed associated with the archaea Thermococcus (Soler et al., 2008). These data indicate that maintaining a robust biofilm is another function of MVs that is potentially conserved across diverse forms of life.
Biofilms are known for their robustness against treatment and for conveying increased levels of resistance to a wide variety of antibiotics as well as antimicrobial peptides. Evidence is mounting that, in addition to being a structural component of the biofilm matrix, MVs can facilitate the horizontal transfer of antibiotic resistance between bacteria (Ciofu et al., 2000; Mashburn-Warren and Whiteley, 2006). β-lactamase has been observed in S. aureus MVs, leading the authors to speculate that Gram-positive cells can employ a MV delivery mechanism to provide transient resistance to neighboring cells (Lee et al., 2009). Similar results were obtained for OMVs from M. catarrhalis which carry β-lactamase and promote survival of Streptococcus pneumoniae and H. influenzae in the presence of the antibiotic amoxicillin (Schaar et al., 2011). Not only can MVs transiently transfer resistance proteins to neighboring cells, but MVs can also facilitate permanent lateral gene transfer and increase long-term drug resistance in a population. It has been demonstrated that resistance to antibiotics can spread from a resistant donor cell to a sensitive recipient cell via the fusion of OMVs and the transfer of DNA for Acinetobacter baumannii, E. coli and N. gonorrhoeae (Dorward et al., 1989; Rumbo et al., 2011; Yaron et al., 2000). Extracellular DNA is also associated with MVs derived from the archaeal species Thermococcus, and this DNA is heat-stable and DNase-resistant (Soler et al., 2008). Although it remains to be experimentally determined, it was hypothesized that archaeal MVs could stabilize DNA against the extreme heat of hydrothermal vents and allow for horizontal gene transfer between cells, even in these denaturing environments.
5. Summary and future directions
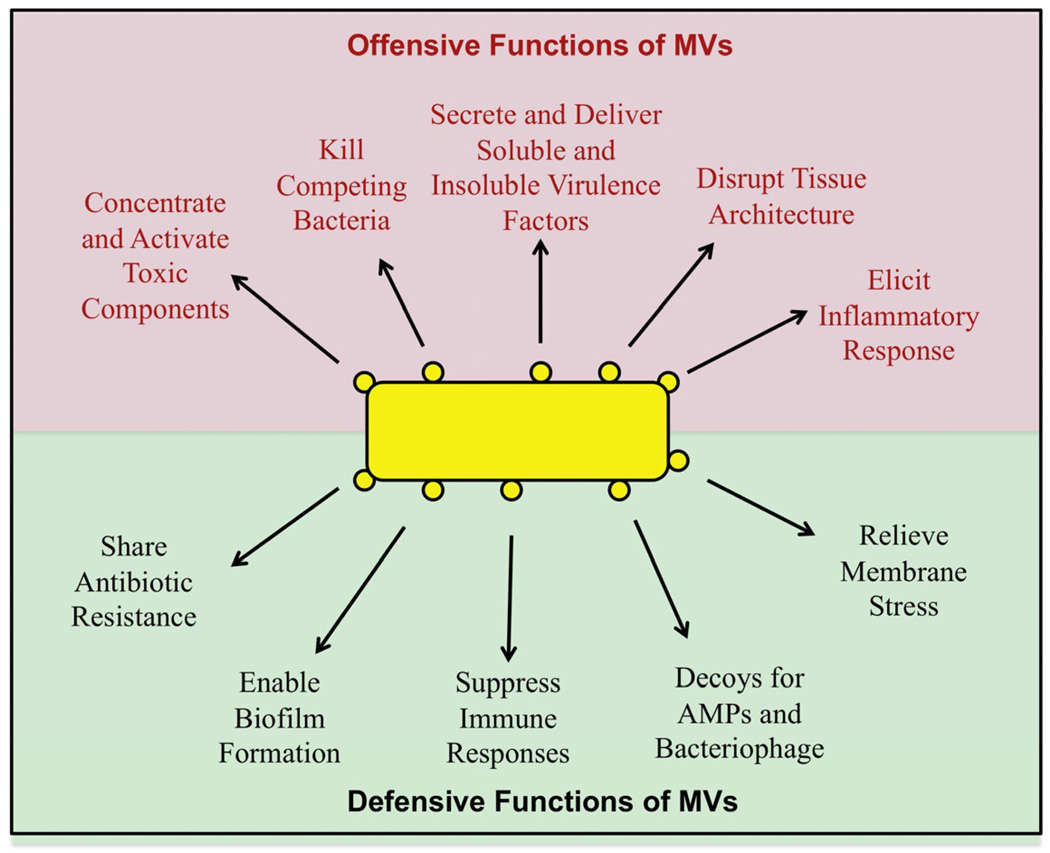
Gram-negative, Gram-positive and archaeal species appear to have evolved MVs as tools which aid in their ability to survive and thrive in diverse environments. MVs can function in both harmful and beneficial ways, by mediating interactions towards either prokaryotes, environmental factors or eukaryotes (Fig 3). Many of the general mechanisms of offensive and defensive action are well conserved amongst bacterial and archaeal species, demonstrating how MV production continues to serve as a common solution to diverse issues despite evolutionary change.
Fig. 3.
A summary diagram of the conserved offensive and defensive functions of MVs produced by Gram-negative bacteria, Gram-positive bacteria and archaea.
In spite of our increasing knowledge of bacterial MV production and their contribution to cell-cell and cell-host interaction, a great deal of research remains to be done in the newly emergent field of MV production in Gram-positive and archaeal species. Our knowledge of Gram-negative OMV production is by no means complete either. Major questions still to be addressed include identifying what is upregulated in the cell under OMV-inducing conditions, how MV cargo is selected, what contribution specific cargo plays in virulence and whether OMV production can be modified to decrease their antitherapeutic effects.
Our knowledge of MVs is not limited only to bacteria, but may have broader implications for understanding how eukaryotes have evolved to form and utilize extracellular vesicles. Higher order vesicle budding has been observed in eukaryotic cells as the direct budding of the plasma membrane (microvesicles) or as exosomes released from multivesicular body fusion with the plasma membrane. As stated above, archaeal MV biogenesis is likely mediated by ESCRT III and Vps4 proteins. Eukaryotic multivesicular body formation uses homologous proteins to pinch off the intralumenal vesicles. The discussion of how microvesicles are formed at the plasma membrane may be informed by similarities to the mechanism of Gram-positive MV formation.
Not only are the mechanisms of MV formation conserved between prokaryotes and eukaryotes, but there is substantial overlap in function. Like prokaryotic MVs, eukaryotic exosomes or microvesicles were demonstrated to function in immune suppression and activation, as a way to transfer genetic material, send signaling molecules at a distance and function as a means to remove stress caused by waste accumulation (van der Pol et al., 2012). Although much more work remains to be done in the field of bacterial MV biogenesis and function, it is impressive to see how strategies for MV formation remain conserved as life has evolved.
Acknowledgements
The authors acknowledge support from NIH grants R01-AI64464, R01-GM098642 and R01-GM099471 and Katherine Bonnington for contributing to Fig. 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ian A. MacDonald, Email: Ian.macdonald@duke.edu.
Meta J. Kuehn, Email: Meta.kuehn@duke.edu.
References
- Antranikian G, Herzberg C, Mayer F, Gottschalk G. Changes in the cell envelope structure of Clostridium sp. strain EM1 during massive production of α-amylase and pullulanase. FEMS Microbiol. Lett. 1987;41:193–197. [Google Scholar]
- Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes Infect. 2006;8:2400–2408. doi: 10.1016/j.micinf.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman SJ, Kuehn MJ. Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol. 2009;9:26. doi: 10.1186/1471-2180-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck BD, Arscott PG, Jacobson A. Novel properties of bacterial elongation factor Tu. Proc. Natl. Acad. Sci. U.S.A. 1978;75:1250–1254. doi: 10.1073/pnas.75.3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadac A, Gavioli M, Lazzaroni JC, Raina S, Lloubes R. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 1998;180:4872–4878. doi: 10.1128/jb.180.18.4872-4878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Makin SA, Kadurugamuwa JL, Li Z. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 1997;20:291–303. doi: 10.1111/j.1574-6976.1997.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielig H, Rompikuntal PK, Dongre M, Zurek B, Lindmark B, Ramstedt M, Wai SN, Kufer TA. NOD-like receptor activation by outer membrane vesicles from Vibrio cholerae non-O1 non-O139 strains is modulated by the quorum-sensing regulator HapR. Infect. Immun. 2011;79:1418–1427. doi: 10.1128/IAI.00754-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell DC, Cota-Robles EH. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J. Bacteriol. 1967;93:427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman JD, Leung E, Atkins KL, O'Seaghdha MN, Lango L, Bernado P, Bagby S, Svergun DI, et al. Interaction of human complement with Sbi, a staphylococcal immunoglobulin-binding protein: indications of a novel mechanism of complement evasion by Staphylococcus aureus. J. Biol. Chem. 2008;283:17579–17593. doi: 10.1074/jbc.M800265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi B, Qi M, Kuramitsu HK. Role of dentilisin in Treponema denticola epithelial cell layer penetration. Res. Microbiol. 2003;154:637–643. doi: 10.1016/j.resmic.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Choi DS, Kim DK, Choi SJ, Lee J, Choi JP, Rho S, Park SH, Kim YK, et al. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics. 2011;11:3424–3429. doi: 10.1002/pmic.201000212. [DOI] [PubMed] [Google Scholar]
- Chutkan H, Kuehn MJ. Context-dependent activation kinetics elicited by soluble versus outer membrane vesicle-associated heat-labile enterotoxin. Infect. Immun. 2011;79:3760–3769. doi: 10.1128/IAI.05336-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofu O, Beveridge TJ, Kadurugamuwa J, Walther-Rasmussen J, Hoiby N. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2000;45:9–13. doi: 10.1093/jac/45.1.9. [DOI] [PubMed] [Google Scholar]
- Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012;80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatherage BL, Lara JC, Bergsbaken T, Rassoulian Barrett SL, Lara S, Cookson BT. Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 2009;72:1395–1407. doi: 10.1111/j.1365-2958.2009.06731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth DR, James D, Kowashi Y, Kato S. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell Microbiol. 2003;5:111–121. doi: 10.1046/j.1462-5822.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- Donato GM, Goldsmith CS, Paddock CD, Eby JC, Gray MC, Hewlett EL. Delivery of Bordetella pertussis adenylate cyclase toxin to target cells via outer membrane vesicles. FEBS Lett. 2012;586:459–465. doi: 10.1016/j.febslet.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward DW, Garon CF. DNA Is Packaged within Membrane-Derived Vesicles of Gram-Negative but Not Gram-Positive Bacteria. Appl. Environ. Microbiol. 1990;56:1960–1962. doi: 10.1128/aem.56.6.1960-1962.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward DW, Garon CF, Judd RC. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J. Bacteriol. 1989;171:2499–2505. doi: 10.1128/jb.171.5.2499-2505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L, Yoshioka M, Chandad F, Grenier D. Loss of lipopolysaccharide receptor CD14 from the surface of human macrophage-like cells mediated by Porphyromonas gingivalis outer membrane vesicles. Microb Pathog. 2004;36:319–325. doi: 10.1016/j.micpath.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Durand V, Mackenzie J, de Leon J, Mesa C, Quesniaux V, Montoya M, Le Bon A, Wong SY. Role of lipopolysaccharide in the induction of type I interferon-dependent cross-priming and IL-10 production in mice by meningococcal outer membrane vesicles. Vaccine. 2009;27:1912–1922. doi: 10.1016/j.vaccine.2009.01.109. [DOI] [PubMed] [Google Scholar]
- Ellen AF, Albers SV, Huibers W, Pitcher A, Hobel CF, Schwarz H, Folea M, Schouten S, et al. Proteomic analysis of secreted membrane vesicles of archaeal Sulfolobus species reveals the presence of endosome sorting complex components. Extremophiles. 2009;13:67–79. doi: 10.1007/s00792-008-0199-x. [DOI] [PubMed] [Google Scholar]
- Ellen AF, Zolghadr B, Driessen AM, Albers SV. Shaping the archaeal cell envelope. Archaea. 2010;2010:608243. doi: 10.1155/2010/608243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TN, Leiman SA, Kuehn MJ. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect Immun. 2010;78:3822–3831. doi: 10.1128/IAI.00433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Garaguso I, Adu-Bobie J, Doro F, Taddei AR, Biolchi A, Brunelli B, Giuliani MM, et al. Outer membrane vesicles from group B Neisseria meningitidis delta gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics. 2006;6:1856–1866. doi: 10.1002/pmic.200500164. [DOI] [PubMed] [Google Scholar]
- Fiocca R, Necchi V, Sommi P, Ricci V, Telford J, Cover TL, Solcia E. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J Pathol. 1999;188:220–226. doi: 10.1002/(SICI)1096-9896(199906)188:2<220::AID-PATH307>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Fricke B, Parchmann O, Kruse K, Rucknagel P, Schierhorn A, Menge S. Characterization and purification of an outer membrane metalloproteinase from Pseudomonas aeruginosa with fibrinogenolytic activity. Biochim Biophys Acta. 1999;1454:236–250. doi: 10.1016/s0925-4439(99)00040-x. [DOI] [PubMed] [Google Scholar]
- Galdiero F, de L'ero GC, Benedetto N, Galdiero M, Tufano MA. Release of cytokines induced by Salmonella typhimurium porins. Infect Immun. 1993;61:155–161. doi: 10.1128/iai.61.1.155-161.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero M, Folgore A, Molitierno M, Greco R. Porins and lipopolysaccharide (LPS) from Salmonella typhimurium induce leucocyte transmigration through human endothelial cells in vitro. Clin Exp Immunol. 1999;116:453–461. doi: 10.1046/j.1365-2249.1999.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-del Portillo F, Stein MA, Finlay BB. Release of lipopolysaccharide from intracellular compartments containing Salmonella typhimurium to vesicles of the host epithelial cell. Infect Immun. 1997;65:24–34. doi: 10.1128/iai.65.1.24-34.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatypova EV, Zlygostev SA, Kaloshin AA, Mikhailova NA. [Immunobiological properties of recombinant L peptide from the Pseudomonas aeruginosa outer membrane] Vestn Ross Akad Med Nauk. 2009:25–28. [PubMed] [Google Scholar]
- Gurung M, Moon DC, Choi CW, Lee JH, Bae YC, Kim J, Lee YC, Seol SY, et al. Staphylococcus aureus produces membrane-derived vesicles that induce host cell death. PLoS One. 2011;6:e27958. doi: 10.1371/journal.pone.0027958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselblatt H, Kurzbauer R, Wilken C, Krojer T, Sawa J, Kurt J, Kirk R, Hasenbein S, et al. Regulation of the sigmaE stress response by DegS: how the PDZ domain keeps the protease inactive in the resting state and allows integration of different OMP-derived stress signals upon folding stress. Genes Dev. 2007;21:2659–2670. doi: 10.1101/gad.445307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurat MF, Aduse-Opoku J, Rangarajan M, Dorobantu L, Gray MR, Curtis MA, Feldman MF. Selective sorting of cargo proteins into bacterial membrane vesicles. J Biol Chem. 2011;286:1269–1276. doi: 10.1074/jbc.M110.185744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heczko U, Smith VC, Mark Meloche R, Buchan AM, Finlay BB. Characteristics of Helicobacter pylori attachment to human primary antral epithelial cells. Microbes Infect. 2000;2:1669–1676. doi: 10.1016/s1286-4579(00)01322-8. [DOI] [PubMed] [Google Scholar]
- Hong GE, Kim DG, Park EM, Nam BH, Kim YO, Kong IS. Identification of Vibrio anguillarum outer membrane vesicles related to immunostimulation in the Japanese flounder, Paralichthys olivaceus. Biosci Biotechnol Biochem. 2009;73:437–439. doi: 10.1271/bbb.80580. [DOI] [PubMed] [Google Scholar]
- Horstman AL, Bauman SJ, Kuehn MJ. Lipopolysaccharide 3-deoxy-D-manno-octulosonic acid (Kdo) core determines bacterial association of secreted toxins. J Biol Chem. 2004;279:8070–8075. doi: 10.1074/jbc.M308633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J Biol Chem. 2000;275:12489–12496. doi: 10.1074/jbc.275.17.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstman AL, Kuehn MJ. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J Biol Chem. 2002;277:32538–32545. doi: 10.1074/jbc.M203740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Beveridge TJ. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol. 1996;178:2767–2774. doi: 10.1128/jb.178.10.2767-2774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Beveridge TJ. Delivery of the non-membrane-permeative antibiotic gentamicin into mammalian cells by using Shigella flexneri membrane vesicles. Antimicrob Agents Chemother. 1998;42:1476–1483. doi: 10.1128/aac.42.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Beveridge TJ. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other gram-negative bacteria. Microbiology. 1999;145(Pt 8):2051–2060. doi: 10.1099/13500872-145-8-2051. [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Lam JS, Beveridge TJ. Interaction of gentamicin with the A band and B band lipopolysaccharides of Pseudomonas aeruginosa and its possible lethal effect. Antimicrob Agents Chemother. 1993;37:715–721. doi: 10.1128/aac.37.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Mayer A, Messner P, Sara M, Sleytr UB, Beveridge TJ. S-layered Aneurinibacillus and Bacillus spp. are susceptible to the lytic action of Pseudomonas aeruginosa membrane vesicles. J Bacteriol. 1998;180:2306–2311. doi: 10.1128/jb.180.9.2306-2311.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EH, Gebru E, Kim MH, Cheng H, Park SC. EstA protein, a novel virulence factor of Streptococcus pneumoniae, induces nitric oxide and pro-inflammatory cytokine production in RAW 264.7 macrophages through NF-kappaB/MAPK. Microb Pathog. 2009;47:196–201. doi: 10.1016/j.micpath.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Keenan J, Day T, Neal S, Cook B, Perez-Perez G, Allardyce R, Bagshaw P. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol Lett. 2000;182:259–264. doi: 10.1111/j.1574-6968.2000.tb08905.x. [DOI] [PubMed] [Google Scholar]
- Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004;23:4538–4549. doi: 10.1038/sj.emboj.7600471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox KW, Vesk M, Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966;92:1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp A, Kuehn MJ. Recognition of beta-strand motifs by RseB is required for sigma(E) activity in Escherichia coli. J Bacteriol. 2011;193:6179–6186. doi: 10.1128/JB.05657-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapinet JA, Scapini P, Calzetti F, Perez O, Cassatella MA. Gene expression and production of tumor necrosis factor alpha, interleukin-1beta (IL-1beta), IL-8, macrophage inflammatory protein 1alpha (MIP-1alpha), MIP-1beta, and gamma interferon-inducible protein 10 by human neutrophils stimulated with group B meningococcal outer membrane vesicles. Infect Immun. 2000;68:6917–6923. doi: 10.1128/iai.68.12.6917-6923.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ, Park KS, Lee JO, et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics. 2007;7:3143–3153. doi: 10.1002/pmic.200700196. [DOI] [PubMed] [Google Scholar]
- Lee EY, Choi DS, Kim KP, Gho YS. Proteomics in gram-negative bacterial outer membrane vesicles. Mass Spectrom Rev. 2008;27:535–555. doi: 10.1002/mas.20175. [DOI] [PubMed] [Google Scholar]
- Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- Lee JC, Lee EJ, Lee JH, Jun SH, Choi CW, Kim SI, Kang SS, Hyun S. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol Lett. 2012;331:17–24. doi: 10.1111/j.1574-6968.2012.02549.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Clarke AJ, Beveridge TJ. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J Bacteriol. 1998;180:5478–5483. doi: 10.1128/jb.180.20.5478-5483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011;11:258. doi: 10.1186/1471-2180-11-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maredia R, Devineni N, Lentz P, Dallo SF, Yu J, Guentzel N, Chambers J, Arulanandam B, et al. Vesiculation from Pseudomonas aeruginosa under SOS. ScientificWorldJournal. 2012;2012:402919. doi: 10.1100/2012/402919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn-Warren L, Howe J, Brandenburg K, Whiteley M. Structural requirements of the Pseudomonas quinolone signal for membrane vesicle stimulation. J Bacteriol. 2009;191:3411–3414. doi: 10.1128/JB.00052-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn-Warren L, Howe J, Garidel P, Richter W, Steiniger F, Roessle M, Brandenburg K, Whiteley M. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol Microbiol. 2008;69:491–502. doi: 10.1111/j.1365-2958.2008.06302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn-Warren LM, Whiteley M. Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol. 2006;61:839–846. doi: 10.1111/j.1365-2958.2006.05272.x. [DOI] [PubMed] [Google Scholar]
- Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- Mayer F, Gottschalk G. The bacterial cytoskeleton and its putative role in membrane vesicle formation observed in a Gram-positive bacterium producing starch-degrading enzymes. J Mol Microbiol Biotechnol. 2003;6:127–132. doi: 10.1159/000077243. [DOI] [PubMed] [Google Scholar]
- McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol. 2006;188:5385–5392. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63:545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon KJ, Castelli ME, Garcia Vescovi E, Feldman MF. Biogenesis of outer membrane vesicles in Serratia marcescens is thermoregulated and can be induced by activation of the Rcs phosphorelay system. J Bacteriol. 2012;194:3241–3249. doi: 10.1128/JB.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee JB, Tamber S, Bains M, Maier E, Gellatly S, Lo A, Benz R, Hancock RE. The major outer membrane protein OprG of Pseudomonas aeruginosa contributes to cytotoxicity and forms an anaerobically regulated, cation-selective channel. FEMS Microbiol Lett. 2009;296:241–247. doi: 10.1111/j.1574-6968.2009.01651.x. [DOI] [PubMed] [Google Scholar]
- Meyer DH, Fives-Taylor PM. Characteristics of adherence of Actinobacillus actinomycetemcomitans to epithelial cells. Infect Immun. 1994;62:928–935. doi: 10.1128/iai.62.3.928-935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DC, Choi CH, Lee JH, Choi CW, Kim HY, Park JS, Kim SI, Lee JC. Acinetobacter baumannii outer membrane protein A modulates the biogenesis of outer membrane vesicles. J Microbiol. 2012;50:155–160. doi: 10.1007/s12275-012-1589-4. [DOI] [PubMed] [Google Scholar]
- Mudrak B, Rodriguez DL, Kuehn MJ. Residues of heat-labile enterotoxin involved in bacterial cell surface binding. J Bacteriol. 2009;191:2917–2925. doi: 10.1128/JB.01622-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mug-Opstelten D, Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim. Biophys. Acta. 1978;508:287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- Namork E, Brandtzaeg P. Fatal meningococcal septicaemia with "blebbing" meningococcus. Lancet. 2002;360:1741. doi: 10.1016/S0140-6736(02)11721-1. [DOI] [PubMed] [Google Scholar]
- Nordstrom T, Blom AM, Forsgren A, Riesbeck K. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2. J. Immunol. 2004;173:4598–4606. doi: 10.4049/jimmunol.173.7.4598. [DOI] [PubMed] [Google Scholar]
- Nordstrom T, Blom AM, Tan TT, Forsgren A, Riesbeck K. Ionic binding of C3 to the human pathogen Moraxella catarrhalis is a unique mechanism for combating innate immunity. J. Immunol. 2005;175:3628–3636. doi: 10.4049/jimmunol.175.6.3628. [DOI] [PubMed] [Google Scholar]
- Olofsson A, Vallstrom A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, Haas R, Backert S, et al. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol. Microbiol. 2010;77:1539–1555. doi: 10.1111/j.1365-2958.2010.07307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit RK, Judd RC. The interaction of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae with normal human serum. Mol. Microbiol. 1992;6:729–734. doi: 10.1111/j.1365-2958.1992.tb01522.x. [DOI] [PubMed] [Google Scholar]
- Prangishvili D, Holz I, Stieger E, Nickell S, Kristjansson JK, Zillig W. Sulfolobicins, specific proteinaceous toxins produced by strains of the extremely thermophilic archaeal genus Sulfolobus. J. Bacteriol. 2000;182:2985–2988. doi: 10.1128/jb.182.10.2985-2988.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey DM, Wozniak DJ. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 2005;56:309–322. doi: 10.1111/j.1365-2958.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Ren D, Nelson KL, Uchakin PN, Smith AL, Gu XX, Daines DA. Characterization of extended co-culture of non-typeable Haemophilus influenzae with primary human respiratory tissues. Exp. Biol. Med. 2012;237:540–547. doi: 10.1258/ebm.2012.011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KC, Bayles KW. Molecular control of bacterial death and lysis. Microbiol. Mol. Biol. Rev. 2008;72:85–109. doi: 10.1128/MMBR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19002–19007. doi: 10.1073/pnas.1008843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbo C, Fernandez-Moreira E, Merino M, Poza M, Mendez JA, Soares NC, Mosquera A, Chaves F, et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011;55:3084–3090. doi: 10.1128/AAC.00929-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabra W, Lunsdorf H, Zeng AP. Alterations in the formation of lipopolysaccharide and membrane vesicles on the surface of Pseudomonas aeruginosa PAO1 under oxygen stress conditions. Microbiology. 2003;149:2789–2795. doi: 10.1099/mic.0.26443-0. [DOI] [PubMed] [Google Scholar]
- Schaar V, de Vries SP, Perez Vidakovics ML, Bootsma HJ, Larsson L, Hermans PW, Bjartell A, Morgelin M, et al. Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell Microbiol. 2011;13:432–449. doi: 10.1111/j.1462-5822.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- Schaar V, Nordstrom T, Morgelin M, Riesbeck K. Moraxella catarrhalis outer membrane vesicles carry beta-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob. Agents Chemother. 2011;55:3845–3853. doi: 10.1128/AAC.01772-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertzer JW, Whiteley M. A bilayer-couple model of bacterial outer membrane vesicle biogenesis. MBio. 2012;3:e00297–e00311. doi: 10.1128/mBio.00297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 2006;188:5945–5957. doi: 10.1128/JB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooling SR, Hubley A, Beveridge TJ. Interactions of DNA with biofilm-derived membrane vesicles. J. Bacteriol. 2009;191:4097–4102. doi: 10.1128/JB.00717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz H, Hume J, de Zhang S, Gioannini TL, Weiss JP. A novel role for the bactericidal/permeability increasing protein in interactions of gram-negative bacterial outer membrane blebs with dendritic cells. J. Immunol. 2007;179:2477–2484. doi: 10.4049/jimmunol.179.4.2477. [DOI] [PubMed] [Google Scholar]
- Schurr MJ, Yu H, Martinez-Salazar JM, Boucher JC, Deretic V. Control of AlgU, a member of the sigma E-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J. Bacteriology. 1996;178:4997–5004. doi: 10.1128/jb.178.16.4997-5004.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe SW, Kuehn MJ, Mason KM. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect. Immun. 2011;79:4361–4369. doi: 10.1128/IAI.05332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoberg RJ, Thomas DD. Specific adherence of Borrelia burgdorferi extracellular vesicles to human endothelial cells in culture. Infect. Immun. 1993;61:3892–3900. doi: 10.1128/iai.61.9.3892-3900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Su YC, Riesbeck K. Vitronectin in bacterial pathogenesis: a host protein used in complement escape and cellular invasion. Mol. Microbiol. 2010;78:545–560. doi: 10.1111/j.1365-2958.2010.07373.x. [DOI] [PubMed] [Google Scholar]
- Slevogt H, Zabel S, Opitz B, Hocke A, Eitel J, N'Guessan PD, Lucka L, Riesbeck K, et al. CEACAM1 inhibits Toll-like receptor 2-triggered antibacterial responses of human pulmonary epithelial cells. Nat. Immunol. 2008;9:1270–1278. doi: 10.1038/ni.1661. [DOI] [PubMed] [Google Scholar]
- Sohn J, Grant RA, Sauer RT. Allosteric activation of DegS, a stress sensor PDZ protease. Cell. 2007;131:572–583. doi: 10.1016/j.cell.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Soler N, Marguet E, Verbavatz JM, Forterre P. Virus-like vesicles and extracellular DNA produced by hyperthermophilic archaea of the order Thermococcales. Res. Microbiol. 2008;159:390–399. doi: 10.1016/j.resmic.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Specka U, Spreinat A, Antranikian G, Mayer F. Immunocytochemical Identification and Localization of Active and Inactive alpha-Amylase and Pullulanase in Cells of Clostridium thermosulfurogenes EM1. Appl. Environ. Microbiol. 1991;57:1062–1069. doi: 10.1128/aem.57.4.1062-1069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisatjaluk R, Doyle RJ, Justus DE. Outer membrane vesicles of Porphyromonas gingivalis inhibit IFN-gamma-mediated MHC class II expression by human vascular endothelial cells. Microb. Pathog. 1999;27:81–91. doi: 10.1006/mpat.1999.0287. [DOI] [PubMed] [Google Scholar]
- Srisatjaluk R, Kotwal GJ, Hunt LA, Justus DE. Modulation of gamma interferon-induced major histocompatibility complex class II gene expression by Porphyromonas gingivalis membrane vesicles. Infect. Immun. 2002;70:1185–1192. doi: 10.1128/IAI.70.3.1185-1192.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DS, Edwards KM, Morris F, McGee ZA. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J. Infect. Dis. 1982;146:568. doi: 10.1093/infdis/146.4.568. [DOI] [PubMed] [Google Scholar]
- Storey DG, Ujack EE, Rabin HR, Mitchell I. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect. Immun. 1998;66:2521–2528. doi: 10.1128/iai.66.6.2521-2528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H, Ogawa T, Yoshimura F, Otsuka K, Kokeguchi S, Kato K, Umemoto T, Kotani S. Immunobiological activities of a porin fraction isolated from Fusobacterium nucleatum ATCC 10953. Infect. Immun. 1988;56:855–863. doi: 10.1128/iai.56.4.855-863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TT, Morgelin M, Forsgren A, Riesbeck K. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J. Infect. Dis. 2007;195:1661–1670. doi: 10.1086/517611. [DOI] [PubMed] [Google Scholar]
- Tashiro Y, Sakai R, Toyofuku M, Sawada I, Nakajima-Kambe T, Uchiyama H, Nomura N. Outer membrane machinery and alginate synthesis regulators control membrane vesicle production in Pseudomonas aeruginosa. J. Bacteriol. 2009;191:7509–7519. doi: 10.1128/JB.00722-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufano MA, Rossano F, Catalanotti P, Liguori G, Capasso C, Ceccarelli MT, Marinelli P. Immunobiological activities of Helicobacter pylori porins. Infect. Immun. 1994a;62:1392–1399. doi: 10.1128/iai.62.4.1392-1399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufano MA, Rossano F, Catalanotti P, Liguori G, Marinelli A, Baroni A, Marinelli P. Properties of Yersinia enterocolitica porins: interference with biological functions of phagocytes, nitric oxide production and selective cytokine release. Res Microbiol. 1994b;145:297–307. doi: 10.1016/0923-2508(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Unal CM, Schaar V, Riesbeck K. Bacterial outer membrane vesicles in disease and preventive medicine. Semin. Immunopathol. 2011;33:395–408. doi: 10.1007/s00281-010-0231-y. [DOI] [PubMed] [Google Scholar]
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- Wai SN, Lindmark B, Soderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell. 2003;115:25–35. doi: 10.1016/s0092-8674(03)00754-2. [DOI] [PubMed] [Google Scholar]
- Walburger A, Lazdunski C, Corda Y. The Tol/Pal system function requires an interaction between the C-terminal domain of TolA and the N-terminal domain of TolB. Mol. Microbiol. 2002;44:695–708. doi: 10.1046/j.1365-2958.2002.02895.x. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell. 2003;113:61–71. doi: 10.1016/s0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- Wood LF, Ohman DE. Independent regulation of MucD, an HtrA-like protease in Pseudomonas aeruginosa, and the role of its proteolytic motif in alginate gene regulation. J. Bacteriol. 2006;188:3134–3137. doi: 10.1128/JB.188.8.3134-3137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LF, Ohman DE. Use of cell wall stress to characterize sigma 22 (AlgT/U) activation by regulated proteolysis and its regulon in Pseudomonas aeruginosa. Mol. Microbiol. 2009;72:183–201. doi: 10.1111/j.1365-2958.2009.06635.x. [DOI] [PubMed] [Google Scholar]
- Yaron S, Kolling GL, Simon L, Matthews KR. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl. Environ. Microbiol. 2000;66:4414–4420. doi: 10.1128/aem.66.10.4414-4420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai K, Hanawa T, Kamiya S. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 2009;9:197. doi: 10.1186/1471-2180-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Kim KS. YgfZ contributes to secretion of cytotoxic necrotizing factor 1 into outer-membrane vesicles in Escherichia coli. Microbiology. 2012;158:612–621. doi: 10.1099/mic.0.054122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Srisatjaluk R, Justus DE, Doyle RJ. On the origin of membrane vesicles in gram-negative bacteria. FEMS Microbiol. Lett. 1998;163:223–228. doi: 10.1111/j.1574-6968.1998.tb13049.x. [DOI] [PubMed] [Google Scholar]