Systematic Review: The emerging interplay between bile acids, gastrointestinal tract, and incretins in the pathogenesis of diabetes and nonalcoholic fatty liver disease (original) (raw)
. Author manuscript; available in PMC: 2013 Nov 1.
Published in final edited form as: Aliment Pharmacol Ther. 2012 Oct 11;36(10):909–921. doi: 10.1111/apt.12084
SUMMARY
Background
Recent research has led to an interest in the role of the gut and liver in type 2 diabetes mellitus (T2DM).
Aim
To review the role of the gastrointestinal system in glucose homeostasis, with particular focus on the effects of incretin hormones, hepatic steatosis, and bile acids.
Methods
PubMed and Google Scholar were searched using terms such as incretin, glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), dipeptidyl peptidase-4 (DPP-4), hepatic steatosis, bile acid, and gastric bypass. Additional relevant references were identified by reviewing the reference lists of articles.
Results
Perturbations of incretin hormones and bile acid secretion contribute to the pathogenesis of T2DM, leading to their potential as therapeutic targets. The incretin hormones (GIP and GLP-1) are deactivated by DPP-4. GLP-1 agonists and DPP-4 inhibitors improve glycemic control in patients with T2DM. Hepatic steatosis, along with insulin resistance, may precede the development of T2DM, and may benefit from antidiabetes medications. Bile acids play an important role in glucose homeostasis, with effects mediated via the farnesoid X receptor (FXR) and the cell surface G protein-coupled receptor TGR5. The bile acid sequestrant colesevelam has been shown to be effective in improving glycemic control in patients with T2DM. Altered gastrointestinal anatomy after gastric bypass surgery may also affect enterohepatic recirculation of bile acids and contribute to improved glycemic control. Conclusions: Research in recent years has led to new pathways and processes with a role in glucose homeostasis, and new therapeutic targets and options for T2DM.
Keywords: bile acid, colesevelam, gastric bypass, hepatic steatosis, incretins
INTRODUCTION
Diabetes mellitus (DM) refers to a group of endocrine diseases that are all characterized by hyperglycemia. It is one of the most rapidly rising diseases in the United States, affecting 25.8 million people (about 8.3% of the population), and is now the seventh leading cause of death in the United States.1 Because of the glycemic dysregulation related to these diseases, the secondary pathophysiological effects of DM on multiple organ systems impose an additional burden on the health care system. DM is the leading cause of end-stage renal disease, lower extremity amputations, and blindness in adults, as well as a known aggravator of cardiovascular disease. Type 2 DM (T2DM), the most common type of DM, is classically described as a heterogeneous group of disorders that is characterized by a decline in insulin-producing pancreatic β cells, an increase in peripheral insulin resistance, an increase in hepatic glucose production, or a combination of these factors.
For decades, therapies for T2DM have focused on this “triad” of characteristics, acting on the liver, pancreas, muscle, and adipose tissue to reduce hepatic glucose production (e.g. biguanides), increase insulin secretion (e.g. sulfonylureas), and improve insulin sensitivity (e.g. thiazolidinediones). However, recent research suggests that other organs and processes play a vital role in glucose homeostasis and the pathogenesis of T2DM, and that these new pathways have potent therapeutic potential. Of particular interest has been the role of the gut in glucose homeostasis, through the direct action of incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP).
Processes occurring in the liver may also play a role in the pathogenesis of T2DM, although a causal link has not yet been definitively demonstrated. Hepatic steatosis (an excess of triglyceride accumulation in hepatocytes) is often observed in the presence of insulin resistance, including in T2DM. Emerging data suggest that hepatic insulin resistance and hepatic steatosis precede the development of T2DM. Diet and pharmacologic therapies used in the management of T2DM have been shown to decrease hepatic triglyceride content, in addition to improving glycemic control.
Emerging research also shows the importance of bile acids in glucose homeostasis, which may lead to an understanding of how bariatric surgery mediates its immediate effect on T2DM. Both the liver and intestines are involved in bile acid metabolism.
Various components of the gastrointestinal system contribute to the pathogenesis of T2DM and may be potential therapeutic targets. In the 2011 American Association of Clinical Endocrinologists clinical practice guidelines for DM, incretin therapy has been recommended in controlling fasting glucose in patients with DM.2 It is likely that incretin therapies will play a much wider role in the treatment of T2DM in the future. In this article, the role of the gastrointestinal system in glucose homeostasis will be reviewed, with particular focus on the effects of incretin hormones, hepatic steatosis, and bile acids.
PubMed and Google Scholar were searched using terms such as incretin, GIP, GLP-1, dipeptidyl peptidase-4 (DPP-4), hepatic steatosis, bile acid, and gastric bypass. Additional relevant references were identified by reviewing the reference lists of articles.
INCRETIN HORMONES
Incretins, which are hormones released by the gastrointestinal tract in response to nutrients, augment glucose-mediated insulin secretion.3 Their existence was suspected over a hundred years ago,4 but was not confirmed until researchers found that the oral administration of glucose resulted in a greater increase in insulin and a more sustained response when compared to glucose administered intravenously.5, 6 An estimated 50%–70% of insulin secretion after glucose ingestion is attributable to this observation, which is now known as the “incretin effect.”7
To date, only two hormones fulfill the definition of an incretin hormone.7 The first incretin hormone to be identified was gastric inhibitory polypeptide, or GIP. Initially, GIP was shown to inhibit gastric acid secretion when tested on dogs.8 However, further work on more purified samples in humans revealed that GIP augmented insulin secretion.9 As a result, the hormone was renamed to glucose-dependent insulinotropic polypeptide to maintain the original acronym but to more accurately describe its function.7 GLP-1 was discovered later, during the sequencing of mammalian genes. When mapping the proglucagon gene, it was noted that, in addition to glucagon, two more peptides were encoded.10, 11 These two peptides were largely homologous to glucagon and hence were named GLP-1 and glucagon-like peptide-2 (GLP-2). However, only GLP-1 was found to stimulate insulin release.12 Both GIP and GLP-1 potentiate the augmentation of glucose-mediated insulin response in an additive manner and together explain the incretin effect observed in humans.13, 14
Secretion of Incretin Hormones
Interestingly, the two incretin hormones are synthesized independently by distinct cell types that are mainly organized in two different regions of the human gut. GIP is synthesized and released from K cells located in the duodenum and proximal jejunum,15 whereas GLP-1 is produced and released from L cells that are primarily located in the distal jejunum and ileum, though fewer numbers are found scattered throughout the small intestine.16 They are both released in response to ingestion of nutrients, especially to glucose, carbohydrates, and fats.17-19 During fasting, the circulating levels of GIP and GLP-1 are low, but both increase rapidly with the ingestion of nutrients.17, 19 Furthermore, their release is dependent on the size of the meal, i.e., the ingestion of large meals leads to secretion of higher amounts of both GIP and GLP-1 when compared to smaller meals.20 Neither one is affected by intravenous administration of glucose.
In humans, both fat and protein markedly stimulate GIP secretion.21 The release of GIP is directly related to the rate of nutrient absorption rather than the presence of ingested material in the gut lumen.7 Hence, in patients with malabsorption, serum GIP levels are low.22 No other stimulator of GIP, besides nutrient absorption, has yet been found.
Serum levels of GLP-1 rise rapidly after ingestion of a meal, and its release occurs in a biphasic pattern.19 Peak levels occur within approximately 5 to 15 minutes after a meal, even though the ingested material has not yet reached the L cells in the distal jejunum and ileum at that point. This phase is followed by a longer 30–60-minute second phase. This early response to ingested material suggests that an indirect stimulation occurs via endocrine or neural mediators. This effect has been described as a proximal-distal neuroendocrine loop that relays stimulation to ingested foods from the proximal duodenum to distally located L cells that release GLP-1.23, 24 Several studies have shown that the autonomic nervous system, through the neurotransmitters gastrin-releasing peptide (GRP) and acetylcholine, contributes to the rapid release of GLP-1.25-27 Furthermore, in rats where the vagus nerve was severed, there was no initial peak in release of GLP-1 after a diet rich in fat.23 Atropine, a muscarinic antagonist, diminished GLP-1 secretion in humans, further bolstering the theory that GLP-1 secretion is stimulated by neural mediators.28 In several rat studies, GIP plays a role in GLP-1 secretion through vagal afferent-efferent pathways and the release of GRP.23, 25 However, GIP does not stimulate the secretion of GLP-1 in humans.29 The second phase of GLP-1 release is due to the direct response of L cells to ingested food in the lumen.30
Biological Actions of Incretin Hormones
The incretin hormones mediate their insulinotropic effects mainly in the pancreas. However, GIP and GLP-1 have receptors in many extrapancreatic tissues that contribute to glucose homeostasis. The GLP-1 receptor has also been found in the liver, kidney, stomach, heart, lung, intestines, skeletal muscle, adipose tissue, nodose ganglion neurons of the vagus nerve, and the brain, including the brainstem and hypothalamus.31-35 GIP receptors are also expressed in a range of tissues in addition to the pancreas. They have been found in the stomach, small intestine, heart, lung, adipose tissue, adrenal cortex, and the brain, including the cerebral cortex, hippocampus, and olfactory bulb.36 In addition, GIP has indirect effects on the liver, although no GIP receptors have been found in the liver and the mechanism for an indirect route of action has not been elucidated.7, 36 Table 1 summarizes the role of the incretin hormones in various tissues.
Table 1.
Effects of GLP-1 and GIP on peripheral tissues7, 35, 37-42
| GLP-1 | GIP | |
|---|---|---|
| Brain | ||
| Neuroprotection | Increased | No effect |
| Appetite | Decreased | No effect |
| Progenitor cell proliferation | No effect | Increased |
| Heart | ||
| Cardioprotection | Increased | No effect |
| Cardiac function | Increased | No effect |
| Stomach | ||
| Gastric emptying | Decreased | No effect |
| Liver | ||
| Glucose production | Decreased | Decreased |
| Pancreas | ||
| Insulin secretion | Increased | Increased |
| Glucagon secretion | Decreased | No effect |
| Insulin biosynthesis | Increased | Increased |
| β-cell proliferation | Increased | Increased |
| β-cell apoptosis | Increased | Decreased |
| Bone | ||
| Bone formation | No effect | Increased |
| Bone resorption | No effect | Decreased |
| Muscle | ||
| Glucose uptake | Increased | Increased |
| Glucagon uptake and storage | Increased | No effect |
| Adipose tissue | ||
| Lipogenesis | No effect | Increased |
| Glucose uptake | Increased | Increased |
In the pancreas, both GLP-1 and GIP stimulate glucose-dependent insulin secretion43, 44 and β-cell proliferation,45, 46 inhibit β-cell apoptosis,47, 48 and increase insulin production.49, 50 In addition, GLP-1 inhibits glucagon production,51 whereas GIP stimulates glucagon secretion.52 However, glucagon secretion by GIP only occurs under basal glucose concentrations, and GIP may play a role in feedback control of glucose homeostasis, as the most profound augmentation of insulin secretion from GIP is seen under hyperglycemic conditions.52
The role of the incretins in the extrapancreatic tissues is diverse. Both GLP-1 (in the liver and/or kidney) and GIP (in the liver, presumably via an indirect mechanism) inhibit glucagon-stimulated glucose production.41, 42 In the gut, GLP-1 impedes gastric emptying and hence delays the rise in glucose after eating.53-55 In the central nervous system, GLP-1 has been shown to decrease appetite and food intake,51 which is mediated through GLP-1 receptors found on the nodose ganglion of the afferent vagus nerve.56
In the muscle and adipose tissue, GLP-1 and GIP stimulate glucose uptake.35, 38-40 Although there is some understanding of the release of incretins and which organs they affect, the way in which they control glucose homeostasis is poorly understood. In this case, GLP-1 has been better studied than GIP. There are two proposed mechanisms through which GLP-1 is hypothesized to mediate its effects: 1) the endocrine pathway and 2) the neural pathway.57 In the endocrine pathway, GLP-1 is released directly into the systemic circulation after the L cells are stimulated by gut nutrients. It then binds to receptors in target organs such as the pancreas, where it increases intracellular cAMP and stimulates glucose-dependent insulin secretion.58, 59 GLP-1 also increases β-cell insulin stores through promoting insulin gene expression and stabilizing transcription as well as stimulating β-cell proliferation and neogenesis.45, 46
Neurons in the central nervous system contain GLP-1 and GLP-1 receptors—the first hint that GLP-1 has a neural pathway through which it mediates some of its actions. The predominant region of the brain that contains GLP-1 receptors is the nodose ganglion of abdominal vagal afferent nerve that terminates in the nucleus of the solitary tract.33 GLP-1 promotes satiety and decreases food intake,32, 34, 51, 60 and GLP-1 agonists have led to weight loss in human studies.61
Given these two pathways, it may be that the GLP-1 insulin potentiation may occur as a combination of the two pathways. More than half of the GLP-1 secreted is inactivated before it reaches the systemic circulation.62 Furthermore, GLP-1 is metabolized in the liver, leaving only a small amount that actually reaches the pancreas.62, 63 It is now presumed that GLP-1 must use local neurons as intermediaries to signal the pancreas.64
Incretin Hormones in Patients with T2DM
The incretin effect is severely reduced in patients with T2DM.65 Although the cause of this is likely multifactorial, studies evaluating the secretion levels of incretins and physiological response to their exogenous administrations have shown two salient findings. There is a likely impaired secretion of GLP-1 and decreased activity of GIP.37 In patients with T2DM, GIP secretion is normal or even increased in basal and postprandial conditions.37 However, its insulinotropic activity has been shown to be greatly diminished in patients with T2DM, with response being 54% lower than that of normal controls.66 In contrast to GIP, the response to GLP-1 in patients with T2DM was similar to that of controls. However, plasma levels of GLP-1 at meal time appear to be at least modestly diminished.20, 67-70 It is noteworthy to point out, however, that a few studies have demonstrated increased or unaltered levels of GLP-1.71-74 Finally, patients with secondary DM, such as those with DM secondary to chronic pancreatitis, have similar inhibition of their incretin activity, suggesting that this is a consequence of T2DM rather than a cause of it.75
Synthetic GLP-1 agonists [exenatide (Amylin Pharmaceuticals, Inc., San Diego, CA, USA) and liraglutide (Novo Nordisk A/S, Bagsvaerd, Denmark)] are available for the treatment of T2DM when used as monotherapy or in combination therapy requiring subcutaneous administration.76, 77 These agents improve glycemic control and promote weight loss, and are associated with low rates of severe hypoglycemia.78-80 However, there have been reports of an association between GLP-1 agonist use and acute pancreatitis in patients with T2DM.81, 82
The major pharmaceutical target influencing incretins has been DPP-4.83 DPP-4, which cleaves GLP-1 and GIP, is found in many tissues including the gastrointestinal tract, biliary tract, liver, spleen, lungs, pancreas, kidneys, and activated T lymphocytes.84-86 The protease resides on the surface of endothelial cells of blood vessels from the intestines; hence, it is in a perfect position to rapidly deactivate more than half of secreted incretins.62 DPP-4 knockout mice are associated with increased GIP and GLP-1, as well as enhanced insulin secretion after oral glucose administration.87 Interestingly, such mice are also resistant to the development of obesity induced by a high-fat diet.88 Four DPP-4 inhibitors are currently on the market: linagliptin (Boehringer Ingelheim International GmbH, Ingelheim, Germany), saxagliptin (Bristol-Myers Squibb, Princeton, NJ, USA), sitagliptin (Merck & Co., Inc., Whitehouse Station, NJ, USA), and vildagliptin (Novartis, Basel, Switzerland; approved in various countries in Europe, Asia Pacific, Africa and Latin America). The DPP-4 inhibitors are generally well tolerated, weight neutral, and not associated with hypoglycemia,89-92 and are associated with a rise in plasma incretins after meals.93, 94 Glucose-mediated insulin secretion was enhanced, which was consistent with improved pancreatic β-cell function.93, 94 They significantly lower blood glucose and hemoglobin A1C levels, and are used either as monotherapy or in combination therapy.95-98
NON-ALCOHOLIC FATTY LIVER DISEASE AND INSULIN RESISTANCE
Alcohol consumption and metabolic syndrome are the two main causes of hepatic steatosis. However, there are many potential causes of hepatic steatosis, including abetalipoproteinemia, acute fatty liver of pregnancy, malnutrition and refeeding syndrome, medications (e.g. amiodarone, methotrexate, tamoxifen), and environmental hepatotoxins (e.g. wild mushroom poisoning).99 Hepatic steatosis that is associated with metabolic syndrome is commonly called non-alcoholic fatty liver disease (NAFLD). Insulin resistance and obesity are associated with an increased risk of NAFLD.100 In patients with NAFLD, the prevalence of obesity is 30%–100% and the prevalence of T2DM is 10%–75%.99 NAFLD is the most common cause of abnormal liver enzymes in the clinical population of the US and affects approximately 20% of the population.101 In patients with NAFLD, the accumulation of triglycerides in hepatocytes can eventually develop into inflammation (non-alcoholic steatohepatitis [NASH]), fibrosis, cirrhosis, and even hepatocellular carcinoma. The molecular and physiological changes that lead to NAFLD have been extensively studied and reviewed.102, 103 The current hypothesis for its development is that obesity and insulin resistance increase the release of free fatty acids (FFAs) from adipocytes.104 Once these FFAs reach the liver, they are either oxidated to generate adenosine triphosphate (ATP) or esterified to produce triglycerides. Triglycerides either become part of very-low-density lipoprotein particles that are exported to the serum, or they are stored within the hepatocyte itself. Defects in any of these processes can result in excessive triglyceride accumulation in the hepatocyte and eventual cell damage and inflammation.
Emerging data suggest that hepatic insulin resistance and hepatic steatosis precede the development of T2DM.105, 106 Elevated serum alanine aminotransferase and fatty liver on ultrasound predict the occurrence of diabetes.106, 107 A low-calorie diet is the primary therapy for insulin resistance and hepatic steatosis. Moderate diet-induced weight loss (5–10% of body weight) can decrease hepatic triglyceride content, improve glycemic control, and improve hepatic and muscle insulin sensitivity.108, 109 A meta-analysis of randomized trials of treatments for NAFLD found that weight loss was safe and improved histological disease activity in NASH in a dose-dependent fashion.110 However, more than 50% of patients failed to achieve target weight loss, and it remains unclear whether patients were able to maintain the weight loss.
Pioglitazone (Takeda Pharmaceutical Company Limited, Osaka, Japan), a thiazolidinedione, has also shown beneficial effects in patients with nonalcoholic steatohepatitis and impaired glucose tolerance or T2DM; in addition to metabolic improvements, treatment with pioglitazone was associated with reductions in hepatic fat content and corresponding improvements in histological findings.111 Although an early meta-analysis of randomized trials for the treatment of NAFLD showed that thiazolidinediones improved histological steatosis and inflammation, but not fibrosis,110 a later meta-analysis that only included randomized, placebo-controlled trials (and hence excluded two open-label trials) showed that there was also an improvement in fibrosis.112 However, patients treated with thiazolinediones had significant weight gain.
There are also multiple randomized control trials suggesting a possible benefit of metformin in nonalcoholic steatohepatitis, in addition to its glycemic effects in T2DM.113-116 Three trials showed a histological improvement in inflammation, steatosis, and fibrosis after treatment with metformin.113, 115, 117 Thiazolidinediones and metformin have both been shown to phosphorylate liver kinase B1 (LKB1), which promotes its nuclear export. LKB1, in turn, activates adenosine monophosphate–activated protein kinase (AMPK) in the liver,118, 119 leading to inhibition of anabolic cellular processes such as hepatic lipogenesis and gluconeogenesis, in addition to stimulating catabolic processes such as glycolysis, fatty acid oxidation, and mitochondrial biogenesis.119, 120 It should be noted that more recent studies on metformin did not find a benefit in hepatic steatosis, serum markers, or insulin resistance when compared with lifestyle modification.121-123 However, these results remain controversial, since the studies were done in small populations, and there were differences in duration and dose of treatment, and variable time periods between pre- and post-treatment biopsies.116
In addition, enzymes that increase oxidation of FFAs, which can prevent accumulation of hepatic triglycerides, can play a role in the treatment of hepatic steatosis, obesity, and other associated metabolic disorders. AMPK tightly regulates mitochondrial long-chain fatty acid oxidation through the inhibition of acetyl-CoA carboxylase 2 (ACC2).124 A product of ACC2 is malonyl-CoA, which is a potent inhibitor of carnitine palmitoyltransferase 1 (CPT1), a mitochondrial membrane enzyme that controls beta-oxidation. Cardiac endothelial cells also oxidize fatty acids in a carnitine-dependent manner, suggesting that this enzyme plays a role in preventing inflammation and coagulation associated with metabolic syndrome and heart disease.125 As a result, both CPT1 and ACC2 have become potential therapeutic targets.126
THE ROLE OF BILE ACIDS IN GLUCOSE HOMEOSTASIS
Recent studies show that bile acids play a much larger role in glucose homeostasis than previously thought. After being released by the gallbladder into the intestines, nearly all of the bile acids (95%) get reabsorbed in the terminal ileum, decreasing the need for de novo bile acid synthesis.127, 128 Hence, there is frequent cycling of the bile acids (i.e. bile acid pool) between the intestines and the liver in the enterohepatic circulation. Bile acids are endogenous ligands to several receptors, including farnesoid X receptor (FXR) and pregnane X receptor (PXR), constitutive androstane receptor (CAR), vitamin D receptor (VDR), and the G-protein-coupled receptor TGR5. Through various signaling pathways, bile acids regulate cholesterol, fasting and mealtime glucose, and metabolism/energy homeostasis as well as their own synthesis and blood levels in the enterohepatic circulation.127, 128 The composition of the bile acid pool has been shown to be altered in patients with T2DM.129 In this section, we will discuss two bile acid signaling pathways, the FXR- and TGR5-mediated changes in homeostasis, in detail.
Characterization of several nuclear receptors has led to a better understanding of how hepatic metabolism can be altered by nutrition from the gut. Nuclear receptors have a ligand-binding domain and a DNA-binding domain. Once activated by a ligand, they can induce a transcriptional change in target genes. They provide important means through which cells can maintain homeostasis in response to changes in their environmental stimuli, such as diet. Studies of several nuclear receptors that were previously thought to be orphaned now reveal that they respond to metabolites such as fatty acids and oxysterols, in addition to digestive enzymes, such as bile acids.130, 131 FXR was a previously orphaned nuclear receptor until bile acids were discovered to be their ligands.132
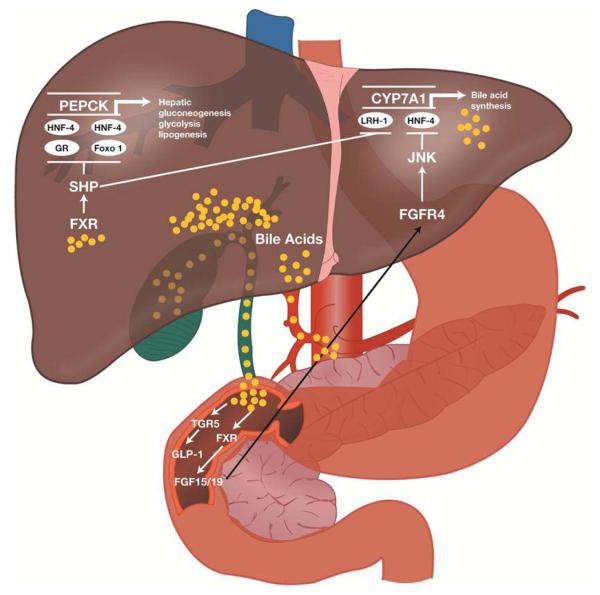
FXR is primarily found in the liver, kidney, and intestines, and overall inhibits hepatic de novo bile acid production.133-135 Bile acids are produced when cholesterol is oxidized in the liver. The “classical” pathway of bile acid production is via 7-α hydroxylation of cholesterol by a rate-limiting enzyme, cholesterol 7-α hydroxylase (CYP7A1). An “alternate” pathway, with 27-hydroxylase in the mitochondria of extrahepatic tissues (e.g. endothelial cells), can also produce bile acids. FXR inhibits de novo bile acid formation with the induction of small heterodimer partner (SHP). SHP plays an essential role in feedback regulation of bile acid biosynthesis through repression of CYP7A1 by inhibiting two nuclear receptors: liver receptor homolog-1 (LRH-1) and hepatocyte nuclear factor-4α (HNF-4α; see Figure 1).137 CYP7A1 is a critical regulatory gene in bile acid synthesis. By inhibiting CYP7A1, FXR inhibits the classic pathway of de novo bile acid formation and moves this process to extrahepatic tissues.
Figure 1.
Potential mechanism(s) of action for the glycemic effects of a bile acid sequestrant. FGF, fibroblast growth factor; FGFR, FGF receptor; FXR, farnesoid X receptor; GLP-1, glucagon-like peptide-1; GR, glucocorticoid receptor; HNF-4, hepatocyte nuclear factor-4; JNK, c-Jun N-terminal kinase; LRH-1, liver receptor homolog-1; SHP, small heterodimer partner. Reprinted with permission from Wright WL. The management of type 2 diabetes mellitus: a novel approach for addressing glycemic and lipid control with colesevelam HCl. Adv Nurse Pract. 2009;17 (11):suppl 1-16.136
Earlier studies have shown that FXR plays an important role in lipoprotein metabolism,138 and FXR knockout mice exhibited elevated plasma triglyceride and cholesterol levels as well as steatohepatitis.139 More recent studies have shown that FXR is also important to glucose homeostasis. FXR knockout mice showed impaired glucose tolerance and decreased insulin sensitivity.140 The role of FXR in glucose homeostasis was further bolstered when FXR synthetic agonists or induced overexpression of FXR repressed hepatic gluconeogenesis and enhanced glycogen synthesis and storage, inducing an overall lower blood glucose level.135 FXR activation induces expression and secretion of fibroblast growth factor (FGF)19 and FGF21 in the intestine (Figure 1).141 Administration of FGF19 and FGF21 to diet-induced obesity mice increased their energy expenditure and caused weight loss and, as a result, improved insulin sensitivity.142, 143 Alterations in bile salts passing through the intestines have strong effects on metabolism by modulating FXR-mediated FGF19 and FGF21 secretion. Because FXR agonists can play a role in both treating steatohepatitis and improving glucose homeostasis, they have become an area of intense research as potential pharmacotherapy for T2DM and nonalcoholic fatty liver disease.
Another mechanism through which bile acids affect glucose metabolism is a novel cell surface G-protein-coupled receptor, TGR5 (Figure 1).144, 145 TGR5 is found in brown adipose tissue, the liver, and intestines.146 Bile acid stimulation of TGR5 increases intracellular cAMP. The effect of this rise in cAMP differs depending on the cell expressing TGR5. For example, in brown adipose tissue, bile acid induction of TGR5 results in a cascade of reactions that eventually converts inactive thyroid hormone (T4) to its active form (T3), hence modulating energy expenditure.147 The role of bile acids on glucose homeostasis was reinforced when studies showed that murine enteroendocrine cell lines, when activated by bile acids, secreted GLP-1 via activation of TGR5.148
Whether TGR5 actually plays a role in glucose homeostasis was not elucidated until a few years later. Thomas et al. showed that the TGR5 agonist INT-777 (Intercept Pharmaceuticals, Inc., New York, NY, USA; a bile acid mimetic) induced GLP-1 secretion in human L-cell lines by increasing intracellular levels of cAMP and altering the ATP/adenosine diphosphate (ADP) ratio and causing an influx of calcium, which, in turn, induced GLP-1 secretion.149 TGR5 overexpression potentiated GLP-1 secretion, whereas TGR5 RNA interference blunted it. Activation of TGR5 by INT-777 in obese mice also prevented weight gain, preserved pancreatic function, and improved insulin sensitivity.
Given these findings, a more complete picture of the normal physiology of meal ingestion and bile acid glucose homeostasis can be formed. After ingestion of a meal, the gallbladder contracts and the amount of bile acids in the intestine increases. This, in turn, causes nutrient-induced secretion of GLP-1 from L cells via activation of TGR5. T2DM dampens gallbladder motility, leading to reduced flow of bile acids to the intestine. With reduced bile acids, there is decreased activation of TGR5 in L cells of the intestine, leading to lower secretion of GLP-1 and poor glucose homeostasis with decreased insulin secretion.150
Despite growing literature on the direct role that bile acids can play in increasing GLP-1 by activating TGR5 in L cells, there is a paradox in that the main therapeutic target thus far has been to diminish the amount that can act in the terminal ileum. Bile acid sequestrants, such as colesevelam hydrochloride (Daiichi Sankyo, Inc., Parsippany, NJ, USA), form non-absorbable complexes with bile acids in the gastrointestinal tract preventing reabsorption and promoting their fecal excretion.146 Although bile acid sequestrants have been used for control of hyperlipidemia for decades, they have shown to be effective in improving glycemic control in patients with T2DM. Studies show that colesevelam resulted in a reduction of hemoglobin A1C of 0.5% when compared with placebo.151-153 Significantly greater reductions in fasting glucose levels were seen following 16–26 weeks of treatment with colesevelam compared to placebo, in addition to insulin-, metformin-, and sulfonylurea-based therapy, further suggesting that bile acids play a role in fasting plasma glucose control.
An explanation for this paradox was proposed by Hofmann in a letter to the journal Hepatology.154 Passage of fatty acids into the ileum (because micellar solubilization is not yet completed in the jejunum) may lead to increased GLP-1 release from the ileal L cells. This is supported by the finding that administration of colesevelam to rats with diet-induced obesity and insulin resistance led to improvements in glucose tolerance and insulin resistance that were accompanied by increased plasma GLP-1 levels; changes in these parameters were not seen in rats administered SC-435 (which decreases bile absorption in the ileum, leading to inactivation of FXR).155 Similarly, in patients with T2DM, the addition of colesevelam to a regimen comprising a sulfonylurea and/or metformin was associated with improvements in glycemic control together with increased plasma levels of GLP-1 and GIP, compared with placebo.156 This could be the mechanism through which glycemic control improves in patients with T2DM with the bile acid sequestrant colesevelam. In addition, this mechanism could explain improvements in glycemic control following intestinal transposition or other bariatric surgery, as discussed in the next section.
THE EFFECTS OF GASTRIC BYPASS SURGERY ON GLUCOSE HOMEOSTASIS
A majority of patients who undergo Roux-en-Y gastric bypass (RYGB) surgery are either completely cured of T2DM or have a large improvement despite initial negligible weight loss. A meta-analysis of 136 bariatric surgery studies reported that, of individuals who underwent gastric bypass, T2DM was resolved in 83.7%, and resolved or improved in 93.2%.157 These patients achieve normal fasting plasma glucose and hemoglobin A1C levels.158-161 These changes occur before the patient has lost significant amounts of weight,160, 161 suggesting that the improvements in glycemic control following RYGB may be caused by effects other than weight loss. Since the primary objective of these procedures is to manipulate and reorient the gut, it is presumed that this improvement is mediated or potentiated by the gut.
A potential explanation for the improvements in glycemic control seen following RYGB is altered secretion of GLP-1 secondary to gastrointestinal manipulation and reorientation. Patients who have undergone RYGB have significantly increased postprandial GLP-1 and insulin secretion, compared with obese and lean controls, and patients who had lost an equivalent amount of weight by gastric banding.162 This could be because more ingested material, especially fats and carbohydrates, are reaching the ileum and causing an increase in GLP-1 release from L cells.
Interestingly, altered gastrointestinal anatomy after RYGB may also affect enterohepatic recirculation of bile acids and contribute to improved glycemic control. A study of patients who had undergone RYGB revealed that total fasting serum bile acids increased two-fold when compared to overweight or morbidly obese control participants.163 A closer analysis showed that multiple bile acid subfractions, including both primary and secondary bile acids, reached statistical significance. Total serum bile acids were inversely correlated with 2-hour postprandial glucose levels and positively correlated with GLP-1 levels. Similarly, in another study in Japanese adults who had undergone laparoscopic bariatric surgery, a positive correlation was observed between the changes in serum concentration of primary bile acids and plasma levels of GIP 1 month after surgery.164 The mechanism by which bile acids in the enterohepatic circulation increase is not fully understood; Patti et al. proposed that their observation that both primary and secondary bile acid levels are increased in the systemic circulation suggests that this occurs via increased uptake of bile acids in the intestines.163 This increase could mediate its effects on GLP-1 increases through TGR5 receptors.
CONCLUSION
The understanding of the pathogenesis of T2DM is continuing to evolve. Increasingly, there is renewed interest in the gut’s neuroendocrine response to nutrition. Although weight loss and diet remain the mainstay of treatment for T2DM, and are the safest, a large majority of patients are either unable to lose a sufficient amount of weight or maintain their lifestyle modification to have a sustained recovery. There is strong indication that bariatric surgery for the morbidly obese who have T2DM helps patients attain a cure of T2DM by affecting the gut’s neuroendocrine response to food. Research of the gut’s response to nutrition in recent years has led to new pathways and processes with a role in glucose homeostasis (such as the incretin hormones and the bile acid pathway) and, as a result, new therapeutic targets and treatment options. Further understanding of the gastrointestinal response to nutrition will help lead a new wave of novel pharmaceutical therapies to treat T2DM and NAFLD.
ACKNOWLEDGMENTS
AZ is funded by the National Institutes of Health T32 DK07202 training grant.
This work is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association to R.L. This work was also supported in part by the NIDDK (K23 DK090303 to RL).
Footnotes
STATEMENT OF INTERESTS Declaration of personal interests: Guarantor of the article is Rohit Loomba. All authors report that no conflicts of interest exist. Declaration of funding interests: The sponsor(s) had no role in the design, collection, analysis, interpretation of the data, and/or drafting of the manuscript.
Writing support was provided by Lucy Whitehouse and Sushma Soni of _in_Science Communications, Springer Healthcare, and funded by Daiichi Sankyo, Inc.
REFERENCES
- 1.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 2.Handelsman Y, Mechanick JI, Blonde L, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(Suppl. 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 4.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. 1902;28:325–53. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elrick H, Stimmler L, Hlad CJ, Jr., Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–82. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet. 1964;2:20–1. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- 7.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Pederson RA, Brown JC. Inhibition of histamine-, pentagastrin-, and insulin-stimulated canine gastric secretion by pure “gastric inhibitory polypeptide”. Gastroenterology. 1972;62:393–400. [PubMed] [Google Scholar]
- 9.Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973;37:826–8. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- 10.Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983;302:716–8. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- 11.Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304:368–71. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- 12.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84:3434–8. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993;76:912–7. doi: 10.1210/jcem.76.4.8473405. [DOI] [PubMed] [Google Scholar]
- 14.Vilsboll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003;114:115–21. doi: 10.1016/s0167-0115(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 15.Buchan AM, Polak JM, Capella C, Solcia E, Pearse AG. Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry. 1978;56:37–44. doi: 10.1007/BF00492251. [DOI] [PubMed] [Google Scholar]
- 16.Eissele R, Goke R, Willemer S, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22:283–91. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 17.Crockett SE, Cataland S, Falko JM, Mazzaferri EL. The insulinotropic effect of endogenous gastric inhibitory polypeptide in normal subjects. J Clin Endocrinol Metab. 1976;42:1098–103. doi: 10.1210/jcem-42-6-1098. [DOI] [PubMed] [Google Scholar]
- 18.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–66. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann C, Goke R, Richter G, Fehmann HC, Arnold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–26. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 20.Vilsboll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 21.Carr RD, Larsen MO, Winzell MS, et al. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E779–84. doi: 10.1152/ajpendo.90233.2008. [DOI] [PubMed] [Google Scholar]
- 22.Besterman HS, Cook GC, Sarson DL, et al. Gut hormones in tropical malabsorption. Br Med J. 1979;2:1252–5. doi: 10.1136/bmj.2.6200.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687–94. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- 24.Roberge JN, Brubaker PL. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology. 1993;133:233–40. doi: 10.1210/endo.133.1.8319572. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann-Rinke C, Voge A, Hess M, Goke B. Regulation of glucagon-like peptide-1 secretion from rat ileum by neurotransmitters and peptides. J Endocrinol. 1995;147:25–31. doi: 10.1677/joe.0.1470025. [DOI] [PubMed] [Google Scholar]
- 26.Persson K, Gingerich RL, Nayak S, Wada K, Wada E, Ahren B. Reduced GLP-1 and insulin responses and glucose intolerance after gastric glucose in GRP receptor-deleted mice. Am J Physiol Endocrinol Metab. 2000;279:E956–62. doi: 10.1152/ajpendo.2000.279.5.E956. [DOI] [PubMed] [Google Scholar]
- 27.Miguel JC, Abdel-Wahab YH, Green BD, Mathias PC, Flatt PR. Cooperative enhancement of insulinotropic action of GLP-1 by acetylcholine uncovers paradoxical inhibitory effect of beta cell muscarinic receptor activation on adenylate cyclase activity. Biochem Pharmacol. 2003;65:283–92. doi: 10.1016/s0006-2952(02)01482-x. [DOI] [PubMed] [Google Scholar]
- 28.Balks HJ, Holst JJ, von zur Muhlen A, Brabant G. Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab. 1997;82:786–90. doi: 10.1210/jcem.82.3.3816. [DOI] [PubMed] [Google Scholar]
- 29.Chia CW, Carlson OD, Kim W, et al. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes. 2009;58:1342–9. doi: 10.2337/db08-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberge JN, Brubaker PL. Secretion of proglucagon-derived peptides in response to intestinal luminal nutrients. Endocrinology. 1991;128:3169–74. doi: 10.1210/endo-128-6-3169. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Letters. 1995;358:219–24. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- 32.Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007;1149:118–26. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu I, Hirota M, Ohboshi C, Shima K. Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology. 1987;121:1076–82. doi: 10.1210/endo-121-3-1076. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa A, Satake H, Nakabayashi H, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci Basic Clin. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Egan JM, Montrose-Rafizadeh C, Wang Y, Bernier M, Roth J. Glucagon-like peptide-1(7-36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3-L1 adipocytes: one of several potential extrapancreatic sites of GLP-1 action. Endocrinology. 1994;135:2070–5. doi: 10.1210/endo.135.5.7956929. [DOI] [PubMed] [Google Scholar]
- 36.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133:2861–70. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- 37.Gautier JF, Choukem SP, Girard J. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab. 2008;34(Suppl. 2):S65–72. doi: 10.1016/S1262-3636(08)73397-4. [DOI] [PubMed] [Google Scholar]
- 38.Luque MA, Gonzalez N, Marquez L, et al. Glucagon-like peptide-1 (GLP-1) and glucose metabolism in human myocytes. J Endocrinol. 2002;173:465–73. doi: 10.1677/joe.0.1730465. [DOI] [PubMed] [Google Scholar]
- 39.O’Harte FP, Gray AM, Flatt PR. Gastric inhibitory polypeptide and effects of glycation on glucose transport and metabolism in isolated mouse abdominal muscle. J Endocrinol. 1998;156:237–43. doi: 10.1677/joe.0.1560237. [DOI] [PubMed] [Google Scholar]
- 40.Asmar M, Simonsen L, Madsbad S, Stallknecht B, Holst JJ, Bulow J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes. 2010;59:2160–3. doi: 10.2337/db10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann H, Ebert R, Creutzfeldt W. Insulin-dependent inhibition of hepatic glycogenolysis by gastric inhibitory polypeptide (GIP) in perfused rat liver. Diabetologia. 1986;29:112–4. doi: 10.1007/BF00456120. [DOI] [PubMed] [Google Scholar]
- 42.Prigeon RL, Quddusi S, Paty B, D’Alessio DA. Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab. 2003;285:E701–7. doi: 10.1152/ajpendo.00024.2003. [DOI] [PubMed] [Google Scholar]
- 43.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–4. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 44.Nauck M, Schmidt WE, Ebert R, et al. Insulinotropic properties of synthetic human gastric inhibitory polypeptide in man: interactions with glucose, phenylalanine, and cholecystokinin-8. J Clin Endocrinol Metab. 1989;69:654–62. doi: 10.1210/jcem-69-3-654. [DOI] [PubMed] [Google Scholar]
- 45.Trumper A, Trumper K, Trusheim H, Arnold R, Goke B, Horsch D. Glucose-dependent insulinotropic polypeptide is a growth factor for beta (INS-1) cells by pleiotropic signaling. Mol Endocrinol. 2001;15:1559–70. doi: 10.1210/mend.15.9.0688. [DOI] [PubMed] [Google Scholar]
- 46.Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–32. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 47.Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–58. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 48.Kim SJ, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH. Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem. 2005;280:22297–307. doi: 10.1074/jbc.M500540200. [DOI] [PubMed] [Google Scholar]
- 49.Alarcon C, Wicksteed B, Rhodes CJ. Exendin 4 controls insulin production in rat islet beta cells predominantly by potentiation of glucose-stimulated proinsulin biosynthesis at the translational level. Diabetologia. 2006;49:2920–9. doi: 10.1007/s00125-006-0433-y. [DOI] [PubMed] [Google Scholar]
- 50.Pamir N, Lynn FC, Buchan AM, et al. Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. Am J Physiol Endocrinol Metab. 2003;284:E931–9. doi: 10.1152/ajpendo.00270.2002. [DOI] [PubMed] [Google Scholar]
- 51.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–20. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meier JJ, Gallwitz B, Siepmann N, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798–801. doi: 10.1007/s00125-003-1103-y. [DOI] [PubMed] [Google Scholar]
- 53.Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7-36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–32. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- 54.Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2719–25. doi: 10.1210/jc.2003-030049. [DOI] [PubMed] [Google Scholar]
- 55.Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981–8. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 56.Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–31. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Burcelin R, Cani PD, Knauf C. Glucagon-like peptide-1 and energy homeostasis. J Nutr. 2007;137:2534S–8S. doi: 10.1093/jn/137.11.2534S. [DOI] [PubMed] [Google Scholar]
- 58.Holz GGt, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37) Nature. 1993;361:362–5. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidtler J, Schepp W, Janczewska I, et al. GLP-1-(7-36) amide, -(1-37), and -(1-36) amide: potent cAMP-dependent stimuli of rat parietal cell function. Am J Physiol. 1991;260:G940–50. doi: 10.1152/ajpgi.1991.260.6.G940. [DOI] [PubMed] [Google Scholar]
- 60.Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541–4. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 61.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 62.Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–63. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 63.Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol. 1996;271:E458–64. doi: 10.1152/ajpendo.1996.271.3.E458. [DOI] [PubMed] [Google Scholar]
- 64.Balkan B, Li X. Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1449–54. doi: 10.1152/ajpregu.2000.279.4.R1449. [DOI] [PubMed] [Google Scholar]
- 65.Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 66.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mannucci E, Ognibene A, Cremasco F, et al. Glucagon-like peptide (GLP)-1 and leptin concentrations in obese patients with Type 2 diabetes mellitus. Diabet Med. 2000;17:713–9. doi: 10.1046/j.1464-5491.2000.00367.x. [DOI] [PubMed] [Google Scholar]
- 68.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 69.Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 70.Muscelli E, Mari A, Casolaro A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–8. doi: 10.2337/db07-1315. [DOI] [PubMed] [Google Scholar]
- 71.Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87:415–23. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550–9. doi: 10.1152/ajpendo.00326.2004. [DOI] [PubMed] [Google Scholar]
- 73.Ahren B, Larsson H, Holst JJ. Reduced gastric inhibitory polypeptide but normal glucagon-like peptide 1 response to oral glucose in postmenopausal women with impaired glucose tolerance. Eur J Endocrinol. 1997;137:127–31. doi: 10.1530/eje.0.1370127. [DOI] [PubMed] [Google Scholar]
- 74.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–87. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 75.Knop FK, Vilsboll T, Hojberg PV, et al. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes. 2007;56:1951–9. doi: 10.2337/db07-0100. [DOI] [PubMed] [Google Scholar]
- 76.Byetta (exenatide) injection: US prescribing information (revised December 2011) Amylin Pharmaceuticals, Inc.; San Diego, CA: 2011. [Google Scholar]
- 77.Victoza (liraglutide [rDNA origin] injection) solution for subcutaneous use: US prescribing information (revised April 2012) Novo Nordisk Inc; Princeton, NJ: 2012. [Google Scholar]
- 78.Riddle MC, Henry RR, Poon TH, et al. Exenatide elicits sustained glycaemic control and progressive reduction of body weight in patients with type 2 diabetes inadequately controlled by sulphonylureas with or without metformin. Diabetes Metab Res Rev. 2006;22:483–91. doi: 10.1002/dmrr.646. [DOI] [PubMed] [Google Scholar]
- 79.Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 80.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 81.Anderson SL, Trujillo JM. Association of pancreatitis with glucagon-like peptide-1 agonist use. Ann Pharmacother. 2010;44:904–9. doi: 10.1345/aph.1M676. [DOI] [PubMed] [Google Scholar]
- 82.Lee PH, Stockton MD, Franks AS. Acute pancreatitis associated with liraglutide. Ann Pharmacother. 2011;45:e22. doi: 10.1345/aph.1P714. [DOI] [PubMed] [Google Scholar]
- 83.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem FEBS. 1993;214:829–35. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 84.Heike M, Mobius U, Knuth A, Meuer S, Meyer zum Buschenfelde KH. Tissue distribution of the T cell activation antigen Ta1. Serological, immunohistochemical and biochemical investigations. Clin Exp Immunol. 1988;74:431–4. [PMC free article] [PubMed] [Google Scholar]
- 85.Hegen M, Niedobitek G, Klein CE, Stein H, Fleischer B. The T cell triggering molecule Tp103 is associated with dipeptidyl aminopeptidase IV activity. J Immunol. 1990;144:2908–14. [PubMed] [Google Scholar]
- 86.Fox DA, Hussey RE, Fitzgerald KA, et al. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984;133:1250–6. [PubMed] [Google Scholar]
- 87.Marguet D, Baggio L, Kobayashi T, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A. 2000;97:6874–9. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conarello SL, Li Z, Ronan J, et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:6825–30. doi: 10.1073/pnas.0631828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649–55. doi: 10.2337/dc08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9:194–205. doi: 10.1111/j.1463-1326.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 91.Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naive patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–8. doi: 10.1016/j.diabres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 92.Taskinen MR, Rosenstock J, Tamminen I, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13:65–74. doi: 10.1111/j.1463-1326.2010.01326.x. [DOI] [PubMed] [Google Scholar]
- 93.Ahren B, Foley JE, Ferrannini E, et al. Changes in prandial glucagon levels after a 2-year treatment with vildagliptin or glimepiride in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2010;33:730–2. doi: 10.2337/dc09-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612–9. doi: 10.1210/jc.2006-1009. [DOI] [PubMed] [Google Scholar]
- 95.Tradjenta (linagliptin) tablets: US prescribing information (revised May 2012) Boehringer Ingelheim Pharmaceuticals Inc; Ridgefield, CT: 2012. [Google Scholar]
- 96.Onglyza (saxagliptin) tablets: US prescribing information (revised December 2011) Bristol-Myers Squibb; Princeton, NJ: 2011. [Google Scholar]
- 97.Januvia (sitagliptin) tablets: US prescribing information (revised April 2012) Merck & Co; Whitehouse Station, NJ: 2012. [Google Scholar]
- 98.European Medicines Agency Summary of opinion (post authorisation) - Galvus (vildagliptin) Dec 15, 2011.
- 99.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 100.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 101.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 103.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–57. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 104.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davis RC, Castellani LW, Hosseini M, et al. Early hepatic insulin resistance precedes the onset of diabetes in obese C57BLKS-db/db mice. Diabetes. 2010;59:1616–25. doi: 10.2337/db09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sung KC, Kim SH. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1093–7. doi: 10.1210/jc.2010-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang Y, Ryu S, Sung E, Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin Chem. 2007;53:686–92. doi: 10.1373/clinchem.2006.081257. [DOI] [PubMed] [Google Scholar]
- 108.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–8. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738–45. doi: 10.1002/hep.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 111.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 112.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–82. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garinis GA, Fruci B, Mazza A, et al. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes (Lond) 2010;34:1255–64. doi: 10.1038/ijo.2010.40. [DOI] [PubMed] [Google Scholar]
- 115.Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 116.Mazza A, Fruci B, Garinis GA, Giuliano S, Malaguarnera R, Belfiore A. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012:716404. doi: 10.1155/2012/716404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.de Oliveira CP, Stefano JT, de Siqueira ER, et al. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008;38:159–65. doi: 10.1111/j.1872-034X.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 118.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–5. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 119.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haukeland JW, Konopski Z, Eggesbo HB, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–60. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 122.Omer Z, Cetinkalp S, Akyildiz M, et al. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22:18–23. doi: 10.1097/MEG.0b013e32832e2baf. [DOI] [PubMed] [Google Scholar]
- 123.Nar A, Gedik O. The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetologica. 2009;46:113–8. doi: 10.1007/s00592-008-0067-2. [DOI] [PubMed] [Google Scholar]
- 124.Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obesity Rev. 2010;11:380–8. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- 125.Schoonderwoerd K, Stam H. Lipid metabolism of myocardial endothelial cells. Mol Cell Biochem. 1992;116:171–9. doi: 10.1007/BF01270585. [DOI] [PubMed] [Google Scholar]
- 126.Kuhajda FP, Ronnett GV. Modulation of carnitine palmitoyltransferase-1 for the treatment of obesity. Curr Opin Investig Drugs. 2007;8:312–7. [PubMed] [Google Scholar]
- 127.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 128.Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32(Suppl. 2):S237–45. doi: 10.2337/dc09-S355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Andersen E, Karlaganis G, Sjovall J. Altered bile acid profiles in duodenal bile and urine in diabetic subjects. Eur J Clin Invest. 1988;18:166–72. doi: 10.1111/j.1365-2362.1988.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 130.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–41. [PubMed] [Google Scholar]
- 131.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–24. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 133.Kobayashi M, Ikegami H, Fujisawa T, et al. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes. 2007;56:239–47. doi: 10.2337/db06-0353. [DOI] [PubMed] [Google Scholar]
- 134.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–9. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–11. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wright WL. The management of type 2 diabetes mellitus: a novel approach for addressing glycemic and lipid control with colesevelam HCl. Adv Nurse Pract. 2009;17(suppl):1–16. [PubMed] [Google Scholar]
- 137.Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 138.Edwards PA, Kast HR, Anisfeld AM. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res. 2002;43:2–12. [PubMed] [Google Scholar]
- 139.Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–22. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cariou B, van Harmelen K, Duran-Sandoval D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–49. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 141.Holt JA, Luo G, Billin AN, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–91. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 143.Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–9. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–9. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 145.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 146.Staels B, Handelsman Y, Fonseca V. Bile acid sequestrants for lipid and glucose control. Curr Diabetes Rep. 2010;10:70–7. doi: 10.1007/s11892-009-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 148.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–90. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 149.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Knop FK. Bile-induced secretion of glucagon-like peptide-1: pathophysiological implications in type 2 diabetes? Am J Physiol Endocrinol Metab. 2010;299:E10–3. doi: 10.1152/ajpendo.00137.2010. [DOI] [PubMed] [Google Scholar]
- 151.Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med. 2008;168:1975–83. doi: 10.1001/archinte.168.18.1975. [DOI] [PubMed] [Google Scholar]
- 152.Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479–84. doi: 10.2337/dc08-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531–40. doi: 10.1001/archinte.168.14.1531. [DOI] [PubMed] [Google Scholar]
- 154.Hofmann AF. Bile acid sequestrants improve glycemic control in type 2 diabetes: a proposed mechanism implicating glucagon-like peptide 1 release. Hepatology. 2011;53:1784. doi: 10.1002/hep.24100. [DOI] [PubMed] [Google Scholar]
- 155.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298:G419–24. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- 156.Beysen C, Murphy EJ, Deines K, et al. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia. 2012;55:432–42. doi: 10.1007/s00125-011-2382-3. [DOI] [PubMed] [Google Scholar]
- 157.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 158.Pories WJ, MacDonald KG, Jr., Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55:582S–5S. doi: 10.1093/ajcn/55.2.582s. [DOI] [PubMed] [Google Scholar]
- 159.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–50. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–84. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Clements RH, Gonzalez QH, Long CI, Wittert G, Laws HL. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. [PubMed] [Google Scholar]
- 162.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–7. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–7. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]