Scribble is required for normal epithelial cell–cell contacts and lumen morphogenesis in the mammalian lung (original) (raw)
Abstract
During lung development, proper epithelial cell arrangements are critical for the formation of an arborized network of tubes. Each tube requires a lumen, the diameter of which must be tightly regulated to enable optimal lung function. Lung branching and lumen morphogenesis require close epithelial cell–cell contacts that are maintained as a result of adherens junctions, tight junctions and by intact apical–basal (A/B) polarity. However, the molecular mechanisms that maintain epithelial cohesion and lumen diameter in the mammalian lung are unknown. Here we show that Scribble, a protein implicated in planar cell polarity (PCP) signalling, is necessary for normal lung morphogenesis. Lungs of the Scrib mouse mutant Circletail (Crc) are abnormally shaped with fewer airways, and these airways often lack a visible, ‘open’ lumen. Mechanistically we show that Scrib genetically interacts with the core PCP gene Vangl2 in the developing lung and that the distribution of PCP pathway proteins and Rho mediated cytoskeletal modification is perturbed in Scrib Crc/Crc lungs. However A/B polarity, which is disrupted in Drosophila Scrib mutants, is largely unaffected. Notably, we find that Scrib mediates functions not attributed to other PCP proteins in the lung. Specifically, Scrib localises to both adherens and tight junctions of lung epithelia and knockdown of Scrib in lung explants and organotypic cultures leads to reduced cohesion of lung epithelial cells. Live imaging of Scrib knockdown lungs shows that Scrib does not affect bud bifurcation, as previously shown for the PCP protein Celsr1, but is required to maintain epithelial cohesion. To understand the mechanism leading to reduced cell–cell association, we show that Scrib associates with β-catenin in embryonic lung and the sub-cellular distribution of adherens and tight junction proteins is perturbed in mutant lung epithelia. Our data reveal that Scrib is required for normal lung epithelial organisation and lumen morphogenesis by maintaining cell–cell contacts. Thus we reveal novel and important roles for Scrib in lung development operating via the PCP pathway, and in regulating junctional complexes and cell cohesion.
Keywords: Scribble, Lung, Branching morphogenesis, Lumen, PCP, Polarity
Highlights
► Scrib signals via the PCP pathway to regulate lung branching morphogenesis. ► Knockdown of Scrib leads to reduced cohesion of lung epithelial cells. ► Scrib is required for normal epithelial organisation and lumen morphogenesis in embryonic lung.
Introduction
Lung organogenesis involves the formation of a network of epithelial tubes with an extensive surface area to support postnatal respiration. New tubes are formed by budding of groups of polarised epithelial cells from an existing tube (Andrew and Ewald, 2009; Hogan and Kolodziej, 2002; Nelson, 2003). In the mouse, the spatial pattern of lung branches is remarkably stereotypical and is generated by three modes of local branching, named domain branching and planar and orthogonal bifurcation (Metzger et al., 2008).
Establishment and maintenance of a central lumen within each epithelial tube is a key step in tubulogenesis that allows efficient transport of liquids or gases (Andrew and Ewald, 2009; Chung and Andrew, 2008; Paul et al., 2003). Moreover, lumen diameter must be carefully regulated to facilitate optimal organ function (Datta et al., 2011). Current understanding of the molecular mechanisms of mammalian lumen morphogenesis is limited, yet disrupted lumen diameter is a feature of many human diseases such as polycystic kidney disease, hypertension and ischemic injury. In the lung, understanding the mechanisms used to establish and maintain lumen size may be important for treatment of cystadenomatoid malformations, pulmonary hypertension and even asthma, in which narrowing of the upper airways occurs.
Preserving sufficient lumen diameter requires maintenance of close contacts between epithelial cells through adherens junctions and tight junctions (Chung and Andrew, 2008; Lubarsky and Krasnow, 2003; Martin-Belmonte and Mostov, 2008). Formation of these junctional complexes is underpinned by the establishment of A/B polarity, characterised by similarly aligned cells with their basal sides immediately adjacent to the basement membrane and their apical sides adjacent to the lumen (Martin-Belmonte and Mostov, 2008; Martin-Belmonte et al., 2008). In lung branching morphogenesis, lumina are not formed de novo, but instead, new tubes arise from clefting or budding of existing tubes containing polarised epithelial cells so that the lumen of the new bud/branch is continuous with the lumen of the existing branch (Andrew and Ewald, 2009; Chung and Andrew, 2008; Hogan and Kolodziej, 2002). Initially, the lumen has a narrow diameter and this subsequently widens as the tube matures to its optimal size (C.D. unpublished observations). Although it is known that establishment of ion channels and secretion of fluid into the luminal space in utero play a role in regulating lung lumen diameter (Wilson et al., 2007), epithelial cells must first establish and preserve A/B polarity, undergoing considerable dynamic cell shape changes, mediated by the cytoskeleton, in order to adopt the morphology necessary to encompass a lumen. Moreover, it is essential that strong cell–cell interactions be maintained, to preserve the luminal space (Andrew and Ewald, 2009).
Scribble is a large cytoplasmic protein containing multiple domains including 4 PDZ domains (Bilder and Perrimon, 2000; Nakagawa and Huibregtse, 2000; Nakagawa et al., 2004). In Drosophila, Scrib is initially located at the basolateral membranes of epithelial cells and later in development becomes more restricted to septate junctions (Bilder and Perrimon, 2000). In mammalian cells in vitro, Scrib is observed at the plasma membrane where it has been shown to influence certain adherens and tight junction proteins including E-cadherin, β-catenin, ZO-1 and ZO-2 (Ivanov et al., 2010a; Metais et al., 2005; Navarro et al., 2005; Qin et al., 2005; Yoshihara et al., 2011). However these studies have reported divergent data concerning the interaction of Scrib with junctional proteins and to date, the mechanism is still unclear. It is notable that mice have only one Scrib gene, in contrast to many of the major apical–basal and planar polarity proteins which are represented by multiple family members.
Scribble acts as a tumour suppressor (Etienne-Manneville, 2009): Drosophila Scrib null mutants exhibit disorganization of epithelial tissues, leading to neoplastic growth and multilayering of epithelial cells (Bilder et al., 2000; Bilder and Perrimon, 2000) and SCRIB expression is decreased in a number of human cancers (Gardiol et al., 2006; Ivanov et al., 2010a; Navarro et al., 2005; Pearson et al., 2011; Thomas et al., 2005). Related to its tumour suppressor role, Scrib has been shown to play a part in maintaining contacts between epithelial cells (Dow et al., 2007; Qin et al., 2005) and in regulating the assembly of tight junctions in intestinal epithelium (Ivanov et al., 2010a).
Drosophila Scrib is required to maintain A/B polarity as part of a polarity protein complex, along with lethal giant larvae (Lgl) and discs large (Dlg); knockdown of Scrib disrupts Drosophila A/B polarity (Humbert et al., 2008). In contrast, most mammalian investigations have shown that Scrib operates within the PCP pathway, to regulate planar cell polarity (Montcouquiol and Kelley, 2003; Montcouquiol et al., 2003; Murdoch et al., 2003; Vandenberg and Sassoon, 2009; Wansleeben et al., 2010). In addition, Scrib has previously been shown to genetically interact with Vangl2; double heterozygotes exhibit defects such as craniorachischisis and disrupted stereociliary bundle orientation that are indicative of planar polarity pathway defects (Montcouquiol et al., 2003; Murdoch et al., 2001). Interestingly, a recent study revealed that Scrib does play a role in establishing PCP in Drosophila, in addition to its well-characterized role in A/B polarity (Courbard et al., 2009), and one study demonstrated mild A/B polarity defects in mammary epithelial cells (Courbard et al., 2009; Zhan et al., 2008). In fact, Drosophila studies show that PCP and A/B polarity pathways are closely linked at the molecular level (Courbard et al., 2009; Djiane et al., 2005) and it may be that many epithelial tissues require both A/B polarisation and planar polarisation for optimal organisation and function.
Given the known functions of Scrib in cell polarity and epithelial organisation along with our previous studies showing the importance of PCP proteins in lung development, we investigated lung morphogenesis in the Scrib mouse mutant Circletail. Here we show that Scrib Crc/Crc lungs are irregularly shaped and contain fewer epithelial branches. Branches are comprised of disorganised epithelial cells with a narrow lumen diameter or, frequently, no lumen at all. Molecular analysis reveals no overt disruption to A/B polarity but significant perturbation of the actin–myosin cytoskeleton. Moreover, there are reduced levels of active RhoA and altered localisation of the PCP proteins Vangl2 and Celsr1, consistent with Scrib operating within the PCP pathway during lung development. We also show a genetic interaction between Scrib and the core PCP gene Vangl2 in embryonic lung. Additionally, our studies reveal unique roles for Scrib that have not been attributed to other previously studied PCP genes in lung development. Time-lapse imaging of lung branching morphogenesis in the presence of Scrib antisense morpholinos reveals reduced cohesion between epithelial cells. Moreover, in vivo, Scrib interacts with the adherens protein β-catenin in lung tissue. Further functional studies show mislocalisation of some tight and adherens junction proteins in Scrib Crc/Crc lungs. These defects in epithelial tubulogenesis are mimicked in vitro, where Scrib knockdown in organotypic cultures results in cysts comprised of disordered cells, small or absent lumina and disrupted sub-cellular localisation of β-catenin, ZO-2 and ZO-1. Our data reveal the importance of Scrib function during normal mammalian lung tubulogenesis, particularly in sustaining lumen diameter.
Materials and methods
Mouse strains and genotyping
Scrib Crc mice, originally described in Rachel et al.( 2000) were maintained on a C3H/HeH background. Scrib Crc mice carry a single base insertion (Murdoch et al., 2003) and were genotyped by PCR amplification of flanking SNPs at 74.88 and 76 Mb (primer sequences available on request) with an annealing temperature of 62 °C and 38 cycles, followed by pyrosequencing. Using limb morphology as an indicator of developmental age, we found no evidence of developmental delay in homozygous mutant embryos compared to wildtype.
Morphometric analysis
Transverse sections of E14.5 or E18.5 left lung lobes stained with H&E were used to measure the width and number of airways. Sections were obtained from equivalent levels along the rostral–caudal lung axis. Airway widths were calculated by measuring the diameter of each airway at its widest point, using the scale bar tool from Zeiss Axiovision software. The mean width of airways was then determined from all of the airways in a field of view taken from 6 sections per sample and using an n of 4 individual samples per genotype. The number of airways was determined by counting the total number of airways within a section from 6 sections per lung sample from an n of 4 individual embryos per genotype.
The ratios of lumen area versus total epithelial airway area at E14.5 were calculated using Velocity 5.4.1 software. Calculations were obtained by measuring the airways present in 5 mutant and 5 wildtype H&E stained transverse lung sections taken from equivalent regions along the rostral–caudal axis of at least 4 separate lungs. The data presented show the percentage of the airway occupied by a lumen. For cell density calculations, the ratio of the number of nuclei: basal airway perimeter in μM was calculated for 33 wildtype and 34 mutant airways from equivalent regions of 4 individual lungs per genotype.
Antibodies, immunostaining and immunoblotting
Four micrometer paraffin sections or 10 μm cryosections were stained with haematoxylin and eosin or immunostained using antibodies as previously described (Dean et al., 2005; Yates et al., 2010) or with antibodies to: Celsr1 1:1000 (Formstone et al., 2010); Scrib 1:200 (C-20 or H-300); aquaporin-5 1:400; ZO-2 1:200, CC-10, 1:1000; using antigen retrieval, Santa Cruz; ZO-1 1:125; rhodamine phalloidin 1:40, Invitrogen; E-cadherin 1:1000, Cell Signaling Technology; Claudin-18 Zymed Laboratories. Incubations were overnight at 4 °C.
Immunoblotting was carried out using 10 μg/lane of E13.5 wildtype and Scrib Crc/Crc (_n_=at least 3 per genotype) whole lung protein extract using antibodies as above or to phospho-PKC 1:1000, Cell signalling technology, GAPDH-HRP 1:3000 or anti-β tubulin 1:10000 for loading controls.
Immunoprecipitation and Rho pull-down assay
Whole lung extracts were prepared by lysing the tissue in 50 mM Tris pH8, 150 mM NaCl, 1% NP-40 substitute, Complete EDTA-free protease inhibitors (Roche) and PhosSTOP phosphatase inhibitors (Roche). Immunoprecipitation of β-catenin complexes from whole lung extracts was performed using Dynabeads-Protein G Immunoprecipitation kit (Invitrogen) according to the manufacturer's instructions. 4 μg of anti-β-catenin antibody (Abcam ab16051) was incubated with 50 μl of Dynabeads-Protein G and allowed to bind for 10 min. After washing, antibody/beads complexes were added to 1 mg of tissue extract and incubated for 2 h at room temperature. Immunocomplexes were then washed and eluted in electrophoresis LDS loading buffer (Invitrogen).
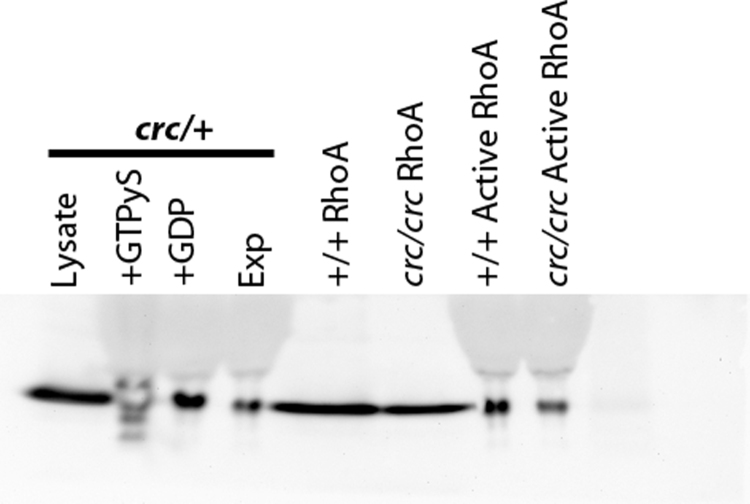
Activated Rho was pulled down from wildtype and Scrib _Crc/Cr_c lungs using the Active Rho pull-down and detection kit (Pierce) with modifications as described (Karner et al., 2009), followed by immunoblotting for RhoA using 8 μl/lane of E13.5 wildtype and Scrib Crc/Crc whole lung protein extract with total RhoA antibody (Cell Signaling 1:1000). Quantification was calculated as the ratio of total RhoA/loading control: active RhoA/loading control from 4 samples per genotype, run on three separate blots. All controls recommended in the kit were run on each Western blot.
Explant cultures
Left lung lobes were isolated from E11.5 mice and cultured as described (Dean et al., 2005).
Organotypic culture and Scrib morpholino knock-down
E12.5 lungs were dissected, removing the primary bronchi and digested in 2 mls 0.3% trypsin, 45 min 37 °C. Lungs were transferred into 2 ml 1:1 DMEM:F12 Invitrogen and 10% FBS, non-essential amino acids, 200 mM glutamine and 50 μg/ml Penicillin–streptomycin (Sigma). Lung tissue was disassociated into a single cell suspension with a 23-gauge needle and centrifuged at 1000 rpm 5 min. Cells were re-suspended, counted and diluted 1:1 in Geltrex (Invitrogen) before adding control (CCTCTTACCTCAGTTACAATTTATA 3′ fluorescein) or Scrib (GAGCGGGATGCACTTCAGCATGATG 3′ fluorescein) morpholinos, MO (Gene Tools) to a final concentration of 10 μM and then plated in a slide chamber (Lab Tek II). After 24 or 48 h cultures were fixed and immunostained.
Morpholino knock-down of Scrib function and time-lapse movies
Intact E11.5 lungs from transgenic mice expressing GFP from the beta-actin promoter (Hadjantonakis et al., 1998) were cultured as described (Yates et al., 2010). Specific morpholino oligonucleotides against Scrib or control (above, Gene-Tools) were added to the media at final concentration of 15 μM at day zero (Dean et al., 2005). Lung explants were cultured for 48 h and then either fixed for immunofluorescence or used for time-lapse imaging on a Zeiss LSM 510 confocal microscope equipped with a controlled stage incubator. Images were taken every 4 min over a period of 24 h. Acquired time-lapse images were exported using LSM software and saved as Quick Time movies using 20 frames/s.
Statistical analysis
All statistics and image analyses were computed using Excel, Graphpad, Velocity and Imaris software. Mann–Whitney _U_-test was conducted for image analysis shown in Fig. 4 comparing the orientation of cell migration in lung explants. Error bars represent standard error of mean and significance was scored using unpaired two-tailed _t_-tests.
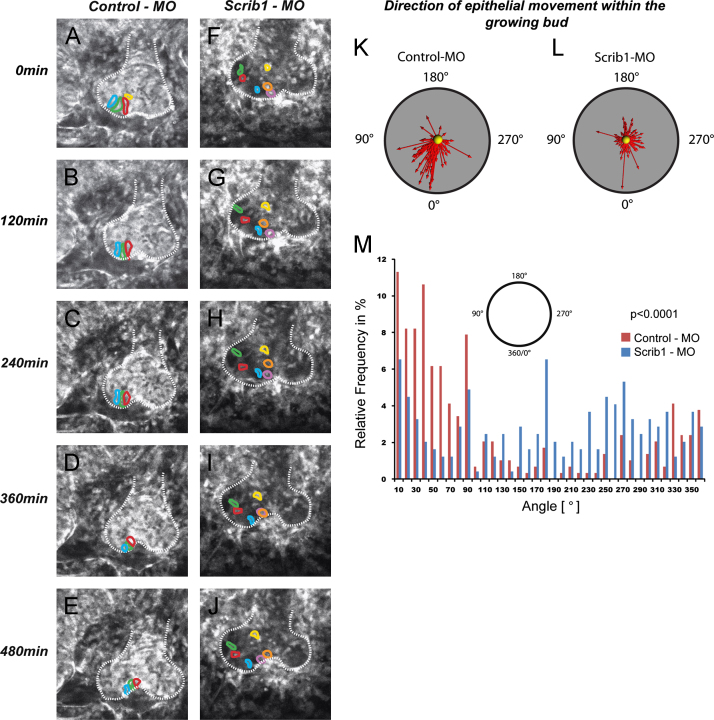
Fig. 4.
Morpholino knock-down of Scrib1 leads to misalignment of epithelial cells in ex vivo lung culture. E11.5 lung explants from β-actin promoter driven GFP embryos were cultured for 48 h with control or Scrib1 morpholinos and subsequently imaged over a period of 24 h (see Movies 1 and 2). During this 24 h time period, Scrib protein levels reduce from 80% of wildtype to 50%. Five time points from this series are depicted. Selected cells from control (A–E) or Scrib1 MO (F–J) treated explants were highlighted with different colours and their movements tracked over time. Epithelial cells from control MO treatment maintain a close neighbor–neighbor relationship, whereas with Scrib1 MO treatment, the cells are more loosely associated and often change their position (in particular, compare the relationship between the cells marked in red and green and in purple and blue and in purple and orange). The distribution of the direction of epithelial cell movements revealed by tracking ∼150 cells within the distal epithelium shows a close to random pattern in _Scrib1_-MO treated lung explants (L) compared to control (K) which shows more directional cell movements. Graphical representation showing the frequency with which cells migrate at each angle (M). P<0.0001, Mann–Whitney U test.
Results
Scrib is required for normal lung development
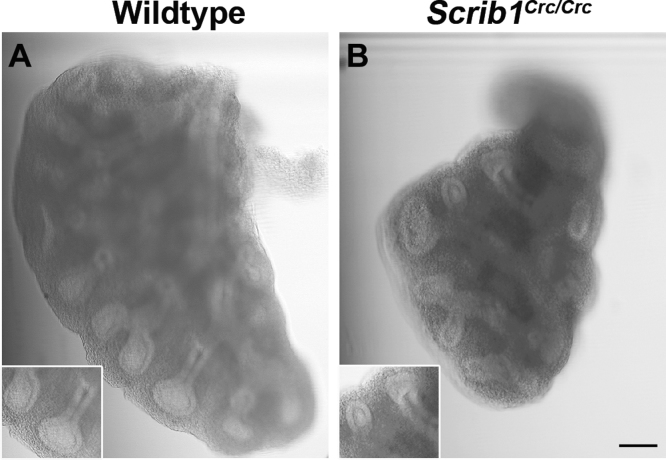
The Circletail mutation disrupts the function of mouse Scribble (Murdoch et al., 2003). Lungs from Scrib Crc/Crc mutant embryos show gross developmental abnormalities (Fig. 1). Murine lungs initiate from the ventral foregut at approximately E9.5 and by E11.5 all five primordial lobes are visible. Gross morphology of Scrib Crc/Crc lungs appeared unaltered at E11.5 but was markedly disturbed at E14.5 as the lung lobes were misshapen and smaller than controls (Fig. 1A and B, Fig. S1,A and B Supplementary material). At E18.5 the distorted shape and reduced size of Scrib Crc/Crc lungs was pronounced (Fig. 1D compared to wildtype in C).
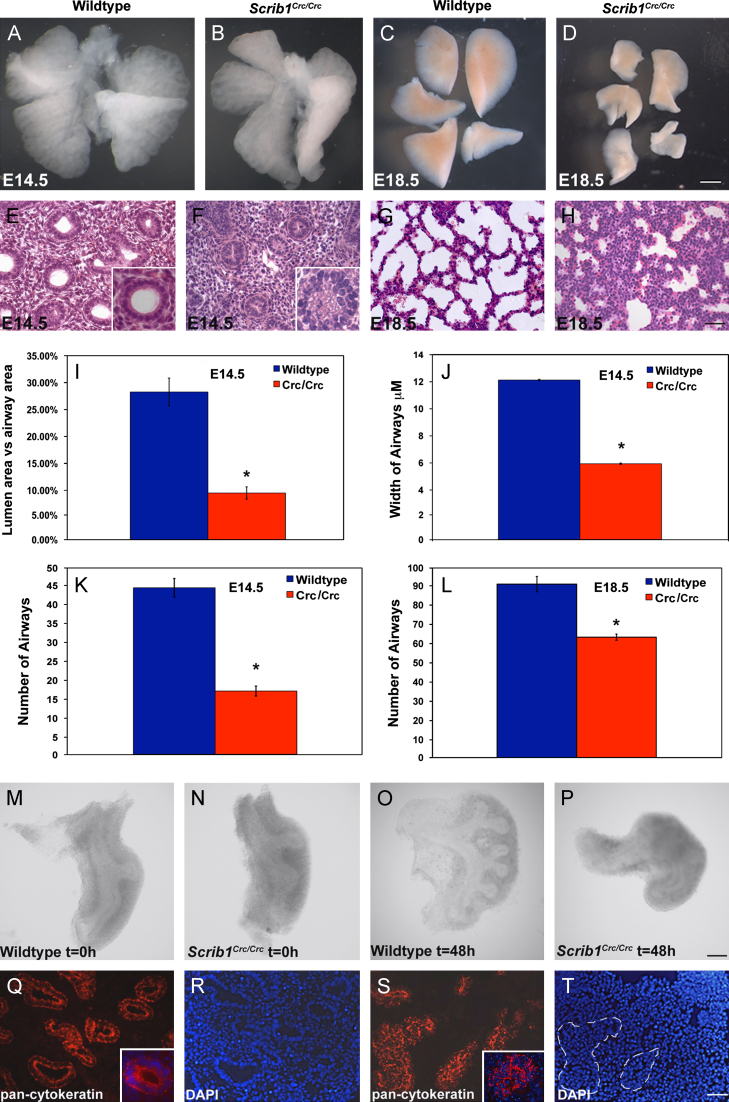
Fig. 1.
Scrib1 is required for normal lung development. E14.5 ((A) and (B)) and E18.5 ((C) and (D)) wildtype ((A) and, (C)) and Scrib1 Crc/Crc lungs ((B) and, (D)) show mutant lung lobes are smaller and misshapen. Histological analysis of E14.5H&E stained sections ((E) and (F)) shows fewer, less organised epithelial airways in Scrib1 Crc/Crc lungs ((F), (K): wildtype mean=44.42 μM, ±2.43, Crc mean=17.17 μM, ±1.64), with reduced lumen area ((I): wildtype mean=28.2%, ±2.93, _n_=39, Crc mean=9.50% ±1.12, _n_=35), compared with wildtype ((E), (I) and (K)). Quantification of airway width highlights the reduced diameter of Scrib1 Crc/Crc lumina ((J): Crc mean=5.94 μM, ±0.05 compared to wildtype mean=12.13 μM, ±0.05). By E18.5 severe hypoplasia is evident, with significant reduction in airway number in Scrib1 Crc/Crc lungs ((H), (L): mean=63.33 μM, ±1.61) compared to wildtype ((G), (L): mean=90.89 μM, ±3.97). E11.5 left lung lobes from wildtype (M) and Scrib1 Crc/Crc (N) with identical numbers of buds at _t_=0 were cultured ex vivo. After 48 h Scrib1 Crc/Crc lungs had developed far fewer new buds (P) than wildtype lungs (O); representative images for each timepoint are shown. Immunostaining of E14.5 transverse cryosections with anti-pan-cytokeratin ((Q) and (S)) highlights disorganisation of epithelial cells in Scrib1 Crc/Crc lungs (S) compared to controls (Q). Epithelial cells are difficult to distinguish by DAPI staining in Scrib1 Crc/Crc (T) but are easily distinguished in wildtype (R). Scale bars; A–D 62.5 μM, E–H 25 μM, M–P 63 μM, Q–T 25 μM, insets in E, F 5 μM; *P<0.05.
Histological analysis showed that lung epithelial and mesenchymal cells were densely packed within the mutant tissue; most notably Scrib homozygous lungs contained far fewer visibly open lumina at E14.5 (Fig. 1E and F). Quantification revealed a 33% reduction in lumen area as a percentage of total airway area in Scrib Crc/Crc (Fig. 1I), and a significant reduction in both the width (Fig. 1J) and total number of airways (Fig. 1K) compared to wildtype littermate lungs. Epithelial cell organization within airways was altered from simple columnar epithelium predominant in wildtype lung sections to multilayered, apparently pseudostratified epithelium in Scrib homozygotes. By E18.5, H&E staining revealed reduced septation resulting in fewer, narrower sacculae (Fig. 1G, H and L).
As Scrib Crc/Crc mutant embryos ultimately have a restricted intrathoracic space, which can impact branching morphogenesis, we performed ex vivo culture to compare development of wildtype and Scrib Crc/Crc lungs without this possible secondary effect. Starting with the same number of terminal buds in wildtype and mutant E11.5 lungs at _t_=0 (Fig. 1M and N), after 48 h culture Scrib Crc/Crc lungs were smaller with significantly fewer end buds (wildtype mean 8.5, ±0.33, _n_=13; Crc mean 2.75,±0.75 _n_=4, _p_=0.0001; Fig. 1O and P). Thus the Scrib mutation in Crc alters lung morphology resulting in fewer epithelial airways with absent or narrower diameter lumina and later on, to fewer, narrower sacculae. We were unable to examine postnatal lung function in _Scrib_Crc/Crc mice as they die at birth from neural tube defects, though it is likely that the defects observed would severely impact lung function.
Disruption of Scrib perturbs epithelial tube formation
To explore the cellular basis for the Scrib Crc/Crc lung defects, we analyzed the expression of markers of lung epithelial differentiation and determined whether cell proliferation or cell death was altered. Immunostaining of E14.5 lung sections with pan-cytokeratin to visualize epithelial airways and DAPI to detect the nuclei showed in wildtype lungs that epithelial cells were easily distinguished from mesenchyme and that the simple columnar epithelial cells were regularly arranged around a distinct central lumen (Fig. 1Q and R). In contrast, Scrib Crc/Crc epithelial cells were not properly aligned with respect to each other and in the majority of cases a lumen was barely visible (Fig. 1S and T), indicating severe defects in epithelial tube structure, consistent with data above showing that many Scrib Crc/Crc airways have narrow lumina and the epithelial cells appear pseudostratified.
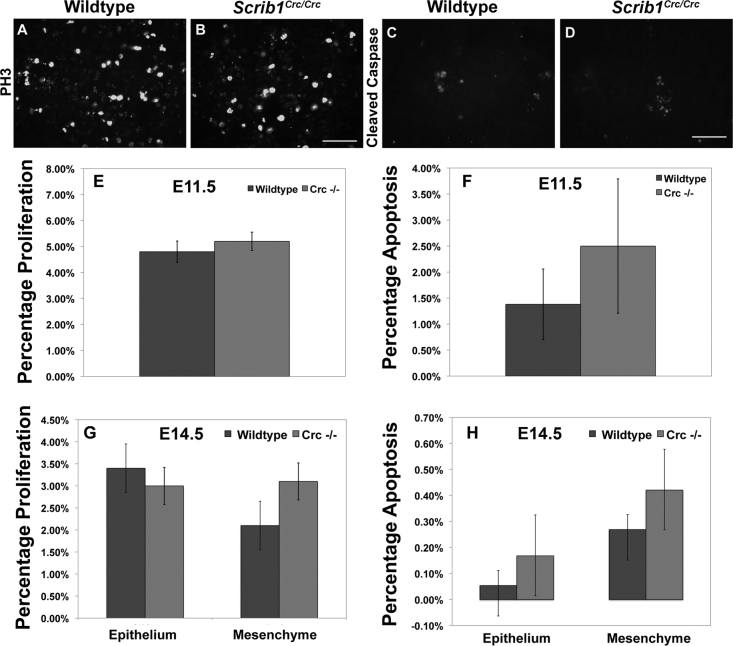
As Scrib Crc/Crc lungs were smaller than controls, we examined cell proliferation and apoptosis at both E11.5 and E14.5. No significant differences relative to wildtype were detected at either time-point in the percentage of cells positive for phospho-histone H3 (PH3) (Fig. S2A, B, E and G) or cleaved caspase 3 (Fig. S2C, D, F and H), indicating the reduced size of Scrib Crc/Crc lungs is not due to changes in proliferation or apoptosis. Calculation of the mean number of nuclei per airway area, however, revealed that cell density was significantly increased in mutant lungs from 0.10±0.003 (_n_=33) in wildtype, to 0.14±0.004 (_n_=34) in Scrib Crc/Crc (_p_=<0.001). This suggests that disrupted cellular organisation and a loss of lumen/air space, may account for the reduction in lung size.
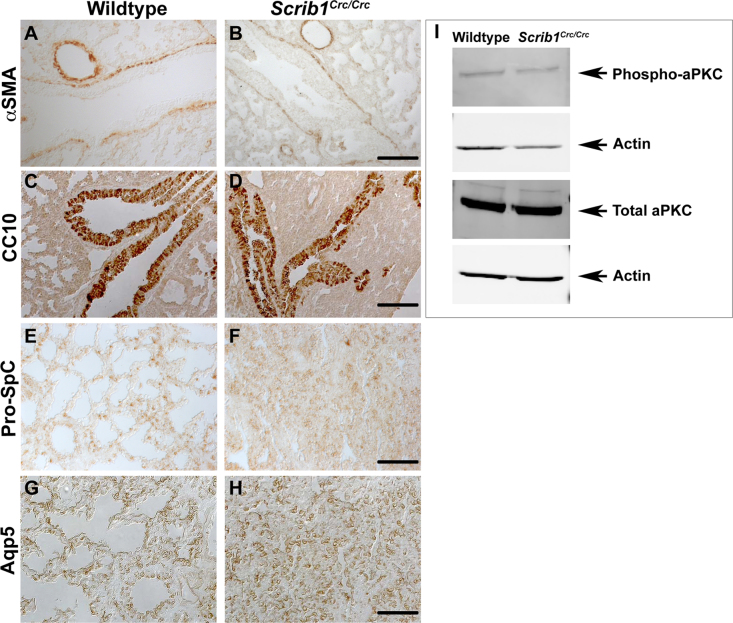
To assess whether cellular differentiation was affected, E18.5 lung sections were immunostained with α-smooth muscle actin (_α_–SMA, proximal airway marker), the Clara cell marker, CC-10, pro-Surfactant protein C (pro SP-C, a marker of distal type II alveolar cells) and aquaporin-5 (type I alveolar cells (Fig. S3 Supplementary material). No differences in expression of these markers were observed between Scrib Crc/Crc and wildtype littermates. Thus it appears that the morphological changes evident in Scrib Crc/Crc lungs do not result from altered proliferation, apoptosis or differentiation but instead result from a disruption in epithelial organization, leading to reduced air space volume.
Scrib Crc/Crc lung epithelia display cytoskeletal defects but A/B polarity is not affected
Our previous studies showed that Celsr1 and Vangl2 proteins signal via the PCP pathway to regulate cytoskeleton dynamics that are necessary for lung branching morphogenesis (Yates et al., 2010). Since Scrib can effect both PCP and A/B polarity (Bilder and Perrimon, 2000; Courbard et al., 2009; Montcouquiol et al., 2003; Zhan et al., 2008), we wished to ascertain the relevant pathway(s) through which Scrib was acting to regulate lung development.
We carefully assessed whether the Circletail mutation leads to disrupted A/B polarity in E14.5 transverse lung sections. Immunostaining for the apical membrane marker aPKCζ and the basement membrane component Laminin revealed no major disruption to A/B polarity (Fig. 2B compared to wildtype in Fig. 2A). This was confirmed by triple-labelling with aPKCζ, DAPI and the Golgi marker GM-130 (Fig. 2C and D). Western blotting revealed no difference in levels of either total or phospho- aPKC between wildtype and Scrib Crc/Crc lungs, indicating that the activity of this apical protein was unaffected (Fig. S3 Supplementary material). In addition, there was no evidence of aberrant A/B polarity by TEM analysis of wildtype and Scrib Crc/Crc lung epithelium (Fig. 2E and F).
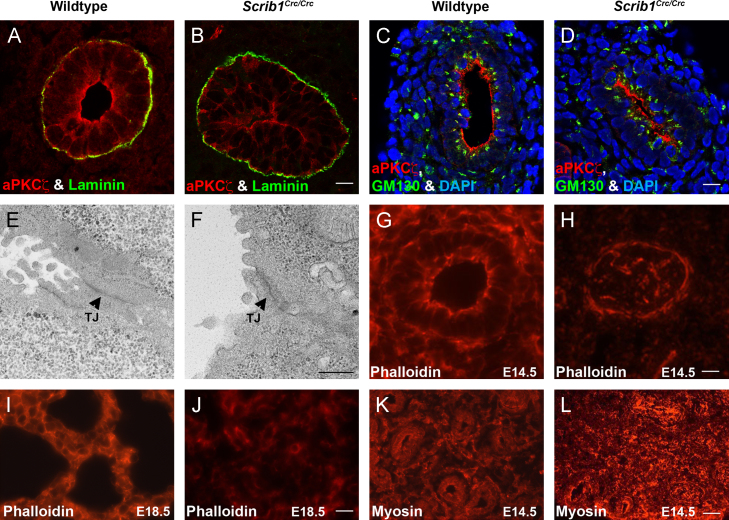
Fig. 2.
Apical–basal polarity appears unaffected but the cytoskeleton is disrupted in Scrib1 Crc/Crc lungs. Immunostaining of E11.5 transverse lung cryosections revealed no obvious defects in A/B polarity in Scrib1 Crc/Crc airways. aPKCς, is present at the apical surface in both Scrib1 Crc/Crc (red (B) and (D)) and wildtype sections (red (A) and (C)). Laminin localises to the basal side of the airways in both wildtype and mutant (green (A) and (B)). The Golgi marker GM130 (green (C) and (D)) is present on the apical side of DAPI-stained (blue (C) and (D)) nuclei in both Scrib1 Crc/Crc (D) and wildtype (C) lung sections. T.E.M. analysis of E16.5 lung revealed no apparent disruption to A/B polarity; in Scrib1 Crc/Crc (F) and wildtype controls (E), airway epithelial cells were orientated with their apical surface (recognised by the presence of microvilli) towards the lumen and apparently intact tight junctions (arrowheads). (TJ), tight junction. E14.5 ((G) and (H)) and E18.5 ((I) and (J)) Phalloidin-stained cryosections reveal severe disruption to the actin cytoskeleton in Scrib1 Crc/Crc ((H) and (J)) lungs; cortical actin was discontinuous and frequently not visible. In wildtype littermates, cortical actin was visible around the cell membranes (G, I). Immunostaining for non-muscle myosin IIA (K, L) also revealed disrupted distribution in Scrib1 Crc/Crc (L) lungs compared to wildtype (K). A–D 125 μM plus ×3 zoom, E–F 5 μM, G–J 5 μM K and L 25 μM.
The end result of activity of the PCP pathway is modification of the cytoskeleton across groups of cells, usually epithelia, to direct coordinated cell movement/organization. To examine the cytoskeleton, lung sections were stained with phalloidin to reveal the filamentous F-actin network. At E14.5, F-actin enrichment normally observed in both the apical and basolateral membranes of wildtype airway epithelial cells (Fig. 2G) was not visible in Scrib Crc/Crc lungs (Fig. 2G and H). Rather than a continuous band of cortical actin highlighting the sub-apical surface, the F-actin in mutant airways was observed in multiple discontinuous patches. At E18.5, phalloidin staining showed further disruption to the F-actin network in Scrib Crc/Crc lungs (Fig. 2I and J). Notably, the fluorescence intensity of phalloidin was lower in the homozygous epithelium and mesenchyme, suggesting a reduction in polymerised actin. Distribution of another critical cytoskeletal protein, non-muscle myosin IIA, was also perturbed in Scrib Crc/Crc (Fig. 2L) compared to control (Fig. 2K). Thus, Scrib mutant lungs show a severe perturbation in cytoskeletal organisation.
Scrib modulates localisation of the core PCP proteins Celsr1 and Vangl2 in lung epithelium and genetically interacts with Vangl2
The relatively normal A/B polarity but disrupted cytoskeleton suggested that Scrib might operate through the PCP signalling pathway in mammalian embryonic lungs. Consistent with this hypothesis, detection of activated RhoA (Rho GTP) by pull-down assay revealed a 60% reduction in levels of Rho GTP, a downstream effector of the PCP pathway, in Scrib Crc/Crc mutant versus wildtype lungs (Fig. 3A, B and Fig. S6 supplemental material).
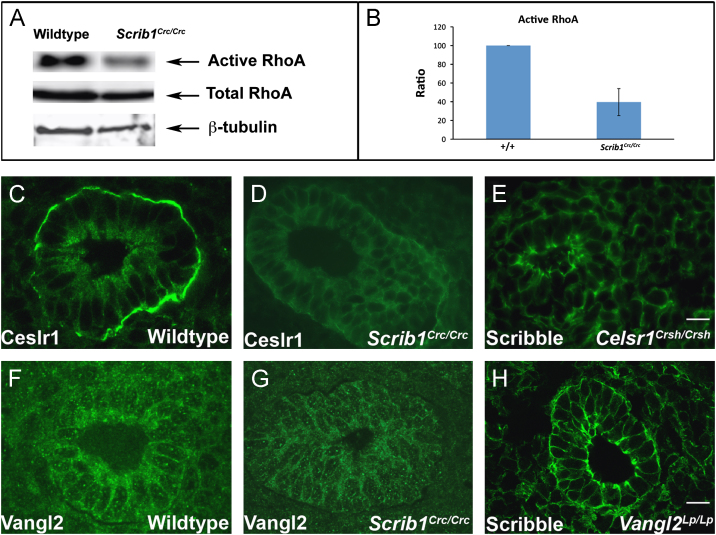
Fig. 3.
Active RhoA is reduced in Scrib1 Crc/Crc lungs and Scrib1 is required for correct localisation of Celsr1 and Vangl2 in lung epithelium. Western blotting (A) reveals a 60% reduction in the relative levels of active RhoA in E13.5 Scrib1 Crc/Crc lungs compared to wildtype littermates following active Rho pull-down and detection with RhoA (21KDa) antibody; (B) relative levels in wildtype: 100; Scrib1 Crc/Crc: 39.6, _p_=0.01, _n_=4 for each genotype. No alteration in levels of total RhoA was observed between Scrib1 Crc/Crc and wildtype (A). Levels of total and RhoA GTP were normalised to loading control levels detected with β-tubulin (50 kDa). E14.5 wildtype transverse lung cryosections immunostained with anti-Celsr1 shows localisation to epithelial cell membranes, at the apical and particularly around the basal side of airways in the basement membrane (C). In Scrib1 Crc/Crc sections, the basement membrane localisation is lost (D). In the Celsr1 Crsh/Crsh mutant, Scrib1 is weaker around epithelial membranes and appears more diffuse (E, compare to Scrib1 localization in wildtype lung I). At E14.5 Scrib1 is localised to the plasma membrane of epithelial and mesenchymal cells and is highly enriched towards the apical surface of airways (I). Scrib1 is absent in E14.5 Scrib1 Crc/Crc lungs (J). Punctate Vangl2 immunostaining is enriched towards the apical surface in wildtype sections (F) but this enrichment is less prominent in Scrib1 Crc/Crc (G). In Vangl2 Lp/Lp airways, Scrib1 immunostaining is unaffected (H). Scale bars; C–G, J 125 μM plus ×3 zoom, H 125 μM plus 2.5 zoom, I 125 μM plus 2 zoom.
To look for additional evidence that Scrib acts within the PCP pathway in lung, we examined whether Scrib modulates the localisation of either Celsr1 or Vangl2 proteins. In E14.5 Scrib Crc/Crc lungs, Celsr1 localization was markedly altered, being redistributed from the polarised, membrane-bound basal location observed in wildtype (Fig. 3C) to a more diffuse distribution around the periphery of epithelial cells as well as in the cytoplasm (Fig. 3D). Staining in the basement membrane was markedly diminished, although the basement membrane was present in Scrib Crc/Crc lung epithelia, and is detected by anti-laminin immunostaining (see Fig. 2B). Vangl2 enrichment towards the apical side of wildtype airways (Fig. 3F) was not observed in Scrib Crc/Crc (Fig. 3G), indicating a subtle change in spatial localisation of Vangl2, in agreement with previous studies (Phillips et al., 2007). In contrast, Scrib protein appeared largely unaltered in Celsr1 Crsh/Crsh and Vangl2 Lp/Lp mutant lungs (Fig. 3E and H) relative to wildtype (Fig. 3I). Scrib Crc/Crc lung tissue immunostained with anti-Scrib almost completely lacked Scrib expression, indicating both specificity of the antibody and that Scrib protein is essentially absent in Circletail lung (Fig. 3J). Thus, in Scrib mutants the asymmetric localization of both Celsr1 and Vangl2 is altered, providing further evidence that Scrib affects the PCP pathway. As further evidence that Scrib acts via the PCP pathway in lung development we intercrossed Scrib Crc/+ and Vangl2 Lp/+ mice (Fig. S4 Supplemental material) and compared the embryonic lungs to wildtype littermate controls (Fig. S4A) and to both Scrib Crc/+ (Fig. S4B) and Vangl2 Lp/+ (Fig. S4C) heterozygotes. Lungs from these compound heterozygotes (Fig. S4B) displaying the open neural tube defect, craniorachischisis, also showed lung defects. The airways of compound heterozygotes displayed a severe phenotype with narrow or absent lumina compared to either wildtype littermates or to Scrib Crc/+ or Vangl2 Lp/+ heterozygotes; although we did observe mild defects in the Vangl2 Lp/+ airways that were distinct from either wildtype or Scrib Crc/+ heterozygotes. The epithelial disorganisation and lung lumen defects present in Scrib Crc/+ ; Vangl2 Lp/+ lungs phenocopied those of Scrib Crc/Crc homozygotes (Fig. 1F) thereby indicating a genetic interaction.
Real-time imaging reveals uncoordinated shifting between lung epithelial cells in the presence of Scrib morpholinos
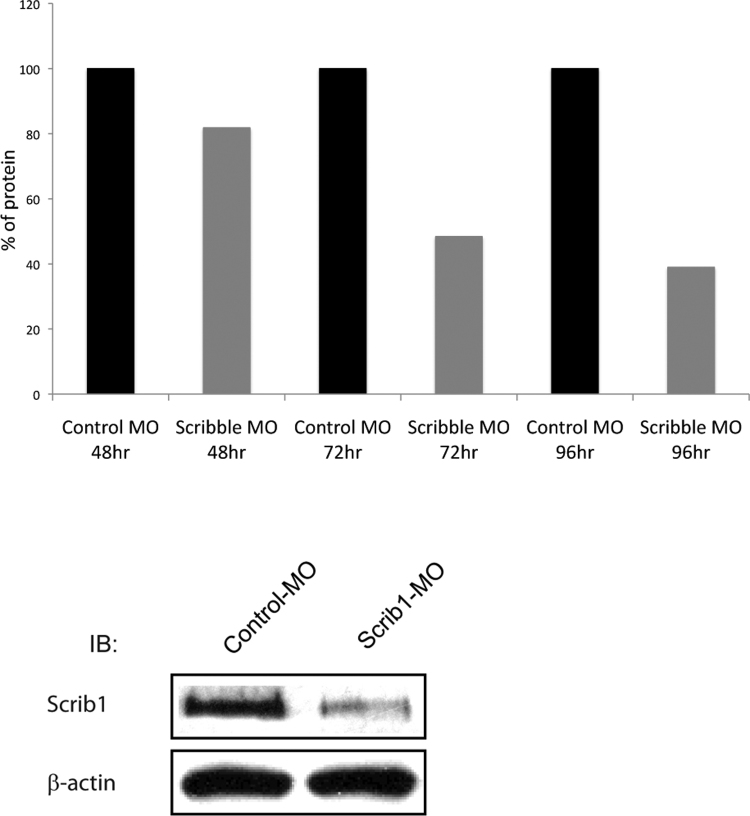
Static images of lung development cannot reveal the dynamic behaviour of cells as they undergo lung morphogenesis. To gain insight into how Scrib regulates epithelial tube morphology we conducted time-lapse video microscopy of wildtype lung explants treated either with control or Scrib morpholinos (MO). Knock-down efficiency was validated by Western blotting to determine the level of Scrib protein in explants after 48, 72 and 96 h in culture (Fig. S5 Supplementary material).
Culture of E11.5 wildtype lungs with Scrib MO resulted in a striking reduction in epithelial cohesion. We observed considerable shifting of individual distal epithelial cells relative to one another, leading to changes in relative positioning of neighbouring cells within forming buds (Fig. 4 and Movie 2, Supplementary material). In control MO lung explants, the epithelial cells maintained their position with respect to their neighbours (Fig. 4 and Movie 1, Supplementary material). Moreover, the direction of epithelial cell movements within the distal epithelium showed a random pattern in Scrib MO treated lung explants relative to the directional cell movements in control MO treated (Fig. 4K, L and M). Thus, upon loss of Scrib function, alignment and close association of epithelial cells were disrupted, suggesting that Scrib functions to regulate epithelial contacts and thereby maintain epithelial integrity and the luminal space.
The following is the Supplementary material related to this article Video 1, Video 2
Video 1
Time-lapse movies of control MO treated β-actin driven GFP lung explants. After 48 h of culture, lungs were transferred to a Zeiss LSM 510 confocal microscope equipped with controlled stage incubator. Images were taken every 4 min over a 24 h period. Acquired time-lapse series were exported using LSM software and saved as Quick Time movies using 20 frames/s.
Video 2
Time-lapse movies of Scribble MO treated β-actin driven GFP lung explants. After 48 h of culture, lungs were transferred to a Zeiss LSM 510 confocal microscope and imaged as described for Movie 1.
Scrib is localised to the cell membranes and associates with junctional proteins
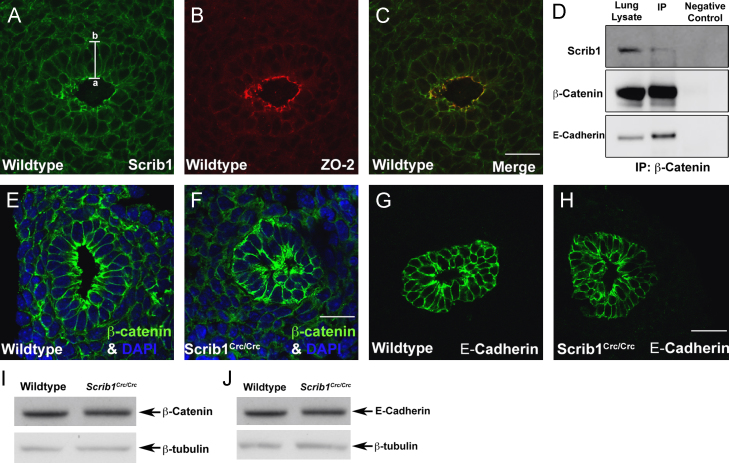
To explore further the mechanism of Scrib function, we first determined the spatial localisation of Scrib in the developing lung. Immunostaining of E11.5 transverse wildtype lung sections showed strongly positive staining towards the apical surface of airway epithelial cells and this co-localized with the tight junction (TJ) marker ZO-2 (Fig. 5A and C). Apical localisation of Scrib is consistent with previous reports in other cells and tissues (Bilder and Perrimon, 2000; Ivanov et al., 2010a; Metais et al., 2005). In addition, lower levels of Scrib were observed around the entire plasma membrane of epithelial and mesenchymal cells (Fig. 5A). To determine which junctional components Scrib physically interacts with in the developing lung we conducted a series of immunoprecipitation experiments using E13.5/14.5 mouse lung lysates. We found a specific interaction between β-catenin and Scrib, indicating that these two proteins physically interact in endogenous lung tissue (Fig. 5D). In contrast we were unable to detect an interaction between ZO-2 and Scrib following separate immunoprecipitation experiments with antibodies to either of these proteins.
Fig. 5.
Scrib1 is localised to the cell membrane and tight junctions and interacts with β-catenin. Transverse cryosection of E11.5 ((A)–(C)) wildtype lung immunostained for Scrib1 ((A) and (C)) shows Scrib1 is localised to membranes of epithelial and mesenchymal cells; strong staining is also observed apically, where Scrib1 co-localises with ZO-2 ((B) and (C)). Immunoprecipitation with β-Catenin antibody revealed an interaction with Scrib1 in endogenous lung tissue, suggesting these proteins physically interact in the lung (D). A thin band of β-catenin is observed around the basolateral membranes of E14.5 wildtype airways and is predominant at the apical membrane (E). In Scrib1 Crc/Crc airways, β-catenin appears diffuse and unevenly distributed around cells (F). E-cadherin distribution is largely unaltered in Scrib1 Crc/Crc airways (G) compared to controls (H). Western blotting shows no significant difference in the quantity of β-catenin (95 kDa) (I) or E-cadherin (120 kDa) protein (J) between E14.5 wildtype and Scrib1 Crc/Crc whole lung. Scale bars; A–C 125 μM plus ×2 zoom, E, F, G and H 125 μM plus ×3 zoom a, apical, b, basal.
Scrib contributes to adherens and tight junction integrity
The interaction of Scrib with β-catenin and its co-localisation with ZO-2 suggested that Scrib contributes to adherens and/or tight junction integrity in lung epithelial cells. We therefore investigated whether adherens and tight junction proteins were normally localised in homozygous mutant airways. E14.5 transverse lung cryosections immunostained for β-catenin showed a thin band of β-catenin around the entire basolateral membrane of wildtype airway epithelial cells, with particularly strong immunostaining towards the apical surface, where the lumen is expanding (Fig. 5E). In Scrib Crc/Crc airways, β-catenin distribution was markedly altered and variable. Thick bands of protein were localised around the basal and lateral sides of many airway cells, whereas in other cells, localisation to lateral membranes was almost absent or restricted to a portion of the lateral membranes only (Fig. 5F). In contrast, E-cadherin localisation appeared largely unaltered in Scrib Crc/Crc versus wildtype airways, although we occasionally noted some subtle but variable changes in E-cadherin such as uneven distribution around the basolateral membranes (Fig. 5G and H). Quantification of β-catenin and E-cadherin by Western blot revealed no change in overall levels of these proteins in Scrib Crc/Crc compared to wildtype lungs (Fig. 5I and J).
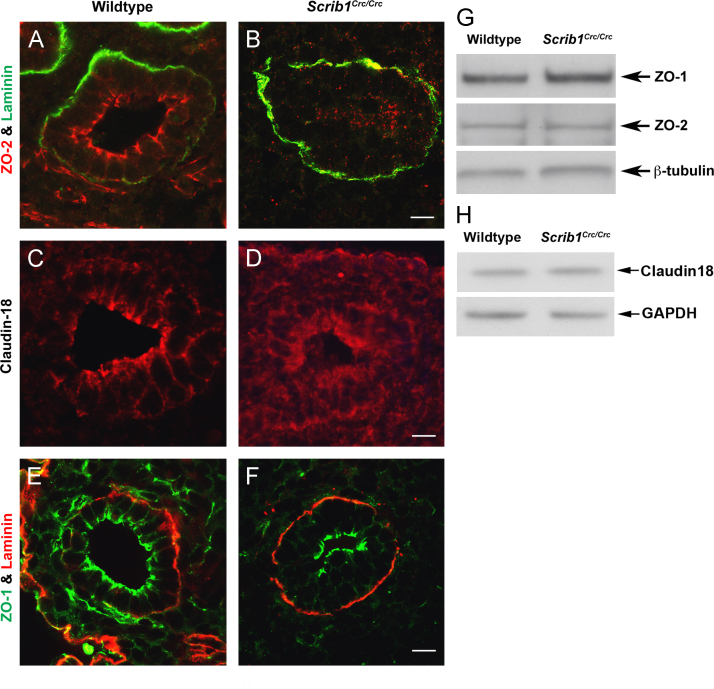
Because the stability of tight junctions is strongly linked to that of adherens junctions and we had previously observed co-localisation of Scrib and the tight junction protein ZO-2 (Fig. 5C), this prompted us to examine the distribution of ZO-2 and additional TJ proteins, ZO-1 and Claudin-18 in Scrib Crc/Crc lung tissue. The sub-cellular distribution of ZO-2 was severely perturbed in Scrib Crc/Crc lung epithelia, although Western blotting revealed no difference in the overall level of ZO-2 between wildtype and mutant lung (Fig. 6A, B and G). The distribution, but not the overall levels, of Claudin-18 was also markedly perturbed in Scrib Crc/Crc lungs. Claudin-18 staining appears more diffuse throughout the mutant epithelium and mesenchyme rather than being predominantly restricted towards the apical surface of wildtype epithelial cells where the tight junctions are located (Fig. 6C, D and H). Interestingly, we did not detect disruption to another zonula occludens protein, ZO-1, in Scrib Crc/Crc lungs (Fig. 6E, F and G), suggesting that the interaction between Scrib and TJ proteins is selective.
Fig. 6.
Specific tight junction proteins are disrupted in Scrib1 Crc/Crc lungs. E14.5 transverse wildtype ((A), (C) and (E)) and Scrib1 Crc/Crc ((B), (D) and (F)) lung sections immunostained for tight junction markers. Apical localisation of ZO-2 is maintained in Scrib1 Crc/Crc lungs (B), but its localisation is abnormal compared to wildtype (A). Claudin-18 is also mislocalised in Scrib1 Crc/Crc epithelial airways (D) compared to wildtype (C). ZO-1 appears normal in Scrib1 Crc/Crc lungs ((E) and (F)). ((G) and (H)) Levels of ZO-1 (220 kDa), ZO-2 (160 kDa) (G) and Claudin-18 (23 kDa) (H) were similar in wildtype and Scrib1 Crc/Crc lungs by Western blot. Scale bars; A–F 125 μM plus ×3 zoom.
Intact adherens and tight junctions are necessary to maintain proper epithelial cell–cell contacts (Gooding et al., 2004; Shin et al., 2006). Our observations that Scrib is localised to both the cell membranes and tight junctions, along with disruption of some tight and adherens junction proteins in Scrib Crc/Crc epithelial airways, supports the hypothesis that Scrib plays a role in maintaining correct cell–cell adhesion. Our data suggest that in Crc mice, disruption of key junctional proteins destabilises airway epithelial cells so that they are unable to maintain their proper alignment and this lack of epithelial cohesion leads to a collapse of the epithelial cells into the lumen and a reduction in luminal area.
Organotypic cultures reveal a role for Scrib in formation and correct organisation of epithelial cysts
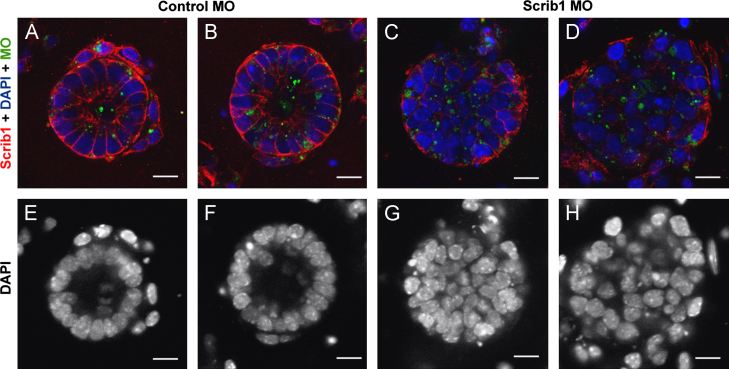
Our data indicate that Scrib plays a role in the maintenance of junctional complexes that are necessary for proper epithelial organisation. To determine whether Scrib is required for the formation of de novo epithelial cell–cell contacts, we performed organotypic cultures using single cell suspensions of mixed epithelial and mesenchyme cells from E12.5 wildtype mouse lung in the presence of either control or Scrib MO. After 48 h culture with control MO, well-ordered cysts had formed, each of which contained a simple columnar epithelium surrounding a visible centrally located lumen (Fig. 7A, B, E and F). In contrast, cells cultured with Scrib MO formed cysts containing disorganized epithelial cells and lacked a visible lumen or had a very narrow diameter lumen (Fig. 7C, D, G and H). Knock-down was confirmed by immunostaining for Scrib in control MO-treated (Fig. 7A and B) and Scrib MO-treated (Fig. 7C and D) organotypic cultures. These findings are consistent with our in vivo results showing that Scrib Crc/Crc lungs contain fewer epithelial branches with a disorganized epithelium and narrow or absent lumina.
Fig. 7.
Scrib1 knockdown prevents normal epithelial cyst formation in organotypic cultures. Morpholino (MO, A–D, green) knockdown of Scrib1 ((C) and (D)) in organotypic cultures inhibits re-association of epithelial cells into organized cysts with a centrally located lumen. Scrib1 ((A)–(D), red) and DAPI ((A)–(H), blue/white) staining reveal organised epithelial cells surrounding a centrally located lumen in cultures containing control MO ((A), (B), (E) and (F)). In the presence of Scrib1 MO, cysts are comprised of misaligned epithelial cells and either a small non-central lumen or, frequently no visible lumen ((C), (D), (G) and (H)). Scale bars; A–H 125 μM ×2 zoom.
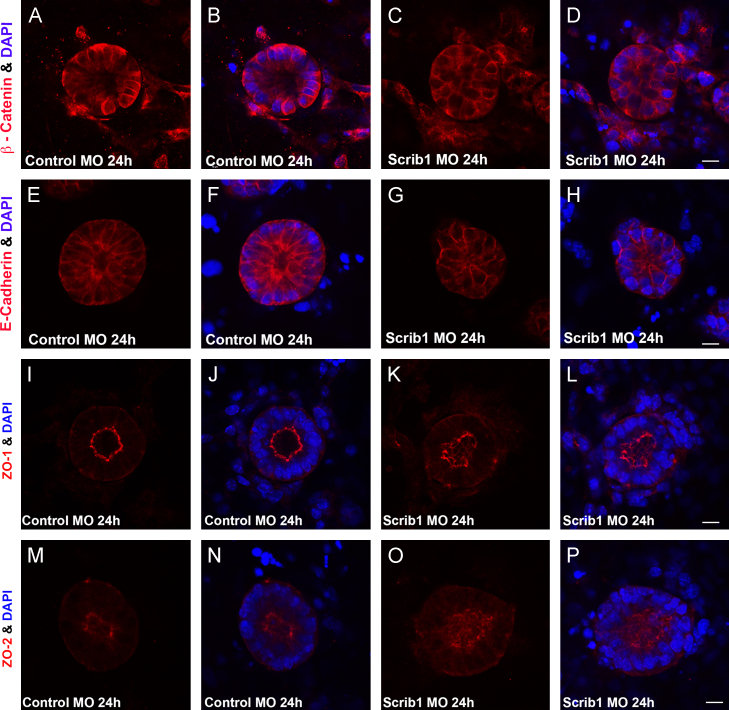
In addition we compared the distribution of junctional proteins in control and Scrib MO-treated organotypic cultures. β-catenin (Fig. 8A–D) and ZO-2 (Fig. 8M–P) displayed significant changes in sub-cellular localisation upon Scrib knockdown whilst E-cadherin (Fig. 8E–H) did not appear to be visibly altered. ZO-1 (Fig. 8I–L) localisation also appeared to be disrupted though the changes were significantly milder than seen for β-catenin or ZO-2. These organotypic culture results are in agreement with our in vivo data and indicate that Scrib loss perturbs the localization of some junctional proteins and disrupts cell–cell contacts necessary to maintain epithelial integrity.
Fig. 8.
Scrib1 knockdown in organotypic cultures disrupts β-catenin and ZO-2 localization. Morpholino (MO) knockdown of Scrib1 ((C),(D), (G), (H), (K), (L), (O) and (P)) in organotypic cultures compared with control MO ((A), (B), (E), (F), (I), (J), (M) and (N)), followed by immunostaining for junctional proteins revealed selective disruption to key junctional proteins,. β-catenin ((A)–(D) red) and ZO-2 ((M)–(P) red) sub-cellular localisation was significantly disrupted upon Scrib1 knockdown ((C), (D) and (O), (P) compared with (A), (B) and (M), (N)). E-cadherin ((E)–(H) red) did not appear to be visibly altered (compare (G) and (H) with control MO (E) and (F)). ZO-1 ((I)–(L) red) localisation in Scrib1 MO cultures ((K) and (L)) appeared mildly disrupted compared with control MO ((I) and (J)). DAPI staining highlights nuclei ((B), (D), (F), (H), (J), (L), (N) and (P) blue). Scale bars; A–P 5 μM plus 6_x_ zoom.
Discussion
Scrib is required for normal lung branching morphogenesis
Our investigation reveals that Scrib is required for normal lung branching morphogenesis.
The Scrib loss-of-function mutation in Circletail mice leads to smaller and abnormally shaped lung lobes. Lung epithelial branches show abnormal distribution of the actin–myosin cytoskeleton and considerable cellular disorganisation; the number and width of airways is reduced and they often lack a visible lumen or contain only a narrow diameter lumen. The finding that Scrib is required to maintain proper lumen diameter in utero provides novel mechanistic insight into the regulation of lumen morphogenesis in the lung. To date, investigations into how lung lumen diameter is regulated have focused on the roles of foetal breathing movements and on the establishment of ion channels, which facilitate the regulation of lumen size via pumping of fluid into the luminal space (Inanlou et al., 2005; Wilson et al., 2007). The studies presented in this manuscript show that perturbation of Scrib leads to loss of normal cell–cell interactions between airway epithelial cells and that this in turn results in abnormal lumen diameter. Mechanistically, through analysis of mutant lungs, the use of lung explant cultures to dynamically observe lung branching and of organotypic cultures to study lumen organization, we show that Scrib regulates epithelial cell contacts and the sub-cellular distribution of adherens and tight junction proteins, most likely through the association of Scrib with β-catenin in the embryonic lung. Finally, Scrib is required for cytoskeletal re-modelling in the developing lungs, which determines the structure of the epithelial tubes, and is also required for the proper distribution of the PCP proteins Celsr1 and Vangl2, mutations of which also cause defects in lung morphogenesis.
Scrib signals via the PCP pathway to regulate lung development
Scrib is able to signal via the A/B and planar polarity pathways, though the pathways utilised appear largely species-specific. Most Drosophila studies show that Scrib is involved in A/B polarity, whilst in mammals Scrib appears to be required for planar polarity. However, a recent Drosophila study examined the effect of a Scrib hypomorph, in which PDZ domains 3 and 4 are absent (Courbard et al., 2009). This hypomorph exhibits PCP defects whilst A/B polarity is unaffected, indicating that Scrib does contribute to planar polarity in Drosophila. It may be that in the complete absence of Scrib, A/B polarity defects are the predominant phenotype and this masks a role in planar polarity. Interestingly, Laprise et al. (2010) have shown that Scrib also affects tube size during Drosophila tracheal development, however unlike in mouse, Scrib mutation in Drosophila leads to longer tracheal tubes due to a broadening of apical membrane size.
The results presented here indicate that, in the mammalian embryonic lung, Scrib operates via the PCP signalling pathway to modulate epithelial tube anatomy, in part via Rho kinase-mediated organisation of the cytoskeleton. In normal lungs, cytoskeletal re-modelling enables organised and coordinated movement of groups of cells (morphogenesis) necessary for the formation and optimal dimensions of epithelial branches. In Scrib Crc/Crc mutants, lung morphogenesis is disrupted, similar to the lungs of other PCP mutants, including Celsr1, Vangl2 and PTK7 (Paudyal et al., 2010; Yates et al., 2010). We have shown a genetic interaction between Scrib and Vangl2 which leads to lung defects similar to those observed in homozygous mutants of either gene i.e., disordered epithelial organisation in airways and narrow or absent lumina. Consistent with published data in other tissues, we show that the Scrib Crc/Crc mutation affects the localisation of the core PCP proteins Vangl2 and Celsr1 in the lung (Courbard et al., 2009; Montcouquiol and Kelley, 2003). Moreover, our data indicates Scrib operates via Rho signalling in the lung as a significant reduction in active RhoA was observed in Scrib Crc/Crc lungs. Furthermore, Scrib Crc/Crc mutant lungs show severe disruption to the actin–myosin cytoskeleton, which is the target of the PCP signalling pathway. Our data show that A/B polarity is largely unaffected in Scrib Crc/Crc lungs, although we cannot completely rule out subtle alterations. Taken together, these data provide evidence that Scrib operates within the PCP pathway to regulate lung development.
Scrib is required to maintain normal cell–cell contact and epithelial junctions
There are clear similarities between some of the phenotypes observed in Scrib Crc/Crc lungs and those of Celsr1 and Vangl2 mutants, as would be expected from components of the same signalling pathway. However, Scrib Crc/Crc lungs are more severely affected than Vangl2 Lp/Lp and Celsr1 Crsh/Crsh lungs (Yates et al., 2010). Macroscopically, Scrib Crc/Crc lungs are smaller, with profound epithelial organisation defects and most ‘tubes’ lack a lumen. In Celsr1 and Vangl2 mutant lungs, most epithelial tubes have a visible lumen, although frequently, their diameter is considerably narrower than in wildtype lungs. All three of these mouse mutants display actin–myosin cytoskeletal defects that affect the overall structure and morphogenesis of lung epithelia.
Scrib also has distinct functions not shared with Celsr1 and Vangl2. Scrib Crc/Crc lungs display defects in cell junctions and reduced cohesion of epithelial cells that are not observed in Celsr1 or Vangl2 mutants. Real-time imaging of ex vivo Scrib morpholino treated lung explant culture revealed reduced cell–cell contact and increased movement of epithelial cells, suggesting that cell adhesion might be affected. Organotypic cultures of Scrib morpholino treated lung cells also highlight the requirement for Scrib function in epithelial organization and lumen formation. Close epithelial cell–cell contacts, maintained by adherens and tight junctions, are critical for tubulogenesis and a pre-requisite for a correctly sized and positioned lumen at the centre of an airway (Affolter and Zeller, 2009; Andrew and Ewald, 2009; Morrisey and Hogan, 2010).
Previous studies have shown co-localisation between Scrib and the junctional proteins β-catenin and E-cadherin, and Scrib has been shown to influence recruitment and localisation of adherens junction proteins in vitro (Kamei et al., 2007; Navarro et al., 2005; Sun et al., 2009). Here we provide evidence that Scrib interacts with β-catenin in endogenous lung tissue. Consistent with such an interaction, the sub-cellular localisation of β-catenin is perturbed in Scrib Crc/Crc lungs.
The interaction between β-catenin and Scrib appears to be specific because E-cadherin localisation was not notably changed in Scrib Crc/Crc lungs. Structurally, β-catenin is an intracellular protein whereas E-cadherin contains both extra- and intra-cellular domains. Moreover, E-cadherin is involved in very early aspects of adherens junction formation, becoming stabilised at the cell surface where as β-catenin is subsequently recruited along with other proteins to form adherens junction complexes (Hartsock and Nelson, 2008). Importantly, β-catenin directly links the junctional complexes in the plasma membrane with the intracellular cytoskeleton, and this connection considerably strengthens cadherin-mediated adhesion (Yap et al., 1997). In addition to β-catenin mislocalisation, we also observed cytoskeletal disruption in Scrib Crc/Crc lung epithelia, both of which are likely to contribute to destabilisation of the epithelial cells. Thus, differences in the functions of β-catenin and E-cadherin may explain why one and not the other is disrupted in Circletail.
Our immunochemistry studies show that Scrib protein is enriched in the tight junctions of lung epithelial cells, in addition to its localization to the plasma membrane. Tight junctions contribute significantly to epithelial cell stability and intact adherens junctions are believed to be required for tight junction formation (Yoshihara et al., 2011). Scrib protein is composed of multiple domains that mediate protein–protein interactions (Kallay et al., 2006; Metais et al., 2005; Petit et al., 2005; Sun et al., 2009). In vitro, Scrib has been shown to interact with ZO-1 and ZO-2 and the interaction with ZO-2 relies on PDZ domains 3 and 4 of Scrib (Ivanov et al., 2010a; Metais et al., 2005). Interestingly, our studies show that ZO-2 is spatially disrupted in Circletail mutants in which a premature stop codon results in a truncated protein lacking PDZ domains 3 and 4 (Murdoch et al., 2003). This domain-specific interaction between Scrib and ZO-2 may explain why ZO-2 localization was perturbed, whereas that of ZO-1 was largely unaffected in Scrib Crc/Crc lungs. Since TEM analysis of tight junctions showed no obvious physical defect in tight junctions of Scrib Crc/Crc lungs, and the overall levels of tight junction proteins were unaltered, this suggests that the destabilisation of epithelial cells is likely due to mislocalisation of specific proteins within the junctional complexes, rather than an absence or mechanical separation of tight junctions. Taken together with the multi-layered disorganized epithelium, severe reduction of visible lumina and increased cell density in Scrib Crc/Crc airways, we propose that disruption of Scrib leads to reduced cohesion between epithelial cells, causing destabilization of the epithelium and filling of the luminal space. Since a large proportion of the normal lung volume is air space, this loss of luminal space leads to a considerable reduction in overall lung size in Scrib Crc/Crc mice. Further evidence of a role for Scrib in cell–cell cohesion comes from our analysis of de novo epithelial cyst formation in lung organotypic cultures. In these assays, Scrib knockdown results in visible cellular disorganisation and absence of a centrally located, expanded lumen as well as mislocalisation of β-catenin and ZO-2. These data are consistent with previous studies showing that Scrib can act as a tumour suppressor by helping to maintain epithelial organisation and that its expression is downregulated in a number of human cancers (Gardiol et al., 2006; Ivanov et al., 2010b; Navarro et al., 2005; Pearson et al., 2011; Thomas et al., 2005; Zhan et al., 2008).
Whilst the observation that there are intact tight junctions in the Scrib Crc/Crc lungs seen by TEM may at first appear inconsistent with the reduced cohesion of epithelial cells in time-lapse imaging of ex vivo lung culture and mislocalisation of adherens and tight junction proteins, we propose that these data are consistent and in fact the mislocalisation of junctional proteins does not lead to a loss of adherens or tight junctions but instead results in weaker cell–cell interactions (reduced cohesion), allowing dynamic dissociation and subsequent re-formation of cellular junctions. This understanding of the data would not have been reached without the use of real-time imaging in 3D culture along with the more traditional 2D imaging techniques at single timepoints.
The relationships between polarity pathways and lung branching morphogenesis
The importance of the PCP signalling pathway for the generation of glands and organs that undergo branching morphogenesis is beginning to be realised. A number of studies have shown that PCP genes are required for lung and kidney organogenesis (Carroll and Das, 2011; Yates and Dean, 2011). However, in the kidney, mutations in genes that disrupt the PCP pathway frequently lead to increased lumen diameter and cyst formation (Fischer et al., 2006; Karner et al., 2009). PCP function is also necessary in other branched structures like the lacrimal and salivary glands as morphogenetic defects in these tissues are observed in PCP mutant mice (C.D. unpublished observations).
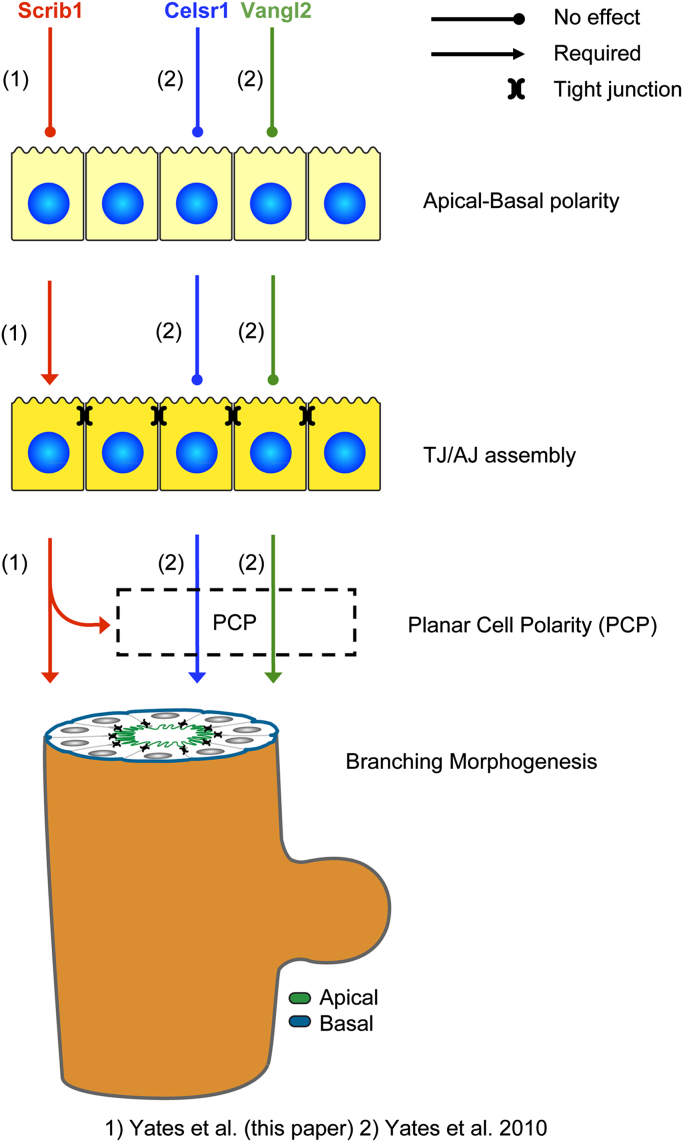
Our investigations into the roles of Celsr1, Vangl2 and Scrib in lung development have allowed us to build an understanding of the relationships between planar polarity, A/B polarity and tight junction formation in lung development (summarised in Fig. 9). Biophysically, these three cellular mechanisms could occur simultaneously and one or more aspects be mediated by the same protein. Our studies indicate that Celsr1, Vangl2 and Scrib are all required for planar polarity and lung branching morphogenesis, However, none of the studied alleles show a disruption in A/B polarity, suggesting that A/B polarity and planar polarity are separate steps required for branching morphogenesis. Tight junction formation may also be a separable step because Scrib loss affects the sub-cellular organisation of tight junction proteins, presumably leading to their disruption, yet these alterations do not appear to affect other A/B polarity markers. This, and other studies, lead us to conclude that A/B polarity is a prerequisite for tight junction assembly (Umeda et al., 2006) and further that tight junction assembly is necessary for branching morphogenesis to occur (Chung and Andrew, 2008; Feigin and Muthuswamy, 2009; Hogan and Kolodziej, 2002; Martin-Belmonte and Mostov, 2008). We have shown that Scrib, like Celsr1 and Vangl2, is required for PCP pathway function, and PCP signalling is necessary for lung branching morphogenesis. Notably though, this and our previous studies have shown that in addition to the similarities in function between Celsr1, Vangl2 and Scrib, there are also clear differences. In particular, we have previously shown that Celsr1 is important for bud bifurcation in lung branching (Yates et al., 2010) whereas Scrib does not affect bifurcation and instead is required for proper epithelial cell cohesion (Fig. 4 and Movies 1 and 2).
Fig. 9.
Model depicting the potential hierarchy between A/B polarity, planar polarity and branching morphogenesis in the lung. The relationships of Scrib1, Celsr1 and Vangl2 to these processes are indicated (based on the results described here and in Yates et al., 2010).
Our results reveal an important role for Scrib in aspects of lung development, specifically, in formation and maintenance of junctional complexes and in lumen morphogenesis. These data broaden our understanding of the mechanisms of lung development and will likely provide important insight into the pathobiology of diseases featuring abnormal epithelial organisation and lumen morphology.
Acknowledgements
We thank Mireille Montcouquiol for Vangl2 antibody, Jonathan Wilde for Scrib quantification in explant cultures and the histology and imaging departments at MRC Harwell; particularly Steve Thomas for assistance with Fig. 9. We also thank staff in the Mary Lyon Centre, for excellent mouse husbandry. Authors' contributions were as follows: CHD conceived of the study; LLY, CS, LC, AP, HH, DB, JNM, CHD conducted experiments; LLY, CS, LH, AP, HH, MRR, JW, JS, CHD performed analysis, CF and JNM provided reagents, LLY, CS, LH and CHD prepared figures LLY, CS, LH, AP, HH, JNM, LAN and AG contributed to writing the manuscript and CHD wrote the manuscript. This work was supported by the Medical Research Council and by NIH HL089431 to LAN. Additional funding for LH was provided by the EU Network of Excellence ENFIN (contract no LSHG-CT-2005–518254). LAN is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Appendix A. Supporting information
Fig. S1.
Confocal DIC images of E14.5 wildtype and Scrib1 Crc/Crc left lung lobes. Confocal DIC images of E14.5 wildtype (A) and Scrib1 Crc/Crc (B) left lung lobes. Homozygous mutant lungs are smaller with fewer branches and narrower lumina (see insets for detail).
Fig. S2.
Proliferation and apoptosis are unaffected in Scrib1 Crc/Crc lungs. E14.5 wildtype ((A) and (C)) and Scrib1 Crc/Crc ((B) and (D)) sections immunostained with anti-phosphohistone-3 (PH3) ((A) and (B)) to assess proliferation or anti-cleaved caspase-3 ((C) and (D)) to assess apoptosis. Quantification at both E11.5 ((E) and (F)) and E14.5 ((G) and (H)) shows no difference in the percentage of proliferating cells ((E) and (G)) or the percentage of cells undergoing programmed cell death ((F) and (H)) between Scrib1 Crc/Crc and wildtype littermates. (E) wildtype _mean_=4.8%±0.38, _n_=9, _Crc mean_=5.2%±0.34, _n_=9. (F), wildtype _mean_=1.38%, ±0.63, _n_=8, Crc _mean_=2.50%±1.18, _n_=6. (G), Wildtype epithelium mean=3.40%, ±0.54, _n_=16, Crc epithelium mean=3.04%, ±0.41, _n_=21; wildtype mesenchyme mean=2.11%, ±0.28, _n_=8, Crc mesenchyme mean=3.05%, ±0.32, _n_=14. (H), wildtype epithelium mean=0.05%, ±0.05, _n_=8, Crc epithelium mean=0.17%, ±0.10, _n_=6; wildtype mesenchyme mean=2.71%, ±0.15, _n_=7, Crc mesenchyme mean=0.42%, ±0.16, _n_=6 Scale bars; A–D 12.5 μM.
Fig. S3.
Differentiation is unaffected in Scrib1 Crc/Crc lungs. Analysis of two proximal airway markers, αSMA ((A) and (B)) and Clara cell-10 ((C) and (D)), and two distal airway markers, Pro-SpC ((E) and(F)) and Aqp5 ((G) and (H)), by immunohistochemistry revealed no obvious differences in differentiation of Scrib1 Crc/Crc lungs ((B), (D), (F) and (H)) compared to wildtype ((A), (C), (E) and (G)). Western blotting revealed the levels of both total and phospho-aPKC remained similar in Scrib1 Crc/Crc lungs compared to wildtype littermates (I). Scale bars; A–H 12.5 μM.
Fig. S4.
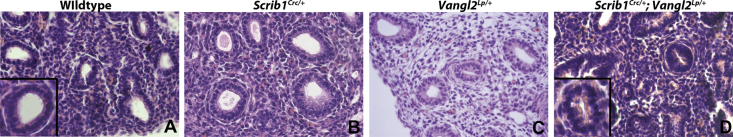
Scrib1 shows genetic interaction with Vangl2. E14.5 paraffin sections from wildtype littermates (A _n_=3), Scrib1 Crc/+ (B _n_=2), Vangl2 Lp/+ (C _n_=3) or Scrib1 Crc/+; Vangl2 Lp/+ double heterozygotes (D _n_=4) were stained with H&E to compare histology. Airways in Scrib1 Cr//+(B) and Vangl2 Lp/+ (C) heterozygous lung sections show open organised airways, similar to those seen in wildtype sections (A) although minor epithelial disorganisation and slight narrowing of lumina is observed in Vangl2 Lp/+. In contrast, the lungs of compound heterozygotes (D) contain disorganised airways with severely reduced or absent lumina. Insets in A and D show a single epithelial airway at high magnification. Scale bars; A and B 25 μM, insets 12.5 μM.
Fig. S5.
Validation of morpholino knockdown. E11.5 lungs were cultured for between 48 and 72 or 96 h in the presence of Scrib1 or control morpholinos (MO) and then protein extracted for Western blotting with anti-Scrib1 antibodies; _n_=3 explants (minimum) for each condition at each timepoint. Quantification of relative protein levels following Western blotting revealed an increasing reduction in Scrib1 protein following Scrib1 morpholino treatment over time. IB, immunoblotting shows an example of data from 96 h culture.
Fig. S6.
Western blot showing comparison of total RhoA and active RhoA in wilddtype and Scrib1 Crc/Crc. Raw data of image shown in Fig. 3A.
References
- Affolter M., Zeller R. From cells to organs: new insight into organ morphogenesis. Curr. Opin. Genetics Dev. 2009;19:421–423. doi: 10.1016/j.gde.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Andrew D.J., Ewald A.J. Morphogenesis of epithelial tubes: insights into tube formation, elongation, and elaboration. Dev. Biol. 2009;341:34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D., Li M., Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Bilder D., Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Carroll T.J., Dashfft A. Planar cell polarity in kidney development and disease. Organogenesis. 2011;7:180–190. doi: 10.4161/org.7.3.18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Andrew D.J. The formation of epithelial tubes. J. Cell Sci. 2008;121:3501–3504. doi: 10.1242/jcs.037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courbard J.R., Djiane A., Wu J., Mlodzik M. The apical/basal-polarity determinant Scribble cooperates with the PCP core factor Stbm/Vang and functions as one of its effectors. Dev. Biol. 2009;333:67–77. doi: 10.1016/j.ydbio.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Bryant D.M., Mostov K.E. Molecular regulation of lumen morphogenesis. Curr. Biol. 2011;21:R126–R136. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C.H., Miller L.A., Smith A.N., Dufort D., Lang R.A., Niswander L.A. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev. Biol. 2005;286:270–286. doi: 10.1016/j.ydbio.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Djiane A., Yogev S., Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Dow L.E., Kauffman J.S., Caddy J., Zarbalis K., Peterson A.S., Jane S.M., Russell S.M., Humbert P.O. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–2282. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Scribble at the crossroads. J. Biol. 2009;8:104. doi: 10.1186/jbiol190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin M.E., Muthuswamy S.K. Polarity proteins regulate mammalian cell–cell junctions and cancer pathogenesis. Curr. Opin Cell Biol. 2009;21:694–700. doi: 10.1016/j.ceb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Legue E., Doyen A., Nato F., Nicolas J.F., Torres V., Yaniv M., Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- Formstone C.J., Moxon C., Murdoch J., Little P., Mason I. Basal enrichment within neuroepithelia suggests novel function(s) for Celsr1 protein. Mol. Cell. Neurosci. 2010;44:210–222. doi: 10.1016/j.mcn.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Gardiol D., Zacchi A., Petrera F., Stanta G., Banks L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int. J. Cancer. 2006;119:1285–1290. doi: 10.1002/ijc.21982. [DOI] [PubMed] [Google Scholar]
- Gooding J.M., Yap K.L., Ikura M. The cadherin–catenin complex as a focal point of cell adhesion and signalling: new insights from three-dimensional structures. Bioessays. 2004;26:497–511. doi: 10.1002/bies.20033. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis A.K., Gertsenstein M., Ikawa M., Okabe M., Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hartsock A., Nelson W.J. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B.L., Kolodziej P.A. Organogenesis: molecular mechanisms of tubulogenesis. Nat. Rev. Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- Humbert P.O., Grzeschik N.A., Brumby A.M., Galea R., Elsum I., Richardson H.E. Control of tumourigenesis by the scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- Inanlou M.R., Baguma-Nibasheka M., Kablar B. The role of fetal breathing-like movements in lung organogenesis. Histol. Histopathol. 2005;20:1261–1266. doi: 10.14670/HH-20.1261. [DOI] [PubMed] [Google Scholar]
- Ivanov A.I., Young C., Beste K.D., Capaldo C.T., Humbert P.O., Brennwald P., Parkos C.A., Nusrat A. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am. J. Pathol. 2010;176:134–145. doi: 10.2353/ajpath.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A.I., Young C., Den Beste K., Capaldo C.T., Humbert P.O., Brennwald P., Parkos C.A., Nusrat A. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am. J. Pathol. 2010;176:134–145. doi: 10.2353/ajpath.2010.090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallay L.M., McNickle A., Brennwald P.J., Hubbard A.L., Braiterman L.T. Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J. Cell Biochem. 2006;99:647–664. doi: 10.1002/jcb.20992. [DOI] [PubMed] [Google Scholar]
- Kamei Y., Kito K., Takeuchi T., Imai Y., Murase R., Ueda N., Kobayashi N., Abe Y. Human scribble accumulates in colorectal neoplasia in association with an altered distribution of beta-catenin. Hum. Pathol. 2007;38:1273–1281. doi: 10.1016/j.humpath.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Karner C.M., Chirumamilla R., Aoki S., Igarashi P., Wallingford J.B., Carroll T.J. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat. Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P., Paul S.M., Boulanger J., Robbins R.M., Beitel G.J., Tepass U. Epithelial polarity proteins regulate Drosophila tracheal tube size in parallel to the luminal matrix pathway. Curr. Biol. 2010;20:55–61. doi: 10.1016/j.cub.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubarsky B., Krasnow M.A. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F., Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr. Opin. Cell Biol. 2008;20:227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F., Yu W., Rodriguez-Fraticelli A.E., Ewald A.J., Werb Z., Alonso M.A., Mostov K. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr. Biol. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metais J.Y., Navarro C., Santoni M.J., Audebert S., Borg J.P. hScrib interacts with ZO-2 at the cell–cell junctions of epithelial cells. FEBS Lett. 2005;579:3725–3730. doi: 10.1016/j.febslet.2005.05.062. [DOI] [PubMed] [Google Scholar]
- Metzger R.J., Klein O.D., Martin G.R., Krasnow M.A. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M., Kelley M.W. Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J. Neurosci. 2003;23:9469–9478. doi: 10.1523/JNEUROSCI.23-28-09469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M., Rachel R.A., Lanford P.J., Copeland N.G., Jenkins N.A., Kelley M.W. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Morrisey E.E., Hogan B.L. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch J.N., Henderson D.J., Doudney K., Gaston-Massuet C., Phillips H.M., Paternotte C., Arkell R., Stanier P., Copp A.J. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum. Mol. Gen. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- Murdoch J.N., Rachel R.A., Shah S., Beermann F., Stanier P., Mason C.A., Copp A.J. Circletail, a new mouse mutant with severe neural tube defects: chromosomal localization and interaction with the loop-tail mutation. Genomics. 2001;78:55–63. doi: 10.1006/geno.2001.6638. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Huibregtse J.M. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 2000;20:8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S., Yano T., Nakagawa K., Takizawa S., Suzuki Y., Yasugi T., Huibregtse J.M., Taketani Y. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br. J. Cancer. 2004;90:194–199. doi: 10.1038/sj.bjc.6601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C., Nola S., Audebert S., Santoni M.J., Arsanto J.P., Ginestier C., Marchetto S., Jacquemier J., Isnardon D., Le Bivic A., Birnbaum D., Borg J.P. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- Nelson W.J. Tube morphogenesis: closure, but many openings remain. Trends Cell Biol. 2003;13:615–621. doi: 10.1016/j.tcb.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudyal A., Damrau C., Patterson V.L., Ermakov A., Formstone C., Lalanne Z., Wells S., Lu X., Norris D.P., Dean C.H., Henderson D.J., Murdoch J.N. The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear. BMC. Dev. Biol. 2010;10:87. doi: 10.1186/1471-213X-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S.M., Ternet M., Salvaterra P.M., Beitel G.J. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130:4963–4974. doi: 10.1242/dev.00691. [DOI] [PubMed] [Google Scholar]
- Pearson H.B., Perez-Mancera P.A., Dow L.E., Ryan A., Tennstedt P., Bogani D., Elsum I., Greenfield A., Tuveson D.A., Simon R., Humbert P.O. SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. J. Clin. Invest. 2011;121:4257–4267. doi: 10.1172/JCI58509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit M.M., Meulemans S.M., Alen P., Ayoubi T.A., Jansen E., Van de Ven W.J. The tumor suppressor Scrib interacts with the zyxin-related protein LPP, which shuttles between cell adhesion sites and the nucleus. BMC Cell Biol. 2005;6:1. doi: 10.1186/1471-2121-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips H.M., Rhee H.J., Murdoch J.N., Hildreth V., Peat J.D., Anderson R.H., Copp A.J., Chaudhry B., Henderson D.J. Disruption of planar cell polarity signaling results in congenital heart defects and cardiomyopathy attributable to early cardiomyocyte disorganization. Circ. Res. 2007;101:137–145. doi: 10.1161/CIRCRESAHA.106.142406. [DOI] [PubMed] [Google Scholar]
- Qin Y., Capaldo C., Gumbiner B.M., Macara I.G. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachel R.A., Murdoch J.N., Beermann F., Copp A.J., Mason C.A. Retinal axon misrouting at the optic chiasm in mice with neural tube closure defects. Genesis. 2000;27:32–47. doi: 10.1002/1526-968x(200005)27:1<32::aid-gene50>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Shin K., Fogg V.C., Margolis B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Sun Y., Aiga M., Yoshida E., Humbert P.O., Bamji S.X. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol. Biol. Cell. 2009;20:3390–3400. doi: 10.1091/mbc.E08-12-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Massimi P., Navarro C., Borg J.P., Banks L. The hScrib/Dlg apico-basal control complex is differentially targeted by HPV-16 and HPV-18 E6 proteins. Oncogene. 2005;24:6222–6230. doi: 10.1038/sj.onc.1208757. [DOI] [PubMed] [Google Scholar]
- Umeda K., Ikenouchi J., Katahira-Tayama S., Furuse K., Sasaki H., Nakayama M., Matsui T., Tsukita S., Furuse M. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Vandenberg A.L., Sassoon D.A. Non-canonical Wnt signaling regulates cell polarity in female reproductive tract development via van gogh-like 2. Development. 2009;136:1559–1570. doi: 10.1242/dev.034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansleeben C., Feitsma H., Montcouquiol M., Kroon C., Cuppen E., Meijlink F. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development. 2010;137:1067–1073. doi: 10.1242/dev.041434. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Olver R.E., Walters D.V. Developmental regulation of lumenal lung fluid and electrolyte transport. Respir. Physiol. Neurobiol. 2007;159:247–255. doi: 10.1016/j.resp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Yap A.S., Brieher W.M., Pruschy M., Gumbiner B.M. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr. Biol. 1997;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- Yates L.L., Dean C.H. Planar polarity: a new player in both lung development and disease. Organogenesis. 2011;7:209–216. doi: 10.4161/org.7.3.18462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L.L., Schnatwinkel C., Murdoch J.N., Bogani D., Formstone C.J., Townsend S., Greenfield A., Niswander L.A., Dean C.H. The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum. Mol. Gen. 2010;19:2251–2267. doi: 10.1093/hmg/ddq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara K., Ikenouchi J., Izumi Y., Akashi M., Tsukita S., Furuse M. Phosphorylation state regulates the localization of Scribble at adherens junctions and its association with E-cadherin–catenin complexes. Exp. Cell Res. 2011;317:413–422. doi: 10.1016/j.yexcr.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Zhan L., Rosenberg A., Bergami K.C., Yu M., Xuan Z., Jaffe A.B., Allred C., Muthuswamy S.K. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135:865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1
Time-lapse movies of control MO treated β-actin driven GFP lung explants. After 48 h of culture, lungs were transferred to a Zeiss LSM 510 confocal microscope equipped with controlled stage incubator. Images were taken every 4 min over a 24 h period. Acquired time-lapse series were exported using LSM software and saved as Quick Time movies using 20 frames/s.
Video 2
Time-lapse movies of Scribble MO treated β-actin driven GFP lung explants. After 48 h of culture, lungs were transferred to a Zeiss LSM 510 confocal microscope and imaged as described for Movie 1.