Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus (original) (raw)
Abstract
In vitro evidence suggests that plasmacytoid dendritic cells (pDCs) are intimately involved in the pathogenesis of lupus. However, it remains to be determined whether these cells are required in vivo for disease development, and whether their contribution is restricted to hyperproduction of type I IFNs. To address these issues, we created lupus-predisposed mice lacking the IFN regulatory factor 8 (IRF8) or carrying a mutation that impairs the peptide/histidine transporter solute carrier family 15, member 4 (SLC15A4). IRF8-deficient NZB mice, lacking pDCs, showed almost complete absence of anti-nuclear, anti-chromatin, and anti-erythrocyte autoantibodies, along with reduced kidney disease. These effects were observed despite normal B-cell responses to Toll-like receptor (TLR) 7 and TLR9 stimuli and intact humoral responses to conventional T-dependent and -independent antigens. Moreover, Slc15a4 mutant C57BL/6-Fas lpr mice, in which pDCs are present but unable to produce type I IFNs in response to endosomal TLR ligands, also showed an absence of autoantibodies, reduced lymphadenopathy and splenomegaly, and extended survival. Taken together, our results demonstrate that pDCs and the production of type I IFNs by these cells are critical contributors to the pathogenesis of lupus-like autoimmunity in these models. Thus, IRF8 and SLC15A4 may provide important targets for therapeutic intervention in human lupus.
Extensive evidence suggests that type I IFNs are major pathogenic effectors in lupus-associated systemic autoimmunity. A well-documented pattern of expression of type I IFN-inducible genes occurs in peripheral blood mononuclear cells of patients with systemic lupus erythematosus (SLE) (1–3), and reduced disease is observed in some lupus-predisposed mice that either lack the common receptor (IFNAR) for these cytokines (4, 5) or have been treated with IFNAR-blocking antibody (6). Consequently, attention has focused on defining the cell subsets and signaling processes involved in type I IFN production, the mechanisms by which these mediators accelerate disease, and approaches to interfere with these pathogenic events.
Early in vitro studies showed that type I IFN production can be induced in normal blood leukocytes by SLE autoantibodies complexed with nucleic acid-containing apoptotic/necrotic cell material, and further work demonstrated that this activity is sensitive to RNase and DNase digestion (7, 8). These results were integrated in a more comprehensive scheme following the demonstration that type I IFN induction by these complexes is mediated by the engagement of endosomal Toll-like receptors (TLRs) (9–11). Similarly, antigenic cargo containing nucleic acids was found to promote B-cell proliferation in a TLR9- or TLR7-dependent manner, with this effect enhanced by type I IFN signaling (9, 12, 13). The contribution of nucleic acid-sensing TLRs to systemic autoimmunity was further corroborated by studies in lupus-predisposed mice lacking or overexpressing TLR7 and/or TLR9 (14-20), and in Unc93b1 (3d) mutant mice in which signaling by endosomal TLRs is extinguished (21).
The cell population involved in type I IFN production in response to lupus-related immune complexes corresponds to natural IFN-producing cells (22, 23). These cells, known as plasmacytoid DCs (pDCs), are the most potent producers of type I IFNs, a functional characteristic attributed to constitutive expression of TLR7, TLR9, and IRF7 and likely signaling from a unique intracellular compartment (24–27). The involvement of pDCs in lupus is further suggested by the reduced frequency of these cells in patient blood together with increases in afflicted organs, presumably caused by the attraction of activated pDCs to inflammatory sites (10). Similar increases have been noted in inflammatory tissues of patients with Sjögren's syndrome (28), rheumatoid arthritis (29, 30), dermatomyositis (31), and psoriasis (32).
Collectively, these results suggest that pDCs, acting through type I IFN hyperproduction, are major pathogenic contributors to lupus. Whether the participation of these cells is obligatory remains to be documented in vivo, however. Here, using congenic lupus-predisposed mice lacking pDCs (as well as other DC subsets) owing to IRF8 deficiency, or exhibiting pDC-specific defects in endosomal TLR signaling and type I IFN production owing to Slc15a4 (feeble) mutation, we provide strong evidence that pDCs are indeed required for disease development, and this effect appears to be mediated by hyperproduction of inflammatory cytokines, most likely type I IFNs.
Results
Absence of pDCs and CD8α+ DCs in IRF8-Deficient NZB Mice.
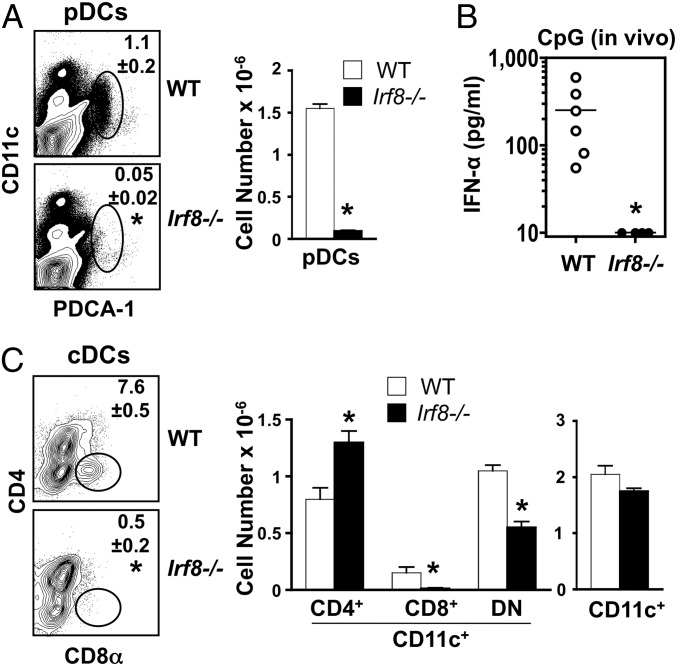
Studies in normal background mice have shown that IRF8, a hematopoietic cell-specific transcription factor, is essential for the development of pDCs (33–35). Accordingly, assessment of _Irf8_−/− NZB mice at a young age (3 mo) showed almost complete absence of pDCs (CD11clowCD11b–B220+Siglec-H+PDCA1+) in spleen (Fig. 1_A_), and this correlated with undetectable in vivo production of type I IFNs in response to CpG-DNA (Fig. 1_B_). Moreover, CD8α+ conventional DCs (cDCs) were absent and CD4–CD8– cDCs were reduced in _Irf8_−/−mice (Fig. 1_C_), consistent with the levels of IRF8 expression in these subsets of normal mice (35). Total cDC numbers were unaffected, however, likely owing to compensatory increases in CD4+ cDCs (Fig. 1_C_), which do not express IRF8 (35). As noted previously with _Irf8_−/− normal background mice (36, 37), IRF8-deficient NZB mice also showed expansion of marginal zone and CD21lowCD23+ follicular and transitional B cells, but total B-cell and T-cell numbers were unchanged (data not shown).
Fig. 1.
Absence of pDCs and CD8α+ cDCs in young IRF8-deficient NZB mice. Mutant and WT NZB mice (age 3 mo; n = 3–6/group) were analyzed for cellular differences in spleen and in vivo responses to CpG challenge. (A) pDC frequency and numbers. Spleen cells were assessed by flow cytometry using anti-CD11c and anti-PDCA-1 antibodies. Similar results were obtained when cells were stained with antibodies to B220, SiglecH, and CD11b (data not shown). (B) In vivo type I IFN production in response to TLR9 engagement. Serum IFN-α levels in CpG-challenged mice were determined by ELISA. (C) cDC frequency and numbers. Spleen cells were analyzed by flow cytometry after gating on CD11c+ cells to identify CD4+, CD8+, and CD4–CD8– cDC subsets. Numbers within the flow cytometry plots correspond to average frequencies of the indicated subsets. Error bars in graphs indicate SD. Asterisks indicate statistical significance (P < 0.05).
Reduced Autoimmunity in IRF8-Deficient NZB Mice.
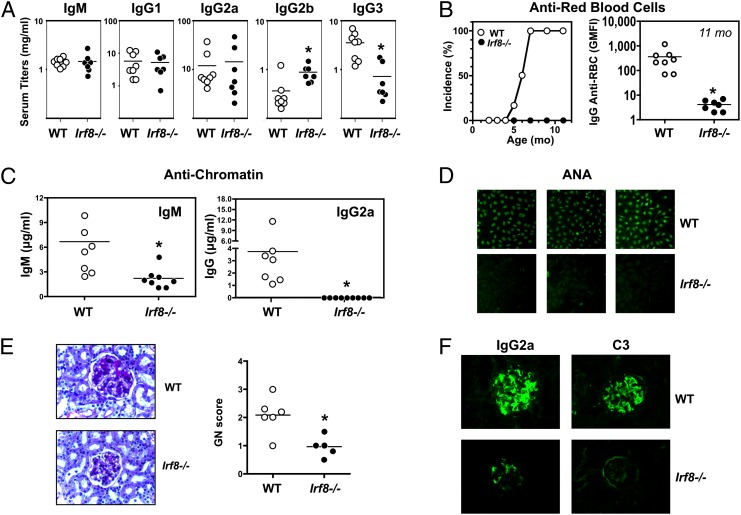
At age 11 mo, serum levels of polyclonal IgM and IgG, as well as IgG1 and IgG2a, were similar in _Irf8_−/− and WT NZB mice, whereas IgG2b was slightly increased and IgG3 was decreased in the mutant mice (Fig. 2_A_). Strikingly, however, autoantibody production was almost completely suppressed in the IRF8-deficient mice. Thus, IgG anti-erythrocyte autoantibodies, which are typically detectable in 100% of WT mice by age 7–8 mo, were undetectable in _Irf8_−/− mice even at 11 mo (Fig. 2_B_). Moreover, IgG2a anti-chromatin autoantibodies (the dominant subclass in WT NZB mice) and IgG anti-nuclear autoantibodies (ANA) were reduced to background levels, and IgM anti-chromatin autoantibodies were decreased substantially (Fig. 2 C and D). Mirroring these serologic changes, kidney disease was significantly ameliorated in _Irf8_−/− mice, as evidenced by reductions in both glomerulonephritis scores (Fig. 2_E_) and immune (IgG2a, C3) deposits (Fig. 2_F_).
Fig. 2.
Reduced autoimmunity in IRF8-deficient NZB mice. Mutant and WT NZB mice (age 11 mo; n = 5–8/group) were examined for serologic and histological disease characteristics. (A) Polyclonal serum Ig levels. (B) Anti-erythrocyte autoantibodies. Individuals with a geometric mean fluorescence intensity >50 were considered positive for anti-RBC autoantibodies. (C) Anti-chromatin autoantibodies. (D) ANAs, with representative individuals shown. (E) Representative PAS-stained kidney sections and glomerulonephritis scores. (F) Kidney deposits (IgG2a and C3), with representative immunofluorescence images shown. Asterisks indicate statistical significance (P < 0.05).
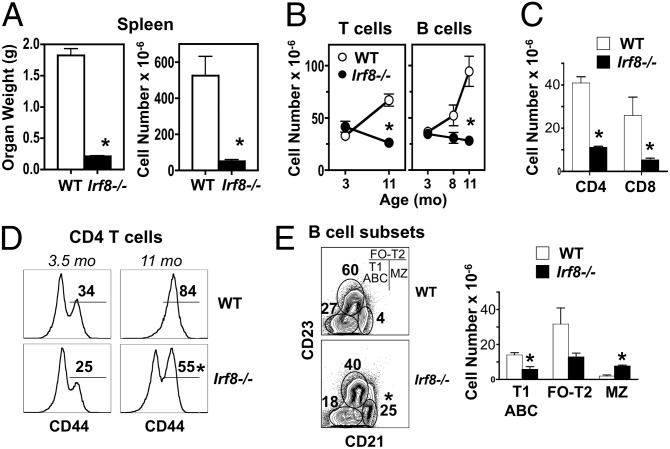
Splenomegaly and accumulation of T cells and B cells, observed with aging in WT controls, were also decreased in _Irf8_−/− NZB mice (Fig. 3 A and B). The reduction in T-cell numbers affected both CD4+ and CD8+ subsets and was accompanied by a decrease in activated CD4+ T cells (Fig. 3 C and D), whereas the reduction in B cells was primarily in the CD21–CD23– subset (Fig. 3_E_), which includes the so-called “age-associated” B cells (38, 39). In contrast, the expanded population of marginal zone B cells detected in young _Irf8_−/− mice was retained with aging (Fig 3_E_), whereas the frequency of peritoneal B1 cells was unchanged. Thus, IRF8 deficiency in NZB mice was associated with decreased humoral and histological disease manifestations, along with reduced activation/expansion of T cells and B cells.
Fig. 3.
Reduced T-cell and B-cell expansion in older IRF8-deficient NZB mice. Mutant and WT NZB mice (age 8–11 mo; n = 3/group) were analyzed for cellular changes in spleen and peritoneal cavity. (A) Spleen weight and cellularity at 11 mo. (B) T-cell and B-cell numbers in spleen at age 11 mo. Cells were analyzed by flow cytometry after gating on TCRβ+ (T cell) or B220+ (B cell) populations. (C) CD4+ and CD8+ T-cell subsets in spleen at age 11 mo. (D) Frequency of activated CD4+ T cells in spleen at age 11 mo. Gated CD4+ T cells were assessed for expression of CD44. (E) B-cell subsets in spleen at age 8 mo. Gated B220+IgM+ cells were analyzed for transitional T2 and follicular (T2-FO), marginal zone (MZ), and T1 immature and age-associated (T1-ABC) B cells. Numbers within flow cytometry plots correspond to average frequencies of the indicated subsets. Error bars in graphs indicate SD. Asterisks indicate statistical significance (P < 0.05).
Defective cDC Responses to Endosomal TLR Ligands in IRF8-Deficient NZB Mice.
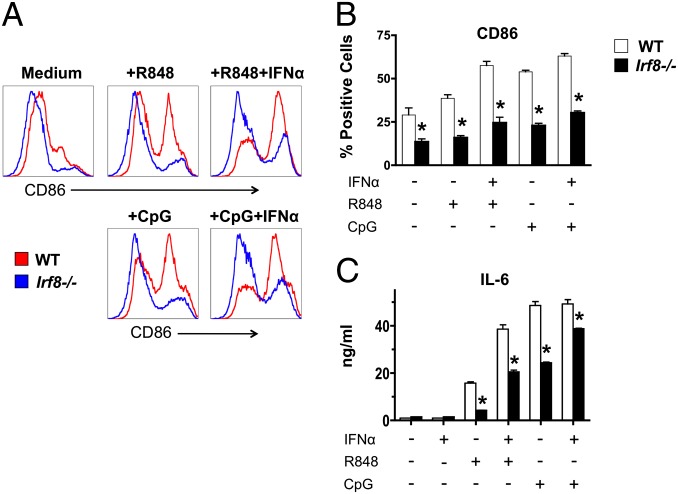
Because engagement of endosomal TLRs is thought to be a major mechanism of DC activation in lupus, we also examined the response of cDCs to TLR7 and TLR9 ligands. As noted above, _Irf8_−/− NZB mice did not produce detectable amounts of IFN-α after CpG-DNA injection, indicating that the cDC subtypes present in these mice could not compensate for the absence of pDCs in response to this TLR9 stimulus. Furthermore, CD11c+ cDCs derived from GM-CSF–differentiated bone marrow (BM) cells showed reduced CD86 up-regulation and IL-6 production after in vitro stimulation with TLR7 or TLR9 ligands in the presence or absence of IFN-α (Fig. 4). Thus, in addition to pDC deficiency, _Irf8_−/− NZB mice exhibited defective cDC responses to endosomal TLR stimulation.
Fig. 4.
Defective in vitro responses by cDCs from IRF8-deficient NZB mice. cDCs were differentiated from BM cells from WT and mutant mice (age 3 mo; pools of 2–3 mice) in the presence of GM-CSF, then stimulated with endosomal TLR ligands in the presence or absence of IFN-α. (A and B) CD86 up-regulation defined by flow cytometry after gating on CD11c+ cells. (C) IL-6 production determined by ELISA. One representative of two independent experiments is shown. Error bars in graphs indicate SD of samples analyzed in triplicate. Asterisks indicate statistical significance (P < 0.05).
B-Cell Responses to TLR Ligands and Exogenous Antigens Are Not Compromised in IRF8-Deficient NZB Mice.
IRF8 is known to affect B-cell differentiation and germinal center formation (40), changes that might contribute to reduced autoimmunity in IRF8-deficient mice. To address this possibility, we assessed in vitro B-cell responses to TLR or BCR engagement and in vivo antibody responses to exogenous antigens. In vitro responses of purified _Irf8_−/− B cells to TLR7 and TLR9 ligands in the presence or absence of IFN-α were largely conserved, as indicated by efficient up-regulation of CD86 and H2-Kd, as well as production of IL-6 and IL-10 (Fig. S1 A–C). Similarly, activation by anti-IgM and anti-CD40 cross-linking was comparable in mutant and WT B cells (Fig. S1 D and E). Moreover, immunization of WT and _Irf8_−/− NZB mice with a thymus-independent antigen (TNP-LPS) or a thymus-dependent antigen (TNP-KLH) produced anti-hapten antibody responses of similar titers and affinities (Fig. S2). Thus, despite the numerical changes, B-cell responses to endosomal TLR ligands and to conventional exogenous antigens were largely uncompromised in IRF8-deficient NZB mice, suggesting that reduced autoimmunity likely is not due to B-cell functional defects.
Feeble Mutation of Slc15a4 Prevents Autoimmunity in Congenic C57BL/6-Fas lpr Mice.
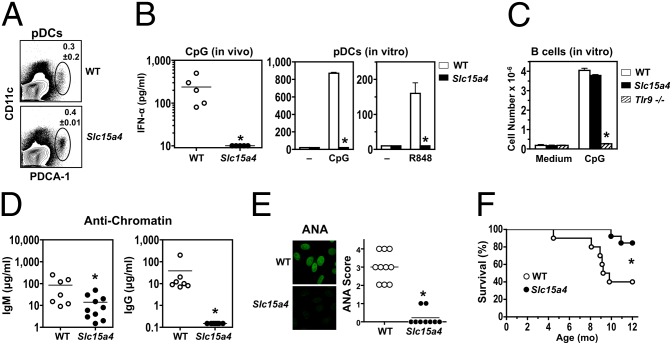
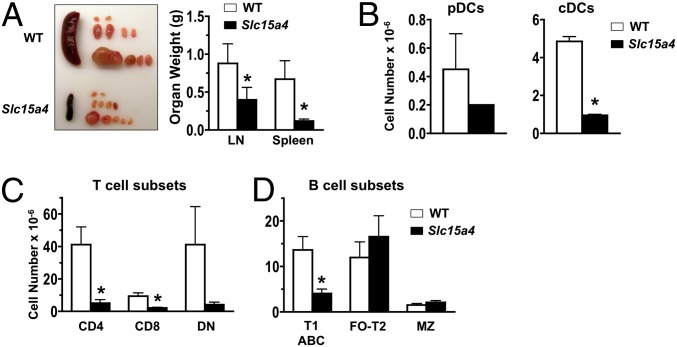
To directly examine how pDCs contribute to systemic autoimmunity, we developed congenic C57BL/6-Fas lpr mice carrying the feeble mutation of Slc15a4. In normal background mice with this mutation, development of both pDCs and cDCs is intact, but cytokine production, particularly type I IFNs, in response to TLR7 or TLR9 ligands is absent only in pDCs (27). Accordingly, in Slc15a4 mutant C57BL/6-Fas lpr mice, pDCs were present at normal frequencies in spleen (Fig. 5_A_) and differentiated efficiently after Flt3L treatment of BM cells, but did not produce type I IFNs in response to TLR7 or TLR9 ligands both in vivo and in vitro (Fig. 5_B_). In contrast, B cells from Slc15a4 mutant C57BL/6-Fas lpr mice showed normal in vitro proliferation to TLR9 stimulation (Fig. 5_C_) and efficient humoral responses to T-dependent and T-independent antigens (Fig. S3). Remarkably, at age 8 mo, these mutants had significantly reduced IgM anti-chromatin and an almost complete absence of IgG anti-chromatin autoantibodies and ANA (Fig. 5 D and E). In addition, the mutants had significantly decreased hypergammaglobulinemia (8.4 ± 2.9 mg/mL IgG vs 23.6 ± 14.8 mg/mL in WT controls; P < 0.05) and extended survival (Fig. 5_F_). Reductions were also observed in lymphadenopathy and splenomegaly (Fig. 6_A_), with corresponding numerical decreases in cDCs, T cells (CD4+ and CD8+), and B cells (CD21–CD23–), whereas pDCs and double-negative T cells were reduced, but not significantly (Fig. 6 B and C). These findings strongly indicate that pDCs, likely through type I IFN hyperproduction, are major contributors to the pathogenesis of systemic autoimmunity.
Fig. 5.
Reduced autoimmunity in Slc15a4 mutant C57BL/6-Fas lpr mice. Mutant and WT mice were analyzed for pDC development and function at age 4 mo (n = 3–5/group), disease manifestations at age 8 mo (n = 7–10/group), and survival (n = 10–13/group). (A) Frequency of pDCs in spleen. Cells were assessed by flow cytometry using anti-CD11c and anti–PDCA-1 antibodies. Similar results were obtained using antibodies to B220, SiglecH, and CD11b (data not shown). (B) In vivo and in vitro type I IFN production in response to endosomal TLR stimulation. Serum IFN-α levels in CpG-challenged mice, and IFN-α production by BM-differentiated pDCs stimulated with CpG or R848, were determined by ELISA. (C) In vitro B-cell proliferation in response to TLR9 engagement. Purified B cells from mutant and WT mice were stimulated in vitro with CpG. B cells from _Tlr9_−/− C57BL/6 mice were used as negative controls. (D) Anti-chromatin autoantibodies. (E) ANA. (F) Survival. Error bars in graphs indicate SD. Asterisks indicate statistical significance (P < 0.05).
Fig. 6.
Cellular changes in Slc15a4 mutant C57BL/6-Fas lpr mice. Spleen and LNs of mutant and WT mice were examined at age 8 mo (n = 3/group). (A) Spleen and LN organ weights. (B) pDC and cDC numbers in spleen. Flow cytometry was used to identify pDCs (PDCA-1+CD11clow) and cDCs (CD11c+). (C) T-cell numbers in spleen. CD4+, CD8+, and CD4–CD8– (double-negative) T cells were identified by flow cytometry after gating on TCRβ+ cells. (D) B-cell numbers in spleen. Spleen cells were examined by flow cytometry as in Fig 3_E_. Error bars in graphs indicate SD. Asterisks indicate statistical significance (P < 0.05).
Discussion
We have used two types of genetic modification in mouse models of spontaneous lupus-like disease to define the in vivo role of pDCs in the pathogenesis of systemic autoimmunity. In the first instance, we created NZB mice deficient for IRF8, a transcription factor that regulates the development of pDCs, among other effects, whereas in the second instance, we created C57BL/6-Fas lpr mice carrying a mutation in SLC15A4, a peptide/histidine transporter critical for endosomal TLR signaling and type I IFN production by pDCs. The evidence with the Irf8 mutants strongly suggests that pDCs are required for lupus development, whereas the results with the Slc15a4 mutants infer that the contribution of these cells is mediated by endosomal TLR-dependent production of proinflammatory cytokines, primarily type I IFNs.
The biological effects of IRF8, also known as IFN consensus binding protein, have been investigated extensively (40–42). IRF8 is an IFN-inducible transcription factor expressed in pDCs, CD8α+ cDCs, CD4–CD8α– cDCs, macrophages, and B cells. IRF8 forms heterodimeric complexes with several molecular partners and binds to various IFN stimulatory response elements, thereby governing expression of a large set of genes with broad effects in innate and adaptive immune functions. Nonetheless, a dominant characteristic of mice lacking IRF8 is the absence of pDCs.
We found that the age-associated splenomegaly and expansion of T and B cells typical of NZB mice were largely abrogated in _Irf8_−/− congenics, accompanied by significant decreases in CD4+ T-cell activation, autoantibody production, and kidney disease. Interestingly, reductions encompassed not only the classical ANA, but also the dominant antierythrocyte response of this strain. The reductions in antinuclear specificities, particularly in the IgG isotype, likely are related to the absence of pDCs and minimal, if any, endosomal TLR-dependent induction of type I IFNs by self-nucleic acids and related immune complexes. This is consistent with previous findings with lupus-predisposed mice deficient in type I IFN or endosomal TLR signaling, which showed decreases in these autoantibodies and other disease manifestations (11).
In terms of the role of pDCs in anti-RBC responses, although RBCs lose nuclei and mitochondria and thus lack DNA that might engage TLR9, these cells contain RNA species that might engage TLR7. Alternatively, the initial trigger for the anti-RBC response might be provided by uptake of nucleated erythroid precursors or of expelled nuclei, which are surrounded by an intact plasma membrane displaying phosphatidylserine as an “eat-me” signal (43–45). Any one of these processes could provide both antigenic cargo and nucleic acids for efficient activation of pDCs and antigen presentation by cDCs.
It is of interest that, in contrast to reductions or even absence of autoantibodies, responses to conventional T-dependent and T-independent antigens were of normal magnitude and quality in _Irf8_−/− NZB mice, as reported for normal background mice with B-cell–specific conditional Irf8 deletion (37). A possible explanation for this finding is that with lupus-associated autoantigens, adjuvanticity is provided by endosomal TLR engagement by self-nucleic acids, whereas adjuvanticity of complete (CFA) and incomplete (IFA) Freund's adjuvants, or alum in response to conventional exogenous antigens is mediated by endosomal TLR-independent pathways (46–48).
We have attributed the disease-reducing effects of IRF8 deficiency to the absence of pDCs. However, IRF8-deficient mice also lacked CD8α+ cDCs and had diminished in vitro responses to endosomal TLR stimulation in the retained cDC subsets. These modifications also may contribute to disease reduction. CD8α+ cDCs have several functional properties of potential relevance to autoimmunity, including ingestion of materials from dying cells, antigen cross-presentation, and production of IL-12, a cytokine involved in Th1 differentiation (49). Regardless, however, these cells are unlikely to promote disease, because they do not express TLR7, the engagement of which appears to be a major pathogenic contributor in several lupus-predisposed mouse strains (14, 15, 21), and do not produce significant amounts of type I IFNs in response to ligands or viruses that engage endosomal TLRs or cytosolic sensors for nucleic acids (50–54). Similarly, the partial defects in TLR7 and TLR9 responses by the IRF8-deficient CD4+ and CD4–CD8α– cDCs are likely insufficient to explain disease reduction, given that these subsets have been reported to produce meager amounts of type I IFNs when stimulated with endosomal TLR ligands, including lupus-associated nucleic acid-containing immune complexes (53). Nonetheless, considering our present findings and previous findings of others demonstrating disease reduction in lupus-prone mice lacking all DC subsets (including pDCs) (55), targeted deletion/inactivation and/or reconstitution of specific cDC subsets is needed to definitively clarify the exact role of cDCs, as opposed to pDCs, in systemic autoimmunity.
An additional open question is whether modifications in the B-cell compartment caused by IRF8 deficiency also might contribute to disease reduction, given that ablation of IRF8 has been shown to affect B-cell differentiation and germinal center organization, as well as to induce expansion of marginal zone and follicular B cells (40). However, as reported previously (37) and reproduced here with IRF8-deficient NZB mice, humoral responses to exogenous antigens remain unmodified. Furthermore, we have shown that _Irf8_−/− B cells mounted normal in vitro responses to TLR7 and TLR9 ligands, which could be enhanced with IFN-α. Our results suggest that B-cell activation by endosomal TLRs, although necessary, by itself is not sufficient to drive autoimmunity, with type I IFN hyperproduction resulting from the concomitant engagement of these TLRs in pDCs, required as well. This scheme of endosomal TLR engagement in both B cells and pDCs fits our previously outlined model for the induction of systemic autoimmunity (56).
Combined with the foregoing considerations, the data from IRF8-deficient mice provide strong support for an indispensable in vivo disease-promoting effect of pDCs. However, IRF8 deficiency prevents us from stating a definite conclusion as to whether pDCs contribute to disease solely through hyperproduction of type I IFNs or other functions, such as antigen presentation (57). The recent identification of the mutant strain feeble, in which defective expression of the peptide/histidine transporter SLC15A4 leads to absence of endosomal TLR signaling and severely reduced production of proinflammatory cytokines, including type I IFNs, specifically in pDCs (27), has provided an experimental tool to address this question. We found that lupus-predisposed C57BL/6-Fas lpr mice carrying this mutation exhibited striking reductions in disease manifestations and extended survival, despite efficient B-cell responses to both endosomal TLR stimuli and conventional exogenous antigens. These findings support the critical role of type I IFN production by pDCs in systemic autoimmunity. Disease reduction in Slc15a4 mutant C57BL/6-Fas lpr mice, as well as in Unc93b1 mutant C57BL/6-Fas lpr mice (21), is at variance with the reported disease enhancement in IFNAR-deficient MRL-Fas lpr mice (58). There is no clear explanation for this variance, but possibilities include genetic contributions beyond the Fas defect, exemplified by differences in disease severity between these strains (59), and complete absence of signaling in _Ifnar1_-deleted mice versus pDC-specific absence of type I IFN production in Slc15a4 mutant mice. Creation of MRL-Fas lpr mice congenic for the Slc15a4 mutation (and possibly the Unc93b1 mutation) will likely clarify this issue.
The exact mechanism by which SLC15A4 deficiency specifically compromises endosomal TLR signaling and proinflammatory cytokine production in pDCs remains to be defined. Nevertheless, the defect seems to be related not to inefficient uptake of TLR ligands or secretion of type I IFNs, but rather to inefficient signaling by endosomal TLRs, given that intracellular Ifna transcripts and corresponding proteins could not be detected in stimulated mutant pDCs even in the presence of exogenous IFN-α (27). A reported function of SLC15A4 is to transport, in a low pH-dependent manner, free histidine from the endosome to the cytosol (60, 61), a process that might facilitate the cathepsin-mediated cleavage of endosomal TLRs required for efficient signaling (62–65). Alternatively, SLC15A4 may be localized in a unique pDC-associated lysosome-related organelle, where it contributes to the establishment of an optimal microenvironment required for the assembly of functional TLR signaling complexes and hyperproduction of type I IFNs (27, 66).
In summary, based on our studies with _Irf8_-deleted and Slc15a4 mutant mice, we report strong evidence that pDCs are essential for the initiation of abnormal innate immune responses leading to systemic autoimmunity, likely through high production of type I IFNs. These events appear to be instrumental for DC activation, efficient antigen presentation by DCs and B cells, and production of disease-inducing isotype-switched autoantibodies. Similar processes are likely involved in the pathogenesis of human SLE; interestingly, IRF8 and SLC15A4 have been identified as susceptibility loci in certain populations with this condition (67, 68). Our present results further suggest that pharmacologic inhibition of IRF8, and particularly interference with the effects of SLC15A4 on pDCs, may be of utility in the treatment of SLE and other inflammatory conditions in which type I IFNs are major pathogenic effectors.
Materials and Methods
Mice.
NZB mice and C57BL/6-Fas lpr mice were obtained from Jackson Laboratory or The Scripps Research Institute Animal Facility. C57BL/6 mice deficient for IRF8 (_Irf8_−/−) or TLR9 (_Tlr9_−/−) or carrying the feeble mutation of the Slc15a4 gene have been reported (27, 35), and congenic _Irf8_−/− NZB and Slc15a4 mutant C57BL/6-Fas lpr mice were generated by standard marker-assisted breeding as described previously (4). The WT and _Irf8_−/− NZB mice used in this study were littermates generated by crossing heterozygous Irf8+/− mice. All mice were housed under specific pathogen-free conditions. The experimental protocols were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by The Scripps Research Institute’s Animal Care and Use Committee.
Statistical Analysis.
Group comparisons were analyzed using the unpaired two-tailed Student t test. Survival was analyzed by Kaplan–Meier plots and the log-rank test. P < 0.05 was considered significant.
Additional Methods.
Procedures for in vivo and in vitro studies, cell preparations, flow cytometry, analysis of ANA and anti-erythrocyte autoantibodies, kidney pathology, and immunohistology are described in SI Materials and Methods.
Supplementary Material
Supporting Information
Acknowledgments
We thank Carrie N. Arnold for assistance with the B cell proliferation assays and Anthony Nguyen for excellent technical support. This work was supported by National Institutes of Health Grants AR53228, AR31203, AR39555, 1U19-AI100627-01, and 2P01-AI070167-06A1. A.L.B. was supported by The Irvington Institute Fellowship Program of the Cancer Research Institute. This is article number 22092 from The Scripps Research Institute, Department of Immunology and Microbial Sciences.
Footnotes
The authors declare no conflict of interest.
References
- 1.Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol. 2004;16(6):801–807. doi: 10.1016/j.coi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Kirou KA, et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 3.Obermoser G, Pascual V. The interferon-alpha signature of systemic lupus erythematosus. Lupus. 2010;19(9):1012–1019. doi: 10.1177/0961203310371161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santiago-Raber ML, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197(6):777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun D, Geraldes P, Demengeot J. Type I interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20(1):15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 6.Baccala R, et al. Anti-IFN-α/β receptor antibody treatment ameliorates disease in lupus-predisposed mice. J Immunol. 2012;189(12):5976–5984. doi: 10.4049/jimmunol.1201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Båve U, Alm GV, Rönnblom L. The combination of apoptotic U937 cells and lupus IgG is a potent IFN-alpha inducer. J Immunol. 2000;165(6):3519–3526. doi: 10.4049/jimmunol.165.6.3519. [DOI] [PubMed] [Google Scholar]
- 8.Lövgren T, Eloranta ML, Båve U, Alm GV, Rönnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50(6):1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 9.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: The role of Toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 10.Rönnblom L. The type I interferon system in the etiopathogenesis of autoimmune diseases. Ups J Med Sci. 2011;116(4):227–237. doi: 10.3109/03009734.2011.624649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theofilopoulos AN, Kono DH, Beutler B, Baccala R. Intracellular nucleic acid sensors and autoimmunity. J Interferon Cytokine Res. 2011;31(12):867–886. doi: 10.1089/jir.2011.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 13.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202(9):1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25(3):417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Nickerson KM, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184(4):1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312(5780):1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian S, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA. 2006;103(26):9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deane JA, et al. Control of Toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27(5):801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairhurst AM, et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur J Immunol. 2008;38(7):1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santiago-Raber ML, et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol. 2008;181(2):1556–1562. doi: 10.4049/jimmunol.181.2.1556. [DOI] [PubMed] [Google Scholar]
- 21.Kono DH, et al. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci USA. 2009;106(29):12061–12066. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vallin H, Blomberg S, Alm GV, Cederblad B, Rönnblom L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-alpha) production acting on leucocytes resembling immature dendritic cells. Clin Exp Immunol. 1999;115(1):196–202. doi: 10.1046/j.1365-2249.1999.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Båve U, Vallin H, Alm GV, Rönnblom L. Activation of natural interferon-alpha producing cells by apoptotic U937 cells combined with lupus IgG and its regulation by cytokines. J Autoimmun. 2001;17(1):71–80. doi: 10.1006/jaut.2001.0519. [DOI] [PubMed] [Google Scholar]
- 24.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8(8):594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 25.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: Recent progress and open questions. Annu Rev Immunol. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434(7036):1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 27.Blasius AL, et al. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci USA. 2010;107(46):19973–19978. doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Båve U, et al. Activation of the type I interferon system in primary Sjögren’s syndrome: A possible etiopathogenic mechanism. Arthritis Rheum. 2005;52(4):1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 29.Takakubo Y, et al. Distribution of myeloid dendritic cells and plasmacytoid dendritic cells in the synovial tissues of rheumatoid arthritis. J Rheumatol. 2008;35(10):1919–1931. [PubMed] [Google Scholar]
- 30.Lebre MC, et al. Rheumatoid arthritis synovium contains two subsets of CD83−DC-LAMP− dendritic cells with distinct cytokine profiles. Am J Pathol. 2008;172(4):940–950. doi: 10.2353/ajpath.2008.070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberg SA, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 2005;57(5):664–678. doi: 10.1002/ana.20464. [DOI] [PubMed] [Google Scholar]
- 32.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202(1):135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiavoni G, et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med. 2002;196(11):1415–1425. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol. 2003;170(3):1131–1135. doi: 10.4049/jimmunol.170.3.1131. [DOI] [PubMed] [Google Scholar]
- 35.Aliberti J, et al. Essential role for ICSBP in the in vivo development of murine CD8alpha+ dendritic cells. Blood. 2003;101(1):305–310. doi: 10.1182/blood-2002-04-1088. [DOI] [PubMed] [Google Scholar]
- 36.Qi CF, et al. Differential expression of IRF8 in subsets of macrophages and dendritic cells and effects of IRF8 deficiency on splenic B cell and macrophage compartments. Immunol Res. 2009;45(1):62–74. doi: 10.1007/s12026-008-8032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng J, et al. IFN regulatory factor 8 restricts the size of the marginal zone and follicular B cell pools. J Immunol. 2011;186(3):1458–1466. doi: 10.4049/jimmunol.1001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118(5):1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubtsov AV, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118(5):1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Morse HC., 3rd IRF8 regulates myeloid and B lymphoid lineage diversification. Immunol Res. 2009;43(1-3):109–117. doi: 10.1007/s12026-008-8055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozato K, Tailor P, Kubota T. The interferon regulatory factor family in host defense: Mechanism of action. J Biol Chem. 2007;282(28):20065–20069. doi: 10.1074/jbc.R700003200. [DOI] [PubMed] [Google Scholar]
- 42.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 43.Ji P, Murata-Hori M, Lodish HF. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol. 2011;21(7):409–415. doi: 10.1016/j.tcb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keerthivasan G, Wickrema A, Crispino JD. Erythroblast enucleation. Stem Cells Int. 2011;2011:139851. doi: 10.4061/2011/139851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida H, et al. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437(7059):754–758. doi: 10.1038/nature03964. [DOI] [PubMed] [Google Scholar]
- 46.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science. 2006;314(5807):1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seubert A, et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci USA. 2011;108(27):11169–11174. doi: 10.1073/pnas.1107941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234(1):18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 50.Asselin-Paturel C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2(12):1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 51.Björck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98(13):3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- 52.Nakano H, Yanagita M, Gunn MD. CD11c(+)B220(+)Gr-1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194(8):1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yasuda K, et al. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J Immunol. 2007;178(11):6876–6885. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- 54.Luber CA, et al. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity. 2010;32(2):279–289. doi: 10.1016/j.immuni.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Teichmann LL, et al. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33(6):967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13(5):543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 57.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29(3):352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173(3):2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 59.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 60.Yamashita T, et al. Cloning and functional expression of a brain peptide/histidine transporter. J Biol Chem. 1997;272(15):10205–10211. doi: 10.1074/jbc.272.15.10205. [DOI] [PubMed] [Google Scholar]
- 61.Bhardwaj RK, Herrera-Ruiz D, Eltoukhy N, Saad M, Knipp GT. The functional evaluation of human peptide/histidine transporter 1 (hPHT1) in transiently transfected COS-7 cells. Eur J Pharm Sci. 2006;27(5):533–542. doi: 10.1016/j.ejps.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 62.Park B, et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9(12):1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ewald SE, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456(7222):658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto F, et al. Cathepsins are required for Toll-like receptor 9 responses. Biochem Biophys Res Commun. 2008;367(3):693–699. doi: 10.1016/j.bbrc.2007.12.130. [DOI] [PubMed] [Google Scholar]
- 65.Sasawatari S, et al. The solute carrier family 15A4 regulates TLR9 and NOD1 functions in the innate immune system and promotes colitis in mice. Gastroenterology. 2011;140(5):1513–1525. doi: 10.1053/j.gastro.2011.01.041. [DOI] [PubMed] [Google Scholar]
- 66.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329(5998):1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lessard CJ, et al. BIOLUPUS Network GENLES Network Identification of IRF8, TMEM39A, and IKZF3-ZPBP2 as susceptibility loci for systemic lupus erythematosus in a large-scale multiracial replication study. Am J Hum Genet. 2012;90(4):648–660. doi: 10.1016/j.ajhg.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han JW, et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet. 2009;41(11):1234–1237. doi: 10.1038/ng.472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information