Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0 (original) (raw)
. Author manuscript; available in PMC: 2013 Mar 6.
Published in final edited form as: Curr Opin Neurobiol. 2010 Aug;20(4):474–480. doi: 10.1016/j.conb.2010.08.005
Abstract
Microglia are increasingly recognized as critical in the pathogenesis of pain hypersensitivity caused by injury to peripheral nerves. The core signalling pathway is through P2X4 purinergic receptors on the microglia which, via the release brain derived neurotrophic factor, cause disinhibition of nociceptive dorsal horn neurons by raising intracellular chloride levels. This disinhibition works in synergy with enhanced excitatory synaptic transmission in the dorsal horn to transform the output of the nociceptive network. There is increased discharge output, unmasking of responses to innocuous peripheral inputs, and spontaneous activity in neurons that otherwise only signal nociception. Together the changes caused by microglia-neuron signalling may account for the main symptoms neuropathic pain in humans.
Introduction
Neuropathic pain and inflammatory pain are the two principal types of chronic pain1. Inflammatory pain arises as a consequence of tissue damage/inflammation and neuropathic pain from nervous system lesions. In contrast to inflammatory pain hypersensitivity, which usually returns to normal if the disease process is controlled, neuropathic pain persists long after the initiating event has healed. Both types of chronic pain are characterized by hypersensitivity at the site of damage and in adjacent normal tissue. Pain may appear to arise spontaneously, in that stimuli that would never normally produce pain begin to do so (allodynia) and noxious stimuli evoke a greater and more prolonged pain (hyperalgesia). Chronic pain, in contrast to acute pain, serves no known defensive, or any other helpful, function. Neither the intensity nor the quality of chronic pain is obviously related to tissue damage, and indeed chronic pain may persist long after any tissue damage, which may have caused acute pain, has abated. Chronic pain is not just acute pain that lasts a long time. Rather, chronic pain has a fundamentally different neurobiological basis than does acute pain. Acute pain is produced by the physiological functioning of the normal nervous system whereas chronic pain is a reflection of aberrant functioning of a pathologically altered nervous system.
Chronic pain reflects not only increases in the sensory input into the spinal cord, but also pathological amplification of these inputs within the nociceptive processing networks in the CNS1. The somatosensory gateway in the CNS is in the spinal cord dorsal horn, which is not a simple relay station. Rather, the dorsal horn contains a complex nociceptive processing network through which inputs from the periphery are transduced and modulated by local, as well as descending, excitatory and inhibitory control mechanisms2. The output of this network is transmitted to areas of the CNS involved in sensory, emotional, autonomic and motor processing. Normally, the output is balanced by excitatory and inhibitory processes. But in pathological pain states the output of the dorsal horn nociceptive network is greatly increased by suppressing inhibitory mechanisms and enhancing excitatory synaptic transmission.
In the case of chronic neuropathic pain, a rapidly growing body of evidence has established that the enhanced output of the dorsal horn nociceptive network is not solely dependent upon neuron-neuron transmission but that glia-neuron interactions are critical in establishing and maintaining pain hypersensitivity. Microglia in particular have emerged as key players in enhancing nociceptive output. Here we develop a renewed framework for understanding microglia-neuron interactions in the dorsal horn in neuropathic pain states by focusing on a specific microglial phenotypic state characterized by upregulation of a subtype of purinergic receptor, the P2X4 receptor, following peripheral nerve injury (PNI). Converging lines of evidence have shown that this P2X4R+ state is critical for subsequent pain hypersensitivity.
Microglia-neuronal signalling in neuropathic pain hypersensitivity 1.0
The previously conventional view that microglia subserve solely immune and housekeeping roles in the CNS has changed radically in the last half decade, in particular for the role of microglia in pain resulting from PNI. In the healthy CNS microglia are not dormant3,4 as thought until several years ago, but instead are in continuous surveillance of the local environment. By this continuous surveillance microglia detect and respond to various stimuli that threaten physiological homeostasis. The responding microglia undergo stereotypic programs of changes in morphology, gene expression, function and number5. Previously, microglia were considered to respond monolithically to all stimuli. But it is now clear that there are programs of response that are differentially engaged in by specific stimuli6,7.
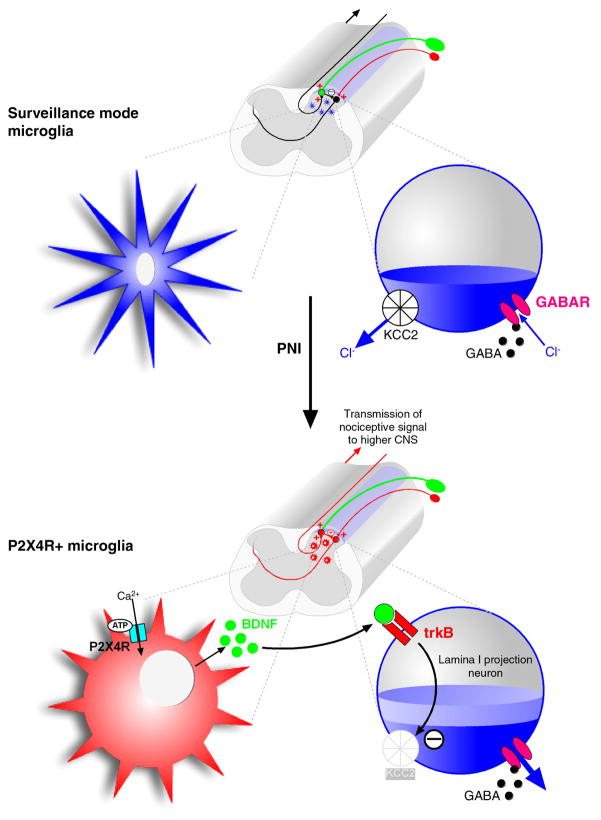
Our initial conceptual model for the microglial response in the dorsal horn following injury to a peripheral nerve and for the microglia-neuron signalling that is critical for pain hypersensitivity is illustrated in Figure 1. This model stemmed from a series of studies that came together around 2005 and led to the identification of the core P2X4R− BDNF-KCC2-Cl− cascade.
Figure 1. Microglia-neuronal signalling 1.0.
Conceptual model developed in 2005 illustrating the changes seen in the P2X4R – BDNF-KCC2–Cl− pathway. The normal situation is shown in the top panel. Microglia maintain a surveillance mode and do not express P2X4 receptors. Dorsal horn neurons, shown enlarged on the right, express KCC2 and have normal chloride extrusion capacity, thus maintaining low intracellular chloride concentration. Consequently, GABA activation produces a net inward flux of chloride and therefore inhibition.
Following peripheral nerve injury, microglia in the spinal dorsal horn adopt a P2X4R+ phenotype. Activation of these receptors causes release of BDNF which subsequently downregulates neuronal KCC2. Unable to extrude chloride, intracellular concentrations rise, impairing the inhibitory ability of the neuron.
Evidence that P2X4Rs are necessary and sufficient for pain hypersensitivity
Many studies had shown a correlation between responses of microglia in the dorsal horn and signs of pain hypersensitivity8–10. However, definitive evidence that microglia in the spinal dorsal horn play a causal role in maintaining neuropathic pain behaviours resulting from PNI was the discovery that PNI-induced pain behaviours are reversed by blocking P2X4Rs which, in the spinal cord, are specifically expressed by microglia after PNI9. An important line of evidence for the necessity of P2X4Rs, obtained subsequently, is that PNI does not cause mechanical hypersensitivity in mice lacking P2X4R11,12. Sufficiency of P2X4R stimulation in microglia for development of allodynia was demonstrated by ‘microglia transfer experiments’ in which microglia in primary culture are administered intrathecally in animals that were otherwise naïve9,13. When the microglia are stimulated in vitro by ATP, to activate P2X4Rs, the animals progressively develop mechanical hypersensitivity over the course of the 3–5 hours following the administration. In contrast, transferring unstimulated microglia does not cause allodynia nor does administering vehicle or ATP-only controls.
P2X4R-induced pain hypersensitivity is mediated by BDNF from microglia
To evoke pain behaviours the P2X4Rs in microglia must initiate signalling that is communicated to the nociceptive neurons in the dorsal horn, which then transmit information to the brain14. The core signalling pathway for this microglia-neuron communication was elucidated through the discovery that stimulating P2X4Rs in microglia with ATP evokes release of brain-derived neurotrophic factor (BDNF) which disinhibits neurons in lamina I (LI) of the dorsal horn, a major group of nociceptive output neurons15. The disinhibition is produced by BDNF acting on its cognate receptor, trkB, known to be expressed on LI neurons16, causing an increase in intracellular [Cl−] in the lamina I neurons. With the increase in [Cl−], opening GABAA or glycine channels is much less effective in producing inhibition, and in some lamina I neurons [Cl−] is increased to such an extent that in approximately one-third of GABA becomes excitatory13,15. The rise in [Cl−] within the dorsal horn neurons is mediated by suppressing KCC2, the main Cl− transporter in these cells.
Microglia–neuronal signalling in neuropathic pain hypersensitivity 2.0
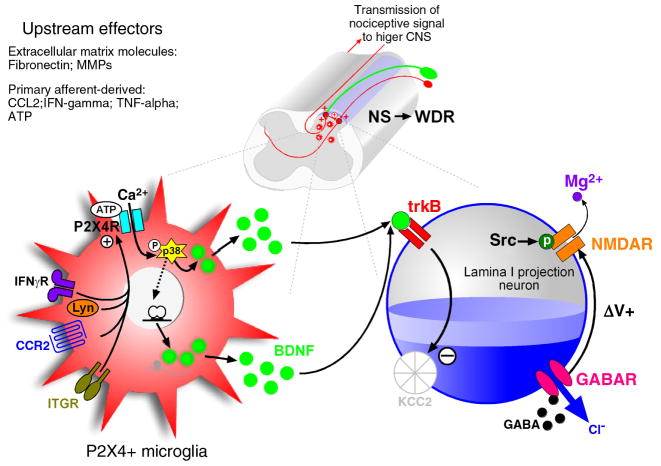
In the time since we formulated our initial model a wealth of evidence has accumulated reinforcing the concept of the key role for signalling between neurons and microglia in pain hypersensitivity after PNI. Further details of the signalling have been elucidated and new key molecular players identified. By integrating this recent information with the core signalling cascade identified previously we have now formulated a new conceptual framework that is illustrated in Figure 2.
Figure 2. Microglia-neuronal signalling 2.0.
The current hypothesis, combining new evidence that builds on the original model. Several factors have now been demonstrated to upregulate microglial P2X4 following peripheral nerve injury. These include the extracellular matrix molecule fibronectin, the cytokine IFNγ and the chemokine CCL2 (MCP-1). Activation of P2X4Rs increases both BDNF synthesis and release that is p38MAPK-dependent. BDNF-dependent downregulation of KCC2 changes the membrane properties of dorsal horn lamina I neurons which can then affect neuronal excitability via NMDAR potentiation. These changes are sufficient to cause a transformation in the firing properties of identified lamin I projection neurons in vivo such that normally nociceptive-specific neurons change phenotype to wide dynamic range, providing a substrate for the behavioural aspects of pain hypersensitivity: hyperalgesia, allodynia and spontaneous pain behaviours.
Cellular and molecular pathways leading to increased expression of P2X4Rs in microglia in the dorsal horn
A major unresolved issue in the original model was the virtually complete lack of information on the pathway, or pathways, by which injury to a nerve signals to the dorsal horn causing increased transcription of P2X4R mRNA leading to increased expression of receptor protein9. Propagation of action potentials in primary sensory neurons into the spinal cord is likely a critical step as the microglia response per se requires action potentials in primary afferents17. The subsequent signalling to spinal microglia has been suggested to involve a number of ligand/receptor systems, including fibronectin/integrin18,19, MCP1/CCR220, interferon/IFG receptor signalling21. Also critical for increasing expression of P2X4Rs is the tyrosine kinase Lyn22. Thus several key signalling elements necessary for the upregulation of P2X4Rs have been identified, and thus the next major challenges will be to determine how these elements are causally connected, and whether these encompass the entirety of the necessary pathways, or where other pathways are also required.
Cellular pathways for P2X4R-induced release of BDNF in microglia
Another large gap that has recently been filled is determining how the secretion of BDNF is effected by the stimulation of microglial P2X4Rs. Stimulation of microglial P2X4Rs in vitro increased both BDNF accumulation and release, indicating de novo synthesis of BDNF23. In P2X4 null mutants, BDNF accumulates in microglia, indicating that P2X4R activation is required for BDNF release11. Further studies investigating the intracellular mechanisms responsible showed that ATP stimulation of P2X4Rs caused a SNARE-dependent vesicular release of BDNF dependent upon p38-MAPK activation23. This work provides a parsimonious means to place p38MAPK in the core signalling cascade, thereby unifying observations that had previously appeared disparate. 24. These results indicate that p38-dependent P2X4R signalling has a critical role for the actions of BDNF in PNI-induced pain hypersensitivity.
Transforming the output of the dorsal horn nociceptive network
Whether the depolarizing shift in chloride reversal potential and the subsequent disinhibition of lamina I neurons may actually change the activity and responses of these neurons to account for pain hypersensitivity is a key question emanating from the core signalling cascade. Dorsal horn projection neurons in lamina I are modality specific. In the naïve animal the majority are nociceptive specific, with a small minority having a wide dynamic range. For hypersensitivity to occur, a change in the modality specificity of the output neurons must occur, i.e. the firing pattern of the output neurons switches such that innocuous input from the periphery is transformed and encoded by these previously nociceptive specific neurons. In a series of in vivo electrophysiological experiments this was shown to occur25. The same phenotypic switch in identified lamina I projection neurons was also achieved either by directly disrupting the chloride homeostasis pharmacologically, by blocking KCC2 activity, or by administering ATP-stimulated microglia. Thus, in the context of an intact dorsal horn nociceptive network, the ultimate consequence initiated by P2X4R stimulation in microglia is increased discharge output, unmasking responses to innocuous peripheral inputs, and causes spontaneous activity. Together these changes in neuronal discharge can account for the main symptoms neuropathic pain in humans, hyperalgesia, allodynia and spontaneous pain25. Thus, disinhibition by microglia-neuron signalling in the dorsal horn does not only alter the motor responses evoked by innocuous peripheral inputs, as was known previously. But disinhibition also increases the ascending output of the dorsal horn nociceptive network.
State-dependent potentiation of NMDA receptor function by PNI
In addition to suppressed inhibition, the output of the nociceptive dorsal horn network may be increased by facilitating excitatory transmission at dorsal horn glutamatergic synapses. This enhancement is mediated by intracellular signalling networks involving serine/threonine and tyrosine kinases within nociceptive transmission neurons26. Key for this enhancement is facilitation of NMDA receptor function by the non-receptor tyrosine kinase Src. Src is anchored within the NMDA receptor complex by the protein ND227. Disrupting the ND2-Src interaction in vivo attenuates behavioural pain hypersensitivity without the deleterious consequences of directly blocking NMDARs28. NMDAR currents are well-known to be strongly depressed by blockade of the channel pore by Mg2+. The blockade by Mg2+ is highly voltage-dependent and thus NMDAR currents will be amplified by disinhibition. Thus, we hypothesize that the essential elements of the core cascade within dorsal horn lamina I neurons are comprised of interacting disinhibition and facilitation of excitation: upon depolarization of the chloride reversal potential feeds forward with reduction and Mg2+ blockade of Src-potentiated NMDARs. In this way membrane potential changes due to altered chloride homeostasis from microglia-neuron signalling may gate the function of NMDARs.
On microglial “markers”, proliferation, infiltration and neuropathic pain
Our framework focuses on the P2X4R+ response state of dorsal horn microglia given the dramatic upregulation in the expression of these receptors after PNI and the multiple lines of evidence that these receptors are necessary and sufficient for inducing pain hypersensitivity. But what about other molecules widely considered as ‘markers’ of one or more response state of microglia? There are a plethora of studies demonstrating a correlation between a microglial response, as judged by one or more these markers, and pain hypersensitivity. Commonly microglial responses to PNI are assessed using changes in expression of membrane-associated markers, e.g. iba1 and cd11b/CR3, along with changes in microglia morphology. Whether the changes in markers and morphology are necessary for microglia to be able participate in pain hypersensitivity is not known, having never been tested directly. It is clear however, that these changes are not sufficient to produce pain hypersensitivity: the most striking evidence of this comes from P2X4R null mutant mice in which the increase in iba1 and the morphological changes induced by PNI are indistinguishable from the wild type, but there is no mechanical hypersensitivity in the P2X4R nulls11. It is possible that iba1 and the morphological change are upstream of P2X4Rs. But it is alternatively conceivable that these changes are in alternative or additional cellular pathways unrelated to the core cascade that produces pain hypersensitivity. A key step going forward, therefore, will be to determine whether ‘marker’ proteins are in-line in the signalling cascades for PNI-induced pain hypersensitivity. If so, then use of these markers can be justified. But if not, then it will be critical to assess microglia responses in terms of elements that are known to be involved, such as P2X4Rs.
Likewise there is abundant evident that PNI induces proliferation of microglia in the dorsal horn11,20,29. But there has been no attempt to determine whether this proliferation is in any way linked to a pain phenotype. Does the proliferative response represent an explicit reaction to axotomy of the peripheral axons of primary afferents whose central terminal fields determine the spatial extent of that proliferative response? The proliferative response might therefore be a distinct microglial phenotype that marks the initial response of the CNS to peripheral injury which is presumably initiated with an afferent barrage and associated release of various factors, and culminates in a phagocytic activity to remove axonal debris. Whether any of these activities is associated with PNI-induced pain remains to be determined.
In addition to alteration in molecular phenotype, morphology and proliferation induced by PNI, there is growing evidence that injury to peripheral nerves leads to the infiltration of monocytes from the circulation20. A population of circulating monocytes express P2X4Rs basally30. It is as yet uncertain whether it is these cells that enter the dorsal horn or whether P2X4R− monocytes infiltrate and then proceed to differentiate into microglia and adopt a P2X4+ phenotype. A recent interesting twist to the concept of infiltrating cells from the blood the demonstration that T cells infiltrate the dorsal horn parenchyma following PNI and release IFNγ31, in turn inducing an upregulation of microglia P2X4R expression21. As the infiltration monocytes and T cells target the region of the spinal cord dorsal horn in which output neurons become hypersensitivity is not only mechanistically intriguing but also provides potential for novel diagnostic and therapeutic approaches.
Conclusions
The microglia-neuronal signalling framework represents the unification of several converging lines of evidence demonstrating sufficiency and necessity in the initiation and expression of pain hypersensitivity following peripheral nerve injury. Many putative microglial inhibitors have been postulated, what is now needed is how they fit into the pathway, or to demonstrate additional pathways also necessary for maintaining behavioural sensitivity. Without defined specificity, generic “microglial inhibitors” are undesirable. Contradictory roles of microglia –protective v pathological have been demonstrated in neuroinflammatory responses to CNS injury32,33, suggesting complex phenotypic changes dependent upon the stimulus. Furthermore in the non-pathological CNS microglia have important roles within the immune system. Without a more complete understanding of the stimulus-dependent phenotypic changes of spinal microglia to PNI, attempting to inhibit their function may have deleterious as well as beneficial effects. Finally, evidence is accumulating for astrocytic involvement in spinal modulation of neuropathic pain states34–36 although the specific signalling pathways to neurons and how they interact with microglia/disinhibition remain to be established.
Acknowledgments
Work of the authors is supported by grants from the Canadian Institutes of Health Research (CIHR; grant number MT-12682), the Neuroscience Canada Brain Repair Program, the Krembil Foundation and the Ontario Neurotrauma Foundation to MWS. MWS is an International Research Scholar of the Howard Hughes Medical Institute and holds a Canada Research Chair (Tier I) in Neuroplasticity and Pain.
References
- 1.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 2.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 4.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 5.Perry VH. Modulation of microglia phenotype. Neuropathol Appl Neurobiol. 1994;20:177. [PubMed] [Google Scholar]
- 6.Hanisch U, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 7.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 8.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 9.Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 10.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 11.Ulmann L, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuda M, et al. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coull JAM, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 14.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Coull JAM, et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 16.Slack SE, Grist J, Mac Q, McMahon SB, Pezet S. TrkB expression and phospho-ERK activation by brain-derived neurotrophic factor in rat spinothalamic tract neurons. J Comp Neurol. 2005;489:59–68. doi: 10.1002/cne.20606. [DOI] [PubMed] [Google Scholar]
- 17.Suter MR, Berta T, Gao Y, Decosterd I, Ji R. Large A-fiber activity is required for microglial proliferation and p38 MAPK activation in the spinal cord: different effects of resiniferatoxin and bupivacaine on spinal microglial changes after spared nerve injury. Mol Pain. 2009;5:53. doi: 10.1186/1744-8069-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasu-Tada K, Koizumi S, Tsuda M, Kunifusa E, Inoue K. Possible involvement of increase in spinal fibronectin following peripheral nerve injury in upregulation of microglial P2X4, a key molecule for mechanical allodynia. Glia. 2006;53:769–775. doi: 10.1002/glia.20339. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda M, et al. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56:579–585. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuda M, et al. IFN-gamma receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc Natl Acad Sci USA. 2009;106:8032–8037. doi: 10.1073/pnas.0810420106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuda M, et al. Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia. 2008;56:50–58. doi: 10.1002/glia.20591. [DOI] [PubMed] [Google Scholar]
- 23.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin S, Zhuang Z, Woolf CJ, Ji R. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu XJ, Salter MW. Glutamate receptor phosphorylation and trafficking in pain plasticity in spinal cord dorsal horn. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gingrich JR, et al. Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondrial protein NADH dehydrogenase subunit 2. Proc Natl Acad Sci USA. 2004;101:6237–6242. doi: 10.1073/pnas.0401413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu XJ, et al. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325–1332. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun. 2007;21:624–633. doi: 10.1016/j.bbi.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29:2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costigan M, et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29:14415–14422. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- 33.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Y, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki Y, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuang Z, Gerner P, Woolf CJ, Ji R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]