Hormone replacement therapy improves contractile function and myonuclear organization of single muscle fibres from postmenopausal monozygotic female twin pairs (original) (raw)
Abstract
Ageing is associated with a decline in muscle mass and strength leading to increased physical dependency in old age. Postmenopausal women experience a greater decline than men of similar age in parallel with the decrease in female sex steroid hormone production. We recruited six monozygous female twin pairs (55–59 years old) where only one twin pair was on hormone replacement therapy (HRT use = 7.8 ± 4.3 years) to investigate the association of HRT with the cytoplasmic volume supported by individual myonuclei (myonuclear domain (MND) size,) together with specific force at the single fibre level. HRT use was associated with a significantly smaller (∼27%; P < 0.05) mean MND size in muscle fibres expressing the type I but not the IIa myosin heavy chain (MyHC) isoform. In comparison to non-users, higher specific force was recorded in HRT users both in muscle fibres expressing type I (∼27%; P < 0.05) and type IIa (∼23%; P < 0.05) MyHC isoforms. These differences were fibre-type dependent, i.e. the higher specific force in fast-twitch muscle fibres was primarily caused by higher force per cross-bridge while slow-twitch fibres relied on both a higher number and force per cross-bridge. HRT use had no effect on fibre cross-sectional area (CSA), velocity of unloaded shortening (_V_0) and relative proportion of MyHC isoforms. In conclusion, HRT appears to have significant positive effects on both regulation of muscle contraction and myonuclei organization in postmenopausal women.
Key points
- The ageing-related impairment of muscle function and consequent falls and fall-related injuries have severe negative effects on morbidity and mortality in old age, with women being more negatively affected than men.
- The effects of hormone replacement therapy (HRT) on regulation of muscle contraction and myonuclear organization were investigated in monozygous postmenopausal twin pairs where only one twin was an HRT-user.
- HRT treatment improved single fibre force-generating capacity (specific force), without affecting fibre size and speed of contraction, due to fibre type-specific effects on force and number of force-generating cross-bridges.
- HRT had a significant effect on the myonuclear organization in slow-twitch muscle fibres, improving the synthetic capacity of the myonuclei and optimizing transport of proteins.
- Significant positive effects on regulation of muscle contraction and myonuclear organization were observed at the cellular level in response to HRT with consequences for quality of life in postmenopausal women.
Introduction
Sarcopenia is the ageing-related progressive change in skeletal muscle quantity and quality leading to decline in strength and mobility (Frontera et al. 2000). The changes at the whole muscle level reflect ageing-related changes in structure and function at the motor unit, muscle cell and motor protein levels, resulting in a decline in muscle mass, force-generating capacity and contractile speed (Larsson et al. 1997; Yu et al. 2007). These changes are attributed to a complex interface of many factors ultimately leading to a progressive loss of mobility in old age (Ryall et al. 2008).
Current theories attribute an altered endocrine activity as an important contributor to the ageing-related muscle dysfunction. In this context, the most dramatic event in women is the menopause, resulting in an additional 15% loss in muscle mass (Phillips et al. 1993) making older women more vulnerable to fall and fall-related injuries (Frontera et al. 1991). Hence, hormone replacement therapy has been extensively used to partially counteract the deleterious effects on muscles (Skelton et al. 1999; Sipila et al. 2001). However, the beneficial impacts of HRT on muscle function are still in debate, mainly due to experimental limitations, such as genetic and lifestyle differences among HRT users and non-users (Onambele-Pearson, 2009). To overcome this limitation, a case control study was recently performed in postmenopausal monozygotic (MZ) twin pairs where only one of the twins was an HRT user (Ronkainen et al. 2009; Finni et al. 2011). In that study, HRT users had better walking speed and jumping height (Ronkainen et al. 2010) as well as in vivo force production (Finni et al. 2011) compared with their non-user genetically identical co-twins. Similarly, a recent meta-analysis reported a 5% increase in muscle strength with HRT use in postmenopausal women compared with age-matched controls (Greising et al. 2009).
The cellular and molecular mechanisms underlying the in vivo improvements in response to HRT treatment remain unclear. It has been speculated, on one hand, that oestrogen improves muscle directly by affecting actomyosin interactions (Phillips et al. 1993; Lowe et al. 2010). On the other hand, oestrogen has been suggested to boost satellite cell activation, attenuating exercise-induced muscle damage and creating a pro-anabolic environment in muscles of postmenopausal women (Enns et al. 2008; Dieli-Conwright et al. 2009_a_,b). Recently, a positive anti-catabolic effect and an improved regulatory action on the cytoskeleton and extracellular matrix were observed in response to HRT treatment in MZ twin pairs, leading to better muscle quality (Ronkainen et al. 2010; Ahtiainen et al. 2012). While some or all of the above mechanisms may influence skeletal muscle function, little is known about the action of oestrogen on the regulation of muscle contraction at the cell and motor protein levels in humans. An investigation of the actomyosin interactions at the cell and motor protein levels is forwarded as a relevant experimental model to improve our understanding of the mechanisms underlying the effects of HRT and in the identification of potential future pharmacological intervention strategies aimed at improving muscle function in postmenopausal women in general and/or in specific conditions such as postmenopausal rehabilitation. Further, myonuclear organization is affected by ageing and we have recently shown that myonuclear domain (MND) size is linked to specific force and the quantity of the molecular motor protein myosin in skeletal muscles fibres (Cristea et al. 2010; Qaisar et al. 2012)
It is hypothesized that the altered in vivo muscle function related to HRT treatment observed in MZ twin pairs (Ronkainen et al. 2009; Finni et al. 2011) is caused by the combined effect of an altered actomyosin interaction, contractile protein expression and myonuclear organization. This study aims at unravelling the effects of HRT treatment by studying a subset of the unique group of HRT-discordant postmenopausal MZ twin pairs described above by studying the force generation capacity, contractile speed and the 3-D myonuclear organization in single muscle fibre segments.
Methods
Ethical approval
The ethics committee of the Central Finland Health Care District approved the study, and it was conducted according to the guidelines laid down by the World Medical Association in the Declaration of Helsinki (2000). Written informed consent was provided by the participants before taking the biopsy and the measurements.
Study design and subjects
This study is part of a larger study, ‘Sarcopenia – Skeletal Muscle Adaptation to Postmenopausal Hypogonadism and Effects of Hormone Replacement Therapy and Physical Activity in Older Women: a Genetic and Molecular Biological Study on Estrogen-related Pathways’ (SAWEs). The study design, subject recruitment and exclusion criteria have been described previously (Ronkainen et al. 2009). Subjects with chronic musculoskeletal diseases, type 1 diabetes, type 2 diabetes with medication, diagnosed mental disorder, asthma with oral cortisol treatment, acute cancer, known drug or alcohol abuse/dependence, or Crohn's disease were excluded. All measurements were done on the same day in a given twin pair. Briefly, after screening and confirmation for monozygosity by multiple genetic markers, a total of six MZ twin pairs, clearly postmenopausal and discordant for HRT, were chosen for the current study from participants in the Finnish Twin Cohort Study. The mean age of the 12 subjects was 56.6 ± 1.3 years (range 55–59 years). Oestradiol and progesterone were the effective agents given as pills in three HRT users while oestradiol was given alone in another three subjects. The mean duration of HRT use was 7.8 ± 4.3 years (range 4–16 years).
Muscle biopsies
Bergstrom needles were used to obtain biopsies from the right vastus lateralis muscle with the understanding and consent of the subjects. The biopsy specimens typically contained segments of 200–800 muscle fibres and weighed 50–120 mg. Specimens were placed in relaxing solution at 4°C, and bundles of ∼50 fibres were carefully dissected free and then tied with surgical silk to glass capillary tubes at slightly stretched lengths. The muscle fibre bundles were chemically skinned for 24 h in relaxing solution containing 50% (v/v) glycerol at 4°C, cryoprotected (Frontera & Larsson, 1997) and subsequently stored at –180°C before use. The relaxing solution contained (in mm): 4 MgATP, 1 free Mg2+, 20 imidazole, 7 EGTA, 14.5 creatine phosphate and sufficient KCl to adjust the ionic strength to 180. The pH was adjusted to 7.0. The free Ca2+ concentration, expressed as pCa (–log[Ca2+]), was 10−9 m. The apparent stability constant for Ca2+-EGTA was corrected for temperature and ionic strength (Fabiato & Fabiato, 1979).
Muscle biopsy samples from 13 healthy male control subjects (25–89 years) were included for comparison of myosin protein post-translational modifications (PTMs).
Single fibre contractile recordings
The experimental procedure has been described in detail elsewhere (Larsson & Moss, 1993). Briefly, membrane-permeabilized muscle fibres were used with an average segment length of 1.60 ± 0.20 mm (mean ± SD, range 1.00–2.00 mm) exposed to the solution between the connectors of the force transducer and servomotor. The sarcomere length (SL) of the single-fibre segment was set to 2.77 ± 0.05 μm (range 2.71–2.85 μm) by adjusting the overall segment length. Fibre CSA was calculated from the width and depth, assuming an elliptical circumference. Specific tension (ST) was calculated as maximum tension (_P_0) normalized to CSA, and was corrected for the 20% swelling that is known to occur during skinning (Moss, 1979).
Maximum unloaded shortening velocity (V_0; Musle length (ML)/s) was measured by the slack test procedure (Edman, 1979). Fibres were activated at pCa 4.5 and, once steady-state tension was reached, various amplitudes of slack (Δ_L) were rapidly introduced (within 1–2 ms) at one end of the fibre. The time (Δ_t_) required to take up the imposed slack was measured from the onset of the length step to the beginning of the tension redevelopment. For each amplitude of Δ_L_ the fibre was re-extended while relaxed to minimize non-uniformity of the sarcomere length. A straight line was fitted to a plot of Δ_L vs.Δ_t using a least-squares regression, and the slope of the line was recorded as _V_0 for that fibre. Relaxing and activating solutions were prepared as previously described (Larsson & Moss, 1993). All contractile measurements were carried out at 15°C. The contractile recordings were accepted in subsequent analyses only if _P_0 did not change more than 10% from first to final activation, if SL during isometric tension development did not change by more than 0.10 μm compared with SL when the fibre was relaxed or if the _V_0 value based on linear regression included four or more data points, and the data were discarded if the coefficient of reliability (_r_2) for the fitted line was less than 0.96 (Moss, 1979).
Stiffness
Once steady-state isometric force was reached, small-amplitude sinusoidal changes in length (Δ_L_: ±0.2% of fibre length) were applied at 500 Hz at one end of the fibre (Martyn et al. 2007). The resultant force response (Δ_F_) was measured, and the mean of 20 consecutive readings of Δ_L_ and Δ_F_ was used to determine stiffness. The actual elastic modulus (E) was calculated as the difference between E in activating solutions and resting E measured in the same segment in the relaxing solution. E was determined as follows (McDonald & Fitts, 1995):
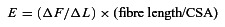
Fluorescent labelling, image acquisition and analyses of myonuclear organization
Skinned single fibre segments were mounted at a fixed sarcomere length corresponding to optimal filament overlap for force generation. Actin and myonuclei were stained with Rhodamine Phalloidin and 4′,6-diamidino-2-phenylindole (DAPI), respectively. Confocal images were analysed by means of a novel algorithm. The volume G of a general elliptical cylinder was developed and used to calculate the volumes of the MNDs and the CSA of the fibre. The 3-D parameters of every nucleus were determined manually and the MND size determined by means of automatic image analysis. A detailed description of procedures is given elsewhere (Cristea et al. 2010).
Single fibre gel electrophoresis
The procedure is described in detail elsewhere (Larsson & Moss, 1993). In short, the MyHC composition of single fibres was determined by SDS-PAGE. The total acrylamide and bis concentrations were 4% (w/v) in the stacking gel and 6% in the running gel, and the gel matrix included 30% glycerol. Polymerization was activated by adding tetramethylethylenediamine (TEMED) to the stacking (0.1%) and separation gels (0.07%). Sample loads were kept small to improve the resolution of the MyHC bands and electrophoresis was performed at 120 V for 22–24 h with a Tris-glycine electrode buffer (pH 8.3) at 10°C.
Post-translational modifications
Vastus lateralis muscle biopsy cryo-sections from HRT using and their non-using co-twins as well as from controls were run on 6% SDS-PAGE gel. Gel bands corresponding to type I, IIa and IIx MyHC isoforms were extracted. Samples were digested in-gel, separated with a 40 min gradient RP-nanoHPLC and analysed online using a 7 tesla LTQ-FT Ultra tandem mass spectrometer (MS; Thermo Fisher Scientific Pittsburgh, PA, USA) modified with a nano electrospray ion source (Proxeon Biosystems Odense, Denmark). High-resolution survey scan followed by low-resolved MS/MS scans of the five most abundant peaks was used. Peptide identification was performed using the Mascot search engine allowing two missed cleavages and a set of variable PTMs (i.e. multiple oxidations, methylations and phosphorylations (Artemenko et al. 2011).
Statistical analysis
Dfferences between the means in body composition parameters from HRT using and non-using co-twins were tested using Wilcoxon's signed rank test. Intra-pair differences are expressed as percentages (IPD%) and calculated as follows: (HRT user – non-user)/(non- user) × 100. In addition, the 95% confidence interval (95% CI) was calculated for each IPD%. The paired sample t test was used to compare fibre CSA, MND and contractile recordings between HRT using and their non-using co-twins. The data normality was assessed by the Kolmogorov–Smirnov test. Values are expressed as mean ± standard error of mean (SEM) with the exception of body composition values which are expressed as means ± standard deviations (SD). Statistical significance was set at P < 0.05 for all analysis.
Results
Lifestyle characteristics and body composition
Lifestyle characteristics and body composition of the original study population with 15 54- to 62-year-old postmenopausal MZ twin pairs discordant for long-term HRT have been described in detail by Ronkainen et al. (2009). In summary, there were no differences in the leisure or work physical activity, occupation, smoking behaviour, alcohol use or daily energy intake between the HRT using and non-using co-twins. Furthermore, body composition in terms of body weight, body mass index (BMI), waist or hip circumference as well as body fat percentage did not differ between HRT using and their non-using co-twins either in the original (_n_= 15 pairs) nor in the current study population with six twin pairs (Table 1).
Table 1.
Anthropometry and body composition in HRT-using twins and their non-using co-twins (_n_= 6 pairs)
| Variable | HRT users | HRT non-users | IPD% (95% CI) | P value |
|---|---|---|---|---|
| Body height (cm) | 162.7 ± 2.1 | 162.2 ± 2.2 | 0.31 (−0.11 to 0.74) | 0.250 |
| Body weight (kg) | 70.3 ± 4.5 | 78.4 ± 6.8 | −9.0 (−19.6 to 1.6) | 0.156 |
| BMI (kg m−2) | 26.7 ± 2.0 | 30.1 ± 3.2 | −9.5 (−20.6 to 1.6) | 0.156 |
| Waist circumference (cm) | 89.4 ± 4.1 | 98.0 ± 5.5 | −8.3 (−16.3 to 0.3) | 0.094 |
| Hip circumference (cm) | 102.3 ± 3.0 | 106.6 ± 4.2 | −3.8 (−8.7 to 1.1) | 0.156 |
| Body fat (%) | 33.1 ± 3.5 | 37.7 ± 3.8 | −12.8 (−26.2 to 0.59) | 0.094 |
Single fibre cross-sectional area and MyHC isoform expression
The CSA of individual muscle fibres was measured in a total of 326 fibre segments from HRT non-users (_n_= 162) and users (_n_= 164) at a fixed sarcomere length assuming an elliptical circumference (Table 2). Statistical analysis was restricted to fibres expressing the type I (_n_= 176) and type IIa MyHC isoforms (_n_= 89) because of the scarcity and unequal distribution across subjects of fibres expressing the type IIx MyHC isoform (_n_= 5), co-expressing type I and type IIa (_n_= 29) or type IIa and IIx (_n_= 27) MyHC isoforms. No statistically significant difference was found in the CSA of fibres expressing the type I or type IIa MyHC isoform between twin pairs, resulting in a similar type IIa/I fibre area ratio between twin sisters. In young adults, type II fibres are typically larger than type I fibres independent of sex demonstrating that the preferential type II fibre atrophy reported in old age in skinned muscle fibres (Larsson et al. 1997; Cristea et al. 2010) or from enzyme-histochemically stained sections (Larsson, 1978) becomes manifest already at 50–59 years of age.
Table 2.
Cross-sectional area (CSA), specific tension (ST), stiffness and maximum velocity of unloaded shortening (_V_0) in skinned single muscle fibres expressing different MyHC isoforms in HRT using and non-using co-twins
| Type I | Type I/IIa | Type IIa | Type IIax | Type IIx | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HRT non-users | HRT users | HRT non-users | HRT users | HRT non-users | HRT users | HRT non-users | HRT users | HRT non-users | HRT users | |
| (_N_= 6) | (_N_= 6) | (_N_= 4) | (_N_= 6) | (_N_= 6) | (_N_= 6) | (_N_= 4) | (_N_= 6) | (_N_= 2) | (_N_= 1) | |
| CSA (μm2) | 2550 ± 110 | 2460 ± 140 | 2630 ± 450 | 1860 ± 190 | 2240 ± 170 | 2260 ± 130 | 1850 ± 120 | 1560 ± 280 | 2050 ± 100 | 1320 |
| (_n_= 97) | (_n_= 79) | (_n_= 13) | (_n_= 16) | (_n_= 39) | (_n_= 50) | (_n_= 10) | (_n_= 17) | (_n_= 3) | (_n_= 2) | |
| ST (N cm−2) | 28.5 ± 2 | 36.4 ± 2.7* | 30.1 ± 5.3 | 39.1 ± 4 | 31.2 ± 2.2 | 38.2 ± 1.3* | 29.3 ± 3 | 31.8 ± 4.8 | 23 ± 2.3 | 30.8 |
| (_n_= 71) | (_n_= 48) | (_n_= 9) | (_n_= 11) | (_n_= 25) | (_n_= 39) | (_n_= 9) | (_n_= 15) | (_n_= 3) | (_n_= 2) | |
| Stiffness (N cm−2) | 2550 ± 40 | 2870 ± 170 | 2450 ± 900 | 2880 ± 460 | 2140 ± 130 | 2500 ± 70* | 2200 ± 270 | 2050 ± 230 | ||
| (_n_= 71) | (_n_= 48) | (_n_= 7) | (_n_= 8) | (_n_= 20) | (_n_= 32) | (_n_= 8) | (_n_= 6) | |||
| _V_0 (ML[LJ29] s−1) | 1 ± 0.1 | 0.90 ± 0.1 | 1.6 ± 0.6 | 1.8 ± 0.3 | 2.1 ± 0.3 | 2.3 ± 0.2 | 2.5 ± 0.2 | 2.7 ± 0.1 | 2.3 ± 0.1 | 2.5 |
| (_n_= 59) | (_n_= 46) | (_n_= 8) | (_n_= 10) | (_n_= 20) | (_n_= 32) | (_n_= 7) | (_n_= 12) | (_n_= 3) | (_n_= 2) |
The proportion of MyHC isoforms expressed in dissected single muscle fibres used in contractile measurements and in muscle biopsy cross-sections are presented in Table 3. In short, no statistically significant difference was found in the relative proportion of different MyHC isoforms between twin sisters, either in the analysed muscle fibres or at the muscle biopsy level.
Table 3.
MyHC isoform expression measured in single muscle fibres and biopsy cross-sections from HRT using and non-using co-twins
| Total number of fibres | Type I (%) | Type I/IIa (%) | Type IIa (%) | Type IIax (%) | Type IIx (%) | |
|---|---|---|---|---|---|---|
| HRT non-users | 162 | 60 (47 ± 6) | 8 | 24 (51 ± 6) | 6 | 2 (12 ± 1) |
| HRT users | 164 | 49 (43 ± 5) | 10 | 30 (52 ± 4) | 10 | 1 (15 ± 3) |
Phenotypical observations
Individual myonuclei typically had a rounded or elliptical appearance and both shapes were frequently observed in the same fibre segments irrespective of MyHC isoform and HRT status (Fig. 1). The longitudinal axis of elliptical nuclei was parallel with the longitudinal axis of muscle fibre in most, but not all, myonuclei. Deviations from the common rounded or elliptical shapes were rare, but a small number of nuclei were observed with ‘notches’. In accordance with our previous observations (Cristea et al. 2010), fibres expressing the type I MyHC isoforms frequently presented with groove-like structures with long chains of aggregated nuclei (Fig. 1), leading to an increased MND size variability. This type of spatial organization of myonuclei was typically observed in fibres expressing the type I MyHC isoform while it was relatively scarce in fibres expressing the type IIa MyHC isoform where myonuclei showed a more ordered organization (Fig. 1_C_ and D). These observations were independent of HRT status.
Figure 1. Confocal microscopy images of single muscle fibres and their representative myonuclei from HRT users and HRT non-users.
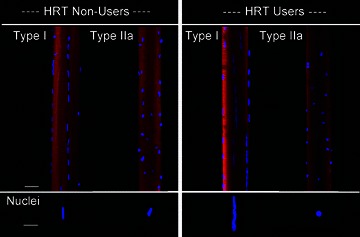
Type I fibres are typically characterized by deep groove-like structures harbouring long chains of nuclei. Type IIa fibres have a more ordered organization of both spherical and elliptical myonuclei. DAPI stained myonuclei (blue) while rhodamine labelled actin (red). Horizontal bars denote 50 μm (fibre) and 8 μm (nuclei).
Internal nuclei were rare, but a small number was observed in muscle fibres expressing the type I MyHC isoform in the HRT non-users (3 out of 31 fibre segments) and users (3 out of 29 fibre segments). Further, internal nuclei were infrequent in type I/IIa (1 of 7, in HRT user only) and in type IIa fibres (2 of 26, in non-users only), but they were not observed in fibres expressing the type IIx MyHC or co-expressing type IIa and IIx isoforms. When present, internal nuclei constituted 2–12% of all nuclei in the fibre segment.
Nuclei number per unit length and MND size
The MNDs at the terminal part of the fibre segment may extend outside the fibre segment and give rise to erroneously small MND sizes, therefore half of the terminal nuclei were randomly included in the analysis and half were excluded. Only the fibres expressing type I (_n_= 61) and type IIa (_n_= 36) MyHC isoforms were considered for statistical analysis, because of the small number of fibres expressing other MyHC isoforms or a combination of MyHC isoforms. No significant difference was found in myonuclear number per unit length in muscle fibres expressing type I or type IIa MyHC isoforms between the HRT user and non-user groups (Fig. 2_B_).
Figure 2. Myonuclear domain size (A), nuclei number per unit length (B), and specific tension and stiffness recordings (C) in type I and type IIa fibres in vastus lateralis muscles from HRT using and HRT non-using co-twins.
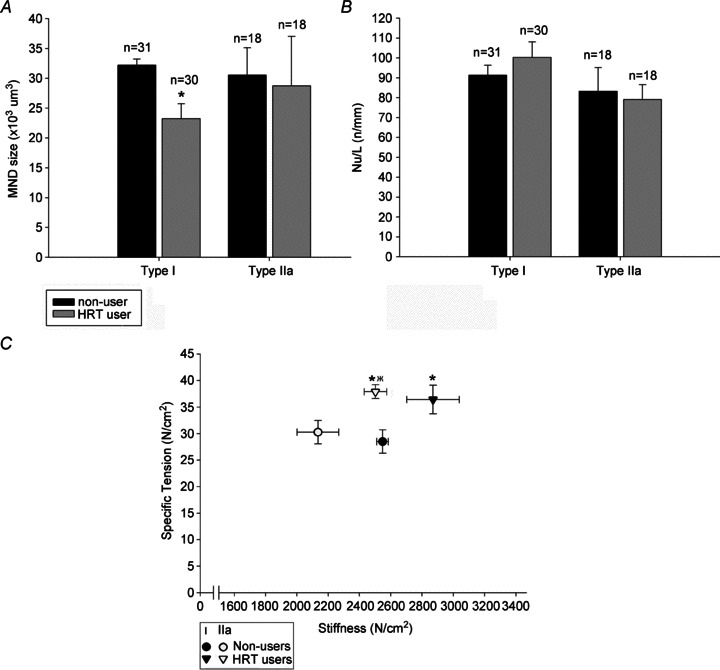
In A and B, * denotes statistically significant difference from non-users (P < 0.05). In C, * and † denote statistically significant difference for the specific force and stiffness, respectively (P < 0.05). All values are mean ± SEM.
In muscle fibres expressing the type I MyHC isoform, myonuclear domain size was 27% smaller (P < 0.05) in HRT users than their non-user counterparts (Fig. 2_A_), due to the combined effect of small trends, not statistically significant, towards both smaller fibres and extra myonuclei in the HRT users. In fibres expressing the type IIa MyHC isoform, MND size did not differ significantly between HRT users and non-users.
Contractile properties
A total of 216 fibres expressing type I (_n_= 129) and type IIa (_n_= 83) MyHC isoforms met the strict criteria for acceptance and were included in the analysis of contractile properties (Table 2). Fibres expressing the type IIx MyHC isoform (_n_= 5), co-expressing type I and type IIa (_n_= 20) or type IIa and type IIx (_n_= 21) MyHC isoforms were omitted from the statistical analysis due to paucity of these fibre types. Maximum force normalized to muscle fibre cross-sectional area, i.e. specific force was higher in the HRT using than in their non-using co-twins both in muscle fibres expressing the type I MyHC isoforms ∼27% (P < 0.05) and in fibres expressing the type IIa MyHC isoform ∼23% (P < 0.05) (Fig. 2_C_, Table 4). The higher specific force may accordingly reflect HRT-associated differences in the regulation of muscle contraction, i.e. a larger number of strongly attached cross-bridges in series or force produced by each cross-bridge (Regnier et al. 2004). Stiffness recordings (_E_0) represent a good index of the number of strongly attached cross-bridges in series. In muscle fibres expressing the type I MyHC isoform, the slightly higher stiffness (∼13%) in the HRT group was not statistically significant (Fig. 2_C_). Accordingly, it is unlikely that the higher total number of cross-bridges is the major source underlying the 27% higher specific force suggesting that the force per cross-bridge is higher in type I fibres from HRT users. In fibres expressing the type IIa MyHC isoform, on the other hand, the ∼17% higher stiffness (P < 0.05) suggests that a larger number of cross-bridges contribute significantly to ∼23% higher specific force among HRT users (Fig. 2_C_). Maximum velocity of unloaded shortening did not differ between users and non-users, independent of MyHC isoform expression.
Table 4.
Specific force and MND size in the six twin pairs
| Twins | Type I fibres | Type IIa fibres | ||||
|---|---|---|---|---|---|---|
| Twin pairs | HRT status | Total number of fibres | ST (N cm−2) | MND (×103μm3) | ST (N cm−2) | MND (×103μm3) |
| Pair 1 | HRT non-user | 42 | 21.9 ± 2.3 | 35.7 ± 4.7 | 22.3 ± 2.1 | 26.3 ± 2.3 |
| (_n_= 23). | (_n_= 8). | (_n_= 9). | (_n_= 2). | |||
| HRT user | 34 | 28.4 ± 4.3 | 34.3 ± 5.1 | 33.4 ± 6.7 | 12.7 ± 3.1 | |
| (_n_= 18) | (_n_= 4) | (_n_= 7) | (_n_= 3) | |||
| Pair 2 | HRT non-user | 26 | 32.5 ± 5.7 | 29.5 ± 4.3 | 27.2 ± 3.7 | 53.1 ± 7.5 |
| (_n_= 9). | (_n_= 7). | (_n_= 7). | (_n_= 3). | |||
| HRT user | 25 | 39.9 ± 4.9 | 16.9 ± 3.1 | 40.7 ± 8.3 | 27.7 ± 2.5 | |
| (_n_= 9) | (_n_= 5) | (_n_= 5) | (_n_= 2) | |||
| Pair 3 | HRT non-user | 21 | 34.9 ± 6.4 | 33.1 ± 3.5 | 34.7 ± 3.7 | 20.9 ± 2.7 |
| (_n_= 8). | (_n_= 4). | (_n_= 6). | (_n_= 2). | |||
| HRT user | 24 | 38.7 ± 4.1 | 19.8 ± 3.1 | 40.5 ± 5.3 | 60.6 ± 8.2 | |
| (_n_= 9) | (_n_= 3) | (_n_= 7) | (_n_= 3) | |||
| Pair 4 | HRT non-user | 22 | 23.4 ± 4.1 | 31.7 ± 7.1 | 32.1 ± 5.5 | 27.4 ± 4.4 |
| (_n_= 8). | (_n_= 4). | (_n_= 5). | (_n_= 5). | |||
| HRT user | 27 | 29.1 ± 6.6 | 18.7 ± 4.4 | 41.6 ± 9.1 | 22.7 ± 4 | |
| (_n_= 7) | (_n_= 7) | (_n_= 6) | (_n_= 3) | |||
| Pair 5 | HRT non-user | 19 | 29.9 ± 4.1 | 29.4 ± 5.1 | 36 ± 3.9 | 26.6 ± 3.9 |
| (_n_= 6). | (_n_= 4). | (_n_= 5). | (_n_= 4). | |||
| HRT user | 26 | 46.3 ± 6.3 | 24.3 ± 5.4 | 35.3 ± 3.3 | 19.9 ± 3.1 | |
| (_n_= 7) | (_n_= 4) | (_n_= 8) | (_n_= 4) | |||
| Pair 6 | HRT non-user | 32 | 28.3 ± 5.3 | 33.7 ± 7.3 | 34.5 ± 4.1 | 28.9 ± 3.1 |
| (_n_= 17). | (_n_= 4). | (_n_= 9). | (_n_= 2). | |||
| HRT user | 28 | 35.9 ± 5 | 25.4 ± 2.1 | 37.8 ± 4.2 | 27.7 ± 1.6 | |
| (_n_= 8) | (_n_= 7) | (_n_= 9) | (_n_= 3) |
Post-translational myosin modifications
In an attempt to improve our understanding of the mechanisms underlying the HRT-induced effects on myosin function, a mass spectrometry approach was taken to determine myosin post-translational modifications (PTMs) in response to HRT treatment. Analyses were restricted to the type I and IIa MyHC isoform due to the paucity of the type IIx MyHC isoform in some of the HRT twin pairs. Type I and IIa MyHC isoforms were separated on 6% SDS-PAGE gels extracted and screened for acetylation, carbonylation, deamidation, glucosylation, methylation, nitration, ubiquitination, and phosphorylation by liquid chromatography–mass spectrometry (LC/MS).
Eight myosin modifications were observed in both HRT users and non-users. Although none of the myosin PTMs were specific to HRT treatment, three out of four carbonylations dominated in the HRT group (Table 5). Deamidations, on the other hand, dominated among non-users and one of these deaminations (position 1079) was only observed among the older controls and not in middle-aged or young controls (Table 5). One modification was situated in the myosin motor domain (carbonylation amino acid (aa) 413), and was more prominent in the non-users; the seven others were located in the tail region of the myosin. In addition, six modifications were specific for the type IIa MyHC isoform (carbonylation aa 853, deamidation aa 940, 1079, 1293 and methylation aa 1449, 1493) and the two other modifications were found in both type I and IIa myosin isoforms (carbonylation aa 413 and 1623/1627).
Table 5.
Myosin protein post-translational modifications in HRT using (U) and non-using (NU) monozygotic twin pairs
| Modification type | Peptide sequence | Amino acid modified | HRT user or non-user | Myosin isoforms | Amino acid position | Domain |
|---|---|---|---|---|---|---|
| Carbonylation | KMEGDLNEMEIQLNHANR | D/K | 5U 1NU | I/IIa | 1623/1627 | Tail domain |
| Carbonylation | LQTESGEFSR | F | 5U 2NU | IIa | 1293 | Tail domain |
| Carbonylation | SAETEKEMATMKEEFQK | K | 4U 2NU | IIa | 853 | Tail domain |
| Carbonylation | VKVGNEFVTK | F | 3U 6NU | I/IIa | 413 | S1 myosin head (CM-loop) |
| Deamidation | AEDEEEINAELTAK | N | 1U 5NU | IIa | 940 | Tail domain |
| Deamidation | LAQESIMDIENEK | N | 2U 4NU | IIa | 1079 | Tail domain* |
| Methylation | QAEEAEEQANTNLSK | E | 4U 1NU | IIa | 1893 | Tail domain |
| Methylation | TNAACAALDKK | D | 2U 4NU | IIa | 1449 | Tail domain |
Discussion
The results from this study favour a beneficial effect of hormone replacement therapy on skeletal muscle in postmenopausal women and the major findings from this study are as follows: (i) HRT preserves the specific force without affecting fibre CSA; (ii) stiffness values reflected the change in specific force in fibres expressing type IIa MyHC isoform, but not in type I fibres; (iii) smaller MND size was observed in fibres expressing the type I MyHC isoform in response to hormone use while MND size was unaffected in type IIa fibres; (iv) no significant change was observed in the velocity of unloaded shortening (_V_0) and myonuclei number with HRT use.
Fibre CSA and MyHC isoform expression
The higher specific tension in muscle from the HRT user could be secondary to a myosin isoform switching towards a faster phenotype or an increase in the relative area of muscle fibres expressing the fast myosin isoform, since human muscle fibres expressing fast myosin isoforms generate higher specific forces than fibres expressing the slow MyHC isoform (Medler, 2002; Korhonen et al. 2006; Yu et al. 2007). This in part is due to a higher force-generating capacity of the human fast MyHC isoform (Li & Larsson, 2010). However, fibre CSA or the proportion of MyHC isoforms did not differ between HRT using and non-using co-twins (Tables 2 and 3). These observations are in accordance with a previous publication comparing hormone replacement with non-replacement in postmenopausal women (Widrick et al. 2003).
HRT affects myosin function to preserve single fibre force-generating capacity
The two prime determinants of specific force are the fraction of strongly attached cross-bridges and the force produced by individual cross-bridges. The results from this study suggest that both factors contribute to the higher specific force in HRT users, but the relative contribution appears to be MyHC dependent.
In muscle fibres expressing the β/slow MyHC isoform, stiffness recordings suggest that only ∼50% of the higher specific force in the HRT user is attributed to an increased fraction of strongly attached cross-bridges (Fig. 2_C_), indicating that the force/cross-bridge account for the remaining increase in specific force. In fibres expressing the type IIa MyHC isoform, on the other hand, specific force is in good agreement with stiffness measurements demonstrating that the lower specific force in the non-user is primarily due to a smaller fraction of strongly attached cross-bridges (Fig. 2_C_). This conforms with previous hypotheses (Phillips et al. 1993) and experimental results using electron paramagnetic resonance (EPR) spectroscopy in ovariectomized rats treated with oestrogen (Moran et al. 2007). Thus, the contractile recordings indicate a fibre type-specific effect of the HRT treatment in the postmenopausal women where the positive effects are mainly due to a quantitative effect in fibres expressing the fast myosin isoform while the effect is both quantitative and qualitative in fibres expressing the slow myosin isoform. The higher metabolic rate, mitochondrial density and formation of reactive oxidative species in slow versus fast muscle fibres suggest that post-translational protein modifications are a significant source underlying the qualitative changes in the force generation capacity of the type I fibres in postmenopausal women (McArdle et al. 2002).
The concentration of oestrogen receptors is higher in slow- than in fast-twitch fibres (Saartok, 1984; Meeuwsen et al. 2000; Lemoine et al. 2002) and oestrogen has anti-oxidant properties (Persky et al. 2000). The increased post-translational myosin modification by free radicals is one of the mechanisms leading to ageing-related contractile dysfunction (Lowe et al. 2001, 2004). HRT may accordingly reduce the impaired myosin function in postmenopausal women more efficiently in type I fibres by a specific protection against post-translational modifications. In this context it is interesting to note that the only ageing-specific modification observed among the twin-pairs (deamidation in the 1079 position in the tail region of the motor protein) dominated among non-users, but this PTM was specific for the IIa MyHC isoform. The modifications specific for type I and IIa MyHC isoforms, carbonylations in the 1623/1627 and 413 positions, dominated in the users or non-users, respectively. Overall, the PTMs were mainly situated on the more accessible tail region of the myosin. Despite not interacting directly with actin, the myosin rod is essential for the molecular motor function of myosin and mutations in this region have been shown to disrupt the structure of the protein, resulting in myopathies (Meredith et al. 2004; Tajsharghi et al. 2005; Armel & Leinwand, 2010). The PTMs we observed in young individuals tended to be preserved in HRT users while non-users gained new PTMs found only in aged individuals. However, multiple PTMs may go undetected and become lost during enzymatic digestion when analysed by mass spectrometry (MS). Post-translational modifications may also involve protein O-GlcNAcylation via which several cellular processes are regulated. This process is comparable and partially competitive to protein phosphorylation and acts as a cellular sensor for nutritional status and glucose metabolism. Concerning the effects of HRT on protein O-GlcNAc modification Pöllänen et al. (2007) observed that the gene expression of the OGT enzyme catalysing O-GlcNAcylation was down-regulated in skeletal muscle of early postmenopausal women during one year of HRT intervention in comparison to the placebo-using controls. Consequently, other protein modifications undetected by MS may have affected regulation of muscle contraction at the contractile protein level.
Maximum velocity of unloaded shortening did not differ between the HRT-discordant twin pairs irrespective of MyHC isoform expression. This indicates that the greater in vivo muscle power reported among the HRT-using co-twins (Ronkainen et al. 2009) was primarily due to the higher specific force.
HRT reduces myonuclear domain size in slow-twitch fibres but not in fast-twitch fibres
In accordance with our recent findings in older women (84 ± 8 yrs.; Cristea et al. 2010) average MND size did not differ between type I and type IIa fibre types in the non-user group (Fig. 2). This is probably related to the ageing-related preferential type IIa fibre atrophy (Tomonaga, 1977; Larsson et al. 1978, 1982) since we previously found no change in myonuclei count per unit fibre length with ageing (Cristea et al. 2010). In the HRT user group, MND size was significantly smaller in muscle fibres expressing the type I MyHC isoform (Fig. 2_A_), but it did not differ between users and non-users in fibres expressing the type IIa MyHC isoform (Fig. 2_B_). A possible explanation for this discrepancy is the observation that the ageing-related oxidative stress has a more profound effect on slow-twitch fibres (McArdle et al. 2002) in part due to a decreased production of HSP70 (Broome et al. 2006). Slow-twitch fibres are also transcriptionally more active than fast-twitch fibres (Habets et al. 1999). Oxidative damage reduces transcriptional capacity leading to a reduced specific force and a need for smaller myonuclear domains. It is therefore possible that due to a higher concentration of its receptors in slow-twitch fibres, oestrogen not only arrests the ageing-related oxidative damage but also reduces myonuclear domain size to restore force-generating capacity in ageing fibres. This also optimizes transport distances for cellular proteins and synthetic capacity of the ageing myonucleus. Further, recent experimental results from our group in ‘double-muscle mice’ have shown that MND size does play an important role for maintenance of specific force (Qaisar et al. 2012). The smaller MND size in HRT users may accordingly compensate for ageing-related changes in myonuclear organization (Cristea et al. 2010).
In the fibres expressing the type IIa MyHC isoform, on the other hand, HRT seems to have no effect on MND size. We have recently reported an ageing-related decline in CSA and MND size in type IIa fibres (Cristea et al. 2010) and it is assumed that HRT usage impacts on existing nuclei to optimize their transcriptional and translational efficiency to restore functional capacity without a need for smaller domains or additional myonuclei.
Gene set enrichment analyses of the muscle transcriptome of these postmenopausal monozygotic twins have revealed subtle, but significant differences in expressions in nine gene sets including ‘regulation of anatomical structure and morphogenesis’ (Ronkainen et al. 2010). In order to investigate the contribution of HRT to muscle transcriptome changes, we are currently running analysis on muscle miRNA arrays to find out whether post-transcriptional regulation via miRNAs is one mechanism by which oestrogen-containing hormone replacement therapy regulates muscle gene expression and possibly also the enzyme-catalysed post-translational modification of proteins.
Conclusion
There is growing interest in exploring the effects of HRT on skeletal muscle and the possible mechanisms by which it can assert its influence on muscle mass and strength. Results from our study indicate that HRT has significant positive effects on the ability of single muscle fibres to generate more force without a change in size. This effect is obtained by modulation and direct influence on actin–myosin interactions and the number of such interactions as well as altered myonuclear organization. These effects are fibre-type dependent and the force per actin–myosin interaction plays a stronger role in fast- than in slow-twitch fibres, with slow-twitch fibres relying on both the number and force per cross-bridge. These findings open a venue for future pharmacological interventions aiming at enhancing muscle mass and function in old age.
Acknowledgments
We are very grateful to Rebeca Corpeno and Ann-Marie Gustafson for their excellent technical assistance, to Dr Julien Ochala for his invaluable suggestions and support throughout this project and to Ms Hannah Ogilvie for English editing. This study was supported by grants from the Swedish Research Council (8651), STINT, King Gustaf V Research Foundation, the Swedish Center for Sports Research Council and the European Commission (MyoAge, EC Fp7 CT-223756 and COST CM1001) to L.L., STINT and the Swedish Research Council (621-2011-4423) to J.B., and the Higher Education Commission of Pakistan to R.Q. J.K. was supported by the Academy of Finland Center of Excellence in Complex Disease Genetics (grant numbers: 213506, 129680) and V.K. by the Academy of Finland and Finnish Ministry of Education. Gerontology Research Center is a joint effort between the University of Jyväskylä and the University of Tampere in Finland.
Glossary
CSA
cross-sectional area
HRT
hormone replacement therapy
MZ twins
monozygous twins
MND
myonuclear domain
MyHC
myosin heavy chain
PTM
post-translation modification
Author contributions
All experiments were performed in the University of Uppsala, Sweden while biopsies were taken at the University of Jyväskylä in Finland. R.Q., S.S., V.K. and L.L. designed the experiments. R.Q., Y.H. and G.R. performed the analyses. K.A. and J.B. conducted the high resolution nLC-FTICR mass spectrometry (MS), interpreted the MS and revised the paper. R.Q. analysed the data and R.Q. and L.L. wrote the paper. E.P., P.R., J.K., M.A., S.S. and V.K. revised the paper. All authors approved the final version of the manuscript.
References
- Ahtiainen M, Pollanen E, Ronkainen PH, Alen M, Puolakka J, Kaprio J, Sipila S, Kovanen V. Age and estrogen- based hormone therapy affect systemic and local IL-6 and IGF-1 pathways in women. Age (Dordr) 2012;34:1249–1260. doi: 10.1007/s11357-011-9298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armel TZ, Leinwand LA. Mutations at the same amino acid in myosin that cause either skeletal or cardiac myopathy have distinct molecular phenotypes. J Mol Cell Cardiol. 2010;48:1007–1013. doi: 10.1016/j.yjmcc.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artemenko KA, Bergstrom Lind S, Elfineh L, Mayrhofer C, Zubarev RA, Bergquist J, Pettersson U. Optimization of immunoaffinity enrichment and detection: toward a comprehensive characterization of the phosphotyrosine proteome of K562 cells by liquid chromatography-mass spectrometry. Analyst. 2011;136:1971–1978. doi: 10.1039/c0an00649a. [DOI] [PubMed] [Google Scholar]
- Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 2006;20:1549–1551. doi: 10.1096/fj.05-4935fje. [DOI] [PubMed] [Google Scholar]
- Cristea A, Qaisar R, Edlund PK, Lindblad J, Bengtsson E, Larsson L. Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell. 2010;9:685–697. doi: 10.1111/j.1474-9726.2010.00594.x. [DOI] [PubMed] [Google Scholar]
- Dieli-Conwright CM, Spektor TM, Rice JC, Sattler FR, Schroeder ET. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J Appl Physiol. 2009a;107:853–858. doi: 10.1152/japplphysiol.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieli-Conwright CM, Spektor TM, Rice JC, Sattler FR, Schroeder ET. Influence of hormone replacement therapy on eccentric exercise induced myogenic gene expression in postmenopausal women. J Appl Physiol. 2009b;107:1381–1388. doi: 10.1152/japplphysiol.00590.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf) 2008;194:81–93. doi: 10.1111/j.1748-1716.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Finni T, Noorkoiv M, Pollanen E, Ronkainen PH, Alen M, Kaprio J, Kovanen V, Sipila S. Muscle function in monozygotic female twin pairs discordant for hormone replacement therapy. Muscle Nerve. 2011;44:769–775. doi: 10.1002/mus.22162. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Larsson L. Contractile studies of single human skeletal muscle fibers: a comparison of different muscles, permeabilization procedures, and storage techniques. Muscle Nerve. 1997;20:948–952. doi: 10.1002/(sici)1097-4598(199708)20:8<948::aid-mus3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071–1081. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets PE, Franco D, Ruijter JM, Sargeant AJ, Pereira JA, Moorman AF. RNA content differs in slow and fast muscle fibers: implications for interpretation of changes in muscle gene expression. J Histochem Cytochem. 1999;47:995–1004. doi: 10.1177/002215549904700803. [DOI] [PubMed] [Google Scholar]
- Korhonen MT, Cristea A, Alen M, Hakkinen K, Sipila S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol. 2006;101:906–917. doi: 10.1152/japplphysiol.00299.2006. [DOI] [PubMed] [Google Scholar]
- Larsson L. Morphological and functional characteristics of the ageing skeletal muscle in man. A cross-sectional study. Acta Physiol Scand Suppl. 1978;457:1–36. [PubMed] [Google Scholar]
- Larsson L. Physical training effects on muscle morphology in sedentary males at different ages. Med Sci Sports Exerc. 1982;14:203–206. [PubMed] [Google Scholar]
- Larsson L, Li X, Yu F, Degens H. Age-related changes in contractile properties and expression of myosin isoforms in single skeletal muscle cells. Muscle Nerve Suppl. 1997;5:S74–78. [PubMed] [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Lemoine S, Granier P, Tiffoche C, Berthon PM, Rannou-Bekono F, Thieulant ML, Carre F, Delamarche P. Effect of endurance training on oestrogen receptor alpha transcripts in rat skeletal muscle. Acta Physiol Scand. 2002;174:283–289. doi: 10.1046/j.1365-201x.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Li M, Larsson L. Force-generating capacity of human myosin isoforms extracted from single muscle fibre segments. J Physiol. 2010;588:5105–5114. doi: 10.1113/jphysiol.2010.199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DA, Baltgalvis KA, Greising SM. Mechanisms behind estrogen's beneficial effect on muscle strength in females. Exerc Sport Sci Rev. 2010;38:61–67. doi: 10.1097/JES.0b013e3181d496bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:C540–C547. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- Lowe DA, Warren GL, Snow LM, Thompson LV, Thomas DD. Muscle activity and aging affect myosin structural distribution and force generation in rat fibers. J Appl Physiol. 2004;96:498–506. doi: 10.1152/japplphysiol.00842.2003. [DOI] [PubMed] [Google Scholar]
- McArdle A, Vasilaki A, Jackson M. Exercise and skeletal muscle ageing: cellular and molecular mechanisms. Ageing Res Rev. 2002;1:79–93. doi: 10.1016/s0047-6374(01)00368-2. [DOI] [PubMed] [Google Scholar]
- McDonald KS, Fitts RH. Effect of hindlimb unloading on rat soleus fiber force, stiffness, and calcium sensitivity. J Appl Physiol. 1995;79:1796–1802. doi: 10.1152/jappl.1995.79.5.1796. [DOI] [PubMed] [Google Scholar]
- Martyn DA, Smith L, Kreutziger KL, Xu S, Yu LC, Regnier M. The effects of force inhibition by sodium vanadate on cross-bridge binding, force redevelopment, and Ca2+ activation in cardiac muscle. Biophys J. 2007;92:4379–4390. doi: 10.1529/biophysj.106.096768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medler S. Comparative trends in shortening velocity and force production in skeletal muscles. Am J Physiol Regul Integr Comp Physiol. 2002;283:R368–R378. doi: 10.1152/ajpregu.00689.2001. [DOI] [PubMed] [Google Scholar]
- Meeuwsen IB, Samson MM, Verhaar HJ. Evaluation of the applicability of HRT as a preservative of muscle strength in women. Maturitas. 2000;36:49–61. doi: 10.1016/s0378-5122(00)00132-8. [DOI] [PubMed] [Google Scholar]
- Meredith C, Herrmann R, Parry C, Liyanage K, Dye DE, Durling HJ, Duff RM, Beckman K, de Visser M, van der Graaff MM, Hedera P, Fink JK, Petty EM, Lamont P, Fabian V, Bridges L, Voit T, Mastaglia FL, Laing NG. Mutations in the slow skeletal muscle fiber myosin heavy chain gene (MYH7) cause Laing early-onset distal myopathy (MPD1) Am J Hum Genet. 2004;75:703–708. doi: 10.1086/424760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol. 2007;102:1387–1393. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- Moss RL. Sarcomere length–tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onambele-Pearson GL. HRT affects skeletal muscle contractile characteristics: a definitive answer. J Appl Physiol. 2009;107:4–5. doi: 10.1152/japplphysiol.00448.2009. [DOI] [PubMed] [Google Scholar]
- Persky AM, Green PS, Stubley L, Howell CO, Zaulyanov L, Brazeau GA, Simpkins JW. Protective effect of estrogens against oxidative damage to heart and skeletal muscle in vivo and in vitro. Proc Soc Exp Biol Med. 2000;223:59–66. doi: 10.1046/j.1525-1373.2000.22308.x. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- Pöllänen E, Ronkainen PH, Suominen H, Takala T, Koskinen S, Puolakka J, Sipilä S, Kovanen V. Muscular transcriptome in postmenopausal women with or without hormone replacement. Rejuvenation Res. 2007;10:485–500. doi: 10.1089/rej.2007.0536. [DOI] [PubMed] [Google Scholar]
- Qaisar R, Renaud G, Morine K, Barton ER, Sweeney HL, Larsson L. Is functional hypertrophy and specific force coupled with the addition of myonuclei at the single muscle fiber level. FASEB J. 2012;26:1077–1085. doi: 10.1096/fj.11-192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys J. 2004;87:1815–1824. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronkainen PH, Kovanen V, Alen M, Pollanen E, Palonen EM, Ankarberg-Lindgren C, Hamalainen E, Turpeinen U, Kujala UM, Puolakka J, Kaprio J, Sipila S. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: a study with monozygotic twin pairs. J Appl Physiol. 2009;107:25–33. doi: 10.1152/japplphysiol.91518.2008. [DOI] [PubMed] [Google Scholar]
- Ronkainen PH, Pollanen E, Alen M, Pitkanen R, Puolakka J, Kujala UM, Kaprio J, Sipila S, Kovanen V. Global gene expression profiles in skeletal muscle of monozygotic female twins discordant for hormone replacement therapy. Aging Cell. 2010;9:1098–1110. doi: 10.1111/j.1474-9726.2010.00636.x. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- Saartok T. Steroid receptors in two types of rabbit skeletal muscle. Int J Sports Med. 1984;5:130–136. doi: 10.1055/s-2008-1025894. [DOI] [PubMed] [Google Scholar]
- Sipila S, Taaffe DR, Cheng S, Puolakka J, Toivanen J, Suominen H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: a randomized placebo-controlled study. Clin Sci (Lond) 2001;101:147–157. [PubMed] [Google Scholar]
- Skelton DA, Phillips SK, Bruce SA, Naylor CH, Woledge RC. Hormone replacement therapy increases isometric muscle strength of adductor pollicis in post-menopausal women. Clin Sci (Lond) 1999;96:357–364. [PubMed] [Google Scholar]
- Tajsharghi H, Darin N, Rekabdar E, Kyllerman M, Wahlstrom J, Martinsson T, Oldfors A. Mutations and sequence variation in the human myosin heavy chain IIa gene (MYH2. Eur J Hum Genet. 2005;13:617–622. doi: 10.1038/sj.ejhg.5201375. [DOI] [PubMed] [Google Scholar]
- Tomonaga M. Histochemical and ultrastructural changes in senile human skeletal muscle. J Am Geriatr Soc. 1977;25:125–131. doi: 10.1111/j.1532-5415.1977.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Maddalozzo GF, Lewis D, Valentine BA, Garner DP, Stelzer JE, Shoepe TC, Snow CM. Morphological and functional characteristics of skeletal muscle fibers from hormone-replaced and nonreplaced postmenopausal women. J Gerontol A Biol Sci Med Sci. 2003;58:3–10. doi: 10.1093/gerona/58.1.b3. [DOI] [PubMed] [Google Scholar]
- Yu F, Hedstrom M, Cristea A, Dalen N, Larsson L. Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol (Oxf) 2007;190:229–241. doi: 10.1111/j.1748-1716.2007.01699.x. [DOI] [PubMed] [Google Scholar]