In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria (original) (raw)
. Author manuscript; available in PMC: 2013 Jul 29.
Published in final edited form as: Kidney Int. 2010 Jan;77(1):57–64. doi: 10.1038/ki.2009.399
Abstract
Systematic study of the course of renal function decline and progression to proteinuria in patients with type 1 diabetes and new onset microalbuminuria has not been reported. From the 1080 participants with normoalbuminuria enrolled in the 1st Joslin Kidney Study, we identified 109 who developed new onset microalbuminuria in the first four years of observation and followed 79 for a subsequent 12.4±1.4 years to estimate glomerular filtration rate by the four-variable MDRD formula (GFRMDRD) and the course of microalbuminuria. 12–year cumulative risk of advanced chronic kidney disease (CKD)[defined by GFRMDRD<60ml/min/1.73m2] and proteinuria were 23(29%) and 21(27%). However, concordance between these outcomes was weak. Only 12 of the 23 subjects (52%) who developed advanced CKD had progression to proteinuria. Furthermore, this progression generally did not precede but rather accompanied the development of advanced CKD. The remaining 11(48%) subjects who developed advanced CKD experienced only persistent microalbuminuria (8 subjects) or regressed to normoalbuminuria (3 subjects). In conclusion, risk of advanced CKD approaches one-third early after microalbuminuria onset and its development is not conditional on the presence of proteinuria. Contrary to the existing concept of early nephropathy in type 1 diabetes, less emphasis should be placed on the mechanisms of progression to proteinuria and more on the mechanisms initiating and promoting early renal function decline that leads to advanced CKD.
INTRODUCTION
Microalbuminuria has become firmly entrenched as the primary predictive marker of risk for eventual end stage renal disease. The genesis of this current model can be traced to three small studies published in the 1980s.1–3 In total 30 patients with prevalent microalbuminuria, defined by urinary albumin excretion approximately in the range of 30–300 micrograms per minute, were followed for 7 to 14 years, and advanced nephropathy developed in 60–90% of them. In these studies, the outcome was defined by worsening of urinary albumin excretion to proteinuria (frequently referred to as ‘macroalbuminuria’) but not by the development of advanced chronic kidney disease (advanced CKD). The findings of extraordinary high risk of progression to proteinuria together with reports in cross-sectional clinical studies of association between renal function impairment with proteinuria gave plausibility to a simple model of diabetic nephropathy comprising three sequential stages: Microalbuminuria heralds proteinuria, which after long-term exposure initiates the process of renal function loss which leads to end stage renal disease.4 This model has become the paradigm for research on diabetic nephropathy and for the development of its preventive and therapeutic protocols.5,6
Accordingly, therapy in patients with microalbuminuria focused on prevention of proteinuria as the means to prevent renal function decline. However, the evidence base for this use of proteinuria as a surrogate biomarker for declining renal function was not available. That had to await a means to evaluate changes in renal function as well as the natural history of abnormalities in urinary albumin excretion. Half of the story, the natural history of albumin excretion in the microalbuminuria range, was clarified recently.7 Contrary to the three early studies, in a large prospective systematic study microalbuminuria proved to be a dynamic process that was more likely to remit to normal albumin excretion than to progress to proteinuria. The 6-year cumulative incidence of remission was approximately 50%, whereas the risk of progression to proteinuria was only 15%.7 Similar findings were reported by others8–11
However, the other half of the story – the impact of these changes in microalbuminuria on the risk of renal function loss - was studied only partially. In the subsequent 8 year follow-up study using slopes of renal function change over time estimated on the basis of serum cystatin C concentrations, we demonstrated that patients with microalbuminuria had frequent early progressive renal function decline 12. That study, however, combined prevalent cases with new onset cases of microalbuminuria and due to short follow-up was not able to study the relationship between progression to proteinuria and declining renal function to advanced CKD, an outcome considered more clinically meaningful than the slope of change in GFR over time.
To explore this relationship, the simultaneous tracking of renal function change and urinary albumin excretion in a cohort of patients with new onset microalbuminuria is required. Absence of the knowledge of the precise timing of microalbuminuria onset would compromise the assessment of subsequent renal function because a collection of prevalent cases detected by a single screening examination would not only lack early GFR measures but would be biased by the under-representation of patients who had rapid progression from microalbuminuria to proteinuria or to advanced CKD.
Guided by these considerations, we examined further the cohort of patients with new onset microalbuminuria 13 that was included in the previous publication12, by extending follow-up to 12 years and using the measurements of serum creatinine which were many times more frequently performed than measurements of serum cystatin C. In this report we characterized the courses of urinary albumin excretion changes and the development of advanced CKD during 12 years of follow-up.
RESULTS
Characteristics of the Study Subjects According to Development of Advanced CKD
The 79 subjects with new onset microalbuminuria and normal renal function were followed for a mean of 12.4±1.4 years in which they had 15±8 GFRMDRD determinations. Advanced- CKD, defined by GFRMDRD <60 ml/min/1.73m2 (stage 3 or 4 chronic kidney disease) or end stage renal disease requiring dialysis or transplant (stage 5 chronic kidney disease), developed in 23 (29%) of the 79 subjects. Six of these cases developed end stage renal disease over a mean of 10.6 years (range 8.8 to 11.8 years) from the time of microalbuminuria onset. The remaining 17 cases developed stage 3 or 4 chronic kidney disease, corresponding to a GFRMDRD <60 ml/min/1.73m2, and had a total follow-up time of 12.2±1.4 years. In none of the cases was chronic kidney disease owing to non-diabetic causes recognized. The remaining 56 controls (71%) maintained a GFRMDRD of 60 ml/min/1.73m2 or greater over 12.5±1.4 years of follow-up.
The baseline characteristics of the 79 study subjects at the time of microalbuminuria onset are summarized in Table 1 according to case-control status at the end of observation. A significantly older age at baseline, and excess of women was observed among cases. No differences in age at diabetes diagnosis, diabetes duration, smoking, or blood pressure were seen among controls and cases. Of the biochemical variables, glycosylated hemoglobin A1c was significantly higher in cases. However, neither cholesterol, the degree of urinary albumin excretion, nor the level GFRMDRD or GFRCYSTATIN C reflected future case status.
Table 1.
Baseline* Characteristics of the 79 Subjects According To Case-Control Status at the End of Follow-up
| Characteristic | CONTROLS (N=56) | C ASES (N=23) | ||
|---|---|---|---|---|
| GFR > 60ML/MIN/1.73 m2 “STAGES 1–2 CKD’ | GFR ≤ 60ML/MIN/1.73 m2 “STAGES 3–4 CKD” (N=17) | ESRD “STAGE 5 CKD” (N=6) | P† | |
| Age at Diabetes Diagnosis (yr) | 13 ± 7 | 14 ± 10 | 15 ± 10 | 0.38 |
| Women (%) | 33 (59%) | 14 (82%) | 5 (82%) | 0.05 |
| Age at Baseline (yr) | 30 ± 8 | 36 ± 7 | 30 ± 10 | 0.02 |
| Diabetes duration (yr)‡ | 17 ± 9 | 22 ± 8 | 15 ± 4 | 0.57 |
| Current/Past Smoking (%) | 60 | 65 | 40 | 0.61 |
| Systolic BP (mmHg) | 122 ± 16 | 122 ± 17 | 128 ± 16 | 0.75 |
| Diastolic BP (mmHg) | 76 ± 8 | 76 ± 8 | 79 ± 9 | 0.74 |
| HbA1c (%) | 9.0 ± 1.4 | 9.5 ± 1.8 | 11.5 ± 1.1 | 0.01† |
| Total Cholesterol (mg/dL)§ | 203 ± 38 | 210 ± 43 | 232 ± 35 | 0.41 |
| Albumin Excretion(μg/min) | ||||
| Median | 49 | 40 | 46 | 0.33 |
| Interquartile Range | 34 – 68 | 34 – 45 | 33 – 69 | |
| Serum creatinine (mg/dl) | 0.86 ± 0.19 | 0.84 ± 0.24 | 0.86 ± 0.20 | 0.77 |
| Serum cystatin C (mg/L) | 0.70 ± 0.16 | 0.67 ± 0.18 | 0.69 ± 0.17 | 0.45 |
| GFRMDRD (ml/min/1.73m2)¶ | 107 ± 26 | 98 ± 23 | 101 ± 25 | 0.77 |
| GFRCYSTATIN C (ml/min/1.73m2)¶ | 123 ± 26 | 126 ± 18 | 122 ± 41 | 0.78 |
Magnitude of Renal Function Decline
Despite a mean GFRMDRD for the 79 participants of 104 ±25 ml/min/1.73m2 at baseline evaluation, by the last examination window the mean value was 73 ±23 ml/min/1.73m2. Table 2 illustrates the magnitude of renal function change according to cases and controls. Although controls maintained levels of GFRMDRD ≥60ml/min/1.73m2, their mean value decreased from 107 to 84 ml/min/1.73m2 over 12.5 years, representing a mean decline in renal function of approximately 20 percent far exceeding expected age-related decline.14 By definition, cases had a much greater magnitude of decline in GFRMDRD, of approximately 50–75% over twelve years.
Table 2.
Estimated Glomerular Filtration Rate and Urinary Albumin Excretion at Baseline and in the Final 2-year Evaluation Interval.*
| Characteristic | CONTROLS (N=56) | CASES (N=23) | |||
|---|---|---|---|---|---|
| GFR > 60ML/MIN/1.73m2 “CKD STAGES 1–2’ (N=56) | GFR ≤ 60ML/MIN/1.73m2 “CKD STAGES 3–4” (N=17) | ESRD “CKD STAGE 5” (N=6) | P† | ||
| GFRMDRD (ml/min/1.73m2) | |||||
| Mean[Min, Max] | |||||
| Baseline | 107 [60, 164] | 98 [73, 156] | 101 [69, 134] | 0.77 | |
| Final Interval* | 84 [61, 126] | 50 [22,59] | 27 [4, 58] | - | |
| Urinary Albumin Excretion Rate (in μg/min) | |||||
| Median[interquartile range] | |||||
| Baseline | 49 [34, 68] | 40 [34, 45] | 46 [33, 69] | 0.33 | |
| Final Interval* | 35 [16, 71] | 52 [18, 241] | 1285 [856, 2805] | 0.06 | |
| Albumin Excretion Changes during 12 years of follow- up: | |||||
| Remission to Normoalbuminuria | 28 (50%) | 3 (18%) | 0 (0%) |  |
0.001‡ |
| Persistent Microalbuminuria | 19 (34%) | 8 (47%) | 0 (0%) | ||
| Progression to Proteinuria | 9 (16%) | 6 (35%) | 6 (100%) | ||
| 56 (100%) | 17 (100%) | 6 (100%) | |||
| Treatment with Reno- Protective Drugs during the final interval* | |||||
| ACE-Inhibitor and ARB§ | 41 (73%) | 11(65%) | 5(83%) | 0.45 |
Relationship Between the Development of Advanced CKD and Progression to Proteinuria
The mean urinary albumin excretion rate at baseline and in the final interval of follow-up is shown in the second section of Table 2 for controls and cases. The only marked increase in AER occurred in the subset of cases that developed ESRD.
Subjects in the study were also classified into 3 categories according to changes in AER during the 12 years of follow-up: those in whom microalbuminuria regressed to normoalbuminuria, those with persistent microalbuminuria, and those in whom microalbuminuria progressed to proteinuria. Distribution of controls and cases according to these categories is shown in Table 2. Half of the controls had microalbuminuria which regressed to normoalbuminuria, 34% of them had persistent microalbuminuria and 16% of them developed proteinuria. The pattern was different in cases. All six cases of end stage renal disease developed proteinuria, However, among cases of Stage 3–4 of CKD only 35% developed proteinuria – these cases were most likely to have persistent microalbuminuria (47%) or regress to normoalbuminuria (18%) despite significant loss of renal function. Overall, the distributions of the categories of AER change during the 12 years of follow-up between controls and cases were highly statistically significant (P<0.001). The data presented in Table 2, however, did not address the issue timing of the development of proteinuria relative to the development of advanced CKD in the half of cases (12 of 23) that developed both.
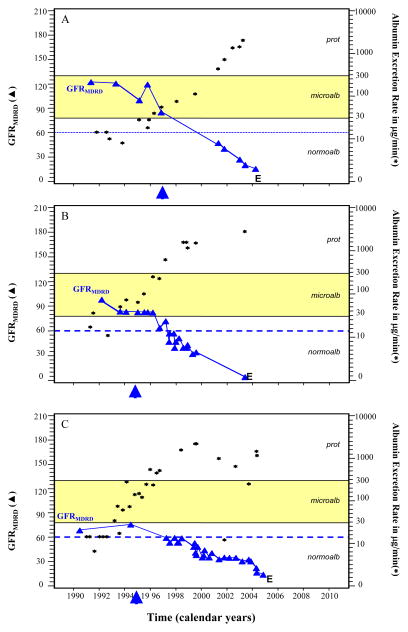
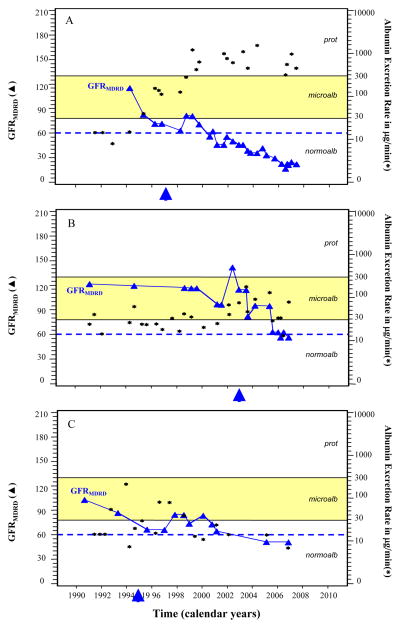
To further explore this question, we plotted the sequence of urinary albumin excretion and GFRMDRD for representative examples from the 6 cases with end stage renal disease (Figure 1). Five of the six cases, despite having GFRMDRD near the normal range at the beginning of follow-up, appeared to have loss of renal function at the time that microalbuminuria was progressing to proteinuria (the two processes were developing concurrently). Two such examples are shown in Panels A and B. During the course of microalbuminuria for these examples, GFRMDRD levels clearly began to decline even prior to exposure to proteinuria. In only one of the 6 cases could the timing of initiation of decline not be determined relative to the onset of proteinuria owing to infrequent GFRMDRD measures (Panel C). Similar examples from the 17 cases that developed Stage 3–4 chronic kidney disease are shown in Figure 2. It is important to emphasize that in the majority of these patients the development of advanced CKD occurred in the absence of proteinuria.
Figure 1. Serial Estimates of GFRMDRD (3) in ml/min/1.73m2 and of Urinary Albumin Excretion Rate (▲) in μg per minute for Three Representative Cases of End Stage Renal Disease.
Panel A describes the clinical course of an individual who was followed for approximately four years before the onset of microalbuminuria. After 8.8 years from the onset of microalbuminuria, end stage renal disease occurred (indicated by “E”). Although end stage renal disease was preceded by proteinuria, the fifth estimate of GFRMDRD indicates that renal function had already begun to decline soon after the onset of microalbuminuria. The sixth estimate of GFRMDRD was below 60 ml/min/1.73m2 despite short duration of exposure to proteinuria. Panel B describes a case that had decline in GFRMDRD to a level near 60 ml/min/1.73m2 during the course of microalbuminuria. Panel C describes another case of end stage renal disease preceded by proteinuria. However, after only very short exposure to proteinuria the GFRMDRD had declined to a level below 60 ml/min/1.73m2. Evidence that decline in GFRMDRD had begun soon after the onset of microalbuminuria was evident for 5 of the 6 cases of end stage renal disease (as in Panels A and B). The arrows indicate the time of initiation of angiotensin converting enzyme inhibitor agents.
Figure 2. Serial Estimates of GFRMDRD (3) in ml/min/1.73m2 and of Urinary Albumin Excretion Rate (▲) in μg per minute for Three Representative Cases of Stage 3–4 Chronic Kidney Disease.
Panel A shows the clinical course of GFRMDRD for a case of stage 4 chronic kidney disease that developed proteinuria. Panel B describes the course for a subject who developed Stage 3 chronic kidney disease but who had stable levels of urinary albumin excretion rate in the microalbuminuria range without developing proteinuria. Panel C describes the course of a subject with Stage 3 chronic kidney disease despite remission of urinary albumin excretion to normoalbuminuria levels. In all of these three examples, the initiation of renal function decline clearly occurred during microalbuminuria-range albumin excretion. The arrows indicate the time of initiation of angiotensin converting enzyme inhibitor agents.
Relationship Between Angiotensin Converting Enzyme Inhibitor Use and Advanced CKD
By the end of follow-up, the use of angiotensin converting enzyme inhibitor agents was common in both cases and controls (Table 2). Of the 12 cases who developed proteinuria, these agents were initiated prior to proteinuria onset in 10 (83%). Significant differences could not be detected between cases and controls in the proportion receiving angiotensin converting enzyme inhibitor agents, the years of exposure, the proportion of observation time exposed, or in the time of delay between the diagnosis of microalbuminuria and initiation of therapy. Use of angiotensin receptor blocking agents was rare, and is described in the legend to Table 2.
DISCUSSION
In a cohort of 79 subjects with type 1 diabetes, normal renal function, and the documented new onset of microalbuminuria, the12-year risk of advanced-CKD corresponding to Stage 3–5 was high (23 cases, 29%). Higher glycosylated hemoglobin A1c and the development of proteinuria were strongly associated with risk of advanced-stage kidney disease. However, nearly half of cases never developed proteinuria and, in those cases that did, the vast majority began to lose renal function years prior to the development of proteinuria independent of such factors as the use of angiotensin converting enzyme inhibitor agents. Our study showed un-coupling of risk of advanced chronic kidney disease and progression to proteinuria in patients with Type 1 diabetes and new onset microalbuminuria.
Presence of advanced-CKD has been observed in women with type 1 diabetes patients without proteinuria,15–17. However, its occurrence had not been confirmed systematically in subjects with new onset of microalbuminuria and type 1 diabetes. The traditional view is that end stage renal disease is secondary to exposure to proteinuria.18–23 Although previously suspected,24–28 a recent report from our research group confirmed the existence of early renal function decline, quantified its risk, and further proposed a method for its accurate estimation.12 The systematic study, conducted in a large cohort of patients that included both incident and prevalent microalbuminuria cases, estimated serial GFRCYSTATIN C to determine trends in renal function during microalbuminuria. Serum cystatin C, unlike creatinine-based estimates, is a valid assay for renal function prediction when it is in the normal or elevated range.29–32 By calculating the linear slope of GFRCYSTATIN C, our study demonstrated that the process of renal function decline begins during the microalbuminuria stage in a third of subjects, and that renal function may be elevated above normal when the process starts.12 The current study builds upon this notion by measuring the long-term risk of stage 3–5 chronic kidney disease, a more clinically-relevant late-stage renal outcome, specifically in those with the documented new onset of microalbuminuria. Our findings of un-coupling of the development of advanced CKD from the exposure to proteinuria have fundamental implications for the traditional model of diabetic nephropathy that have previously placed the time of initiation of renal function decline at the stage of clinical proteinuria. A new model for diabetic nephropathy emerges: The onset of microalbuminuria heralds, in a subset of approximately one-third of individuals, a process of progressive early renal function decline leading to advanced CKD and ESRD that occurs irrespective or in parallel of the progression of microalbuminuria to proteinuria. Microalbuminuria and early renal function decline may thus represent two phenotypes that have separate underlying etiological processes. This finding in part reconciles the results of recent clinical trials that demonstrated a failure of therapies designed to attenuate urinary albumin excretion on reducing clinically meaningful renal function and renal morphology endpoints.33,34,35
Similar to the findings observed in the earlier studies,15–17 we found an excess of advanced CKD in women as compared to men. The magnitude of this excess, however, is uncertain. In the course of study, 9 women and 21 men were lost to follow-up from the new onset microalbuminuria cohort (see methods). In the group of men during the limited follow-up we observed 2 cases of advanced CKD and 2 deaths due to CAD. It is possible that a few more such cases occurred in men but they were not ascertained. It should be recognized that this limitation does not impact the validity of the conclusion of our study about the un-coupling of risk of advanced CKD from risk of progression to proteinuria. Our study has some other limitations. We used estimates of GFRMDRD rather than direct measurement – a measure more accurate for GFR in the normal or elevated ranges, such as cystatin C, might reveal that the initiation of renal function decline occurs even earlier in the course of microalbuminuria than was observed in this analysis. In preliminary analysis using the limited number of measurements of cystatin C available, we observed higher GFRCYSTATIN values and steeper slopes of renal function decline in some of the cases shown in Figure 1 and 2. Long-term systematic follow-up with cystatin C measurements is the focus of a more recently-accrued cohort (the 2nd Joslin Kidney Study). Owing to limited power for predictor variables, we cannot conclude a lack of a protective association of rennin angiotensin system inhibitor agents against advanced-stage kidney disease.5,9,36 That renal function was commonly seen to decline regardless of such anti-proteinuric therapy, though, does not diminish the important implications of this work in further refining the natural history of microalbuminuria and early renal function decline.35
The emergence of this new model has fundamental implications for research on the pathological mechanisms and on potential biomarkers of diabetic nephropathy. Although the presence of microalbuminuria infers a 10-year risk of progression to proteinuria in the range of 15–25%7–11 - though the 12-year risk of advanced-stage kidney disease approximates one-third -the degree or course of urinary albumin excretion is not a sufficiently robust surrogate marker for the development of chronic kidney disease in type 1 diabetes. First, microalbuminuria is a dynamic process – when it develops, it can remain static, advance toward proteinuria, but most frequently it regresses toward normal levels of albumin excretion.7,8 Second, although the course of microalbuminuria is generally correlated with risk of renal function decline,12 the predictive value is insufficient: Decline occurs frequently without the presence of overt proteinuria. This new model of diabetic nephropathy demands that emphasis be placed on research into better biomarkers that could identify those at risk of advanced-stage kidney disease ten to fifteen years prior to its development. Such biomarkers could enhance the prediction of the conventional markers for early nephropathy (microalbuminuria, serum creatinine) or replace them.
CONCISE METHODS
Study Participants
Patients with normoalbuminuria who developed microalbuminuria in the first four years of follow-up in the 1st Joslin Kidney Study were eligible for the current project.7,13,37,38 The protocol and consent procedures were approved by the Committee on Human Studies of the Joslin Diabetes Center.
Urine samples from every second patient with type 1 diabetes who was 15 to 44 years of age and seen at the Joslin Clinic between January 1991 and April 1992 were examined (1602 patients). During the first two years, repeat urine samples were examined to establish the albumin excretion status. Members of the cohort with microalbuminuria at baseline (n=312) and proteinuria or end-stage renal disease at baseline (n=347) were excluded from the current analysis. Within four years of initial evaluation, new onset microalbuminuria developed in 109 out of 1080 patients with normoalbuminuria at baseline and these patients with microalbuminuria were the focus of this report. They were examined biennially, and additional urinary albumin excretion and creatinine measurements were obtained from clinical visits. We selected all individuals with at least five (on average thirteen) creatinine measures spanning a minimum of ten years after microalbuminuria onset. Seventy-nine of the 109 patients (72%) were available for analysis according to these criteria.
Among 30 patients (9 women and 21 men) who were excluded from the current study, 7 did not have any follow-up visit except for the examination at enrollment. The remaining 23 patients were lost from the observation between the 2nd and 10th year of follow-up (median 5 years). In these individuals mean GFRMDRD at baseline was 102±25 ml/min/1.73m2, very similar to those included in analysis. In this group of 23 patients, during the limited follow-up 17 subject regressed to normoalbuminuria of which one reached advanced CKD as of the last examination; five subjects remained microalbuminuric of which none reached advanced CKD; one subject progressed to proteinuria and this individual developed advanced-CKD but before proteinuria occurred. None of the 30 excluded individuals developed ESRD according to registration in the United States Renal Data System (USRDS) registry as of the end of 2005. According to the National Death Index as of the end of 2005, two subjects died (both men, due to coronary artery disease). One of these deaths occurred 8 years after the onset of microalbuminuria in a patient who had regression from microalbuminuria to normoalbuminuria. The second death occurred 9 years after microalbuminuria onset in a patient who had persistent microalbuminuria.
Assessment of Urinary Albumin Excretion and Exposure Variables
The albumin excretion rate (in μg per minute) was estimated from the albumin-to-creatinine ratio in random urine samples.7,37,38 Individual values for the albumin-to-creatinine ratio (measured in milligrams per gram) were transformed to a (base-10) logarithmic scale for analysis and converted to albumin excretion rates (in micrograms per minute) by the formula log(AER) = 0.44 + (0.85)log(ACR) − (0.13)sex, where AER is the albumin excretion rate, ACR is the albumin-to-creatinine ratio, and sex is assigned a value of1 for female patients and 0 for male patients.37,38 This conversion formula was derived from an independent sample of patients with type 1 diabetes who underwent simultaneous determinations of the albumin-to-creatinine ratio and the albumin excretion rate based on a three-hour daytime collection (Pearson correlation coefficient, 0.97). Follow-up observation was organized into two-year intervals, and patients were assigned an albumin excretion status for each interval according to the geometric mean of their determinations in that interval (on average 3): normoalbuminuria was defined as an excretion rate less than 30 μg per minute; microalbuminuria 30 to 299 μg per minute; and proteinuria 300 μg per minute or greater.
Estimation of Glomerular Filtration Rate and Definition of Advanced-CKD Cases
The “four-variable” estimating equation derived from the Modification of Diet in Renal Disease Study Group was applied to estimate glomerular filtration rate in ml/min/1.73m2, termed GFRMDRD, using the serum creatinine level (in mg/dL), age, gender and ethnicity.39,40 Serum creatinine was measured throughout the study by a modified picrate method of Jaffe on a Ciba Corning Express Plus Chemistry Analyzer. Although well validated in chronic kidney disease, the equation underestimates GFR in those with levels above 60 ml/min/1.73m2.41–42 As such, we defined advanced-stage kidney disease cases as those who attained a level below this threshold (Stage 3 chronic kidney disease) or below 30 ml/min/1.73m2 (Stage 4 Chronic Kidney Disease). Stage 5 chronic kidney disease (end stage renal disease) was designated if participants required dialysis or renal transplantation in their clinical care. Subjects who maintained GFRMDRD levels ≥ 90 ml/min/1.73m2 (Stage 1 chronic kidney disease) or 60 to 89 ml/min/1.73m2 (Stage 2 chronic kidney disease) were classified as controls.40 Clinical records were reviewed by a nephrologist (B.R.) to determine cause of renal disease.
For descriptive purposes, we also estimated GFR during the first years of microalbuminuria from the measurement of serum cystatin C (GFRCYSTATIN C) on a BN Prospec™ System nephelometer (Dade Behring Incorporated, Newark, DE, USA) using a validated conversion formula defined by GFRCYSTATIN C in ml/min/1.73m2 = (86.7/cystatin C concentration) − 4.2.29,43
Analysis
Analyses were performed in SAS (version 9.1 for Windows). Differences between cases and controls at baseline were assessed using χ2 tests for categorical variables or t-tests for continuous variables. Significance was based on an α-level of 0.05. Individual determinations of the albumin excretion rate were transformed to the logarithmic scale (base 10) for analysis. The risk of advanced-CKD was estimated by cumulative incidence.
Acknowledgments
This research was supported by NIH grants DK041526, DK067638 (to ASK) and the Joslin Diabetes Center. B.A.P is a Canadian Diabetes Association Scholar and was supported by the Banting and Best Diabetes Center.
Footnotes
DISCLOSURE:
Statements of Competing Financial Interests
All authors have no competing interests to declare.
Contributor Information
Bruce A Perkins, Division of Endocrinology, University of Toronto, Toronto, Canada.
Linda H Ficociello, Section on Genetics and Epidemiology, Joslin Diabetes Center, Boston, USA.
Bijan Roshan, Division of Nephrology, Beth Israel-Deaconess Medical Center and Joslin Diabetes Center, Boston, USA.
James H Warram, Section on Genetics and Epidemiology, Joslin Diabetes Center, Boston, USA.
Andrzej S Krolewski, Section on Genetics and Epidemiology, Joslin Diabetes Center, Boston, USA
References
- 1.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, et al. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–2. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 2.Parving HH, Oxenboll B, Svendsen PA, et al. Early detection of patients at risk of developing diabetic nephropathy: A longitudinal study of urinary albumin excretion. Acta Endocrinol (Copenh) 1982;100:550–5. doi: 10.1530/acta.0.1000550. [DOI] [PubMed] [Google Scholar]
- 3.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 4.Williams ME. Diabetic nephropathy: the proteinuria hypothesis. Am J Nephrol. 2005;25:77–94. doi: 10.1159/000084286. [DOI] [PubMed] [Google Scholar]
- 5.ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors?. A meta-analysis of individual patient data. Ann Intern Med. 2001;134:370–9. doi: 10.7326/0003-4819-134-5-200103060-00009. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association Clinical Practice Guidelines. Nephropathy in Diabetes. Diabetes Care. 2004;27:S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 7.Perkins BA, Ficociello LH, Silva KH, et al. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–93. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 8.Giorgino F, Laviola L, Cavallo Perin P, et al. Factors associated with progression to macroalbuminuria in microalbuminuric Type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia. 2004;47:1020–8. doi: 10.1007/s00125-004-1413-8. [DOI] [PubMed] [Google Scholar]
- 9.Ficociello LH, Perkins BA, Silva KH, et al. Determinants of progression from microalbuminuria to proteinuria in patients who have type 1 diabetes and are treated with angiotensin-converting enzyme inhibitors. Clin J Am Soc Nephrol. 2007;2:461–9. doi: 10.2215/CJN.03691106. [DOI] [PubMed] [Google Scholar]
- 10.Hovind P, Tarnow L, Rossing P, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ. 2004;328:1105. doi: 10.1136/bmj.38070.450891.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ. 2008;336:697–701. doi: 10.1136/bmj.39478.378241.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk of early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353–61. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 13.Scott LJ, Warram JH, Hanna LS, et al. A nonlinear effect of hyperglycemia and current cigarette smoking are major determinants of the onset of microalbuminuria in type 1 diabetes. Diabetes. 2001;50:2842–9. doi: 10.2337/diabetes.50.12.2842. [DOI] [PubMed] [Google Scholar]
- 14.Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–285. doi: 10.1111/j.1532-5415.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 15.Lane PH, Steffes MW, Mauer SM. Glomerular structure in IDDM women with low glomerular filtration rate and normal urinary albumin excretion. Diabetes. 1992;41:581–6. doi: 10.2337/diab.41.5.581. [DOI] [PubMed] [Google Scholar]
- 16.Tsalamandris C, Allen TJ, Gilbert RE, et al. Progressive decline in renal function function in diabetic patients with and without albuminuria. Diabetes. 1994;43:649–55. doi: 10.2337/diab.43.5.649. [DOI] [PubMed] [Google Scholar]
- 17.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52:1036–40. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 18.Viberti GC, Bilous RW, Mackintosh D, et al. Monitoring glomerular function in diabetic nephropathy: A prospective study. Am J Med. 1983;74:256–64. doi: 10.1016/0002-9343(83)90624-1. [DOI] [PubMed] [Google Scholar]
- 19.Zeller K, Whittaker E, Sullivan L, et al. Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1991;324:78–84. doi: 10.1056/NEJM199101103240202. [DOI] [PubMed] [Google Scholar]
- 20.Biesenbach G, Janko O, Zazgornik J. Similar rate of progression in the predialysis phase in type I and type II diabetes mellitus. Nephrol Dial Transplant. 1994;9:1097–1102. doi: 10.1093/ndt/9.8.1097. [DOI] [PubMed] [Google Scholar]
- 21.Mathiesen ER, Feldt-Rasmussen B, Hommel E, et al. Stable glomerular filtration rate in normotensive IDDM patients with stable microalbuminuria. A 5-year prospective study. Diabetes Care. 1997;20:286–9. doi: 10.2337/diacare.20.3.286. [DOI] [PubMed] [Google Scholar]
- 22.Hovind P, Rossing P, Tarnow L, et al. Progression of diabetic nephropathy. Kidney Int. 2001;59:702–9. doi: 10.1046/j.1523-1755.2001.059002702.x. [DOI] [PubMed] [Google Scholar]
- 23.Costacou T, Ellis D, Fried L, et al. Sequence of progression of albuminuria and decreased GFR in persons with type 1 diabetes: a cohort study. Am J Kidney Dis. 2007;50:721–32. doi: 10.1053/j.ajkd.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Feldt-Rasmussen B, Mathiesen ER, Jensen T, et al. Effect of improved metabolic control on loss of kidney function in type 1 (insulin-dependent) diabetic patients: an update of the Steno studies. Diabetologia. 1991;34:164–70. doi: 10.1007/BF00418270. [DOI] [PubMed] [Google Scholar]
- 25.Rudberg S, Osterby R. Decreasing glomerular filtration rate—an indicator of more advanced diabetic glomerulopathy in the early course of microalbuminuria in IDDM adolescents? Nephrol Dial Transplant. 1997;12:1149–54. doi: 10.1093/ndt/12.6.1149. [DOI] [PubMed] [Google Scholar]
- 26.Dahlquist G, Stattin EL, Rudberg S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dial Transplant. 2001;16:1382–6. doi: 10.1093/ndt/16.7.1382. [DOI] [PubMed] [Google Scholar]
- 27.Bangstad HJ, Osterby R, Rudberg S, et al. Kidney function and glomerulopathy over 8 years in young patients with Type I (insulin-dependent) diabetes mellitus and microalbuminuria. Diabetologia. 2002;45:253–61. doi: 10.1007/s00125-001-0744-y. [DOI] [PubMed] [Google Scholar]
- 28.Amin R, Turner C, van Aken S, et al. The relationship between microalbuminuria and glomerular filtration rate in young type 1 diabetic subjects: The Oxford Regional Prospective Study. Kidney Int. 2005;68:1740–49. doi: 10.1111/j.1523-1755.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 29.Premaratne E, MacIsaac RJ, Finch S, et al. Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care. 2008;31:971–3. doi: 10.2337/dc07-1588. [DOI] [PubMed] [Google Scholar]
- 30.Tan GD, Lewis AV, James TJ, et al. Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: reproducibility and accuracy compared with standard measures and iohexol clearance. Diabetes Care. 2002;25:2004–9. doi: 10.2337/diacare.25.11.2004. [DOI] [PubMed] [Google Scholar]
- 31.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:404–12. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins BA, Nelson RG, Krolewski AS. Cystatin C and the risk of death. N Engl J Med. 2005;353:842–4. [PubMed] [Google Scholar]
- 33.Mauer M. The Renin Angiotensin System Study (RASS): Effects of Enalapril and Losartan on Diabetic Renal and Retinal Lesions in Normotensive, Normoalbuminuric Type I Diabetic Patients. Invited Lecture presented in “Late-Breaking Interventions in Glomerular Disease and Diabetic Nephropathy”. American Society of Nephrology Renal Week. 2007 [Google Scholar]
- 34.Mann JF, Schmieder RE, McQueen M, et al. for the ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomized, double-blind, controlled trial. Lancet. 2008;16:547–53. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 35.Jerums G, Panagiotopoulos S, Premaratne E, et al. Lowering of proteinuria in response to antihypertensive therapy predicts improved renal function in late but not in early diabetic nephropathy: a pooled analysis. Am J Nephrol. 2008;28:614–27. doi: 10.1159/000117461. [DOI] [PubMed] [Google Scholar]
- 36.Steinke JM, Sinaiko AR, Kramer MS, et al. International Diabetic Nephopathy Study Group. The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes. 2005;54:2164–71. doi: 10.2337/diabetes.54.7.2164. [DOI] [PubMed] [Google Scholar]
- 37.Krolewski AS, Laffel LMB, Krolewski M, et al. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;332:1251–5. doi: 10.1056/NEJM199505113321902. [DOI] [PubMed] [Google Scholar]
- 38.Warram JH, Scott LJ, Hanna LS, et al. Progression of microalbuminuria to proteinuria in type 1 diabetes: nonlinear relationship with hyperglycemia. Diabetes. 2000;49:94–100. doi: 10.2337/diabetes.49.1.94. [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, Greene T, Kusek JW, et al. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:A0828. [Google Scholar]
- 40.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 41.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 42.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–37. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 43.Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49:1686–9. doi: 10.1007/s00125-006-0275-7. [DOI] [PubMed] [Google Scholar]