Glyceraldehyde-3-Phosphate Dehydrogenase of Streptococcus pneumoniae Is a Surface-Displayed Plasminogen-Binding Protein (original) (raw)
Abstract
The recruitment of plasminogen endows the bacterial cell surface of Streptococcus pneumoniae with proteolytic activity. In this study we demonstrate specific plasmin- and plasminogen-binding activity for the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which is located in the cytoplasm as well as on the surface of pneumococci. GAPDH exhibits a high affinity for plasmin and a significantly lower affinity for plasminogen.
A prerequisite for the invasiveness of a pathogen is the pathogen's ability to breach epithelial as well as endothelial barriers in order to gain access to the submucosa and blood. A successful strategy used by pathogenic bacteria to degrade the extracellular matrix and to promote invasiveness is the recruitment of proteolytic activity to the bacterial cell surface (19, 21). Streptococcus pneumoniae, a common etiologic agent of respiratory tract diseases and life-threatening invasive diseases, is able to capture plasminogen on the bacterial cell surface. Subsequent activation by tissue-type and urokinase-type eukaryotic plasminogen activators allows the bacteria to acquire surface-associated proteolytic activity (10, 17). Plasminogen, a glycoprotein, is the zymogen of the serine protease plasmin, which is a key enzyme of the fibrinolytic pathway (7). The acquired plasmin activity promotes dissemination and transmigration of the pathogen through reconstituted basement membranes (6, 10, 21).
Analysis of plasminogen binding to viable pneumococci by the method described by Scatchard (25) indicated the presence of more than one plasminogen receptor on the pneumococcal cell surface. A blot overlay with soluble radioiodinated human plasminogen showed binding to eight pneumococcal components (2). α-Enolase (Eno) was identified as the major plasminogen- and plasmin-binding protein of S. pneumoniae (2). A further peptide sequence, obtained by an N-terminal sequence analysis of excised proteins positive for plasminogen binding, showed 100% homology to the N-terminal sequence of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; GapA) in S. pneumoniae strains (http://www.tigr.org/tigr-scripts/CMR2/CMRGenomes.spl) (16, 26). These results identified the GAPDH of S. pneumoniae, which is an essential enzyme of the glycolytic pathway, as a putative plasminogen-binding protein. For expression cloning of gapA, the gene was amplified by PCR using the chromosomal DNA of S. pneumoniae serotype 2 (ATCC 11733) and oligonucleotide primers SB 13 (5′-GGATCCTTGCTTGCACACTTGTTGAAATAC-3′), incorporating an in-frame BamHI restriction site at the 5′ end, and SB 14 (5′-AAGCTTTTATTTAGCAATTTTTGCGAAGTATT-3′), incorporating an in-frame HindIII restriction site at the 3′ end. The BamHI- and HindIII-digested PCR product was cloned into the similarly digested expression vector pQE30 (Qiagen). The purification of His-tagged GAPDH under native conditions was conducted according to a standard protocol (Qiagen). In order to identify the subcellular localization of GAPDH by immunoblot analysis, polyclonal antibodies against purified recombinant GAPDH were raised in rabbits by routine immunogenic procedures (Eurogentec). Results indicated the presence of GAPDH in both the cytoplasmic fraction and the cell surface protein fraction of S. pneumoniae (Fig. 1). A sequence comparison performed with the algorithm of Lipman and Pearson (18) revealed 89.0% identity to the group A streptococcal GAPDH Plr/streptococcal surface dehydrogenase and 85.2% identity to GapC in Streptococcus equisimilis (14, 20, 24). Plasminogen- and plasmin-binding activity, which is most likely mediated via C-terminal lysyl residues (28), to both streptococcal proteins has been reported. The pneumococcal GAPDH contains two C-terminal lysine amino acids which are separated by isoleucine and alanine. The C-terminal sequences (>40 amino acids) of the above-mentioned GAPDH proteins are 100% identical. Moreover, sequence comparison revealed identical C termini in the GAPDHs of Streptococcus agalactiae, Streptococcus dysgalactiae, and Streptococcus mutans (Table 1). Interestingly, the GAPDHs of streptococci of groups A, C, and G were recently identified as extracellular targets for a broad spectrum of extracellular matrix proteins and especially for plasmin and plasminogen (20, 22).
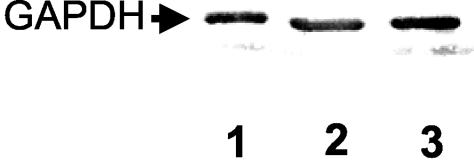
FIG. 1.
Identification of the cellular distribution of the pneumococcal GAPDH. Immunoblot analysis, using anti-GAPDH antibodies, of the cell wall fraction (lane 1) and soluble fraction (lane 2) of S. pneumoniae R6x and of the soluble fraction of an S. pneumoniae eno internal deletion mutant expressing α-enolase with mutated plasminogen binding sites (lane 3).
TABLE 1.
Comparison of GAPDH sequences and C-terminus identities among different streptococcal species
| Species | Protein | % Identity | C-terminal sequence | Accession no. | Reference(s) |
|---|---|---|---|---|---|
| S. pneumoniae | GapA | 100 | VRTLEYFAKIAK | AJ505822 | This paper |
| S. pyogenes | Plr/SDH | 89.0 | VRTLEYFAKIAK | M95569 | 20, 24 |
| S. agalactiae | GapC | 86.4 | VRTLEYFAKIAK | AF421899 | 13 |
| S. dysgalactiae | GapC | 89.6 | VRTLEYFAKIAK | AF375662 | EMBL |
| S. equisimilis | GapC | 85.2 | VRTLEYFAKIAK | X97788 | 14 |
| S. mutans | GapC | 88.7 | VRTLEYFAKIAK | AE014883 | 1 |
In order to demonstrate the plasminogen-binding activity of the pneumococcal GAPDH, total proteins of the serotype 2 strain and the serotype 2 eno internal deletion (_eno_int/del) mutant were incubated with immobilized plasminogen. The mutant expresses an α-enolase with a deletion of the C-terminal lysyl residues and amino acid substitutions in the internal plasminogen-binding motif of Eno. The Enoint/del mutant exhibited substantially reduced plasminogen-binding activity (3) and was used in order to avoid binding of the α-enolase to plasminogen. Plasminogen (obtained from Sigma or provided by K. Preissner, Giessen, Germany) was immobilized on a polyvinylidine fluoride membrane, and GAPDH binding was detected with polyclonal anti-GAPDH antiserum followed by horseradish peroxidase-conjugated anti-rabbit antibody. Results demonstrated a concentration-dependent GAPDH-plasminogen interaction, in which the protein-protein interaction depends on both the amount of immobilized plasminogen and the amount of soluble GAPDH (Fig. 2).
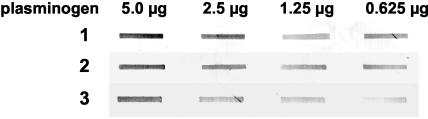
FIG. 2.
Binding of pneumococcal GAPDH to plasminogen. Human plasminogen was immobilized in the different amounts indicated. GAPDH was purified under native conditions from the cytosolic compartment of the serotype 2 _eno_int/del mutant pneumococci (ATCC 11733). Rows 1 to 3, binding of GAPDH applied in serial dilutions (1:2).
In an attempt to visualize the subcellular localization of GAPDH and the binding of plasminogen to GAPDH protein, preembedding, labeling studies were carried out as recently demonstrated for Eno (2). The unencapsulated pneumococcal strain R6x and the encapsulated pneumococcal strains of serotypes 2 (ATCC 11733) and 35A (NCTC 10319) were prelabeled with polyclonal protein A-purified anti-GAPDH immunoglobulin G and 15-nm-diameter gold particles coupled to protein A before embedding. Ultrathin sections revealed that the GAPDH of S. pneumoniae resembles the α-enolase located on the bacterial cell surface of unencapsulated (Fig. 3B) and encapsulated (Fig. 3A and C) strains. These results indicate that the common surface disposition of GAPDH is independent of the state of capsular polysaccharide expression. The surface-located glycolytic enzymes of the Embden-Meyerhof-Parnas pathway and other surface-displayed proteins like PavA of S. pneumoniae (15) and FBP54 of Streptococcus pyogenes (8, 9) lack the classical secretion and anchoring mechanisms (11, 12, 27, 29) and thus constitute a novel class of exported proteins of gram-positive bacteria (5). The conditions and factors required for protein secretion, as well as the mechanism of anchoring these proteins, are not known. How plasminogen is bound to GAPDH or Eno through the capsule also remains unknown.
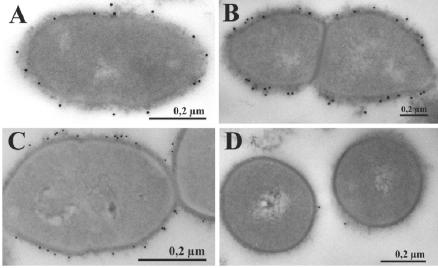
FIG. 3.
Immunoelectron microscopic visualization of GAPDH on the surface of S. pneumoniae ATCC 11733 (type 2) (A), unencapsulated R6x (B), and S. pneumoniae NCTC 10319 (type 35A) (C). (A to C) GAPDH was visualized on the bacterial cell surfaces of ultrathin sections of preembedding labeled samples by using anti-GAPDH antibodies and protein A-15-nm-diameter gold particle studies. (D) Visualization of unspecific binding of protein A-15-nm-diameter gold particles to type 2 pneumococci.
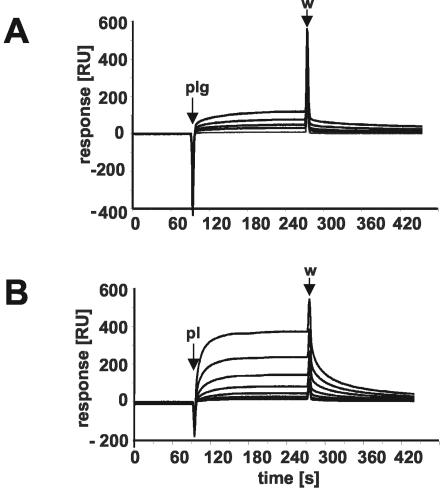
The dynamics of GAPDH-plasminogen and GAPDH-plasmin complex formation and their dissociation were analyzed by surface plasmon resonance (SPR) technique. GAPDH was covalently immobilized on a BIAcore CM5 sensor chip as previously described (3, 23). The association and dissociation kinetics of Glu-plasminogen and plasmin (Sigma) to GAPDH were evaluated according to the heterogeneous ligand model (A + B1 ↔ AB1; A + B2 ↔ AB2). This revealed two equilibrium constants; for plasminogen, KD1 was equal to 4.3 × 10−7 M and KD2 was equal to 1.6 × 10−10 M, and for plasmin, KD1 was equal to 2.8 × 10−8 M and KD2 was equal to 5.2 × 10−8 M. Studies using SPR showed that the pneumococcal GAPDH had a higher affinity for the serine protease plasmin than for its zymogen plasminogen (Fig. 4), as has also been shown previously for the Plr/streptococcal surface dehydrogenase of group A streptococci (20). The differences between equilibrium constants are probably the result of conformationally dependent structure recognition by Plr of the plasmin or plasminogen molecule (4). The interaction of GAPDH and plasminogen produced lower equilibrium constants than the Eno-plasminogen dissociation and complex formation did (KD1, 8.6 × 10−8 M; KD2, 5.5 × 10−10 M) in S. pneumoniae (3), indicating a lower affinity of GAPDH for plasminogen. Pathogenic bacteria acquire proteolytic activity by the recruitment and subsequent activation of plasminogen. A recent study indicated that the nonglycolytic property of α-enolase contributes to the pathogenesis of pneumococci (3). The concerted action of both α-enolase and GAPDH on the bacterial cell surface might, therefore, result in an enhancement of the pathogen's ability to degrade the extracellular matrix and to invade host tissues.
FIG. 4.
SPR measurements of GAPDH-plasminogen (A) and GAPDH-plasmin (B) interactions. Purified GAPDH protein was immobilized on a BIAcore CM5 sensor chip, and plasmin and plasminogen were used as the analyte. Binding kinetics and concentration dependence of binding of plasminogen and plasmin to GAPDH are shown as changes in plasmin resonance. Plasmin and plasminogen were used in concentrations of 500 (for plasminogen only), 250, 125, 62.5, 31.25, 15.625, 7.8, and 3.9 nM. The blank run was subtracted from the sensorgram. Pl and Plg, starts of injection; w, stop of injection; RU, relative units.
Acknowledgments
We are grateful to U. Hentschel and J. Reidl for critical readings of the manuscript.
This work was partially supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 587/479, Teilprojekt A6/A7 to S.H., and Ro 2407/1 to M.R.) and the Bundesministerium für Bildung und Forschung (CAPNetz to S. H.).
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002.Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99**:**14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40**:**1273-1287. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, S., D. Wild, O. Diekmann, R. Frank, D. Bracht, G. S. Chhatwal, and S. Hammerschmidt. 2003. Identification of a novel plasmin(ogen)-binding motif in surface displayed α-enolase of Streptococcus pneumoniae. Mol. Microbiol. 49**:**411-423. [DOI] [PubMed] [Google Scholar]
- 4.Broder, C. C., R. Lottenberg, and M. D. P. Boyle. 1989. Mapping of the human plasmin domain recognized by the unique plasmin receptor of group A streptococci. Infect. Immun. 57**:**2597-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chhatwal, G. S. 2002. Anchorless adhesins and invasins of Gram-positive bacteria: a new class of virulence factors. Trends Microbiol. 10**:**205-208. [DOI] [PubMed] [Google Scholar]
- 6.Coleman, J. L., E. J. Roemer, and J. L. Benach. 1999. Plasmin-coated Borrelia burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect. Immun. 67**:**3929-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collen, D., and M. Verstraete. 1975. Molecular biology of human plasminogen. II. Metabolism in physiological and some pathological conditions in man. Thromb. Diath. Haemorrh. 34**:**403-408. [PubMed] [Google Scholar]
- 8.Courtney, H. S., J. B. Dale, and D. L. Hasty. 1996. Differential effects of the streptococcal fibronectin-binding protein, FBP54, on adhesion of group A streptococci to human buccal cells and HEp-2 tissue culture cells. Infect. Immun. 64**:**2415-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtney, H. S., Y. Li, J. B. Dale, and D. L. Hasty. 1994. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect. Immun. 62**:**3937-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberhard, T., G. Kronvall, and M. Ullberg. 1999. Surface bound plasmin promotes migration of Streptococcus pneumoniae through reconstituted basement membranes. Microb. Pathog. 26**:**175-181. [DOI] [PubMed] [Google Scholar]
- 11.Fischetti V. A., V. Pancholi, and O. Schneewind. 1991. Common characteristics of the surface proteins from gram-positive cocci, p. 290-294. In G. M. Dunny, P. P. Cleary, and L. L. McKay (ed.), Genetics and molecular biology of streptococci, lactococci, and enterococci. American Society for Microbiology, Washington, D.C.
- 12.Fischetti, V. A., V. Pancholi, and O. Schneewind. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol. Microbiol. 4**:**1603-1605. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine, M. C., J. Perez-Casal, X.-M. Song, J. Shelford, P. J. Willson, and A. A. Potter. 2002. Immunisation of dairy cattle with recombinant Streptococcus uberis GapC or a chimeric CAMP antigen confers protection against heterologous bacterial challenge. Vaccine 20**:**2278-2286. [DOI] [PubMed] [Google Scholar]
- 14.Gase, K., A. Gase, H. Schirmer, and H. Malke. 1996. Cloning, sequencing and functional overexpression of the Streptococcus equisimilis H46A gapC gene encoding a glyceraldehyde-3-phosphate dehydrogenase that also functions as a plasmin(ogen)-binding protein. Purification and biochemical characterization of the protein. Eur. J. Biochem. 239**:**42-51. [DOI] [PubMed] [Google Scholar]
- 15.Holmes, A. R., R. McNab, K. W. Millsap, M. Rohde, S. Hammerschmidt, J. L. Mawdsley, and H. F. Jenkinson. 2001. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol. Microbiol. 41**:**1395-1408. [DOI] [PubMed] [Google Scholar]
- 16.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D.-J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P.-M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183**:**5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuusela, P., M. Ullberg, O. Saksela, and G. Kronvall. 1992. Tissue-type plasminogen activator-mediated activation of plasminogen on the surface of group A, C, and G streptococci. Infect. Immun. 60**:**196-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipman, D. J., and W. R. Pearson. 1985. Rapid and sensitive protein similarity searches. Science 227**:**1435-1441. [DOI] [PubMed] [Google Scholar]
- 19.Lottenberg, R. 1997. A novel approach to explore the role of plasminogen in bacterial pathogenesis. Trends Microbiol. 5**:**466-468. [DOI] [PubMed] [Google Scholar]
- 20.Lottenberg, R., C. C. Broder, M. D. P. Boyle, S. J. Kain, B. L. Schroeder, and R. Curtiss III. 1992. Cloning, sequence analysis, and expression in Escherichia coli of a streptococcal plasmin receptor. J. Bacteriol. 174**:**5204-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lottenberg, R., D. Minning-Wenz, and M. D. Boyle. 1994. Capturing host plasmin(ogen): a common mechanism for invasive pathogens? Trends Microbiol. 2**:**20-24. [DOI] [PubMed] [Google Scholar]
- 22.Modun, B., and P. Williams. 1999. The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect. Immun. 67**:**1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nice, E. C., T. L. McInerney, and D. C. Jackson. 1996. Analysis of the interaction between a synthetic peptide of influenza virus hemagglutinin and monoclonal antibody using an optical biosensor. Mol. Immunol. 33**:**659-670. [DOI] [PubMed] [Google Scholar]
- 24.Pancholi, V., and V. A. Fischetti. 1992. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. J. Exp. Med. 176**:**415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scatchard, G. 1949. The attraction of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 51**:**660-672. [Google Scholar]
- 26.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293**:**498-506. [DOI] [PubMed] [Google Scholar]
- 27.von Heijne, G. 1986. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 14**:**4683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winram, S. B., and R. Lottenberg. 1998. Site-directed mutagenesis of streptococcal plasmin receptor protein (Plr) identifies the C-terminal Lys334 as essential for plasmin binding, but mutation of the plr gene does not reduce plasmin binding to group A streptococci. Microbiology 144**:**2025-2035. [DOI] [PubMed] [Google Scholar]
- 29.Yother, J., and J. M. White. 1994. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176**:**2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]