Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells (original) (raw)
. Author manuscript; available in PMC: 2013 Sep 3.
Published in final edited form as: Nat Immunol. 2001 May;2(5):415–422. doi: 10.1038/87720
Abstract
The rules that govern memory T cell differentiation are not well understood. This study shows that after antigenic stimulation naïve CD8+ T cells become committed to dividing at least seven times and differentiating into effector and memory cells. Once the parental naïve CD8+ T cell had been activated, this developmental process could not be interrupted and the daughter cells continued to divide and differentiate in the absence of further antigenic stimulation. These data indicate that initial antigen encounter triggers an instructive developmental program that does not require further antigenic stimulation and does not cease until memory CD8+ T cell formation.
The main features that provide antigen-specific memory CD8+ T cells with a protective advantage over naïve CD8+ T cells are that memory CD8+ T cells persist for extended periods of time, are present in larger numbers and respond more rapidly to antigen than naïve CD8+ T cells do1,2. Although it is known that these characteristics are acquired as activated CD8+ T cells differentiate into memory CD8+ T cells, the mechanisms that guide this process are not clearly understood.
The course of memory CD8+ T cell development occurs over two phases after infection or vaccination1,2. The first phase begins when peripheral naïve CD8+ T cells encounter antigen, become activated and differentiate into effector cytotoxic T lymphocytes (CTLs)1,2. Antigen recognition also initiates T cell proliferation that can be tightly coupled to changes in gene expression3,4. As naïve CD8+ T cells differentiate into effector CTLs they gain the ability to produce antiviral cytokines, such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), and cytotoxic molecules, such as perforin and granzymes, and rapidly eliminate the infectious pathogen5–10. After antigen removal, a second phase of T cell development ensues, whereby most of the antigen-specific effector CD8+ T cells die by apoptosis and the surviving effectors differentiate into memory CD8+ T cells1,2,5,8,11–13.
Several studies have shown that the strength and duration of T cell antigen receptor (TCR) and costimulatory receptor signaling are important parameters regulating T cell activation14–16. For example, naïve CD4+ T cells require signaling through both the TCR and costimulatory receptors for ∼20 h in vitro before committing to cellular proliferation15. However, it is not known whether these minimal requirements, which cause T cells to commit to proliferate in vitro, are also sufficient to induce naïve T cells to differentiate into effectors and subsequently into memory cells in vivo.
Cell division may be an important parameter that regulates formation of memory CD8+ T cells because progression through the cell cycle can provide a window of opportunity to remodel chromatin and gene expression patterns to create new cellular phenotypes3,17. For example, the expression of cytokines such as IFN-γ and interleukin 4 (IL-4) appears to be dependent on specific numbers of cell divisions in activated CD4+ T cells. A similar phenomenon has been observed for expression of IFN-γ and certain surface receptors in CD8+ T cells3,4,6,9. Extensive CD8+ T cell proliferation has been observed during viral and intracellular bacterial infections and it is possible that the qualitative changes that occur as naïve CD8+ T cells differentiate into memory cells are instilled with each successive cell division so that a certain number of divisions are required for these cellular changes to be permanently embedded8,11,18–23. In support of this idea, one study reported that development of memory CD8+ T cells required at least five cell divisions and that naïve CD8+ T cells that divided fewer than five times after antigenic stimulation did not differentiate into memory cells5.
We wanted to examine more closely how the salient characteristics of memory CD8+ T cells—such as the ability to persist, to respond rapidly to antigen and to confer protective immunity—are influenced by the number of times a naïve CD8+ T cell divides after antigenic stimulation. We assumed that the number of cell divisions could be controlled by exposing naïve CD8+ T cells to low, intermediate or high amounts of antigen and that the cells would divide to a lesser or greater extent according to these conditions. Instead, we found that varying the antigen dose affected the number of naïve CD8+ T cells recruited into the immune response, but that all the recruited CD8+ T cells divided at least seven times after initial antigen encounter. These cells also differentiated into fully functional effector CTLs that secreted IFN-γ, exhibited cytolytic activity and continued to further differentiate into protective memory CD8+ T cells that were long-lived. In addition, we found that further exposure to antigen, after the initial stimulus, was not necessary to sustain cell proliferation and differentiation. These data suggest a model in which activated CD8+ T cells become committed to an instructive developmental program that drives them to divide many times and differentiate into effector and memory CD8+ T cells.
Results
Activated CD8+ T cells committed to divide extensively
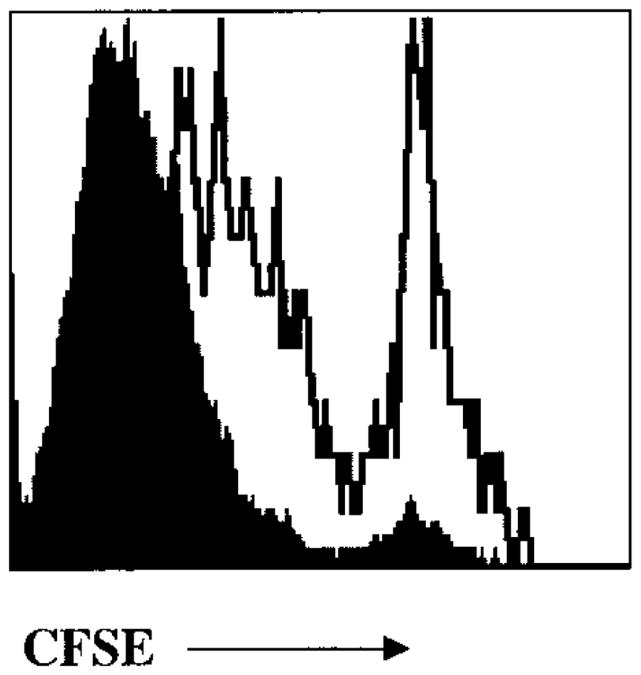
Initially, we attempted to design a system in which we could control the number of cell divisions that activated CD8+ T cells underwent by varying the dose of antigen that was administered in vivo. To visualize T cell proliferation in vivo we used P14 transgenic CD8+ T cells that express a TCR specific for the H-2Db– restricted GP(33–41) epitope of the lymphocytic choriomeningitis virus (LCMV) glyoprotein24,25. These cells are referred to hereafter as P14 CD8+ T cells. The P14 CD8+ T cells were labeled with the fluorescent, cell-permeable, dye carboxyl fluorescein succinimidyl ester (CFSE) to quantify the number of cell divisions. CFSE is partitioned equally during cell division, which results in the sequential halving of cellular fluorescent intensity with each successive generation. Using this technique one can visually monitor up to seven cell divisions before the cells become CFSEneg.
Approximately 1×106–2×106 naïve CFSE-labeled P14 CD8+ T cells were adoptively transferred into C57BL/6 (B6) mice that were subsequently infected with high (3×104 colony-forming units, or CFU), intermediate (3×103 CFU) or low (100 CFU) doses of a recombinant Listeria monocytogenes bacterial strain (LM-GP33) that expresses the GP(33–41) epitope. As expected, the kinetics of bacterial clearance corresponded to the dose administered. In mice that received a low dose of LM-GP33, low amounts of bacteria (1×103–3×103 CFU/spleen) were present during the first 2 days of infection, but became undetectable by day 3. In mice that received a high dose, high amounts of bacteria (∼4×106–8×106 CFU/spleen) were still present at day 3, but were undetectable by day 7 (data not shown). Also, as expected, the size of the primary P14 CD8+ T cell response at the peak of the immune response, day 7, correlated with the amount antigen administered (Fig. 1a–c). Mice infected with high doses of LM-GP33 had ∼20×106 P14 CD8+ T cells/spleen, those infected with the intermediate dose had ∼7×106 P14 CD8+ T cells/spleen and those infected with low doses had ∼1×106 P14 CD8+ T cells/spleen. It should be noted that, at the time of infection, these chimeric mice had ∼1×105 naïve P14 CD8+ T cells, as the engraftment of adoptively transferred T cells into intact mice was only ∼5–10%. Based on this, there were ∼200-, ∼70- and ∼tenfold increases in the number of antigen-specific P14 CD8+ T cells after challenge with the high, intermediate and low doses of LM-GP33, respectively.
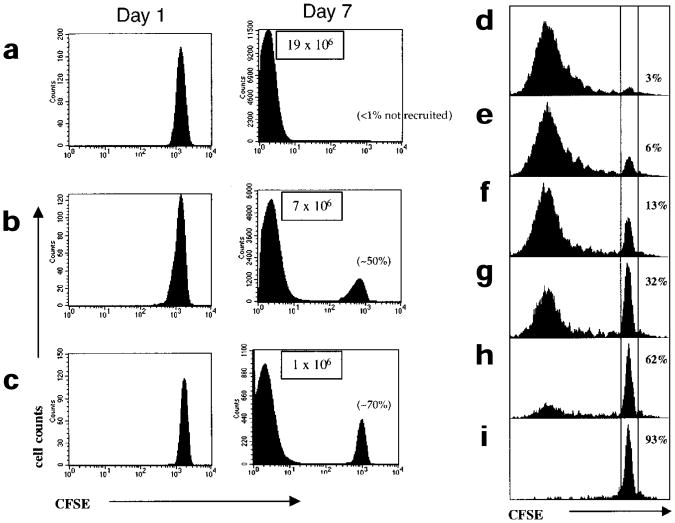
Figure 1. Varying the antigen dose affects the magnitude of the CD8+ T cell response and the recruitment of naïve CD8+ T cells, but all recruited cells divide extensively.
(a–c) CFSE-labeled naïve P14 CD8+ T cells (∼1–2×106) were adoptively transferred into B6 mice and these chimeric mice were then infected with (a) high (3×104 CFU) (b) intermediate (3×103 CFU) or (c) low (100 CFU) doses of LM-GP33. On days 1 and 7 after infection the CFSE fluorescence of splenic P14 CD8+ T cells was analyzed by flow cytometry by staining with anti-CD8α and Db-GP(33–41) tetramer. Histograms show the CFSE intensity of CD8+Db-GP(33–41)+ T cells. Note that on day 7 after intermediate- and low-dose infections, the P14 CD8+ T cells are found in only two CFSE peaks: cells that had not divided and cells that had divided more than seven times and were CFSEneg. The percentage of P14 CD8+ T cells not recruited into the immune response are shown in parentheses. The total number of activated CFSEneg P14 CD8+ T cells in a representative experiment are in boxes. (d–i) CFSE-labeled P14 CD8+T cells (50,000) were cultured for 4 days in vitro with (d) 10,000 (e) 5000 (f) 1000 (g) 200 (h) 100 or (i) 0 GP(33–41)-pulsed splenocytes. The cells were stained with anti-CD8α and Db-GP(33–41) tetramers and CFSE fluorescence analyzed was by flow cytometry. The percentage of cells that were not activated and did not proliferate are indicated.
As the burst size is a function of the number of CD8+ T cells recruited into the immune response and the number of times the CD8+ T cells divide, we examined both these parameters. Varying the antigen dose had a substantial effect on the number of P14 CD8+ T cells recruited to the immune response. During high-dose LM-GP33 infections all the naïve P14 CD8+ T cells were recruited, whereas only ∼50% and 30% of the P14 CD8+ T cells were recruited during the intermediate- and low-dose infections, respectively (Fig. 1a–c). The nonrecruited P14 CD8+ T cells had not undergone any proliferation 7 days after infection because the intensity of CFSE staining in these cells was equal to that observed in CFSE-labeled P14 CD8+ T cells from uninfected mice (data not shown). The number of antigen-presenting cells (APCs) that efficiently present the GP(33–41) epitope are most likely limited during the intermediate- and low-dose LM-GP33 infections and this reduces the number of CD8+ T cells that are activated and recruited to the immune response. The number of times the recruited P14 CD8+ T cells divided during the high-, intermediate- or low-dose LM-GP33 infections were similar. In each case, the recruited P14 CD8+ T cells were CFSEneg by day 7, indicating that these cells had divided at least seven times (Fig. 1a–c). Even when antigen was most limiting and ≥70% of the naïve P14 CD8+ T cells were not recruited into the response, the recruited cells still divided multiple times; CD8+ T cells that had stalled after one or two divisions were never detected.
To further observe the effects of limited antigen exposure on CD8+ T cell recruitment and cell division, we examined the responses of P14 CD8+ T cells stimulated with decreasing numbers of APCs in cell culture. A constant number of naïve P14 CD8+ T cells were CFSE-labeled and stimulated with decreasing numbers of GP(33–41)–pulsed APCs for four days (Fig. 1d–i). As we had seen in vivo, decreasing the number of APCs in vitro led to a decrease in the percentage of P14 CD8+ T cells recruited; yet again, the recruited P14 CD8+ T cells divided to a similar extent. Taken together, these results show that decreasing the number of APCs greatly affects the number of CD8+ T cells recruited into the response, but all the recruited cells become committed to dividing multiple times.
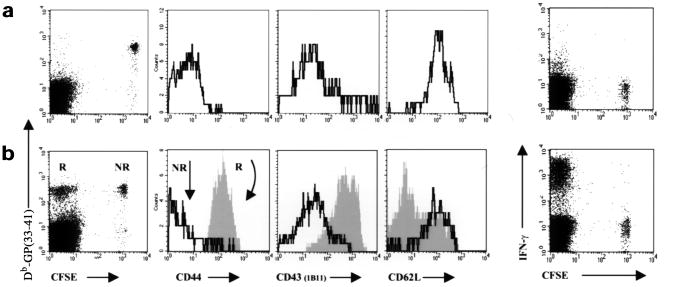
Because T cell differentiation is coupled to cell proliferation, we next analyzed the extent to which the proliferating P14 CD8+ T cells differentiated into effector CTLs. Differentiation into effector CTLs was measured by the ability of these cells to secrete cytokines such as IFN-γ, TNF-α and IL-2, their ability to kill cells expressing GP(33–41) peptide and by the altered expression of several activation markers such as CD44, CD43 and CD62L (L-selectin). By 7 days after low-dose LM-GP33 infection, the CFSEneg P14 CD8+ T cells were CD44hiCD43hiCD62Llo, as normally observed for activated CD8+ T cells (Fig. 2)6,26. In contrast, the nonrecruited CFSEpos P14 CD8+ T cells showed a naïve phenotype and were CD44loCD43loCD62Lhi (Fig. 2). In addition, only the CFSEneg P14 CD8+ T cells secreted IFN-γ, TNF-α and IL-2 when restimulated with GP(33–41) peptide (Fig. 2 and data not shown). We also examined whether the CD8+ T cells recruited to the immune response during the low-dose infection acquired cytolytic activity in response to GP(33–41) peptide–pulsed cells. Direct ex vivo cytotoxic killing was observed in standard 51Cr-release assays, which indicated that the effector CTLs produced after a low-dose LM-GP33 infection exhibited all the qualities of fully differentiated effector CTLs (data not shown)7,8. Similar to the effects on cell division, it seems that the activated CD8+ T cells are incapable of stalling in an intermediate state of differentiation. This “all-or-nothing” phenomenon suggested that once a CD8+ T cell passes a threshold of activation, supplied by both sufficient TCR and costimulatory signals, a developmental program is initiated that causes the T cells to divide multiple times and fully differentiate into effector CTLs.
Figure 2. CD8+ T cells become committed to differentiate fully into effector CTLs after initial activation.
CFSE-labeled naïve P14 CD8+ T cells (2×106) were adoptively transferred into B6 mice that were then infected with LM-GP33 (100 CFU). On (a) day 1 and (b) day 7 after infection splenic P14 CD8+ T cells were identified using fluorescent Db-GP(33–41) tetramers and the expression of proteins associated with T cell activation were analyzed with anti-CD44, anti-CD43 (clone 1B11), anti-CD62L and anti–IFN-γ. The expression of CD44, CD43 and CD62L on the recruited (R, filled histograms) and nonrecruited (NR, solid-line histograms) P14 CD8+ T cells. IFN-γ production by P14 CD8+ T cells 1 and 7 days after infection was analyzed by flow cytometry after 5 h of GP(33–41) peptide stimulation and then CD8+ surface and IFN-γ intracellular staining. Dot-plots represent CD8+ T cells; note that only CFSEneg cells produce IFN-γ on day 7 after infection.
Antigen-independent division and differentiation
The previous data suggest a model whereby activated CD8+ T cells execute an instructive developmental program that drives naïve CD8+ T cells to proliferate and differentiate into effector CTLs. The essence of the model is that once the parental naïve CD8+ T cell has been stimulated with antigen it will divide multiple times and that the daughter cells do not need to “see” antigen again to continue dividing. To more rigorously test this model, we did two series of experiments to show that activated CD8+ T cells continue to proliferate in the absence of antigen. The presence of any residual antigen in these experiments was monitored with the use of two congenic strains of P14-transgenic mice that are polymorphic at the Thy1 locus. P14 CD8+ T cells from these mice express either the Thy1.1 or the Thy1.2 isoform and can be distinguished using the isoform-specific anti-Thy1.1 and anti-Thy1.2.
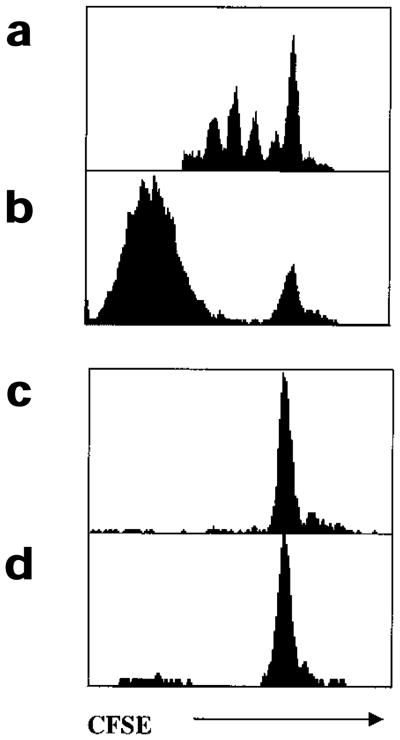
In the first set of experiments, CD8+ T cell responses were observed in vitro by stimulating naïve CFSE-labeled Thy1.1+ P14 CD8+ T cells with a limited number of GP(33–41) peptide-pulsed APCs in cell cultures (Fig. 3). At 48 h after stimulation the activated CD8+ T cells had divided one to five times. The cells continued dividing and by 96 h after stimulation the majority of the recruited cells had divided multiple times and become CFSEneg (Fig. 3a). To check whether the recruited CD8+ T cells continued to proliferate in the absence of antigen between 48 and 96 h, additional naïve or memory CFSE-labeled Thy1.2+ P14 CD8+ T cells were added to the cultures at 48 h and their proliferation analyzed 2 days later. At 96 h, no cell division was observed in either the naïve or memory P14 CD8+ T cell populations that had been spiked at 48 h. This showed that although insufficient amounts of GP(33–41) peptide remained in the culture after 48 h, the activated Thy1.1+P14 CD8+ T cells continued to divide (Fig. 3b).
Figure 3. Activated CD8+ T cells continue to divide in the absence of antigenic stimulation in vitro.
(a,b) CFSE-labeled Thy1.1+ naïve P14 CD8+ T cells (50,000) were stimulated with GP(33–41) peptide–pulsed splenocytes (5000 from Thy1.2+ mice) for 4 days in vitro. (a) At 48 h and (b) 96 h, cells were removed from the cultures and stained with anti-Thy1.1 and Db-GP(33–41) tetramers. Histograms were gated on Thy1.1+Db-GP(33–41)+ T cells. After 48 h, CFSE-labeled (c) naïve or (d) memory Thy1.2+P14 CD8+ T cells were added to the cultures described above. After an additional 48 h, the cells were stained with anti-Thy1.2 and Db-GP(33–41) tetramers. Histograms were gated on Thy1.2+Db-GP(33–41)+ T cells.
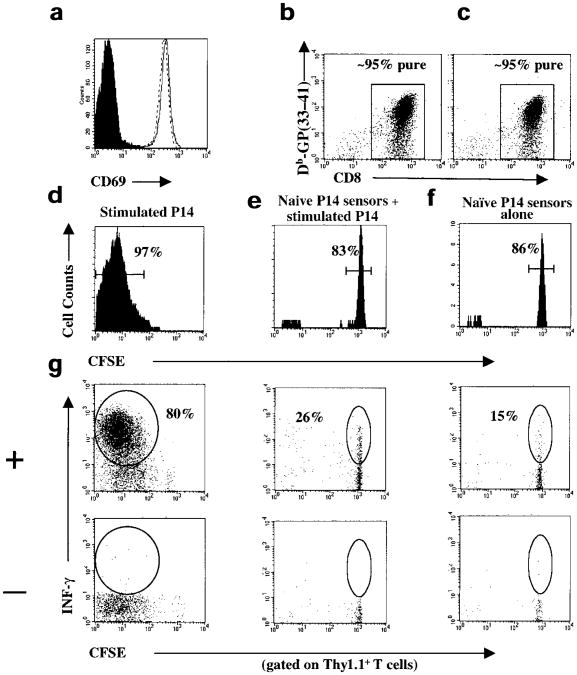
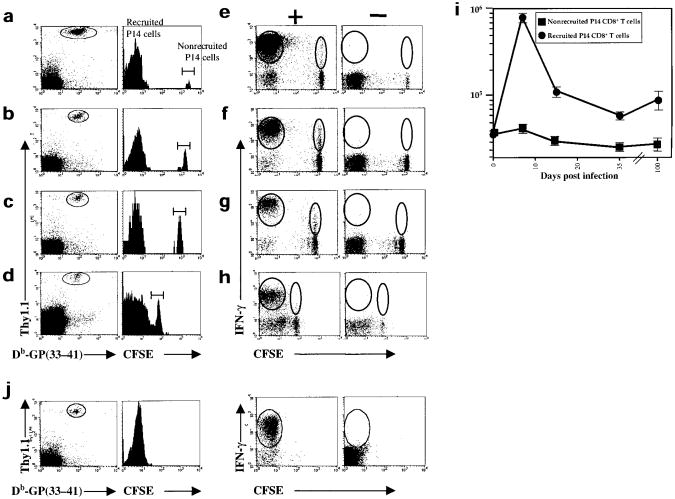
In the second series of experiments, we purified CD8+ T cells after initial antigenic stimulation to effectively remove APCs, adoptively transferred these activated CD8+ T cells into naïve B6 mice and monitored the proliferation and differentiation of these cells in the absence of antigen in vivo. Thy1.1+ or Thy1.2+ naïve P14 CD8+ T cells were stimulated with GP(33–41) peptide–pulsed APCs for 24 h in vitro. After 24 h, all the P14 CD8+ T cells from both strains of mice were CD69hi, which indicated that they had been activated (Fig. 4a). Anti-CD8α magnetic beads were used to purify the activated CD8+ T cells to ∼95% and the Thy1.1+ P14 CD8+ T cells were labeled with CFSE and transferred into naïve (Thy1.2+) B6 mice (Fig. 4b,c). Five to six days after adoptive transfer, the extent of proliferation and effector CTL differentiation in vivo was measured. During this time, the stimulated P14 Thy1.1+CD8+ T cells had divided more than seven times and were CFSEneg (Fig. 4d). The cells also produced IFN-γ upon restimulation and became CD44hi, which indicated that these cells had differentiated into effector CD8+ T cells (Fig. 4g and data not shown).
Figure 4. Repeated exposure to antigen is not necessary for activated CD8+ T cells to divide several times and differentiate into effector CTLs in vivo.
(a) Thy1.1+ and Thy1.2+ P14 CD8+ T cells were stimulated for 24 h with GP(33–41) peptide and stained with anti-CD8α + anti-CD69 and Db-GP(33–41) tetramers. Histograms are gated on CD8+Db-GP(33–41)+ T cells and show the amount of CD69 on stimulated Thy1.1+(solid line), Thy1.2+(dashed line) or unstimulated (filled histogram) P14 CD8+ T cells. After 24 h of GP(33–41) peptide stimulation the (b) Thy1.1+ or (c) Thy1.2+P14 CD8+ T cells were purified using CD8α magnetic beads and the purity analyzed by staining for CD8+ and Db-GP(33–41)–specific T cells. More than 95% of the purified cells were CD8+. (d) The purified Thy1.1+–stimulated P14 CD8+ T cells were CFSE-labeled and injected into B6 mice. (e) The purified Thy1.2+-stimulated P14 CD8+ T cells were not CFSE-labeled but were injected into a second set of B6 mice along with the naïve CFSE-labeled Thy1.1+P14 CD8+ T cells that served as sensors for presence of residual antigen. (f) A third set of B6 mice received the sensor P14 CD8+ T cells alone. (d–g) Five to six days after transfer, the extent of proliferation and differentiation of the Thy1.1+-stimulated or sensor P14 CD8+ T cells was analyzed. Splenocytes from the recipient mice were stained with anti-Thy1.1 and Db-GP(33–41) tetramers. The histograms, which show CFSE fluorescence, are gated on Thy1.1+Db-GP(33–41)+ T cells. The percentage of CFSEpos or CFSEneg P14 CD8+ T cells is shown. (g) IFN-γ production in Thy1.1+ stimulated or sensor P14 CD8+ T cells was analyzed by flow cytometry after a 5-h incubation with (+) or without (–) GP(33–41) peptide. The splenocytes were stained for surface Thy1.1 and intracellular IFN-γ and dot-plots are gated on Thy1.1+ T cells. The percentage of Thy1.1+ T cells producing IFN-γ are indicated.
To verify that this proliferation and differentiation occurred in the absence of antigen, a control set of mice was used that had been injected with stimulated Thy1.2+ P14 CD8+ T cells and naïve Thy1.1+ P14 CD8+ T cells. The stimulated Thy1.2+ P14 CD8+ T cells were purified to ∼95% purity and injected into B6 mice along with CFSE-labeled naïve Thy1.1+ P14 CD8+ T cells. The CFSE-labeled naïve Thy1.1+ P14 CD8+ T cells served as sensors for contaminating GP(33–41)-presenting APCs, as activation would cause these cells to proliferate and lose CFSE fluorescence. No proliferation was detected in the naïve CFSE-labeled Thy1.1+ sensors over the 5–6-day period (Fig. 4e). This indicated that antigen was efficiently removed when the stimulated CD8+ T cells were purified. In addition, the naïve Thy1.1+ P14 CD8+ T cells that were mixed with the stimulated Thy1.2+ P14 CD8+ T cells did not secrete any more than background amounts of IFN-γ: usually ∼15–20% of naïve P14 CD8+ T cells secrete IFN-γ upon stimulation with GP(33–41) peptide (Fig. 4g and S. Kaech, unpublished data). These results support the model whereby fully activated CD8+ T cells can proiferate and complete a developmental program that manifests in the formation of functional effector CTLs independent of continued exposure to antigen.
IL-2 enhancement of antigen-independent proliferation
It is possible that a fully activated CD8+ T cell is self-sufficient and contains all the information required to divide multiple times in the absence of further antigenic stimulation. Alternatively, activated CD8+ T cells may rely on signals from growth-promoting cytokines such as IL-2 to propel antigen-independent CD8+ T cell proliferation. To evaluate the role of IL-2, CFSE-labeled P14 CD8+ T cells were stimulated for the first 24 h with a limited number of GP(33–41) peptide-pulsed APCs (Fig. 3). Over the next 72 h the activity of IL-2 was blocked by the addition of antibodies to IL-2 and the IL-2 receptor α chain (CD25). Similar amounts of antibodies to IgG of the same isotype were added to control cultures. After 96 h the cultures were analyzed and a general reduction in the extent of proliferation was observed in cultures where IL-2 was blocked (Fig. 5). These data support the idea that fully activated CD8+ T cells become committed to proliferate but rely on extrinsic signals, such as IL-2, for optimal proliferation.
Figure 5. IL-2 is important for activated CD8+ T cells proliferating in the absence of continuous antigenic stimulation.
Naïve CFSE-labeled P14 CD8+ T cells were stimulated with GP(33–41) peptide– pulsed cells for 4 days in culture, as described in Fig. 3. After the first 24 h of stimulation, either anti–IL-2 + anti-CD25 (open histogram) or IgG isotype control antibodies (filled histogram) were added to the cultures every 12 h over the next 3 days. Histograms are gated on CD8+Db-GP(33–41)+ T cells.
Programmed memory CD8+ T cell development
Memory CD8+ T cells are defined by two important phenotypes: their ability to persist and their ability to rapidly regain effector CTL functions when re-exposed to antigen. These salient properties provide long-term immunological protection to reinfection and/or disease2. We next determined whether the developmental program that is initiated when naïve CD8+ T cells are stimulated with antigen for a brief period of time was sufficient to drive the necessary cellular changes that generate memory CD8+ T cells. P14 CD8+ T cells that were recruited to the immune response after infection with the low-dose of LM-GP33 were still present 100 days later (Fig. 6a–d, i). More importantly, however, these CD8+ T cells were capable of rapidly producing IFN-γ (within 5 h) upon stimulation with antigen (Fig. 6e–h). This rapid cytokine response is a characteristic feature of memory T cells. Note that the naïve P14 CD8+ T cells that were not recruited into the response are not efficient cytokine producers.
Figure 6. Activated CD8+ T cells are programmed to differentiate into long-lived, functional memory CD8+ T cells.
Naïve CFSE-labeled Thy1.1+ P14 CD8+ T cells (2×106) were adoptively transferred into naïve B6 (Thy1.2+) mice, which were then infected with LM-GP33 (∼100 CFU). At days (a, e) 7 (b, f) 15 (c, g) 35 and (d, h) 100 the extent of proliferation and differentiation of the P14 CD8+ T cells was examined by staining splenocytes with anti-Thy1.1 and Db-GP(33–41) tetramer. (a–d) Histograms are gated on Thy1.1+Db-GP(33–41)-specific T cells. The CFSE intensity of nonrecruited P14 CD8+ T cells decreases with time due to intrinsic quenching. (e–h) Splenocytes were incubated for 5 h either with (+) or without (−) GP(33–41) peptide and then Thy1.1 surface and IFN-γ intracellular staining was done. The dot-plots are gated on Thy1.1+ T cells.(i)The absolute numbers of recruited (CFSEneg) and nonrecruited (CFSEpos) P14 CD8+ T cells in the spleen were quantified on days 7–100 after low-dose LM-GP33 infection. (j) Thy1.1+ P14 CD8+ T cells stimulated with GP(33–41) peptide for 24 h in vitro were purified to ∼95%, CFSE-labeled and transferred into naïve B6 (Thy1.2+) mice that were devoid of contaminating GP(33–41)-expressing APCs, as described in Fig. 4. The number of Thy1.1+ P14 CD8+T cells persisting in a functional state was assessed, as described in a, 43 days after transfer.
We also followed the memory CD8+ T cell development of naïve P14 CD8+ T cells that had been stimulated with antigen for 24 h in vitro and then adoptively transferred into B6 mice. We have shown earlier that these stimulated and purified CD8+ T cells differentiated into effector cells in the absence of antigen (Fig. 4). We found that these cells were also able to differentiate into functional memory CD8+ T cells and persist in vivo (Fig. 6j).
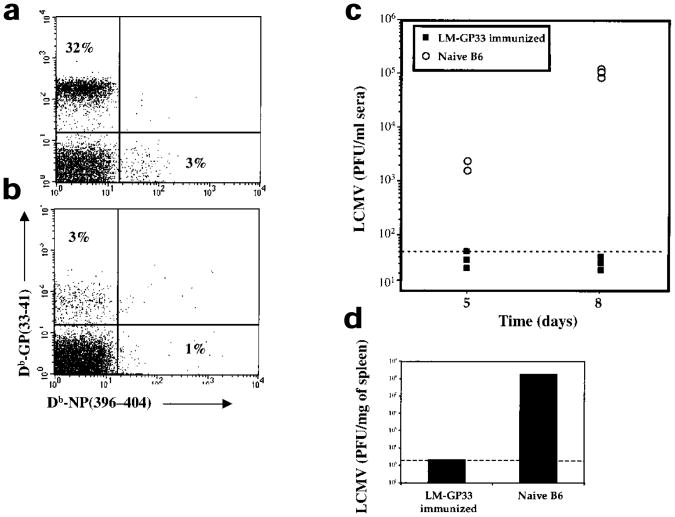
The hallmark of memory CD8+ T cells is their ability to confer protective immunity. We tested this by challenging mice vaccinated with low doses of LM-GP33 (90 days after immunization) with a virulent strain of LCMV, the clone 13 strain, which causes chronic infection in naïve mice27,28. The vaccinated mice showed a vigorous antiviral CD8+ T cell response and rapidly cleared the infection (Fig. 7). The magnitude of the Db-GP(33–41)-specific CD8+ T cell response in the LM-GP33–immunized mice was substantially greater (∼tenfold higher) than the responses observed in naïve B6 mice (Fig. 7a,b). This correlated with more rapid clearance of LCMV from the sera and spleens of LM-GP33–immunized mice than those of naïve mice. In LM-GP33–immunized mice, virus was essentially cleared from the serum by day 5 and from the spleen by day 8 (Fig. 7c,d). In contrast, viral titers remained high in both the sera and spleens of naïve mice (Fig. 7c,d). These data clearly show that initial antigen contact triggers a cellular differentiation program in naïve CD8+ T cells that culminates in the formation of memory CD8+ T cells that persist and confer immunological protection.
Figure 7. Memory CD8+ T cells generated in antigen-limiting conditions can confer protective immunity.
Naïve CFSE-labeled P14 CD8+ T cells (2×106) were adoptively transferred into naïve B6 mice that were then infected with LM-GP33 (∼100 CFU). (a) LM-GP33–immunized mice and (b) naïve B6 mice were intravenously challenged 90 days later with LCMV clone 13 (2×106 PFU). Eight days after clone 13 infection the splenocytes were stained with anti-CD8α + Db-GP(33–41) and Db-NP(396–404). The dot-plots are gated on CD8+ T cells and the percentage of tetramers GP(33–41)- or NP(396–404)–specific CD8+ T cells is shown. (c) At days 5 and 8 after LCMV clone 13 infection viral titers were determined by plaque assay analysis of the sera of LM-GP33-immunized and naïve B6 mice. (d) LCMV viral titers in the spleen were determined by plaque assay analysis 8 days after infection.
Discussion
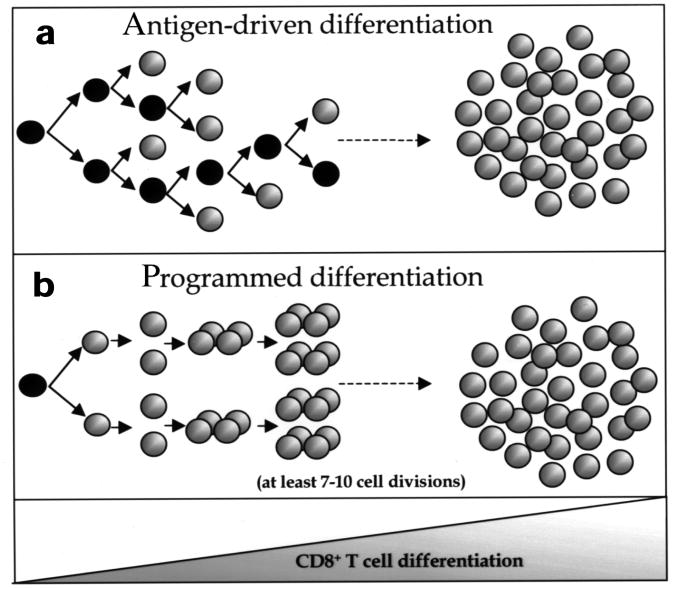
To understand the generation of any cell type it is critical to identify the signals and mechanisms that initiate and drive cells to terminally differentiate. It is well established that TCR engagement with peptide–MHC class I complexes induces CD8+ T cells to differentiate into effector and memory T cells, however, the signals or mechanisms that drive CD8+ T cells to complete this process are less well defined2. Two different mechanisms may govern memory CD8+ T cell development. It is possible that CD8+ T cells are induced to divide and progressively differentiate into effector CTLs and memory CD8+ T cells after every encounter with antigen (Fig. 8a). Based on this model, activated CD8+ T cell division and differentiation is dependent on continual antigenic stimulation and the cells would stop upon antigen removal. If the number of APCs are limiting and the cells are short-lived, then the CD8+ T cells may not divide the necessary number of times to instill phenotypic changes and may reside in partially differentiated states. Alternatively, it may be that CD8+ T cells are developmentally programmed to proliferate and differentiate into effector CTLs and memory CD8+ T cells (Fig. 8b). This developmental program is initiated by activation of the “parental” CD8+ T cell, which instruct the daughter cells to continue to divide and differentiate into effector and memory CD8+ T cells independent of further antigenic stimulation. This model predicts that if antigen is not abundant—for example, during certain vaccines and infections—then the CD8+ T cells that become activated will still be able to develop into memory CD8+ T cells.
Figure 8. Models for proliferation and differentiation of naïve CD8+ T cells.
(a) CD8+ T cell proliferation is dependent on repeated encounters with antigen. Each cell that is stimulated by antigen divides and progressively differentiates into effector CTLs and memory CD8+ T cells with each successive cell division. According to this model, it is essential that each daughter cell be stimulated with antigen, otherwise CD8+ T cell division, and possibly differentiation, would be halted upon antigen removal. (b) Naïve CD8+ T cells are developmentally programmed to divide at least seven to ten times and to differentiate into effector CTLs and long-lived functional memory CD8+ T cells. Optimal antigenic stimulation of the parental cell triggers this developmental program and the CD8+ T cells become committed to proliferation and differentiation. Further antigenic stimulation of the daughter cells may increase the number of times the activated CD8+ T cells divide, but it is unnecessary for this developmental program to progress.
The results from our experiments favor the second model. We have shown that the size of the CD8+ T cell response correlated with the dose of LM-GP33 administered, but that this was primarily due to the number of naïve CD8+ T cells recruited to the response. It was affected less by the number of times the activated CD8+ T cells divided. All the recruited cells divided at least seven times and, as the second model predicts, we found that after the initial activation of the parental CD8+ T cell, the daughter cells continued to divide and differentiate in the absence of further antigenic stimulation. A potential concern with this model is the finding that T cells absorb MHC-peptide complexes upon engagement with an APC38,39. However, it has been shown that these complexes are extremely short-lived and are degraded in the lysosomes within 3 h38,39. Therefore, the absorption of MHC-peptide complexes by the CD8+ T cells is unlikely to play a role in sustaining an antigenic signal for more than a few hours after a CD8+ T cell encounters an APC.
Our experiments show that fully activated CD8+ T cells divide a minimum of seven times. However, this should not be interpreted to mean that these cells are not capable of a greater number of cell divisions. Instead, we propose that without repeated antigen exposure, CD8+ T cells will divide a minimum of 7–10 times (a minimal developmental program) but that if antigen persisted, the extent of proliferation and the overall burst size would be greater.
The signals that induce the qualitative changes that occur during naïve→effector→memory CD8+ T cell transitions can be either intrinsic or extrinsic. Our data supports the model that CD8+ T cells rely on both intrinsic and extrinsic signals because activated CD8+ T cells can continue to divide and differentiate in the absence of sustained antigenic exposure, although optimal proliferation occurs in the presence of IL-2. Therefore, we propose that CD8+ T cells embody a “blueprint for differentiation” that relies on an initial internal stimulus (TCR signaling) coupled with external signals to be carried out.
Two recent studies support our theory that, after TCR activation, T cells can continue to proliferate and differentiate independent of further antigenic stimulation31,32. In one study CD4+T cells continued to proliferate independently of antigen in vitro and this proliferation was partly dependent on IL-231. In the other study, _L. monocytogenes_–specific CD8+ T cells continued to proliferate and differentiate after the bacterial infection was controlled with the use of ampicillin, although this study did not formally prove that antigen was eliminated from the host concomitant with antibiotic treatment32.
Our findings have an impact on several issues regarding immune responses and the formation of memory CD8+ T cells. Different models of acute viral and bacterial infections indicate that infectious pathogens are typically cleared 2–3 days before the peak of the CD8+ T cell response8,11. Until now it has been unclear why CD8+ T cell numbers continue to rise after viral clearance; now this can be explained by the ability of activated CD8+ T cells to continue dividing in the absence of antigen. The expansion that continues in the absence of antigen may be important in determining the size of the memory CD8+ T cell population because there is a clear correlation between the number of effector CTLs at the peak of the immune response and the number of memory cells generated8,11,34,35. Thus, if every CD8+ T cell division was induced by recurring antigenic stimulation, then the pool of effector CTLs would peak several days earlier, be significantly smaller and, consequently, fewer memory CD8+ T cells would be generated.
A major goal of vaccination is to generate long-lived protective memory T cells. Often vaccines rely on replication-deficient vehicles that consequently limit the number of APCs that present pathogen-derived antigens. However, our results indicate that even if the number of APCs presenting antigen are few, formation of functional memory CD8+ T cells will not be suboptimal because the CD8+ T cells that are activated will proliferate and differentiate into memory CD8+ T cells. Therefore, protective memory CD8+ T cells can be generated in the absence of prolonged antigen exposure, although the number of memory CD8+ T cells formed will be dependent on the total number of CD8+ T cells recruited into the immune response. Depending on the virulence and morbidity of the pathogen, different thresholds of memory CD8+ T cells may be needed to confer protection against disease. Our results suggest that the failure of “weak” T cell vaccines is primarily due to quantitative effects and not because of the inability to generate long-lived memory T cells. This finding would suggest that the main effect of vaccine boosters is to increase the number of antigen-specific memory T cells to one that confers better protection. Therefore, we propose that the most effective T cell vaccines will be those that produce the optimal burst of antigen expression and recruit the greatest number of antigen-specific CD8+ T cells into the response. Even if the duration of antigen expression is brief, a single vaccination could produce long-lived protective immunity.
Methods
Mice
B6 and B6.PL-Thy1a/Cy mice were from the Jackson Laboratory (Bar Harbor, ME). The P14 transgenic mice (H-2b and Thy1.2+) have been described13,24. To generate Thy1.1+ P14 transgenic mice, P14 mice were crossed onto a B6.PL-Thy1a/Cy (H-2b and Thy1.1+) background.
Bacterial and viral infection
Splenocytes from P14 transgenic mice, which contained 1×106–2×106 P14 CD8+ T cells, were adoptively transferred into normal (nonirradiated) B6 mice by intravenous injection. Mice were infected intravenously with either 100, 3×103 or 3×104 CFU of recombinant L. monocytogenes strain XFL203 that was engineered from the parental bacterial strain HSL23536 to express the GP(33–41) epitope. For secondary rechallenge experiments, mice were injected intravenously with 2×106 plaque-forming units (PFU) of LCMV clone 13. To generate Thy1.2+ memory P14 CD8+ T cells, 1×106 Thy1.2+ P14 splenocytes were adoptively transferred into naïve B6 hosts and the mice were infected intraperitoneally with 2×105 PFU of LCMV-Armstrong and killed after 6 weeks or longer. Virus titers were quantified as described37.
Cell surface, intracellular staining, flow cytometry and FACS analysis
Preparation of cells and staining has been described8.
Antibodies, H-2Db tetramers and CFSE-labeling
All the antibodies used in these experiments were from PharMingen (San Diego, CA). In particular, the CD43 antibody (clone 1B11) recognizes a specific glycosylated isoform of CD43 that is associated with T cell activation26. The construction and purification of the MHC class I LMCV tetramers Db-GP(33–41) and Db-NP(396–404) have been described8. Cells were labeled with CFSE (Molecular Probes, Eugene, OR) as described9.
Direct ex vivo CTL assays
GP(33–41)–specific CTL activity was determined by a 6 h 51Cr-release assay, as described8.
CD8+ T cell isolation and depletion
CD8+ T cells were enriched by positive selection by magnetic-activated cell sorting (MACS, Miltenyi Biotec, Germany). Briefly, single-cell suspensions of splenocytes were incubated in PBS containing 0.5% bovine serum albumin with anti-CD8α magnetic beads, according to instructions. The cells were washed and separated over charged MACS columns. The columns were washed and the flow-through was collected (CD8-depleted splenocytes). The columns were then removed from the magnet and the CD8+ splenocytes were eluted from the column. Typical purification resulted in 85–97% CD8+ cell purity.
Cell culture and IL-2 activity inhibition
For all in vitro experiments, splenocytes or T cells were cultured in RPMI 1640 supplemented with penicillin (200 μg/ml, Sigma, St Louis, MO) and streptomycin (200 μg/ml, Sigma), glutamine (4 mM, Sigma), 2-mercaptoethanol (50 μM, Sigma) and 10% fetal calf serum (HyClone Laboratories, Logan, UT). CD8+ T cells from Thy1.1+ P14 mice were isolated using MACS and labeled with CFSE. Approximately 50,000 P14 CD8+ T cells were cultured in 96-well flat-bottom plates with the indicated number of APCs. CD8-depleted splenocytes from congenic naïve B6 mice that had been incubated with 0.1 μg/ml of GP(33–41) peptide for 1 h at room temperature and then washed in PBS three times served as APCs. In addition, 1×106 CD8-depleted splenocytes from naïve B6 mice were used as feeder cells in the cultures. The cultures were incubated at 37 °C for 4 days. To show that antigen was removed from the cultures by 48 h, CFSE-labeled Thy1.2+ naïve or memory P14 CD8+ T cells were added to the cultures. Cultures were left to incubate at 37 °C for the next 2 days.
After the first 24-h period of stimulation, the activity of IL-2 was inhibited by the addition of 15 μg/ml of antibodies to purified IL-2 (clone S4B6) and IL-2Rα (CD25, clone 3C7) every 12 h over the next 3 days. The same amount of IgG2a and IgG2b isotype control antibodies were added to a separate set of cultures. To show that IL-2 was effectively blocked, the supernatants were removed and transferred to wells containing CFSE-labeled CTLL-2 cells (a gift of G. Kersh, Emory University). CTLL-2 cells are dependent on IL-2 for growth and, 3 days later, no CTLL-2 cell division was observed when cultured in supernatants containing IL-2-blocking antibodies.
Acknowledgments
We thank R. Antia, B. Evavold, G. Shadel and the Ahmed lab for helpful discussions and critical reading of this manuscript, H. Shen for the recombinant L. monocytogenes strain XFL203 and P. Mahar and K. Madhavi-Krishna for their technical assistance. Supported by National Institutes of Health grant AI30048 (to R. A.) and the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation, DRG-1570 (to S. M. K).
References
- 1.Ahmed R, Biron CA. In: Fundamental Immunology. Paul WE, editor. Lippincott-Raven Publishers; Philadelphia: 1999. pp. 1295–1333. [Google Scholar]
- 2.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 3.Bird JJ, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 5.Opferman JT, Ober BT, Ashton-Rickardt PG. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 1999;283:1745–1748. doi: 10.1126/science.283.5408.1745. [DOI] [PubMed] [Google Scholar]
- 6.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 7.Bachmann MF, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol. 1999;29:291–299. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 9.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 10.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nature Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 11.Busch DH, Pilip IM, Vijh S, Pamer EG. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 12.Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 13.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 14.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 15.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 16.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092–4101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub H, Flint SJ, Leffak IM, Groudine M, Grainger RM. The generation propagation of variegated chromosome structures. Cold Spring Harb Symp Quant Biol. 1978;42:401–407. doi: 10.1101/sqb.1978.042.01.042. [DOI] [PubMed] [Google Scholar]
- 18.Pantaleo G, et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994;370:463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- 19.Wills MR, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T- cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callan MF, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nature Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 21.Sunil-Chandra NP, Arno J, Fazakerley J, Nash AA. Lymphoproliferative disease in mice infected with murine γ herpesvirus 68. Am J Pathol. 1994;145:818–826. [PMC free article] [PubMed] [Google Scholar]
- 22.Mullbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata K, et al. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell Immunol. 1996;173:96–107. doi: 10.1006/cimm.1996.0255. [DOI] [PubMed] [Google Scholar]
- 24.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman C, Brduscha-Riem K, Blaser C, Zinkernagel RM, Pircher H. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med. 2000;191:1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matloubian M, Somasundaram T, Kolhekar SR, Selvakumar R, Ahmed R. Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. J Exp Med. 1990;172:1043–1048. doi: 10.1084/jem.172.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel RM. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med. 1997;185:1241–1251. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarozinski CC, Welsh RM. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J Exp Med. 1997;185:1629–1639. doi: 10.1084/jem.185.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jelley-Gibbs DM, Lepak NM, Yen M, Yen M, Swain SL. Two distinct stages in the transition from naive CD4 T cells to effectors, early antigen-dependent and late cytokine-driven expansion and differentiation. J Immunol. 2000;165:5017–5026. doi: 10.4049/jimmunol.165.9.5017. [DOI] [PubMed] [Google Scholar]
- 32.Mercado R, et al. Early Programming of T cell Populations Responding to Bacterial Infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 33.Biron CA. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr Opin Immunol. 1994;6:530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 34.Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 35.Vijh S, Pamer EG. Immunodominant and subdominant CTL responses to Listeria monocytogenes infection. J Immunol. 1997;158:3366–3371. [PubMed] [Google Scholar]
- 36.Shen H, et al. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang JF, et al. TCR-mediated internalization of peptide-MHC-complexes acquired by T cells. Science. 1999;286:952–954. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 39.Hwang I, et al. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–1148. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]