Immunology Taught by Humans (original) (raw)
. Author manuscript; available in PMC: 2013 Sep 4.
Abstract
After a half-century of mouse-dominated research, human immunology is making a comeback. Informed by mouse studies and powered by new techniques, human immune research is both advancing disease treatment and providing new insights into basic biology.
The field of immunology had its origins in observations about the relationship between human beings and infectious diseases, particularly the many virulent diseases such as smallpox or measles that would periodically sweep through a community killing many. From the Greek historian Thucydides—who noted that survivors of the plague of Athens were unscathed when that plague returned years later—to Jenner's close study of milkmaids—which suggested that the much milder cowpox disease seemed to confer immunity to smallpox—the study of human disease has not only improved our basic understanding of the human immune system but also led to the development of medical therapies such as attenuated vaccines, which are still one of the most effective medical interventions ever devised in terms of lives saved. In this issue of Science Translational Medicine, Clark et al. remind us of the value of coupling basic and disease-related human immunology research: The authors identify fundamental physiological characteristics of antigen-experienced (“memory”) T lymphocytes in the skin through a study of two different memory T cell malignancies (1).
Mighty Mouse?
In the last half-century or so, much of the heavy lifting in immunology research has been done by laboratory animals, especially inbred mice. The homogeneity and ease of manipulation of this “super” model of modern immunology, and indeed much of biology, have led to elegant solutions to some of the most perplexing problems in the field. Inbred mice have enlightened us about the nature of antibody diversity, how T cells “see” antigens, and the elaboration of innate immunity in mammals. One indicator of the importance of this work has been that most of the Nobel prizes in immunology for the past 30 years have been for research performed with mouse models. This dominance has led many to conclude that mice possess the only immune system worth studying.
However, mice, for all their charm and ease of use, have a number of serious flaws as a model system. For one, almost all mouse work is done on inbred strains. Inbreeding removes potentially confounding genetic variation, but the resulting lack of heterozygosity also limits the adaptability that is critical to fitness in the wild. Indeed, hybrid crops and animals are generally robust, whereas inbreeding outside of the lab is frequently a fast track to individual and familial extinction.
Another limitation of the mouse model is researchers' desire to shelter mice from any sort of disease. Disease introduces hard-to-control variables, but the immune system has evolved to handle a continuous onslaught of infectious diseases. The relative (and sometimes extreme) sterility of mouse-colony housing could have a significant impact on the development and function of the inhabitants' immune systems: The work of Virgin and colleagues has shown that the presence of endogenous viruses very similar to those found in most humans changes the immune systems of inbred mice to make them more like those of humans (2). Another limitation of mouse models, as Casanova has pointed out (3), is that many mouse models of disease are far from spontaneous and involve often-problematic manipulations that only approximate the human disease that is being modeled.
Selective Amnesia
Fortunately, modern experimental methods and the knowledge of basic immunology gleaned from inbred mouse models have made possible sophisticated studies of human immunology. The findings of Clark et al. (1) represent an excellent illustration of this phenomenon in that they not only reveal some remarkable T cell biology but also show a new and promising therapy for a life-threatening cancer.
Extensive investigation in both mice and humans has shown that the antigen-specific T cells that respond to a given antigen first become effector cells (from what is thought to be a naïve state) (Fig. 1). These effector cells divide rapidly and either help stimulate an antibody response or become cytotoxic T cells that can kill pathogen-infected cells directly. After a pathogen is cleared, many of these T cells die, but others become memory T cells (4), which respond more rapidly and in greater numbers to a recurring pathogen relative to the T cell response during the initial infection. Effective vaccines are thought to work because they stimulate memory T and B cell formation so that an infection that might have been fatal before vaccination can go unnoticed afterward because it is cleared so rapidly.
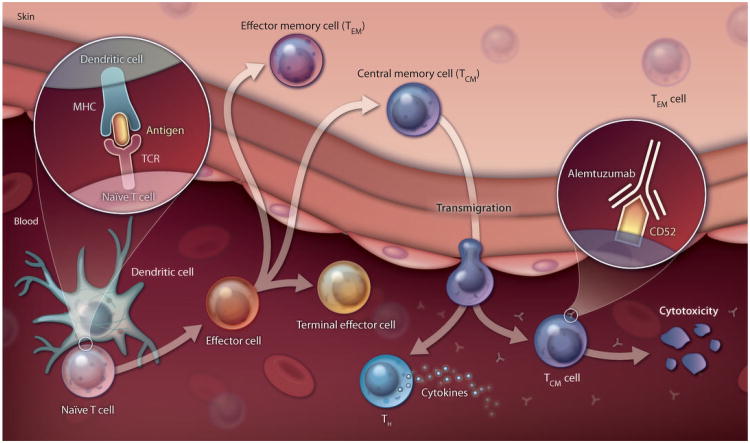
Fig. 1. Travel advisory.
TCM cells leave the skin and enter the circulation, making them susceptible to antibody annihilation. TEM cells stay in the skin and are protected from assault by alemtuzumab therapy . While the illustration shows only the cytotoxic T cell lineage, it is believed that helper T cells (TH) follow a similar course. TCR, T cell receptor; MHC, major histocompatibility complex.
Previously, Kupper and colleagues found that human skin contains about one million memory T cells per square centimeter (5). The finding that skin harbors a high density of memory T cells indicates the importance of the skin as an immunological barrier for highly specific adaptive immune responses as well as the more generic ones linked to the innate immune system. In the new study, Clark, Kupper, and colleagues investigated a type of skin cancer called leukemic-cutaneous T cell lymphoma (L-CTCL)—one form of which is Sézary syndrome. L-CTCL is an often fatal disease with a median life expectancy of 3 years after diagnosis, primarily because patients die from infectious diseases for reasons that remain unclear. The malignant T cells in the skin of L-CTCL patients have the cell surface characteristics of central memory T cells (TCM cells), which are the classical type of memory T cells that persist in the absence of ongoing infection. TCM cells are thought to be the most important of memory T cell subsets in terms of defending against viral and bacterial infections (6). In contrast, a much less malignant form of skin cancer, mycosis fungoides, is caused by the oncogenic transformation of another subset of memory T cells, the effector memory T cells (TEM cells).
Memory T cells circulate in the blood but are also widely distributed in specific tissues, with TCM cells generally thought to be more important in many responses. Clark et al. showed that alemtuzumab, an antibody to CD52 that binds to all circulating B and T lymphocytes and removes them from circulation, also clears (on a somewhat longer time scale) L-CTCL T cells from the skin in 100% of patients. This observation suggests that skin resident TCM cells do not remain in the skin but instead recirculate in the body, making them vulnerable to alemtuzumab treatment. However, TEM cells may not recirculate: Clark et al. found that alemtuzumab treatment left TEM cells in the skin untouched and that mycosis fungoides, the TEM cell-related skin malignancy, was not affected by alemtuzumab therapy.
Remarkably, patients who received alemtuzumab therapy did not appear to be susceptible to common infections, suggesting that the skin resident TEM cells that remain represent an effective barrier to at least some pathogens. It is also probable that TEM cells may be retained in other tissues that are directly exposed to pathogens, such as the lungs or the gastrointestinal tract, as the authors point out. The one patient in this study who did have a pulmonary infection had undergone repeated irradiation of the lungs, which is likely to have depleted lung resident TEM cells. Taken together, these data suggest that TEM cells have been underappreciated as a first line of defense against recurring infections and that the immunology of human skin is more complex than previously thought. The study by Clark et al. represents a real translational tour de force by showing how cancer treatments can be developed by understanding the circulatory habits of host immune cells.
A Human Revival?
This study may be a harbinger of things to come for the immunologists who have come to expect that most new knowledge will arise from mouse models. Although studies in humans have been limited by ethical and technical strictures, a wealth of data can be gleaned by observation and from interventional trials. New technologies, such as increasingly sophisticated cell labeling and phenotypic and functional analyses (7) as well as the hundreds of characterized cell surface markers, are starting to make increasingly sophisticated analyses of clinical materials possible. For example, using systems biology approaches (8) that integrate many data sets to characterize disease and vaccine responses in humans, Pulendran and colleagues found that expression of certain genes correlates with a robust immune response to the yellow fever vaccine (9). Furthermore, the work of Casanova and colleagues (10) has shown that children with a deficiency in the MyD88 gene have a much more narrowly restricted and transient defect in resisting pathogens than inbred mice with a similar lesion. The presence of alternative mechanisms in humans that are lacking in mice is consistent with the much greater evolutionary investment in an individual human compared with a mouse, both in generation time and numbers, and suggests that there are additional immunological mechanisms to discover in Homo sapiens. These and other ongoing studies suggest that a resurgence in human immunology is under way and will be well worth watching.
Acknowledgments
I thank R. M. Zinkernagel for inspiring the title [Immunology taught by viruses. Science 271, 173–178 (1996)] and the Howard Hughes Medical Institute, NIH, and the Bill and Melinda Gates Foundation for support.
References and Notes
- 1.Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, LeBoeuf NR, Carter JB, Fisher DC, Kupper TS. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra7. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: Natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 5.Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: Mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Bendall SC, Simonds EF, Qiu P, Amir ED, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe'er D, Tanner SD, Nolan GP. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MM. A prescription for human immunology. Immunity. 2008;29:835–838. doi: 10.1016/j.immuni.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Aróstegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yagüe J, Antón J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Maródi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]