Autoimmune disease preceding amyotrophic lateral sclerosis: An epidemiologic study (original) (raw)
Abstract
Objective:
To study whether the risk of amyotrophic lateral sclerosis (ALS) is increased in people with prior autoimmune disease.
Methods:
An all-England hospital record-linkage dataset spanning 1999–2011 was used. Cohorts were constructed of people with each of a range of autoimmune diseases; the incidence of ALS in each disease cohort was compared with the incidence of ALS in a cohort of individuals without prior admission for the autoimmune disease.
Results:
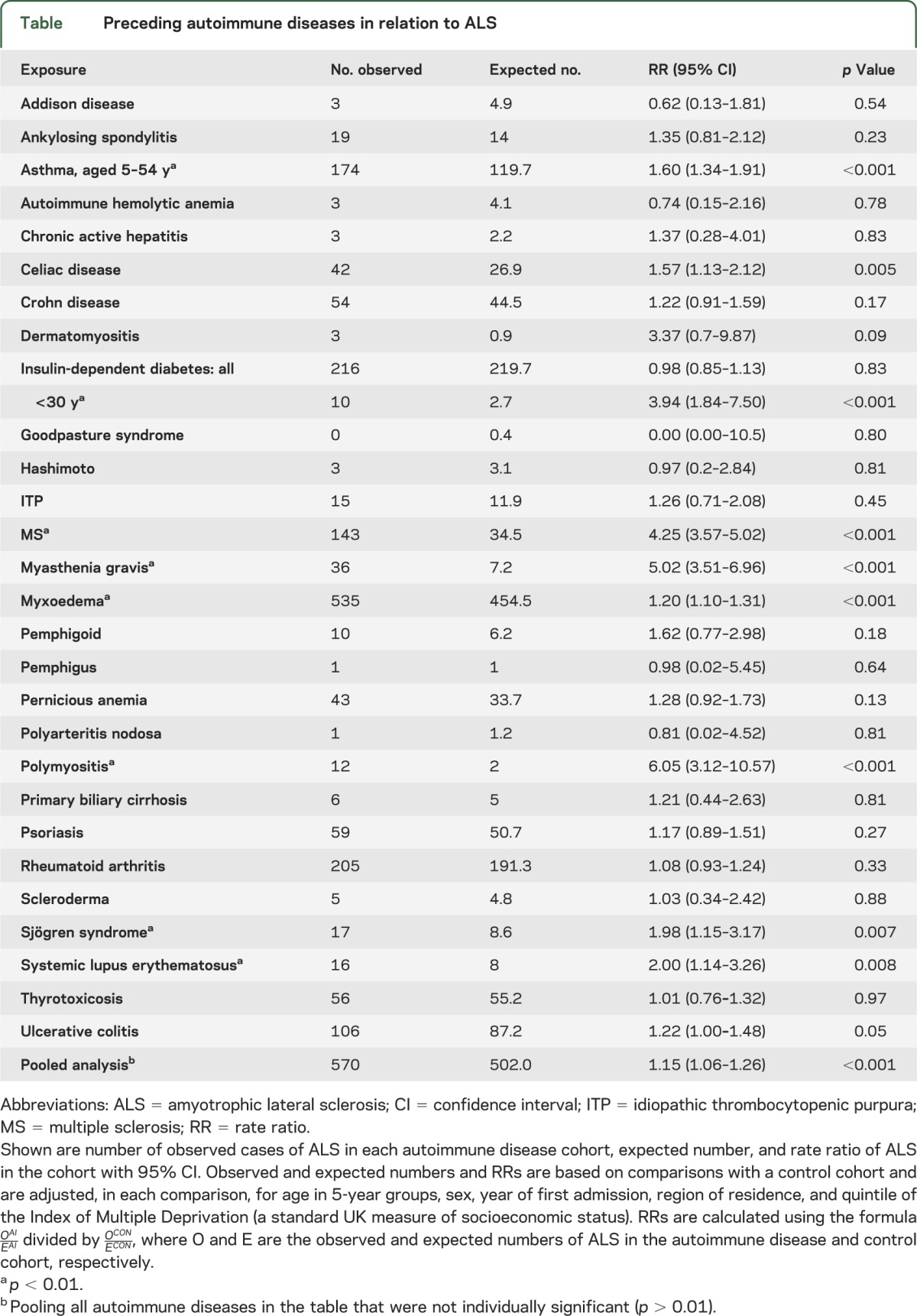
There were significantly more cases than expected of ALS associated with a prior diagnosis of asthma, celiac disease, younger-onset diabetes (younger than 30 years), multiple sclerosis, myasthenia gravis, myxedema, polymyositis, Sjögren syndrome, systemic lupus erythematosus, and ulcerative colitis.
Conclusions:
Autoimmune disease associations with ALS raise the possibility of shared genetic or environmental risk factors.
Understanding the risk factors for neurodegenerative disorders, including any pathogenic pathways shared with other diseases, will improve the chances of effective therapeutic development. Amyotrophic lateral sclerosis (ALS) is among the most rapidly progressive forms of neurodegenerative disease, involving a heterogeneous mixture of upper and lower motor neuron loss in conjunction with involvement of extramotor pathways that have clinicopathologic overlap with frontotemporal dementia (FTD). Median survival is 3 to 5 years from symptom onset and 20% survive more than 5 years. The disorder is apparently sporadic in 95% of patients.1
There is a perception that patients with ALS might be premorbidly “fitter” than the general population, with higher rates among varsity athletes2 and professional soccer players,3 although there are many potential confounders relating to increased leisure-time physical activity,4 lower body mass index,5 and reduced cardiovascular risk,6,7 all noted among those with ALS. There have been few studies of comorbidities among patients with ALS. One case-control study showed no significant difference in the frequency of a range of broad disease categories (including endocrine).8 In a cohort of 514 patients with ALS,9 the prevalence of cardiovascular diseases and cardiovascular risk factors was significantly lower than their prevalence in the general population, confirming previously published findings (discussed in reference 10). Autoimmunity in the pathogenesis of ALS, or perhaps as a mediator of heterogeneity, has long been postulated.11 There are no clear human lymphocyte antigen associations.12
METHODS
An all-England national record-linkage dataset of Hospital Episode Statistics and mortality data (1999–2011)13 was used to undertake studies of cohorts of people with each of a range of autoimmune disorders, compared with a control cohort without these diseases, to determine the risk of subsequent ALS in each cohort. A similar analysis was undertaken on a regional record-linkage dataset, the Oxford record-linkage study, using its data from an earlier period (1963–1998).14 Details of the methods are given elsewhere.13,14
RESULTS
Observed and expected numbers and rate ratios comparing ALS in each autoimmune group with the control cohort are shown in the table. The rate ratios were significantly high (p < 0.01) for ALS associated with a prior diagnosis—at least 1 year before ALS—of asthma, celiac disease, multiple sclerosis (MS), myasthenia gravis, myxoedema, polymyositis, Sjögren syndrome, and systemic lupus erythematosus. The rate ratios were high for each of these for both males and females although not invariably significantly so (subdivision by sex lowered statistical power). The rate ratio for people with a coded diagnosis of insulin-dependent diabetes was not increased considering people of all ages; however, it was increased for people with a first recorded diagnosis of diabetes aged less than 30 years. The rate ratio for ulcerative colitis was borderline significant (p = 0.05). In a pooled analysis combining all the diseases in the table that were not individually significant (p < 0.01), the rate ratio was significantly but modestly elevated. Numbers in the Oxford record-linkage study were much smaller than all-England but there were 2 conditions with significantly high rate ratios: that for MS was 10.9 (95% confidence interval 5.4–19.8, based on 11 observed cases) and for myxoedema was 3.1 (95% confidence interval 1.1–6.7, based on 6 observed cases).
Table.
Preceding autoimmune diseases in relation to ALS
DISCUSSION
A previous case-control study found no significant excess of antecedent diseases, including diabetes and thyroid disease.15 The hospital record-linkage approach has the advantage of large numbers and removal of recall bias that may be a factor in some case-control studies. It is, however, dependent on accurate coding. We suggest that the apparent association of inflammatory muscle disease and myasthenia gravis with ALS is most likely to represent an artifact of diagnostic mimicry. It is also recognized that our study only includes patients for whom hospital admission was required, which may be a source of unmeasurable bias (e.g., younger-onset diabetes).
An association between ALS and MS has been previously suggested. A familial study separately using index cases of ALS and MS reported increased association,16 and a case series of coincident MS in cases of ALS was associated with expansions of the C9orf72 GGGGCC repeat.17 A population-based study of 810 ALS cases observed only 2 with a prior diagnosis of MS, which was believed to be no more than expected by chance, and neither had a C9orf72 GGGGCC repeat expansion.18 This latter result would suggest that our findings in relation to MS and ALS may also be confounded by misdiagnosis.
Another hospital record-linkage study considered autoimmune diseases among relatives and spouses of ALS cases. An association was found for MS, but also Behçet disease, ulcerative colitis, and Wegener granulomatosis in the offspring of patients with ALS.19 However, the basis of these observations in relation to shared pathogenesis remains unclear. Immunologic intervention for ALS, including bone marrow transplant,20 has so far not been effective, despite a large body of evidence linking neuroinflammatory processes to pathogenesis in ALS, in particular oligodendrocyte dysfunction.21
In a descriptive study of 1,200 patients with ALS,22 comorbid diabetes (type unspecified) was reported by 9%, and was the most frequent (42%) among diseases reported in first- and second-order relatives, followed by arthritis (unspecified) 35%, allergies (unspecified) 19%, thyroid disease (unspecified) 17%, Alzheimer disease 12%, Parkinson disease 8%, collagen vascular diseases (unspecified) 3%, demyelinating disease (unspecified) 4%, and myasthenia gravis <1%. The issue of recall bias and lack of a control group make these data difficult to interpret. However, the alterations in energy metabolism consistently observed in patients with ALS23 may have overlap with the wider concept of insulin resistance.
The observed number of ALS cases in the thyrotoxicosis cohort was very similar to the expected number, but, as noted above, there was an excess of ALS in the myxoedema cohort. Two large retrospective studies observed that approximately one-fifth of patients with ALS reported a personal or family history of thyroid disease, or had biochemical evidence of such.22,24 A case-control study demonstrated a significantly increased incidence of hereditary thyroid disease in the relatives of patients with ALS, with a reported odds ratio for thyroid disease among patients ranging from 1.5 to 2.3 and for another autoimmune disease, pernicious anemia, the ratio was higher at 3.0 to 3.6.25
ALS and some forms of FTD are unified by the presence of cytoplasmic inclusions of the transactive response DNA-binding protein 43 (TDP-43). A case-control study of the prevalence of autoimmune diseases across the phenotypic range of TDP-43–associated FTD revealed significant associations among those with semantic variant primary progressive aphasia, and familial cases linked to PRGN mutations. Although small numbers of patients were involved, several of the autoimmune diseases we describe in association with ALS were specifically noted, including diabetes, celiac disease, Sjögren syndrome, systemic lupus erythematosus, thyroid disease, and colitis.26
Finally, multifocal motor neuropathy (MMN) with conduction block is a rare autoimmune disorder that may mimic ALS in its initial presentation, but it does not have similar progression or obvious CNS pathology. However, a case-control study in MMN showed that type 1 diabetes, Hashimoto thyroid disease, and celiac disease were all significantly more prevalent in family members of patients.27 We have previously reported a case of progressive weakness without sensory involvement and striking corticospinal tract MRI changes in the context of celiac disease, which improved with dietary exclusion of gluten.28 The significance of increased premorbid celiac disease in those with ALS, and in family members of patients with MMN remains unclear at present.
Evidence is presented that several preexisting autoimmune disorders are associated with a small increased risk of ALS. The pathogenesis of neurodegenerative disorders more widely seems likely to involve multiple susceptibilities, stochastic events, and environmental triggers. These disease associations raise the possibility of shared genetic or environmental risk factors, or clues to modifiable triggers that might thereby reduce the incidence of ALS.
GLOSSARY
ALS
amyotrophic lateral sclerosis
FTD
frontotemporal dementia
MMN
multifocal motor neuropathy
MS
multiple sclerosis
TDP-43
transactive response DNA-binding protein 43
AUTHOR CONTRIBUTIONS
M.R.T. planned the study and drafted the manuscript. R.G. undertook the analysis and edited the manuscript. S.R. and K.T. edited the manuscript. M.J.G. planned and designed the study and edited the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
M. Turner is funded by the Medical Research Council/Motor Neurone Disease Association Lady Edith Wolfson Fellowship. The Oxford Motor Neuron Disease Care & Research Centre receives funding from the Motor Neurone Disease Association UK Care Centre Program. R. Goldacre and S. Ramagopalan report no disclosures. K. Talbot: the Oxford Motor Neuron Disease Care & Research Centre receives funding from the Motor Neurone Disease Association UK Care Centre. M. Goldacre: the Unit of Health-Care Epidemiology, University of Oxford, was funded by the National Institute for Health Research to undertake the record-linkage studies (RNC/035/002). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet 2011;377:942–955 [DOI] [PubMed] [Google Scholar]
- 2.Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Premorbid weight, body mass, and varsity athletics in ALS. Neurology 2002;59:773–775 [DOI] [PubMed] [Google Scholar]
- 3.Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain 2005;128(pt 3):472–476 [DOI] [PubMed] [Google Scholar]
- 4.Huisman MH, Seelen M, De Jong SW, et al. Lifetime physical activity and the risk of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry Epub 2013 Feb 16. [DOI] [PubMed]
- 5.Gallo V, Wark PA, Jenab M, et al. Prediagnostic body fat and risk of death from amyotrophic lateral sclerosis: the EPIC cohort. Neurology 2013;80:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner MR, Wotton C, Talbot K, Goldacre MJ. Cardiovascular fitness as a risk factor for amyotrophic lateral sclerosis: indirect evidence from record linkage study. J Neurol Neurosurg Psychiatry 2012;83:395–398 [DOI] [PubMed] [Google Scholar]
- 7.Sutedja NA, van der Schouw YT, Fischer K, et al. Beneficial vascular risk profile is associated with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2011;82:638–642 [DOI] [PubMed] [Google Scholar]
- 8.Kurtzke JF, Beebe GW. Epidemiology of amyotrophic lateral sclerosis: 1. A case-control comparison based on ALS deaths. Neurology 1980;30:453–462 [DOI] [PubMed] [Google Scholar]
- 9.Koerner S, Kollewe K, Ilsemann J, et al. Prevalence and prognostic impact of comorbidities in amyotrophic lateral sclerosis. Eur J Neurol 2013;20:647–654 [DOI] [PubMed] [Google Scholar]
- 10.Turner MR. Increased premorbid physical activity and amyotrophic lateral sclerosis: born to run rather than run to death, or a seductive myth? J Neurol Neurosurg Psychiatry Epub 2013 Feb 27. [DOI] [PubMed]
- 11.Appel SH, Smith RG, Engelhardt JI, Stefani E. Evidence for autoimmunity in amyotrophic lateral sclerosis. J Neurol Sci 1993;118:169–174 [DOI] [PubMed] [Google Scholar]
- 12.Bartfeld H, Pollack MS, Cunningham-Rundles S, Donnenfeld H. HLA frequencies in amyotrophic lateral sclerosis. Arch Neurol 1982;39:270–271 [DOI] [PubMed] [Google Scholar]
- 13.Seminog OO, Goldacre MJ. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax 2013;68:171–176 [DOI] [PubMed] [Google Scholar]
- 14.Fois AF, Wotton CJ, Yeates D, Turner MR, Goldacre MJ. Cancer in patients with motor neuron disease, multiple sclerosis and Parkinson's disease: record linkage studies. J Neurol Neurosurg Psychiatry 2010;81:215–221 [DOI] [PubMed] [Google Scholar]
- 15.Armon C, Kurland LT, O'Brien PC, Mulder DW. Antecedent medical diseases in patients with amyotrophic lateral sclerosis: a population-based case-controlled study in Rochester, Minn, 1925 through 1987. Arch Neurol 1991;48:283–286 [DOI] [PubMed] [Google Scholar]
- 16.Etemadifar M, Abtahi SH, Akbari M, Maghzi AH. Multiple sclerosis and amyotrophic lateral sclerosis: is there a link? Mult Scler 2012;18:902–904 [DOI] [PubMed] [Google Scholar]
- 17.Ismail A, Cooper-Knock J, Highley JR, et al. Concurrence of multiple sclerosis and amyotrophic lateral sclerosis in patients with hexanucleotide repeat expansions of C9ORF72. J Neurol Neurosurg Psychiatry 2013;84:79–87 [DOI] [PubMed] [Google Scholar]
- 18.van Doormaal PT, Gallo A, van Rheenen W, Veldink JH, van Es MA, van den Berg LH. Amyotrophic lateral sclerosis is not linked to multiple sclerosis in a population based study. J Neurol Neurosurg Psychiatry 2013;84:940–941 [DOI] [PubMed] [Google Scholar]
- 19.Hemminki K, Li X, Sundquist J, Sundquist K. Familial risks for amyotrophic lateral sclerosis and autoimmune diseases. Neurogenetics 2009;10:111–116 [DOI] [PubMed] [Google Scholar]
- 20.Appel SH, Engelhardt JI, Henkel JS, et al. Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology 2008;71:1326–1334 [DOI] [PubMed] [Google Scholar]
- 21.Philips T, Bento-Abreu A, Nonneman A, et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain 2013;136(pt 2):471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population: validation of a scoring system and a model for survival prediction. Brain 1995;118(pt 3):707–719 [DOI] [PubMed] [Google Scholar]
- 23.Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol 2011;10:75–82 [DOI] [PubMed] [Google Scholar]
- 24.Appel SH, Stockton-Appel V, Stewart SS, Kerman RH. Amyotrophic lateral sclerosis: associated clinical disorders and immunological evaluations. Arch Neurol 1986;43:234–238 [DOI] [PubMed] [Google Scholar]
- 25.Gunnarsson LG, Bodin L, Soderfeldt B, Axelson O. A case-control study of motor neurone disease: its relation to heritability, and occupational exposures, particularly to solvents. Br J Ind Med 1992;49:791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller ZA, Rankin KP, Graff-Radford NR, et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. J Neurol Neurosurg Psychiatry Epub 2013 Mar 30. [DOI] [PMC free article] [PubMed]
- 27.Cats EA, Bertens AS, Veldink JH, van den Berg LH, van der Pol WL. Associated autoimmune diseases in patients with multifocal motor neuropathy and their family members. J Neurol 2012;259:1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner MR, Chohan G, Quaghebeur G, Greenhall RC, Hadjivassiliou M, Talbot K. A case of celiac disease mimicking amyotrophic lateral sclerosis. Nat Clin Pract Neurol 2007;3:581–584 [DOI] [PubMed] [Google Scholar]