Chemokine responsiveness of CD4+ CD25+ regulatory and CD4+ CD25− T cells from atopic and nonatopic donors (original) (raw)
. Author manuscript; available in PMC: 2013 Oct 25.
Published in final edited form as: Allergy. 2009 Feb 7;64(8):10.1111/j.1398-9995.2008.01962.x. doi: 10.1111/j.1398-9995.2008.01962.x
Abstract
Background
Allergic inflammation is associated with Th2-type T cells, which can be suppressed by CD4+ CD25+ regulatory T cells (Tregs). Both express chemokine receptors (CCR) 4 and CCR8, but the dynamics of expression and effect of atopic status are unknown.
Objective
To examine the expression of chemokine receptors by CD4+ CD25+ and CD4+ CD25− T cells from atopic and nonatopic donors, and document response to allergen stimulation in vitro.
Methods
Chemokine receptor expression was examined by flow cytometry and quantitative PCR of CD4+ CD25hi and CD4+ CD25− T cells from atopics and nonatopics. Responsiveness to chemokines was by actin polymerization. Dynamics of chemokine receptor expression in 6-day allergen-stimulated cultures was analysed by carboxyfluoroscein succinimidyl ester labelling.
Results
CD4+ CD25hi Tregs preferentially expressed CCR3, CCR4, CCR5, CCR6 and CCR8. CD4+ CD25hi Tregs responded to the chemokine ligands for CCR4, CCR6 and CCR8 (CCL17, 22, 20 and 1 respectively), with no differences between atopic and nonatopic donors. Over 6-day allergen stimulation, CD4+ CD25+ T-cells downregulated CCR4 and upregulated CCR7, in contrast to CD4+ CD25− effector cells, which downregulated CCR7 and upregulated CCR4.
Conclusions
CCR4, CCR6 and CCR8 have potential roles in localization of both CD4+ CD25+ regulatory and CD4+ CD25− effector T cells to sites of allergic inflammation. Upregulation of CCR7 and downregulation of CCR4 upon allergen stimulation of Tregs may allow their recirculation from sites of inflammation, in contrast to retention of effector T cells.
Keywords: allergic inflammation, atopy, chemokine receptors, chemokines, regulatory T cells
Allergic inflammation is characterized by the presence of T cells expressing a T helper 2 (Th2)-type pattern of cytokine expression (1). Chemokines are thought to be important in the recruitment and retention of specific T-cell subsets to sites of inflammation (2). Chemokine receptor expression by T cells is flexible and differs on naïve, memory and effector T cells (3), depending on their activation status. This is thought to determine homing to secondary lymphoid organs, different tissues and sites of inflammation, together with the interaction with particular antigen presenting cell types, including dendritic cell and B cells. In particular, Th2 lines or clones were shown to express preferentially CCR3 (4, 5), CCR4 (5) and CCR8 (6, 7) when compared to Th1-polarized T cells. This was born out by studies showing CCR3 and CCR4 expression by IL-4-producing airway T cells in asthmatics (8), and we have previously reported expression of CCR4 by allergen specific cells from the airway of atopic children (9). In addition, expression of CCR4 and CCR8 by T-cells infiltrating asthmatic airways was reported after allergen challenge (10, 11) together with the expression of the CCR4 ligands CCL17 and CCL22 (12).
Recently, attention has focused on regulatory T-cell populations, in particular CD4+ CD25+ T cells, which can suppress activation of effector cells in vitro and prevent a variety of inflammatory pathology in in vivo mouse models. We and others have previously reported relative deficiency in suppression of allergen-stimulated T-cell responses in vitro by peripheral blood CD4+ CD25+ T cell from atopic individuals when compared to cells from those without allergic sensitization (13). In addition, CD4+ CD25+ T cells could prevent eosinophilic inflammation and airway hyper-responsiveness in a mouse model of allergen challenge (14). Several reports have described expression of chemokine receptors by CD4+ CD25+ regulatory T cells (Tregs) and their responsiveness to chemokines (15). These Tregs have been described to express a wide variety of chemokine receptors with preferential expression of CCR4 (16–18), CCR5 (19), CCR6 (20) and CCR8 (16, 17) compared with CD4+ CD25− effector T cells. CCR4 has been implicated in homing of CD4+ CD25+ T cells to skin (21), and CCR8 has also been described as functional in homing of human CD4+ CD25+ T cells from peripheral blood and thymus (16, 17, 22).
The role of chemokines in recruitment and retention of CD4+ CD25+ T cells to sites of allergic inflammation is unknown. One hypothesis for Th2 cell infiltration in allergic inflammation would be a deficiency in chemokine receptor expression by Tregs, leading to a failure of recruitment and thus reduced suppression in the tissues. Here, we examined chemokine receptor expression and chemokine responsiveness by peripheral blood CD4+ CD25+ and CD4+ CD25− T cells from atopic and nonatopic donors and determined changes in receptor expression during the course of in vitro culture with allergen.
Methods
Participants
We recruited nonatopic and atopic volunteers by advertisement and from staff of the National Heart and Lung Institute, Imperial College London. All volunteers completed a standard questionnaire about allergic symptoms and, to establish presence or absence of atopy, had skin prick tests to common aeroallergens, such as cat, house dust mite, grass pollen and aspergillus (ALK, Horsham, Denmark). A positive skin prick test was defined as 3 mm or larger diameter than that elicited by the negative control. Atopic donors were defined on the basis of a positive skin prick test to at least one aeroallergen and a history of allergic symptoms, whereas nonatopic donors had no history of allergic symptoms and negative skin prick tests. All studies were approved by the Ethics Committee of the Royal Brompton and Harefield Hospitals NHS Trust and all volunteers gave written informed consent.
Reagents and antibodies
The following flow cytometry antibodies and their matched isotype controls were used in this study: anti-human CCR1-PE, CCR2-APC, CCR3-PE, CCR4-APC, CCR5-PE, CCR6-APC, CCR7-PE, CCR8-APC, CXCR1-PE, CXCR2-PE, CXCR4-APC, CXCR6-APC (all from R&D Systems, Abingdon, UK), CD4-FITC, CD4-APC, CD25-PE, CD25-PECy5, CD127-PE (all from BD Pharmingen, Cowley, UK), CD3-TexasRed (Caltag Medsystems, Buckingham, UK) and FoxP3-FITC (Ebiosciences, San Diego, CA, USA). cDNA isolated from Th1 and Th2 polarized cell lines was a kind gift from Dr David Cousins, Kings College London (23).
Cell isolation
CD4+, CD4−, CD4+ CD25+ and CD4+ CD25− T cells were isolated from peripheral blood by a combination of density-gradient centrifugation and positive and negative immunomagnetic bead selection using MACS (Miltenyi Biotech, Bisley, UK), as previously described (13). CD4+ T isolation provided 94–97% purity, and CD4+ CD25+ T cells had a purity of 70–90%.
Flow cytometry
CD4+ T cells were washed with ice cold staining buffer and incubated with the indicated monoclonal antibodies or matched isotype controls for 30 min. Cells were then washed twice using ice-cold staining buffer. Samples were analysed using a FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA, USA) and analysed with cellquest software (BD Biosciences, San Jose, CA, USA). Lymphocytes were gated using forward scatter vs side scatter, CD25+ cells were gated using the appropriate isotype control and CD25high cells were gated to include only the high CD25− expressing population (approximately 2% of CD4+ T cells), as described previously (24).
Intracellular actin polymerization
The responses of CD4+ T cells to chemokine stimulation were measured using an intracellular actin polymerization assay as previously described (25). Briefly, freshly isolated CD4+ T cells were labelled with CD25-PE and CD4-APC as described above. Cells were incubated for 10 min at 37°C in RPMI 1640 at a concentration of 5 × 106 cells/ml. Then, chemokine at 100 ng/ml was added to the cell suspension, and every 15 s 0.5 × 106 cells were removed and mixed with 400 μl of 10−7 M FITC-phalloidin, 0.125 mg/ml l-α-lysophosphatidylcholine and 4.0% paraformaldehyde in phosphate buffer saline (PBS). Cells were incubated for 10 min at 37°C, washed twice in PBS and then analysed by flow cytometry. The responses to CCL1, CCL5, CCL11, CCL17, CCL20, CCL21, CCL22 and CXCL12 (all purchased from Peprotech Inc, Rocky Hill, NJ, USA) were measured using this system.
RNA isolation and Taqman RT-PCR
RNA was extracted from CD4+ CD25+ and CD4+ CD25− cells using the Absolutely RNA RT-PCR Miniprep kit from Stratagene (La Jolla, CA, USA) according to the manufacturer’s instructions. cDNA was generated from extracted RNA using the High Capacity cDNA Archive Kit from Applied Biosystems (Foster City, CA, USA). A total of 25 μl PCR reactions were performed in triplicate with Taqman universal PCR mastermix (Applied Biosystems). FOXP3, CCR8, T-bet and GATA-3 specific primers were purchased from Applied Biosystems. Samples were standardized to 18s (Applied Biosystems) and quantified using the comparative threshold for detection method (http://appliedbiosystems.com).
Allergen-driven cell culture
The CD4+ CD25− T-cell population was labelled with the cellmembrane dye carboxyfluoroscein succinimidyl ester (CFSE) and washed extensively prior to cell culture. Cells were cultured at a density of 2 × 106/ml in triplicates in 96-well flat bottomed plates in RPMI 1640, supplemented with penicillin and streptomycin and 5% human AB serum (Sigma, Poole, UK) with 100 μg/ml cat or grass pollen allergen extracts (depending on which elicited a positive skin prick test response in the donors) for 6 days. Cultures were of CFSE-labelled CD4+ CD25− cells with or without added CD4+ CD25+ cells in a ratio of 2 : 1, as previously described (13). In all cultures of isolated T cells, irradiated CD4− cells were added as antigen-presenting cells (APC) (2 × 105 cells/well). On days 2, 4 and 6 of the cell culture period, the cells from six wells were removed and pooled prior to CD4+ T-cell isolation using MACS. This second CD4+ cell isolation was performed to remove the CD4− cell population used as APCs during cell culture, leaving a pure CD4+ T-cell population for further analysis using flow cytometry. Cell populations were gated using forward scatter and side scatter in addition to CFSE expression. CD4+ CD25− proliferating cells were gated using their increased forward and side scatter coupled to being CFSE low. Nonproliferating CD4+ CD25− cells were gated on being CFSE high, and CD4+ CD25+ cells were gated using their normal forward scatter vs side scatter profile coupled to being CFSE low. Flow cytometry plots from these experiments are shown in Fig. S1.
Statistical analysis
Statistical analysis of chemokine receptor expression and chemokine responsiveness of different cell types was performed using a Wilcoxon-matched pairs test. Statistical comparison between patient groups was performed using a Mann–Whitney _U_-test. Statistical analysis of Taqman PCR experiments was performed on ΔCt values.
Results
Chemokine receptor expression by CD4+ CD25hi T cells compared to CD4+ CD25− T cells
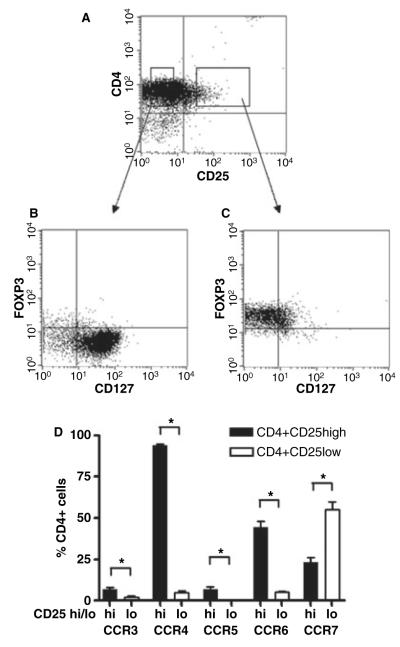
Previous reports have suggested preferential expression of certain chemokine receptors by human peripheral blood CD4+ CD25+ T cells. Regulatory cells within this population may be identified by high expression of CD25, expression of the transcription factor FoxP3 and lack of expression of the IL-7R alpha chain or CD127 (26, 27). We therefore gated on CD4+ CD25hi T cells (Fig. 1A) and initially confirmed that these cells were FoxP3+ and CD127lo, in contrast to their CD4+ CD25− T cell counterparts (Fig. 1B,C). We then examined chemokine receptor expression by the CD4+ CD25hi T-cell population and demonstrated preferential expression of CCR3, CCR4, CCR5 and CCR6 with reduced expression of CCR7 when compared to CD4+ CD25− T cells (Fig. 1D).
Figure 1.
Differential chemokine receptor expression on CD4+ CD25hi and CD4+ CD25− T cells. Chemokine receptor expression by CD4+ CD25hi, FoxP3+, CD127lo and CD4+ CD25− human peripheral blood T cells. Gated CD4+ CD25hi T cells were confirmed to be FoxP3+ and CD127−, whilst CD4+ CD25− T cells were FoxP3− and CD127+ (A–C). These gates were then used to determine chemokine receptor expression by CD4+ CD25hi (black bars) and CD4+ CD25− T cells (white bars) using flow cytometry. Results shown are from n = 7 donors. *P < 0.05. Chemokine receptor expression is shown as percent positive for CD4+ CD25hi and CD4+ CD25lo T cells (D).
Chemokine responsiveness of CD4+ CD25hi T cells does not differ between atopic and nonatopic donors
To compare chemokine responsiveness of CD4+ T-cell subsets from atopic and nonatopic donors, we used an actin polymerization assay (25). This revealed preferential responses of CD4+ CD25hi Tregs to CCL17, CCL22 and CCL20, with no differences between cells from atopic and nonatopic donors (Fig. 2 and Table SI). Responses to CCL5 were low and variable, but the trend was for greater responses from CD4+ CD25hi T cells than from CD4+ CD25− T cells, and this did not differ between atopic and nonatopic donors (Table SI). No responses to CCL11 were detected for any of the cell types tested.
Figure 2.
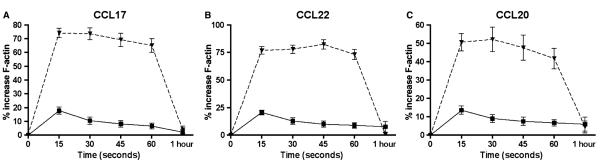
Chemokine responsiveness of CD4+ CD25+, CD4+ CD25− and CD4+ CD25high T cells. The percentage increase in actin polymerization over baseline was measured for chemokine-stimulated CD4+ T cells at 15-s intervals up to 1 min. The chemokine responsiveness of CD4+ CD25− (solid line), CD4+ CD25+ (dashed line) and CD4+ CD25high T cells (dotted line) to the chemokines CCL17 (A), CCL22 (B) and CCL20 (C) is shown for n = 13 donors.
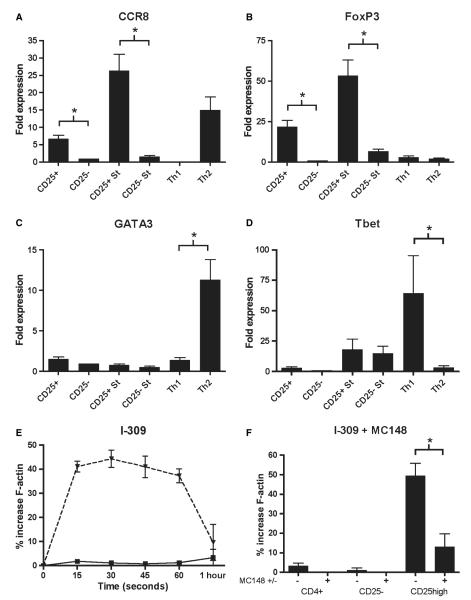
CCL1 acts on CD4+ CD25+ T cells
In contrast to previous reports, we could not detect CCR8 expression by peripheral blood CD4+ CD25+ T cells from either atopic or nonatopic donors using monoclonal antibodies (16, 17) (Table 1). Quantitative PCR did however detect mRNA for CCR8 from resting and activated CD4+ CD25+ T cells, as well as polarized Th2 T-cell lines (Fig. 3A). In addition, we confirmed preferential expression of FoxP3 by Tregs, GATA3 by Th2 cells and T-bet by Th1 cells in this system (Fig. 3B–D). Using the actin polymerization assay, we consistently detected preferential responsiveness of CD4+ CD25hi T cell from both atopic and nonatopics to CCL1, and this was blocked by MC148 (Fig. 3E,F).
Table 1.
Chemokine receptor expression on CD4+ CD25+ and CD4+ CD25− T cells from atopic and nonatopic donors
| CD25+/− | Atopic(median and range %) | Nonatopic(median and range %) | |
|---|---|---|---|
| CCR1 | + | < 2 (0.0–0.6) | < 2 (0.0–0.4) |
| − | < 2 (0.0–0.7) | < 2 (0.0–0.3) | |
| CCR2 | + | < 2 (0.0–8.8) | < 2 (0.0–5.0) |
| − | < 2 (0.0–3.4) | < 2 (0.0–3.9) | |
| CCR3 | + | 4.82 (0.8–10.6) | 5.50 (2.8–9.1) |
| − | 4.01 (0.4–9.3) | 3.85 (1.6–6.7) | |
| CCR4 | + | 51.38 (28.0–67.4) | 50.40 (35.4–63.8) |
| − | 12.48 (5.5–25.3) | 14.43 (9.5–22.0) | |
| CCR5 | + | 3.06 (0.0–9.6) | < 2 (0.3–4.3) |
| − | < 2 (0.0–3.1) | < 2 (0.0–4.9) | |
| CCR6 | + | 31.71 (12.1–45.7) | 26.96 (18.9–41.5) |
| − | 12.61 (3.3–25.9) | 11.52 (5.5–21.9) | |
| CCR7 | + | 36.54 (15.2–62.3) | 35.26 (17.1–66.1) |
| − | 68.44 (46.1–83.7) | 63.73 (35.7–90.1) | |
| CCR8 | + | < 2 (0.0–2.1) | < 2 (0.0–1.7) |
| − | < 2 (0.0–1.6) | < 2 (0.0–2.4) | |
| CXCR1 | + | 3.75 (0.0–17.6) | 4.26 (0.0–22.0) |
| − | < 2 (0.0–2.9) | < 2 (0.0–4.6) | |
| CXCR2 | + | < 2 (0.0–10.8) | < 2 (0.0–1.5) |
| − | 2.26 (0.0–12.7) | < 2 (0.0–1.6) | |
| CXCR4 | + | 3.22 (0.0–17.7) | 2.27 (0.0–9.6) |
| − | 4.84 (0.0–37.7) | 6.75 (0.0–21.0) | |
| CXCR6 | + | < 2 (0.0–1.2) | < 2 (0.0–0.6) |
| − | < 2 (0.0–0.2) | < 2 (0.0–0.7) |
Figure 3.
CD4+ CD25+ T cells express CCR8 mRNA and respond to its ligand CCL1. mRNA expression of CCR8 (A), FoxP3 (B), GATA3 (C) and Tbet (D) was measured in freshly isolated CD4+ CD25+ and CD4+ CD25) T cells and in anti-CD3 stimulated T cells from n = 6 donors. mRNA expression in Th1 and Th2 polarized cell lines is also shown. The chemokine responsiveness of CD4+ CD25-, CD4+ CD25+ and CD4+ CD25high T cells to stimulation with CCL1 in the actin polymerization assay is shown for n = 13 donors (E). This response was blocked in the presence of MC148 (10 μg/ml) (F). *P < 0.05; St, anti-CD3 stimulated cells.
Allergen reactive CD4+ CD25+ and CD4+ CD25− T-cells modulate chemokine receptor expression profiles
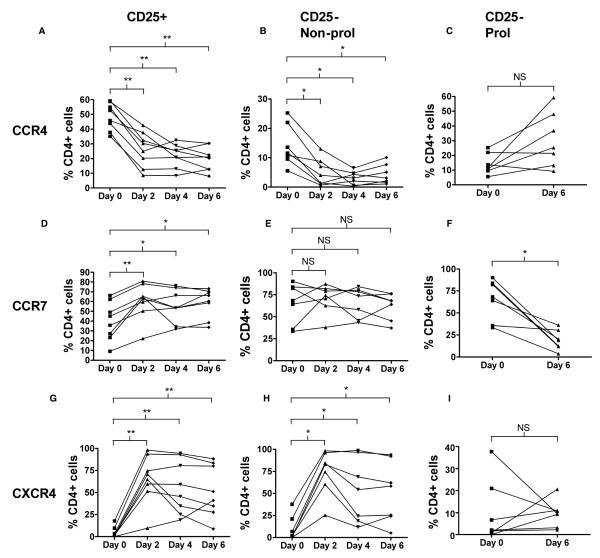
T cells are thought to alter their chemokine receptor expression profile depending on antigen stimulation. We therefore labelled CD4+ CD25− T cells and tracked chemokine receptor expression of these cells and co-cultured MACS-separated CD4+ CD25+ T cells (which contain the CD25hi subset) over the course of a 6 day in vitro allergen-stimulated assay (Fig. 4A). CD4+ CD25+ T cells from both atopics and nonatopics significantly downregulated CCR4 and upregulated CCR7 and CXCR4, during the 6 days of culture (Fig. 4 and Table 2). Proliferating CD4+ CD25− T cells from both atopics and nonatopics showed downregulation of CCR7 expression and a trend for upregulation of CCR4 (P = 0.078), over the course of the 6-day culture (Fig. 4B–D). Other chemokine receptors did not show consistent changes (Table 2). There were no differences between the dynamics of receptor expression between cells from atopic and nonatopic donors (data not shown). Co-culture with CD4+ CD25+ T cells reduced the proportion of proliferating CD4+ CD25− T cells, as judged by the loss of CFSE, but did not affect the changes in chemokine receptor expression (Fig. 4B−D). Nonproliferating CD4+ CD25− T-cells downregulated CCR4 and upregulated CXCR4 during the 6 days of culture, but CCR7 expression did not change.
Figure 4.
Modulation of CD4+ CD25+ and CD4+ CD25− T cell chemokine receptor expression during allergen-driven cell culture. Graphs A–I showing expression of CCR4 (A–C), CCR7 (D–F) and CXCR4 (G–I) on CD4+ CD25+ (A,D,G), nonproliferating CD4+ CD25− (B,E,H) and proliferating CD4+ CD25− T cells (C,F,I) on days 0, 2, 4 and 6 of allergen-stimulated cell culture. Due to the very low numbers of proliferating cells in culture on days 2 and 4, only the expression on days 0 and 6 is shown for proliferating CD4+ CD25− T cells (C,F,I). Results are shown for n = 8 different donors. *P < 0.05; **P < 0.01.
Table 2.
Modulation of CD4+ CD25+ and CD4+ CD25− T-cell chemokine receptor expression during allergen-driven cell culture
| Day 0CD25+ | Day 2CD25+ | Day 4CD25+ | Day 6CD25+ | Day 0CD25– | Day 2CD25– NonProl | Day 4CD25– NonProl | Day 6CD25– NonProl | Day 6CD25– Prol | |
|---|---|---|---|---|---|---|---|---|---|
| CCR3 | 4.4 (0.8–10.6 | 3.0 (0.0–16.5) | 1.5 (0.0–6.1) | 4.7 (0.5–18.5) | 3.4 (0.4–6.7) | 0.9 (0.0–2.6) | 1.6 (0.0–5.1) | 1.2 (0.3–1.9) | 8.8 (4.2–21.2) |
| CCR4 | 48.6 (35.2–59.4) | 26.2 (8.6–42.7) | 21.6 (8.4–32.4) | 19.8 (8.1–30.4) | 12.3 (5.5 25.2) | 4.5 (0.7–13.0) | 2.7 (0.4–6.5) | 3.9 (1.1–10.1) | 26.6 (9.2–59.2) |
| CCR5 | 1.6 (0.0–4.6) | 0.8 (0.0–3.0) | 2.3 (0.0–7.5) | 2.1 (0.0–5.1) | 0.8 (0.0–3.2) | 1.1 (0.0–2.2) | 0.9 (0.0–1.7) | 1.2 (0.0–3.6) | 4.3 (0.8–20.7) |
| CCR6 | 30.0 (9.9–45.7) | 27.1 (14.6–42.4) | 17.0 (5.2 22.9) | 16.4 (4.8–31.9) | 9.2 (3.6–25.9) | 12.0 (6.5–27.5) | 7.0 (3.0–13.4) | 6.4 (1.5–11.3) | 8.6 (3.4–19.2) |
| CCR7 | 39.7 (9.2–66.2) | 60.5 (22.2 (80.8) | 55.2 (31.7–76.0) | 58.7 (38.4–73.1) | 57.2 (33.5–90.1) | 61.7 (37.8–87.2) | 57.8 (43.4–84.2) | 54.3 (37.1–76.2) | 16.8 (3.8–36.2) |
| CXCR4 | 4.6 (0.0–17.7) | 65.3 (9.6–98.1) | 56.3 (18.9–94.3) | 51.8 (8.8–88.2) | 8.8 (0.0 37.7) | 65.2 (25.3–98.3) | 46.6 (11.7–98.8) | 45.1 (5.1–93.9) | 8.3 (2.3–20.6) |
Discussion
Here, we confirm that human CD4+ CD25+ Tregs preferentially express the chemokine receptors CCR4, CCR6 and CCR8 and respond to ligands for these receptors. In addition, we found no differences in chemokine responsiveness between Tregs from atopic individuals when compared to nonatopics. In response to allergen stimulation, CD4+ CD25+ T-cells downregulated CCR4 and increased CCR7 and CXCR4 expression, whereas allergen-responsive CD4+ CD25− T-cells upregulated CCR4 and downregulated CCR7 expression.
Here, we have extended information of chemokine receptor expression by human Tregs by examining CD4+ CD25hiFoxP3+ CD127lo T cells to show that they preferentially express CCR3, CCR4, CCR5, CCR6 and CCR8. This profile of chemokine receptor expression is broadly in agreement with previous reports. Iellem et al. (16) showed preferential expression of CCR4 and CCR8 together with responsiveness to CCL17 or CCL22 and CCL1, by human CD4+ CD25+ Tregs. In addition, Annunziato et al. (17) detected CCR8 expression by human CD4+ CD25+ thymocytes. We could not detect surface staining for CCR8 on peripheral blood CD4+ CD25+ T cells using flow cytometry, but did show increased mRNA expression and in vitro responsiveness to CCL1 by CD4+ CD25hi T cells, which was blocked by the viral CCR8 antagonist MC148. It is of note that relative responsiveness to CCL1 was less than that to CCL17 or CCL22 in our system, in keeping with results from previous reports, and it may be that CCR8 receptor expression is markedly less than that for CCR4. The relative importance of these two ligands in tissue localization of Th2 effector cells and Tregs remains uncertain. In addition, neither CCR4 blockade (28) nor CCR8 gene deficient animals (29, 30) show marked effects in allergen-induced airway hyper-responsiveness and inflammation, and there may be redundancy between these two receptors in allergic inflammation. CCL17 and CCL22 are expressed in asthmatic airways, whereas CCL1 expression was minimal.
Previously, CCR5 has been implicated in chemoattraction of CD4+ CD25+ Tregs, particularly in their interactions with B cells (31). We found increased expression of CCR5 by CD25hi T cells compared with CD25− cells, but minimal in vitro responsiveness to the CCR5 ligand CCL5. This difference may relate to species or tissue differences. In addition, the majority of CD4+ CD25+ T cells in human peripheral blood bear memory markers such as CD45RO, and this may explain differences from cells isolated from antigen naïve mice. In addition, although CD4+ CD25hi T cells compared with CD4+ CD25− T cells preferentially expressed CCR3, the percentage of cells expressing the receptor was low. Further, there was no significant CCL11 responsiveness of these subsets. It will be of interest to define whether there are functional differences between CCR3 and CCR5 expressing and negative sub-populations of Tregs.
We did not see any differences in chemokine receptor expression or responsiveness of CD4+ CD25+ T cells between atopic and nonatopic donors. In particular, CCR4/CCL22 has been implicated in interactions between Tregs and mature dendritic cells. One possible explanation for our previous finding of deficient regulatory activity of CD4+ CD25+ Tregs from atopic donors in allergen stimulated cultures when compared with cells from nonatopic donors would be a difference in chemokine receptor expression affecting T–T or T–dendritic cell interactions. However, the present data do not support this hypothesis. Similarly, there was no deficiency in chemokine receptor expression by CD4+ CD25+ T cells from atopic donors compared with nonatopics, thus suggesting there is no defect in tissue recruitment of Tregs in atopy.
We have previously described increased expression of CCR4 by allergen-reactive T cells derived from the adenoids of atopic and nonatopic children (9). Here, we extend those findings to allergen-reactive CD4+ CD25− T cells from peripheral blood of adults showing increased expression of CCR4 in dividing cells together with downregulation of CCR7 as expected for effector T cells (32). Interestingly, the CD4+ CD25+ T cells in allergen stimulated cultures downregulated CCR4 and increased expression of CCR7 and CXCR4. We cannot be certain what proportion of the CD4+ CD25+ T-cell population at 6 days remains from the cells added at day 0, although our previous data indicated that there is relatively little apoptosis or cell division in this population in a 6-day MLR culture (Clare Notley PhD thesis, University of London). Traffcking of CXCR4+ Tregs to bone marrow has previously been described (33), but whether upregulation of CXCR4 in culture reflects ageing cells, which would in vivo be destined for apoptosis in the bone marrow (34) or whether these cells have a regulatory function in the marrow remains to be established. It is possible that allergen reactive CD4+ CD25+ Tregs downregulate CCR4 and upregulate CCR7 to allow egress from the site of allergic inflammation and traffcking to lymphoid organs, where they might suppress activation of allergen-responsive naïve T cells. Tracking experiments in animal model systems will be required to further elucidate this process.
In conclusion, we confirm the potential importance of CCR4, CCR6, CCR7 and CCR8 and their ligands in traffcking of human CD4+ CD25+ T cells, and show that these may be involved in both recruitment and recirculation of allergen stimulated T cells of both effector and suppressive phenotypes. These findings suggest that strategies aimed at blocking chemokine receptors may impact both on natural regulatory mechanisms as well as pro-inflammatory Th2 cells.
Acknowledgments
This work was funded by the Welcome Trust UK.
Abbreviations
APC
antigen-presenting cell
CCR
CC chemokine receptor
CFSE
carboxyfluoroscein succinimidyl ester
CXCR
CXC chemokine receptor
Treg
regulatory T cell
Footnotes
Supporting Information Additional Supporting Information may be found in the online version of this article:
Figure S1. Measurement of allergen-stimulated CD4+ CD25− T cell proliferation by CFSE dilution and inhibition of proliferation by CD4+ CD25+ T cells. CD4+ CD25− T cells were isolated and stained with CFSE prior to culture in the absence (culture A) or presence (culture B) of CD4+ CD25+ T cells. On days 2, 4 and 6, CD4+ cells were isolated and stained with CD25-PE and analysed by flow cytometry. Dot plots a and c showing CFSE dilution and CD25 expression of CD4+ cells from culture condition A and B respectively. Corresponding histograms b and d showing CD4+ cells from each cell culture condition. Blue = undivided, Orange = 1 division, Turquoise = 4 divisions, yellow = 5 divisions, Clear = CD4+ CD25+ T cells. Data from one representative experiment of 12.
Table S1. Chemokine-responsiveness of CD4+ CD25high and CD4+ CD25low T cells from atopic and nonatopic donors.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 2.Pease JE, Williams TJ. Chemokines and their receptors in allergic disease. J Allergy Clin Immunol. 2006;118:305–318. doi: 10.1016/j.jaci.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 5.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, et al. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- 7.D’Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 8.Morgan AJ, Symon FA, Berry MA, Pavord ID, Corrigan CJ, Wardlaw AJ. IL-4-expressing bronchoalveolar T cells from asthmatic and healthy subjects preferentially express CCR 3 and CCR 4. J Allergy Clin Immunol. 2005;116:594–600. doi: 10.1016/j.jaci.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 9.Banwell ME, Robinson DS, Lloyd CM. Adenoid-derived TH2 cells reactive to allergen and recall antigen express CC chemokine receptor 4. J Allergy Clin Immunol. 2003;112:1155–1161. doi: 10.1016/j.jaci.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nouri-Aria KT, Wilson D, Francis JN, Jopling LA, Jacobson MR, Hodge MR, et al. CCR4 in human allergen-induced late responses in the skin and lung. Eur J Immunol. 2002;32:1933–1938. doi: 10.1002/1521-4141(200207)32:7<1933::AID-IMMU1933>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Pilette C, Francis JN, Till SJ, Durham SR. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur Respir J. 2004;23:876–884. doi: 10.1183/09031936.04.00102504. [DOI] [PubMed] [Google Scholar]
- 13.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, et al. Relation of CD4+ CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 14.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+ CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei S, Kryczek I, Zou W. Regulatory T cell compartmentalization and traffcking. Blood. 2006;108:426–431. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, et al. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor PA, McKinnon KP, Su L, et al. Critical role for CCR5 in the function of donor CD4+ CD25+ regulatory T cells during acute graftversus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- 21.Iellem A, Colantonio L, D’Ambrosio D. Skin-versus gut-skewed homing receptor expression and intrinsic CCR4 expression on human peripheral blood CD4+ CD25+ suppressor T cells. Eur J Immunol. 2003;33:1488–1496. doi: 10.1002/eji.200323658. [DOI] [PubMed] [Google Scholar]
- 22.Colantonio L, Iellem A, Sinigaglia F, D’Ambrosio D. Skin-homing CLA+ T cells and regulatory CD25+ T cells represent major subsets of human peripheral blood memory T cells migrating in response to CCL1/I-309. Eur J Immunol. 2002;32:3506–3514. doi: 10.1002/1521-4141(200212)32:12<3506::AID-IMMU3506>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J Immunol. 2002;169:2498–2506. doi: 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- 24.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 25.Francis JN, Jacobson MR, Lloyd CM, Sabroe I, Durham SR, Till SJ. CXCR1+ CD4+ T cells in human allergic disease. J Immunol. 2004;172:268–273. doi: 10.4049/jimmunol.172.1.268. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conroy DM, Jopling LA, Lloyd CM, Hodge MR, Andrew DP, Williams TJ, et al. CCR4 blockade does not inhibit allergic airways inflammation. J Leukoc Biol. 2003;74:558–563. doi: 10.1189/jlb.0103030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung CD, Kuo F, Kumer J, Motani AS, Lawrence CE, Henderson WR, Jr, et al. CCR8 is not essential for the development of inflammation in a mouse model of allergic airway disease. J Immunol. 2003;170:581–587. doi: 10.4049/jimmunol.170.1.581. [DOI] [PubMed] [Google Scholar]
- 30.Goya I, Villares R, Zaballos A, Gutierrez J, Kremer L, Gonzalo JA, et al. Absence of CCR8 does not impair the response to ovalbumin-induced allergic airway disease. J Immunol. 2003;170:2138–2146. doi: 10.4049/jimmunol.170.4.2138. [DOI] [PubMed] [Google Scholar]
- 31.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 32.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, et al. Bone marrow is a reservoir for CD4+ CD25+ regulatory T cells that traffc through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 34.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]