Proapoptotic Bax and Bak Proteins Form Stable Protein-permeable Pores of Tunable Size (original) (raw)
Background: During apoptosis Bax/Bak release differently sized proteins out of the mitochondria.
Results: The size of Bax/Bak pores depends on protein concentration.
Conclusion: Bax/Bak form stable toroidal pores tunable in size.
Significance: Pore size-tuning constitutes a new level for the regulation of Bax/Bak activity.
Keywords: Apoptosis, Bcl-2 Proteins, Confocal Microscopy, Membrane Biophysics, Vesicles
Abstract
The Bcl-2 proapoptotic proteins Bax and Bak mediate the permeabilization of the mitochondrial outer membrane during apoptosis. Current models consider that Bax and Bak form pores at the mitochondrial outer membrane that are responsible for the release of cytochrome c and other larger mitochondrial apoptotic factors (i.e. Smac/DIABLO, AIF, and endoglycosidase G). However, the properties and nature of Bax/Bak apoptotic pores remain enigmatic. Here, we performed a detailed analysis of the membrane permeabilizing activity of Bax and Bak at the single vesicle level. We directly visualized that cBid-activated Bax and BakΔC21 can form membrane pores large enough to release not only cytochrome c, but also allophycocyanine, a protein of 104 kDa. Interestingly, the size of Bax and BakΔC21 pores is not constant, as typically observed in purely proteinaceous channels, but evolves with time and depends on protein concentration. We found that Bax and BakΔC21 formed long-lived pores, whose areas changed with the amount of Bax/BakΔC21 but not with cardiolipin concentration. Altogether, our results demonstrate that Bax and BakΔC21 follow similar mechanisms of membrane permeabilization characterized by the formation of protein-permeable pores of dynamic size, in agreement with the proteolipidic nature of these apoptotic pores.
Introduction
The Bcl-2 proteins are multifunctional proteins with a major role in apoptosis regulation. Moreover, they are involved in other processes like mitochondrial dynamics, the regulation of mitochondrial energy metabolism, cellular Ca2+ homeostasis, autophagy, or the unfolded protein response at the endoplasmic reticulum (1). The best understood function of the Bcl-2 proteins is the regulation of programmed cell death. Proapoptotic Bax-type proteins (Bax and Bak) are thought to be the executioners of mitochondrial outer membrane (MOM)7 permeabilization during apoptosis (reviewed in Refs. 2–6). Their activity is regulated by two other factions of the Bcl-2 family: proapoptotic BH3-only proteins such as Bid, which play an activating role, and anti-apoptotic Bcl-2 type proteins, including Bcl-2, Bcl-xL, and Mcl-1, which inhibit proapoptotic partners.
In cells, Bid, Bax, Bcl-xL, and Mcl-1 behave as amphitropic proteins that shuttle between soluble and MOM-inserted conformations depending on the physiological status of the cell, whereas Bak and Bcl-2 are membrane-integral proteins mainly localized at the MOM (e.g. (7–11)). According to currently popular models, after a proapoptotic stimulus, Bax translocates to the MOM and therein, both Bax and Bak undergo a set of conformational changes leading to protein oligomerization and ultimately, assembly of MOM-perforating pores (e.g. Refs. 12–16). In this context, the mitochondrial lipid CL has been proposed to play a role in the MOM permeabilization process elicited by Bax/Bak (15, 17–19). However, despite intense research, the exact mechanism by which Bax/Bak mediate MOM permeabilization and the properties of the pores they form remain poorly understood.
The MOM permeabilization process that occurs during apoptosis usually leads to the release into the cytosol of multiple mitochondrial apoptogenic proteins that are crucial for triggering downstream events in the apoptotic cascade. The best studied mitochondrial protein is cytochrome c (Cyt c, 12 kDa) essential for the assembly of the apoptosome, but larger mitochondrial proteins like Smac/Diablo (a dimer of 54 kDa), Omi/HtrA2 (48 kDa), AIF (62 kDa), and endoglycosidase G (23 kDa) are also released. Early cellular studies indicated that MOM permeabilization leads to simultaneous release of all mitochondrial apoptogenic proteins (20, 21). Additional evidence indicated that deficiencies in Bax/Bak impair the release of Smac/Diablo or larger molecules more readily than that of Cyt c (22–24)), suggesting that scenarios may exist in living cells in which limitations in Bax/Bak amounts restrict the release of certain mitochondrial apoptogenic factors, but not others. On top of this, it is even debated whether the pore-forming functions of Bax and Bak are by themselves sufficient for the release of all these mitochondrial apoptogenic proteins into the cytosol (25).
In the last years, we and others have studied the mechanism of pore formation by Bax-type proteins using minimalist model systems (14–19, 26–30). We gathered different lines of evidence supporting that active forms of Bax and Bak form toroidal pores of proteolipidic nature. On the one hand, we found that pore formation by Bax and Bak is linked to changes in the intrinsic monolayer curvature and line tension of the membrane, two physical properties known to be critical for toroidal pore formation (17, 18, 26, 31, 32). On the other hand, x-ray diffraction experiments demonstrated that a peptide derived from the central helix of Bax (Bax α5) forms pores in supported bilayers containing lipid molecules in their lumen (33), consistent with functional and structural information obtained with this peptide in a variety of model membrane systems (34–36, 41–43). Nevertheless, other evidence supports the view that Bax and Bak permeabilize membranes by acting as typical ion channels, which form purely proteinaceous pores rather than proteolipidic pores (27–29). Thus, at present there is insufficient evidence to enable a clear consensus on the precise nature of the pores formed by Bax-type proteins.
Here, we performed a detailed analysis of the pore-forming activities of Bax and Bak, using the single giant unilamellar vesicle (GUV) methodology. We directly visualized that active Bax and BakΔC21 form stable protein-permeable pores in GUVs. Interestingly, we found that the sizes of Bax/BakΔC21 pores can be tuned by protein concentration: at low Bax/BakΔC21 concentrations and under equilibrium conditions the pore size is only sufficient for release of Cyt c (12.5kDa), whereas at higher Bax/BakΔC21 concentrations the pore expands and allows the release of APC (104 kDa). Furthermore, CL concentration varies the propensity for Bax/BakΔC21 pore formation, without altering the actual pore size and the number of pores per GUV. Based in these observations, we propose that during apoptosis the release of the complete set of mitochondrial apoptogenic proteins into the cytosol occurs via protein-permeable proteolipidic pores formed by Bax/Bak, which can be finely tuned by changes in the concentration of the active forms of Bax/Bak or the CL concentration.
EXPERIMENTAL PROCEDURES
Protein Production and Labeling
Full-length mouse Bid, full-length human Bax, Bak lacking the carboxyl-terminal 21 amino acids (BakΔC21), and mice Mcl-1 lacking the N-terminal 151 amino acids and the C-terminal 23 amino acids (Mcl-1ΔN151ΔC23) were expressed in Escherichia coli. Bax, BakΔC21, and Mcl-1ΔN151ΔC23 were purified as described in Refs. 14, 18, and 37–39. From Bid, cBid was cleaved and purified as described in Refs. 12 and 14. Bovine Cyt c and allophycocyanin (APC) were purchased at Sigma. Cyt c was labeled at lysines with Alexa 488 as described in Ref. 19.
Composition of the Lipid Mixtures
All lipids were purchased from Avanti Polar Lipids.
The lipid mixture mimicking the mitochondrial outer membrane composition was prepared as in Refs. 10 and 16 with 49% egg l-α-phosphatidylcholine (PC), 27% egg l-α-phosphatidylethanolamine (PE), 10% bovine liver l-α-phosphatidylinositol (PI), 10% 18:1 phosphatidylserine (PS), and 4% CL (all percentages mol/mol). The CL-enriched lipid mixtures were prepared with 70% egg PC, 30% CL, or with 80% egg PC and 20% CL (mol/mol). In the case of GUV experiments <0.05% of DiI (1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine perclorate; DiIC18) was added to the lipid mixture.
GUV Permeabilization Experiments
GUVs were produced by electroformation and the experiments were done as described in Refs. 10 and 19. Briefly, 5 μg of lipid mixture dissolved in chloroform were spread on platinum electrodes in the electroformation chamber and allowed to dry, before immersion in 300 mm sucrose. Electroformation proceeded for 2 h at 10 Hz, followed by 30 min at 2 Hz. Cyt _c_488, APC, and the Bcl-2 proteins were mixed in LabTec chambers (NUNC) with PBS at the desired concentrations and 75 μl of GUVs suspension were then added to a final volume of 300 μl. In pore stability experiments, Alexa 555 was added at time 0, whereas 50 μl of a Cyt _c_488/APC mixture were added after 45 min incubation for Bax or 2 h for BakΔC21. Images were collected 15 min later. Timing is crucial in pore stability experiments. Pores opening between Cyt _c_488/APC additions and imaging are considered as pores producing noise. To minimize this noise, pictures were taken directly before Cyt _c_488/APC addition and 15 min later. The percentage of GUVs permeabilized for Alexa 555 were similar in both cases (data not shown) indicating that this “noise” is not affecting the experimental results.
Confocal Microscopy and Fluorescence Correlation Spectroscopy
All experiments were performed on an LSM710 microscope with a C-Apochromat ×40 N.A. 1.2 water immersion objective (Zeiss). Excitation light came from argon ion (488 nm), HeNe (561 nm), or HeNe lasers (633 nm). A spectral beam guide was used to separate emitted fluorescence. Images were processed with ImageJ as described below. Fluorescence correlation spectroscopy experiments were performed as described in Ref. 40.
Image Analysis
The analysis was done as described in Ref. 19.
The degree of GUV filling was calculated as,
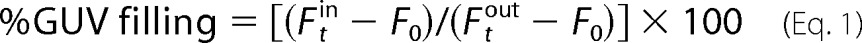
where F _t_in and F _t_out are the average fluorescence intensities inside and outside a GUV at time t, and _F_0 is the background fluorescence. We arbitrarily set the threshold for classifying GUVs as non-permeabilized to <20%. Several hundred GUVs were analyzed per experiment (>100 per condition and experiment).
In the kinetics experiments, images were recorded every 20 (for Bax) or 30 s (for BakΔC21). Changes in the fluorescence intensity inside GUVs were analyzed over time as,
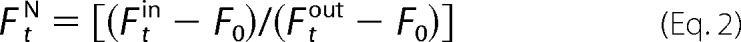
where F _t_N is the normalized fluorescence intensity at time t.
To analyze the data of the kinetics, two approaches have been used. A monoexponential fitting to study the permeabilized area (A) from the experiments in equilibrium and a multiexponential fitting based on the reduction of the permeabilized area from the pre-equilibrium experiments (19, 41–43).
To calculate the permeabilized area A from the filling kinetics under equilibrium conditions, we used a monoexponential fitting using Equation 3,

where the influx rate τflux equates to,

where V is the vesicle volume, D is the diffusion coefficient of the dye, and m the membrane thickness (pore length).
In the equilibrium kinetics, the intensity of the background signal (Cyt _c_488 and APC) through the sample volume fluctuates in parallel to the dye entrance and this process has to be taken into account. The kinetics of diffusive dilution of the dyes can be modeled as an exponential decay,
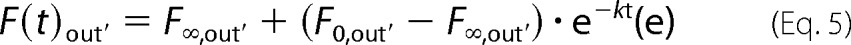
where, F(t)out′ represents the time-dependent fluorescence outside the vesicle, F(t)0′ the initial fluorescence value, F(t)∞′ the equilibrium fluorescence value, and k the rate constant. By including this expression in Equation 3, we obtain the corresponding exponential change of fluorescence inside the vesicle, which is fitted to the non-normalized data.
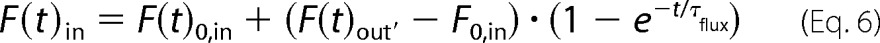
From the fitted parameters we obtain τflux, from which we calculate the permeabilized area as described in Equation 4.
For pre-equilibrium kinetics we take into account that the permeabilized area is decreasing with time, and accordingly the initial pore size relaxes to a smaller structure (19, 41–43). It was modeled to an exponential decay according to,

where, _A_0 and _A_relax are the initial and relaxed permeabilized areas of individual GUVs, and τrelax is the relaxation time. This means that the τflux is also time-dependent,
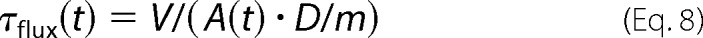
which can now be included in Equation 3, resulting in a multiexponential fitting.
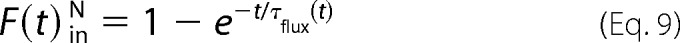
Membrane thickness was assumed to be 4.5 nm (44). The diffusion coefficient of Cyt _c_488 and APC were 196 (196 ± 27 μm2/s) and 59 μm2/s (59 ± 7 μm2/s), respectively, as calculated by fluorescence correlation spectroscopy.
Calcein Release Assays
Large unilamellar vesicles (LUVs) were prepared as in Ref. 35. Lipids were mixed in chloroform, dried to thin films, and resuspended at 4 mg/ml in 80 mm calcein (Sigma) neutralized with NaOH, followed by repeated freezing and thawing cycles, and extrusion through 100-nm polycarbonate membranes. Non-encapsulated calcein was removed with PD10 columns (GE Healthcare). Calcein release was measured with an Infinite M200 plate reader (TECAN) by the increase in fluorescence intensity.
RESULTS
Novel Minimalist Assay to Visualize and Analyze Protein Permeation Across Bax/Bak Pores
To attempt direct visualization of protein permeation across Bax and/or Bak pores and directly examine how the size of such putative pores formed by Bax and Bak may be regulated, we implemented a novel minimalist system. This system is based on four components: (i) functional recombinant Bcl-2 proteins (14, 18, 19, 39), (ii) GUV containing a lipid mixture mimicking the MOM, (iii) proteinaceous and non-proteinaceous fluorescent size markers, and (iv) a time-lapse confocal fluorescence microscope.
In the initial experiments, we found that cBid-activated BakΔC21 was substantially less potent permeabilizing GUVs than cBid-activated Bax (Fig. 1). To compensate this difference between the two pore-forming proteins, longer incubation times and higher protein concentrations were used for experiments with BakΔC21. To our knowledge, nobody (including us) has yet succeeded purifying wild type recombinant full-length Bak in a native form, although a recent study presented results using a recombinant version of Bak containing a mutagenized helix 9 (45). In cells, BakΔC21 has been described to display reduced proapoptotic activity relative to full-length Bak (11).
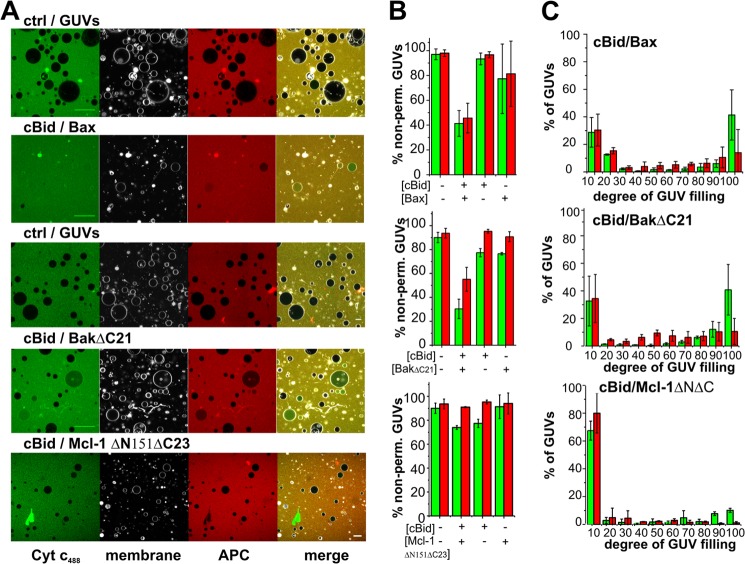
FIGURE 1.
Activated Bax and BakΔC21 form pores in GUV allowing permeation of large molecular weight proteins. A, representative images of GUVs (gray) in a solution of 12-kDa Cyt _c_488 (green) and 104-kDa APC (red) incubated simultaneously in the absence or presence of 20 nm cBid plus 100 nm Bax, 15 nm cBid plus 300 nm BakΔC21, or 15 nm cBid plus 300 nm Mcl-1ΔN151ΔC23. Images were taken 1 h (for Bax) or 2 h (for BakΔC21 and Mcl-1ΔN151ΔC23) after mixing the components. The internalization of Cyt _c_488 and/or APC to the lumen of GUVs corresponds to permeabilized vesicles. GUV composition was 49% PC, 27% PE, 10% PI, 10% PS, 4% CL for Bax (MOM mix), and 80% PC, 20% CL for BakΔC21, and Mcl-1ΔN151ΔC23 (20% CL). B, fraction of non-permeabilized GUVs in the presence or absence of the indicated Bcl-2 proteins, green bars correspond to Cyt _c_488 and red bars to APC. C, distribution of the degree of filling of individual GUVs to Cyt _c_488 (green bars) and APC (red bars) after treatment with the indicated Bcl-2 proteins. In each of three experiments, a minimum of 250 vesicles were analyzed per condition. Error bars represent S.D.
Several lipid mixtures have been used to model the lipid composition of the MOM. On the one hand, the average lipid composition of the MOM contains low CL concentrations (4% of CL, mol/mol). On the other hand, higher CL concentrations have also been used (e.g. see Refs. 18 and 32; up to 30% mol/mol) considering that the amounts of CL at the MOM increase during apoptosis and that cBid concentrates at the contact sites between MOM and MIM, which are enriched in CL (46). Here, the role of CL in Bax-induced pores has been studied comparing a mixture mimicking the MOM (MOM mixture (16)) and a CL-enriched lipid composition. In the case of BakΔC21, we used a lipid mixture containing 20% CL, because BakΔC21 does not bind to the MOM mixture (18).
Both Bax and Bak have been proposed to form pores big enough to release high molecular weight proteins from mitochondria during apoptosis. As a novelty in studies of pore formation by Bax/Bak, Cyt _c_488 (Alexa 488-labeled cytochrome c, 12 kDa (19)) and APC (104 kDa; far-red fluorescent protein; see Fig. 2A) were used simultaneously as fluorescent markers to probe the Bax/Bak pore size. We confirmed that the Cyt _c_488 and APC used here neither bind nor permeabilize the GUVs (see Fig. 1A, left column). Fluorescence correlation spectroscopy measurements also confirmed that the calculated diffusion coefficients and hydrodynamic radii of Cyt _c_488 and APC were in line with the expected molecular sizes for monomeric proteins (see Fig. 2B).
FIGURE 2.
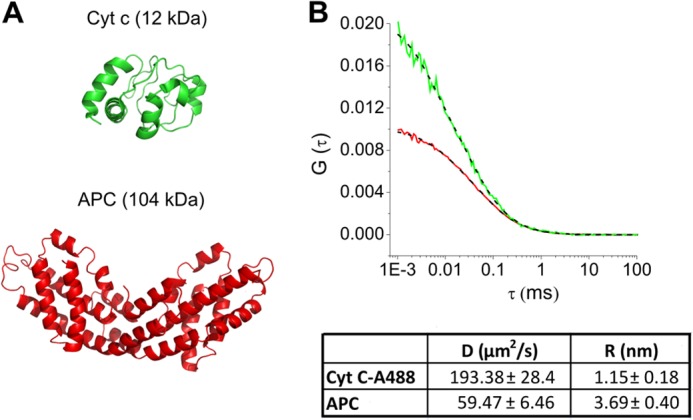
Diffusion coefficient and radius of Cyt _c_488 and APC. A, model of soluble Cyt c (PDB 2B4Z (48)) (green) and APC (PDB 1ALL (47) (red). B, representative autocorrelation analysis of Cyt _c_488 (green line) and APC (red line) in solution. The fitted autocorrelation curves are shown in dashed black lines. In the table, diffusion coefficient and hydrodynamic radius (± S.D.) for Cyt _c_488 and APC are shown.
Bax and BakΔC21 Form Pores in GUVs That Allow the Passage of Cyt c488 and APC
In a first set of experiments, we examined the effect of a single dose of Bax/BakΔC21 on GUV permeability. To this aim, we mixed Cyt _c_488, APC, and the Bcl-2 proteins of choice in buffer, added the GUVs, and incubated the components 1 (Bax) or 2 h (BakΔC21) at RT before analysis by confocal microscopy (see Fig. 1A). To discriminate between “permeabilized” and “non-permeabilized” GUVs, we arbitrarily classified GUVs with permeabilization values lower than 20% as non-permeabilized. As shown in Fig. 1, cBid/Bax and cBid/BakΔC21 mixtures effectively induced the internalization of both Cyt _c_488 and APC into GUVs. Internalization of the two fluorescent size markers was negligible in the presence of Bax, BakΔC21, or cBid proteins alone. As a control, we found that the antiapoptotic protein Mcl-1ΔNΔC combined with cBid did not induce substantial internalization of Cyt _c_488 and APC into the GUVs, despite this protein binds to these type of liposomes (39).
Next, we calculated the “degree of filling” of individual GUVs in the vesicle population (Fig. 1C). In this analysis two situations can be distinguished that are directly linked to the mechanism of membrane permeabilization. In the “all or none” mechanism, the vesicles in the sample exhibit two states: impermeable or totally permeabilized. Alternatively, the individual GUVs in the sample can exhibit a varying percentage of filling degrees according to the “graded” mechanism. For both cBid-activated Bax and BakΔC21, Cyt _c_488 followed an all or none mechanism (Fig. 1C), whereas APC followed a graded mechanism of permeabilization. This suggests that, at the concentrations tested, the pores induced by Bax and BakΔC21 have similar sizes in the range of one APC molecule. From fluorescence correlation spectroscopy experiments we calculated the hydrodynamic radius of APC to be 3.69 ± 0.40 nm (Fig. 2; x-ray data showed a rod shaped protein in the same size range (47)).
Pre-equilibrium Permeabilization Kinetics Induced by Bax/BakΔC21 Show That the Total Porated Area Per Vesicle Depends on the Protein Concentration
Next, we examined the permeabilization kinetics of single vesicles incubated with low or high Bax and BakΔC21 concentrations, measuring the entrance of each marker into the GUV as a function of time (Fig. 3A). Two different experimental setups were designed. In the first approach, the size markers were added to the protein mixture before the GUVs, and thus the initial pores and their progressions were studied (pre-equilibrium kinetics). In the second approach, the size markers were added after the GUVs had been incubated with Bax/Bak for a time period long enough to allow completion of the Bax/Bak permeabilization process (equilibrium kinetics). In both setups the size of pores at equilibrium was calculated. Here, we present the first strategy and the second one is described later in the text.
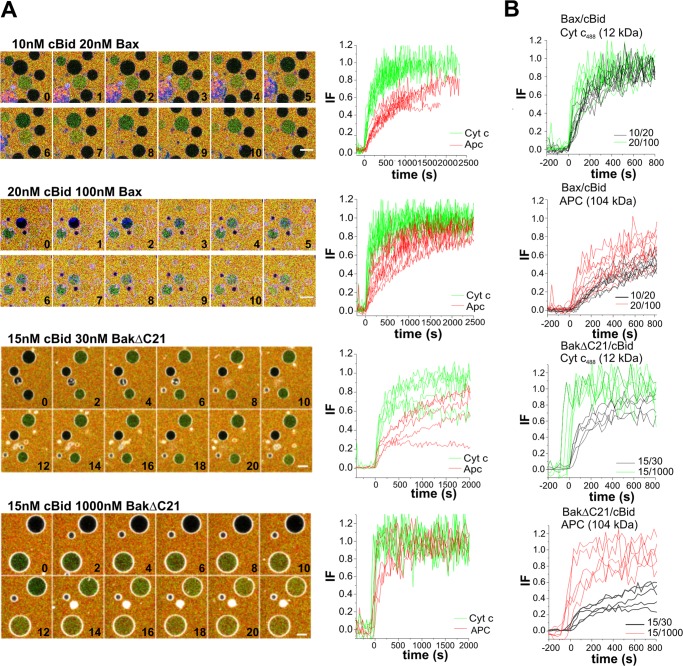
FIGURE 3.
Bax/BakΔC21 pores can grow in size. A, time lapse merged images of GUVs (gray) treated with increasing concentrations of Bax/BakΔC21 plus cBid, in the presence of Cyt _c_488 (green) and APC (red). Scale bar, 20 μm. Time is indicated in minutes. Images were taken every 20 or 30 s during 90 min (Bax conditions) and 2 h (BakΔC21 conditions) and the corresponding normalized data of representative leak-in kinetics of Cyt _c_488/APC into individual GUVs after treatment with different doses of Bax/BakΔC21. GUV permeabilization happens stochastically, but was aligned to the starting point of permeabilization for clarity. In B, representative leak-in kinetics of Cyt _c_488 or APC are compared for the different Bax/BakΔC21 concentrations.
For Cyt _c_488, the filling rates of all GUVs were fast and always reached 100%. However, at high Bax/BakΔC21 concentrations the leak-in kinetics were more rapid than at low protein concentrations (Fig. 3B). Interestingly, GUV filling with APC was generally slower than with Cyt _c_488, and clear differences between high and low protein concentrations were observed (Fig. 3, A and B). At high protein concentrations, the majority of GUVs were permeable to both markers, whereas at lower concentrations only a minority of the vesicles was totally filled with APC. This indicates that at lower protein concentration the pores are too small to allow free APC passage (see Fig. 4A).
FIGURE 4.
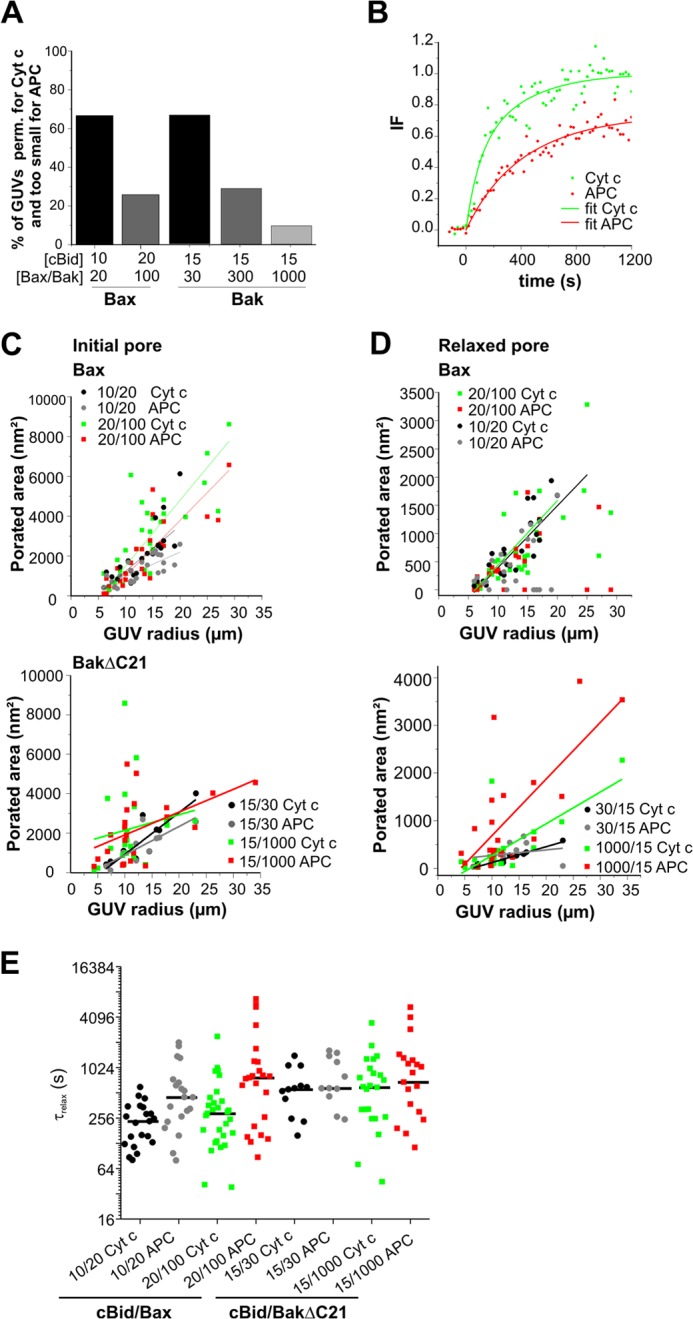
Data analysis of the Bax/BakΔC21 pores. A, fraction of analyzed GUVs showing >80% degree of filling for Cyt _c_488 and <50% degree of filling for APC, after treating vesicles with different amounts of Bax/BakΔC21. B, example of leak in kinetics (dots) and the fitted curves (lines) for Cyt _c_488 (green), and APC (red). C, initial, and D, relaxed estimated porated areas (nm2) calculated from a multiexponential fitting of the normalized data of leak-in kinetics for both dyes and for different concentrations of cBid/Bax (upper panel) and cBid/BakΔC21 (lower panel). Data were obtained from at least three independent experiments, and a minimum of 10 kinetics of individual GUVs were analyzed for each condition. E, presentation of the _t_relax values calculated for the different conditions.
From the data of pre-equilibrium kinetics we calculated the initial pore area and the area in equilibrium (19, 41–43). For clarity the equilibrium pores estimated here are called relaxed pores, to distinguish them from the (equilibrium) pores estimated in the equilibrium kinetic approach presented later. Note that relaxed and equilibrium pores describe the same pore states, but were analyzed by different methods. An example of the exponential fit to the experimental data are shown in Fig. 4B.
The calculated total porated area corresponds to the sum of the areas of all the pores that are formed in a single GUV, being equivalent for one large pore of x size or for several pores having different sizes which sum is x size. As shown in Fig. 4C, for both cBid-activated Bax and BakΔC21 the effective size of the initially permeabilized area is similar and this parameter increases with protein concentration.
We next calculated the pore sizes at relaxed conditions. The analysis revealed that the relaxed porated area is smaller than the initial one. Moreover, at higher Bax/BakΔC21 concentrations the calculated relaxed pore areas are larger than at lower protein concentration (Fig. 4D). Notably, under all conditions examined a relationship between the porated area (initial or relaxed) and GUV size was found (see Fig. 4, C and D). The simplest explanation for this would be that the Bax/Bak density per μm2 GUV surface somehow sets or limits the pore size and larger GUVs have a higher number of pores. This idea is in line with the experimental finding that big GUVs with big pore areas were often not completely filled with APC, demonstrating that the increase in total permeabilized area corresponds to an increase in pore number and not in pore size.
Additionally, τrelax was estimated from the fitting kinetics. This parameter reflects the time at which the initial permeabilized area relaxes to a smaller area. For both Bax and BakΔC21, we obtained times between 2 and 20 min independent of the protein concentration (see Fig. 4E). Thus, the Bax/Bak pore area, but not τrelax, seems to be related to protein concentration.
Bax/BakΔC21 Pores under Equilibrium Allow APC Passage Only at High Concentrations
Because of the inherent limitations of estimating the relaxed permeabilized area from fitting APC leak-in kinetics with a multiexponential function, we decided to use an independent experimental approach to analyze the size of Bax/BakΔC21 pores under equilibrium conditions (equilibrium pores). GUVs and Bcl-2 proteins were incubated with Alexa 555 (1 kDa) long enough to allow pore formation and relaxation (2 h). Subsequently, the size markers Cyt _c_488 and APC were added and the entry of these dyes into individual GUVs that had been initially permeabilized to Alexa 555 was followed for another 2 h. For BakΔC21, we were able to follow this procedure at low and high protein concentrations (see Fig. 5). However, at high Bax concentrations most GUVs were destroyed during the long duration of the experiments, and thus shorter times were used for Bax (see below).
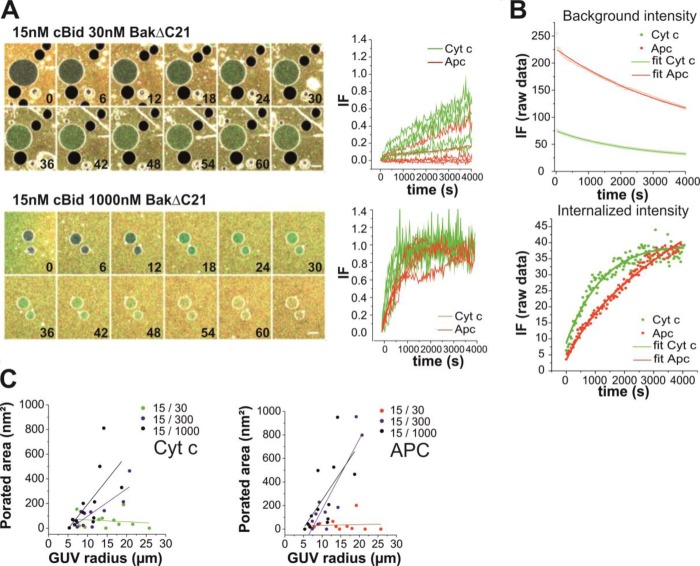
FIGURE 5.
Equilibrium BakΔC21 pores are stably open. A, time lapse merge images and leak-in kinetics of 20% CL GUVs containing 0.05% DiI (intense gray) in a solution of free Alexa 555 (gray) with 30 nm or 1 μm BakΔC21 and 15 nm cBid. After 2 h of incubation, Cyt _c_488 (green)/APC (red) were added and images were recorded every 30 s during 2 h more. Scale bar, 20 μm. The time is indicated in minutes. The curves represent normalized data of representative leak-in kinetics of individual GUVs. Cyt _c_488 and APC passage are shown in green and red, respectively. B, examples of the fitted curves in equilibrium conditions; average curves of three background intensity fluorescence kinetics were modeled as an exponential decay following Equation 5 (upper panel) and, non-normalized intensity of fluorescence of leak-in kinetics were fitted with Equation 6 (lower panel). Dots and lines correspond to raw data and the fitted curves, respectively. C, estimated permeabilized area (nm2) related to the GUV radius under equilibrium conditions. Cyt _c_488 is shown in the left panel and APC in the right panel. Data were obtained from at least three independent experiments, and a minimum of ten kinetics of individual GUVs were analyzed for each condition.
The analysis of permeabilization under equilibrium conditions showed that the pores induced by cBid-activated BakΔC21 were stable and their size clearly changed at low and high BakΔC21 concentrations (see Fig. 5). Concretely, at the lowest BakΔC21 concentration APC internalization was rare and Cyt _c_488 passage occurred in most GUVs with slow kinetics (Fig. 5A). This can be explained by a pore diameter similar to the size of Cyt _c_488 (∼3 nm diameter, see Fig. 2 and x-ray data (48)). In contrast, at the highest BakΔC21 concentration both size markers freely crossed the membrane of the individual GUVs, indicating that the size of BakΔC21 pores under these conditions is larger than APC (>8 nm diameter) (Fig. 5A). Examples of the exponential fits to the raw data for the fluorescence intensity in the background and inside of a vesicle are shown in Fig. 5B. We found that the porated area was smaller than estimated for initial or relaxed pores in the pre-equilibrium experiments and, again a relationship between pore area and GUV size was found (see Fig. 5C).
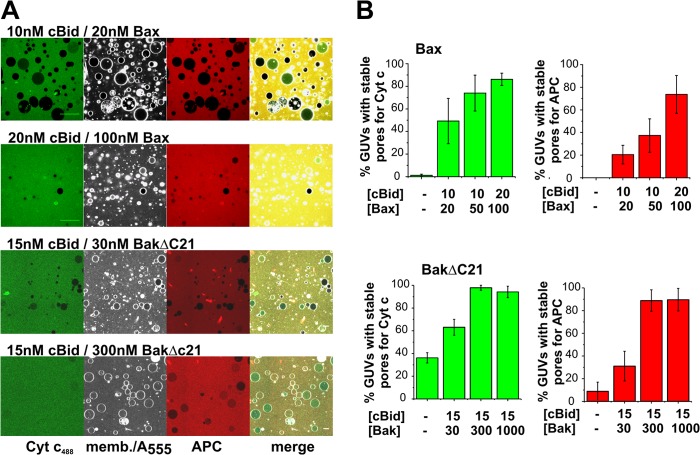
To study the size of Bax pores in equilibrium, we needed to use an approach with a shorter experimental duration. To this aim, we used the same experimental setup, but added Cyt _c_488 and APC after 45 min (a time point at which most GUVs were permeabilized at the higher Bax concentration) and 15 min later the GUVs were imaged (Fig. 6A). Only GUVs filled >80% with Alexa 555 were considered. From those, we calculated the percentage of stably permeabilized GUVs big enough to allow >50% entry of Cyt _c_488 or APC (Fig. 6B). At low concentrations about half of the permeabilized GUVs had stable pores through which Cyt _c_488, but not APC, passage was possible and even Cyt _c_488 passage was hindered (Fig. 6B). At high protein concentrations, the pore size was increased, so that almost all GUVs were permeable to Cyt _c_488 and APC (Fig. 6B), in line with the equilibrium kinetics (Fig. 5A). Importantly, this indicates that high amounts of Bax/BakΔC21 in the membrane stabilize pores of larger sizes.
FIGURE 6.
Large pores formed by activated Bax and BakΔC21 are long-lived. A, representative images of GUVs (dark gray) in a solution of free Alexa 555 (soft gray) incubated simultaneously with different concentrations of Bax/cBid in MOM GUVs containing <0.05% DiI or BakΔC21/cBid in 20% CL GUVs containing <0.05% DiI. After 45 min (Bax conditions) or 2 h (BakΔC21 conditions), Cyt _c_488 (green) and APC (red) were added. Images were taken 15 min (Bax) or 2 h (BakΔC21) after incubation with Cyt _c_488 and APC. Scale bar, 20 μm. B, percentage of permeabilize GUVs for Cyt c488 (green) and APC (red) that were permeable for free Alexa 555 at different concentrations of Bax/cBid in MOM GUVs, and BakΔC21/cBid in 20% CL GUVs. Data were obtained from at least three independent experiments and a minimum of 250 vesicles were analyzed per condition. Error bars represent S.D.
CL Concentration Affects Bax Binding to GUVs but Not the Bax Pore Size
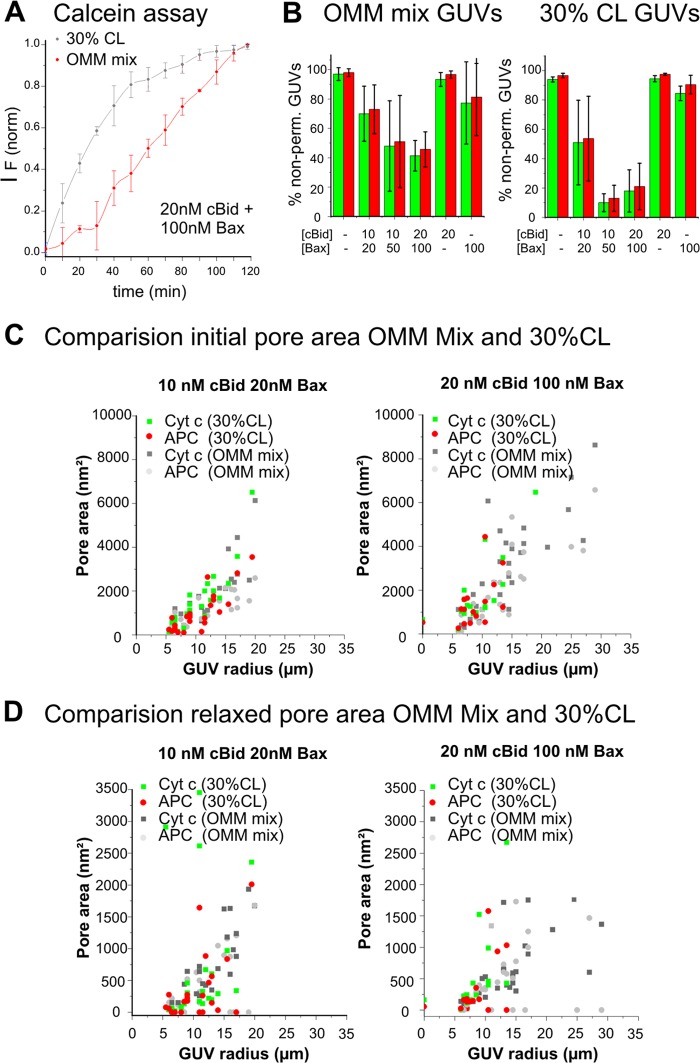
In the experiments described above, we used a lipid mixture presenting the average composition of the MOM. However, we observed recently that cBid, Bax, and BakΔC21 binding to membranes is increased at higher CL concentrations (18, 19). To clarify the relevance of CL, we examined the impact of the CL concentration on pore size and stability (MOM mix versus a mixture containing 30% CL; about 7-fold increase in CL). At 30% CL concentration, the average lag time for individual vesicle permeabilization became shorter (Fig. 7A, and data not shown) and more GUVs became permeabilized (Fig. 7B). This indicates that raising the CL content of the membrane somehow augments the Bax-induced membrane permeabilization process.
FIGURE 7.
CL does not affect Bax pore size. A, comparison of Bax-induced permeabilization of LUVs composed of MOM-like mixture or containing 30% CL. LUVs encapsulating calcein were incubated with 100 nm Bax and 20 nm cBid. Membrane permeabilization was monitored by the increase in fluorescence due to the release of quenched calcein from the LUVs. Fluorescence intensity was normalized. The lipid:protein ratio was ∼500 to 1. B, comparison of Bax-induced GUV permeabilization using GUV composed of the MOM mixture or containing 30% CL. The fraction of non-permeabilized GUVs (filled <20% with size marker) in the presence or absence of cBid/Bax at the indicated concentration is shown. Green bars correspond to Cyt _c_488 and red bars to APC. Images were taken 1 h after mixing the components. C, initial porated area (nm2), and D, relaxed porated area (nm2) calculated from a multiexponential fitting of the normalized data of leak-in kinetics for both dyes using different concentrations of cBid/Bax and different lipid mixtures. Data were obtained from at least three independent experiments, and a minimum of 10 kinetics of individual GUVs were analyzed for each condition.
Interestingly, a comparison of the total porated areas estimated for individual GUVs for the two lipid mixtures showed very similar size distributions for both initial pores and relaxed pores (Fig. 7C). This suggests that CL does not affect the average size and number of Bax pores per GUV, but rather that it affects the propensity and the lag time for Bax pore opening.
DISCUSSION
In this work, we present a detailed analysis of pores formed by Bax/BakΔC21 using a novel minimalist system of physiological relevance based in the single GUV methodology and the simultaneous addition of different proteinaceous fluorescent size markers. This approach allowed us to directly visualize the passage of two differently sized proteins, Cyt c (12.5 kDa) and APC (104 kDa), across Bax/BakΔC21 pores, as well as to investigate how different parameters and conditions affect Bax/BakΔC21 pore size. From our results, we conclude that the sizes of Bax/BakΔC21 pores are not fixed as typically observed in purely proteinaceous channels, but rather evolve with protein concentration and time, being larger immediately after pore opening than when the pores are relaxed. We also found that increasing the CL content does not vary the size of the Bax pore, but decreases the lag time between protein addition and pore formation. In summary, our results demonstrate that activated Bax and BakΔC21 form stable protein-permeable pores of tunable size.
Bax and Bak have been described to be mostly redundant in the permeabilization of the MOM in cells (49–51). In this study we show that cBid-activated Bax and BakΔC21 form pores of similar sizes and behaviors. In both cases the pore size increases with protein concentration. At low protein concentrations the initial diameter of each pore is ≥8 nm (APC), whereas in equilibrium the pore size is smaller and in the size range of a Cyt _c_488 molecule (diameter 3 nm, area of a circular pore ∼7 nm2). Of note, previous data obtained with a synthetic peptide representing the amphipatic Bax α5, which forms lipid-containing toroidal pores, showed an initial pore diameter of ∼11 nm (35), which relaxed to a pore of ∼5 nm diameter (42). Moreover, other studies performed with antimicrobial amphipatic peptides also known to form toroidal pores such as melitin (52) and magainin (53) showed a qualitatively similar behavior regarding the decrease in pore size.
At high protein concentrations the pore size is always ≥8 nm (area of a circular pore >50 nm2), as the APC passage is barely restricted. Thus, although the overall porated area is diminished from the initial to the equilibrium states, the final size of individual Bax/BakΔC21 pores in equilibrium is clearly bigger at high than at low protein concentrations. Our results can be explained by stably opened or flickering pores. Considering that none of the kinetic curves indicates any flickering we assume that the pores remain stably opened.
The calculated Bax/BakΔC21 pore areas are big and stable enough to allow the total release of all proteins released during apoptosis (cytochrome c, Smac/Diablo, Omi/HtrA2, AIF, and endoglycosidase G), as all these proteins are smaller than APC. Our finding that the pore size is tunable can reconcile the contradictory literature describing a simultaneous or a differential release of Cyt c, Smac/Diablo, and AIF (21, 25). A pore tunable in size adds a new regulatory element in the apoptosis process, which may lead to different pore sizes in different apoptotic scenarios or different cell types.
To date, there is no precedent for a purely proteinaceous channel that undergoes changes in size in a concentration-dependent manner as described here for cBid-activated Bax and BakΔC21. In contrast, toroidal or proteolipidic pores described for several amphipatic antimicrobial peptides can be regulated by the protein:lipid ratio, as well as by membrane lipid composition (41). Indeed, it was already suggested by us and others that Bax/BakΔC21 permeabilize membranes by forming toroidal proteolipidic pores (17, 18, 26, 33–35, 42).
Mechanistically, pore formation could happen as follows. The asymmetric attack induced by insertion of Bax/BakΔC21 into the outer membrane leaflet would generate an increase in surface area in the outer monolayer and therefore create membrane tension. This decreases the energy barrier to form a toroidal pore (reviewed in Ref. 41). In line with this, higher Bax/BakΔC21 concentrations should induce higher membrane tension, leading to bigger initial porated areas as experimentally found. Then, pore formation allows the equilibration of Bax/Bak molecules between the two leaflets and the relaxation of the membrane tension. Toroidal pores are metastable structures that, once the membrane tension is released, tend to close due to the high line tension present at the rim of the pore. To induce stable pores, Bax/Bak decrease the line tension through changes in membrane monolayer curvature and/or by localizing themselves at or near the pore rim (26, 54, 55). In this context, the initial pores induced by Bax and BakΔC21 shrink to a final size that is stabilized by protein molecules. In agreement with this, higher Bax/BakΔC21 concentrations stabilize bigger pores due to the higher amount of Bax/Bak present in the membrane. In line with this interpretation, a peptide derived from helix 5 from Bax was previously shown to reduce the line tension of membrane openings (56) and to induce a toroidal pore (33).
The estimated size of equilibrium pores is bigger when analyzed from pre-equilibrium kinetics compared with equilibrium kinetics (compare Figs. 4D and 5C). These findings are likely due to technical difficulties in the pre-equilibrium kinetics analysis. First, GUVs that do not reach 100% filling are assumed to contain pores closed for the marker, although they could simply contain pores smaller than the marker, leading to an underestimation of the pore size. Second, GUVs completely filled with the marker can be filled either during the initial stage before relaxing takes place, during, or after the relaxation process. Only the third subgroup is well described by the fitting algorithm, whereas in the other two cases the size of the relaxed pore is overestimated. However, the fitting of the pre-equilibrium kinetics describe well the major characteristics of the system: pore relaxation, the correlation of pore size and protein concentrations, and the pore stability. For a quantitative analysis of the pore size, the equilibrium kinetics are better suited.
Our equilibrium pores are smaller than many of the Bax/BakΔC21 pores visible in cryo-EM pictures of LUVs (14, 18, 30). We find two possible explanations for this difference. First, the different conditions used in the cryo-EM experiments (different lipid curvature and different lipid to protein ratio) could lead to different protein densities in the bilayer and therefore the pores could be bigger. Second, studying the cryo-EM pictures in detail one finds many pores in the size of our initial pores and some in the size of our equilibrium pores. The images present the two-dimensional projection of a three-dimensional object. Thus, big pores are always visible, whereas small pores are only visible when they are on certain positions of the LUVs (e.g. small pores on the top or bottom of the vesicle are invisible). This leads to an overestimation of the mean pore size. Taking this into account the pore sizes found in cryo-EM and in the experimental setups used here are not antagonistic. Our equilibrium pores are bigger than pores recently found in cryo-EM experiments with nanodiscs containing Bax that describe a preliminary step of pore formation (57). This result is possibly due to the size limitation set by the nanodisc (reviewed in Ref. 58).
From our previous experiments (18, 19) we know that an increase in CL content has a big impact on cBid, Bax, and BakΔC21 binding to vesicles, although it is not yet clear whether under these conditions Bax and BakΔC21 are inserted into the membrane or only loosely bound to it. The experiments presented here showed two changes in Bax membrane activity upon CL enrichment. First, CL enrichment led to shorter lag times for pore formation, and second, more vesicles were permeabilized during the experiment. The second difference is probably a direct consequence of the first. Thus, the CL content affects protein binding and the initiation of pore opening, which is probably limited by the rate of Bax insertion and/or oligomerization in the membrane. Our results demonstrate that after this initial stage, the pore size and pore number per GUV are similar in both CL concentrations. This result may seem at odds with our finding that the pore size increases with the Bax/BakΔC21 concentration. But this is simply explained because the number of Bax/BakΔC21 molecules per GUV at low protein concentration and 30% CL is still smaller than in the experiments using high Bax/BakΔC21 concentrations and MOM mixture. Moreover, the high cBid concentration at the membrane at the high CL concentration may result into many cBid ·Bax complexes, which are transient intermediates in Bax/Bak activation (59–61). These complexes may inhibit Bax/Bak oligomerization as long as they exist (60, 61). However, CL has been shown to be crucial for Bax/Bak activation (15, 18, 37). We suggest that CL could act like a chaperone promoting Bax insertion into the membrane by steric optimization of the Bax-cBid-membrane interaction, similarly to what we proposed before for Bak (18). The high propensity of CL to adopt non-bilayer configurations (63) may also promote Bax insertion into CL-enriched membranes (32, 39, 62).
To summarize, here we carried out a quantitative analysis of Bax and BakΔC21 pores activity at the single vesicle level. We demonstrate that Bax and BakΔC21 open pores of similar sizes that induce stable vesicle permeabilization during long times. The pore size and its stability are tunable by the concentration of Bax/BakΔC21, whereas the propensity for pore opening can be regulated by protein concentration or CL content. These two elements offer useful mechanisms for cells to fine-tune the timing of the proapoptotic factor release and apoptotic progression and might explain several controversial findings described in literature.
Acknowledgment
We (S.B. and A.J.G.S.) thank Carolin Stegmueller for excellent technical assistance.
Footnotes
7
The abbreviations used are:
MOM
mitochondrial outer membrane
GUV
giant unilamellar vesicle
CL
cardiolipin
APC
allophycocyanin
PC
l-α-phosphatidylcholine
PE
l-α-phosphatidylethanolamine
PI
l-α-phosphatidylinositol
PS
phosphatidylserine
DiI
1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine perclorate
LUV
large unilamellar vesicle
Cyt c
cytochrome c.
REFERENCES
- 1.Danial N. N., Gimenez-Cassina A., Tondera D. (2010) Homeostatic functions of BCL-2 proteins beyond apoptosis. Adv. Exp. Med. Biol. 687, 1–32 [DOI] [PubMed] [Google Scholar]
- 2.García-Sáez A. J., Fuertes G., Suckale J., Salgado J. (2010) Permeabilization of the outer mitochondrial membrane by Bcl-2 proteins. Adv. Exp. Med. Biol. 677, 91–105 [DOI] [PubMed] [Google Scholar]
- 3.Basañez G., Soane L., Hardwick J. M. (2012) A new view of the lethal apoptotic pore. PLoS Biol. 10, e1001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Sáez A. J. (2012) The secrets of the Bcl-2 family. Cell Death Differ. 19, 1733–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender T., Martinou J. C. (2013) Where killers meet. Permeabilization of the outer mitochondrial membrane during apoptosis. Cold Spring Harbor Perspect. Biol. 5, a011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llambi F., Green D. R. (2011) Apoptosis and oncogenesis: give and take in the BCL-2 family. Curr. Opin. Genet. Dev. 21, 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schellenberg B., Wang P., Keeble J. A., Rodriguez-Enriquez R., Walker S., Owens T. W., Foster F., Tanianis-Hughes J., Brennan K., Streuli C. H., Gilmore A. P. (2013) Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol. Cell 49, 959–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlich F., Banerjee S., Suzuki M., Cleland M. M., Arnoult D., Wang C., Neutzner A., Tjandra N., Youle R. J. (2011) Bcl-xL retrotranslocates Bax from the mitochondria into the cytosol. Cell 145, 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolter K. G., Hsu Y. T., Smith C. L., Nechushtan A., Xi X. G., Youle R. J. (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleicken S., García-Sáez A. J., Conte E., Bordignon E. (2012) Dynamic interaction of cBid with detergents, liposomes and mitochondria. PloS One 7, e35910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer P. E., Frederick P., Gulbis J. M., Dewson G., Kluck R. M. (2012) Translocation of a Bak C-terminus mutant from cytosol to mitochondria to mediate cytochrome. Implications for Bak and Bax apoptotic function. PLoS One 7, e31510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desagher S., Osen-Sand A., Nichols A., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J. C. (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144, 891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskes R., Desagher S., Antonsson B., Martinou J. C. (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20, 929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bleicken S., Classen M., Padmavathi P. V., Ishikawa T., Zeth K., Steinhoff H. J., Bordignon E. (2010) Molecular details of Bax activation, oligomerization, and membrane insertion. J. Biol. Chem. 285, 6636–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwana T., Mackey M. R., Perkins G., Ellisman M. H., Latterich M., Schneiter R., Green D. R., Newmeyer D. D. (2002) Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111, 331–342 [DOI] [PubMed] [Google Scholar]
- 16.Lovell J. F., Billen L. P., Bindner S., Shamas-Din A., Fradin C., Leber B., Andrews D. W. (2008) Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135, 1074–1084 [DOI] [PubMed] [Google Scholar]
- 17.Terrones O., Antonsson B., Yamaguchi H., Wang H. G., Liu J., Lee R. M., Herrmann A., Basañez G. (2004) Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J. Biol. Chem. 279, 30081–30091 [DOI] [PubMed] [Google Scholar]
- 18.Landeta O., Landajuela A., Gil D., Taneva S., Di Primo C., Sot B., Valle M., Frolov V. A., Basañez G. (2011) Reconstitution of proapoptotic BAK function in liposomes reveals a dual role for mitochondrial lipids in the BAK-driven membrane permeabilization process. J. Biol. Chem. 286, 8213–8230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleicken S., Wagner C., García-Sáez A. J. (2013) Mechanistic differences in the membrane activity of Bax and Bcl-xL correlate with their opposing roles in apoptosis. Biophys. J. 104, 421–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz-Pinedo C., Guío-Carrión A., Goldstein J. C., Fitzgerald P., Newmeyer D. D., Green D. R. (2006) Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary in duration. Proc. Natl. Acad. Sci. U.S.A. 103, 11573–11578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rehm M., Düssmann H., Prehn J. H. (2003) Real-time single cell analysis of Smac/DIABLO release during apoptosis. J. Cell Biol. 162, 1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhola P. D., Mattheyses A. L., Simon S. M. (2009) Spatial and temporal dynamics of mitochondrial membrane permeability waves during apoptosis. Biophys. J. 97, 2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips D. C., Martin S., Doyle B. T., Houghton J. A. (2007) Sphingosine-induced apoptosis in rhabdomyosarcoma cell lines is dependent on pre-mitochondrial Bax activation and post-mitochondrial caspases. Cancer Res. 67, 756–764 [DOI] [PubMed] [Google Scholar]
- 24.Flanagan L., Sebastià J., Tuffy L. P., Spring A., Lichawska A., Devocelle M., Prehn J. H., Rehm M. (2010) XIAP impairs Smac release from the mitochondria during apoptosis. Cell Death Dis. 1, e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnoult D., Parone P., Martinou J. C., Antonsson B., Estaquier J., Ameisen J. C. (2002) Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J. Cell Biol. 159, 923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basañez G., Nechushtan A., Drozhinin O., Chanturiya A., Choe E., Tutt S., Wood K. A., Hsu Y., Zimmerberg J., Youle R. J. (1999) Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. U.S.A. 96, 5492–5497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito M., Korsmeyer S. J., Schlesinger P. H. (2000) BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat. Cell Biol. 2, 553–555 [DOI] [PubMed] [Google Scholar]
- 28.Roucou X., Rostovtseva T., Montessuit S., Martinou J. C., Antonsson B. (2002) Bid induces cytochrome _c_-impermeable Bax channels in liposomes. Biochem. J. 363, 547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Caballero S., Dejean L. M., Kinnally M. S., Oh K. J., Mannella C. A., Kinnally K. W. (2009) Assembly of the mitochondrial apoptosis-induced channel, MAC. J. Biol. Chem. 284, 12235–12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer B., Quispe J., Choudhary V., Chipuk J. E., Ajero T. G., Du H., Schneiter R., Kuwana T. (2009) Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. Mol. Biol. Cell 20, 2276–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etxebarria A., Terrones O., Yamaguchi H., Landajuela A., Landeta O., Antonsson B., Wang H. G., Basañez G. (2009) Endophilin B1/Bif-1 stimulates BAX activation independently from its capacity to produce large scale membrane morphological rearrangements. J. Biol. Chem. 284, 4200–4212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montessuit S., Somasekharan S. P., Terrones O., Lucken-Ardjomande S., Herzig S., Schwarzenbacher R., Manstein D. J., Bossy-Wetzel E., Basañez G., Meda P., Martinou J. C. (2010) Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 142, 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian S., Wang W., Yang L., Huang H. W. (2008) Structure of transmembrane pore induced by Bax-derived peptide. Evidence for lipidic pores. Proc. Natl. Acad. Sci. U.S.A. 105, 17379–17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-Sáez A. J., Coraiola M., Dalla Serra M., Mingarro I., Menestrina G., Salgado J. (2005) Peptides derived from apoptotic Bax and Bid reproduce the poration activity of the parent full-length proteins. Biophys. J. 88, 3976–3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-Sáez A. J., Coraiola M., Serra M. D., Mingarro I., Müller P., Salgado J. (2006) Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. FEBS J. 273, 971–981 [DOI] [PubMed] [Google Scholar]
- 36.Epand R. F., Martinou J. C., Montessuit S., Epand R. M. (2003) Transbilayer lipid diffusion promoted by Bax. Implications for apoptosis. Biochemistry 42, 14576–14582 [DOI] [PubMed] [Google Scholar]
- 37.Bleicken S., Zeth K. (2009) Conformational changes and protein stability of the pro-apoptotic protein Bax. J. Bioenerg. Biomembr. 41, 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terrones O., Etxebarria A., Landajuela A., Landeta O., Antonsson B., Basañez G. (2008) BIM and tBID are not mechanistically equivalent when assisting BAX to permeabilize bilayer membranes. J. Biol. Chem. 283, 7790–7803 [DOI] [PubMed] [Google Scholar]
- 39.Etxebarria A., Landeta O., Antonsson B., Basañez G. (2008) Regulation of antiapoptotic MCL-1 function by gossypol. Mechanistic insights from in vitro reconstituted systems. Biochem. Pharmacol. 76, 1563–1576 [DOI] [PubMed] [Google Scholar]
- 40.García-Sez A. J., Ries J., Orzáez M., Pérez-Payà E., áSchwille P. (2009) Membrane promotes tBID interaction with BCL(XL). Nat. Struct. Mol. Biol. 16, 1178–1185 [DOI] [PubMed] [Google Scholar]
- 41.Fuertes G., Giménez D., Esteban-Martín S., Sánchez-Muñoz O. L., Salgado J. (2011) A lipocentric view of peptide-induced pores. Eur. Biophys. J. 40, 399–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuertes G., García-Sáez A. J., Esteban-Martín S., Giménez D., Sánchez-Muñoz O. L., Schwille P., Salgado J. (2010) Pores formed by Baxα5 relax to a smaller size and keep at equilibrium. Biophys. J. 99, 2917–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuertes G. (2011) Baxα5 at Lipid Membranes: Structure, Assembly and Pore Formation, Ph.D. thesis, Universitat de València, Valencia, Italy [Google Scholar]
- 44.Kucerka N., Tristram-Nagle S., Nagle J. F. (2005) Structure of fully hydrated fluid phase lipid bilayers with monounsaturated chains. J. Membr. Biol. 208, 193–202 [DOI] [PubMed] [Google Scholar]
- 45.Leshchiner E. S., Braun C. R., Bird G. H., Walensky L. D. (2013) Direct activation of full-length proapoptotic BAK. Proc. Natl. Acad. Sci. U.S.A. 110, E986–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ardail D., Privat J. P., Egret-Charlier M., Levrat C., Lerme F., Louisot P. (1990) Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 265, 18797–18802 [PubMed] [Google Scholar]
- 47.Brejc K., Ficner R., Huber R., Steinbacher S. (1995) Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the cyanobacterium Spirulina platensis at 2.3 Å resolution. J. Mol. Biol. 249, 424–440 [DOI] [PubMed] [Google Scholar]
- 48.Mirkin N., Jaconcic J., Stojanoff V., Moreno A. (2008) High resolution X-ray crystallographic structure of bovine heart cytochrome c and its application to the design of an electron transfer biosensor. Proteins 70, 83–92 [DOI] [PubMed] [Google Scholar]
- 49.Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. (2001) Proapoptotic BAX and BAK. A requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zong W. X., Lindsten T., Ross A. J., MacGregor G. R., Thompson C. B. (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15, 1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindsten T., Ross A. J., King A., Zong W. X., Rathmell J. C., Shiels H. A., Ulrich E., Waymire K. G., Mahar P., Frauwirth K., Chen Y., Wei M., Eng V. M., Adelman D. M., Simon M. C., Ma A., Golden J. A., Evan G., Korsmeyer S. J., MacGregor G. R., Thompson C. B. (2000) The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell 6, 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuzaki K., Yoneyama S., Miyajima K. (1997) Pore formation and translocation of melittin. Biophys. J. 73, 831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamba Y., Yamazaki M. (2009) Magainin 2-induced pore formation in the lipid membranes depends on its concentration in the membrane interface. J. Phys. Chem. B 113, 4846–4852 [DOI] [PubMed] [Google Scholar]
- 54.Mihajlovic M., Lazaridis T. (2010) Antimicrobial peptides bind more strongly to membrane pores. Biochim. Biophys. Acta 1798, 1494–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang H. W., Chen F. Y., Lee M. T. (2004) Molecular mechanism of peptide-induced pores in membranes. Phys. Rev. Lett. 92, 198304. [DOI] [PubMed] [Google Scholar]
- 56.García-Sáez A. J., Chiantia S., Salgado J., Schwille P. (2007) Pore formation by a Bax-derived peptide. Effect on the line tension of the membrane probed by AFM. Biophys. J. 93, 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu X. P., Zhai D., Kim E., Swift M., Reed J. C., Volkmann N., Hanein D. (2013) Three-dimensional structure of Bax-mediated pores in membrane bilayers. Cell Death Dis. 4, e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bayburt T. H., Sligar S. G. (2010) Membrane protein assembly into Nanodiscs. FEBS Lett. 584, 1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Czabotar P. E., Westphal D., Dewson G., Ma S., Hockings C., Fairlie W. D., Lee E. F., Yao S., Robin A. Y., Smith B. J., Huang D. C., Kluck R. M., Adams J. M., Colman P. M. (2013) Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531 [DOI] [PubMed] [Google Scholar]
- 60.Dai H., Smith A., Meng X. W., Schneider P. A., Pang Y. P., Kaufmann S. H. (2011) Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J. Cell Biol. 194, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moldoveanu T., Grace C. R., Llambi F., Nourse A., Fitzgerald P., Gehring K., Kriwacki R. W., Green D. R. (2013) BID-induced structural changes in BAK promote apoptosis. Nat. Struct. Mol. Biol. 20, 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinou J. C., Youle R. J. (2011) Mitochondria in apoptosis. Bcl-2 family members and mitochondrial dynamics. Dev. Cell 21, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis R. N., McElhaney R. N. (2009) The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim. Biophys. Acta 1788, 2069–2079 [DOI] [PubMed] [Google Scholar]