Prediction of Outcomes in Crescentic IgA Nephropathy in a Multicenter Cohort Study (original) (raw)
Abstract
Crescentic IgA nephropathy (IgAN), defined as >50% crescentic glomeruli on kidney biopsy, is one of the most common causes of rapidly progressive GN. However, few studies have characterized this condition. To identify risk factors and develop a prediction model, we assessed data from patients≥14 years old with crescentic IgAN who were followed ≥12 months. The discovery cohort comprised 52 patients from one kidney center, and the validation cohort comprised 61 patients from multiple centers. At biopsy, the mean serum creatinine (SCr) level ± SD was 4.3±3.4 mg/dl, and the mean percentage of crescents was 66.4%±15.8%. The kidney survival rates at years 1, 3, and 5 after biopsy were 57.4%±4.7%, 45.8%±5.1%, and 30.4%±6.6%, respectively. Multivariate Cox regression revealed initial SCr as the only independent risk factor for ESRD (hazard ratio [HR], 1.32; 95% confidence interval [CI], 1.10 to 1.57; _P_=0.002). Notably, the percentage of crescents did not associate independently with ESRD. Logistic regression showed that the risk of ESRD at 1 year after biopsy increased rapidly at SCr>2.7 mg/dl and reached 90% at SCr>6.8 mg/dl (specificity=98.5%, sensitivity=64.6% for combined cohorts). In both cohorts, patients with SCr>6.8 mg/dl were less likely to recover from dialysis. Analyses in additional cohorts revealed a similar association between initial SCr and ESRD in patients with antiglomerular basement membrane disease but not ANCA-associated systemic vasculitis. In conclusion, crescentic IgAN has a poor prognosis, and initial SCr concentration may predict kidney failure in patients with this disease.
IgA nephropathy (IgAN) is one of the most common GN worldwide.1 On immunohistological examination, it is characterized by the presence of glomerular IgA deposition.2 IgAN is now recognized as an autoimmune renal disease that occurs as a consequence of increased circulating levels of IgA1 with galactose-deficient hinge region _O_-glycans and antiglycan autoantibodies.3 The clinical and pathologic manifestations of IgAN are diverse. The clinical course of the disease ranges from isolated hematuria to rapidly progressive renal failure,4 and kidney biopsy findings vary from mild mesangial proliferation to diffuse crescent formation.2 Extracapillary proliferation, usually characterized by noncircumferential crescents, occurs in up to 20%–30% of IgAN patients.2,5 Crescentic IgAN, usually defined as the presence of crescents in over 50% of the glomeruli, is a rare phenotype, and it often presents as rapidly progressively kidney failure.2,6
Crescentic IgAN affects only a minority of IgAN patients and has not been widely investigated, except in a few small studies.5,7,8 The recent Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for GN recommended that crescentic IgAN be treated using an immunosuppressive therapy regimen analogous to the regimen used for ANCA vasculitis.6 Until now, most studies on crescentic IgAN have been case series or small studies with less than 30 patients.7,8 Little is known about the long-term outcomes and risk factors that influence kidney recovery in patients with crescentic IgAN,9 especially those patients who have undergone initial aggressive immunosuppressive therapy. We, therefore, examined the long-term outcomes of a large cohort of 113 patients who were diagnosed with crescentic IgAN using the criterion of crescents in more than 50% of glomeruli on biopsy specimens. In addition, we developed a concise model for predicting recovery from kidney failure.
Results
Patient Characteristics
This study included 113 Chinese patients with crescentic IgAN recruited from eight kidney centers across China. Most of the patients (58.4%) were men (Table 1). The mean initial urine protein excretion was 4.44±2.63 g/d, the serum creatinine (SCr) level was 4.3±3.4 mg/dl, and the mean percentage of crescents was 66.4%±15.8% (percentages of cellular, fibrocellular, and fibrous crescents were 24.3%±19.5%, 31.6%±19.5%, and 8.3%±14.5%, respectively). The patients were followed up for 22.6±23.9 months (range=12–102 months). Overall, 88 patients (78%) presented with rapidly progressive GN, which was defined as an increase in SCr to more than 1.5 mg/dl; 12 of the remaining patients showed nephrotic range proteinuria (>3.5 g/d), and 12 patients had hematuria and proteinuria.
Table 1.
Baseline characteristics of patients with diffuse crescentic IgAN (discovery and validation groups), crescentic anti-GBM disease, or crescentic AASV
| Parameter | IgAN | Anti-GBM Disease | AASV | |||
|---|---|---|---|---|---|---|
| Entire Population | Discovery Cohort | Validation Cohort | Pa | |||
| N of patients | 113 | 52 | 61 | 38 | 42 | |
| Baseline | ||||||
| Men (N) | 66; 58.5% | 34; 65% | 32; 52.5% | 0.17 | 26; 68.4% | 19; 45.2% |
| Age (yr) | 36.3±15.1 | 35.4±15.8 | 37.0±14.6 | 0.47 | 34.3±15.8 | 59.2±14.1 |
| History (mo) | 10.4±23.2 | 12.8±28.7 | 7.8±15.2 | 0.26 | 2.6±3.1 | 32.6±52.9 |
| Macrohematuria (N) | 26; 23% | 17; 32.7% | 9; 14.8% | 0.03 | 13; 34.2% | 4; 9.1% |
| MABP (mmHg) | 118.1±19.7 | 119.0±20.2 | 117.3±19.3 | 0.53 | 99.4±15.2 | 95.5±14.2 |
| Hypertension (N) | 79; 69.9% | 46; 88.8% | 33; 54.1% | <0.001 | 20; 52.6% | 19; 43.2% |
| SCr (mg/dl) | 4.3±3.3 | 4.7±3.4 | 3.9±3.2 | 0.19 | 7.6±3.7 | 6.8±3.2 |
| Oliguria (N) | 9; 8.0% | 3; 5.8% | 6; 9.8% | 0.43 | 11; 28.9% | 5; 11.4% |
| Proteinuria (g/24 h) | 4.44±2.63 | 4.84±3.13 | 4.00±2.13 | 0.33 | 3.92±3.71 | 2.55±1.94 |
| Nephrotic syndrome (N) | 38; 33.6% | 18; 34.6% | 20; 32.8% | 0.84 | 8; 21.0% | 9; 20.5% |
| Pathology | ||||||
| Total glomeruli (N) | 23.5±12.0 | 26.1±12.1 | 21.1±11.6 | 0.008 | 24.3±10.4 | 26.7±12.1 |
| Total crescent (%) | 66.4±15.8 | 68.5±15.4 | 64.2±16.0 | 0.24 | 91±13 | 76.8±16.6 |
| Cellular crescent (%) | 25.0±20.1 | 25.6±20.7 | 24.3±19.5 | 0.81 | 55.8±30.0 | 28.8±25.5 |
| Fibrocellularcrescent (%) | 30.0±19.1 | 28.5±18.7 | 31.6±19.5 | 0.42 | 32.0±28.3 | 19.2±16.6 |
| Fibrouscrescent (%) | 11.4±15.0 | 14.4±15.0 | 8.3±14.5 | 0.004 | 2.9±6.7 | 28.9±28.4 |
| Global glomerulosclerosis (%) | 7.9±10.6 | 7.9±9.8 | 7.9±11.7 | 0.99 | ||
| Normal glomeruli (%) | 20.7±14.8 | 21.5±15.1 | 19.8±14.4 | 0.59 | ||
| Tubular atrophy/interstitial fibrosis (N) | 0.28 | |||||
| 0% | 0 | 0 | 0 | |||
| 1%–50% | 43; 38.1% | 17; 32.7% | 26; 42.6% | |||
| >50% | 66; 58.4% | 33; 63.5% | 33; 54.1% | |||
| Interstitial inflammation (N) | 0.26 | |||||
| 1%–20% | 2; 1.8% | 0; 0 | 2; 3.3% | |||
| 20%–50% | 7; 6.2% | 2; 3.8% | 5; 8.2% | |||
| >50% | 100; 88.5% | 48; 92.3% | 52; 85.2% | |||
| Follow-up time (mo) | 23.7±22.8 | 24.6±21.4 | 22.9±24.1 | 0.27 | 1.8±6.4 | 22.6±26.7 |
| Treatment | ||||||
| Plasma exchange | 0 | 0 | 0 | 21 | 2 | |
| Steroids + CTX | 51 | 32 | 19 | 12 | 37 | |
| Steroids + MMF/leflunomide | 6 | 3 | 3 | |||
| Steroids alone | 21 | 10 | 11 | 4 | 1 | |
| CTX or CysA alone | 3 | 0 | 3 | |||
| No immunosuppressive treatment | 32 | 7 | 25 | 1 | 4 | |
| ESRD (N) | 63; 55.8% | 28; 53.8% | 35; 57.4% | 0.71 | 35; 92.1% | 29; 69.0% |
| ESRD at 1 yr (N) | 48; 42.5% | 22; 42.3% | 26; 42.6% | 0.97 | 35; 92.1% | 22; 52.4% |
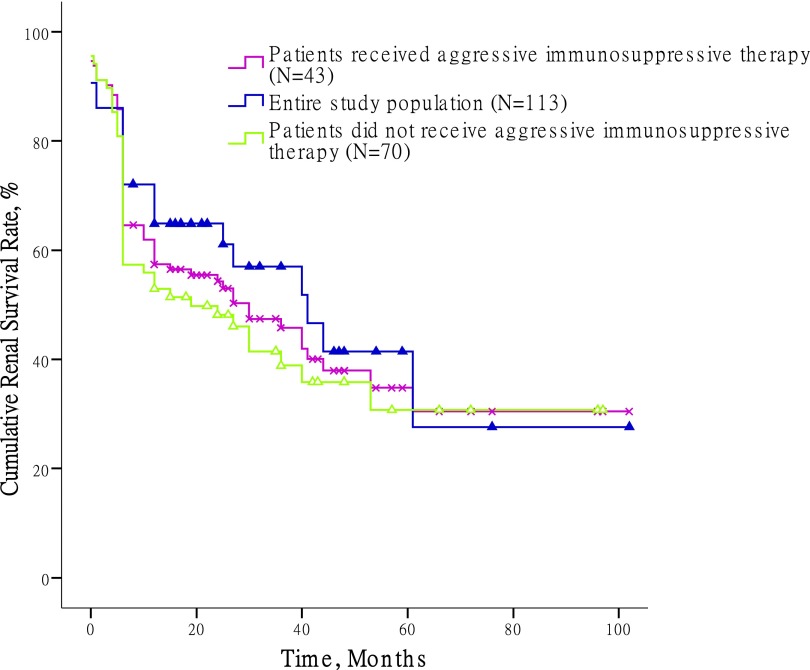
A total of 57 patients (50.4%) were treated with steroids and immunosuppressive agents, including 43 patients (38.1%) who received pulse methylprednisolone plus cyclophosphamide therapy (Supplemental Table 1). By the time of study completion, 63 patients (55.8%) had developed ESRD. The cumulative kidney survival rates in the first, third, and fifth years were 57.4%±4.7%, 45.8%±5.1%, and 30.4%±6.6%, respectively. In 43 patients who received aggressive immunosuppressive therapy, which was defined as high-dose pulse methylprednisolone (7–15 mg/kg per day) plus cyclophosphamide therapy, the kidney survival rates in the first, third, and fifth years were 64.9%±7.3%, 57.0%±8.3%, and 27.6%±13.0%, respectively (Figure 1).
Figure 1.
Renal survival of crescentic IgA nephropathy treated with different regimens. Aggressive immunosuppressive therapy was defined as high-dose pulse methylprednisolone (7–15 mg/kg per day) plus cyclophosphamide therapy.
Predictors of Kidney Survival
In this study, 52 patients from one large renal center were considered the discovery cohort, and the remaining 61 patients from multiple centers formed the validation cohort. The baseline clinical and pathologic characteristics did not significantly differ between these two groups (Table 1).
We investigated whether the clinical characteristics of the patients at the time of disease onset were associated with subsequent ESRD. In the discovery cohort, univariate Cox regression revealed that the initial SCr (mg/dl) concentration (hazard ratio [HR], 1.37; 95% confidence interval [95% CI], 1.20 to 1.56; P<0.001), macrohematuria (HR, 0.36; 95% CI, 0.14 to 0.95; _P_=0.04), mean BP (HR, 1.03; 95% CI, 1.01 to 1.05; _P_=0.001), percentage of global glomerulosclerosis (HR, 173.65; 95% CI, 5.79 to 5205.46; _P_=0.003), percentage of normal glomeruli (HR, 0.03; 95% CI, <0.01 to 0.43; _P_=0.01), and interstitial fibrosis/tubular atrophy (HR, 5.85; 95% CI, 1.74 to 19.68; P<0.001) were risk factors for the development of ESRD. However, on multivariate regression, only SCr concentration (HR, 1.34; 95% CI, 1.07 to 1.67; _P_=0.009) was independently associated with ESRD events. The percentage of glomeruli showing crescent formation was not found to be a risk factor for ESRD on univariate or multivariate Cox regression (Table 2).
Table 2.
Factors that were found to affect renal survival in patients with crescentic IgAN on univariate and multivariate Cox regression analyses
| Study Group | Validation Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Women | 1.08 | 0.50 to 2.32 | 0.85 | 0.45 | 0.23 to 0.92 | 0.03 | 0.83 | 0.30 to 2.30 | 0.72 | |||
| Age (yr) | 1.01 | 0.98 to 1.03 | 0.64 | 0.99 | 0.96 to 1.01 | 0.21 | ||||||
| Proteinuria (g/24 h) | 1.05 | 0.95 to 1.16 | 0.39 | 0.98 | 0.83 to 1.16 | 0.88 | ||||||
| SCr (mg/dl) | 1.37 | 1.20 to 1.56 | <0.001 | 1.34 | 1.08 to 1.67 | 0.009 | 1.36 | 1.23 to 1.50 | <0.001 | 1.51 | 1.25 to 1.82 | <0.001 |
| Serum albumin (g/L) | 0.96 | 0.90 to 1.03 | 0.25 | 1.00 | 0.96 to 1.05 | 0.93 | ||||||
| Macrohematuria | 0.36 | 0.14 to 0.95 | 0.04 | 1.27 | 0.36 to 4.46 | 0.71 | 0.32 | 0.08 to 1.35 | 0.12 | |||
| Oliguria | 0.63 | 0.09 to 4.65 | 0.65 | 1.31 | 0.40 to 4.31 | 0.66 | ||||||
| Hypertension | 3.98 | 0.54 to 29.39 | 0.18 | 3.34 | 1.48 to 7.55 | 0.007 | ||||||
| Mean BP (mmHg) | 1.03 | 1.01 to 1.05 | 0.002 | 0.10 | 0.97 to 1.02 | 0.78 | 1.03 | 1.01 to 1.04 | 0.004 | 1.01 | 0.99 to 1.04 | 0.40 |
| Total crescent (%) | 2.30 | 0.18 to 29.03 | 0.52 | 10.36 | 0.65 to 166.48 | 0.10 | ||||||
| Cellular/fibrocellularcrescent (%) | 1.49 | 0.45 to 4.96 | 0.52 | 3.27 | 0.43 to 24.74 | 0.25 | ||||||
| Global glomerulosclerosis (%) | 173.65 | 5.79 to 5205.46 | 0.003 | 5.17 | 0.09 to 295.42 | 0.43 | 77.20 | 2.46 to 2423.68 | 0.01 | 0.79 | 0.01 to 57.30 | 0.92 |
| Normal glomeruli (%) | 0.03 | <0.01 to 0.43 | 0.01 | 4.52 | 0.12 to 164.86 | 0.41 | <0.01 | 0.00 to 0.12 | 0.002 | 0.17 | <0.01 to 30.97 | 0.51 |
| Tubular atrophy/interstitial fibrosis (N; %, study/validation group) | ||||||||||||
| 1%–50% (17; 33%/26; 43%) | Reference | Reference | Reference | |||||||||
| >50% (33; 64%/33; 54%) | 5.85 | 1.74 to 19.68 | <0.001 | 3.53 | 0.69 to 17.99 | 0.13 | 4.65 | 2.00 to 10.81 | <0.001 | 3.25 | 0.69 to 15.38 | 0.14 |
| Interstitial inflammation (N; %, study/validation group) | ||||||||||||
| 1%–20% (0; 0%/2; 3%) | Reference | |||||||||||
| 20%–50% (2; 4%/5; 8%) | NA | 52,791.69 | 0.00 to 2.42×e148 | 0.95 | ||||||||
| >50% (48; 92%/52; 85%) | 24.21 | 0.03 to 19,252 | 0.35 | 71,865.22 | 0.00 to 3.29×e148 | 0.95 |
Model for ESRD Prediction
In the discovery cohort, we used the initial SCr concentration as the variable and calculated the cumulative probability of ESRD at 1 year after renal biopsy using logistic regression. The logistic curve fitted a typical S shape, with two clear turning points (Figure 2A). When the initial SCr concentration was lower than 3.6 mg/dl, the cumulative probability of ESRD at 1 year after renal biopsy was lower than 20%. However, the risk increased rapidly with increase in the SCr concentration. When the concentration was higher than 6.5 mg/dl, the cumulative probability of ESRD plateaued at above 90%. In the patients in the discovery cohort who had received aggressive immunosuppressive therapy (_n_=27), the model for predicting kidney mortality yielded a similar S-shaped curve (Figure 2B), with two turning points at 3.7 and 6.6 mg/dl. Thus, we identified that an SCr concentration of 6.8 mg/dl (600 μmol/L) at the time of renal biopsy was a nonreturn point for the occurrence ESRD, and these patients were less likely to recover from dialysis. Indeed, in the discovery cohort, all 17 patients with initial SCr concentration>6.8 mg/dl (600 μmol/L) progressed to ESRD at the end of 1 year. The specificity and sensitivity of this SCr cutoff for predicting ESRD at 1 year were 100% and 72.7%, respectively. During follow-up, the discriminatory capacity of this SCr concentration for ESRD prediction remained good, with 100% of patients with initial SCr>6.8 mg/dl (600 μmol/L) developing ESRD (P<0.001) (Figure 3A). In the discovery cohort, the specificity and sensitivity of SCr>6.8 mg/dl (600 μmol/L) in predicting ESRD during follow-up were 100% and 57.1%, respectively.
Figure 2.
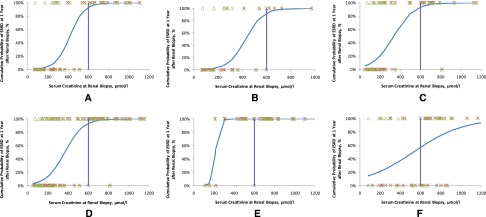
The logistic regression curves for crescentic GN due to IgAN, Anti-GBM disease, and AASV. Blue line, logistic curve; green triangles, renal survival during follow-up; purple line, SCr concentration=6.8 mg/dl (600 μmol/L); red crosses, renal survival at 1 year after renal biopsy. A value of 0% means that the patient did not develop ESRD, whereas 100% means that the patient developed ESRD. (A) Logistic curve in the discovery cohort (_n_=52). The curve is S-shaped. In patients with SCr>6.8 mg/dl (600 μmol/L), the cumulative probability of ESRD at 1 year is >90%. (B) A similar result is observed in the discovery cohort patients under aggressive immunosuppressive therapy. (C) Logistic curve in the validation cohort (_n_=61). The threshold for predicting renal mortality was identical to the value calculated for the discovery cohort. (D) Logistic curve of the entire crescentic IgAN cohort. (E) The logistic curve of anti-GBM disease is S-shaped. (F) The logistic curve of AASV is nearly linear.
Figure 3.
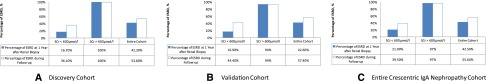
The prediction ability of SCr threshold (6.8mg/dl) for ESRD event. (A) Percentage of patients who developed ESRD at 1 year after renal biopsy and during follow-up in the discovery cohort (_n_=52). (B) Percentage of patients who developed ESRD at 1 year after renal biopsy and during follow-up in the validation cohort (_n_=61). (C) Percentage of patients who developed ESRD at 1 year after renal biopsy and during follow-up in the entire crescentic IgAN cohort (_n_=113). The SCr threshold of 6.8 mg/dl (600 μmol/L) showed good power in predicting renal mortality at 1 year and during follow-up.
Validation of Prediction Model
To determine the risk factors for and test the prediction model of crescentic IgAN, we further confirmed the above results in a validation cohort of 61 patients. On univariate analyses, women (HR, 0.45; 95% CI, 0.23 to 0.92), hypertension (HR, 3.34; 95% CI, 1.48 to 7.55), mean BP (HR, 1.03; 95% CI, 1.01 to 1.04), SCr concentration (HR, 1.36; 95% CI, 1.23 to 1.50), percentage of global glomerulosclerosis (HR, 77.20; 95% CI, 2.46 to 2423.678; _P_=0.01), percentage of normal glomeruli (HR, <0.01; 95% CI, 0.00 to 0.12; _P_=0.002), and interstitial fibrosis/tubular atrophy (HR, 4.65; 95% CI, 2.00 to 10.81) were risk factors for the development of ESRD. SCr concentration was identified as the only determinant of ESRD on multivariate analyses (HR, 1.51; 95% CI, 1.25 to 1.82; _P_<0.001). The logistic curve of the association between the probability of ESRD and SCr concentration was S-shaped (Figure 2C), with turning points at 2.3 and 6.3 mg/dl (Figures 2C and 3B). The specificity and sensitivity of SCr>6.8 mg/dl (600 μmol/L) in predicting ESRD at 1 year were 97.1% and 57.7%, respectively, whereas the specificity and sensitivity during follow-up were 96.2% and 42.9%, respectively. We also performed a sensitivity analysis of this model in patients with cellular/fibrocellular crescents>50% of the total glomeruli (_n_=66) or >50% of the total crescents (_n_=88). These analyses did not change the overall results (Supplemental Tables 1 and 2). The interstitial fibrosis/tubular atrophy was found to be independently associated with kidney failure in the entire cohort analysis (_n_=113), although it was not found to be associated in the discovery or validation cohort separately.
We next analyzed the entire cohort to further develop the prediction model. We calculated the model for the prediction of ESRD at 1 year after renal biopsy as follows:
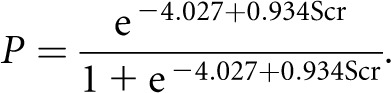
The two turning points of initial SCr concentration for predicting ESRD were 2.7 and 6.4 mg/dl (Figure 2D). In the entire cohort (_n_=113), 32 patients had an initial SCr concentration higher than 6.8 mg/dl (600 μmol/L), and 31 of these patients (97.0%) developed ESRD during follow-up (Figure 3C). The specificity and sensitivity of SCr>6.8 mg/dl (600 μmol/L) in predicting ESRD within 1 year were 98.5% and 64.6%, respectively, whereas the specificity and sensitivity during the entire follow-up period were 98.0% and 49.2%, respectively.
Comparison with ANCA-Associated Systemic Vasculitis and Antiglomerular Basement Membrane Disease
The association between ESRD and initial SCr concentration was also assessed for two other types of crescentic glomerulonephritides, namely antiglomerular basement membrane (anti-GBM) disease and ANCA-associated systemic vasculitis (AASV). The logistic curve for anti-GBM disease fitted a typical S shape (Figure 2E); however, in the case AASV, the initial SCr concentration and ESRD risk showed a nearly linear association (Figure 2F). All anti-GBM disease patients with SCr>6.8 mg/dl (600 μmol/L) progressed to ESRD, whereas of 25 AASV patients with SCr>6.8 mg/dl, 24% of patients (6/25) recovered from dialysis after aggressive immunosuppressive therapy.
Discussion
In this large study, we found that crescentic IgAN had a poor prognosis, and nearly 70% of these patients progressed to ESRD in 5 years, including those patients who had received immunosuppressive therapy. The initial SCr concentration was the strongest risk factor for kidney failure; interestingly, the percentage of crescents was not independently associated with the occurrence of ESRD in this multicenter study. Using logistic curves, we developed a simple model based on SCr concentration to predict the outcomes of crescentic IgAN. In this model, a plot of the association between the probability of ESRD and the initial SCr formed an S-shaped curve. Patients with early stage disease (SCr<2.7 mg/dl; the first turning point) had a good prognosis, with less than 25% of patients progressing to ESRD during follow-up. In contrast, patients with SCr>6.8 mg/dl (600 μmol/L; the second turning point) were less likely to recover from chronic dialysis, even after aggressive immunosuppressive therapy.
The strengths of this study are its large sample size and the multicenter validation of the reported results. To the best of our knowledge, this study is the largest study on crescentic IgAN reported to date. IgAN with diffuse crescent formation is a rare but severe pathologic manifestation of IgAN. Although crescentic IgAN often rapidly progresses to kidney failure, only a few small-scale studies have characterized this condition. In a Japanese study with 25 crescentic IgAN patients, 75% of the patients developed ESRD in 10 years.5 In another study with 25 Chinese patients who had IgAN with diffuse crescent formation, most of the patients exhibited rapid deterioration of kidney function.8 With immunosuppressive therapy, approximately 67% of these patients maintained sufficient kidney function to avoid renal replacement therapy. However, the high incidence of loss to follow-up (10 of 25 patients) greatly limited the study results.8 Because of the small sample size and limited study power of the above studies, neither of them could assess the risk factors for crescentic IgAN. In the recent Oxford classification of IgAN, patients with rapidly progressive kidney failure were excluded, and thus, crescentic IgAN was not evaluated.2 In the present large multicenter cohort study, we have described the long-term outcomes of patients with diffuse crescent formation and found that recovery from illness could be simply predicted using the initial SCr concentration. Interestingly, the percentage of crescents was not independently associated with the occurrence of ESRD in this multicenter study. In IgAN, counting the number of crescents is not a precise measurement, because it is affected by the size of the biopsy sample and number of histologic sections examined. Although the interstitial fibrosis/tubular atrophy was independently associated with kidney progression in the whole population, it was not found to be a risk factor of renal failure in the analysis in the discovery or validation cohort. Our data will be of importance for clinical therapeutic strategy.
The critical and unique value of the study is that we have developed a simple model to predict kidney recovery in patients with crescentic IgAN. This prediction model can also be used for anti-GBM disease; in both cases, the association between ESRD development and initial SCr concentration formed S-shaped curves. In the case of AASV, however, kidney outcomes and SCr concentration showed a nearly linear association. On the basis of three small observational studies,5,7,8 the recent KDIGO guidelines for GN recommend steroid and cyclophosphamide therapy for crescentic IgAN, which is analogous to the therapy for AASV.6 Given the limitation of cohort study design, results were not comparable between patients with and without immunosuppressive therapy. Thus, we could not evaluate the effect of immunosuppressive treatment in crescentic IgAN. Our results only showed that there is a trend that immunosuppressive therapy reduced the risk of ESRD in patients with dominant active crescents (cellular or firocellular crescents>50%, _P_=0.08) (Supplemental Figure 1A) but not patients with high chronic lesions (tubular atrophy/interstitial fibrosis>50%, _P_=0.37) (Supplemental Figure 1B). However, among the patients who had undergone immunosuppressive therapy, few patients with SCr>6.8 mg/dl (600 μmol/L) recovered from dialysis. In contrast, 24% of the AASV patients with SCr>6.8 mg/dl could be weaned off dialysis. There is good evidence to support the use of plasma exchange in patients with anti-GBM disease or severe AASV.10,11 Future studies should evaluate plasma exchange therapy in crescentic IgAN patients with a severe decline in kidney function. One anecdotal report involving five patients with crescentic IgAN and one clinical trial have indicated a benefit of using plasma exchange combined with immunosuppressive therapies.12,13
In conclusion, crescentic IgAN has a poor prognosis. SCr concentration was the strongest predictor of kidney failure, whereas the percentage of crescents was not associated with ESRD in this study. The association between ESRD and initial SCr concentration formed an S-shaped curve. Patients with SCr>6.8 mg/dl (600 μmol/L) were less likely to recover from dialysis, even after aggressive immunosuppressive therapy. Alternative approaches are required to reduce the occurrence of ESRD in patients with advanced crescentic IgAN.
Concise Methods
Patients and Study Design
Eight tertiary kidney centers and clinics in China participated in this study, which was conducted from June of 2011 to March of 2012. The same inclusion and exclusion criteria were used in all participating renal centers: patients who were older than 14 years and had primary crescentic IgAN were eligible for inclusion. The histologic criterion of crescentic IgAN was the presence of crescents in more than 50% of the glomeruli. Patients were followed up for at least 12 months or until they progressed to ESRD if it occurred within 1 year. The exclusion criteria included (1) a biopsy specimen containing less than eight glomeruli, (2) secondary IgAN (e.g., caused by lupus, liver cirrhosis, or Henoch–Schonlein purpura), and (3) positive serum tests for ANCA or anti-GBM autoantibodies. ANCA assays were performed using indirect immunofluorescence (EUROIMMUN, Lübeck, Germany). Antigen-specific ELISA was performed by antibodies against purified myeloperoxidase and proteinase 3. Anti-GBM assays were performed using ELISA with purified α(IV)NC1 (noncollagenous-1 domain of type IV collagen). Six patients identified as serum ANCA-positive (antimyeloperoxidase or antiproteinase 3) were excluded from the current study.14
We have re-evaluated renal histologic lesions, especially the interstitial/tubular lesions, according to the histologic scoring for AASV.15 The crescents (cellular/fibrous) were calculated as the percentage of the total number of glomeruli in a biopsy. Interstitial and tubular lesions were scored semiquantitatively on the basis of the percentage of the tubulointerstitial compartment that was affected: interstitial infiltrates (−, 0%; +, 0%–20%; ++, 20%–50%; +++, >50%) and interstitial fibrosis/tubular atrophy (−, 0%; +, 0%–50%; ++, >50%).
To develop a prediction model for ESRD in crescentic IgAN, we carried out a two-stage cohort study. In the discovery stage, we identified risk factors and developed a prediction model for crescentic IgAN using the cohort from Peking University First Hospital IgA Nephropathy Database (http://ckd.edc-china.com.cn/login.jsp, discovery cohort; _n_=52). We further validated this model using another cohort consisting of patients from the remaining seven hospitals included in this study (validation cohort; _n_=61). All patients who received kidney biopsy from 1999 to 2010 were followed for at least 1 year. Subsequently, we analyzed the entire cohort and developed the model for the prediction of ESRD in crescentic IgAN.
To determine the characteristics of crescentic IgAN, we compared the results obtained with the data for crescentic GN caused by anti-GBM disease (_n_=38) and AASV (_n_=42). The inclusion criteria for these patients were the presence of at least eight glomeruli on the renal biopsy specimen and crescents affecting more than 50% of the glomeruli. Patients were followed up for at least 12 months or until they progressed to ESRD if it occurred within 1 year.
This study was reviewed and approved by the central ethics committee of Peking University First Hospital, and the tenets of the Declaration of Helsinki (Finland) were followed.
Treatments
Crescentic IgAN patients were administered immunosuppressive therapy as part of the routine intervention strategy in local renal units unless they had contraindications to immunosuppressive therapy, such as active infection or fibrosis of most crescents. The aggressive immunosuppression regimen included pulse methylprednisolone (7–15 mg/kg per day for 3 days) followed by oral prednisone (0.8–1 mg/kg per day; maximum dose=60 mg/d) combined with other immunosuppressive agents, such as cyclophosphamide (2 mg/kg per day) or mycophenolate mofetil (1–2 g/d). The regimen was adjusted according to renal function and histologic lesions.
Follow-Up and Outcome Measures
The eight participating renal institutes used the same data collection methods and forms. The collected data included sex, age, BP, SCr concentration, and details about proteinuria, histologic lesions, therapy, and follow-up. The patient’s current status was also confirmed by a telephone call at the end of this study. The primary outcomes of interest were renal survival as defined by the occurrence of ESRD and patient mortality. ESRD was diagnosed in patients who had received chronic dialysis for at least 6 months or undergone renal transplantation.
Statistical Analyses
Continuous variables were expressed as mean and SD or median and range as appropriate. Categorical variables were summarized as frequency and percentage. Differences among the test groups were assessed using the chi-squared test in the case of qualitative parameters and the nonparametric Wilcoxon rank sum test in the case of quantitative parameters. The Kaplan–Meier survival curve was used to calculate the cumulative renal survival rates. The log-rank test was used to compare the differences between renal survival curves. Univariate analysis followed by multivariate Cox regression analysis with a stepwise variable selection procedure was applied to identify independent factors associated with ESRD. All baseline variables were entered in the initial model and maintained if P<0.10. The cumulative probability of ESRD at 1 year was calculated using logistic regression. The function to calculate the probability of this event was as follows:
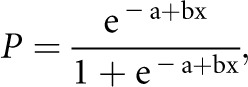
where P is the probability of ESRD at 1 year after renal biopsy, and x is the baseline SCr concentration (mg/dl) that was identified as an independent risk factor for ESRD on multivariate Cox analysis. Using this equation, we calculated the values of SCr at which the probability of ESRD was 20% and 90%. The high-risk threshold of the initial SCr concentration was the value at which the cumulative probability of ESRD at 1 year reached 90%. The low-risk threshold of initial SCr concentration was the value at which the cumulative probability of ESRD at 1 year was less than 20% (Supplemental Figure 2). All statistical analyses were performed using SAS 9.2 (Cary, NC).
Disclosures
None.
Acknowledgments
We thank all the patients and their families who participated in this study.
This work was supported in part by National Natural Science Foundation (NSF) Grant 81270795 (to J.L.), Program for New Century Excellent Talents in University from the Ministry of Education of China Grant NCET-12-0011 (to J.L.), Capital Clinical Research Grant Z12110700100000, 2011-4021-06 (to J.L.), Peking University Clinical Research Program Grant PUCRP201102 (to H.Z.), and NSF for Innovative Research Groups of China Grant 81021004 (to M.Z.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.D’Amico G: Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol 24: 179–196, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barratt J, Feehally J: IgA nephropathy. J Am Soc Nephrol 16: 2088–2097, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Abe T, Kida H, Yoshimura M, Yokoyama H, Koshino Y, Tomosugi N, Hattori N: Participation of extracapillary lesions (ECL) in progression of IgA nephropathy. Clin Nephrol 25: 37–41, 1986 [PubMed] [Google Scholar]
- 6.Kidney Disease : Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group: KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012 [Google Scholar]
- 7.Tumlin JA, Lohavichan V, Hennigar R: Crescentic, proliferative IgA nephropathy: Clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol Dial Transplant 18: 1321–1329, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Tang Z, Wu Y, Wang QW, Yu YS, Hu WX, Yao XD, Chen HP, Liu ZH, Li LS: Idiopathic IgA nephropathy with diffuse crescent formation. Am J Nephrol 22: 480–486, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Pankhurst T, Lepenies J, Nightingale P, Howie AJ, Adu D, Harper L: Vasculitic IgA nephropathy: Prognosis and outcome. Nephron Clin Pract 112: c16–c24, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, Mirapeix E, Savage CO, Sinico RA, Stegeman CA, Westman KW, van der Woude FJ, de Lind van Wijngaarden RA, Pusey CD, European Vasculitis Study Group : Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 18: 2180–2188, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Johnson JP, Moore J, Jr, Austin HA, 3rd, Balow JE, Antonovych TT, Wilson CB: Therapy of anti-glomerular basement membrane antibody disease: Analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine (Baltimore) 64: 219–227, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Coppo R, Basolo B, Roccatello D, Giachino O, Lajolo D, Martina G, Rollino C, Amore A, Costa M, Piccoli G: Plasma exchange in progressive primary IgA nephropathy. Int J Artif Organs 8[Suppl 2]: 55–58, 1985 [PubMed] [Google Scholar]
- 13.Chalopin JM, Rifle G for the French Co-operative Group: Plasma exchange (PE) and corticosteroids in severe IgA nephropathy: A prospective randomised trial, interim analysis. Presented at the 2nd International Congress of World Apheresis Association, Ottowa, ON, Canada, May 18–20, 1988 [Google Scholar]
- 14.Bantis C, Stangou M, Schlaugat C, Alexopoulos E, Pantzaki A, Memmos D, Ivens K, Heering PJ: Is presence of ANCA in crescentic IgA nephropathy a coincidence or novel clinical entity? A case series. Am J Kidney Dis 55: 259–268, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC, European Vasculitis Study Group (EUVAS) : Renal histology in ANCA-associated vasculitis: Differences between diagnostic and serologic subgroups. Kidney Int 61: 80–89, 2002 [DOI] [PubMed] [Google Scholar]