Immunological off-target effects of standard treatments in gastrointestinal cancers (original) (raw)
The effects on immune cells of standard cancer treatments (chemotherapeutic or biologic agents, interventional radiologic procedures) have become better appreciated. Likewise the contribution of the immune system towards the effectiveness of these treatments. This knowledge can potentially lead to novel applications of existing standard of care therapies in addition to potentiating their effect.
Keywords: pancreatic, immune, myeloid-derived suppressor cells, colorectal, CTLA4
Abstract
The effects on immune cells and the inflammatory microenvironment of commonly applied cancer treatments (chemotherapeutic or biologic agents, interventional radiologic procedures) have become better appreciated. Likewise, the contribution of the immune system toward the effectiveness of these treatments is clearer. The relevance of immune evasion by developing tumors is endorsed by its inclusion as one of the (updated) hallmarks of cancer. A greater understanding of this dimension can potentially lead to novel applications of existing standard of care therapies, in addition to potentiating their effect. This review summarizes the immune aspects of currently employed therapies—cytotoxic chemotherapeutics, biologic agents and interventional radiologic procedures—in solid tumor malignancies with a particular focus on those agents used in gastrointestinal cancers.
introduction
Recent decades have brought undoubted advances in understanding the molecular events driving tumor growth. These events—mainly aberrant activity through a particular signal transduction node or activating mutations—have resulted in much-heralded treatments, often termed ‘targeted therapies’. The underlying assumption has been that cancer is driven by deranged signaling, and that the most effective treatments will be those which best interrupt signaling, causing a direct antitumor effect. Increasingly, however, ‘off target’ effects of agents have been appreciated, particularly their effects on immune cells and the inflammatory microenvironment. In addition, the role of the immune system in enabling cancer cell death caused by chemotherapy has been appreciated to the extent that in certain situations, it may be the major determinant of efficacy. For example, it has been noted by many investigators across multiple murine cancer models that tumors respond better to treatment when implanted in an immunocompetent as opposed to immune-deficient host [1].
A greater understanding of the immune effects of treatments can lead to novel applications of existing standard drugs, in addition to potentiating their effect. In time, this will also help us decide how best to combine standard treatments with the immunotherapies in development and which are showing promise [2–6]. Appreciation of the role in developing tumors of immune evasion is evidenced by its inclusion as one of the (updated) hallmarks of cancer [7].
No immune-based therapy has been approved in gastrointestinal (GI) cancer, although it should be remembered that in colon cancer two of the much-heralded, targeted therapeutic approaches—anti-VEGF and anti-EGFR treatment—are antibodies with a capacity, through their Fc portion, to activate the immune system. This review summarizes the immune aspects of standard-of-care therapies in GI malignancies and discusses future applications.
chemotherapy-induced cytotoxicity requires an intact immune system
The traditional paradigm for drug development in oncology dates from early successful attempts to treat germ cell tumors and lymphomas, whereupon combination regimens were employed to counteract drug resistance. It seemed to make sense that for these regimens a maximally tolerated dose be established. In this approach, the immune system is seen as a barrier to giving higher drug dosages, the main limiting toxicities being haematologic. However, there is gathering evidence that certain chemotherapeutics can activate rather than suppress the immune system, and that a robust immune response is a necessary component determining tumor response [8].
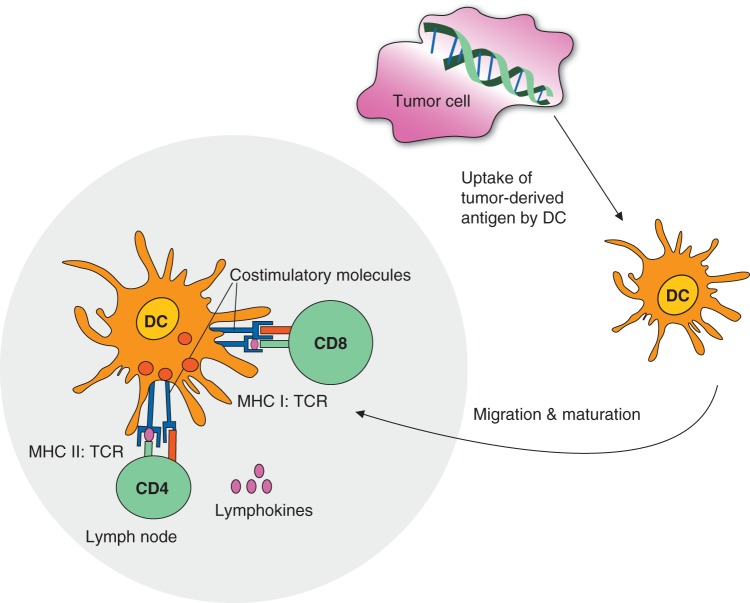
While it is beyond the scope of this review to summarize our knowledge of tumor immunology, it is worth reviewing some basic immunological concepts. Tumors can be recognized by the adaptive immune system, which consists of B and T cells as well as the innate immune system (NK cells). Cytotoxic CD8+ T cells recognize specific tumor antigens presented as peptides on MHC class I molecules (MHC class II in the case of CD4+ T cells). In order to prime a CD8+ T-cell response, professional antigen-presenting cells such as dendritic cells need to pick up antigens from dying tumor cells and present them with the support of CD4+ helper T cells to CD8+ T cells, a mechanism called ‘cross-priming’, believed to occur in the lymph node. Finally, cytotoxic CD8+ T cells travelling through the blood stream can kill tumor cells (Figure 1). Tumors have developed multiple mechanisms of evading immune responses, both by forming a suppressive microenvironment containing macrophages, regulatory T cells and myeloid-derived suppressor cells (MDSCs). They secrete cytokines with various functions, including IL-10, TGF-β and other. Finally, molecules important for the function of CD8+ T cells such as PD1, CD28 can be affected by tumors.
Figure 1.
Priming of CD8+ T cell response. Professional antigen presenting cells such as dendritic cells pick up antigens from dying tumor cells and present them with the support of CD4+ helper T cells to CD8+ T cells, a mechanism called “cross-priming”. Cytotoxic CD8+ T cells travelling through the blood stream can recognize and kill tumor cells.
Drug-induced cell death itself triggers an immune response [9]. It was demonstrated in the 1970s that an intact immune system was required for the antitumor effect of anthracyclines [10]. Chemotherapy causes apoptotic cell death, which can be immunogenic [10]. The resultant antigen release has been shown to activate T cells [11]. A requirement for this is calreticulin and phosphatidylserine exposure on the cell surface of the dying cell [12, 13]. Further, Apetoh et al. demonstrated that release of a protein called HMGB1which acts as a ligand for toll-like receptor (TLR)-4, promoting dendritic cell activation—is a necessary supplement to this initial signal [14]. Intriguingly, these authors also report that in breast cancer patients undergoing anthracycline-based chemotherapy, the presence of a polymorphism in TLR4—and by implication a less immunogenic drug-induced cell death—was associated with an inferior prognosis.
specific chemotherapeutic agents
Commonly used chemotherapeutic reagents either directly affect T cells, antigen-presenting cells or mechanisms associated with immunosuppression such as Tregs or MDSC [15] (Figure 2). A limited number of chemotherapeutics have demonstrated efficacy in the treatment of GI cancers, and there is considerable overlap in their use across cancer subtypes. Table 1 summarizes the main chemotherapeutics used in GI oncology and the evidence regarding their immune-mediated effects.
Figure 2.
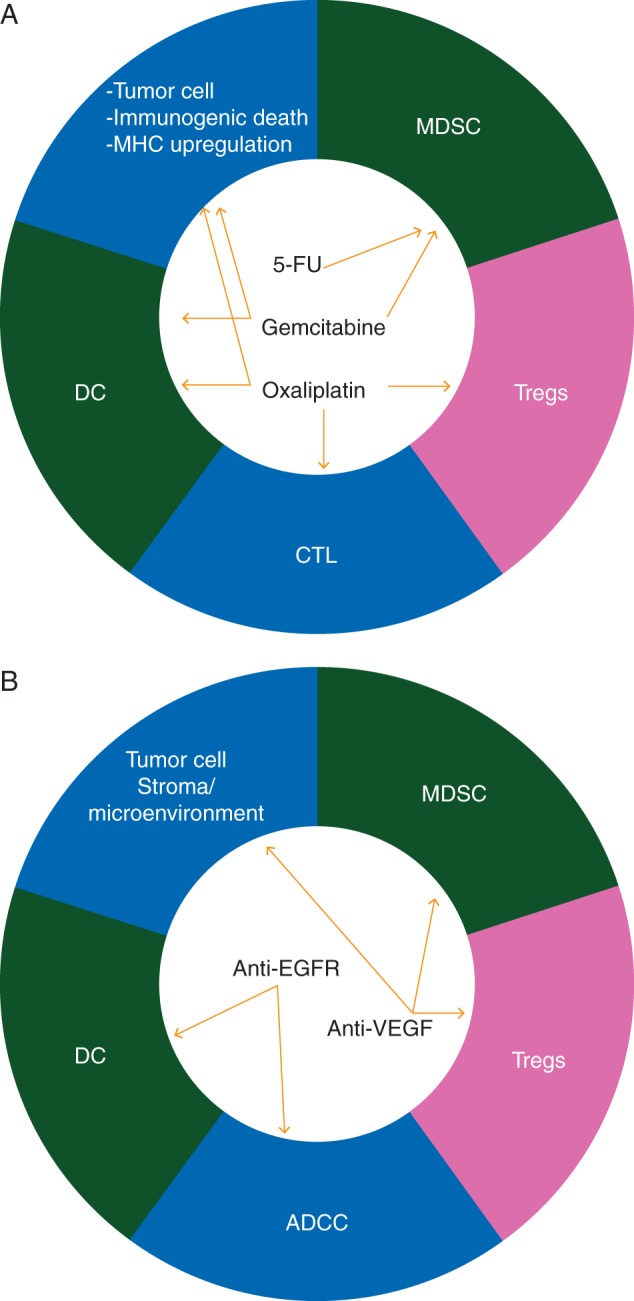
Both commonly used chemotherapeutic reagents (panel A) and so-called targetted therapies (panel B) have been shown to have immunological effects, either directly by making tumor cells more immunogenic and enhancing the cytotoxic T lymphocyte (CTL) response or stimulating antigen presenting cells or affecting mechanisms associated with immunosuppression such as Tregs or MDSC.
Table 1.
Commonly used drugs in gastrointestinal (GI) oncology: preclinical and clinical evidence of immune effects
| Intervention | Use | Preclinical data | Clinical data |
|---|---|---|---|
| Gemcitabine | Pancreaticobiliary cancers | Enhanced cellular immunity: in vitro T-lymphocyte recall responses to hemagglutinin, increased cross-presentation of antigen to CD8 cells following apoptosis; increase in MHC-I expression in tumor cells; augmented DC-maturation and function [8, 11]. | No significant proportional changes in B- or T-cell populations; decreased memory T cells, increased naïve activated cells; Boosting of cellular and humoral responses to the vaccinated peptides [18, 28]. |
| Selective reduction of MDSC [24] | |||
| Oxaliplatin | Colorectal, gastric, pancreatic cancer | Augmented DC-maturation; induces immunogenic cell death in various cell lines through production of HMGB1 [8, 14]; | TLR4 polymorphisms predictive of clinical benefit from oxaliplatin; |
| reduced expression of PD-L2 resulting in enhanced antigen-specific proliferation and Th1 cytokine secretion [32]; increase in the CD8+/T regulatory cell ratio and a reduction in MDSC in tumor microenvironment. | |||
| Irinotecan | Colorectal, esophago-gastric, pancreatic cancer | Improved the proficiency of DC vaccine [35]; | Increase in both CD4 and CD8 cells in patients treated [34] |
| Lowering of MDSC and Tregs | |||
| 5-Fluorouracil | Colorectal, esophago-gastric, pancreatic cancer | Selective reduction of MDSC [25] | – |
| Paclitaxel, docetaxel | Esophago-gastric, pancreatic cancer | Modulate T-cell, B-cell, and natural killer (NK) cell subsets in addition to enhancing CD8+ function [41]; | Increased IL-6, GM-CSF and IFN-γ levels (in breast cancer patients) [40]. |
| reduction of MDSC [43] | |||
| Cetuximab, panitumumab, trastuzumab | Colorectal, gastric cancer | Induce ADCC [52–54]; | FcgammaR polymorphisms predictive in colon cancer patients treated with cetuximab [56] |
| Cetuximab promotes colon cancer cell opsonization and phagocytosis by human dendritic cells [58] | |||
| Anti-VEGF tx (bevacizumab, aflibercept, sorafenib, regorafenib) | Colorectal, HCC | reduction in MDSC96 (at low doses of anti-VEGF antibody [69]). | Increased proportion of mature dendritic cells [67]; |
| Reduced the percentage of tumor-infiltrating Tregs (preoperative sorafenib [79]). |
gemcitabine
The immune effects of gemcitabine have been studied perhaps more than for any other drug used in GI cancer. Gemcitabine is a nucleoside analog that is part of the standard treatment of pancreatico-biliary malignancies [16, 17]. Its effects on the immune system are diverse. Nowak et al. [18] demonstrated that while treatment of tumor-bearing mice with gemcitabine resulted in massive depletion of lymphocyte numbers, this disproportionately affected B cells. Cellular immunity—as measured by in vitro T-lymphocyte recall responses to hemagglutinin—was enhanced. Treatment of tumor-bearing mice with gemcitabine increased cross-presentation of antigen to CD8 cells resulting in their increased proliferation and functionality [11]. Furthermore, apoptosis primed the host for a strong antitumor response, resulting in markedly decreased tumor growth upon challenge. Similarly, Liu et al. demonstrated that gemcitabine causes an increase in MHC-I expression in tumor cells, resulting in increased killing by T cells [8]. As cytotoxic T-cells are MHC-restricted, tumors lacking this antigen may become undetectable to immune cells. Furthermore, the authors found that T-cell function, as indicated by increased proliferation, was enhanced as supernatants derived from tumors treated with chemotherapy augmented dendritic cell (DC) function [8]. It has also been suggested that chemotherapy agents such as gemcitabine may expand the immune response to lesser, subdominant epitopes [19].
In addition to these direct effects, gemcitabine enhances immunogenicity indirectly by alleviating the suppression of the antitumor immune response. MDSCs are a heterogenous population comprising immature myeloid cells, which suppress the effector response [20–23]. We and others have shown that they are elevated in GI—as well as other cancers—where they contribute toward a suppressive network allowing evasion of the antitumor response [20]. Mundy-Bosse et al. showed that elevated MDSCs in a murine adenocarcinoma model reduced immune responsiveness to interferon, and that responsiveness could be restored by gemcitabine [24]. Similarly, Suzuki et al. found that gemcitabine selectively reduced the number of MDSCs found in the spleens of tumor-bearing mice, with no significant reductions in T cells, NK cells, macrophages or B cells. The loss of MDSCs was accompanied by increased antitumor T-cell activity. Likewise, Vincent et al. [25] observed that the treatment of tumor-bearing mice with gemcitabine was selective for MDSCs.
The effect of gemcitabine on the immunity of patients has been less clearly elucidated. Daikeler et al. [26] found that in patients (n = 16) treated with gemcitabine, T- and B-cell populations were relatively unchanged, as were CD4:CD8 ratio and NK cells. Plate et al. found that, in patients undergoing gemcitabine treatment, although there was an initial decrease in the absolute lymphocyte numbers, this stabilized on subsequent treatments [27]. Potential applications of gemcitabine in immune-based approaches have been shown in a number of studies. Yanagimoto et al. [28] evaluated the safety and clinical/immune responses of combining peptide vaccination with gemcitabine in patients with pancreatic cancer. Boosting of cellular/humoral responses to the vaccinated peptides was observed in the majority of patients tested, with some clinical activity which correlated with immune response. A similar example of the potential immune-based role for gemcitabine was shown by Beatty et al. who combined it with a CD40 agonist with impressive clinical activity [29].
oxaliplatin
Oxaliplatin is a standard treatment option in GI cancer [30, 31]. Similar to gemcitabine, Liu et al. observed that treating tumors with oxaliplatin resulted in immune activation [8]. This derived not from effects on T cells but rather through effects on antigen presenting cells. Unlike gemcitabine, however, the mechanism for oxaliplatin did not appear to be related to MHC upregulation [8]. The investigators found that culturing DCs in the supernatant derived from tumor cells exposed to oxaliplatin augmented DC maturation. These DCs also manifested improved function, causing an increase in the proliferation of T cells when co-cultured. These results suggest pre-treatment with oxaliplatin can be immune-stimulatory, potentiating DC function [8]. Oxaliplatin was shown to induce immunogenic cell death in various cell lines through the production of HMGB1 by dying cells [14]. This process was dependent upon TLR-4 [14]. The investigators further evaluated oxaliplatin as an inducer of immunogenic cell death in a colon cancer model [1]. Treatment with oxaliplatin acted as a vaccine, inoculating the mice against subsequent challenge. Interestingly, this immunogenic cell death was not seen with cisplatin despite the cells being sensitive to this drug. Of note, cisplatin is not considered an active drug in colon cancer. Immunogenicity appeared to be mediated through calreticulin expression which was produced more efficiently in oxaliplatin-treated tumors and was subsequently abolished by the administration of HMGB1-neutralizing antibodies corroborating the importance of the HMGB1–TLR4 axis suggested in the earlier publication. Of clinical relevance, the authors went on to evaluate the effect of TLR4 polymorphisms in colon cancer patients treated with standard of care oxaliplatin. Patients bearing a normal TLR4 allele manifested an increased progression-free survival and overall survival, compared with patients bearing the loss-of-function allele of TLR4. The possibility of this being a predictive marker of oxaliplatin benefit rather than simply being prognostic was suggested by an analysis of TLR4 polymorphism in stage II patients—for whom oxaliplatin would not be given—in whom the presence or absence of the polymorphism made no difference.
Oxaliplatin may also affect the immunesuppression orchestrated by growing tumors. Lesterhuis et al. found that this may be via the PD-1/PD-L pathway. Initially, these investigators found that DCs exposed to oxaliplatin displayed enhanced T-cell stimulatory capacity, an observation not explained by analysis of MHC expression or costimulatory molecules. They did find, however, that the expression of PD-L2 (and to a lesser extent PD-L1) was profoundly reduced, resulting in enhanced antigen-specific proliferation as well as enhanced recognition of tumor cells by T cells [32]. Further evidence for a possible immune-adjuvant role for oxaliplatin was seen in a colon cancer model where intra-hepatic IL-12 injection—known to stimulate T-lymphocytes and NK cells—was combined with oxaliplatin. The authors found that the combination treatment achieved more efficient elimination of liver metastases and improved protection against tumor rechallenge compared with either treatment alone [33]. Oxaliplatin was associated with a shift in the tumor microenvironment (increased CD8+/Treg ratio, reduction in MDSC). The authors concluded that the addition of oxaliplatin appeared to enhance the immune response against tumors by tipping the balance between effector and regulatory/suppressor cells in favor of effector cells.
irinotecan
There are less data in support of an immune aspect to the efficacy of irinotecan. In an early study, Melichar et al. found an increase in CD4 and CD8 cells with irinotecan in patients who were lymphocytopenic at baseline [34]. No functional studies were carried out.
Kim et al. [35] demonstrated that irinotecan—as part of the FOLFIRI regimen—improved the proficiency of DC vaccination by inhibiting the immunosuppressive environment. They employed a DC vaccine transduced with vector encoding CEA. The addition of FOLFIRI to the DC vaccine increased the tumor-specific immune response, as evidenced by an increase in the number of CEA-specific IFN-γ-secreting lymphocytes compared with vaccine alone. The proposed mechanism was the effect on suppressor cells. FOLFIRI treatment caused a lowering of MDSC and Tregs but with a later rebound. The addition of the vaccine lessened this rebound effect.
5-FU
5-Fluorouracil (5-FU) has been the pre-eminent chemotherapeutic in GI oncology since the 1950s and is the backbone agent in modern colorectal cancer regimens. In addition to its directly cytotoxic actions, 5-FU appears to have specific immune effects. Vincent et al. [25] found that 5FU had an even stronger ability than gemcitabine in selectively depleting MDSC in tumor-bearing mice. The net effect was an increase in IFN-γ production by tumor-specific T cells. Despite this evidence of immune activation—and in contrast with oxaliplatin—5-FU did not trigger immunogenic cell death: calreticulin was not upregulated and the antitumor activity was TLR4-independent. The authors concluded that the 5FU immune effect is mediated mainly by its action on MDSC, establishing an important principle whereby immune activation can occur by ameliorating suppression of anti-tumor immunity. Similarly, Ugel et al. evaluated the effect of 5-FU on improving the effect of a passive immunotherapy model at sub-therapeutic doses [36]. The transfer of T cells into fibrosarcoma-bearing mice had no effect on tumor regression, unless it was preceded by treatment with 5-FU.
taxanes
Paclitaxel (Taxol) has demonstrated efficacy in esophageal cancer and—in its albumin-bound formulation—pancreatic cancer, while docetaxel (Taxotere) is a standard option for gastric cancer [37–39]. Immune effects have been demonstrated for this drug class. Tsavaris et al. [40] found that IL-6, GM-CSF and IFN-γ levels all increased following either taxane, as were results of functional assays. Garnett et al. [41] found that docetaxel enhanced CD8+ function in a tumor model known to be docetaxel-resistant. Combining docetaxel with a vaccine resulted in enhanced immune responses and also broadening of the immune response to antigens distinct from the vaccine (so-called antigen cascade).
Zhu et al. [42] reported that paclitaxel selectively decreased Tregs. Similarly, Kodumudi et al. found that, in tumor-bearing mice, docetaxel decreased MDSC [43]. With this reduction in suppressor cells, there was an increase in the percentage of CD4/CD8+ T-cells in the spleens of animals treated with docetaxel compared with controls and an increase in T-cell activation. The investigators proceeded to determine if enhanced T-cell function was due to loss of suppressive capacity in MDSCs by pre-treating with docetaxel. Interestingly they found that not only did the docetaxel-treated MDSC lose their suppressive ability, they actually became stimulatory. An increase in CTL responses was seen which the authors concluded was accomplished via MDSC modulation. Ramakrishnan et al. tested cancer vaccination and adoptive T-cell transfer in combination with chemotherapy in mice. Paclitaxel made tumor cells more susceptible to the cytotoxic effect of CTLs through a dramatic increase in their permeability [44].
chemotherapy combinations
While it is important to delineate as much as possible the immune effects of individual chemotherapeutics, the reality is that most drugs are used in combination, particularly in the first-line setting. Few studies have studied the net effect on the immune system for drug combinations, much less tailored regimens specifically.
The standard colorectal cancer regimens (FOLFOX/ FOLFIRI) were evaluated by Maeda et al. with regard to their immune consequences, at least in terms of quantitative changes [45]. The authors found that Tregs were significantly reduced in those who had high baseline levels, with no change in the relative proportion of CD4, CD8 or NK cells. Other investigators have shown that oxaliplatin/irinotecan/5-FU combinations can be given to patients with vaccines without abrogation of the immune response [46, 47].
Correale et al. [48] developed the GOLF regimen (comprising gemcitabine, oxaliplatin, leucovorin and 5-fluorouracil) based on preclinical evidence of immunogenicity. They proceeded to test this clinically, combining the regimen with GMCSF and IL-2 to enhance immune stimulation [49]. The regimen was active and clinical autoimmunity occurred in six patients, in whom the outcome was significantly better.
anti-EGFR therapy
Cetuximab and panitumumab have been shown to be active in colorectal cancer and trastuzumab has efficacy in gastric cancer [50, 51]. While the original assumption was that these therapies functioned as signal transduction inhibitors, it is also to be remembered that, as antibodies, they can activate the immune system through their Fc portion. It is also noteworthy that small-molecule EGFR inhibitors—which are not antibodies and lack the IgG1 backbone—have not been shown to have efficacy in colon cancer despite excellent EGFR inhibition.
A number of preclinical studies have shown the ability of cetuximab to induce antibody-dependent cell-mediated cytotoxicity (ADCC) [52–54], a mechanism by which NK cells lyse target cells, which have a specific antibody bound to their surface through a Fc receptor dependent mechanism. Similar properties have been described for trastuzumab [55]. Failure to do this, as a result of Fc-Receptor polymorphisms for example, has been shown to be clinically relevant in patients with irinotecan-refractory colorectal cancer treated with cetuximab [56].
Typically, cetuximab is given in combination with chemotherapy. For its immune effects, sequencing may be important, however. Correale et al. [57] found that EGFR expression in colon cancer cells was upregulated after exposure to chemotherapy, and that this was followed by enhanced susceptibility to ADCC, a mechanism independent of k-ras. Cetuximab promoted cancer cell opsonization and phagocytosis by DCs. This suggests an involvement of CTL-dependent immunity in cetuximab anticancer effects [58]. These data have implication for trial design, suggesting that the sequence of administration is important in priming the immune response. It also implies a role for anti-EGFR therapy even in the presence of kras mutations based on an entirely different mechanism.
Based on these considerations, these investigators developed a rational colorectal chemo-biologic regimen comprising gemcitabine, irinotecan and 5-FU, followed by cetuximab administered on day 3 [59]. PBMC from patients undergoing standard of care FOLFOX was used as control. In the experimental arm, the authors found several changes in immune subsets relative to control in addition to an increase in CEA- and thymidylate synthase-specific CTL precursors.
The role of EGFR tyrosine kinase inhibitors is less prominent in GI malignancies than for other cancers, although erlotinib is FDA approved for pancreatic cancer. The immune effects of these treatments are less clear, although part of the adverse effect profile of erlotinib—including hepatitis as well as interstitial lung disease and, rarely, Stevens–Johnson syndrome—has been reported to be immune-mediated. Luo et al. [60] demonstrated that erlotinib inhibits T-cell-mediated immune responses, and that this immunosuppressive activity was likely a result of inhibition of Raf/ERK cascade and Akt signaling in T cells.
anti-VEGF therapy
Over the past decade, various treatments have been approved in GI cancer whose putative mode of action is inhibition of vascular endothelial growth factor (VEGF) [61, 62]. The dominant hypothesis regarding anti-VEGF therapy has been that it remodels tumor vasculature and enhances drug delivery [63]. Maximizing dose to achieve this, however, has not improved outcome. Efforts to move anti-VEGF therapy into the adjuvant setting have failed [64]. But it has also become apparent that pro-angiogenic factors play an integral role in maintaining an immunosuppressive tumor microenvironment. For example, VEGF stimulates inhibitory myeloid cells and Tregs in addition to producing immunosuppressive cytokines [65–67]. Pro-angiogenic factors suppress immune cell function [68, 69]. Counteracting this modulates the immune environment, enhancing antitumor immunity [70, 71].
Clinically, the data are suggestive. In lung cancer patients post-resection, low tumor cell VEGF-A/VEGFR-2 expression in combination with high CD4/CD8 expression in tumor-related stroma was a strong and independent prognostic marker [72]. VEGF expression inversely correlated with DC numbers in tumors of gastric cancer patients [73, 74]. VEGF-Trap—a fusion protein which binds all isoforms of VEGF-A—significantly increased the proportion of mature DCs, albeit with no improvement in immune responses [75]. A subset analysis of this study in cancer patients did reveal improvement in immune responses in those who had no increase in MDSC, implying that any positive effect by anti-VEGF therapy was overwhelmed by the presence of MDSC. Similarly, Osada et al. showed a decrease in immature progenitor cells (known to be immunosuppressive [76]) and a modest increase in DCs in the peripheral blood of 41 patients with lung, breast and colorectal carcinomas [67].
Dose is important, however. Huang et al. evaluated the effect of differing doses of anti-VEGF therapy on two ostensibly separate biologic processes: vascular normalization and immune activation [69]. They employed an anti-VEGFR2 antibody in both immune-tolerant and immunogenic breast cancer models. They found that anti-VEGF therapy combined with tumor vaccination inhibited tumor growth, but only when the anti-VEGF therapy was given at 25% of the dose. Lower dosage resulted in a reduction in MDSC infiltration compared with higher dose, in addition to polarizing macrophages toward immunostimulative capacity and promoting T-cell infiltration. The lower anti-VEGFR2 dose increased the distribution of functional vasculature compared with higher doses. In other words, this important paper synchronized the two major theories regarding VEGF inhibition: that they normalize the chaotic tumor vasculature and alleviate the immune-suppressed microenvironment.
It is unclear what, if any, immune effects contribute to the effect of small-molecule inhibitors of VEGF, such as sorafenib or regorafanib. Both are multikinase inhibitors with a plurality of targets, including VEGF. Multiple immune effects have been reported with sorafenib, but the net effect is unclear. Sorafenib inhibits DC function while also reduces the induction of antigen-specific T cells [77]. Similarly sorafenib inhibits NK cell effector function [78]. But sorafenib also inhibits immunosuppressive cells in kidney cancer patients, and preoperative treatment significantly reduces the percentage of tumor-infiltrating Tregs [79]. It appears likely that this effect on MDSC is indirect [80].
future directions
Recent years have seen validation of efforts to shape an immune response against cancer. Clinical activity has been seen not only in renal cancer and melanoma—diseases long considered immunogenic—but also prostate cancer and, provisionally, colorectal and lung cancers [5, 6]. The advantages of immune-based treatments are clear: long-lasting responses; lack of resistance; generation of memory; less non-specific toxicity. Yet, immune-based approaches do not work in everyone or in every disease. Efforts to expand both the range of responding patients and the range of ‘immuno-susceptible’ tumors are likely to require combined approaches. This review summarizes the immune effects of commonly used chemotherapeutics. In all cases—even the therapeutic antibodies—development focused first on direct antitumor effect and according to convention maximizing dose. Schedules were developed based on tolerability. As we have discussed, however, these conventional treatments have immune effects. There is now considerable evidence that these treatments can facilitate or provoke an antitumor immune response independent from the antitumor effect.
If immune effects become a major consideration in the development of a particular regimen, the dose necessary for immune modulation is likely to be different and lower, meaning greater flexibility in schedule. Metronomic therapy—the chronic administration of chemotherapeutic agents at low doses—has long been an experimental approach, with its major effects felt to be antiangiogenic as a result of targeting endothelial cells. However, this method of administration has also been shown to have immune effects [81, 82]. Metronomic therapy also activates the immune system by promoting DC maturation [83].
The modern clinical trial should incorporate this type of knowledge into its design, either fundamentally by altering the standard dose and schedule or as a correlative aim by collecting PBMC to carry out prospective immune monitoring. The TLR polymorphism data with regard to oxaliplatin [1] demonstrate that important information can be generated in a study that has no overt immune intent. A related aim is to better identify serologic markers of immunity, which are good surrogates for efficacy. While there is a hierarchy of sorts (eg antigen-specific responses presumed better than quantitative changes), no marker is uniformly employed. On the developmental side, novel agents need to be evaluated in immune competent mice and off-target effects investigated. Rational combinations may be at doses considered sub-optimal according to the traditional cytotoxicity assays. A significant barrier to progress is the difficulty of combining novel agents from competing industry partners. Immune considerations may provide a new or expanded role for agents previously considered inactive in a particular setting. Imaginative clinical trials are needed which will balance the direct anticancer effects of conventional therapies with immune considerations. Accomplishing this will achieve the maximal biologic effect of the treatments we already have and better exploit the immune therapies in development.
funding
Both authors are NIH employees and are therefore funded by the NIH.
disclosure
The authors have declared no conflicts of interest.
acknowledgements
We thank Susanna Ulahannan for her helpful comments and review of the manuscript.
references
- 1.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 5.Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Liu WM, Fowler DW, Smith P, et al. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115–123. doi: 10.1038/sj.bjc.6605465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamrekelashvili J, Kruger C, von Wasielewski R, et al. Necrotic tumor cell death in vivo impairs tumor-specific immune responses. J Immunol. 2007;178:1573–1580. doi: 10.4049/jimmunol.178.3.1573. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell AM, Nowak AK, Lake RA. Contribution of the immune system to the chemotherapeutic response. Semin Immunopathol. 2011;33:353–367. doi: 10.1007/s00281-011-0246-z. [DOI] [PubMed] [Google Scholar]
- 11.Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 12.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 13.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 14.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 15.Zitvogel L, Apetoh L, Ghiringhelli F, et al. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 16.Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33(Suppl 1):S18–S22. doi: 10.1016/s0959-8049(96)00324-3. [DOI] [PubMed] [Google Scholar]
- 17.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 18.Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–2358. [PubMed] [Google Scholar]
- 19.Jackaman C, Majewski D, Fox SA, et al. Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother. 2012;61:2343–2356. doi: 10.1007/s00262-012-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy A, Zhao F, Haile L, et al. Comparative analysis of monocytic and granulocytic myeloid-derived suppressor cell subsets in patients with gastrointestinal malignancies. Cancer Immunol Immunother. 2013;62:299–307. doi: 10.1007/s00262-012-1332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 23.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11:802–807. doi: 10.1016/j.intimp.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mundy-Bosse BL, Lesinski GB, Jaime-Ramirez AC, et al. Myeloid-derived suppressor cell inhibition of the IFN response in tumor-bearing mice. Cancer Res. 2011;71:5101–5110. doi: 10.1158/0008-5472.CAN-10-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 26.Daikeler T, Maas K, Weiss B, et al. The influence of gemcitabine on the CD4/CD8 ratio in patients with solid tumours. Oncol Rep. 1997;4:561–564. doi: 10.3892/or.4.3.561. [DOI] [PubMed] [Google Scholar]
- 27.Plate JM, Plate AE, Shott S, et al. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother. 2005;54:915–925. doi: 10.1007/s00262-004-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagimoto H, Shiomi H, Satoi S, et al. A phase II study of personalized peptide vaccination combined with gemcitabine for non-resectable pancreatic cancer patients. Oncol Rep. 2010;24:795–801. doi: 10.3892/or_00000923. [DOI] [PubMed] [Google Scholar]
- 29.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 Agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 31.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 32.Lesterhuis WJ, Punt CJ, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011;121:3100–3108. doi: 10.1172/JCI43656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Aparicio M, Alzuguren P, Mauleon I, et al. Oxaliplatin in combination with liver-specific expression of interleukin 12 reduces the immunosuppressive microenvironment of tumours and eradicates metastatic colorectal cancer in mice. Gut. 2011;60:341–349. doi: 10.1136/gut.2010.211722. [DOI] [PubMed] [Google Scholar]
- 34.Melichar B, Touskova M, Vesely P. Effect of irinotecan on the phenotype of peripheral blood leukocyte populations in patients with metastatic colorectal cancer. Hepatogastroenterology. 2002;49:967–970. [PubMed] [Google Scholar]
- 35.Kim HS, Park HM, Park JS, et al. Dendritic cell vaccine in addition to FOLFIRI regimen improve antitumor effects through the inhibition of immunosuppressive cells in murine colorectal cancer model. Vaccine. 2010;28:7787–7796. doi: 10.1016/j.vaccine.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 36.Ugel S, Peranzoni E, Desantis G, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2:628–639. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 37.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 38.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 39.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsavaris N, Kosmas C, Vadiaka M, et al. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Liu N, Xiong SD, et al. CD4+Foxp3+ Regulatory T-cell impairment by paclitaxel is independent of toll-like receptor 4. Scand J Immunol. 2011;73:301–308. doi: 10.1111/j.1365-3083.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- 43.Kodumudi KN, Woan K, Gilvary DL, et al. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramakrishnan R, Assudani D, Nagaraj S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeda K, Hazama S, Tokuno K, et al. Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res. 2011;31:4569–4574. [PubMed] [Google Scholar]
- 46.Harrop R, Drury N, Shingler W, et al. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57:977–986. doi: 10.1007/s00262-007-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrop R, Drury N, Shingler W, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res. 2007;13:4487–4494. doi: 10.1158/1078-0432.CCR-07-0704. [DOI] [PubMed] [Google Scholar]
- 48.Correale P, Cusi MG, Del Vecchio MT, et al. Dendritic cell-mediated cross-presentation of antigens derived from colon carcinoma cells exposed to a highly cytotoxic multidrug regimen with gemcitabine, oxaliplatin, 5-fluorouracil, and leucovorin, elicits a powerful human antigen-specific CTL response with antitumor activity in vitro. J Immunol. 2005;175:820–828. doi: 10.4049/jimmunol.175.2.820. [DOI] [PubMed] [Google Scholar]
- 49.Correale P, Tagliaferri P, Fioravanti A, et al. Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 Trial) Clin Cancer Res. 2008;14:4192–4199. doi: 10.1158/1078-0432.CCR-07-5278. [DOI] [PubMed] [Google Scholar]
- 50.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 51.Van Cutsem E, Kang Y, Chung H, et al. Efficacy results from the ToGA trial: A phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC) J Clin Oncol. 2009;27:LBA4509. [Google Scholar]
- 52.Kawaguchi Y, Kono K, Mimura K, et al. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int J Cancer. 2007;120:781–787. doi: 10.1002/ijc.22370. [DOI] [PubMed] [Google Scholar]
- 53.Kimura H, Sakai K, Arao T, et al. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007;98:1275–1280. doi: 10.1111/j.1349-7006.2007.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurai J, Chikumi H, Hashimoto K, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 55.Collins DM, O'Donovan N, McGowan PM, et al. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann Oncol. 2012;23:1788–1795. doi: 10.1093/annonc/mdr484. [DOI] [PubMed] [Google Scholar]
- 56.Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 57.Correale P, Marra M, Remondo C, et al. Cytotoxic drugs up-regulate epidermal growth factor receptor (EGFR) expression in colon cancer cells and enhance their susceptibility to EGFR-targeted antibody-dependent cell-mediated-cytotoxicity (ADCC) Eur J Cancer. 2010;46:1703–1711. doi: 10.1016/j.ejca.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Correale P, Botta C, Cusi MG, et al. Cetuximab±chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer. 2012;130:1577–1589. doi: 10.1002/ijc.26181. [DOI] [PubMed] [Google Scholar]
- 59.Botta C, Bestoso E, Apollinari S, et al. Immune-modulating effects of the newest cetuximab-based chemoimmunotherapy regimen in advanced colorectal cancer patients. J Immunother. 2012;35:440–447. doi: 10.1097/CJI.0b013e31825943aa. [DOI] [PubMed] [Google Scholar]
- 60.Luo Q, Gu Y, Zheng W, et al. Erlotinib inhibits T-cell-mediated immune response via down-regulation of the c-Raf/ERK cascade and Akt signaling pathway. Toxicol Appl Pharmacol. 2011;251:130–136. doi: 10.1016/j.taap.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 62.Reddy GK. The addition of bevacizumab to FOLFOX4 prolongs survival in relapsed colorectal cancer: interim data from the ECOG 3200 trial. Clin Colorectal Cancer. 2005;4:300–301. doi: 10.1016/s1533-0028(11)70131-1. [DOI] [PubMed] [Google Scholar]
- 63.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 64.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Correale P, Cusi MG, Tagliaferri P. Immunomodulatory properties of anticancer monoclonal antibodies: is the ‘magic bullet’ still a reliable paradigm? Immunotherapy. 2011;3:1–4. doi: 10.2217/imt.10.92. [DOI] [PubMed] [Google Scholar]
- 66.Alfaro C, Suarez N, Gonzalez A, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer. 2009;100:1111–1119. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osada T, Chong G, Tansik R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y, Chen X, Dikov MM, et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624–631. doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li B, Lalani AS, Harding TC, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12:6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 71.Shrimali RK, Yu Z, Theoret MR, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donnem T, Al-Shibli K, Andersen S, et al. Combination of low vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 expression and high lymphocyte infiltration is a strong and independent favorable prognostic factor in patients with nonsmall cell lung cancer. Cancer. 2010;116:4318–4325. doi: 10.1002/cncr.25333. [DOI] [PubMed] [Google Scholar]
- 73.Saito H, Tsujitani S, Ikeguchi M, et al. Relationship between the expression of vascular endothelial growth factor and the density of dendritic cells in gastric adenocarcinoma tissue. Br J Cancer. 1998;78:1573–1577. doi: 10.1038/bjc.1998.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsukayama S, Omura K, Yoshida K, et al. Prognostic value of CD83-positive mature dendritic cells and their relation to vascular endothelial growth factor in advanced human gastric cancer. Oncol Rep. 2005;14:369–375. [PubMed] [Google Scholar]
- 75.Fricke I, Mirza N, Dupont J, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 76.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 77.Hipp MM, Hilf N, Walter S, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 78.Krusch M, Salih J, Schlicke M, et al. The kinase inhibitors sunitinib and sorafenib differentially affect NK cell antitumor reactivity in vitro. J Immunol. 2009;183:8286–8294. doi: 10.4049/jimmunol.0902404. [DOI] [PubMed] [Google Scholar]
- 79.Desar IM, Jacobs JH, Hulsbergen-vandeKaa CA, et al. Sorafenib reduces the percentage of tumour infiltrating regulatory T cells in renal cell carcinoma patients. Int J Cancer. 2011;129:507–512. doi: 10.1002/ijc.25674. [DOI] [PubMed] [Google Scholar]
- 80.Kapanadze T, Gamrekelashvili J, Ma C, et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol. 2013;59:1007–1013. doi: 10.1016/j.jhep.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 82.Greten TF, Ormandy LA, Fikuart A, et al. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33:211–218. doi: 10.1097/CJI.0b013e3181bb499f. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka H, Matsushima H, Mizumoto N, et al. Classification of chemotherapeutic agents based on their differential in vitro effects on dendritic cells. Cancer Res. 2009;69:6978–6986. doi: 10.1158/0008-5472.CAN-09-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]