Taxonomy and phylogeny of European Gymnopus subsection Levipedes (Basidiomycota, Omphalotaceae) (original) (raw)
Abstract
The systematic integrity of European Gymnopus subsect. Levipedes is verified based on anatomic-morphological characters with support from DNA sequences of ITS and translation elongation factor 1-alpha genes. Seven species (G. alpinus, G. aquosus, G. dryophilus – including var. lanipes, G. erythropus, G. fagiphilus, G. hybridus, and G. ocior) belonging to this subsection are included. We clarify the concepts of G. dryophilus and G. ocior, which were occasionally confused in older literature. Due to unavailability of previously selected neotype of G. dryophilus the substitute neotype specimen is selected. Gymnopus dryophilus var. lanipes is confirmed as a variety; no important differences from nominotypical variability were detected. All discriminative characters used for identification of these species are discussed in detail. An identification key is also provided.
Keywords: ITS, neotype, nomenclature, translation elongation factor 1-alpha
INTRODUCTION
Gymnopus is a large agaricoid genus distributed world-wide circumscribing c. 300 species (Kirk et al. 2008). It is characterized in basidiocarps collybioid, rarely tricholomatoid or marasmioid with a pileus convex, plano-convex to applanate or slightly concave, dry or slightly viscid, glabrous or innately radially fibrillose, lamellae free, emarginate or adnate, crowded to sometimes fairly distant, a stipe central, non-insititious or insititious, and a spore print white. Basidiospores are ellipsoid to oblong, rarely subglobose to globose or lacrimoid, thin-walled, hyaline, non-amyloid; cheilocystidia often present, cylindrical, flexuous, clavate or irregularly coralloid; pleurocystidia usually absent or in some species well-developed (e.g., G. lodgeae (Singer) J.L. Mata, G. omphalodes (Berk.) Halling & J.L. Mata, G. pseudologeae J.L. Mata; Mata et al. 2004, 2006, Mata & Ovrebo 2009); a pileipellis in the form of a cutis or ixocutis of radially arranged cylindrical hyphae, or interwoven, made up of irregular coralloid terminal elements (Dryophila-structure); hyphae never amyloid or dextrinoid (except for sect. Androsacei with dextrinoid context hyphae, at least in stipe apex), and clamp connections mostly present.
Gymnopus sect. Levipedes subsect. Levipedes (type species: Gymnopus dryophilus) is characterized in having a pileipellis composed of cells inflated, lobed or coralloid (a Dryophila-type cutis), well-developed cheilocystidia, a smooth stipe, and hyphae only rarely becoming green in alkali or not (Antonín & Noordeloos 2010).
In Europe, seven species occur: G. alpinus, G. aquosus, G. dryophilus (with var. lanipes), G. erythropus, G. fagiphilus, G. hybridus, and G. ocior. The DNA-based phylogenetic relations among these species are insufficiently known. Mata et al. (2006) included several species of this subsection from various continents (some of them also from Europe), but in their comprehensive analysis did not cover all European taxa of subsect. Levipedes. Moreover, our preliminary results were in disagreement with some of their interpretation of G. ocior and its placement in the ITS phylogram. Therefore, the aim of this paper is a phylogenetic and taxonomic revision of all European taxa of this subsection.
MATERIAL AND METHODS
Morphological dataset
The specimens studied, especially of the G. dryophilus complex, were selected from various regions of Europe. The macroscopic descriptions are based on fresh basidiocarps, if available, were made by the collectors. Microscopic features were studied under Olympus BX 50 light microscope from dried material mounted in H2O, 5 % KOH solution, Melzer’s reagent and Congo Red. Microscopic characters were studied with emphasis on the most important morphological features for taxon delimitation – shape and dimensions of basidiospores, pileipellis structure and shape and dimensions of cheilocystidia. For basidiospores, the factors E (quotient of length and width in any one spore) and Q (mean of E-values) are used. In each herbarium collection, 20 basidiospores were measured. Authors of fungal names are cited according to the International Plant Names Index Authors website (http://www.ipni.org/ipni/authorsearchpage.do); colour abbreviations are according to Kornerup & Wanscher (1983), and for herbarium acronyms see Thiers (2012; accessed 10 Sept. 2012).
Molecular dataset
DNA extraction and PCR
The DNA was extracted from dried herbarium specimens. The specimens selected for DNA extraction and PCR are listed in Table 1. The two loci: ITS region of ribosomal RNA gene (ITS) and partial sequence of translation elongation factor 1-alpha gene (tefa) – were selected for the analysis. The DNA extraction and PCR of ITS was applied according to Tomšovský et al. (2010). For the amplification of tefa, the primer pair 983F/2218R was used (Rehner & Buckley 2005). PCR for the tefa locus was performed using a following touchdown PCR procedure: The amplifications were initiated with a 2 min denaturation at 94 °C. The annealing temperature in the first amplification cycle was 60 °C, which was subsequently incrementally reduced by 1 °C per cycle over the next 9 cycles. An additional 35 amplification cycles were then performed, each consisting of 30 s denaturation at 94 °C, a 30 s annealing step at 50 °C, and a 1 min extension at 72 °C, concluding with a 10 min incubation at 72 °C. In case of unsuccessful amplification of tefa gene, the nested PCR of this gene region was performed according to Tomšovský et al. (2010).
Table 1.
The specimens sequenced by the authors.
Phylogenetic analysis
The newly obtained sequences were augmented by those published by Lutzoni et al. (2004), Mata et al. (2006), and Antonín et al. (2012). Sequences of each individual locus were aligned using the MAFFT version 6 with selected Q-INS-i option algorithm (Katoh & Toh 2010). The sequences of Gymnopus confluens were selected as an outgroup.
The two separate phylogenetic analyses were performed – the first one including a two-gene dataset of both genetic markers (ITS and tefa) and the second one of newly obtained ITS data alone complemented with the respective sequences from GenBank (mostly published by Mata et al. 2006).
To determine whether the datasets of the different genetic markers (ITS and tefa) were in significant conflict, two methods were applied. The partition homogeneity test in PAUP* 4.0b10 (Swofford 2003) was used between the markers using 100 replicates and the heuristic general search option. The null hypothesis of congruence was rejected if p < 0.01. A test based on maximum agreement subtrees (de Vienne et al. 2007) was further performed.
Phylogenies were generated in MrBayes version 3.2.1 (Ronquist & Huelsenbeck 2003). The substitution models were selected prior to analyses using the MrModeltest 2.3 (Nylander 2008). For ITS+tefa dataset he GTR + I + G (General time reversible model + Proportion of invariant + Gamma) while for the ITS dataset GTR + G (General time reversible model + Gamma) were chosen. Markov chains were initiated from a random tree and were run for 5 000 000 generations; samples were taken every 100th generation. The number of excluded generations determined as burn-in was used by Tracer 1.5 (Rambaut & Drummond 2009); burn-in = 500 000 in both analyses. The Bayesian branch supports were assigned as posterior probabilities (PP) on the consensus trees. In addition, bootstrap branch support values (BS) were estimated in PAUP 4.0b10 using 1 000 replicate datasets with the random addition of sequences during each heuristic search.
Additional, phylogenetic analyses were carried out in PHYML estimating maximum likelihood phylogenies and run at the server Phylogeny.fr (Dereeper et al. 2008) using ‘A la Carte’ mode. The alignments were treated with Gblock, eliminating poorly aligned positions and ambiguous regions, and GTR substitution model was selected for both ITS and LSU datasets. Bootstrap branch support values (BP) were estimated in PHYML 3.0 (Guindon et al. 2010) under the maximum likelihood criterion using default 100 replicates. The alignments and phylograms from the Bayesian analyses were deposited in Treebase (ID 13361).
RESULTS
We obtained 58 new sequences of ITS and 36 of tefa gene regions. A partition homogeneity test and a test of maximum agreement subtrees allowed us to combine the ITS and tefa data. The lengths of datasets, likelihood values and model parameters of Bayesian and Maximum likelihood of both datasets are summarized in Table 2.
Table 2.
The statistics of phylogenetic analyses are summarized. In Bayesian analyses, the mean values of two simultaneous runs are presented.
| Dataset/analysis | ITS+tefa / Bayesian analysis (MrBayes) | ITS+tefa / Maximum likelihood (PHYML) | ITS / Bayesian analysis (MrBayes) | ITS / Maximum likelihood (PHYML) |
|---|---|---|---|---|
| Dataset length/bp | 1294 | 1005 | 773 | 531 |
| Variable positions | 336 | 235 | 188 | 121 |
| Singleton positions | 171 | 140 | 109 | 67 |
| Log-likelihood | −4169.583 | −2942.619 | −2581.831 | −1592.603 |
| Gamma shape parameter (alpha in Bayesian analysis) | 0.914 | 0.555 | 0.09735 | 0.693 |
| Proportion of invariant | 0.368 | 0.382 | N/A | N/A |
| f(A) | 0.22600 | 0.23292 | 0.22900 | 0.22748 |
| f(C) | 0.21200 | 0.21714 | 0.17900 | 0.18814 |
| f(G) | 0.22300 | 0.23595 | 0.21400 | 0.21315 |
| f(T) | 0.33900 | 0.31399 | 0.37800 | 0.37123 |
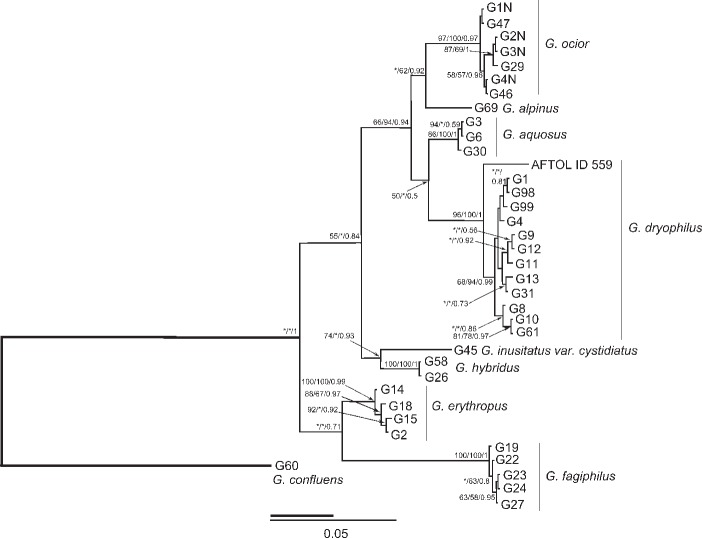
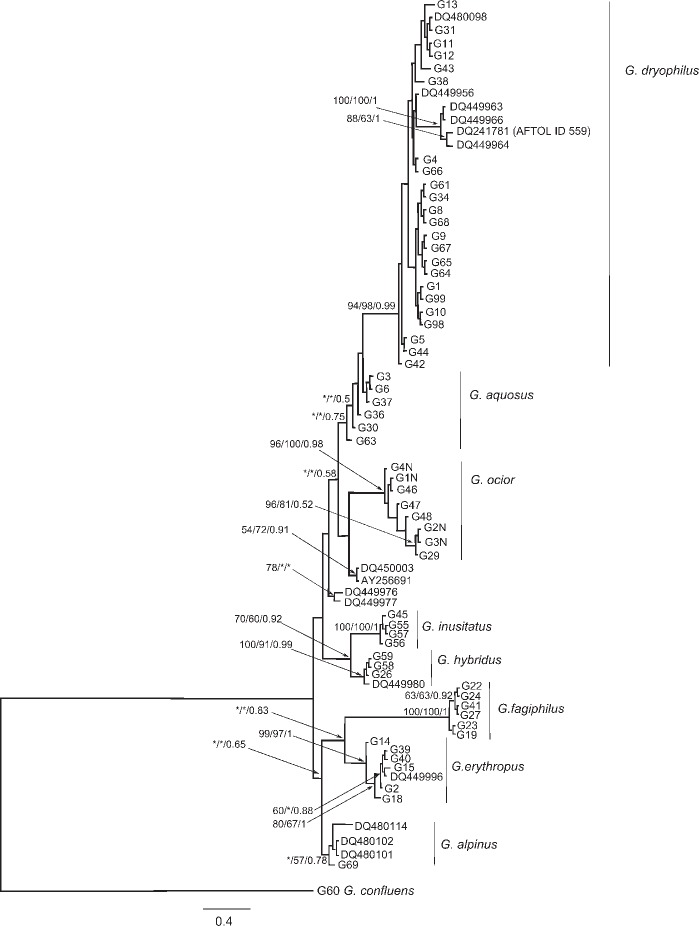
The molecular data (Fig. 1, 2) confirmed sequence homogeneity of G. alpinus, G. aquosus, G. dryophilus, G. erythropus, G. fagiphilus, G. hybridus, and G. ocior. However, four sequences obtained from the Genbank (AY256691, DQ450003, DQ449976-7) formed two unsupported groups proximal to G. ocior or G. aquosus in the ITS phylogram. Gymnopus dryophilus var. lanipes (labelled G13 in the phylogenetical trees) fell unambigously within other sequences of G. dryophilus. Gymnopus hybridus is closely related to G. inusitatus placed in sect. Levipedes subsect. Alkalivirentes Antonín & Noordel. This indicates the current concept of subsections in sect. Levipedes (Antonín & Noordeloos 2010) may not follow phylogenetic relations of the species.
Fig. 1.
The phylogram inferred from the Bayesian analysis of combined dataset tefa and ITS DNA sequences of Gymnopus spp. included in the study. Numbers at branches indicate Maximum likelihood, Bayesian bootstrap values and Bayesian posterior probabilities values higher than 50 %. The bar indicates the number of expected substitutions per position.
Fig. 2.
The phylogram inferred from the Bayesian analysis of ITS gene sequences of Gymnopus spp. included in the study. Numbers at branches indicate Maximum likelihood, Bayesian bootstrap values and Bayesian posterior probabilities values higher than 50 %. The bar indicates the number of expected substitutions per position.
NOTES ON STUDIED TAXA
Gymnopus dryophilus complex
The recent identification of four European species of this complex is based on studies by Vilgalys & Miller (1987) and Vilgalys (1991), who distinguished four species based on anatomic-morphological studies and confirmed the distinctions using mating compatibility tests. The published key (Vilgalys & Miller 1987) relies on the pileus colour, presence of the basal stipe bulb, size of basidiospores and shape of cheilocystidia to distinguish species.
See Antonín & Noordeloos (2010) for monographic details on all taxa.
Gymnopus alpinus (Vilgalys & O.K. Mill.) Antonín & Noordel.
Specimen examined. Latvia, Kemeri National Park, in a Sphagnum stand under Pinus sylvestris and Betula pendula, 23 Aug. 2006, M. Beran, CB 16251.
Notes — Gymnopus alpinus differs from other species of this complex by having a dark red-brown, only weakly hygrophanous pileus, 6.0–7.5 × 3.0–4.0 μm basidiospores, and 14–32 × 7.0–12 μm, clavate, simple, irregular to coralloid cheilocystidia. This species is a widespread but probably rare fungus with unknown distribution in Europe.
Gymnopus aquosus (Bull.: Fr.) Antonín & Noordel.
Specimens examined. Czech Republic, White Carpathian Mts, Sidonie, Sidonie Nature Reserve, beech forest, under Fagus sylvatica, 14 May 2008, V. Antonín 08.07, BRNM 710027; Žd’árské vrchy Mts, Cikháj, Žákova hora National Nature Reserve, in fallen leaves of Acer pseudoplatanus and Acer pseudoplatanus and Fagus sylvatica, 2 July 2004, A. Vágner, BRNM 691431; Útěchov near Brno, Obora forest, in fallen leaves of Quercus and Tilia, A. Vágner, BRNM 665362; Mokrá near Brno, Nad dlouhým (Sivický les) forest, under Carpinus and Picea abies, 21 May 2002, A. Vágner, BRNM 670755; Bílé Karpaty Mts, Suchov, Porážky Nature Reserve, 1 June 2005, A. Vágner, BRNM 695556. – Sweden, Uppsala, Stadsskogen, in moss, 16 June 1984, S. Ryman & R. Vilgalys RV 84/199, DUKE 193407; ibid., 17 June 1984, S. Ryman & R. Vilgalys RV 84/200, DUKE 193408; ibid., amongst herbaceous matter, 17 June 1984, S. Ryman & R. Vilgalys RV 84/197, DUKE 193432; ibid., 17 June 1984, S. Ryman & R. Vilgalys RV 84/201, DUKE 193409; ibid., in moss, 17 June 1984, S. Ryman & R. Vilgalys RV 84/205, DUKE 193412; ibid., in moss, 17 June 1984, S. Ryman & R. Vilgalys RV 84/202, DUKE 193410.
Notes — Gymnopus aquosus is mainly characterized by having a hygrophanous, almost to the centre translucently striate, rather pale coloured, pale yellow to ochre pileus, pallescent to almost white, a distinctly bulbose stipe base with pinkish-ochraceous rhizomorphs, (5.0−)5.5–7.0 × 3.0–4.0(−4.25) μm basidiospores, and 16–51 × (5.0−)7.0–17 μm, clavate, capitate and pedunculate, less frequently subcylindrical or fusoid, simple or coralloid cheilocystidia. It also appears very early in the season (from May, rarely late April). It grows in deciduous, rarely coniferous forests, but also among grass on road-sides in semi-open places, and is widespread all over Europe.
Gymnopus dryophilus (Bull.: Fr.) Murrill s.str.
Lectotypification. Bulliard, Herbier de la France: Historie des champignons de la France, pl. 434 A, B, E, nad F (C and D excluded), 1789; designated here.
Bulliard kept a very broad concept of this species. Excluded pictures C and D show darker, brown to dark brown coloured fungi which may represent other taxa of this species complex (G. ocior, G. alpinus?).
Vilgalys & Miller (1987) proposed the collection from Sweden (Uppsala, Stadsskogen, R. Vilgalys 84/181) as the neotype. However, this neotype specimen was not traced in herbaria BPI, DUKE, VPI, and NY (relocated VPI herbarium) (Halling, Robertson & Vilgalys in litt.). Therefore, the other specimen from the original series of collections (made at the identical locality by the same collectors, and in the same time) was selected as the epitype: Sweden, Uppsala, Stadsskogen, 17 June 1984 leg. S. Ryman & R. Vilgalys 84/204 (DUKE 193411; designated here).
Nomenclature notes — The ITS sequences conspecific to G. dryophilus had been identified as G. ocior (Mata et al. 2006). The name G. ocior was adopted there based on several European specimens. We added two of these sequences (DQ480098 – Duke29 and DQ449956 – TFB 3849, Scotland) in our ITS dataset. These sequences fell in the G. dryophilus clade apart from G. ocior as conceived by us.
To support our concept of G. dryophilus, we examined collections by S. Ryman & R. Vilgalys from Uppsala previously examined by Vilgalys (Vilgalys & Miller 1987, Vilgalys 1991), deposited in the DUKE herbarium. The ITS sequences of these specimens and the morphological characters of G. dryophilus noted by Antonín & Noordeloos (2010) showed that the correct name for this taxon is G. dryophilus. Therefore, we confirm the species concept hypothesized by Vilgalys & Miller (1987) while the concept of G. ocior proposed by Mata et al. (2006) is at variance with our hypothesis. We also determined that European and North American specimens of G. dryophilus are conspecific. The three sequences (DQ449963, DQ449964, DQ449966) included in the North American G. dryophilus group by Mata et al. (2006) formed a well-supported group within our G. dryophilus clade. Also American G. dryophilus included in the AFTOL project (ID 559; Lutzoni et al. 2004) fell in the same group.
Specimens examined. Czech Republic, Krušné hory Mts, Rolava, Velký cínový důl, wet meadows with Sphagnum, 9 June 2004, V. Antonín 04.39, BRNM 691279; Slavkovský les Mts, Nová Ves u Kraslic, Křížky National Nature Monument, serpentine rocks with Calluna and Vaccinium, 10 June 2004, V. Antonín 04.43, BRNM 691282; Doksy, Břehyně-Pecopala National Nature Reserve, under Picea abies and Pinus sylvestris, 17 Aug. 2010, H. Deckerová, Antonín 10.156, BRNM 734758; Třeboňsko, Lomnice nad Lužnicí, Ruda Nature Reserve, 29 Sept. 2005, V. Antonín 05.185, BRNM 695739; Třeboňsko, Lomnice nad Lužnicí, Velký and Malý Tisý National Nature Reserve, 26 Sept. 2005, A. Vít, BRNM 705601; Hluboká u Borovan, Žemlička Nature Monument, under Quercus near a pond, 29 Sept. 2008, A. Vágner, BRNM 712600; Javorníky Mts, Velké Karlovice, Razula National Nature Reserve, under Picea abies, Abies alba, Fagus sylvatica, 28 July 2010, V. Antonín 10.103, BRNM 732938; Moravské Křižánky, Milovy, Malinská skála, 11 June 2005, A. Vágner, BRNM 695586; Mokrá near Brno, Nad dlouhým (Sivický les) forest, under Quercus petraea, Carpinus betulus and Picea abies, 17 Aug. 2006, V. Antonín 06.29, BRNM 704894. – Germany, Mittenwald, Karwendelgrube, alpine meadow, alt. 1250 m, 9 Sept. 2011, P. Karasch, Antonín 11.166, BRNM 737691. – Italy, Ravenna distr., Pineta di San Vitale, Bardello, grass community on dunes, 4 Nov. 2007, V. Antonín 07.399, BRNM 707149. – Norway, Østfold Co., Fredrikstad, Veberg, Sphagnum bog, 10 June 2010, M. Pettersen, BRNM 737692. – Slovakia, Belianské Tatry Mts Bujačí vrch hill, a Homogyne alpina, Poa and Dryas octopetala stand, alt. 1900 m, 28 Aug. 1998, V. Antonín 98.101, BRNM 642393; Vysoké Tatry Mts, Štrbské Pleso, Solisko, Furkotská dolina valley, 7 Aug. 1989, J. Kuthan, BRA 13021; Horná Orava, Námestovo, Klín, Klínské rašeliniště National Nature Reserve, in a Sphagnum stand, 3 June 2002, V. Antonín 02.26, BRNM 670778. – Slovenia, Julian Alps, Triglav National Park, Upper Soča valley, Zadnja Trenta, on the riverbank, among Dryas octopetala and Picea, in grass, 10 Oct. 2001, G. Podgornik, BRNM 695317. – Spain, Málaga, Road Málaga-Colmenar, venta de Garvey, Pinus forest, 10 Nov. 2000, A. Ortega & L. Alcobe, BRNM 670686 ex herb. AH 26980, isoneotype of G. dryophilus var. lanipes. – Sweden, Uppsala, Stadsskogen, in Sphagnum and moss in a mixed forest, 17 June 1984, S. Ryman & R. Vilgalys RV 84/190, DUKE 193401; ibid., in moss, 17 June 1984, S. Ryman & R. Vilgalys RV 84/203, DUKE 193429; ibid., in Sphagnum, 16 June 1984, S. Ryman & R. Vilgalys RV 84/193, DUKE 193403; ibid., in moss, 17 June 1984, S. Ryman & R. Vilgalys RV 84/198, DUKE 193406; ibid., 17 June 1984, S. Ryman & R. Vilgalys RV 84/204, DUKE 193411, neotype, selected here; ibid., 17 June 1984, S. Ryman & R. Vilgalys RV 84/195, DUKE 193405. – Switzerland, Graubünden, Tamins, Reichenau, Ils Aults, in grass and moss in a open place, alt. 650–700 m, 1 Oct. 2004, V. Antonín 04.234, BRNM 693554.
Notes — Gymnopus dryophilus is characterized by having a pale coloured, orange-brown or ochraceous brown, later ochraceous brown, yellow ochraceous to pinkish ochraceous, hygrophanous, translucently striate pileus, white, cream to yellow lamellae (see Discussion), 5.0–7.0(−8.0) × (2.5−)3.0–4.0(−4.5) μm basidiospores, and 17–55 × 4.0–10 μm, (sub)cylindrical, narrowly clavate cheilocystidia, which are mostly coralloid, but also lobate or with apical projections. It grows mostly in deciduous, sometimes also coniferous forests, or in Sphagnum stands, and is widely reported from all over Europe and North America. All studied collections of fungi from this species complex from the alpine habitats represent this species.
Gymnopus dryophilus var. lanipes (Ortega et al. 2003) especially differs from the type variety by having a finely tomentose stipe. It is known from Mediterranean thermophilic forests, especially those containing Quercus ilex, Pinus halepensis, or Cistus. Molecular studies showed that the isoneotype specimen (BRNM 670686) agrees with sequences of G. dryophilus var. dryophilus. Therefore, the proposal to consider it a separate species (Vila & Llimona 2006) is not supported.
Gymnopus ocior (Pers.) Antonín & Noordel.
Specimens examined. Czech Republic, České Švýcarsko National Park, Jetřichovice, Babylon, under Picea abies, Pinus sylvestris, 12 June 2010, V. Antonín 10.50, BRNM 728540; ibid., V. Antonín 10.49, BRNM 728539; České Švýcarsko National Park, Jetřichovice, Starý mlýn, on a woody mulch in a garden, 29 May 2009, V. Antonín 09.19 and S. Komínková, BRNM 714822; Všeteč, on a mulch, 22 May 2010, M. Mikšík, BRNM 728586; Mokrá near Brno, Nad dlouhým (Sivický les) forest, under Larix decidua, Pinus sylvestris and Picea abies, 7 June 2006, A. Vágner, BRNM 699795. – Norway, Østfold Co., Sarpsborg, Hafslundparken, 24 May 2010, Ø. Weholt, BRNM 737695; ibid., 12 June 2010, Ø. Weholt, BRNM 737696; Østfold Co., Fredrikstad, Skjælin, Borge skytterhus, 25 June 2010, Ø. Weholt, BRNM 737697; Østfold Co., Fredrikstad, Veberg, 5 June 2010, Ø. Weholt, BRNM 737693; ibid., 21 June 2010, Ø. Weholt, BRNM 737694. – Slovakia, Podunajské Biskupice, Topolové, Topolové hony Nature Reserve, alluvial forest, under Quercus robur, Acer campestre, Padus racemosa, and Corylus avellana, 19 June 2010, L. Nagy, Antonín 10.82, BRNM 728565.
Notes — Gymnopus ocior is especially characterized by having a non-translucent or only at margin translucently striate, dark red- or orange-brown pileus, pallescent to reddish yellow or pinkish brownish, whitish or yellowish lamellae (see Discussion), (5.0−)5.5–6.5(−7.0) × (2.5−)2.75–3.5(−4.0) μm basidiospores, and 16–60 × 6.0–12 μm, clavate, subcylindrical or subutriform cheilocystidia, often lobate, branched, coralloid or with (apical) projections. It occurs in both deciduous and coniferous forests, on road margins and similar stands. It is a widespread fungus in Europe. The ITS phylogram of Mata et al. (2006) published in f. 4 of the respective publication depicted clade of ‘G. ocior Europe’ with nested subclade of ‘G. dryophilus North Am.’. After co-analysis of selected sequences from this work with our data we revealed sequences of ‘G. ocior Europe’ represent in fact European specimens of G. dryophilus.
Gymnopus erythropus (Pers.: Fr.) Antonín, Halling & Noordel.
Specimens examined. Czech Republic, Český kras, Sv. Jan pod Skalou, J. Burel, BRNM 714784; Moravský kras, Ochoz near Brno, Hornek Nature Reserve, living stem of Crataegus, 3 Oct. 2001, A. Vágner, BRNM 666730; Litovelské Pomoraví, Litovel, Vrapač National Nature Reserve, stump of Quercus, 2 Aug. 2000, A. Vágner, BRNM 664995; Mokrá near Brno, Nad dlouhým (Sivický les) forest, stump of Quercus petraea, 7 Sept. 2005, A. Vágner, BRNM 705224. – Slovakia, Strážovské vrchy Mts, Kšinná, Slávcové hill, on soil under Fagus sylvatica, V. Antonín 07.235, BRNM 706885. – Switzerland, Graubünden, Tamins, Reichenau, Ils Aults, on soil, on a pasture under Pinus sylvestris, 1 Oct. 2004, V. Antonín 04.232, BRNM 693553.
Notes — This species is especially recognizable by having a ± dark red-brown, shining stipe with typically red-brown coloured basal hairs, rather large basidiospores ((5.0−)5.5–8.0(−9.0) × 3.5–4.5(−5.0) μm), and rather narrow (13–40 × 5.0–10(−13) μm), (sub)clavate, subfusoid, irregular to coralloid or apically mucronate cheilocystidia. It usually grows on dead wood or wood debris of various broad-leaved, rarely coniferous (Picea abies, Pinus sylvestris) trees, sometimes also among humus in deciduous woods or on buried wood. It is widely distributed in Europe (Antonín & Noordeloos 2010, Noordeloos 2012). For relevant nomenclature and a detailed description, photograph, and citations of other literature, see Antonín & Noordeloos (2010).
Gymnopus fagiphilus (Velen.) Antonín, Halling & Noordel.
Specimens examined. Czech Republic, České Švýcarsko National Park, Jetřichovice, Babylon, fallen leaves and cupules of Fagus sylvatica, 3 Oct. 2007, V. Antonín 07.320, BRNM 707079; Orlické hory Mts, Horní Rokytnice, Černý důl Nature Reserve, fallen leaves of Fagus sylvatica, V. Antonín 07.310, BRNM 707068; Novohradské hory Mts, Pivonice u Pohorské Vsi, Žofínský prales National Nature Reserve, fallen leaves of Fagus sylvatica, 30 Sept. 2008, V. Antonín 08.245, BRNM 712407; Moravský kras, Vilémovice, Vývěry Punkvy National Nature Reserve, between Suchý and Pustý žleb, decaying leaves of Fagus sylvatica, A. Vágner, BRNM 691489; ibid., Macocha abyss, decaying leaves of Fagus sylvatica, V. Antonín, BRNM 712422. – Slovakia, Javorníky Mts, Dolná Mariková, part Kátlina, fallen leaves of Fagus sylvatica, V. Antonín 05.196, BRNM 695747.
Notes — Gymnopus fagiphilus, known also as Collybia konradiana Singer or Collybia fuscopurpurea sensu Konrad & Maublanc and Kühner & Romagnesi, is characterized by the moderately distant, pinkish brown or pinkish cream coloured lamellae, an apically glabrous, otherwise from a base upwards finely hairy stipe, rather large basidiospores ((6.0−)7.0–9.0 × (3.0−)3.5–4.5 μm), and clavate, irregular to apically almost coralloid cheilocystidia. It is associated with Fagus sylvatica litter, and grows on fallen leaves. It has a scattered distribution in Europe, but details of occurrences are not known. However, at least in Central Europe, it occurs in almost all more or less near-natural and natural beech forests. For a detailed description, photo and citations of other literature, see Antonín & Noordeloos (2010).
Gymnopus hybridus (Kühner & Romagn.) Antonín & Noordel.
Specimens examined. Czech Republic, Petrovice nad Orlicí, Obora forest, U Houkvice Nature Reserve, under Aesculus in an oak stand, 26 Sept. 1993, H. Deckerová, Antonín 93.272, BRNM 576770; Bílé Karpaty Mts, Suchov, Búrová National Nature Monument, 20 Sept. 2006, V. Antonín 06.100, BRNM 704957; Mokrá u Brna, Sivický les, alt. c. 380 m, fallen leaves of Quercus, 5 Oct. 2007, D. Dvořák 393/07, BRNU; Bílovice nad Svitavou, Hádecká planinka National Nature Reserve, alt. c. 405 m, broadleaved forest (Tilia, Quercus, Carpinus), 7 Oct. 2002, D. Dvořák 138/02, BRNU. – Hungary, Börzsöny Mts, Törökmezö, under Quercus and Acer campestre, 27 Oct. 1994, V. Antonín 94.274, BRNM 599209. – Italy, Emilia-Romagna, Borgo val di Taro, Stadielle, on fallen leaves of Quercus robur and Q. cerris, 19 Oct. 2005, V. Antonín 05.230, BRNM 695773.
Notes — Gymnopus hybridus is easily distinguishable by having rather distant, cinnamon-brown lamellae, rather large basidiospores ((6.2−)7.4–9.6 × 3.5–4.8 μm), and only small (18–26 × (3.1−)5.2–6.6 μm), clavate to cylindrical, mostly irregular cheilocystidia. It mostly grows on fallen leaves of Quercus, less frequently on other broad-leaved tree litter, mostly in thermophilic forests, and is widely distributed especially in Central and Western Europe. For a detailed description, photograph and citations of other literature, see Antonín & Noordeloos (2010).
DISCUSSION
The DNA sequences brought light to morphological characters useful for identification of Gymnopus spp. in subsect. Levipedes. Three taxa of subsect. Levipedes in the G. dryophilus complex (G. erythropus, G. fagiphilus, G. hybridus) are clear and easily to identify. Therefore, the most important features of species belonging to the G. dryophilus complex are discussed below.
Colour of lamellae
In the G. dryophilus complex, the lamellae colour is used as an identification character. According to literature, lamellae are white to cream in G. alpinus, G. aquosus, and G. dryophilus, whereas yellowish to yellow, rarely white in G. ocior (e.g. Vilgalys & Miller 1987, Courtecuisse & Duhem 1994, Hausknecht & Krisai-Greilhuber 2000, Gröger 2006, Antonín & Noordeloos 2010). Especially for the latter species, lamellae colour represents an important feature.
Our results show that the lamellae colour agrees with the literature (e.g. Hausknecht & Krisai-Greilhuber 2000, Gröger 2006, Antonín & Noordeloos 2010) in G. alpinus and G. aquosus. On the other hand, the variability is distinctly broader in G. ocior and G. dryophilus. In G. ocior, lamellae may be white to whitish when young and then pale cream coloured (e.g. BRNM 728540 and BRNM 728565) or yellowish when young to pale or light yellow (3A3–4) when old (e.g. BRNM 728539). A surprisingly broad variation was found in G. dryophilus – the lamellae are mostly white to pale cream when young to cream (e.g. BRNM 734758), yellowish white, pale or light yellow (3A2, 3–4A3, 4A3; e.g. BRNM 732938, 642393, and 737691). Even a collection with entirely yellow (yellowish white, pale or light yellow) basidiocarps (pileus 3–4A4, lamellae 2A3, stipe 3–4A4), agreeing macroscopically with G. ocior, belongs here. As summarized, the yellow coloured lamellae may not unambiguously lead to G. ocior. The yellow coloured lamellae are also present in G. junquilleus R.H. Petersen & J.L. Mata (Mata et al. 2006), G. subsulphureus (Peck) Murrill (Vilgalys & Miller 1987, Vilgalys 1991).
Basidiospores
Basidiospores of all taxa of the G. dryophilus group are (broadly) ellipsoid, oblong, pip-shaped or ellipsoid-fusoid. Data on basidiospore measurements are summarized in Table 3.
Table 3.
Basidiospores size of studied specimens.
| Species | Herbarium | Size of basidiospores (μm) | Size average (μm) | E | Q |
|---|---|---|---|---|---|
| G. alpinus | CB 16251 | 6.0–7.5 × 3.0–4.0 | 6.6 × 3.4 | 1.61–2.20 | 1.94 |
| G. aquosus | BRNM 670755 | (5.5−)6.0–7.0 × 3.5–4.5 | 6.3 × 3.9 | 1.43–1.86 | 1.63 |
| BRNM 695556 | (5.75−)6.0–7.0(−7.5) × (3.25−)3.5–4.0(−4.25) | 6.3 × 3.7 | 1.50–1.89 | 1.69 | |
| BRNM 710027 | (5.5−)6.0–6.75 × 3.0–3.5(−3.75) | 6.2 × 3.3 | 1.57–2.09 | 1.89 | |
| BRNM 665362 | 5.75–6.75 × 3.0–3.75 | 6.3 × 3.3 | 1.71–2.09 | 1.91 | |
| BRNM 691431 | (5.0−)5.0–6.0(−6.5) × 3.0–3.75(−4.0) | 5.8 × 3.4 | 1.50–2.00 | 1.74 | |
| DUKE 193407 | 5.0–6.5 × 3.0–3.5(−4.0) | 5.7 × 3.4 | 1.50–1.86 | 1.69 | |
| DUKE 193408 | 5.5–7.0 × 3.5–4.0 | 6.3 × 3.7 | 1.43–1.91 | 1.70 | |
| DUKE 193409 | 5.5–6.5(−6.75) × 3.5–4.0(−4.25) | 6.2 × 3.8 | 1.43–1.86 | 1.61 | |
| DUKE 193432 | 5.75–7.0 × 3.25–4.25 | 6.3 × 3.7 | 1.55–1.88 | 1.70 | |
| DUKE 193412 | 5.5–6.75(−7.0) × 3.5–4.25 | 6.3 × 3.8 | 1.38–1.77 | 1.64 | |
| DUKE 193410 | 5.5–6.0(−6.25) × 3.5–4.0(−4.25) | 5.7 × 3.7 | 1.36–1.71 | 1.56 | |
| G. dryophilus | BRNM 670778 | 6.0–7.5 × 3.0–3.75 | 6.8 × 3.3 | 1.86–2.33 | 2.06 |
| BRNM 695739 | 5.5–6.5(−7.0) × 2.75–3.5 | 6.2 × 3.1 | 1.71–2.25 | 2.03 | |
| BRNM 695586 | (5.75−)6.0–7.0 × (2.75−)3.0–4.0 | 6.4 × 6.2 | 1.71–2.17 | 1.99 | |
| BRNM 691279 | (5.5−)6.0–6.75(−7.0) × (2.5−)2.75–4.0(−4.25) | 6.3 × 3.4 | 1.62–2.20 | 1.86 | |
| BRNM 693554 | 5.5–7.0 × 2.75–3.5(−4.0) | 6,2 × 3,2 | 1.62–2.33 | 1.93 | |
| BRNM 695317 | (5.75−)6.0–8.0 × 3.5–4.5(−5.0) | 7.0 × 4.1 | 1.44–2.03 | 1.73 | |
| BRA 13021 | (5.5−)6.0–7.5 × (3.25−)3.5–4.2 | 6.8 × 3.8 | 1.57–2.03 | 1.79 | |
| BRNM 642393 | 6.0–7.75(−8.0) × 3.0–4.0 | 6.8 × 3.4 | 1.81–2.40 | 2.03 | |
| BRNM 691282 | 6.0–6.75(−7.5) × (2.75−)3.0–3.75 | 6.5 × 3.2 | 1.81–2.22 | 2.00 | |
| BRNM 705601 | (5.0−)5.5–6.5(−6.75) × 3.0–3.5(−4.0) | 6.0 × 3.4 | 1.57–2.03 | 1.77 | |
| BRNM 670686 | (5.0−)5.5–6.0 × 2.75–3.5 | 5.5 × 3.1 | 1.57–2.03 | 1.79 | |
| BRNM 707149 | 5.25–6.0(−6.5) × 3.0–3.75 | 5.7 × 6.6 | 1.57–1.86 | 1.71 | |
| BRNM 734758 | 5.5–6.5(−7.0) × 3.0–3.25 | 6.1 × 3.1 | 1.62–2.17 | 2.00 | |
| BRNM 732938 | 6.0–7.0 × 3.0–4.0 | 6.5 × 3.5 | 1.68–2.17 | 1.86 | |
| BRNM 737692 | (5.0−)5.5–6.25 × 3.25–4.0 | 5.7 × 3.6 | 1.25–1.72 | 1.56 | |
| BRNM 737691 | 5.5–6.5 × 3.5–4.25(−4.5) | 5.9 × 3.8 | 1.33–1.71 | 1.56 | |
| BRNM 704894 | (5.5−)6.5–7.5 × (2.5−)3.0–3.5 | 6.7 × 3.1 | 1.83–2.60 | 2.20 | |
| BRNM 712600 | (5.5−)6.0–7.0 × 3.0–4.0 | 6.3 × 3.4 | 1.62–2.17 | 1.85 | |
| DUKE 193401 | 5.0–5.75(−6.0) × 3.5–4.0 | 5.4 × 3.7 | 1.30–1.63 | 1.47 | |
| DUKE 193403 | 5.0–6.25(−6.5) × (2.75−)3.0–3.75(−4.5) | 5.7 × 3.4 | 1.44–1.88 | 1.68 | |
| DUKE 193405 | (5.0−)5.25–6.0 × (3.0−)3.25–4.0 | 5.7 × 3.5 | 1.43–1.90 | 1.62 | |
| DUKE 193406 | 5.5–6.75(−7.25) × 3.25–4.0 | 6.2 × 3.7 | 1.38–1.86 | 1.69 | |
| DUKE 193411 | 5.0–6.5 × (2.75−)3.25–4.0 | 5.8 × 3.4 | 1.57–1.94 | 1.69 | |
| DUKE 193429 | (5.0−)5.5–6.0 × (2.5−)2.75–3.0 | 5.6 × 3.0 | 1.72–2.00 | 1.86 | |
| G. ocior | BRNM 728565 | 5.25–6.5(−7.0) × 2.75–3.5 | 6.0 × 3.2 | 1.71–2.22 | 1.91 |
| BRNM 728540 | (5.0−)5.5–6.5 × (2.75−)3.0–3.5(−3.75) | 5.9 × 3.1 | 1.62–2.17 | 1.87 | |
| BRNM 699795 | 5.75–6.5(−7.0) × 3.0–3.5 | 6.3 × 3.3 | 1.71–2.17 | 1.92 | |
| BRNM 737695 | (5.0−)5.5–6.5(−7.0) × 3.25–3.75 | 6.0 × 3.4 | 1.43–2.03 | 1.77 | |
| BRNM 737694 | 5.0–6.0(−6.5) × 2.5–3.5 | 5.5 × 2.9 | 1.67–2.20 | 1.89 | |
| BRNM 737696 | 5.5–6.5(−7.0) × 3.25–4.0(−4.25) | 6.1 × 3.5 | 1.50–2.03 | 1.76 | |
| BRNM 737697 | (5.0−)5.5–6.5 × (3.0−)3.25–3.75 | 5.9 × 3.4 | 1.62–1.94 | 1.74 | |
| BRNM 737693 | 5.25–6.5 × (2.5−)2.75–3.5 | 5.8 × 3.1 | 1.57–2.22 | 1.91 | |
| BRNM 728539 | (5.0−)5.5–6.5 × (2.5−)2.75–3.5 | 5.7 × 3.0 | 1.71–2.17 | 1.92 |
The studied specimen of G. alpinus showed slightly smaller basidiospores (6.0–7.5 × 3.0–4.0 μm) than mentioned in the literature ((6.2−)6.5–8.5 × 3.0–4.4 μm; Antonín & Noordeloos 2010). This size is in the lower limit of their variability.
For studied specimens of G. aquosus the basidiospores size ((5.0−)5.5–7.0 × 3.0–4.0(−4.25) μm) also generally agrees with the literature ((5.0−)5.5–8.0 × 2.7–3.5(−4.0) μm; Antonín & Noordeloos 2010) except for the absence of an upper limit (none of basidiospores reached up to 8.0 μm). However, in single specimens we can find a rather large variation. Specimen BRNM 710027 has very narrow (3.0–3.5(−3.75) μm) basidiospores (Q = 1.89), while they were distinctly broader (3.5–4.5 μm, Q = 1.63) in BRNM 670755. However, there is a transition from one extreme to the other and the basidiospore shape may distinctly vary in single specimens as well. The molecular data confirmed identity of both collections.
The results of measurements of G. dryophilus showed large basidiospores (5.0–7.0(−8.0) × (2.5−)3.0–4.0(−4.5) μm), while the literature indicates a larger variability especially in a lower limit ((3.5−)4.0–6.5(−7.0) × 2.5–3.5(−4.0) μm; Antonín & Noordeloos 2010). The differences between single specimens are, however, rather great, and vary between (5.0−)5.5–6.0 × (2.5−)2.75–3.0 μm, Q = 1.86 (DUKE 193429) and (5.75−)6.0–8.0 × 3.5–4.5(−5.0) μm, Q = 1.73 (BRNM 695317). Small basidiospores were also found in the isoneotype specimen of G. dryophilus var. lanipes: (5.0−)5.5–6.0 × 2.75–3.5 μm.
The basidiospore variability in G. ocior ((5.0−)5.5–6.5(−7.0) × (2.5−)2.75–3.5(−4.0) μm, Q = 1.74–1.92) generally agrees with published data ((4.6−)5.1–6.3 × 2.5–3.5(−4.0) μm; Antonín & Noordeloos 2010).
Cheilocystidia
The shape of cheilocystidia is one of the most important identification characters. The first detailed studies of cheilocystidia in the recent literature were made by Vilgalys & Miller (1987). They distinguished inflated-clavate to subglobose or sphaeropendunculate, frequently diverticulate (G. ocior), inconspicuous, diverticulate-filamentous (G. alpinus), inflated-clavate to sphaeropendunculate, somewhat echinulate or lobate-diverticulate (G. aquosus), and sometimes inconspicuous, filamentous cheilocystidia with numerous diverticulate branches (G. dryophilus). However, the variation is even broader.
Cheilocystidia of G. alpinus of the studied specimen (Fig. 3) agree with those drawn by Vilgalys & Miller (1987) and Antonín & Noordeloos (2010). They are clavate, simple, irregular to coralloid.
Fig. 3.
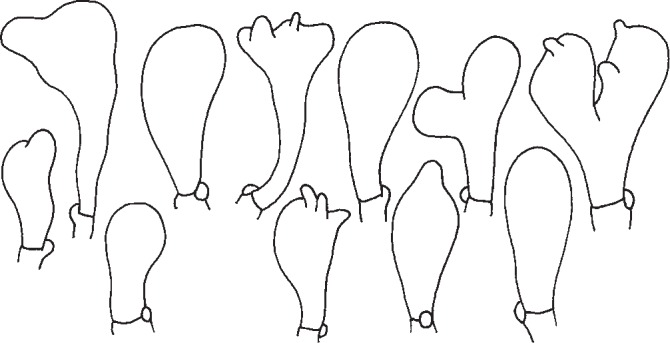
Gymnopus alpinus. Cheilocystidia. — Scale bar = 20 μm.
Gymnopus aquosus has clavate, capitate and pedunculate, less frequently subcylindrical or fusoid, simple or coralloid cheilocystidia (Fig. 4); some of them may even be similar to those of G. dryophilus. Clavate to sphaeropendunculate cheilocystidia drawn by Vilgalys & Miller (1987) represent only the minority of ones found.
Fig. 4.
Gymnopus aquosus. Cheilocystidia. — Scale bar = 20 μm.
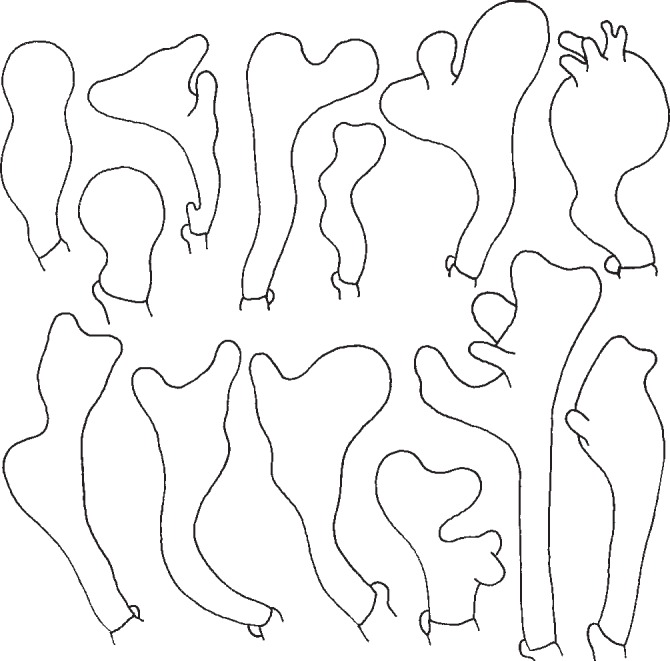
The typical shapes of cheilocystidia of G. dryophilus are (sub)cylindrical, narrowly clavate, mostly coralloid (Fig. 5). Their form agrees with figures by Vilgalys & Miller (1987) and Antonín & Noordeloos (2010). However, we also usually find clavate, simple or slightly irregular cheilocystidia in most of the basidiocarps.
Fig. 5.
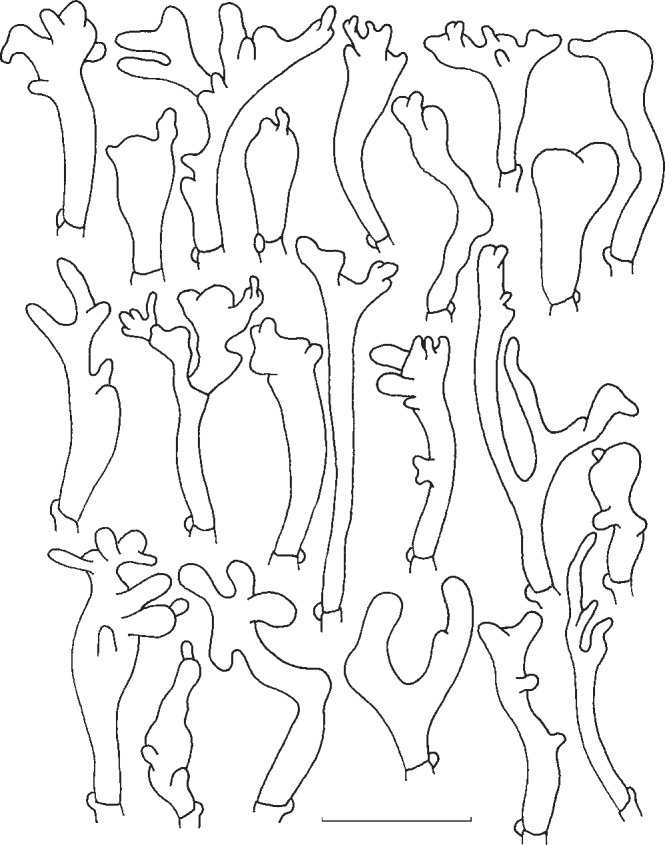
Gymnopus dryophilus. Cheilocystidia. — Scale bar = 20 μm.
The basic shapes of G. ocior cheilocystidia (Fig. 6) are clavate, subcylindrical, or subutriform, but, most of them are lobate, branched, with (apical) projections or coralloid. The form of cheilocystidia drawn by Vilgalys & Miller (1987) represents only a part on their variability. Antonín & Noordeloos (2010) mentioned that forms of G. ocior with yellow lamellae have more distinctly coralloid cheilocystidia. Our studies showed that these yellow forms actually belong to G. dryophilus, and the cheilocystidia fully support that placement. Nevertheless, we can also rarely find cheilocystidia of the G. dryophilus type in typical basidiocarps of G. ocior.
Fig. 6.
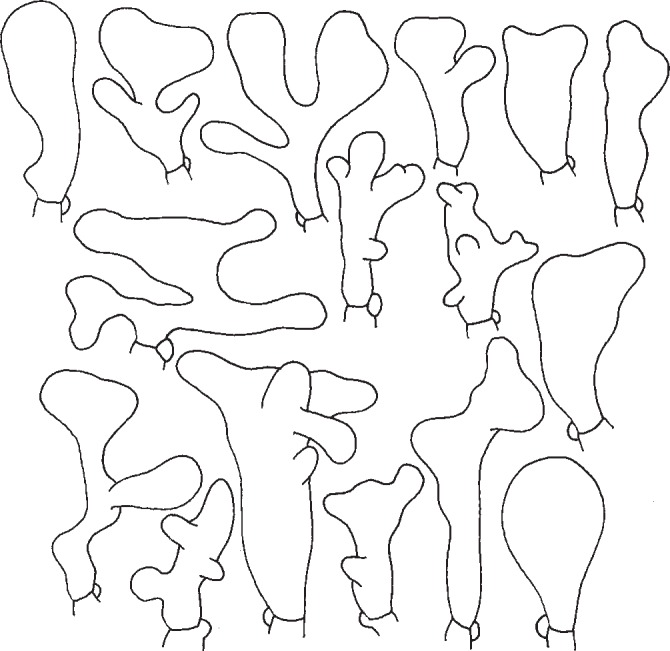
Gymnopus ocior. Cheilocystidia. — Scale bar = 20 μm.
Pileipellis
The pileipellis is a Dryophila-structure in all discussed taxa. In G. alpinus it is composed of cylindrical to inflated, often irregularly coralloid terminal elements with lateral and terminal projections (a poorly developed Dryophila-structure as defined by Antonín & Noordeloos 2010). However, this type of pileipellis structure is useless for identification because of the variability. A poorly to well-developed Dryophila-structure is dependent on the age and development of basidiocarps, and also on the location on the pileus (centre, margin) where the structure is observed.
KEY TO THE EUROPEAN SPECIES OF GYMNOPUS SUBSECTION LEVIPEDES
- 1. Stipe smooth except for basal rhizoids . . . . . . . . . . . . . . 2
- 1. Stipe entirely pubescent, or glabrous only at apex and distinctly finely hairy from a base upwards (up to 2/3 of length) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
- 2. Stipe dark red-brown, shining, with typically red-brown coloured basal hairs; pileus (dark) red-brown at centre, yellow-brown to yellow-red towards margin; lamellae pale cream-coloured; smell sometimes unpleasantly foetid; basidiospores (5.0−)5.5–8.0(−9.0) × 3.5–4.5(−5.0) μm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. erythropus
- 2. Stipe never red-brown, never with red-brown basal hairs 3
- 3. Pileus not translucently striate or at margin only, uniformly pale to dark (reddish, pinkish) brown . . . . . . . . . . . . . . . 4
- 3. Pileus distinctly translucently striate, (pale) yellow, ochraceous yellow, orange-brown, sometimes with darker centre . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
- 4. Spores (5.0−)5.5–6.5(−7.0) × (2.5−)2.75–3.5(−4.0) μm; cheilocystidia 16–60 × 6.0–12 μm, clavate, subcylindrical or subutriform, lobate, branched, coralloid or with (apical) projections; lamellae white, yellowish to yellow . . . G. ocior
- 4. Spores 6.0–7.5 × 3.0–4.0 μm; cheilocystidia 14–32 × 7.0–12 μm, clavate, simple, irregular to coralloid; lamellae white . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. alpinus
- 5. Lamellae rather distant, cinnamon-brown; basidiospores large ((6.2−)7.4–9.6 × 3.5–4.8 μm); cheilocystidia inconspicuous, 18–26 × (3.1−)5.2–6.6 μm, clavate to cylindrical, irregular . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. hybridus
- 5. Lamellae close, white, pale cream-coloured to yellow; basidiospores up to 7.0 × 4.25 μm; cheilocystidia distinct . . . 6
- 6. Basidiospores (5.0−)5.5–7.0 × 3.0–4.0(−4.25) μm; cheilocystidia clavate, capitate and pedunculate, less frequently subcylindrical or fusoid, simple or coralloid; pileus pale yellow, usually without ochre or brown tinges, almost to centre translucently striate; stipe often with distinctly inflated basal part . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. aquosus
- 6. Basidiospores 5.0–7.0(−8.0) × (2.5−)3.0–4.0(−4.5) μm; cheilocystidia (sub)cylindrical, narrowly clavate, mostly coralloid; pileus with ochre-brown tinges, especially at centre, translucently striate up to half the radius; stipe more or less equal . . . . . . . . . . . . . . . . . . . G. dryophilus var. dryophilus
- 7. Lamellae whitish; stipe pubescent; basidiospores (5.0−)5.5–6.0 × 2.75–3.5 μm; Mediterranean thermophilic forests, especially of Quercus ilex, Pinus halepensis, or Cistus . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. dryophilus var. lanipes
- 7. Lamellae pinkish brown or pinkish cream; stipe apically glabrous, otherwise from a base upwards finely hairy; basidiospores (6.0−)7.0–9.0 × (3.0−)3.5–4.5 μm; connected with Fagus sylvatica . . . . . . . . . . . . . . . . . . . . . . . . G. fagiphilus
ACKNOWLEDGEMENTS
The authors are much obliged to the curators of the DUKE herbarium for a loan of herbarium specimens, and Miroslav Beran (České Budějovice, Czech Republic), Øyvind Weholt (Torp, Norway), and Rytas Vilgalys (Durham, USA) for access to their collections and/or collection notes. This publication appeared through financial support provided by the Moravian Museum of the Ministry of Culture of the Czech Republic as part of its long-term conceptual development programme for research institutions (ref. MK000094862) (the 1st author); the Ministry of Environment of the Czech Republic, project no. SP/2D4/59/07 and the European Social Fund and the state budget of the Czech Republic, Project Indicators of trees vitality Reg. No. CZ.1.07/2.3.00/20.0265 (the 2nd and 3th author).
REFERENCES
- Antonín V, Finy P, Tomšovský M. 2012. Taxonomy of the Gymnopus inusitatus group and the new G. inusitatus var. cystidiatus from Hungary. Mycotaxon 119: 219–299 [Google Scholar]
- Antonín V, Noordeloos ME. 2010. A monograph of marasmioid and collybioid fungi in Europe. IHW-Verlag, Eching [Google Scholar]
- Courtecuisse R, Duhem B. 1994. Guide des champignons de France et d’Europe. Delachaux & Niestlé, Lausanne [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research 36 (suppl. 2): W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröger F. 2006. Bestimmungsschlüssel für Blätterpilze und Röhrlinge in Europa. Teil I. Regensburger Mykologische Schriften 13: 1–638 [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate Maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 3: 307–321 [DOI] [PubMed] [Google Scholar]
- Hausknecht A, Krisai-Greilhuber I. 2000. Rüblinge, Schwindlinge und verwandte Taxa in Ostösterreich. Österreichische Zeitschrift für Pilzkunde 9: 31–66 [Google Scholar]
- Katoh K, Toh H. 2010. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 26, 15: 1899–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JS. 2008. Ainsworth & Bisby’s dictionary of fungi, 10th edn CABI, Wallingford [Google Scholar]
- Kornerup A, Wanscher JH. 1983. Methuen handbook of colour. 3rd ed.Methuen, London [Google Scholar]
- Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, et al. 2004. Assembling the fungal tree of life: Progress, classification and evolution of subcellular traits. American Journal of Botany 91, 10: 1446–1480 [DOI] [PubMed] [Google Scholar]
- Mata JL, Halling RE, Petersen RH. 2004. New species and mating system reports in Gymnopus (Agaricales) from Costa Rica. Fungal Diversity 16: 113–129 [Google Scholar]
- Mata JL, Hughes KW, Petersen RH. 2006. An investigation of /omphalotaceae (Fungi: Euagarics) with emphasis of the genus Gymnopus. Sydowia 58: 191–289 [Google Scholar]
- Mata JL, Ovrebo CL. 2009. New reports and illustrations of Gymnopus for Costa Rica and Panama. Fungal Diversity 38: 125–131 [Google Scholar]
- Noordeloos ME. 2012. Gymnopus (Pers.) Roussel. In: Knudsen H, Vesterholt J. (eds), Funga Nordica, Copenhagen: 341–349 [Google Scholar]
- Nylander JAA. 2008. MrModeltest v2.3. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala [Google Scholar]
- Ortega A, Antonín V, Esteve-Raventós F. 2003. Three interesting thermophilic taxa of Gymnopus (Basidiomycetes, Tricholomataceae): G. pubipes sp. nov., G. pubipes var. pallidopileatus var. nov. and G. dryophilus var. lanipes comb. nov. Mycotaxon 85: 67–75 [Google Scholar]
- Rambaut A, Drummond AJ. 2009. Tracer v1.4, Available from http://beast.bio.ed.ac.uk/TracerInstitute of Evolutionary Biology, University of Edinburgh, Edinburgh [Google Scholar]
- Rehner SA, Buckley EP. 2005. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2003. PAUP* Phylogenetic Analysis Using Parsimony 4.0. Version Beta 10. Sinauer Associates, Sunderland, USA [Google Scholar]
- Thiers B. 2012. [continuously updated]: Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium; http://sweetgum.nybg.org/ih/ [Google Scholar]
- Tomšovský M, Sedlák P, Jankovský L. 2010. Species recognition and phylogenetic relationships of European Porodaedalea (Basidiomycota, Hymenochaetales). Mycological Progress 9: 225–233 [Google Scholar]
- Vienne DM de, Giraud T, Martin OC. 2007. A congruence index for testing topological similarity between trees. Bioinformatics 23: 3119–3124 [DOI] [PubMed] [Google Scholar]
- Vila J, Llimona X. 2006. Noves dades sobre el component fúngic de les comunitats de Cistus de Catalunya. II. Revista Catalana de Micologia 28: 167–207 [Google Scholar]
- Vilgalys R. 1991. Speciation and species concepts in the Collybia dryophila complex. Mycologia 83, 6: 758–773 [Google Scholar]
- Vilgalys R, Miller OK. 1987. Morphological studies on the Collybia dryophila group in Europe. Transactions of the British Mycological Society 88, 4: 461–472 [Google Scholar]