Specific DNA rearrangements in synchronously developing nuclei of Tetrahymena (original) (raw)
Abstract
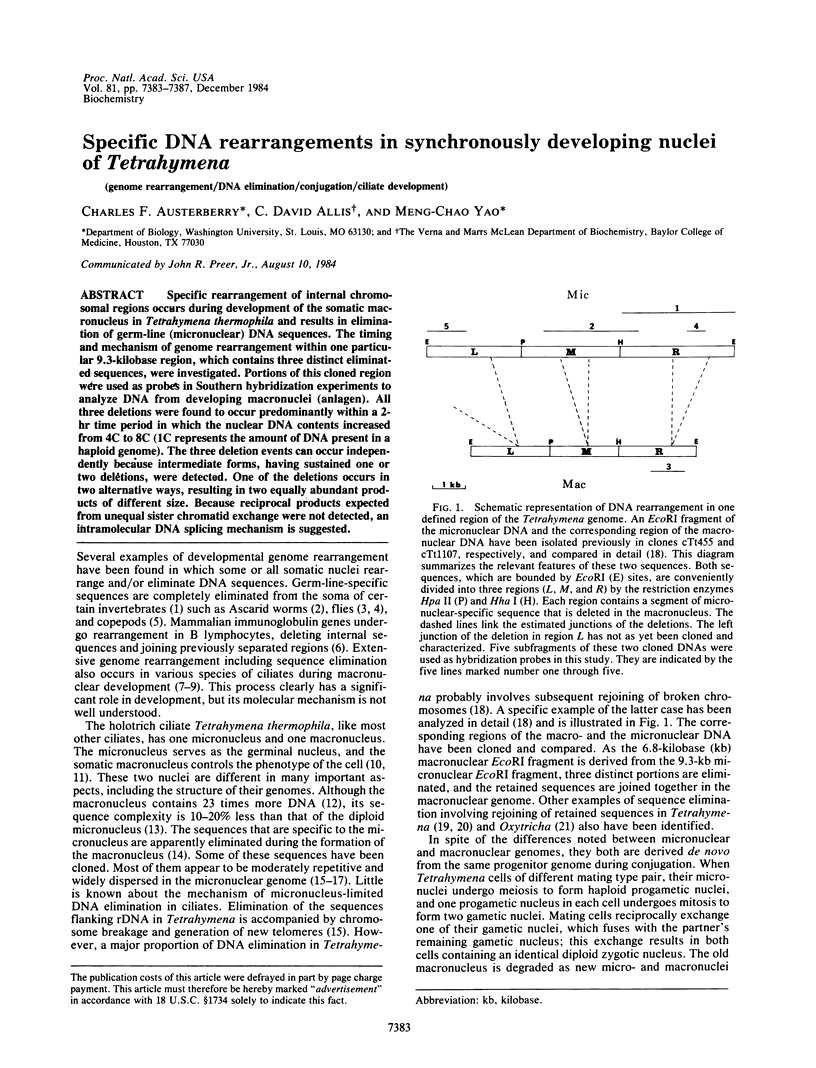
Specific rearrangement of internal chromosomal regions occurs during development of the somatic macronucleus in Tetrahymena thermophila and results in elimination of germ-line (micronuclear) DNA sequences. The timing and mechanism of genome rearrangement within one particular 9.3-kilobase region, which contains three distinct eliminated sequences, were investigated. Portions of this cloned region were used as probes in Southern hybridization experiments to analyze DNA from developing macronuclei (anlagen). All three deletions were found to occur predominantly within a 2-hr time period in which the nuclear DNA contents increased from 4C to 8C (1C represents the amount of DNA present in a haploid genome). The three deletion events can occur independently because intermediate forms, having sustained one or two deletions, were detected. One of the deletions occurs in two alternative ways, resulting in two equally abundant products of different size. Because reciprocal products expected from unequal sister chromatid exchange were not detected, an intramolecular DNA splicing mechanism is suggested.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Dennison D. K. Identification and purification of young macronuclear anlagen from conjugating cells of Tetrahymena thermophila. Dev Biol. 1982 Oct;93(2):519–533. doi: 10.1016/0012-1606(82)90139-7. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Wiggins J. C. Histone rearrangements accompany nuclear differentiation and dedifferentiation in Tetrahymena. Dev Biol. 1984 Feb;101(2):282–294. doi: 10.1016/0012-1606(84)90142-8. [DOI] [PubMed] [Google Scholar]
- Bantock C. R. Experiments on chromosome elimination in the gall midge, Mayetiola destructor. J Embryol Exp Morphol. 1970 Sep;24(2):257–286. [PubMed] [Google Scholar]
- Beams H. W., Kessel R. G. The problem of germ cell determinants. Int Rev Cytol. 1974;39:413–479. doi: 10.1016/s0074-7696(08)60944-4. [DOI] [PubMed] [Google Scholar]
- Beermann S. The diminution of Heterochromatic chromosomal segments in Cyclops (Crustacea, Copepoda). Chromosoma. 1977 Apr 20;60(4):297–344. doi: 10.1007/BF00292858. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Tsao S. G., Diamond C. H., Ohashi P. S., Tsao N. N., Pearlman R. E. Reorganization of unique and repetitive sequences during nuclear development in Tetrahymena thermophila. Can J Biochem. 1982 Sep;60(9):847–853. doi: 10.1139/o82-107. [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Brussard T. B. Pair formation in tetrahymena pyriformis, an inducible developmental system. J Exp Zool. 1974 Jun;188(3):337–344. doi: 10.1002/jez.1401880309. [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Brussard T. B. Positive selection for mating with functional heterokaryons in Tetrahymena pyriformis. Genetics. 1974 Nov;78(3):831–841. doi: 10.1093/genetics/78.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R. C., Shalke G., Gorovsky M. A. Developmental rearrangements associated with a single type of expressed alpha-tubulin gene in Tetrahymena. Cell. 1984 Feb;36(2):441–445. doi: 10.1016/0092-8674(84)90237-x. [DOI] [PubMed] [Google Scholar]
- Crouse H. V., Brown A., Mumford B. C. --chromosome inheritance and the porblem of chromosome "imprinting" in Sciara (Sciaridae, Diptera). Chromosoma. 1971;34(3):324–8. doi: 10.1007/BF00286156. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerder F. P., Debault L. E. Cytofluorimetric analysis of nuclear DNA during meiosis, fertilization and macronuclear development in the ciliate Tetrahymena pyriformis, syngen 1. J Cell Sci. 1975 May;17(3):471–493. doi: 10.1242/jcs.17.3.471. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A. Macro- and micronuclei of Tetrahymena pyriformis: a model system for studying the structure and function of eukaryotic nuclei. J Protozool. 1973 Feb;20(1):19–25. doi: 10.1111/j.1550-7408.1973.tb05995.x. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L., Prescott D. M. Internal sequences are eliminated from genes during macronuclear development in the ciliated protozoan Oxytricha nova. Cell. 1984 Apr;36(4):1045–1055. doi: 10.1016/0092-8674(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Murti K. G. Chromosome structure in ciliated protozoans. Cold Spring Harb Symp Quant Biol. 1974;38:609–618. doi: 10.1101/sqb.1974.038.01.065. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Streeck R. E., Moritz K. B., Beer K. Chromatin diminution in Ascaris suum: nucleotide sequence of the eliminated satellite DNA. Nucleic Acids Res. 1982 Jun 11;10(11):3495–3502. doi: 10.1093/nar/10.11.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Van Ness B. G., Coleclough C., Perry R. P., Weigert M. DNA between variable and joining gene segments of immunoglobulin kappa light chain is frequently retained in cells that rearrange the kappa locus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):262–266. doi: 10.1073/pnas.79.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Woodard J., Kaneshiro E., Gorovsky M. A. Cytochemical studies on the problem of macronuclear subnuclei in tetrahymena. Genetics. 1972 Feb;70(2):251–260. doi: 10.1093/genetics/70.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Choi J., Yokoyama S., Austerberry C. F., Yao C. H. DNA elimination in Tetrahymena: a developmental process involving extensive breakage and rejoining of DNA at defined sites. Cell. 1984 Feb;36(2):433–440. doi: 10.1016/0092-8674(84)90236-8. [DOI] [PubMed] [Google Scholar]
- Yao M. C. Elimination of specific DNA sequences from the somatic nucleus of the ciliate Tetrahymena. J Cell Biol. 1982 Mar;92(3):783–789. doi: 10.1083/jcb.92.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Gorovsky M. A. Comparison of the sequences of macro- and micronuclear DNA of Tetrahymena pyriformis. Chromosoma. 1974;48(1):1–18. doi: 10.1007/BF00284863. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Kimmel A. R., Gorovsky M. A. A small number of cistrons for ribosomal RNA in the germinal nucleus of a eukaryote, Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3082–3086. doi: 10.1073/pnas.71.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C. Ribosomal RNA gene amplification in Tetrahymena may be associated with chromosome breakage and DNA elimination. Cell. 1981 Jun;24(3):765–774. doi: 10.1016/0092-8674(81)90102-1. [DOI] [PubMed] [Google Scholar]
- Yokoyama R. W., Yao M. C. Elimination of DNA sequences during macronuclear differentiation in Tetrahymena thermophila, as detected by in situ hybridization. Chromosoma. 1982;85(1):11–22. doi: 10.1007/BF00344591. [DOI] [PubMed] [Google Scholar]
- Yokoyama R., Yao M. C. Internal micronuclear DNA regions which include sequences homologous to macronuclear telomeres are deleted during development in Tetrahymena. Nucleic Acids Res. 1984 Aug 10;12(15):6103–6116. doi: 10.1093/nar/12.15.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]