Aid-Assisted Decision-Making and Colorectal Cancer Screening: A Randomized Controlled Trial (original) (raw)
. Author manuscript; available in PMC: 2014 Mar 26.
Abstract
Background
Shared decision-making (SDM) is a widely recommended yet unproven strategy for increasing colorectal cancer (CRC) screening uptake. Previous trials of decision aids to increase SDM and CRC screening uptake have yielded mixed results.
Purpose
To assess the impact of decision aid–assisted SDM on CRC screening uptake.
Design
RCT.
Setting/participants
The study was conducted at an urban, academic safety-net hospital and community health center between 2005 and 2010. Participants were asymptomatic, average-risk patients aged 50–75 years due for CRC screening.
Intervention
Study participants (_n_=825) were randomized to one of two intervention arms (decision aid plus personalized risk assessment or decision aid alone) or control arm. The interventions took place just prior to a routine office visit with their primary care providers.
Main outcome measures
The primary outcome was completion of a CRC screening test within 12 months of the study visit. Logistic regression was used to identify predictors of test completion and mediators of the intervention effect. Analysis was completed in 2011.
Results
Patients in the decision-aid group were more likely to complete a screening test than control patients (43.1% vs 34.8%;_p_=0.046) within 12 months of the study visit; conversely, test uptake for the decision aid and decision aid plus personalized risk assessment arms was similar (43.1% vs 37.1%; _p_=0.15). Assignment to the decision-aid arm (AOR 1.48; 95% CI=1.04, 2.10), black race (AOR 1.52, 95% CI=1.12, 2.06) and a preference for a patient-dominant decisionmaking approach (AOR, 1.55; 95% CI=1.02, 2.35) were independent determinants of test completion. Activation of the screening discussion and enhanced screening intentions mediated the intervention effect.
Conclusions
Decision aid–assisted SDM has a modest impact on CRC screening uptake. A decision aid plus personalized risk assessment tool is no more effective than a decision aid alone.
Introduction
Colorectal cancer remains a leading cause of cancer-related morbidity and mortality, despite recent declines in both incidence and mortality.1,2 A compelling body of evidence has accumulated to suggest that screening is the most effective and rational strategy for further reducing the public health burden of this deadly yet potentially preventable disease. Consequently, screening is now endorsed by most, if not all, authoritative groups, including the U.S. Preventive Services Task Force, American Cancer Society, and U.S. Multi-society Task Force on Colorectal Cancer.3,4 These endorsements, combined with more-widespread coverage by medical insurers and heightened public awareness efforts, have contributed to a steady increase in screening prevalence in recent years. Nevertheless, more than one third of age-eligible Americans have never been screened.5
Eliciting patient preference within the context of shared decision-making (SDM) has been advocated as a potentially effective strategy for increasing patient acceptance and adherence to CRC screening recommendations.3,4 Engaging patients to participate in the decision-making process when confronted with preference-sensitive choices related to CRC screening is also fundamental to the concept of patient-centered care.6–8 CRC screening is ideally suited for this approach given the availability of multiple options with distinct advantages and disadvantages, the lack of consensus regarding an optimal cost-effective strategy, and limited effectiveness of the more-traditional paternalistic approach in which providers assume full responsibility for the decision-making process. Further support is derived from studies finding that both patients and providers hold distinct preferences for the various screening options,9–14 that providers often misperceive patient preferences,10 and that many patients endorse an SDM approach for CRC screening.15,16
Despite a compelling rationale, SDM has been difficult to implement in routine clinical practice in part due to lack of time, resources, clinician expertise and suitability for certain patients or clinical situations.17,18 The use of patient-oriented decision aids has been proposed as a potentially effective strategy for circumventing several of these barriers.8,19 Decision aids help patients make informed, value-concordant choices about a particular course of action based on an understanding of potential benefits, risks, probabilities and scientific uncertainty.20 Studies to date have shown that decision aids for CRC screening enable users to identify a preferred screening option,11,16,21–25 reduce decisional conflict22,24 and increase interest in screening.21,23,25,26 The authors have recently shown that decision aids can also facilitate SDM by increasing patient knowledge, increasing satisfaction with the decision-making process, enhancing screening intentions, and improving the quality and efficiency of the patient–provider encounter.16,27
The extent to which decision aids increase CRC screening uptake, however, is less well-defined.21–25,28 Hence, the primary objective of this study was to test the hypothesis that decision-aid users were more likely to complete a CRC screening test than non-users. Unlike previous such studies, effectiveness was evaluated within the context of a shared rather than informed decision-making framework.29 Based on evidence suggesting that individualized risk communication might also increase uptake of screening tests,30 a secondary objective was to test the hypothesis that a modified version of the decision aid which incorporated a validated personalized risk assessment tool for CRC would be more effective than the decision aid alone for increasing test completion. .
Methods
Study Population and Recruitment Process
The study sample was made up of average-risk primary care patients cared for at Boston Medical Center or the South Boston Community Health Center. Patients were deemed eligible if they were aged 50–75 years and due for CRC screening.3,4 Patients meeting any of the following criteria were excluded: (1) prior CRC screening by any method other than fecal occult blood testing (FOBT); (2) high-risk condition (personal history of colorectal cancer or polyps, family history of colorectal cancer or polyps involving one or more first-degree relatives, or chronic inflammatory bowel disease); (3) lack of fluency in written and spoken English; or (4) comorbidities that preclude CRC screening by any recommended method, as determined by primary care provider (PCP). The decision to exclude patients with prior screening other than FOBT was based on concerns that such patients may be more likely to adhere to repeat testing than previously unscreened patients. Conversely, patients with prior FOBT were included because of institutional data suggesting that they were less likely to adhere to repeat testing and thus potentially more likely to consider alternative screening options after reviewing the decision aid.
Three different recruitment strategies were used during the course of the study. The vast majority of patients (_n_=796) were recruited using an “opt-out” approach in which patients due for screening were identified from monthly audits of the electronic medical record 2–4 weeks prior to a scheduled office visit and contacted directly by telephone by a research assistant if deemed appropriate by the patient’s PCP. Those expressing interest were provided with a brief overview of the study, evaluated for eligibility and invited to participate. Two other PCP-mediated strategies, including an “opt-in” electronic flagging approach (_n_=12) and “opt-in” letter approach (_n_=17), were used initially but were discontinued after 6 months due to low enrollment. Details of each of these approaches and their relative cost effectiveness have been previously published.31
Setting
The study was conducted at two urban ambulatory care sites. The first, Boston Medical Center (BMC), is an urban, nonprofit academic medical center affiliated with the Boston University School of Medicine, which serves a mostly low-income, racially/ethnically-diverse patient population. The second, the South Boston Community Health Center (SBCHC), is a community health center affiliated with BMC, which serves a mostly non-Hispanic white, low-income patient population. Both sites use the same electronic medical record system (Centricity™ ). The study protocols were approved for both sites by the Boston University Medical Campus IRB.
Provider Characteristics and Training
Sixty-one primary care providers, including 47 board-certified general internists, 11 board-certified family physicians and three nurse practitioners, practicing at both BMC and the SBCHC participated in the study. Pre-trial training seminars and annual refreshers were conducted at both sites to educate providers about the current status of CRC screening highlighting the recommendation for SDM, provide an overview of the study design, and elicit support. The meetings also provided a venue for informing participating providers about the status of recruitment and addressing any logistic problems that they experienced related to the study. All providers attended at least one of the meetings. By design, no formal training in SDM was undertaken.
Study Design
An RCT was conducted between April 2005 and December 2010 to evaluate the impact of the decision aid on SDM and screening behavior. Eligible patients were instructed to arrive 1 hour before a prearranged chronic care visit with their primary care provider. Each received a pre-visit reminder call to ensure that the patient had no acute medical illnesses that would preclude the CRC screening discussion.
After informed consent was obtained, patients were administered a 10-minute, paper-based pretest that assessed knowledge, beliefs, attitudes and behaviors related to CRC screening, as well as level of desire for participating in decision-making related to CRC screening. The pretest was administered using a structured interviewer format by one of four trained research assistants in a private office located in one of the ambulatory care clinics of the participating sites. After completing the pretest, patients were randomized to one of two intervention arms (decision aid alone or decision aid plus_YourDiseaseRisk_ (YDR; personalized risk assessment tool with feedback) or usual care with stratification by provider. Patients randomized to the usual care arm reviewed a modified online version of “9 Ways to Stay Healthy and Prevent Disease”, which discussed generic lifestyle changes other than screening for minimizing risk of preventable diseases.
Immediately after completing the interactive computer session, patients met with their providers to discuss screening and identify a preferred screening strategy. Providers received written notification hand-delivered by all the patients acknowledging that they were participating in the “CRC decision aid study” at the time of the visit to ensure that screening was discussed; no information was provided regarding preferences or factors influencing choice for patients in the intervention arms. Before leaving the clinic, patients completed a 10-minute, paper-based post-test, again using a structured interviewer format, which assessed whether CRC screening was discussed, whether a screening strategy was chosen, patient satisfaction with the decisionmaking process, and screening intentions; the post-test also reassessed knowledge, beliefs, and attitudes related to CRC screening.
Decision Aid
Details of the decision-aid’s theoretic framework, development, content and usability testing have been previously published16 In brief, the DVD-formatted tool employed an audiovisual and touch-screen design to simplify use for individuals with limited literacy and/or computer skills. The tool consists of a series of modules, in which professional actors playing the role of a black, Hispanic female moderator and a white, non-Hispanic male physician convey relevant information via on-screen video, animation and/or graphics.
The modules include: (1) an introductory segment that briefly discusse the importance of screening, purpose of the tool and instructions in its use; (2) a brief overview of the epidemiology of CRC, natural history, benefits of screening, availability of multiple screening options, and the lack of consensus regarding a best screening method; (3) brief descriptions of five screening methods (FOBT, flexible sigmoidoscopy, the combination of FOBT plus flexible sigmoidoscopy, DCBE and colonoscopy) endorsed at the time the study was initiated32–34; (4) audio and visual comparisons of each method with respect to individual test features; (5) a summary of the different test features for each method with optional links to additional information about the preparation or test itself, as well as vignettes from patients describing their experience with a particular test; and (6) a decision-making module where users are asked to identify a screening preference (including no screening) and rank-order test features influencing their selection; and (7) a concluding segment in which the narrator encourages the user to discuss screening and their preferences with their doctor.
A modified version of the decision aid was also created that incorporated the web-based YDR CRC risk assessment tool (www.yourdiseaserisk.wustl.edu), in order to assess whether personalized 10-year CRC risk feedback influenced decision-making. The risk estimate was conveyed using qualitative framing (“very much below average risk” to “very much above average risk”) with accompanying suggestions for behavior modifications that might reduce risk, including a strong recommendation for screening, regardless of risk. The decision aid took between 20 and 30 minutes to complete, depending on which of the optional segments users chose to review.
Measures
The primary outcome measure was completion of a CRC screening test within 12 months of the study visit. Because of long waiting times (≥3 months) and high cancellation rates (>20%) at the start of the study, the 12-month time frame was selected a priori to allow sufficient time for patients who needed to cancel an endoscopic screening procedure to complete the rescheduled examination. Secondary outcomes included test uptake at 6 months and test ordering at 1, 3, 6 and 12 months post-study visit.
All outcomes were tracked using electronic clinical data reporting systems, which captured results for all endoscopic procedures, imaging studies and FOBT completed at the participating sites, and evaluated using an intention-to-treat analysis. Other outcomes of interest included the identification of predictors of test completion and mediators of the intervention effect. Mediators were defined as measures that: (1) significantly changed as a consequence of the intervention (e.g., screening intentions16); (2) had a significant independent effect on the primary outcome of interest (i.e., test completion); and (3) diminished the intervention’s effect on the primary outcome in the adjusted model.35
Sample Size and Power Considerations
Sample size and power considerations focused on a two-group comparison of the decision aid alone versus control study arms for the primary outcome of CRC screening test completion at 12 months. Based on crude estimates of baseline test uptake, it was determined that a target sample of 275 subjects per arm provided greater than 80% power of detecting a 54% versus 40% difference in the percentage of patients completing a CRC screening test within 12 months of the study visit.
Statistical Analyses
Data analysis was completed in 2011. As a check on randomization, the three study groups were first compared on demographic characteristics, prior FOBT screening, risk perception and desired role in decision-making through the chi-square test of independence. Chi-square tests were also used to compare the percentage of patients in the decision aid –alone group to those in the control group or decision aid plus YDR group who either had a test ordered or completed at each of the designated time points. Logistic regression was used to identify patient-level determinants of test completion and mediators of the intervention effect. Details regarding measurement of patient knowledge, satisfaction with the decision-making process and screening intentions were previously published.16Variables exhibiting a significant association with test completion in univariate analyses at the two-tailed p<0.05 level were included as covariates in the multivariate analyses. Sobel tests were performed to assess significance for the mediation analyses.36 All other analyses were conducted using SAS 9.2.
Results
Patient Characteristics
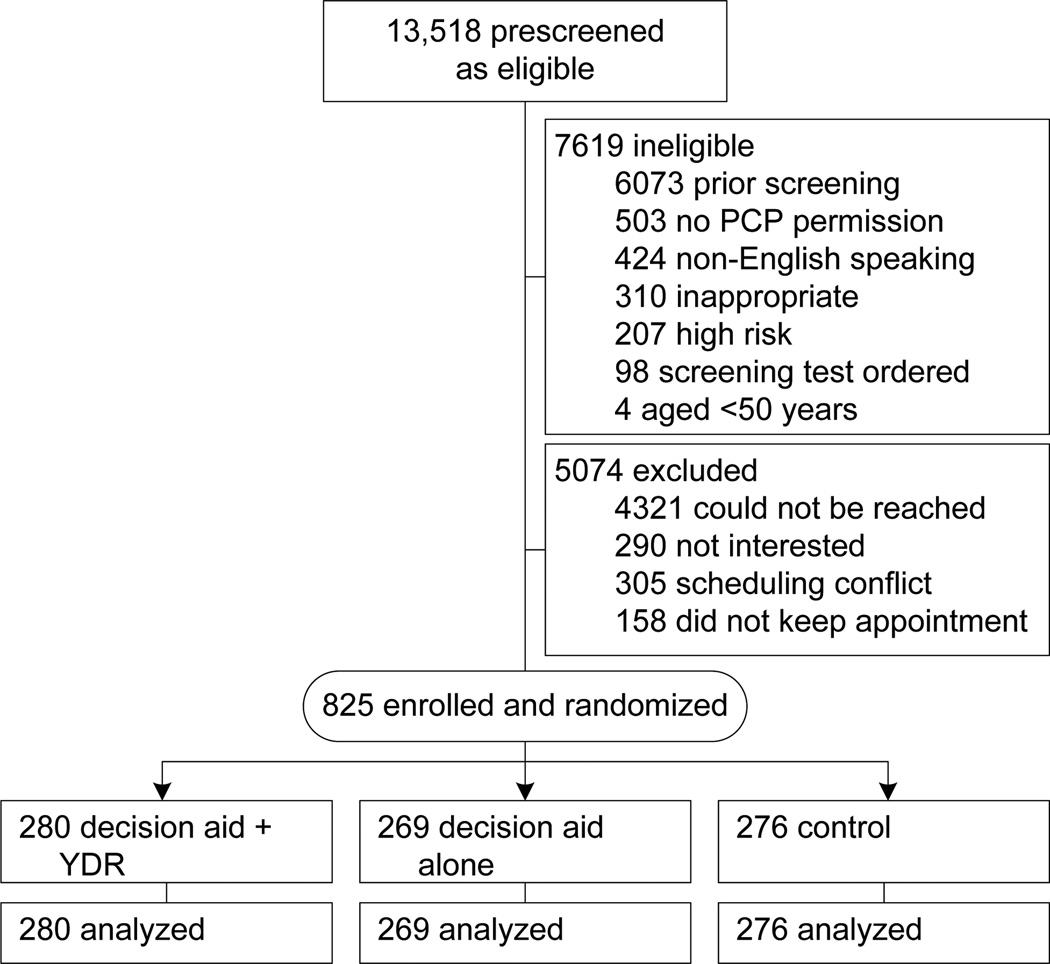
Of the 13,518 patients identified as potentially eligible for screening because of age, 7619 (56%) were deemed ineligible (mostly due to prior screening [_n_=6073]) and 5074 (38%) were excluded (Figure 1). Reasons for exclusion included: inability to contact (_n_=4321); disinterest (_n_=290); scheduling conflict (_n_=305); and failure to keep appointment (_n_=158). The remaining 825 patients (52% of eligible subjects contacted) were enrolled and randomized to decision aid–alone (_n_=269); decision aid plus YDR (_n_=280); or control (_n_= 76) arms.
Figure 1. Study flow diagram.
PCP, primary care provider; YDR, _YourDiseaseRisk_personalized risk assessment tool with feedback
The three study arms were well-balanced with respect to all baseline characteristics, including patient age, gender, ethnicity, race, marital status, education, insurance coverage, prior FOBT, and decision-making preference (Table 1). Overall, the study group was mostly aged <65 years (84%); female (59%); non-Hispanic (95%); and black (62%), with at least a high-school degree (78%). Only 36% were married or living with a partner. Although most had some form of healthcare insurance (98%), nearly two thirds were covered by Medicare, Medicaid or Massachusetts’ “Free Care” (now “Commonwealth Care”) program. Most (87%) had no prior FOBT. The majority preferred a patient-dominant (27%) or shared-decision-making approach (53%) for selecting a preferred CRC screening option.
Table 1.
Characteristics of Study Participants (_n_=825),n (%)
| Characteristic | DecisionAid + YDR(_n_= 280) | Decisionaid(_n_=269) | Control(_n_=276) | _p_-value a |
|---|---|---|---|---|
| Age, years | 0.35 | |||
| < 65 | 232 (83) | 234 (87) | 230 (83) | |
| ≥ 65 | 48 (17) | 35 (13) | 46 (17) | |
| Gender | 0.70 | |||
| Female | 163 (58) | 164 (61) | 159 (58) | |
| Male | 117 (42) | 105 (39) | 117 (42) | |
| Ethnicity | 0.35 | |||
| Non-Hispanic | 262 (94) | 259 (96) | 261 (95) | |
| Hispanic | 18 (6) | 10 (4) | 15 (5) | |
| Race | 0.52 | |||
| Black | 172 (61) | 160 (59) | 180 (65) | |
| White | 99 (35) | 96 (36) | 88 (32) | |
| Asian | 3 (1) | 7 (3) | 2 (1) | |
| Other | 6 (2) | 6 (2) | 6 (2) | |
| Educationb | 0.12 | |||
| ≥ High school | 221 (79) | 213 (80) | 200 (74) | |
| < High school | 58 (21) | 52 (20) | 72 (26) | |
| Marital Status b | 0.40 | |||
| Married | 89 (32) | 72 (27) | 85 (31) | |
| Living with a partner | 12 (4) | 20 (7) | 14 (5) | |
| Other | 176 (64) | 175 (66) | 174 (64) | |
| Insurance | 0.06 | |||
| Private/HMO | 85 (33) | 95 (39) | 76 (30) | |
| Medicare | 79 (31) | 58 (24) | 86 (34) | |
| Medicaid | 70 (28) | 76 (31) | 63 (25) | |
| Free care | 14 (6) | 11 (5) | 16 (6) | |
| None | 6 (2) | 2 (1) | 10 (4) | |
| Prior FOBT b | 0.90 | |||
| Yes | 33 (12) | 35 (13) | 36 (13) | |
| No | 243 (88) | 232 (87) | 239 (87) | |
| Desired role in decision-making | 0.54 | |||
| Mostly patient | 75 (27) | 66 (25) | 81 (29) | |
| Shared | 151 (54) | 149 (55) | 134 (49) | |
| Mostly doctor | 54 (19) | 54 (20) | 61 (22) |
Intervention Effects on Test Ordering and Completion
Patients in the decision aid–alone group were more likely to have a test ordered than the control group at the 1-month (69.1% vs 60.5%, p<0.035); 3-month (71.8% vs 62.3%, _p_=0.019); 6- month (77.0% vs 65.2%, _p_=0.002); and 12-month (80.7% vs 71.4%, _p_=0.011) time points (Table 2). The decision aid–alone group was also more likely to have a test ordered than the decision aid plus YDR group at each of these points, but here the differences were only significant at 1 month (69.1% vs 60.4%; p<0.031); 6 months (77.0% vs 67.1%, p<0.010); and 12 months (80.7% vs 73.6%, _p_=0.048). The pattern of test ordering was similar for the three groups; regardless of patient preferences, colonoscopy was the most commonly ordered test (range, 79%–81%) followed by FOBT (13%–19%); flexible sigmoidoscopy (<2%); and barium enema (<2%).
Table 2.
Patient outcomes by study group, n (%) or % (95% CI)
| Decision aid vs Control | Decision aid vs Decision aid +YDR | |||||
|---|---|---|---|---|---|---|
| Outcome | Decisionaid alone | Control | Difference | Decision aidalone | Decision aid +YDR | Difference |
| Test Ordered | ||||||
| 1 month | 186 (69.1) | 167 (60.5) | 8.6 (0.7, 16.6)* | 186 (69.1) | 169 (60.4) | 8.8 (0.8, 16.7)* |
| 3 months | 193 (71.8) | 172 (62.3) | 9.4 (1.6, 17.3)* | 193 (71.8) | 180 (64.3) | 7.5 (−0.3, 15.2) |
| 6 months | 207 (77.0) | 180 (65.2) | 11.7 (4.2, 19.3)* | 207 (77.0) | 188 (67.1) | 9.8 (2.4, 17.3)* |
| 12 months | 217 (80.7) | 197 (71.4) | 9.3 (2.2, 16.4)* | 217 (80.7) | 206 (73.6) | 7.1 (0.1, 14.1)* |
| TestCompleted | ||||||
| 6 months | 92 (34.2) | 73 (26.4) | 7.8 (0.1, 15.4)* | 92 (34.2) | 84 (30.0) | 4.2 (−3.6, 12.0) |
| 12 months | 116 (43.1) | 96 (34.8) | 8.3 (0.2, 16.5)* | 116 (43.1) | 104 (37.1) | 6.0 (−2.2, 14.2) |
Among the 525 intervention patients expressing a preference, 322 (61%) had their preferred test ordered, 91 (17%) had an alternate test ordered, and 112 (21%) had no test ordered (Table 3). For individual tests, concordance between patient preference and test ordered varied from 81% for colonoscopy to 35% for FOBT, 28% for barium enema, 19% for flexible sigmoidoscopy and 19% for FOBT plus flexible sigmoidoscopy. Patients who preferred tests other than colonoscopy were less likely to have any test ordered than those who preferred colonoscopy (69% vs 85%,p<0.001).
Table 3.
Concordance between patient preference and test ordered or completed at 12 months (intervention groups only a).
| Patient Preference,n (%) | ||||||
|---|---|---|---|---|---|---|
| Outcome | Colonoscopy(_n_=323) | FOBT(_n_=134) | FlexibleSigmoidoscopy(_n_=27) | DCBE(_n_=25) | FOBT + FlexibleSigmoidoscopy(_n_=16) | Overall(_n_=525a) |
| Test Ordered b | ||||||
| Any | 264 (85) d | 93 (69) | 19 (70) | 16 (64) | 11 (69) | 413 (79) |
| Same | 260 (80) | 47 (35) | 5 (19) | 7 (28) | 3 (19) e | 322 (61) |
| Different | 14 (4) | 46 (34) | 14 (52) | 9 (36) | 8 (50) | 91 (17) |
| None | 49 (15) | 41 (31) | 8 (30) | 9 (36) | 5 (31) | 112 (21) |
| TestCompletedc | ||||||
| Same | 138 (53) | 17 (36) | 3 (60) | 3 (43) | 2 (67) | 163 (51) |
| Different | 7 (50) | 31 (67) | 7 (50) | 4 (44) | 4 (50) | 53 (58) |
Test completion, the primary outcome of interest, was higher for the decision aid–alone group than usual-care group at both the 6-month (34.2% vs 26.4%; _p_=0.049) and 12-month (43.1% vs 34.8%; _p_=0.046) time points (Table 2). Test completion for the decision aid–alone group and decision aid plus YDR group was similar at both time points (34.2% vs 30.0% at 6 months, _p_=0.292; 43.1% vs 37.1% at 12 months, _p_=0.153). Among the group of intervention patients who had a test ordered, 12-month uptake was similar when there was concordance or discordance between patient preference and test ordered (51% vs 58%; _p_=0.199; Table 3).
Associations Between Pre-Intervention Patient Characteristics and Test Completion
Table 4 depicts associations between baseline patient characteristics and test completion at 12 months. Assignment to the decision aid–alone study group (AOR 1.48, 95% CI=1.04, 2.10); black race (AOR 1.52, 95% CI=1.12, 2.06); and a preference for a patient-dominant decision-making approach (AOR 1.55; 95% CI=1.02, 2.35) were independent predictors of test completion. No associations were observed for site, age, gender, ethnicity, education, marital status, insurance status, or prior FOBT. Similar results were observed for test completion at 6 months (Appendix A, available online at www.ajpmonline.org), except that black race was no longer significant in the adjusted analyses (AOR 1.18, 95% CI=0.86, 1.63).
Table 4.
Associations between pre-intervention patient characteristics and test completion at 12 months
| n | Test Completion_n_(%) | Unadjusted OR(95% CI)a | AOR(95% CI) ab | |
|---|---|---|---|---|
| Study group | ||||
| Decision aid only | 269 | 116 (43) | 1.42 (1.01 , 2.01) | 1.48 (1.04, 2.10) |
| Decision aid + YDR | 280 | 104 (37) | 1.11 (0.78 , 1.57) | 1.13 (0.80, 1.61) |
| Control | 276 | 96 (35) | --- | --- |
| Site | ||||
| BMC | 763 | 297 (39) | --- | |
| SBCHC | 62 | 19 (31) | 0.69 (0.40 , 1.21) | |
| Age, years | ||||
| < 65 | 696 | 268 (39) | --- | |
| ≥ 65 | 129 | 48 (37) | 0.95 (0.64 , 1.40) | |
| Gender | ||||
| Female | 486 | 188 (39) | 1.04 (0.78 , 1.38) | |
| Male | 339 | 128 (38) | -- | |
| Race | ||||
| White | 283 | 92 (33) | --- | --- |
| Black | 512 | 214 (42) | 1.49 (1.10 , 2.02) | 1.52 (1.12, 2.06) |
| Asian/Other | 30 | 10 (33) | 1.04 (0.47 , 2.31) | 1.00 (0.45, 2.23) |
| Ethnicity | ||||
| Hispanic | 43 | 16 (37) | 0.95 (0.50 , 1.80) | |
| Non-Hispanic | 782 | 300 (38) | --- | |
| Education | ||||
| < high school graduate≥High school graduate | 182 | 65 (36) | --- | |
| 634 | 247 (39) | 1.15 (0.82 , 1.62) | ||
| Marital Status | ||||
| Married/Living with partnerUnmarried/Living alone | 246 | 100 (41) | 1.15 (0.85 , 1.56) | |
| 571 | 213 (37) | --- | ||
| Insurance, n(%) | ||||
| Private/HMO | 256 | 111 (43) | --- | |
| Medicare | 223 | 81 (36) | 0.74 (0.52, 1.08) | |
| Medicaid | 209 | 78 (37) | 0.78 (0.54, 1.13) | |
| Free care | 41 | 18 (44) | 1.02 (0.53, 1.99) | |
| None | 18 | 8 (44) | 1.04 (0.40, 2.74) | |
| Prior FOBT | ||||
| Yes | 104 | 40 (38) | 1.01 (0.66, 1.54) | |
| No | 714 | 273 (38) | --- | |
| Decision-makingpreference | 222 | 97 (44) | 1.52 (1.01 , 2.31) | 1.55 (1.02, 2.35) |
| Patient | 434 | 162 (37) | 1.17 (0.80 , 1.70) | 1.15 (0.79, 1.68) |
| Shared | 169 | 57 (34) | --- | --- |
| Doctor |
Mediators of Intervention Effects
Patients in the decision aid-alone group were more likely than controls to discuss screening at the study visit (93% vs 86%,_p_=0.008) even though all patients were given written prompts to hand to their providers acknowledging their participation in the study. As previously reported,16 other measures of SDM including post-test knowledge, satisfaction with the decisionmaking process, and screening intention scores were also higher for the two intervention groups than they were for controls (Appendix B, available online at www.ajpmonline.org). Measures (Table 5) included: whether or not screening was discussed at the study visit (AOR 3.24, 95% CI=1.73, 6.05) and whether screening intentions (AOR 1.69, 95% CI=1.25, 2.28) were independent post-intervention determinants of test completion after adjustment for study group, race, and decision-making preference. Controlling for both determinants diminished the positive association for the decision aid–alone group (AOR, 1.30, 95% CI=0.90, 1.87), suggesting a mediation effect, which was confirmed using Sobel tests for significance (screening discussion, _p_=0.026; intentions,_p_=0.038). Post-intervention knowledge, satisfaction with the decisionmaking process, patient preferences and concordance between patient preference and test ordered showed no association with test completion.
Table 5.
Associations between potential mediators of intervention effect and test completion at 12 months.
| n | Test Completion_n_(%) | Unadjusted OR(95% CI)c | AOR(95% CI)c,d | |
|---|---|---|---|---|
| Study group | ||||
| Decision aid only | 269 | 116 (43) | 1.42 (1.01, 2.01) | 1.30 (0.90, 1.87) |
| Decision aid + YDR | 280 | 104 (37) | 1.11 (0.78, 1.57) | 1.03 (0.72, 1.48) |
| Control | 276 | 96 (35) | --- | --- |
| Discussed screening at study visit | ||||
| Yes | 740 | 302 (41) | 3.71 (2.02, 6.83) | 3.24 (1.73, 6.05) |
| No | 83 | 13 (16) | --- | |
| Knowledge Post, quartile | ||||
| 1 | 234 | 83 (35) | --- | |
| 2 | 120 | 51 (42) | 1.34 (0.86 , 2.11) | |
| 3 | 189 | 72 (38) | 1.12 (0.75, 1.67) | |
| 4 | 282 | 110 (39) | 1.16 (0.81, 1.67) | |
| Satisfaction with decision-making process, quartile | ||||
| 1 | 218 | 79 (36) | --- | |
| 2 | 186 | 77 (41) | 1.24 (0.83 , 1.86) | |
| 3 | 237 | 95 (40) | 1.18 (0.81, 1.72) | |
| 4 | 153 | 60 (39) | 1.14 (0.74, 1.74) | |
| Intention (How sure are you that you will complete a CRC screening test?) | ||||
| Completely Sure | 447 | 194 (43) | 1.63 (1.22 , 2.17) | 1.69 (1.25, 2.28) |
| Less Sure | 362 | 116 (32) | --- | --- |
| Patient Preference a | ||||
| Colonoscopy | 324 | 145 (45) | --- | |
| FOBT | 134 | 49 (37) | 0.71 (0.47, 1.08) | |
| Other screening test(s) b | 68 | 23 (34) | 0.63 (0.36, 1.09) | |
| Concordancea | ||||
| Patient/Provider Preference Same | 329 | 164 (50) | --- | |
| Patient/Provider Preference | 100 | 53 (53) | 1.13 (0.72 , 1.78) | |
| Different |
Discussion
This study provides new evidence that decision aid–assisted SDM is an effective strategy for increasing CRC screening. Test completion uptake was ∼8% higher among decision-aid users than controls at both 6 and 12 months, suggesting a very modest but sustained impact on screening uptake. Unlike previous such studies, this study also explored the role of individual elements of SDM on screening behavior and found that the positive impact was mediated through activation of the screening discussion and heightened screening intentions rather than increased knowledge, satisfaction with the decision-making process, or concordance between patient preference and test ordered. Because providers received written notification of participation in the study from all patients, the authors speculate that enhanced activation of the screening discussion in the decision-aid group was the result of patient empowerment rather than differential provider behavior in response to the cue.
Additional findings were that use of a decision aid that incorporates a personalized risk assessment tool fails to increase test completion compared to a decision aid lacking the tool. Unlike for conditions where the benefits of screening are less certain, the primary goal of SDM for CRC screening is to enable patients to identify a preferred screening option rather than to decide whether or not to undergo screening.4,7,8Consequently, personalized risk feedback might have a detrimental effect without appropriate framing. Individuals deemed to be at higher risk may be fearful of the potential findings, whereas those at lower risk may feel that screening is unnecessary, especially if they overestimated their lifetime risk of cancer before receiving the feedback.37
Results of the current study contribute to a growing body of evidence suggesting that patient-level interventions alone have a relatively modest impact on CRC screening. The use of tailored educational approaches,38–41patient reminders,42,43 activation strategies44 and, as previously noted, decisions aids,21–25, 28 have demonstrated either no effect or a slight increases in screening uptake that rarely exceed 20% compared to control groups. With the steady rise in screening prevalence nationally,5 this limited effectiveness could partly reflect the challenges of trying to reach a more recalcitrant patient population. Regardless, this experience highlights the need for additional multilevel interventions that address not only patient- but also provider- and system-level barriers to participation.
Several clinical implications of the current study are notable. The findings and feedback from providers attest to the feasibility and validity of using decision aids as a point-of-contact intervention in clinical practice.27 The observation that patients who preferred a patient-dominant decision-making style were more likely to complete screening provides new evidence supporting the importance of assessing a patient’s desire to participate in the decision-making process prior to engaging in SDM.
Conversely, the lack of association between concordance and test completion suggests that complying with patient preferences may be less important in select patients than the provider’s ability to effectively communicate his/her reasoning for recommending a preferred strategy. However, failure to comply with patient preferences negatively influenced test ordering and thus compromised the overall impact of SDM on screening uptake. The observation that blacks were more likely than whites to complete a screening test in a safety-net healthcare system corroborates previously published data suggesting that barriers to access and socioeconomic inequalities rather than cognitive factors may be largely responsible for racial disparities in screening rates.45
Limitations and Strengths
Limitations to this study include the fact that lack of provider blinding may have negatively influenced the magnitude of the interventions’ effect on outcomes of interest. Second, no attempt was made to assess the quality of the patient–provider discussion. Even though satisfaction with the decision-making process was universally high (albeit higher in the intervention groups), recent data suggest that most patient–provider discussions related to CRC screening often fail to incorporate key elements of informed decision-making.15,46
Third, this study did not explore reasons for the large discrepancy between test ordering and test completion for each of the study arms. Although outcome assessment does not preclude the remote possibility that some patients may have completed tests elsewhere, the authors speculate that well-described patient- and system-level barriers to participation are largely responsible.47 Lastly, no attempt was made to assess the cost effectiveness of the intervention from the perspective of the provider or healthcare center.
Despite these limitations, this study has several notable strengths. First, it is the largest study to date to demonstrate that the use of decision aids to promote SDM has a positive impact on screening behavior. Second, the use of an RCT study design, large sample size, and diverse study population enhances both the internal and external validity of its findings. Third, the randomization scheme after stratification by provider, and inclusion of mostly unscreened patients, minimizes potential confounding.
Conclusion
This study finds that decision aid–assisted SDM has a modest impact on CRC screening uptake, even when provider and patient preferences differ. The findings also suggest that decision aids not only enable patients to identify a value-concordant screening preference but also empower them to initiate the screening discussion and heighten screening intentions. Conversely, incorporating personalized risk feedback may have negative consequences on screening behavior in the absence of appropriate messaging that motivates patients to undergo screening regardless of risk. Despite its importance, however, SDM alone is unlikely to have a profound impact on CRC screening uptake unless strategies are in place to address patient- and system-level barriers to participation.
Supplementary Material
01
Acknowledgments
The study was supported by grant RO1HS013912 from the Agency for Healthcare Research and Quality (PI, Paul C. Schroy III). Dr. Pignone’s effort was supported by a National Cancer Institute Established Investigator Award K05CA129166. Dr. Peters’s effort was supported in part by the National Science Foundation (SES-1047757 and -0517770).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, et al. Annual Report to the Nation on the Status of Cancer, 1975–2006, Featuring Colorectal Cancer Trends and Impact of Interventions (Risk Factors, Screening, and Treatment) to Reduce Future Rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous Polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Vital signs: Colorectal cancer screening, incidence, and mortality—U.S., 2002–2010. MMWR Morb Mortal Wkly Rep. 2011;60(26):884–889. [PubMed] [Google Scholar]
- 6.IOM. Crossing the quality chasm: a new health system for the 21st century. Washington DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 7.Sheridan SL, Harris RP, Woolf SH. Shared decision making about screening and chemoprevention. a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26(1):56–66. doi: 10.1016/j.amepre.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Briss P, Rimer B, Reilley B, et al. Promoting informed decisions about cancer screening in communities and healthcare systems. Am J Prev Med. 2004;26(1):67–80. doi: 10.1016/j.amepre.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Pignone M, Bucholtz D, Harris R. Patient preferences for colon cancer screening. J Gen Intern Med. 1999;14:432–437. doi: 10.1046/j.1525-1497.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling BS, Moskowitz MA, Wachs D, Pearson B, Schroy PC. Attitudes toward colorectal cancer screening tests: A survey of patients and physicians. J Gen Int Med. 2001;16:822–830. doi: 10.1111/j.1525-1497.2001.10337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroy PC, 3rd, Lal S, Glick JT, Robinson PA, Zamor P, Heeren TC. Patient preferences for colorectal cancer screening: how does stool DNA testing fare? Am J Manag Care. 2007;13(7):393–400. [PubMed] [Google Scholar]
- 12.Marshall DA, Johnson FR, Phillips KA, Marshall JK, Thabane L, Kulin NA. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health. 2007;10(5):415–430. doi: 10.1111/j.1524-4733.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- 13.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46(9 Suppl 1):S10–S16. doi: 10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 14.Powell AA, Burgess DJ, Vernon SW, et al. Colorectal cancer screening mode preferences among U.S. veterans. Prev Med. 2009;49(5):442–448. doi: 10.1016/j.ypmed.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Wunderlich T, Cooper G, Divine G, et al. Inconsistencies in patient perceptions and observer ratings of shared decision making: the case of colorectal cancer screening. Patient Educ Couns. 2010;80(3):358–363. doi: 10.1016/j.pec.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroy PC, 3rd, Emmons K, Peters E, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011;31(1):93–107. doi: 10.1177/0272989X10369007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legare F, Ratte S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: update of a systematic review of health professionals' perceptions. Patient Educ Couns. 2008;73(3):526–535. doi: 10.1016/j.pec.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Holmes-Rovner M, Valade D, Orlowski C, Draus C, Nabozny-Valerio B, Keiser S. Implementing shared decision-making in routine practice: barriers and opportunities. Health Expect. 2000;3(3):182–191. doi: 10.1046/j.1369-6513.2000.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed decision making: what is its role in cancer screening? Cancer. 2004;101(5 Suppl):1214–1228. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- 20.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews. 2011;(10) doi: 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. A randomized, controlled trial. Ann Intern Med. 2000;133(10):761–769. doi: 10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 22.Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Med Decis Making. 2002;22(2):125–139. doi: 10.1177/0272989X0202200210. [DOI] [PubMed] [Google Scholar]
- 23.Ruffin MTt, Fetters MD, Jimbo M. Preference-based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Prev Med. 2007;45(4):267–273. doi: 10.1016/j.ypmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Smith SK, Trevena L, Simpson JM, Barratt A, Nutbeam D, McCaffery KJ. A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial. BMJ. 2010;341:c5370. doi: 10.1136/bmj.c5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DP, Jr, Spangler JG, Case LD, Goff DC, Jr, Singh S, Pignone MP. Effectiveness of a web-based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed-literacy population. Am J Prev Med. 2011;40(6):608–615. doi: 10.1016/j.amepre.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Whitney A, Hayter S, et al. Development and initial testing of a computer-based patient decision aid to promote colorectal cancer screening for primary care practice. BMC Med Inform Decis Mak. 2005;5:36. doi: 10.1186/1472-6947-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroy PC, 3rd, Mylvaganam S, Davidson P. Provider perspectives on the utility of a colorectal cancer screening decision aid for facilitating shared decision making. Health Expect. 2011 Sep 8; doi: 10.1111/j.1369-7625.2011.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trevena LJ, Irwig L, Barratt A. Randomized trial of a self-administered decision aid for colorectal cancer screening. J Med Screen. 2008;15(2):76–82. doi: 10.1258/jms.2008.007110. [DOI] [PubMed] [Google Scholar]
- 29.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651–661. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 30.Edwards A, Unigwe S, Elwyn G, Hood K. Personalised risk communication for informed decision making about entering screening programs. Cochrane Database Syst Rev. 2003;(1):CD001865. doi: 10.1002/14651858.CD001865. [DOI] [PubMed] [Google Scholar]
- 31.Schroy PC, Glick JT, Robinson P, et al. A cost-effectiveness analysis of subject recruitment strategies in the HIPAA era: results from a colorectal cancer screening adherence trial. Clin Trials. 2009;6:597–609. doi: 10.1177/1740774509346703. [DOI] [PubMed] [Google Scholar]
- 32.Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001--testing for early lung cancer detection. CA Cancer J Clin. 2001;51(1):38–75. doi: 10.3322/canjclin.51.1.38. quiz 77–80. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137(2):129–131. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 34.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124(2):544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 35.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 36.Preacher KJLG. Calculation for the Sobel test: an interactive tool for mediation tests. 2010–2012 http://quantpsy.org/sobel/sobel.htm. [Google Scholar]
- 37.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. How making a risk estimate can change the feel of that risk: shifting attitudes toward breast cancer risk in a general public survey. Patient Educ Couns. 2005;57(3):294–299. doi: 10.1016/j.pec.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 39.Vernon SW, Bartholomew LK, McQueen A, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: sometimes more is just the same. Ann Behav Med. 2011;41(3):284–299. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon U, Belue R, Wahab S, et al. A randomized trial comparing the effect of two phone-based interventions on colorectal cancer screening adherence. Ann Behav Med. 2011;42(3):294–303. doi: 10.1007/s12160-011-9291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sequist TD, Zaslavsky AM, Colditz GA, Ayanian JZ. Electronic patient messages to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2011;171(7):636–641. doi: 10.1001/archinternmed.2010.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denberg TD, Coombes JM, Byers TE, et al. Effect of a mailed brochure on appointment-keeping for screening colonoscopy: a randomized trial. Ann Intern Med. 2006;145(12):895–900. doi: 10.7326/0003-4819-145-12-200612190-00006. [DOI] [PubMed] [Google Scholar]
- 43.Sequist TD, Zaslavsky AM, Marshall R, Fletcher RH, Ayanian JZ. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–371. doi: 10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katz ML, Fisher JL, Fleming K, Paskett Ed. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012;21(1):45–52. doi: 10.1158/1055-9965.EPI-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgess DJ, van Ryn M, Grill J, et al. Presence and correlates of racial disparities in adherence to colorectal cancer screening guidelines. J Gen Intern Med. 2011;26(3):251–258. doi: 10.1007/s11606-010-1575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McQueen A, Bartholomew LK, Greisinger AJ, et al. Behind Closed Doors: Physician-Patient Discussions About Colorectal Cancer Screening. J Gen Intern Med. 2009;24:1228–1235. doi: 10.1007/s11606-009-1108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medina GG, McQueen A, Greisinger AJ, Bartholomew LK, Vernon SW. What would make getting colorectal cancer screening easier? Perspectives from screeners and nonscreeners. Gastroenterol Res Pract. 2012:895807. doi: 10.1155/2012/895807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01