The FIND-CKD study—a randomized controlled trial of intravenous iron versus oral iron in non-dialysis chronic kidney disease patients: background and rationale (original) (raw)
Abstract
Background
Rigorous data are sparse concerning the optimal route of administration and dosing strategy for iron therapy with or without concomitant erythropoiesis-stimulating agent (ESA) therapy for the management of iron deficiency anaemia in patients with non-dialysis dependent chronic kidney disease (ND-CKD).
Methods
FIND-CKD was a 56-week, open-label, multicentre, prospective, randomized three-arm study (NCT00994318) of 626 patients with ND-CKD and iron deficiency anaemia randomized to (i) intravenous (IV) ferric carboxymaltose (FCM) at an initial dose of 1000 mg iron with subsequent dosing as necessary to target a serum ferritin level of 400–600 µg/L (ii) IV FCM at an initial dose of 200 mg with subsequent dosing as necessary to target serum ferritin 100–200 µg/L or (iii) oral ferrous sulphate 200 mg iron/day. The primary end point was time to initiation of other anaemia management (ESA therapy, iron therapy other than study drug or blood transfusion) or a haemoglobin (Hb) trigger (two consecutive Hb values <10 g/dL without an increase of ≥0.5 g/dL).
Results
The background, rationale and study design of the trial are presented here. The study has been completed and results are expected in late 2013.
Discussion
FIND-CKD was the longest randomized trial of IV iron therapy to date. Its findings will address several unanswered questions regarding iron therapy to treat iron deficiency anaemia in patients with ND-CKD. It was also the first randomized trial to utilize both a high and low serum ferritin target range to adjust IV iron dosing, and the first not to employ Hb response as its primary end point.
Keywords: anaemia, ferric carboxymaltose, FIND-CKD, intravenous iron, iron deficiency, non-dialysis CKD
BACKGROUND TO THE FERINJECT® ASSESSMENT IN PATIENTS WITH IRON DEFICIENCY ANAEMIA AND NON-DIALYSIS DEPENDENT CHRONIC KIDNEY DISEASE STUDY
Iron deficiency anaemia: a clinical challenge
Chronic kidney disease (CKD) affects >10% of adults in developed countries [1–3], and the number is increasing as populations age and contributory diseases such as diabetes and hypertension become more common [1]. Only a small proportion require dialysis [2, 3]. For patients with non-dialysis CKD (ND-CKD), management focuses on interventions to ameliorate further loss of kidney function, treating the multiple co-morbidities and managing cardiovascular risk.
Anaemia is a frequent complication of CKD. Prevalence estimates vary widely according to the definition which is applied, but a large U.S. survey observed haemoglobin (Hb) levels <12 g/dL in more than one in four with relatively mild ND-CKD (Stages 1–2), increasing to more than half of those with severe ND-CKD (Stage 4) [4]. In addition to erythropoietin deficiency, iron deficiency is an important cause of anaemia in ND-CKD, arising from multiple factors directly or indirectly related to renal dysfunction [5]. Inflammation is also common and is associated with increased hepcidin levels, which lead to impaired absorption of iron from the gastrointestinal tract and retention of iron in the reticuloendothelial system [6]. Both absolute and functional iron deficiency limit erythropoiesis and are the main cause of hyporesponsiveness to erythropoiesis-stimulating agents (ESAs) [7–9]. In addition, iron is critical for many other cellular functions, such as energy metabolism, muscle performance and immune function [10, 11].
THE CURRENT EVIDENCE BASE
Anaemia management has centred on the use of ESA and iron therapy for the last two decades. However, following safety concerns associated with high-dose ESA therapy and aggressive Hb targets in three large clinical trials in ND-CKD patients (CREATE [12], CHOIR [13, 14] and TREAT [15]) the appropriate management of anaemia has been questioned and interest in the use of iron therapy has heightened. The latest anaemia guideline from the Kidney Disease Improving Global Outcomes (KDIGO) initiative recommends that iron therapy should be used to correct iron deficiency before initiating ESA therapy [16]. It also recommends use of iron therapy if an increase in Hb is desired without starting ESA therapy or a decrease in ESA dose is desired.
The effectiveness of intravenous (IV) iron therapy in correcting anaemia and replenishing iron stores has been demonstrated in ND-CKD populations [17–21]. However, only one randomized trial has compared the effectiveness of IV versus oral iron therapy exclusively in ND-CKD patients not receiving concomitant ESA therapy [22] (Table 1). Limited data from another three randomized studies were derived from ESA-free subpopulation analyses in which the decision whether to initiate ESA therapy was investigator driven [17–19]. In these three subpopulation analyses, 53.2% [17], 29.7% [18] and 59.5% [19] of subjects had an Hb increase of 1 g/dL or more with IV iron therapy alone. Moreover, treatment with IV ferric carboxymaltose (FCM) alone achieved a greater increase in Hb compared with oral iron and a comparable increase in Hb to that observed with oral iron in combination with ESA therapy [17]. This was coupled with significantly greater increases in mean serum ferritin and transferrin saturation (TSAT) levels in FCM-treated subjects versus those randomized to oral iron. In the only trial to have excluded ESA-treated patients, IV ferric gluconate produced a more rapid Hb increase compared with oral iron, although the final increase in Hb was similar with either treatment. Notably, all four trials had short-term follow-up, lasting only a maximum of 6 weeks [17–19, 22], which is insufficient to draw any conclusions regarding longer term safety, and all four trials employed only Hb response as their primary end point. The limited evidence base relating to IV iron therapy without concomitant ESA in patients with ND-CKD has obliged expert groups to extrapolate conclusions from the scientific evidence in dialysis patients to the ND-CKD setting when developing anaemia management guidelines [5, 16, 23, 24].
Table 1.
IV iron therapy versus oral iron in anaemic ND-CKD patients without concomitant ESA therapy in randomized, multicentre studies
| Study | N | IV iron therapy | Oral iron therapy | Follow-up (weeks) | Primary end point | |||
|---|---|---|---|---|---|---|---|---|
| Parameter | IV iron | Oral iron | P-value | |||||
| Qunibi et al. [17] | 111a | FCM: single dose of 1000 mg iron (with up to 2 additional 500 mg iron doses) | Ferrous sulphate: 65 mg iron three times a day | 8 | Hb increase ≥1 g/dL (% patients) | 53.2% | 29.9% | 0.002 |
| Spinowitz et al. [18] | 145b | Ferumoxytol: 2 doses of 510 mg iron | Ferrous fumarate: 100 mg iron twice a day | 5 | Mean (SD) increase in Hb, g/dL | 0.62 (1.02) | 0.13 (0.93) | 0.0045 |
| Van Wyck et al. [19] | 47c | Iron sucrose: 1000 mg iron in 2 or 5 doses | Ferrous sulphate: 65 mg iron three times a day | 6 | Hb increase ≥1 g/dL (% patients) | 59.5% | 41.2% | n/a |
| Agarwal et al. [22] | 75 | Ferric gluconate: 4 doses of 250 mg iron | Ferrous sulphate: 65 mg iron three times a day | 6 | Mean (SD) increase in Hb, g/dL | 0.4 (0.8)* | 0.2 (0.9)d | n.s. |
Although IV iron has been shown to replete iron stores more effectively than oral iron in patients with ND-CKD [17, 25, 26], a potential effect of iron repletion on outcomes other than Hb level is largely unexplored in the ND-CKD population. There is a growing awareness from trials in patients with chronic heart failure, with and without concomitant ND-CKD, that the benefits of IV iron therapy in iron-deficient individuals are not restricted to improved erythropoiesis. The large, randomized FAIR-HF trial recently demonstrated that IV iron therapy significantly improved symptoms, quality of life and functional capacity versus placebo even in non-anaemic patients with iron deficiency [27]. Additionally, this study suggested that the correction of iron deficiency with IV iron might improve renal function in ND-CKD patients with chronic heart failure [27, 28].
USE OF IV IRON IN NON-DIALYSIS CKD
There is currently no widely accepted consensus on whether IV or oral iron should be used as first-line therapy to replete iron stores and treat iron deficiency anaemia in patients with ND-CKD. The recent KDIGO Anemia Guideline, therefore, proposes a 1- to 3-month trial of oral iron therapy in ND-CKD and IV iron in patients receiving dialysis [16]. The recent European Renal Best Practice position statement recommends a minimum 3-month trial of oral iron unless there is gastrointestinal intolerance, oral iron is ineffective, severe anaemia is present, or to preserve vascular access [29]. While it is recognized that some patients may benefit from oral iron therapy, studies comparing oral with IV iron in ND-CKD populations have demonstrated that IV iron is superior to oral iron with regard to replenishing iron stores, and have shown a small but significant superiority to oral iron with regard to increasing Hb [26]. These studies, however, have been relatively small and of short duration, such that the long-term safety and efficacy of either type of therapy has not been fully elucidated.
The potential benefits of oral iron include convenience and low cost per dose. However, intestinal absorption of iron is often poor, particularly in patients with ND-CKD who are at an increased risk of inflammation with elevated C-reactive protein (CRP) and hepcidin levels. Additionally, gastrointestinal complications are frequently observed when ferrous salts such as ferrous sulphate are used, and adherence can therefore often be poor, an issue compounded by high pill burden [30]. IV iron administration bypasses the problem of poor absorption in the gastrointestinal tract and can more rapidly and efficiently provide iron for erythropoiesis and replenishment of iron stores.
Other important issues that remain unresolved are the optimal dose of IV iron and the appropriate target levels for serum ferritin and TSAT when administering iron therapy. No randomized trial has systematically examined the efficacy and safety of dosing based on specific serum ferritin or TSAT ranges, so it has not been possible to develop strong evidence-based guidelines on this issue [16, 31]. In one prospective cohort study of 228 unselected patients receiving haemodialysis treated with ESA therapy and IV iron sucrose which used protocol-stipulated dose adjustments according to both Hb concentration, percentage hypochromic red cells (< 2%) and serum ferritin level (100–500 µg/L), a close inverse correlation was observed between serum ferritin level and the mean dose of ESA that was required [32]. Consistent with this, Descombes et al. observed in a series of 25 patients receiving haemodialysis that adjusting IV iron dose to target a serum ferritin level of 200–600 µg/L achieved ∼30% reduction in ESA dose while maintaining the same mean Hb level [33]. It may therefore be hypothesized that targeting higher serum ferritin levels could permit delayed or reduced ESA dosing in the ND-CKD population. Hence, the Ferinject® assessment in patients with iron deficiency anaemia and non-dialysis dependent chronic kidney disease (FIND-CKD) study assessed whether targeting higher ferritin levels with IV iron is more effective than oral iron or, indeed, targeting lower ferritin levels with IV iron.
CURRENT CLINICAL QUESTIONS
Clinicians managing patients with ND-CKD and iron deficiency, with or without anaemia, currently lack of rigorous data to inform their decision-making regarding the timing and route of administration for iron therapy, the optimal serum ferritin and/or TSAT targets, and when to use ESA therapy. The FIND-CKD study was undertaken to address these important clinical questions and to assist the clinician in managing anaemia in their patients with ND-CKD. The study employed a rigorous study design that compared IV iron therapy targeting pre-defined serum ferritin levels versus oral iron therapy, in terms of preventing or delaying the use of other anaemia management therapies (e.g. ESA therapy, other iron therapy or blood transfusions) and a number of other clinically pertinent secondary end points. The study was also designed to address the question of whether targeting a higher or lower serum ferritin level with IV iron is more effective than oral iron.
THE FIND-CKD STUDY DESIGN
Overview
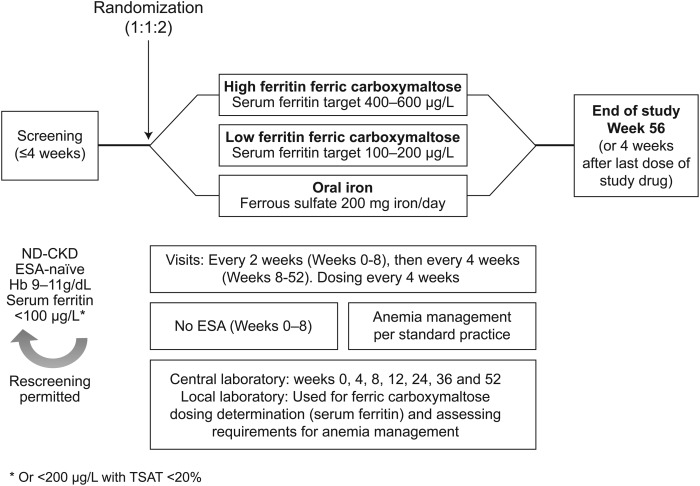
FIND-CKD was a 56-week, open-label, multicentre, prospective, randomized, three-arm study (ClinicalTrials.gov identifier: NCT00994318) (Figure 1). The primary objective of the study was to evaluate the long-term efficacy of IV FCM (using targeted serum ferritin levels to determine dosing) or oral iron to delay and/or reduce use of ESA therapy or other anaemia management options in patients with ND-CKD and iron deficiency anaemia. FIND-CKD was the first randomized trial to compare IV versus oral iron using two different IV iron dosing schemes based on a target range for serum ferritin of 400–600 and 100–200 µg/L—‘high ferritin’ and ‘low ferritin’ FCM dosing groups, respectively. The schedule of study procedures is summarized in Supplementary Material, Appendix S1.
FIGURE 1:
FIND-CKD study design. ESA-naïve was defined no exposure to ESA therapy in the four months prior to randomisation. ESA, erythropoiesis-stimulating agent; FCM, ferric carboxymaltose; ND-CKD, non-dialysis dependent chronic kidney disease.
The FIND-CKD study employed a treatment algorithm that reflects the latest international guidance on anaemia management in CKD from KDIGO [16], which recommends that iron deficiency should be treated before ESA therapy is initiated. The protocol also employed a cautious approach to the introduction of ESA, i.e. ESA therapy was only considered in cases when the Hb level falls <10.0 g/dL, reflecting changes in the prescribing information for ESA therapy from the U.S. Food and Drug Administration (FDA) [34].
FIND-CKD was the longest duration randomized controlled trial of IV iron therapy to be performed to date in patients with ND-CKD. Patients were recruited into the 56-week study at 193 sites in 20 countries: Australia, Austria, Belgium, Czech Republic, Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Poland, Portugal, Romania, Spain, Sweden, Switzerland, Turkey, UK and the USA. The study was conducted according to the principles of the Declaration of Helsinki and the ICH Guidelines for Good Clinical Practice. All patients provided written informed consent.
Selection of study population
The study population was comprised of patients with ND-CKD who had one or more Hb level between 9 and 11 g/dL, and any single serum ferritin level <100 (or <200 µg/L with TSAT <20%), within the 4 weeks prior to randomization (Table 2). These criteria were chosen based on the European Renal Best Practice guidelines at the time of study initiation, which recommended targeting an Hb concentration of 11–12 g/dL with a lower limit of 100 µg/L for serum ferritin and 20% for TSAT [36]. The lower Hb limit of 9 g/dL was selected to exclude patients with severe anaemia, who would be more likely to require blood transfusion within the first 8 weeks of the study and who, if randomized to oral iron, may not have received appropriate iron management [16]. While serum ferritin <100 µg/L indicates the presence of absolute iron deficiency in CKD, functional iron deficiency may be present despite normal serum ferritin levels, i.e. the patient can be regarded as iron-deficient even when serum ferritin exceeds 100 µg/L if inadequate iron is available for erythropoiesis. Therefore, an inclusion criterion of serum ferritin <200 µg/L with TSAT <20% [37] was also used. Additional entry criteria were also applied to ensure that patients did not have rapidly deteriorating renal function, to minimize the risk that a patient would progress to end-stage renal failure during the 12-month study (Table 2). All patients were required to be ‘ESA naïve’, defined as no exposure to ESA therapy in the 4 months prior to randomization. Oral iron therapy (>100 mg iron/day) was to be discontinued at least 1 week prior to randomization.
Table 2.
Key inclusion and exclusion criteria
| Key inclusion criteria | Key exclusion criteria |
|---|---|
| ≥18 years | History of acquired iron overload |
| ND-CKD | Known hypersensitivity reaction to any component of ferrous sulphate or FCM (subjects with hypersensitivity to other forms of iron were permitted to participate) |
| eGFR ≤60 mL/min/1.73 m2 (MDRD [35]) | Documented history of discontinuing oral iron products due to significant gastrointestinal distress |
| eGFR loss ≤12 mL/min/1.73 m2/yeara and predicted | TSAT >40% at screening |
| eGFR ≥15 mL/min/1.73 m2 in 12 monthsb | Known active infection, CRP >20 mg/L |
| Any single Hb level between 9 and 11 g/dL within 4 weeks of randomization | Clinically significant overt bleeding |
| Any single serum ferritin <100, or <200 µg/L with TSAT <20%, within 4 weeks of randomization | Active malignancyc |
| No exposure to ESA therapy in last 4 months prior to randomization | History of chronic alcohol abuse (alcohol consumption >40 g/day). |
| Chronic liver disease and/or screening alanine transaminase or aspartate transaminase greater than three times the upper limit of the normal range | |
| Active HIV infection or AIDS syndrome, or active hepatitis B or C virus infectiond | |
| Anaemia due to reasons other than iron deficiency (e.g. haemoglobinopathy). Subjects with treated vitamin B12 or folic acid deficiency were permitted | |
| IV iron and/or blood transfusion in previous 30 days prior to screening or during screening period | |
| Oral iron therapy at doses >100 mg/day dosing were to be discontinued at least 1 week prior to randomization | |
| Receipt of oral iron therapy (>100 mg/day) for >3 monthse | |
| Immunosuppressive therapy that may lead to anaemia | |
| Currently requiring renal dialysis or anticipated dialysis or transplantation during the study | |
| Anticipated need for surgery that may result in significant bleeding (>100 mL) | |
| Currently suffering from chronic heart failure New York Heart Association Class IV | |
| Poorly controlled hypertension (>160 mmHg systolic pressure or >100 mmHg diastolic pressure) | |
| Acute coronary syndrome or stroke within the 3 months prior to screening | |
| Body weight <35 kg |
Key exclusion criteria are summarized in Table 2.
Randomization
In total, 626 patients were randomized in a 1:1:2 randomization ratio (high ferritin FCM group: low ferritin FCM group: oral iron group). This ratio was selected to ensure a 1:1 randomization ratio for FCM therapy versus oral iron and a 1:1 comparison between the high ferritin and low ferritin FCM groups, with balanced recruitment to FCM or oral iron therapy overall.
Intervention
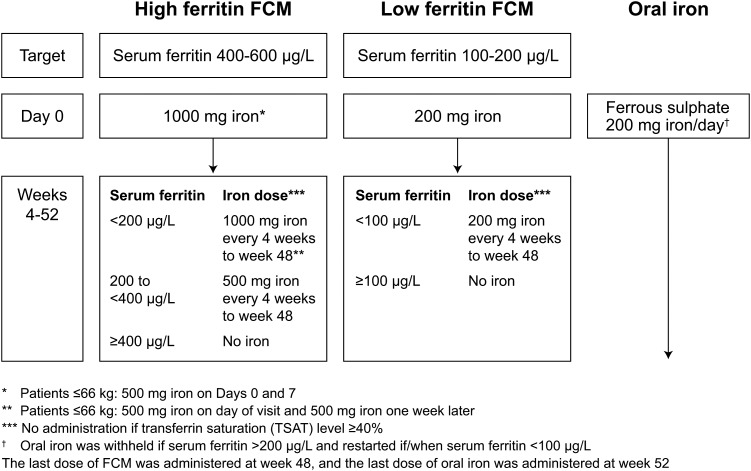
The FCM doses in the high ferritin and low ferritin groups were adjusted to a target serum ferritin level in the range 400–600 and 100–200 µg/L, respectively. An initial dose of 1000 mg iron in the high ferritin group (500 mg iron on Days 0 and 7 in patients weighing ≤66 kg) and 200 mg iron in the low ferritin group was administered on Day 0 if the serum ferritin level was <100 µg/L. During Weeks 4–48, FCM was administered every 4 weeks if the serum ferritin level was <400 µg/L in the high ferritin group or <100 µg/L in the low ferritin group and if TSAT was <40% (Figure 2). For subjects with a TSAT >35% from a prior assessment (completed >10 days prior to the planned day of FCM administration), dosing was withheld until the TSAT level was confirmed to be <40%.
FIGURE 2:
Study treatment. FCM, ferric carboxymaltose.
Oral iron therapy consisted of commercially available ferrous sulphate, administered at a dose of 200 mg iron/day to Week 52. Dosing was suspended if the serum ferritin was 200 µg/L or higher, and was resumed if the serum ferritin level fell below 100 µg/L.
Initiation of other anaemia management
During the first 8 weeks after the start of randomized therapy, subjects were not to receive ESA therapy, red cell transfusion or any other treatment to manage the Hb level unless there was an absolute requirement (e.g. severe or serious adverse reaction to randomized treatment, unable to continue assigned medication or rapid Hb drop requiring ESA therapy or transfusion). After this initial 8-week period, ESA or other therapies could be initiated as per local practice if the Hb level decreased to <10 g/dL, and could include any combination of ESA, iron therapy (oral, IV or intramuscular), blood transfusion or other therapies to achieve and maintain the Hb level within the applicable guideline range. ESA therapy was not permitted in subjects with Hb level ≥10 g/dL. An alternative form of iron therapy was only to be initiated in subjects with Hb level >10 g/dL when the subject could not comply or tolerate the randomized treatment arm.
Study end points
The primary end point of the study was time to initiation of other anaemia management, such as ESA therapy or transfusion or an Hb trigger (two consecutive Hb values <10 g/dL and without an Hb increase ≥0.5 g/dL during Weeks 8–52). Introduction of an alternative iron product, or a change in the protocol-specified dosing schedule or total iron dose to address a lack of efficacy or a treatment-related adverse event, was also regarded as meeting the primary end point. Secondary end points are summarized in Table 3.
Table 3.
FIND-CKD study end points and safety evaluations
| Efficacy end points | Safety evaluations |
|---|---|
| Primary end point | Clinical laboratory tests (haematology, serum chemistry, urinalysis, hormone levels) |
| Time to initiation of other anaemia management (e.g. ESA or transfusion) | Concomitant medication |
| Secondary end points | Adverse events, including severity and relationship to study medication |
| Cumulative ESA dose | Adverse events and serious adverse events leading to study withdrawal |
| Requirement for transfusion | Physical examination (unusual findings) |
| Cumulative iron dose and number of iron administrations | Vital signs (blood pressure, temperature, heart rate and body weight) |
| Increase of Hb ≥1 g/dL prior to any other anaemia management (e.g. ESA or transfusion) | Abnormal features on electrocardiogram |
| Change from baseline to end of study for haematological and iron parameters | |
| Change from baseline to end of study for eGFR (MDRD formula) | |
| Requirement for dialysis | |
| Change in health-related quality of life from baseline to end of study (SF-36) | |
| Health resource utilization over the study period (direct, indirect and total costs from payer's and societal perspective) | |
| Cost effectiveness of treatment options using relevant effectiveness parameters | |
| Percentage of subjects discontinuing the study drug due to intolerance |
Statistical analysis
The primary end point, time to the initiation of other anaemia management or Hb trigger, is being analysed in all intent-to-treat (ITT) subjects based on Kaplan–Meier survival analyses, using the log rank test to compare treatment arms. Three primary comparisons will be made using a hierarchical step-down procedure to preserve the overall α level of 0.05, in the following order: (i) high ferritin FCM versus oral iron, (ii) high ferritin FCM versus low ferritin FCM and (iii) low ferritin FCM versus oral iron. The sample size calculation (Supplementary Material, Appendix S2) indicated that 360 subjects were required to provide 90% power to detect a difference in the primary end point between the high ferritin FCM group and the oral iron group, which is considered to be the primary comparison.
For continuous secondary end points, analysis of covariance models will be used, implementing repeated measures procedure, and including treatment group, visit, pooled country, baseline Hb and/or baseline ferritin as covariates. For categorical end points, survival analyses and logistic regressions will be performed, and odds ratios will be used to compare treatment groups. The significance level will be set at α = 0.05 and no adjustment will be made for testing multiple secondary outcomes.
The ITT population will be comprised of all subjects who received at least one dose of randomized treatment and attended at least one post-baseline visit. The safety population will include all subjects who received at least one dose of randomized treatment. All statistical analyses will be performed using SAS Version 9.0 or later (SAS Institute Inc. SAS/STAT, Cary, NC).
CLINICAL RELEVANCE OF THE FIND-CKD RESULTS
An evidence-based approach to the use of iron therapy for the treatment of iron deficiency anaemia in patients with ND-CKD has been hampered by a lack of randomized, controlled trials of sufficient size and duration. Additionally, the majority of trials have been performed in ND-CKD patients receiving ESA therapy and have used Hb response as their primary end point, and the limitations of this approach have become clear in recent years. A clinical trial such as FIND-CKD is therefore long overdue. The results of FIND-CKD are also likely to receive particular attention in light of safety concerns related to ESA therapy, particularly at high doses [34, 38, 39], and the 2012 KDIGO Anemia Guideline [16].
Unlike in haemodialysis patients where IV iron is universally recommended, there is currently no international consensus regarding which route of administration of iron therapy is more appropriate for the treatment of iron deficiency anaemia in patients with ND-CKD. The choice of route of administration therefore often varies from country to country and from centre to centre, and indeed among individual nephrologists within the same centre. FIND-CKD will provide a robust comparison of the effectiveness and safety of the two routes of administration and help inform clinical practice in patients with ND-CKD not receiving ESA therapy. In addition, the study will examine whether the route of administration and dosing protocol has benefits beyond achieving certain laboratory targets, such as a significant impact on quality of life.
FIND-CKD will also evaluate the potential for IV iron therapy targeting pre-defined serum ferritin levels, and for oral iron, to delay, reduce or avoid ESA administration as well as other anaemia treatment options such as other iron therapy or blood transfusions. Avoiding or decreasing blood transfusions would be welcomed given the recent increase in blood transfusions that has occurred since the publication of CREATE [12], CHOIR [13, 14] and TREAT [15], coupled with the FDA-mandated change in the ESA label, as well as the risk of transfusion-induced sensitization to human leukocyte antigens with adverse effects on waiting time for transplantation and immunological status following transplant [40, 41].
The study protocol specified that ESA therapy should not be initiated until the Hb level was <10 g/dL, which is consistent with the latest guidance from KDIGO [16] and the FDA [34]. Using this contemporary protocol, particular attention will be paid to the proportion of subjects requiring ESA therapy, and whether the IV route of administration utilizing a high ferritin or low ferritin target compared with the oral route confers any additional benefit in terms of a reduction in the requirement for ESA therapy.
Data on the progression of renal function [eGFR (modification of diet in renal disease formula)] will also be of particular interest since there are several studies with conflicting results regarding the impact of iron therapy on renal function. Some small clinical studies have suggested that IV iron therapy may adversely affect renal tubular function and increase proteinuria [42–44]. The effect of IV iron on renal function in FIND-CKD (a secondary end point) will therefore be of great interest. Furthermore, FIND-CKD will allow a direct comparison of the safety profile of IV versus oral iron therapy, although it should be noted that the trial was powered for the primary efficacy end point, not for a safety end point.
Finally, the study will evaluate the cost effectiveness of each treatment arm. A key aspect of anaemia management is the cost of ESA therapy, which represents the largest drug expenditure for ND-CKD patients [45]. In other therapeutic areas, it has been proposed that the use of FCM is cost saving due largely to reduced requirements for ESA [46]. Results of the health economic assessment will help to determine the cost impact of FCM in the setting of ND-CKD and, specifically, if IV iron dosing according to different serum ferritin targets is cost saving compared with oral iron.
Answers to these questions from the FIND-CKD trial are awaited with interest by the nephrology community. Recruitment to the study has been completed, and the first results from the study are expected in late 2013.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
I.M. has received speakers' fees, honoraria and consultancy fees from several ESA and IV iron manufacturers, including Affymax, AMAG, Amgen, Ortho Biotech, Pharmacosmos, Roche, Takeda and Vifor Pharma. A.B. has received speaker's honoraria and consultancy fees from Amgen, Roche and Vifor Pharma. F.C. has no conflicts of interest to declare. K.-U.E. has received speaker's fees and consultancy fees from several ESA and IV iron manufacturers, including Affymax, Amgen, Bayer, Johnson & Johnson, Roche and Vifor Pharma. C.G. has received speakers' fees, honoraria and consultancy fees from several ESA and IV iron manufacturers, including Amgen, Pharmacosmos, Roche, Takeda and Vifor Pharma. D.V.W. is an employee and stockholder of DaVita Healthcare Partners, Inc. S.R. has received speakers' fees, honoraria and consultancy fees from several ESA and IV iron manufacturers, including Amgen, Hoffmann-La Roche, Janssen-Cilag, Novartis, Sandoz, Takeda and Vifor Pharma. B.R. and T.C. are employees of Vifor Pharma Ltd.
AUTHORS’ CONTRIBUTIONS
I.C.M., A.B., F.C., K.-U.E., C.G., D.V.W. and S.D.R. contributed to the study design. B.R. provided biostatistical input. T.C. was the clinical lead and contributed to the study design. All authors critically reviewed and approved the manuscript.
Supplementary Material
Supplementary Data
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. doi:10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.de Jong PE, Halbesma N, Gansevoort RT. Screening for early chronic kidney disease – what method fits best? Nephrol Dial Transplant. 2006;21:2358–2361. doi: 10.1093/ndt/gfl195. doi:10.1093/ndt/gfl195. [DOI] [PubMed] [Google Scholar]
- 3.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17:2275–2284. doi: 10.1681/ASN.2005121273. doi:10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 4.McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501–1510. doi: 10.1185/030079904X2763. doi:10.1185/030079904X2763. [DOI] [PubMed] [Google Scholar]
- 5.Locatelli F, Aljama P, Bárány P, et al. European Best Practice Guidelines Working Group. European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19:ii1–ii47. doi: 10.1093/ndt/gfh1032. doi:10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 6.Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55:726–741. doi: 10.1053/j.ajkd.2009.12.030. doi:10.1053/j.ajkd.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hörl WH. Non-erythropoietin-based anaemia management in chronic kidney disease. Nephrol Dial Transplant. 2002;17:35–38. doi: 10.1093/ndt/17.suppl_11.35. doi:10.1093/ndt/17.suppl_11.35. [DOI] [PubMed] [Google Scholar]
- 8.Fishbane S, Frei GL, Maesaka J. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis. 1995;26:41–46. doi: 10.1016/0272-6386(95)90151-5. doi:10.1016/0272-6386(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 9.Sunder-Plassmann G, Horl WH. Importance of iron supply for erythropoietin therapy. Nephrol Dial Transplant. 1995;10:2070–2076. [PubMed] [Google Scholar]
- 10.Arora NP, Ghali JK. Iron deficiency anemia in heart failure. Heart Fail Rev. 2013;18:485–501. doi: 10.1007/s10741-012-9342-y. doi:10.1007/s10741-012-9342-y. [DOI] [PubMed] [Google Scholar]
- 11.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131:568S–580S. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- 12.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;335:2071–2084. doi: 10.1056/NEJMoa062276. doi:10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 13.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. doi:10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 14.Szczech La, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int. 2008;74:791–798. doi: 10.1038/ki.2008.295. doi:10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeffer MA, Burdmann EA, Chen C-Y, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. New Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. doi:10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease Improving Global Outcomes (KDIGO) Clinical practice guideline for anemia in chronic kidney disease. Kid Int Suppl. 2012;2:292–298. doi:10.1038/kisup.2012.34. [Google Scholar]
- 17.Qunibi WY, Martinez C, Smith M, et al. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26:1599–1607. doi: 10.1093/ndt/gfq613. doi:10.1093/ndt/gfq613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spinowitz BS, Kausz AT, Baptista J, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. doi:10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wyck DB, Roppolo M, Martinez CO, et al. for the United States Iron Sucrose (Venofer) Clinical Trials Group. A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int. 2005;68:2846–2856. doi: 10.1111/j.1523-1755.2005.00758.x. doi:10.1111/j.1523-1755.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 20.Charytan C, Bernardo MV, Koch TA, et al. Intravenous ferric carboxymaltose versus standard medical care in the treatment of iron deficiency anemia in patients with chronic kidney disease: a randomized, active-controlled, multi-center study. Nephrol Dial Transplant. 2013;28:953–964. doi: 10.1093/ndt/gfs528. doi:10.1093/ndt/gfs528. [DOI] [PubMed] [Google Scholar]
- 21.Mircescu G, Gârneata L, Capusa C, et al. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant. 2006;21:120–124. doi: 10.1093/ndt/gfi087. doi:10.1093/ndt/gfi087. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R, Rizkala AR, Bastani B, et al. A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol. 2006;26:445–454. doi: 10.1159/000096174. doi:10.1159/000096174. [DOI] [PubMed] [Google Scholar]
- 23.Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47:S11–S145. doi: 10.1053/j.ajkd.2006.03.010. doi:10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health and Clinical Excellence (NICE) Clinical guideline 114. Anaemia management in people with chronic kidney disease. 2011.
- 25.Liles AM. Intravenous versus oral iron for treatment of iron deficiency in non-hemodialysis-dependent patients with chronic kidney disease. Am J Health Syst Pharm. 2012;69:1206–1211. doi: 10.2146/ajhp110231. doi:10.2146/ajhp110231. [DOI] [PubMed] [Google Scholar]
- 26.Rosen-Zvi B, Gafter-Gvili A, Paul M, et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta-analysis. Am J Kidney Dis. 2008;52:897–906. doi: 10.1053/j.ajkd.2008.05.033. doi:10.1053/j.ajkd.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 27.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. doi:10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 28.Okonko DO, Grzeslo A, Witkowski T, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol. 2008;51:103–112. doi: 10.1016/j.jacc.2007.09.036. doi:10.1016/j.jacc.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 29.Locatelli F, Bárány P, Covic A, et al. Kidney disease: improving global outcomes guidelines on anaemia management in chronic kidney disease: a european renal best practice position statement. Nephrol Dial Transplant. 2013;28:1346–1359. doi: 10.1093/ndt/gft033. doi:10.1093/ndt/gft033. [DOI] [PubMed] [Google Scholar]
- 30.Macdougall IC. Iron supplementation in the non-dialysis chronic kidney disease (ND-CKD) patient: oral or intravenous? Curr Med Res Opin. 2010;26:473–482. doi: 10.1185/03007990903512461. doi:10.1185/03007990903512461. [DOI] [PubMed] [Google Scholar]
- 31.Fishbane S. Upper limit of serum ferritin: misinterpretation of the 2006 KDOQI anemia guidelines. Semin Dial. 2008;21:217–220. doi: 10.1111/j.1525-139X.2007.00420.x. doi:10.1111/j.1525-139X.2007.00420.x. [DOI] [PubMed] [Google Scholar]
- 32.Richardson D, Bartlett C, Will EJ. Optimizing erythropoietin therapy in hemodialysis patients. Am J Kidney Dis. 2001;38:109–117. doi: 10.1053/ajkd.2001.25203. doi:10.1053/ajkd.2001.25203. [DOI] [PubMed] [Google Scholar]
- 33.Descombes E, Fellay G. Improved response to erythropoietin therapy with long-term continuous iron supplementation. Nephron. 2000;84:196–197. doi: 10.1159/000045574. doi:10.1159/000045574. [DOI] [PubMed] [Google Scholar]
- 34.FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. Food and Drug Administration 2011 [Updated June 24, 2011]. http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. 29 January 2013, date last accessed)
- 35.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. doi:10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 36.Locatelli F, Covic A, Eckardt KU, et al. ERA-EDTA ERBP Advisory Board. Anaemia management in patients with chronic kidney disease: a position statement by the anaemia working group of european renal best practice (ERBP) Nephrol Dial Transplant. 2009;24:348–354. doi: 10.1093/ndt/gfn653. doi:10.1093/ndt/gfn653. [DOI] [PubMed] [Google Scholar]
- 37.Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1:S4–S8. doi: 10.2215/CJN.01490506. doi:10.2215/CJN.01490506. [DOI] [PubMed] [Google Scholar]
- 38.Unger EG, Thompson AM, Blank MJ, et al. Erythropoiesis-stimulating agents – time for a reevaluation. New Engl J Med. 2010;362:189–192. doi: 10.1056/NEJMp0912328. doi:10.1056/NEJMp0912328. [DOI] [PubMed] [Google Scholar]
- 39.Singh AK. Does TREAT give the boot to ESAs in the treatment of CKD in anemia? J Am Soc Nephrol. 2010;21:2–6. doi: 10.1681/ASN.2009111127. doi:10.1681/ASN.2009111127. [DOI] [PubMed] [Google Scholar]
- 40.Obrador GT, Macdougall IC. Effect of red cell transfusions on future kidney transplantation. Clin J Am Soc Nephrol. 2013;8:852–860. doi: 10.2215/CJN.00020112. doi:10.2215/CJN.00020112. [DOI] [PubMed] [Google Scholar]
- 41.Macdougall IC, Obrador GT. How important is transfusion avoidance in 2013? Nephrol Dial Transplant. 2013;28:1092–1099. doi: 10.1093/ndt/gfs575. doi:10.1093/ndt/gfs575. [DOI] [PubMed] [Google Scholar]
- 42.Schouten BJ, Hunt PJ, Livesey JH, et al. FGF23 Elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94:2332–2327. doi: 10.1210/jc.2008-2396. doi:10.1210/jc.2008-2396. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal R, Vasavada N, Sachs NG, et al. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65:2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. doi:10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal R, Leehey DJ, Olsen SM, et al. Proteinuria induced by parenteral iron in chronic kidney disease – a comparative randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:114–121. doi: 10.2215/CJN.06020710. doi:10.2215/CJN.06020710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pizzi LT. Economic considerations in a changing anemia environment. Am J Kidney Dis. 2008;52:S29–S33. doi: 10.1053/j.ajkd.2008.09.001. doi:10.1053/j.ajkd.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Luporsi E, Mahi L, Morre C, et al. Evaluation of cost savings with ferric carboxymaltose in anemia treatment through its impact on erythropoiesis-stimulating agents and blood transfusion: French healthcare payer perspective. J Med Econ. 2012;15:225–232. doi: 10.3111/13696998.2011.639823. doi:10.3111/13696998.2011.639823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data