Meiotic germ cells antagonize mesonephric cell migration and testis cord formation in mouse gonads (original) (raw)
. Author manuscript; available in PMC: 2014 Jun 27.
Published in final edited form as: Development. 2003 Oct 15;130(24):5895–5902. doi: 10.1242/dev.00836
Summary
The developmental fate of primordial germ cells in the mammalian gonad depends on their environment. In the XY gonad, Sry induces a cascade of molecular and cellular events leading to the organization of testis cords. Germ cells are sequestered inside testis cords by 12.5 dpc where they arrest in mitosis. If the testis pathway is not initiated, germ cells spontaneously enter meiosis by 13.5 dpc, and the gonad follows the ovarian fate. We have previously shown that some testis-specific events, such as mesonephric cell migration, can be experimentally induced into XX gonads prior to 12.5 dpc. However, after that time, XX gonads are resistant to the induction of cell migration. In current experiments, we provide evidence that this effect is dependent on XX germ cells rather than on XX somatic cells. We show that, although mesonephric cell migration cannot be induced into normal XX gonads at 14.5 dpc, it can be induced into XX gonads depleted of germ cells. We also show that when 14.5 dpc XX somatic cells are recombined with XY somatic cells, testis cord structures form normally; however, when XX germ cells are recombined with XY somatic cells, cord structures are disrupted. Sandwich culture experiments suggest that the inhibitory effect of XX germ cells is mediated through short-range interactions rather than through a long-range diffusible factor. The developmental stage at which XX germ cells show a disruptive effect on the male pathway is the stage at which meiosis is normally initiated, based on the immunodetection of meiotic markers. We suggest that at the stage when germ cells commit to meiosis, they reinforce ovarian fate by antagonizing the testis pathway.
Keywords: Primordial Germ cells, Meiosis, Sex Determination, Gonad, Testis, Sry
Introduction
The mammalian gonad arises as a bipotential primordium with the plasticity to develop into an ovary or a testis. Both somatic and germ cells follow a dimorphic fate once sex determination occurs. Testis fate is determined by the expression of Sry, which initiates the differentiation of Sertoli cells and their structural organization into testis cords (Koopman et al., 1991). Sertoli cells sequester primordial germ cells inside testis cords by 12.5 days post coitum (dpc). By 13.5 dpc, as a result of interactions with Sertoli cells, germ cells arrest in G1 of the mitotic cell cycle and do not enter meiosis until well after birth (McLaren, 1988). Germ cells are not required for the organization of testis cords (McLaren, 1988); however, there is some evidence that germ cells may facilitate this process in the XY gonad (Adams and McLaren, 2002).
In the absence of the Sry gene, ovarian fate proceeds (Gubbay et al., 1992; Hawkins et al., 1992). In contrast to the case in the XY gonad, germ cells are crucial for the formation and maintenance of ovarian structure. In the absence of germ cells, ovarian follicles do not assemble, and when germ cells are lost, ovarian follicles rapidly degenerate (McLaren, 1988). By 13.5 dpc, germ cells in the XX gonad enter meiosis and arrest in prophase I by birth (McLaren, 1988). The timing of germ cell entry into meiosis appears to be based on an intrinsic clock. Germ cells enter meiosis around 13.5 dpc even when they develop in regions outside the gonad such as adrenal glands and the mesonephros (Zamboni and Upadhyay, 1983), or when they are assembled in lung aggregates in culture (McLaren and Southee, 1997).
Several pieces of evidence indicate that the male pathway must be initiated within a narrow window in development. During normal gonad development, male and female fates are mutually exclusive; testis and ovarian structures normally do not co-exist. One exception is the formation of ovotestes in hermaphrodites where the YPOS chromosome from Mus domesticus poschiavinus is crossed onto Mus musculus musculus strains, notably C57BL/6. These ovotestes typically consist of testis cords in the mid-region of the gonad and ovarian structure in the polar regions (Bradbury, 1987). Based on these data, it was hypothesized that there is a requirement for the testis-determining gene to act during a narrow window of time, and above a crucial threshold, to initiate the testis pathway and avert the competing ovarian pathway (Burgoyne and Palmer, 1991; Eicher and Washburn, 1986). Consistent with this idea, recent molecular evidence has provided a strong correlation between delayed and/or lowered expression of Sry, and ovotestis development in C57BL/6 XYPOS mice. If Sry expression is delayed by 24 hours, complete or partial sex reversal occurs in XY gonads (Eicher et al., 1995; Nagamine et al., 1998; Washburn et al., 2001).
Organ culture experiments provide further evidence for a narrow developmental window for the initiation of testis development. Cellular events downstream of Sry, including mesonephric cell migration, can be induced in XX gonads in organ culture during the bi-potential stage. However induction of these elements of the testis pathway can occur in XX gonads only prior to 13.5 dpc (Tilmann and Capel, 1999). These results are consistent with the window for the initiation of testis development predicted on the basis of the C57BL/6 XYPOS ovotestis (Burgoyne and Palmer, 1991; Eicher and Washburn, 1986). These experiments strongly suggested that the timing of the initiation of testis development is crucial because of changes that occur in the gonad that result in loss of the bipotentiality of the organ primordia. What was not clear from these experiments was whether these changes were dependent on somatic or germ cells in the XX gonad.
To test whether somatic or germ cells are responsible for the resistance of XX gonads to induction of the testis pathway after 12.5 dpc, we compared mesonephric cell migration in organ culture assays using XX gonads with or without meiotic germ cells. We also investigated whether XX germ cells or somatic cells interfere with testis cord formation by generating XX↔XY recombinant aggregates in culture. Results of these experiments indicate that the physical presence of germ cells inhibits initiation of the testis pathway. We show that the stage when germ cells from XX gonads inhibit the male pathway is temporally correlated with the time that germ cells spontaneously enter meiosis. We propose that once germ cells commit to meiosis, they are antagonistic to the testis pathway as the result of signaling changes that occur at this stage.
Materials and methods
Mouse strains and matings
CD1 random-bred mouse strains (Charles River) were used for migration assays and immunocytochemistry. GFP transgenic mice [Stock TgN(GFPU)5Nagy, The Jackson Laboratory #003115; (Hadjantonakis et al., 1998)] were used for migration studies. _KitW/W_-v embryos were generated by crossing KitW/+ (WB/ReJ KitW/+, The Jackson Laboratory) and _KitW_-v/+ mice (B6By.Cg-_MitfMi_-_wh KitW_-v T, The Jackson Laboratory). The _KitW/W_-v embryos can be easily identified by their anemic appearance. Timed matings were produced by housing female mice with males overnight and checking for vaginal plugs the next morning [0.5 days post coitum (dpc) = noon of the day when a vaginal plug was found]. The sex of each embryo was determined by Giemsa staining for X chromatin Barr bodies in cells of the amniotic sac (Palmer and Burgoyne, 1991).
Germ cell depletion by busulfan treatment
Pregnant females were injected IP with 100 μl busulfan solution (16 mg/ml of 50% DMSO) or 50% DMSO (control) at 10.5 dpc. Busulfan at this concentration was effective at depleting more than 98% of germ cells in rat (Merchant, 1975) and in mouse gonads based on alkaline phosphatase staining (De Felici et al., 1989). Embryos from the treated females were obtained at 11.5, 13.5 or 14.5 dpc for isolation of the gonad for mesonephric cell migration assays.
Mesonephric cell migration assay
XY gonads from CD1 embryos (12.5 dpc), XX gonads from +/+, _KitW/W_-v embryos or busulfan-treated embryos (11.5, 13.5 or 14.5 dpc), and mesonephroi from 11.5 dpc GFP embryos were obtained and assembled as illustrated in Fig. 3. The recombinant explants were assembled on an 1.5% agar block and cultured for 48 hours in Dulbecco’s Minimal Eagle Medium (DMEM) supplemented with 10% fetal calf serum (Hyclone) and 50 μg/ml ampicillin at 37°C with 5% CO2/95% air (Martineau et al., 1997). Migration images were obtained using a Leica MZFLIII dissecting microscope with a GFP filter. After 48 hours of culture, the explants were fixed and processed for either immunocytochemistry or in situ hybridization.
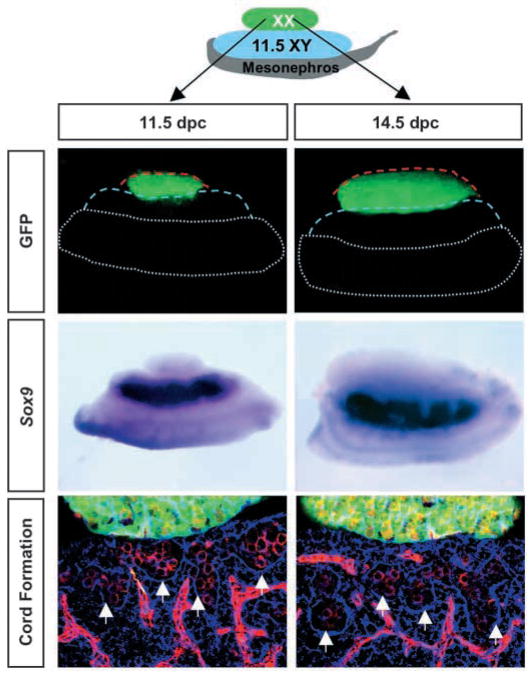
Fig. 3.
Co-culture experiments suggest that meiotic germ cells inhibit the testis pathway via short-range effects. An 11.5 or 14.5 dpc XX GFP gonad (green) was cultured on top of an 11.5 dpc XY gonad for 48 hours. In situ hybridization for Sox9 (dark purple) or immunocytochemical staining for laminin (blue) and germ cells and vasculature (red), reveal no interference with the testis pathway. Red and blue broken lines outline the XX and XY components, respectively. Broken white lines outline the mesonephros. Arrows indicate testis cord boundaries.
Isolation of primordial germ cells and somatic cells from embryonic gonads
Germ cells and somatic cells were separated according to the protocol developed by Pesce and de Felici (Pesce and de Felici, 1995) with minor modification. Briefly, eight pairs of gonads of desired ages were dispersed in 1 ml of trypsin-EDTA at 37°C for 6 minutes followed by brief centrifugation at 2000 g. The supernatant was removed and 1 ml of DNase in PBS (20 μg/ml) was added and incubated for 5 minutes at room temperature. The cell suspension was centrifuged and all but 180 μl of DNase/PBS was removed. 20 μl of TG-1 antibody (mouse IgM,1:10, provided by James Resnick), was added, mixed well, and incubated on ice with shaking for 30 minutes. Cells were spun at 1500 g for 2 minutes at 4°C and washed with DNase/PBS twice. The pellet was resuspended in 180 μl ice-cold DNase/PBS with 20 μl of rat anti-mouse IgM MicroBeads (Miltenyi Biotec). The mixture was incubated on ice for 30 minutes with shaking. The MiniMacs Separation Column (Miltenyi Biotec) was prepared in the magnetic holder according to the manufacturer’s instructions and the cell suspension was applied to the column. The flow-through was collected and reapplied to the column. The second flow-through contained the somatic cell fraction. The column was washed with DNase/PBS three times, removed from the magnet and the germ cell fraction was eluted with 1.5 ml of DNase/PBS. The somatic and germ cell fractions were concentrated by centrifugation at 1500 g for 2 minutes. The pellets were resuspended in 1 ml of prewarmed culture medium.
Generation of recombinant aggregates
After the germ cell and somatic cell fractions were obtained as described above, the cell suspension was centrifuged at 2000 g for 5 minutes and cell pellets were resuspended in 100 μl pre-warmed culture medium. The number of germ cells in the cell suspensions were estimated by counting alkaline phosphatase-positive cells present in aliquots according to De Felici and colleagues (De Felici et al., 1989). Volumes were adjusted such that 50 μl of the somatic cell fraction from 12.5 dpc XY gonads, combined with 50 μl of the germ cell or somatic cell test fractions at different stages, resulted in a cell ratio of ~1.5:1.0. One μl phytohemagglutinin-P (Sigma #L9132, 5 mg/ml in water) was added to the cell mixture. The cell mixture (about 100 μl) was back-loaded into an ordinary sequencing gel loading tip sealed with a flame. The loaded tip was placed in a 15 ml Corning tube and centrifuged at 2000 g for 5 minutes. The sealed end of the tip was cut off and the aggregate was expelled to a groove in an agar block using a filtered mouth pipette. The aggregates were cultured in Dulbecco’s Minimal Eagle Medium (DMEM) supplemented with 10% fetal calf serum (Hyclone) and 50 ug/ml ampicillin for 48 hours at 37°C with 5% CO2/95% air.
Immunocytochemistry
For immunostaining against laminin, samples were fixed overnight in 4% paraformaldehyde in PBS at 4°C. For immunostaining against PECAM-1, phosphorylated H2AX (γH2AX) and SYN/COR, samples were fixed for 1 hour in 1% paraformaldehyde at 4°C. Samples were then processed and cut into 8 μm frozen sections as described (Karl and Capel, 1998). Sections were blocked for 1 hour at room temperature in blocking solution (PBS/10% heat inactivated goat serum/0.1% Triton-X 100). Primary antibody incubations were carried out overnight at 4°C in blocking solution (1:200 dilution of rabbit anti-laminin1 antibody, provided by Harold Erickson; 1:500 dilution of rat anti-PECAM-1 antibody, PharMingen; 1:800 dilution of a rabbit polyclonal antibody against SYN1/COR1, provided by Peter Moens; 1:800 dilution of a rabbit polyclonal antibody against γH2AX, provided by William Bonner). Sections were washed three times in washing solution (PBS/1% heat inactivated goat serum/0.1% Triton-X 100) for 5 minutes each. Secondary antibody incubations were performed overnight at 4°C with 1:500 dilution of fluorescently conjugated secondary antibodies (FITC- or Cy5-conjugated goat anti-rabbit antibody and Cy3-conjugated goat anti-rat antibody, Jackson Immunochemicals). Sections were washed three times for 5 minutes each in washing solution and mounted on glass slides in DABCO. Images were collected on a Zeiss LSM confocal microscope and processed using Adobe Photoshop.
LysoTracker assay for identification of apoptotic cells
Gonads were isolated and incubated in 1 ml DMEM with 5 μl LysoTracker Red (DND-99, Molecular Probes) for 30 minutes at 37°C, washed three times in PBS, then three times in PBT (0.1% Triton X100 in PBS) for 30 minutes per wash. Gonads were fixed overnight in 4% paraformaldehyde and processed for immunocytochemistry.
Whole-mount in situ hybridization
Samples were fixed overnight in 4% paraformaldehyde in PBS at 4°C and processed according to Henrique et al. (Henrique et al., 1995). A digoxigenin labeled RNA probe for Sox9 was detected using an alkaline phosphatase-conjugated anti-digoxigenin antibody.
Results
The presence of germ cells interferes with migration of mesonephric cells into 14.5 dpc XX gonads
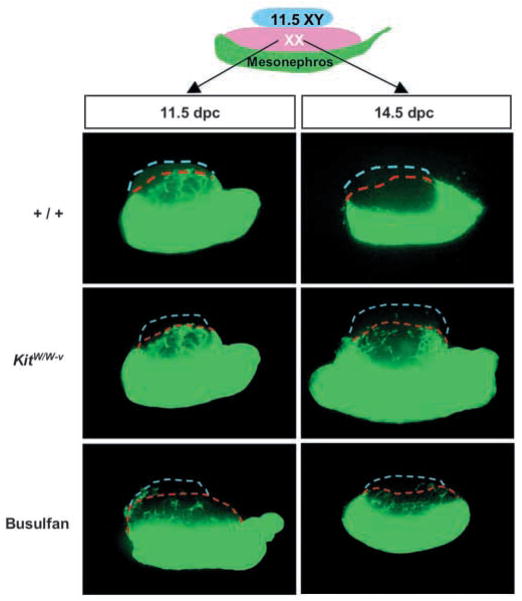
Induction of mesonephric cell migration is an XY-specific event downstream of Sry. We previously showed that mesonephric cells could be induced to migrate into XX gonads when XX gonads were cultured between an 11.5 dpc XY gonad and mesonephros. However, only XX gonads at stages earlier than 13.5 dpc were permissive to migrating cells (Tilmann and Capel, 1999). This observation indicated a change in XX gonads that occurs around 13.5 dpc. To test whether XX somatic cells or germ cells are responsible for the block to mesonephric migration after 13.5 dpc, we assembled a migration assay (Fig. 1) using XX gonads with a normal complement of germ cells or XX gonads depleted of germ cells. We obtained agametic gonads from two sources: from _KitW/W_-v mutants, and from embryos treated with busulfan. In both cases, more than 95% of germ cells were eliminated (see Materials and methods). XX gonads (11.5 or 14.5 dpc) from wild-type, _KitW/W_-v or busulfan-treated embryos were cultured between an 11.5 dpc XY gonad and an 11.5 dpc GFP mesonephros. Mesonephric cell migration was induced into both 11.5 dpc wild-type and 11.5 dpc agametic XX gonads. However XX gonads from wild-type embryos were not permissive for cell migration by 14.5 dpc, as previously shown. By contrast, mesonephric cell migration was induced into both 14.5 dpc _KitW/W_-v and busulfan-treated XX gonads that were depleted of germ cells. These data strongly suggested that germ cells rather than somatic cells in 14.5 dpc XX gonads were responsible for restricting mesonephric cell migration.
Fig. 1.
In the absence of germ cells, mesonephric cells can be induced to migrate into 14.5 dpc XX gonads. An 11.5 or 14.5 dpc XX gonad from a wild type (+/+), _KitW/W_-v embryo or busulfan-treated embryo was cultured between an 11.5 dpc XY gonad and an 11.5 dpc GFP mesonephros for 48 hours. Broken blue lines outline the XY components; broken red lines outline the XX components.
14.5 dpc XX germ cells but not somatic cells interfere with the formation of testis cords
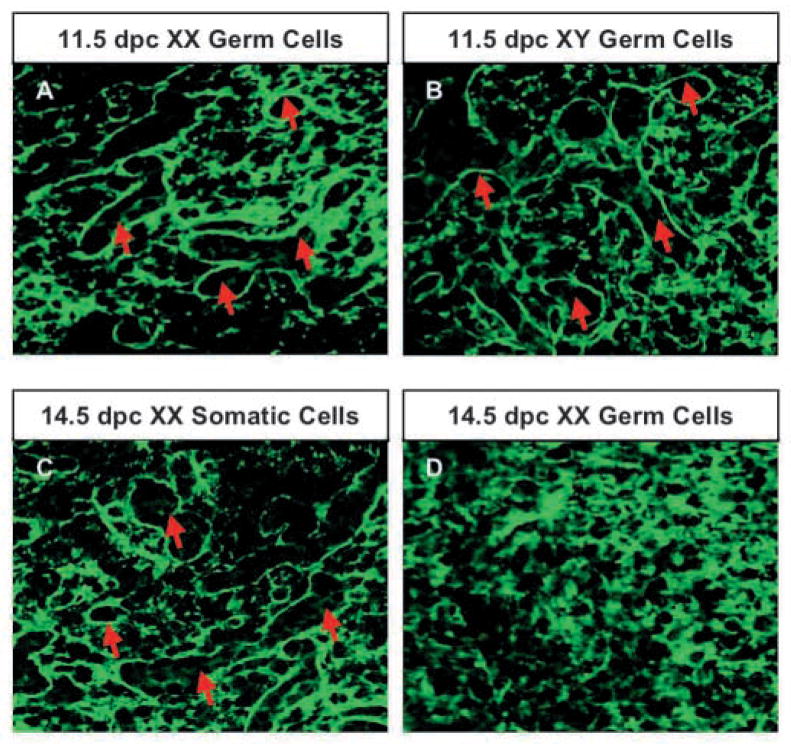
To test whether antagonistic signals from XX somatic cells or germ cells interfere with testis cord formation, we designed an in vitro aggregate culture to recombine XY somatic cells with either purified XX somatic cells or XX germ cells. We separated germ cells from the somatic cell population using an immunomagnetic cell sorting system that yielded a 90–95% purity of germ cell and somatic cell fractions as assayed by the activity of alkaline phosphatase, a germ-cell specific marker (De Felici et al., 1989). In all experiments, we used XY somatic cells from 12.5 dpc gonads. When XY somatic cells were reaggregated with germ cells from 11.5 dpc XY gonads (Fig. 2B) or from 14.5 dpc XY gonads (data not shown), the aggregates efficiently formed testis cord structures after 48 hours of culture. When we recombined a 12.5 dpc XY somatic cell fraction with an 11.5 dpc XX germ cell fraction (Fig. 2A), or a 14.5 dpc XX somatic cell fraction (Fig. 2C), testis cords formed normally as compared with the control. However, when a 12.5 dpc XY somatic cell fraction was recombined with a 14.5 dpc XX germ cell fraction, we found no evidence of testis cord structure in any sample (Fig. 2D, images are representative of three experiments).
Fig. 2.
Meiotic germ cells interfere with cord formation. Germ cells and somatic cells were isolated using an immunomagnetic cell sorting system. Somatic cell fractions from 12.5 dpc XY gonads were aggregated with 11.5 dpc XX germ cell fraction (A), 11.5 dpc XY germ cell fraction (B), 14.5 dpc XX somatic fraction (C) or 14.5 dpc XX germ cell fraction (D) and cultured for 48 hours. Formation of testis cords was shown by immunostaining for laminin (green), a component of the basal lamina of testis cords (red arrows).
In vitro experiments suggest that germ cells interfere with the testis pathway through short-range signals or physical interactions with somatic cells
Previous data are consistent with the idea that after 13.5 dpc, XX germ cells interfere with testis cord formation. Germ cells could secrete a diffusible factor that interferes with testis cord formation, or they could acquire surface properties that are incompatible with aggregation into cords. To test the possibility that germ cells produce a diffusible factor that interferes with the testis pathway, we assembled recombinant gonad explants as illustrated in Fig. 3. We cultured an 11.5 or 14.5 dpc XX gonad on top of an 11.5 dpc XY gonad and mesonephros for 48 hours. Using an XX gonad from a GFP embryo, we determined that there was no cell mixing between GFP XX cells and XY gonadal cells in these cultures. If meiotic germ cells interfered with Sertoli differentiation or testis cord formation via a diffusible factor, we would predict downregulation of Sox9 (a Sertoli-specific marker at this stage) and/or disruption of testis cords in regions of the XY gonad near the margin of the XX gonad segment of the graft. However, we found that neither 11.5 nor 14.5 dpc XX gonads had any effect on the expression of Sox9 or the formation of testis cords in the XY region of the sandwich (Fig. 3).
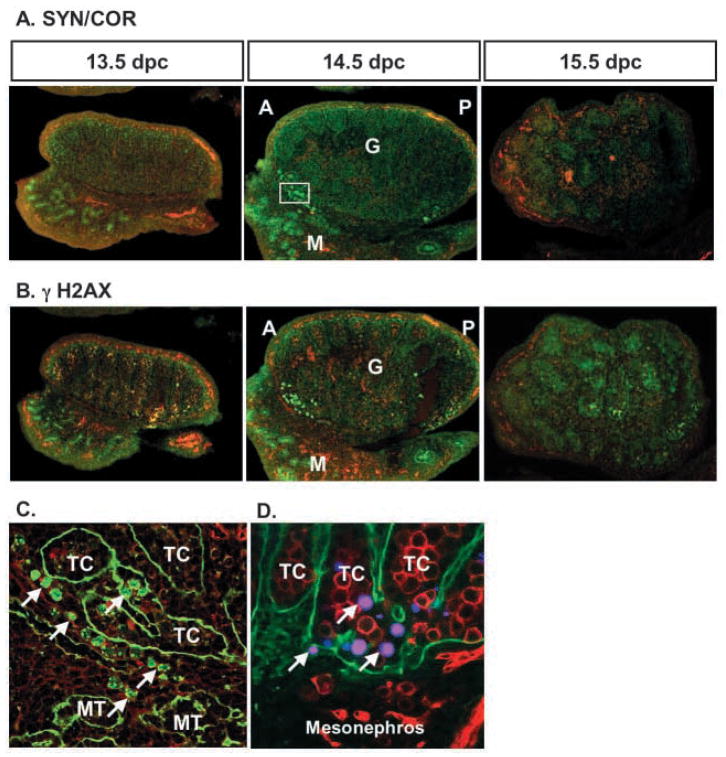
Temporal and spatial patterns of SYN/COR and γH2AX in germ cells of XX mouse gonads
There is still no explanation for why ovarian sectors in ovotestes form in the polar regions of the gonad. We speculated that the temporal and spatial pattern of germ cell entry into meiosis might provide a clue as to why distal regions of the gonad are more vulnerable to sex reversal. To investigate the possibility that germ cells commit to meiosis earlier in polar regions of the gonad, we obtained antibodies that recognize several different components of meiotic chromosomes. Antibodies against SYN/COR are a complex mixture that recognizes elements of the synaptonemal complex of meiotic chromosomes, reportedly during the zygotene-pachytene transition (Dobson et al., 1994). Phosphorylated gamma histone 2AX (γH2AX) is known to associate with the DNA double-strand break points in meiotic chromosomes during leptotene in mouse spermatogenic cells (Mahadevaiah et al., 2001).
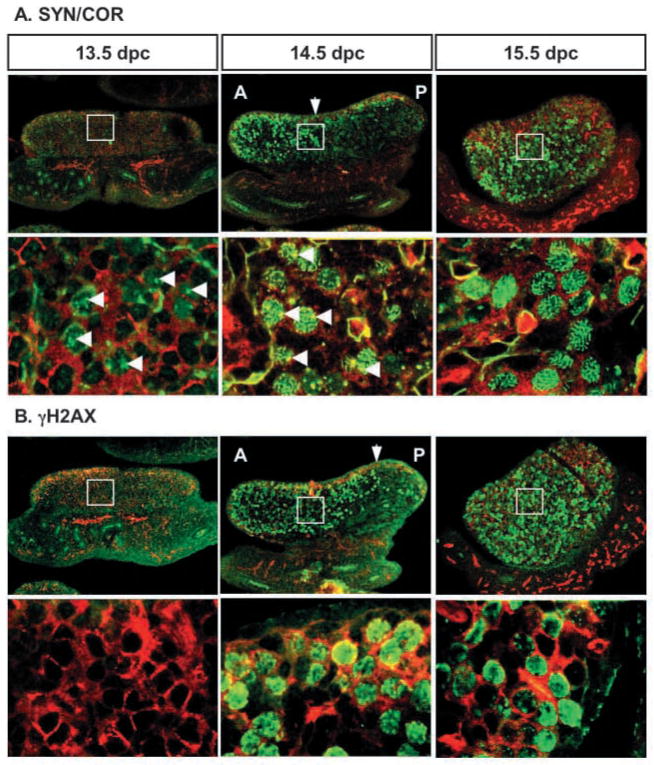
In female gonads, antibodies against SYN/COR detected a punctate pattern in the nuclei of some germ cells at 13.5 dpc (Fig. 4A, green staining), indicating that these germ cells in XX gonads have committed to meiosis by 13.5 dpc. By 14.5 dpc, SYN/COR staining in germ cells showed a filamentous pattern associated with the assembly of homologous chromatid pairs in zygotene/pachytene (Fig. 4A, arrowheads). Germ cells positive for γH2AX were first detected at 14.5 dpc (Fig. 4B). There was no evidence that germ cells in the most distal regions of the gonad entered meiosis earlier than in the central domain. Instead, germ cells in XX gonads became positive for both of these meiotic markers first at the anterior end of the gonad (Fig. 4, arrows). The leading edge of SYN/COR-positive germ cells appeared to follow the leading edge of γH2AX-positive germ cells (Fig. 4; consecutive 8 μm sections are shown). At 15.5 dpc, germ cells throughout the gonad, in the cortex and medulla, were positive for both of these markers. SYN/COR staining was clearly associated with condensed chromosome pairs in all positive cells, whereas γH2AX staining showed a diffuse pattern concentrated at the periphery of some nuclei (Fig. 4B).
Fig. 4.
Two meiotic markers, SYN/COR (A) and γH2AX (B), are expressed in an anterior to posterior pattern in 13.5–15.5 dpc XX gonads: SYN/COR and γH2AX (green); germ cells and vasculature (PECAM-1, red). A higher magnification of the region surrounded by the white rectangle is shown below the corresponding image. Consecutive 8 μm sections were stained with each antibody to define the spatial relationship between SYN/COR and γH2AX. Arrows indicate the leading front of staining at 14.5 dpc. Arrowheads indicate the first detection of SYN/COR at 13.5 dpc and the filamentous pattern associated with the assembly of homologous chromatid pairs in zygotene/pachytene at 14.5 dpc. A, anterior; P, posterior.
SYN/COR and γH2AX are briefly expressed in a few XY germ cells in the region of the rete testis
In XY gonads, staining for SYN/COR and phosphorylated γH2AX was not detected in germ cells at any stage examined with the exception of 14.5 dpc. At 14.5 dpc, 20–30 germ cells positive for both SYN/COR and γH2AX were found in the anterior junction between the gonad and mesonephros (Fig. 5A,B; consecutive 8 μm sections are shown). These SYN/COR- and γH2AX-positive germ cells were located both inside and outside testis cord structures and in the mesonephros (Fig. 5C). SYN/COR and γH2AX positive germ cells appeared transiently in 14.5 dpc gonads and were not found in 15.5 dpc gonads.
Fig. 5.
Meiotic germ cells are present transiently in XY gonads in the region of the rete testis. (A) SYN/COR and (B) γH2AX were detected by immunofluorescence (green) in consecutive 8 μm sections; germ cells were labeled with αPECAM1 (red). (C) A higher magnification of the region surrounded by the white rectangle in A. (D) At 15.5 dpc, apoptotic cells in this region were labeled with LysoTracker (magenta); germ cells were labeled with PECAM (red); the basal lamina of testis cords was stained for laminin (green). Arrows indicate germ cells. A, anterior; P, posterior; G, gonad; M, mesonephros; MT, mesonephric tubule; TC, testis cord.
Based on the model that meiotic germ cells antagonize testis cord development, we investigated the effect of meiotic germ cells in this region of the XY gonad. LysoTracker Red, which marks apoptotic cells, was used in conjunction with antibodies against germ cells and laminin to distinguish somatic and germ cells, and to illuminate testis cord structure in this region of the XY gonad at 13.5–15.5 dpc. Apoptotic cells were concentrated in the region of the XY gonad near the mesonephric boundary, in germ cells and some somatic cells located within and outside testis cords (Fig. 5D, arrows).
Discussion
During the development of the bipotential gonad in all vertebrates, the testis pathway initiates before the ovarian pathway. Early studies on C57BL/6 XYPOS mice that form ovotestes gave rise to the idea that the timing of the initiation of testis development is crucial: a temporal mismatch between the testis and ovarian pathways was proposed to be the cause for testis to ovary sex reversal in B6 XYPOS mice (Burgoyne and Palmer, 1991; Eicher and Washburn, 1986). Although it is known that the testis pathway is initiated by Sry expression in Sertoli cell precursors, the factors that initiate the ovarian pathway are not known. However, it has been proposed that the ovarian pathway is under the control of germ cells (Burgoyne and Palmer, 1991). In previous work, we have shown that elements of the male pathway can be induced in XX gonads prior to 13.5 dpc, but not after. A model consistent with these results is that stable commitment to the ovarian pathway occurs at the stage when germ cells commit to meiosis. If _Sry_-mediated pathways function prior to this time, germ cells are enclosed inside testis cords, and blocked from entry into meiosis. However, if the male pathway is not initiated before germ cells commit to meiosis, the ovarian pathway proceeds and the testis pathway is blocked.
A molecular profile of germ cell meiosis in embryonic gonads
The light microscope has previously been used to monitor the meiotic status of germ cells in embryonic gonads. Using antibodies that detect stages of meiotic progression, we established a molecular profile for meiotic events during early gonad development. These observations show that the commitment of XX germ cells to meiosis occurs by 13.5 dpc. We observed punctate staining for SYN/COR at 13.5 dpc, 24 hours earlier than the first appearance of γH2AX. This pattern probably represents staining of the COR1 proteins, precursors of the lateral elements of the synaptonemal complex that first form as short discrete stretches in leptotene (Mahadevaiah et al., 2001). SYN staining marks the assembly of the synaptonemal complex from zygotene onward (Mahadevaiah et al., 2001). In spermatogenic cells, COR1 and γH2AX appear at the same time (Mahadevaiah et al., 2001). Our results in the XX gonad are slightly different: γH2AX staining was not visible in early leptotene at 13.5 dpc, when COR1 first appeared. However, at 14.5 dpc, when the anterior to posterior pattern is visible, γH2AX staining appears slightly ahead of SYN/COR. This discrepancy may be due to a mechanistic or timing difference in meiotic chromatin assembly between XX and XY germ cells.
Germ cells are believed to enter meiosis based on an intrinsic clock. It is therefore somewhat surprising that they do not enter meiosis simultaneously. Progression of meiosis from anterior to posterior might be expected if there is a regional signaling source that affects the process. Thus far, the evidence has not supported the idea of a local inductive signal, as germ cells enter meiosis with similar timing in all environments (other than the testis) in which they have been studied (McLaren and Southee, 1997). Alternatively, this pattern could result from differences in the time that germ cells populate the anterior versus the posterior of the gonad.
Both SYN/COR and γH2AX markers also detected a small, transient group of meiotic germ cells in XY gonads, at the anterior junction between the gonad and mesonephros. This group of cells has been mentioned in a previous report (McLaren, 1984). Our markers revealed that at 14.5 dpc, these meiotic germ cells were located inside testis cords, outside testis cords and in the mesonephros. By 15.5 dpc, somatic and germ cells in this region of the XY gonad are undergoing apoptosis, and meiotic cells were no longer found. Although the significance of this region of meiotic germ cells in the XY gonad remains obscure, it is nonetheless spatially coincident with the region where disruptions in cord formation contribute to the structural reorganization of the junctions between testis cords, rete testis and efferent ductules. It is possible that meiotic germ cells in this region destabilize the tubules and contribute to their reorganization or, alternatively, that focal disruptions of cord structures trigger germ cell entry into meiosis.
Timing is crucial
In this study, we have directly tested whether XX germ cells or somatic cells inhibit the testis pathway after 14.5 dpc. We provide evidence that 14.5 dpc germ cells can block cell migration into XX gonads and can interfere with the organization of testis cords. Mesonephric cell migration is a male-specific event required for testis cord formation (Buehr et al., 1993; Tilmann and Capel, 1999). In XYPOS mice that form ovotestes, mesonephric cells always migrate into the central testicular region but not into the polar ovarian regions of the ovotestes (Albrecht et al., 2000). The stereotypic compartmentalization of the ovotestes in C57BL/6 XYPOS gonads is not explained by earlier meiosis in distal regions. Instead, this compartmentalization may simply reflect the fact that delayed migration reaches only the regions most proximal to the mesonephros prior to the stage when germ cells become inhibitory.
It would be expected that if the ovarian pathway is initiated by meiotic germ cells, removal of germ cells would eliminate the ovarian pathway and therefore, rescue the sex reversal phenotype in C57BL/6 XYPOS mice. However, the results using mouse mutants devoid of germ cells did not support this notion. In certain alleles of the sterile mutants Kit and Kit ligand (Kitl), the absence of germ cells exacerbated the male to female sex reversal in C57BL/6 XYPOS mice (Burgoyne and Palmer, 1991; Cattanach et al., 1988; Nagamine and Carlisle, 1996). One possible explanation for these results is that germ cells at pre-meiotic stages play a positive role in supporting testis cord formation. Although the presence of germ cells is not required for formation of testis cords, cord formation is delayed in the absence of germ cells in KitW/W_−_v mutants and in other cases where germ cells are lost (H.H.-C.Y. and B.C., unpublished). Recent studies by Adams and McLaren demonstrated that germ cells generate a masculinizing feedback loop by producing prostaglandin D2 which promoted Sertoli cell differentiation (Adams and McLaren, 2002). Loss of germ cells might critically impair the cord forming process in C57BL/6 XYPOS gonads and contribute to failure of Kit mutants to rescue sex reversal in XYPOS mice (Burgoyne and Palmer, 1991).
How do meiotic germ cells interfere with the testis pathway?
We found that when an XX gonad with meiotic germ cells was cultured on top of an XY gonad, there was no inhibitory effect on the adjacent XY tissue: cord formation and the expression of male markers occurred normally in nearby cells. These experiments cannot rule out the possibility that long-range signals from the XX gonad are blocked by signals from somatic or germ cells in the XY gonad. However, these sandwich cultures suggest that the inhibitory effect of meiotic germ cells is mediated through cell-cell interactions or through short-range diffusible factors. We suggest that the adhesion molecules on the surface of germ cells or short-range diffusible factors may be permissive to testis cord formation only until germ cells commit to meiosis (Fig. 6). There are several surface molecules known to exhibit sexually dimorphic patterns as germ cells enter meiosis. Erb2 and Erb3 are present on the germ cell surface between 11.5–13.5 in both sexes, but disappear after 13.5 dpc in XX gonads (Toyoda-Ohno et al., 1999). E-cadherin expression follows a similar pattern with downregulation in XX gonads when germ cells enter meiosis (Di Carlo and de Felici, 2000). However the involvement of these surface molecules in testis development has not been investigated.
Fig. 6.
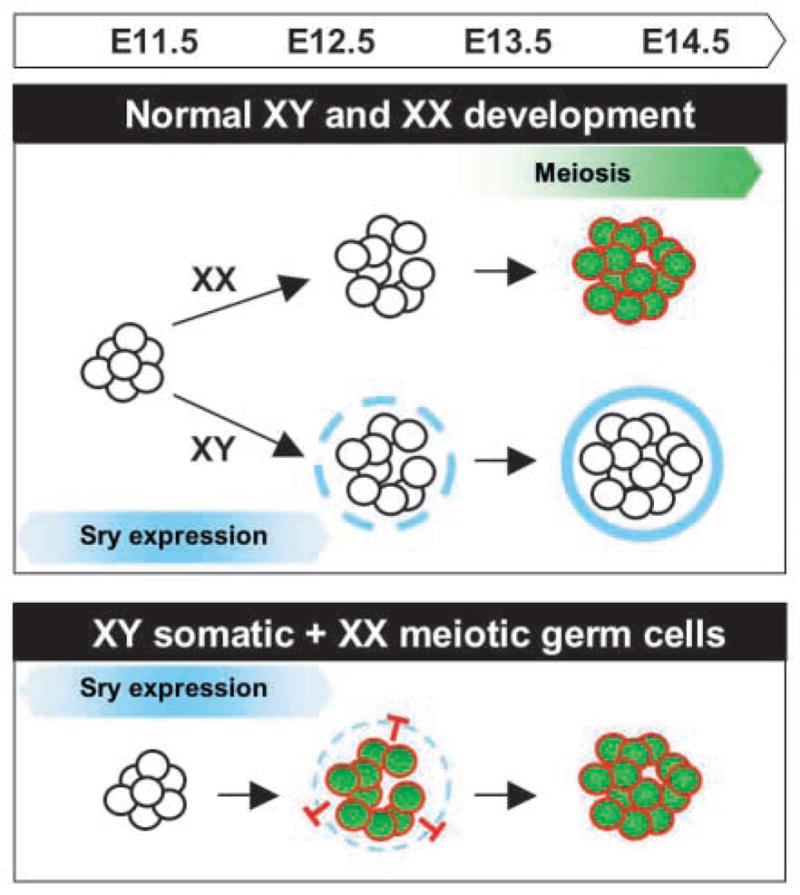
A model for the antagonistic role of meiotic germ cells on the testis pathway. In normal XX gonads, germ cells follow an intrinsic clock and enter meiosis by 13.5 dpc. In normal XY gonads, expression of Sry at 10.5–12.5 dpc initiates cord formation prior to entry of germ cells into meiosis. Cord formation blocks germ cell entry into meiosis. Molecules on the surface of germ cells or short-range diffusible factors from germ cells may be permissive for testis cord formation only until germ cells commit to meiosis when their surface properties may change. A temporal mismatch experimentally created by combining meiotic germ cells with 12.5 dpc XY somatic cells led to failure of testis cord formation.
Our results are consistent with the hypothesis that germ cell entry into meiosis is a competing pathway that opposes _Sry-_induced pathways in the bipotential gonad. According to this hypothesis, the relative timing of these two pathways would determine the fate of the gonad. This mechanism would insure that the ovarian fate is specified when germ cells enter meiosis. This makes intuitive sense in terms of reproductive fitness: once germ cells enter meiosis, their reproductive future is promoted by ovarian but not testis structure because meiotic germ cells in the embryonic testis would be rapidly depleted, leading to sterility. Future experiments to identify the molecular mechanisms elicited by meiotic germ cells will be crucial to uncover the players in the ovarian pathway.
Acknowledgments
We are grateful to Eva Eicher, Anne McLaren and Paul Burgoyne for their insightful experiments and many helpful discussions. Jim Resnick and John Eppig kindly provided protocols and suggestions for germ cell isolation and recombinant gonad assembly. Jim Resnick, Peter Moens, William Bonner and Harold Erickson generously provided antibodies against TG-1, SYN/COR, γH2AX and laminin. We thank Jennifer Brennan, Doug Coveney and Andrea Ross for helpful comments on the manuscript. This work was supported by grants from the NIH to Blanche Capel (GM56757 and HD39963), and from the Lalor Foundation to Humphrey Yao.
References
- Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–1164. doi: 10.1242/dev.129.5.1155. [DOI] [PubMed] [Google Scholar]
- Albrecht KH, Capel B, Washburn LL, Eicher EM. Defective mesonephric cell migration is associated with abnormal testis cord development in C57BL/6J XYMus domesticus mice. Dev Biol. 2000;225:26–36. doi: 10.1006/dbio.2000.9819. [DOI] [PubMed] [Google Scholar]
- Bradbury MW. Testes of XX in equilibrium with XY chimeric mice develop from fetal ovotestes. Dev Genet. 1987;8:207–218. doi: 10.1002/dvg.1020080405. [DOI] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- Burgoyne P, Palmer S. The genetics of XY sex reversal in the mouse and other mammals. Semin Dev Biol. 1991;2:277–284. [Google Scholar]
- Cattanach B, Rasberry C, Beechey CV. Sex reversal and retardation of embryonic development. Mouse News Lett. 1988;82:94. [Google Scholar]
- De Felici M, Dolci S, Siracusa G. Fetal germ cells establish cell coupling with follicle cells in vitro. Cell Differ Dev. 1989;28:65–69. doi: 10.1016/0922-3371(89)90024-5. [DOI] [PubMed] [Google Scholar]
- Di Carlo A, de Felici M. A role for E-cadherin in mouse rimordial germ cell development. Dev Biol. 2000;226:209–219. doi: 10.1006/dbio.2000.9861. [DOI] [PubMed] [Google Scholar]
- Dobson M, Pearlman R, Karaiskakis A, Spyropoulos B, Moens P. Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Biol. 1994;107:2749–2760. doi: 10.1242/jcs.107.10.2749. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Shown EP, Washburn LL. Sex reversal in C57BL/6J-YPOS mice corrected by a Sry transgene. Phil Trans R Soc B. 1995;350:263–269. doi: 10.1098/rstb.1995.0160. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL. Genetic control of primary sex determination in mice. Annu Rev Genet. 1986;20:327–360. doi: 10.1146/annurev.ge.20.120186.001551. [DOI] [PubMed] [Google Scholar]
- Gubbay J, Vivian N, Economou A, Jackson D, Goodfellow P, Lovell-Badge R. Inverted repeat structure of the Sry locus in mice. Proc Natl Acad Sci USA. 1992;89:7953–7957. doi: 10.1073/pnas.89.17.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hawkins JR, Taylor A, Berta P, Levilliers J, van Der Auwere B, Goodfellow PN. Mutational analysis of SRY: Nonsense and missense mutations in XY sex reversal. Hum Genet. 1992;88:471–474. doi: 10.1007/BF00215684. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol. 1984;38:7–23. [PubMed] [Google Scholar]
- McLaren A. Somatic and germ-cell sex in mammals. Philos Trans R Soc Lond. 1988;322:3–9. doi: 10.1098/rstb.1988.0109. [DOI] [PubMed] [Google Scholar]
- McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Dev Biol. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- Merchant H. Rat gonadal and ovarioan organogenesis with and without germ cells. An ultrastructural study. Dev Biol. 1975;44:1–21. doi: 10.1016/0012-1606(75)90372-3. [DOI] [PubMed] [Google Scholar]
- Nagamine C, Capehart J, Carlisle C, Chang D. Ovotestes in B6-XXSxr sex-reversed mice. Dev Biol. 1998;195:24–32. doi: 10.1006/dbio.1997.8826. [DOI] [PubMed] [Google Scholar]
- Palmer S, Burgoyne PS. XY follicle cells in the ovaries of XO/XY and XO/XY/XYY mosaic mice. Development. 1991;111:1017–1020. doi: 10.1242/dev.111.4.1017. [DOI] [PubMed] [Google Scholar]
- Pesce M, de Felici M. Purification of mouse primordial germ cells by MiniMACS magnetic separtation system. Dev Biol. 1995:722–725. doi: 10.1006/dbio.1995.1250. [DOI] [PubMed] [Google Scholar]
- Tilmann K, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- Toyoda-Ohno H, Obinata M, Matsui Y. Members of the ErbB receptor tyrosine kinases are involved in germ cell development in fetal mouse gonads. Dev Biol. 1999;215:399–406. doi: 10.1006/dbio.1999.9482. [DOI] [PubMed] [Google Scholar]
- Washburn LL, Albrecht KH, Eicher EM. C57BL/6J-T-associated sex reversal in mice is caused by reduced expression of a Mus domesticus Sry allele. Genetics. 2001;158:1675–1681. doi: 10.1093/genetics/158.4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni L, Upadhyay S. Germ cell differentiation in mouse adrenal glands. J Exp Zool. 1983;228:173–193. doi: 10.1002/jez.1402280204. [DOI] [PubMed] [Google Scholar]