National trends in antidepressant medication treatment among publicly-insured pregnant women (original) (raw)
. Author manuscript; available in PMC: 2014 Jul 1.
Abstract
Objective
Risk of depression in women is greatest at childbearing age. We sought to examine and explain national trends in antidepressant use in pregnant women.
Methods
Cohort study including pregnant women aged 12–55 who were enrolled in Medicaid during 2000–2007. We examined the proportion of women taking antidepressants during pregnancy by patient characteristics (descriptive), by region (mixed-effects model), and over time (interrupted time-series).
Results
We identified 1,106,757 pregnancies in 47 states; mean age was 23 years and 60% were non-white. Nearly 1 in 12 used an antidepressant during pregnancy. Use was higher for older (11.2% for age ≥30 vs. 7.6% for <30) and white (14.4% vs. 4.0% for non-white) women. There was a 4- to 5-fold difference in rate of antidepressant use among states. Of the 5.3% of women taking antidepressants at conception, 33% and 17% were still on treatment 90 and 180 days, respectively, into pregnancy; an additional 4% began use during pregnancy. Labeled pregnancy-related health advisories did not appear to affect antidepressant use.
Conclusions
Antidepressant use during pregnancy remains high in this population; treatment patterns vary substantially by patient characteristics and region. Comparative safety and effectiveness data to help inform treatment choices are needed in this setting.
Keywords: antidepressants, Medicaid, national cohort, patterns of medication use, pregnancy
INTRODUCTION
The lifetime risk of depression in women is 10–25%, twice that in men [1]. The risk is greatest at childbearing age, with 10–15% of all pregnant women displaying some signs of depression [2]. The strongest risk factor for depression during pregnancy is a history of prior depression [3, 4]. Young, low-income mothers are particularly vulnerable [5]. Use of antidepressant medications in pregnant women has grown steadily over time [6–9].
Although the evidence is less impressive for treatment of mild depression, in patients with moderate to severe depression antidepressants often improve symptoms and can reduce the risk of serious consequences associated with untreated depression for both the mother and her offspring [10]. Untreated depression during pregnancy has been linked to increased risk of self-injurious or suicidal behavior; it has also been reported to be associated with inadequate self-care and poor compliance with prenatal care, miscarriage or preterm birth, poor fetal growth, and impaired fetal and postnatal development [11–15], although findings are not consistent and many studies are inconclusive [16].
However, in recent years there has been increasing concern about the safety of antidepressant use during pregnancy. The risks of several maternal complications, including gestational diabetes, preeclampsia, placental problems, premature rupture of the membranes, bleeding, induced delivery, and the requirement for a cesarean section, have been reported to be increased among women taking antidepressants during pregnancy [17]. First trimester exposure to certain selective serotonin reuptake inhibitors (SSRIs) has been associated with some specific birth defects [18–22], while SSRI use late in pregnancy has been associated with pulmonary hypertension of the newborn (PPHN) [23], prematurity [24–27], low birth weight [26, 27], small size for gestational age [28], and various neonatal complications [24–26, 29]. For several of these outcomes, however, the evidence supporting an association is mixed [16]. Moreover, since most studies did not assess the potential independent effects of medications and depression severity, it remains unclear to what extent such associations are due to biologic or behavioral factors intrinsic to women with mood disorders (such as smoking, substance abuse, or poor diet), to medications used to treat the disorder, or a combination of both. Based on the available evidence, the US Food and Drug Administration (FDA) issued a public health advisory in December 2005 noting an increased risk of congenital malformations associated with first trimester exposure to paroxetine. In July 2006, it issued a health advisory warning regarding exposure to any SSRI after the 20th week of gestation and an increased risk of PPHN. In addition to these pregnancy-specific advisories, the labels of all antidepressants include a black box warning indicating that they are associated with an increased risk of suicidality in children and adolescents (since 2004) and in young adults ages 18–24 (since 2007). When making recommendations for the management of depression during pregnancy, clinicians must weigh the potential risk of untreated or sub-optimally treated depression against the potential risks associated with antidepressant exposure, including medical and obstetric adverse events, teratogenesis, neonatal toxicity and long-term neurobehavioral problems in the offspring.
The objective of our study was to document patterns of antidepressant medication use during pregnancy in a national cohort of Medicaid-enrolled women in the US. In addition to examining variations in use by patient characteristics and geographic region, we sought to evaluate changes in patterns of use throughout pregnancy, as well as temporal trends in light of the various health advisory warnings. Low-income women are at higher risk for depression than women in higher income groups [5], as well as for perinatal complications and morbidity in offspring, making this an important population to study.
METHODS
Data Source and Study Cohort
The study cohort was drawn from the Medicaid Analytic eXtract (MAX) for all US states (except Arizona) and Washington, DC for 2000 – 2007. Medicaid — the joint state and federal health insurance program in the US for low-income people — is the largest health insurance program and covers the medical expenses for more than 40% of births in the US [30]. The MAX dataset is available from the Centers for Medicare and Medicaid Services (CMS), and it contains individual-level demographic and Medicaid enrollment information, as well as healthcare utilization data including physician services and hospitalizations and their accompanying diagnoses and procedures, and all filled medication prescriptions.
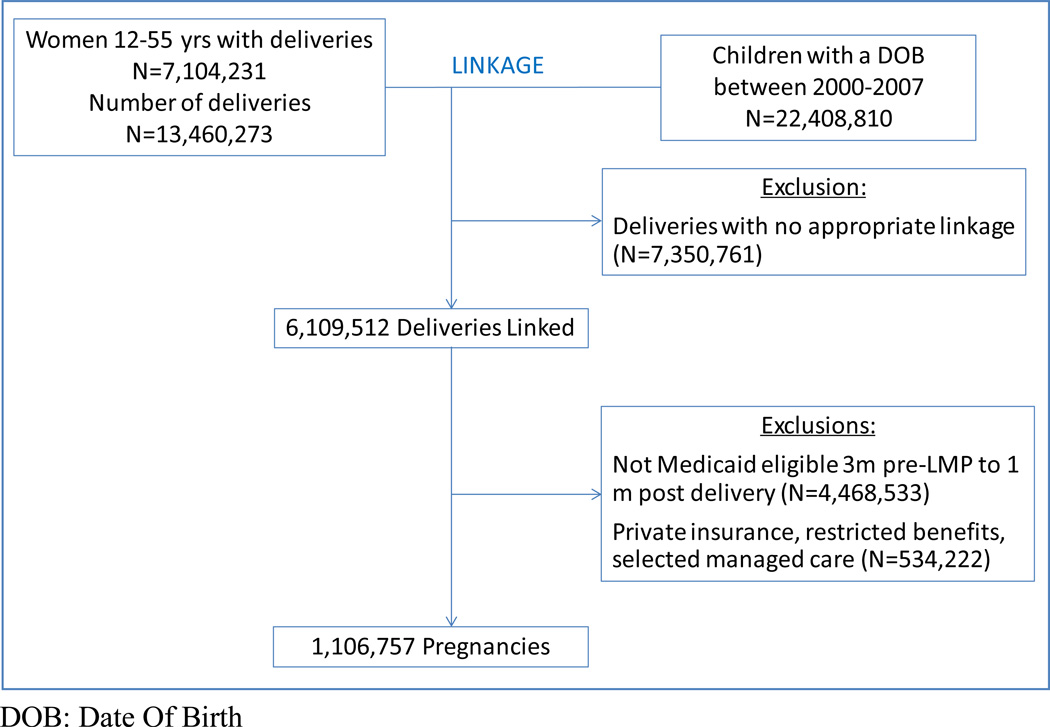
Our study population consisted of a cohort of completed pregnancies, and linked mothers and their infants [31]. We identified all completed pregnancies in women aged 12–55 years using Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) procedure codes for in- and outpatient deliveries. We then linked these pregnancies to live-born infants using the date of birth and the state-specific Medicaid case identification number, which is typically shared by family members. We estimated the date of last menstrual period (LMP) based on the delivery date combined with diagnostic codes indicative of pre-term delivery using a previously validated algorithm [32]. Finally, we required all women who were successfully linked to an infant to be Medicaid eligible from three months before the estimated LMP (to ascertain pre-conception medication use) through one month post delivery. To ensure a complete, longitudinal stream of healthcare claims throughout pregnancy, we excluded women with supplementary private insurance, women with restricted benefits and women in selected capitated managed care plans (Figure 1).
Figure 1. Assembly of the study cohort.
DOB: Date Of Birth
Use of Antidepressant Medications
Maternal exposure to antidepressant medications was derived from pharmacy dispensing records. Antidepressant medications considered include selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and others (e.g., monoaminase oxidase inhibitors (MAOIs), tetracyclic antidepressants (TeCAs)). Exposure status on any given day was based on the dispensing date and number of days supply. We accumulated days supply for consecutive dispensings of the same medication, if overlap occurred.
Data Analyses
We analyzed patterns of antidepressant medication use by patient characteristics, pregnancy stage and by state. To estimate antidepressant use in each state accounting for potential differences in patient characteristics, we fit mixed-effects logistic regression models. The state identifying variable was modeled as a normally distributed random intercept, and the patient characteristics were modeled as fixed effects. Each intercept estimate represents the state-specific prescribing rate (i.e., the proportion of women prescribed an antidepressant) accounting for the other variables in the model. These predicted state-specific prescribing rates are empirical Bayes estimates; that is, they have been shrunk towards the overall mean prescribing rate, with the amount of shrinkage dependent on the relative magnitude of the within-and between-state variance estimates and the number of pregnancies in the state. The ability to account for random variation represents a major advantage of mixed-effects regression analysis over stratified analysis in this context. We used interrupted time-series analysis to explore the impact of FDA Health Advisories on the use of antidepressants during pregnancy from 2000 through 2007, encompassing (1) a no-warning period (January 2000 – May 2003), (2) a suicidality-warning only period (June 2003 – November 2005), and (3) a period during which warnings related to the safety of SSRIs for the newborn were issued (December 2005 – December 2007). The rate of exposure to antidepressants was defined as the number of prescriptions filled in a given calendar month divided by the total number of follow-up days contributed by pregnant women during that month. For each of the three time segments, we estimated the level (intercept, or value at the beginning of the time interval) and trend (slope, or rate of change during the time interval), using least square regression [33].
The study was considered exempt by the Partners Human Research Committee.
RESULTS
We identified 1,106,757 pregnancies linked to a live-born infant in 47 states. Montana and Connecticut were excluded because of inadequate pregnancy-infant linkage based on the Medicaid case identification number; Michigan was excluded because of incomplete claims information. Four states contributed over 40% of pregnancies: California (n=237,628), New York (n=87,587), Illinois (n=87,515), and Tennessee (n=65,748). The cohort was young, with a mean age of 23.2 years (SD: 5.8 years). Non-Hispanic White was the largest racial category (40%), followed by Black (34%), Hispanic (19%), and other (7%; includes Asian, Pacific Islander, American Indian, Alaska Native, >1 race, unknown). Twenty percent of women had a documented diagnosis for which antidepressants are commonly prescribed before or during pregnancy (i.e., depression or other mental health problem, sleep disturbances, pain, chronic fatigue syndrome, smoking cessation – specific diagnostic codes used are listed in eTable 1); 11% had a recorded diagnosis of depression itself.
Overall, 8.1% of women were dispensed an antidepressant medication at some point during their pregnancy; 58.9% of these women were also taking antidepressants during the 3 months before conception whereas 41.1% newly initiated treatment during pregnancy. Antidepressant use was higher among older women (11.2% for ≥30 years versus 7.6% for <30), and among white women (14.4% versus 3.5–6.0% for women of other racial categories). Similar patterns were observed during all trimesters of pregnancy. As expected, use was highest among women with a depression diagnosis (40.3%), followed by women with diagnoses for other potential indications (10.7%), and women without a documented treatment indication during the follow up period (3.3%) (Table 1).
Table 1.
Exposure to antidepressant medications by selected patient characteristics in a cohort of 1,106,757 Medicaid-eligible pregnant women, 2000–2007
| Antidepressant exposure during pregnancy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Before LMP, N (%) | During Pregnancy, N (%) | |||||||||
| −90 to 0 days | Any time | 1st trimester(0 to 90 days) | 2nd trimester(90 to 180 days) | 3rd trimester(+180 days) | |||||||
| Overall | 1,106,757 | 72,134 | (6.5) | 89,980 | (8.1) | 67,273 | (6.1) | 43,047 | (3. 9) | 40,177 | (3.6) |
| Patient subgroups | |||||||||||
| Age | |||||||||||
| <30 | 943,123 | 56,592 | (6.0) | 71,641 | (7.6) | 52,245 | (5.5) | 33,312 | (3.5) | 31,361 | (3.3) |
| ≥30 | 163,634 | 15,542 | (9.5) | 18,339 | (11.2) | 15,028 | (9.2) | 9,735 | (6.0) | 8,816 | (5.4) |
| Race | |||||||||||
| White | 441,524 | 52,177 | (11.8) | 63,632 | (14.4) | 48,693 | (11.0) | 31,701 | (7.2) | 29,603 | (6.7) |
| Black | 373,252 | 9,200 | (2.5) | 13,037 | (3.5) | 8,571 | (2.3) | 5,463 | (1.5) | 5,257 | (1.4) |
| Hispanic | 207,523 | 6,782 | (3.3) | 8,239 | (4.0) | 6,260 | (3.0) | 3,391 | (1.6) | 2,971 | (1.4) |
| Other | 84,458 | 3,975 | (4.7) | 5,072 | (6.0) | 3,749 | (4.4) | 2,492 | (3.0) | 2,346 | (2. 8) |
| Treatment indication | |||||||||||
| Depression | 125,767 | 37,239 | (29.6) | 50,731 | (40.3) | 39,383 | (31.3) | 26,133 | (20. 8) | 24,195 | (19.2) |
| Other* | 92,796 | 8,204 | (8.8) | 9,962 | (10.7) | 7,614 | (8.2) | 4,084 | (4.4) | 3,633 | (3.9) |
| No | 888,194 | 26,691 | (3.0) | 29,287 | (3.3) | 20,276 | (2.3) | 12,830 | (1.4) | 12,349 | (1.4) |
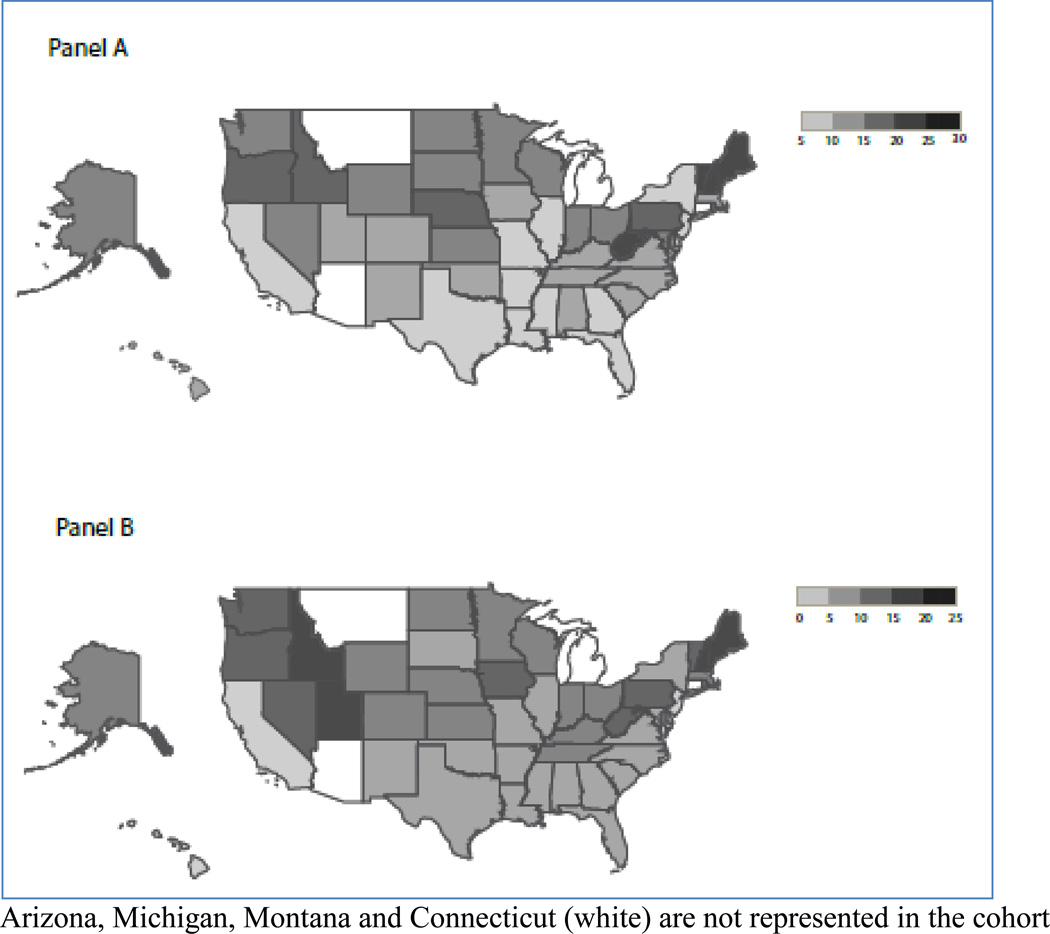
When comparing individual states, the proportion of women with a depression diagnosis ranged from 5.9% (Louisiana) to 29.3% (New Hampshire) (Figure 2, Panel A) and the proportion of women exposed to antidepressants ranged from 2.7% (Hawaii) to 22.6% (Maine) (Figure 2, Panel B). After adjustment for potential between-state differences in patient demographics (age, race), case-mix (treatment indications), and calendar year, and accounting for random variability, the proportion of women exposed to antidepressants ranged from 10.7% to 42.3% among those with a depression diagnosis, and from 1.0% to 5.6% among those without a depression diagnosis (eFigure 1); this represents a 4- to 5-fold difference in use between geographic regions.
Figure 2. Regional variation in the proportion of women with a depression diagnosis (Panel A) and antidepressant medication use (Panel B) in a cohort of 1,106,757 Medicaid-eligible pregnant women, 2000–2007.
Arizona, Michigan, Montana and Connecticut (white) are not represented in the cohort
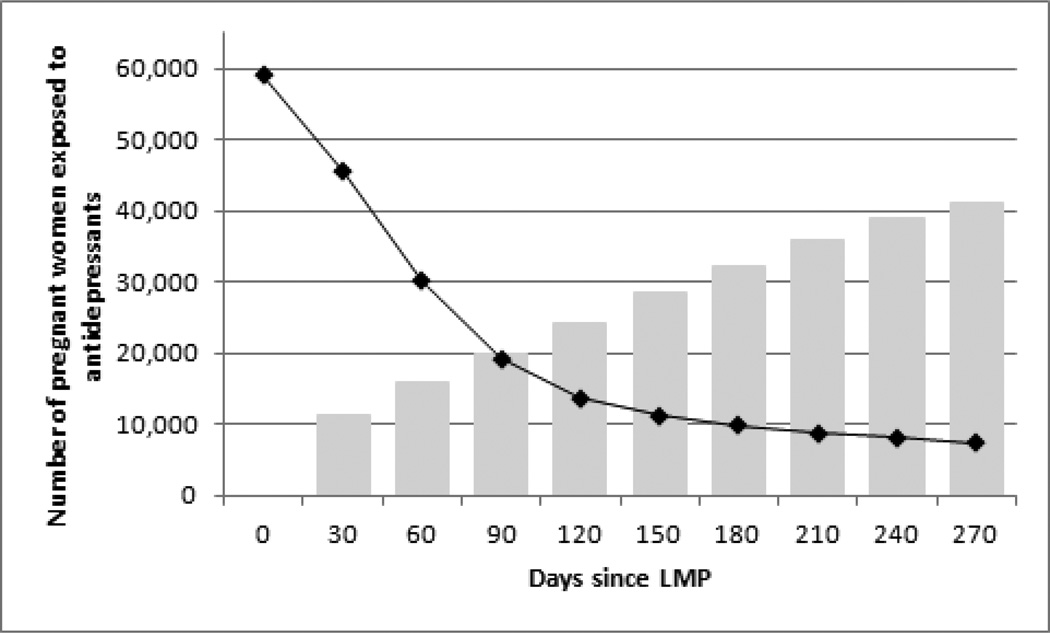
The proportion of women exposed to antidepressants decreased gradually during the first trimester, from 5.3% at the estimated LMP to 2.7% at the end of the first trimester, but it remained relatively stable thereafter. Close to three quarters (73.5%) of women who were taking antidepressants before conception also used antidepressants at some point during their pregnancy. The large majority of patients (about 80%) were treated with SSRIs; roughly equal proportions of patients were exposed to other types of antidepressants (SNRIs, TCAs and other) (eFigure 2). One third of patients (32.5%) who were treated with antidepressants at the estimated LMP were continuously exposed (i.e., no medication gap) throughout the first trimester, 16.6% throughout the second, and 12.6% throughout the third trimester. Among women who were not treated with antidepressants at the time of the estimated LMP, 1.9% (n=19,959) had some exposure by the end of the first trimester, 3.1% (n=32,306) had some exposure by the end of the second trimester, and 3.9% (n=41,031) had some exposure before the end of their pregnancy (Figure 3). Continued use and treatment initiation during pregnancy were highest among women ≥30 years, and among white women (eTable 2).
Figure 3. Changes in antidepressant medication use during pregnancy in a cohort of 1,106,757 Medicaid-eligible pregnant women, 2000–2007.
The black line shows the number of women, among those exposed to antidepressants at the time of the LMP, who were continuously exposed throughout different pregnancy stages.
The grey bars show the cumulative number of women, among those not exposed at the time of the LMP, who had some exposure to antidepressants during pregnancy.
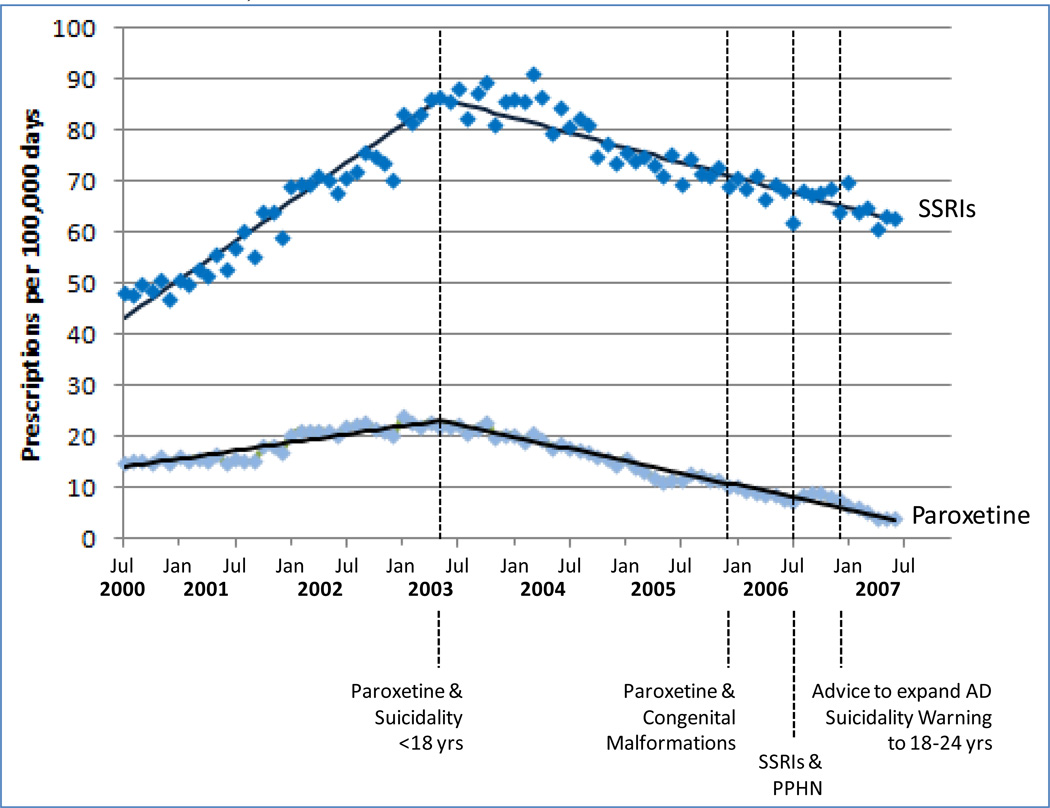
The first antidepressant-related FDA Advisory Warning issued in June 2003 (paroxetine and an increased risk of suicidality in patients younger than 18) was associated with a marked decrease in the use of paroxetine and other SSRIs during pregnancy in our study population. In contrast, no change in the rate of antidepressant use occurred at the time of subsequent warnings related to health risks for the newborn (December 2005 warning related to 1st trimester exposure to paroxetine and risk of congenital malformation; July 2006 warning related to SSRI exposure after gestational week 20 and risk of PPHN), and expansions of the suicidality warning to include all antidepressants and patients 18–24 years old (Figure 4). The rate change at the time of the initial suicidality warning was observed in all age groups (<18, 18–24, >24), and in all racial groups (data not shown).
Figure 4. Impact of FDA Advisory Warnings on antidepressant medication use any time during pregnancy in a cohort of 1,106,757 Medicaid-eligible pregnant women, 2000–2007.
SSRI: Baseline trend: 1.27 (SE: 0.04, p<0.0001)); trend change after Paroxetine & Suicidality warning: −1.76 (SE: 0.06, p<0.0001)
Paroxetine: Baseline trend: 0.27 (SE: 0.01, p<0.0001)); trend change after Paroxetine & Suicidality warning: −0.66 (SE: 0.02, p<0.0001)
DISCUSSION
In this large 47-state cohort of 1,106,757 pregnancies in Medicaid-eligible women between 2000 and 2007, 8.1% of women took an antidepressant medication at some point during their pregnancy. Dispensing of antidepressants during pregnancy was more common among older women, white women and women living in the Midwest (10.4% vs. 5.6% in the West). One-third of women taking antidepressants around the time of conception were still on treatment by the end of the first trimester, and 4% of women not taking antidepressants around the time of conception initiated treatment during pregnancy. Interestingly, the initial suicidality warning in children and adolescents <18 (representing 15.5% of our study cohort), but not subsequent pregnancy-related health advisories or expansions of the suicidadility warning to include women age 18–24 (representing 49.3% of the study cohort), appeared to affect the trend of antidepressant medication use. It is possible, however, that the additional warnings helped to maintain a trend that otherwise could have flattened some years after the initial suicidality warning.
Our findings are largely consistent with those from an earlier, much smaller study using data from Tennessee’s Medicaid program (TennCare) [6]. In this study, 8.7% of women giving birth had exposure to any antidepressant; a marked temporal trend of increasing use during the 5-year period 1999 through 2003 was observed; and older age, white race and more than 12 years of education were significant predictors of antidepressant use. Data from more recent years and a focus on Medicaid enrollees who have higher rates of chronic health conditions, including mental illness, likely contribute to the higher prevalence of antidepressant exposure in these two cohorts than described in earlier studies or in studies of higher income populations [7]. Furthermore, the proportion of women in our study population using antidepressant medications during the first trimester was similar to the proportion who reported taking antidepressants in an interview-based study between 2000 and 2008 (around 6% in both studies) [9]. Variation in the prevalence of depression by state has previously been documented for the general population [34]. Such regional variations might reflect differences in the availability of and access to healthcare services, patterns of reimbursement for mental health care, and associated chronic conditions, such as obesity and diabetes. Demographic characteristics and socio-economic conditions are unlikely to have contributed much to the observed differences in our study (after adjustment for age, race, treatment indications and calendar year) given the homogeneity of the study population - Medicaid-eligible pregnant women - although differences in Medicaid-eligibility criteria between states should be acknowledged.
This large national study cohort of Medicaid eligible pregnant women was assembled without any self-selection, unlike studies based on volunteers that usually include healthier and more educated women. Nevertheless, the necessary restriction to women for whom linkage to a neonatal record was possible and who met our strict inclusion criteria resulted in a reduction in the size of the cohort and could decrease its representativeness of the general Medicaid population. The large study size allowed us to evaluate patterns of use by specific patient characteristics and pregnancy stage, by geographic location, and to examine temporal trends. The racial and ethnic diversity within the cohort revealed a major disparity in antidepressant use during pregnancy between White and non-White women, consistent with earlier findings in the general population [35]. The poverty of Medicaid patients permits documentation of patterns of use in an indigent population typically underrepresented in research. Automated pharmacy dispensing information is usually seen as the gold standard of drug exposure compared to self-reported information [36] or prescribing records in outpatient medical records [37]. Pharmacists fill prescriptions with little room for interpretations, and are reimbursed by insurers on the basis of detailed, complete, and accurate claims submitted electronically [38, 39]. Patient non-response and recall bias are absent from healthcare utilization databases since all data recording is independent of a patient’s memory or agreement to participate in a research study.
Our study also has some limitations, however. Since pregnancies resulting in miscarriage or abortion are not included in the study population, our results cannot be generalized to this subset of pregnant women. Administrative databases typically do not contain information on gestational age or the date of last menstrual period (LMP), yet this date is important for the correct determination of the exposure time window. Nevertheless, the algorithm we used to estimate the LMP date has been shown to correctly classify gestational age within 2 weeks for 93% of term and 70% of preterm pregnancies in one healthcare utilization database [32]. Furthermore, for chronic medications such as antidepressants, it has been shown that misclassification of LMP by a few weeks has little impact on measures of exposure by trimester, as were used in this study [40].
Although we evaluated antidepressant medication use separately in women with and without a recorded diagnosis of depression and evaluated regional differences after adjusting for case-mix variables, depression is under-reported in claims data [41] and no information is available on depression severity, which results in some misclassification of depression status. Exposure status was determined based on filled prescriptions for antidepressant medications. However, filling a prescription does not guarantee that the medication was actually taken as prescribed. In an attempt to address this issue, etiologic studies that aim to assess the association between a given drug exposure and outcome sometimes require one or more refills of a given medication under the assumption that women who refill prescriptions are more likely to actually take them as prescribed. However, since our aim was not to explore a causal relation, but rather to describe patterns of use, we did not impose such a restriction. We considered information on the proportion of women who were prescribed and who filled even a single prescription for antidepressants during pregnancy to be of interest in this context. It has been recommended that 12 data points before and 12 data points after an intervention are needed to conduct interrupted time-series or segmented regression analysis. This number is not based on estimates of power, but has been suggested because it allows the analyst to adequately evaluate seasonal variation [33]. This criterion is met for the paroxetine-related warnings (suicidality and congenital malformations), but not for the subsequent PPHN warning and expansion of the suicidality warning. Nevertheless, there is no indication in the data that an effect would have been observed if longer follow-up after these warnings had been available. Finally, since time-series analyses are ecological analyses correlating the timing of health advisory warnings with temporal trends, causality cannot be assumed. Events coinciding with the warnings (rather than the warnings themselves) could potentially explain the observed trends, although this seems unlikely in this case.
Given the high prevalence of depression in pregnant women, the potential risks of antidepressant medications for the fetus, and the risk of relapse of major depression in untreated patients with serious depression, prescribers need better information on whether and when to discontinue treatment during pregnancy, and which antidepressant to recommend for women of childbearing age if one is required. Adequate information to guide these therapeutic recommendations is currently lacking and likely contributes to the wide unexplained variation in treatment practices. Comparative safety and effectiveness data on different approaches to manage depression in women of childbearing age are needed to help physicians and patients evaluate the potential risks and benefits of these medications for the mother and the developing fetus.
Supplementary Material
01
Acknowledgments
Financial support: This study was supported by Agency for Healthcare Research and Quality (AHRQ) Award R01 HSO18533.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors report no conflict of interest.
References
- 1.Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151(7):979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 2.Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323(7307):257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 4.Dietz P, Williams S, Callaghan W, Bachman D, Whitlock E, Hornbrook M. Clinically identified maternal depression before, during, and after pregnancies ending in live births. American Journal of Psychiatry. 2007;164:1515–1520. doi: 10.1176/appi.ajp.2007.06111893. [DOI] [PubMed] [Google Scholar]
- 5.Holzman C, Eyster J, Tiedje L, Roman L, Seagull E, Rahbar M. A Life Course Perspective on Depressive Symptoms in Mid-Pregnancy. Matern Child Health J. 2006;10(2):127–138. doi: 10.1007/s10995-005-0044-0. [DOI] [PubMed] [Google Scholar]
- 6.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544 e541–544 e545. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194 e191–194 e195. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell A, Gilboa S, Werler M, Kelley K, Louik C, Hernandez-Diaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. American Journal of Obstetrics & Gynecology. 2011;205(1):51.e51–51.e58. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726–735. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- 11.Hirschfeld RM, Keller MB, Panico S, Arons BS, Barlow D, Davidoff F, Endicott J, Froom J, Goldstein M, Gorman JM, et al. The National Depressive and Manic- Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277(4):333–340. [PubMed] [Google Scholar]
- 12.Yonkers K, Wisner K, Stewart D, Oberlander T, Dell D, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31:403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis E, Glynn L, Schetter C, Hobel C, Chics-Demet A, Sandman C. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;(46):737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 14.Leech S, Larkby C, Day R, Day N. Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J Am Acad Child Adolesc Psychiatry. 2006;45:223–230. doi: 10.1097/01.chi.0000184930.18552.4d. [DOI] [PubMed] [Google Scholar]
- 15.Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: a review. Infant Behav Dev. 2006;29:445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413. doi: 10.1016/j.genhosppsych.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart D. Depression during pregnancy. N Engl J Med. 2011;365:1605–1611. doi: 10.1056/NEJMcp1102730. [DOI] [PubMed] [Google Scholar]
- 18.Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. The New England journal of medicine. 2007;356(26):2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- 19.Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356(26):2684–2692. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- 20.Berard A, Ramos E, Rey E, Blais L, St-Andre M, Oraichi D. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol. 2007;80(1):18–27. doi: 10.1002/bdrb.20099. [DOI] [PubMed] [Google Scholar]
- 21.Cole JA, Ephross SA, Cosmatos IS, Walker AM. Paroxetine in the first trimester and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf. 2007;16(10):1075–1085. doi: 10.1002/pds.1463. [DOI] [PubMed] [Google Scholar]
- 22.Wogelius P, Norgaard M, Gislum M, Pedersen L, Munk E, Mortensen PB, Lipworth L, Sorensen HT. Maternal use of selective serotonin reuptake inhibitors and risk of congenital malformations. Epidemiology. 2006;17(6):701–704. doi: 10.1097/01.ede.0000239581.76793.ae. [DOI] [PubMed] [Google Scholar]
- 23.Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. The New England journal of medicine. 2006;354(6):579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 24.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335(14):1010–1015. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 25.Davis RL, Rubanowice D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Platt R. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf. 2007 doi: 10.1002/pds.1462. [DOI] [PubMed] [Google Scholar]
- 26.Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158(4):312–316. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 27.Wen SW, Yang Q, Garner P, Fraser W, Olatunbosun O, Nimrod C, Walker M. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194(4):961–966. doi: 10.1016/j.ajog.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63(8):898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 29.Costei AM, Kozer E, Ho T, Ito S, Koren G. Perinatal outcome following third trimester exposure to paroxetine. Arch Pediatr Adolesc Med. 2002;156(11):1129–1132. doi: 10.1001/archpedi.156.11.1129. [DOI] [PubMed] [Google Scholar]
- 30.Garcia G. Maternal and Child Health (MCH) Update: States Increase Eligibility for Children's Health in 2007. [Accessed March 5, 2012];Electronic citation. Available at: http://www.nga.org/files/live/sites/NGA/files/pdf/0811MCHUPDATE.PDF;jsessionid=7B47A647247DD4E5CB9B709C8F9797AE. [Google Scholar]
- 31.Palmsten K, Huybrechts K, Mogun H, Setoguchi S, Hernandez-Diaz S. Medicaid Analytic eXtract for studies of drug safety during pregnancy. Pharmacoepidemiol & Drug Safety. 2011;20(Supplement 1):S266. [Google Scholar]
- 32.Margulis V, Setoguchi S, Hernandez-Diaz S. A claims-based algorithm to estimate the date of the last menstrual period. Pharmacoepidemiol & Drug Safety. 2010;19(Supplement 1):S300. [Google Scholar]
- 33.Wagner A, Soumerai S, Zhang F, Ross-Degnan D. Segemented regression analysis of interrupted time series studies in medication use research. Journal of Clinical Pharmacy and Therapeutics. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 34.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O'Grady KE, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sclar D, Robison L, Schmidt J, Bowen K, Castillo L, Oganov A. Diagnosis of depression and use of antidepressant pharmacotherapy among adults in the United States: does a disparity persist by ethnicity/race? Clin Drug Investig. 2012;32(2):139–144. doi: 10.2165/11598950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.West S, Savitz D, Koch G, Strom B, Guess H, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. American Journal of Epidemiology. 1995;142(10):1103–1112. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 37.West S, Strom B, Freundlich B, Normand E, Koch G, Savitz D. Completeness of prescription recording in outpatient medical records from a health maintenance organization. Journal of Clinical Epidemiology. 1994;47(2):165–167. doi: 10.1016/0895-4356(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 38.Levy AR, O'Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10(2):67–71. [PubMed] [Google Scholar]
- 39.McKenzie DA, Semradek J, McFarland BH, Mullooly JP, McCamant LE. The validity of medicaid pharmacy claims for estimating drug use among elderly nursing home residents: The Oregon experience. J Clin Epidemiol. 2000;53(12):1248–1257. doi: 10.1016/s0895-4356(00)00259-6. [DOI] [PubMed] [Google Scholar]
- 40.Toh S, Mitchell A, Werler M, Hernandez-Diaz S. Sensitivity and specificity of computerized algorithms to classify gestational periods in the absence of information on date of conception. Am J Epidemiol. 2008;167(6):633–640. doi: 10.1093/aje/kwm367. [DOI] [PubMed] [Google Scholar]
- 41.Noyes K, Liu H, Lyness J, Friedman B. Medicare beneficiaries with depression: comparing diagnoses in claims data with the results of screening. Psychiatr Serv. 2011;62(10):1159–1166. doi: 10.1176/ps.62.10.pss6210_1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01