Influence of Prior Heart Failure Hospitalization on Cardiovascular Events in Patients with Reduced and Preserved Ejection Fraction (original) (raw)
. Author manuscript; available in PMC: 2015 Jul 1.
Abstract
Background
Hospitalization for acute heart failure (HF) is associated with high rates of subsequent mortality and readmission. We assessed the influence of the time interval between prior HF hospitalization and randomization in the CHARM trials on clinical outcomes in patients with both reduced and preserved ejection fraction.
Methods and Results
CHARM enrolled 7,599 patients with NYHA class II-IV heart failure, of whom 5,426 had a history of prior HF hospitalization. Cox proportional hazards regression models were utilized to assess the association between time from prior HF hospitalization and randomization and the primary outcome of cardiovascular death or unplanned admission to hospital for the management of worsening HF over a median of 36.6 months. For patients with HF and reduced (HFrEF) or preserved (HFpEF) ejection fraction, rates of CV mortality and HF hospitalization were higher among patients with prior HF hospitalization than those without. The risk for mortality and hospitalization varied inversely with the time interval between hospitalization and randomization. Rates were higher for HFrEF patients within each category. Event rates for those with HFpEF and a HF hospitalization in the 6 months prior to randomization were comparable to the rate in HFrEF patients with no prior HF hospitalization.
Conclusions
Rates of CV death or HF hospitalization are greatest in those who have been previously hospitalized for HF. Independent of EF, rates of death and readmission decline as time from HF hospitalization to trial enrollment increased. Recent HF hospitalization identifies a high risk population for future clinical trials in HFrEF and HFpEF.
Clinical Trial Registration
URL: http://www.ClinicalTrials.gov. Unique identifier: NCT00634400.
Keywords: heart failure, hospitalization, outcomes
Hospitalization for management of acute decompensated heart failure (HF) is associated with high rates of morbidity, mortality, and re-hospitalization independent of ejection fraction.1,2 Epidemiologic studies and data from clinical trials have shown that the early time period following a hospitalization for HF is a particularly vulnerable interval.3,4,5 While survival rates for all patients with heart failure have improved over the past several decades, the greatest gains have been made in the treatment of patients with heart failure with reduced ejection fraction (HFrEF).6
A better understanding of the impact of hospitalization on the risk of future events is important for several reasons. First, it can help providers target care delivery to those patients at the highest risk of adverse outcomes. Second, it can be leveraged as part of a strategy to inform the design of future clinical trials in heart failure. Future trials, particularly in heart failure with preserved ejection fraction (HFpEF), are needed to further the advances that have been made in reducing HF morbidity and mortality. In addition to the development of new therapies, innovations in the design and implementation of randomized clinical trials, including targeted patient recruitment, will become progressively more important to increase the efficiency of these trials.
We hypothesized that the presence of a recent hospitalization for heart failure can identify a high risk patient population for enrollment in HF trials. We assessed the influence of time between prior HF hospitalization and randomization in the Candesartan in Heart failure: Reduction in Mortality and morbidity (CHARM) program on subsequent mortality and heart failure hospitalization in patients with reduced and preserved ejection fraction.
Methods
Patients
The Candesartan in Heart failure: Reduction in Mortality and morbidity (CHARM) program (ClinicalTrials.gov registration number NCT00634400) consisted of three trials which enrolled 7,599 patients with NYHA class II-IV chronic heart failure and randomized them to candesartan or placebo in addition to standard heart failure therapies. Patients were enrolled in the three trials based upon left ventricular ejection fraction (EF) and treatment with an angiotensin-converting enzyme inhibitor (ACEI). The CHARM trials were approved by an institutional review committee at all sites and all patients gave informed consent for participation in one of the three trials. CHARM-Added enrolled 2,548 patients with LVEF ≤40% who were taking an ACEI at baseline, and had a cardiac hospitalization within 6 months. CHARM-Alternative consisted of 2,028 patients with LVEF ≤40% who were intolerant to ACEI. CHARM-Preserved enrolled 3,023 patients with LVEF>40% (regardless of baseline ACEI use or intolerance), and a prior cardiac hospitalization. Patients were followed for a median of 38 months overall, ranging from 34 months in CHARM-Alternative to 41 months in CHARM-Added.
Ascertainment of time since hospitalization and Outcomes
The time between hospitalization and enrollment was investigator reported. Investigators were asked whether the patient had a documented prior HF hospitalization and the month and year of the most recent prior hospitalization was recorded by the enrolling investigator on the case report form at the baseline visit. The total number of months between prior HF hospitalization and randomization were calculated and used for this analysis. If data was missing on the month of prior hospitalization (n=53) it was imputed as January. If data was missing on the year of prior hospitalization that individual was excluded from this analysis (n=4). Two patients were excluded due to inconsistent dates. The primary outcome was the composite of cardiovascular (CV) death or HF hospitalization in each of the individual trials, and all-cause mortality in the overall CHARM program. All first major CV endpoints including HF hospitalization, myocardial infarction, stroke, and resuscitated sudden death, as well as all deaths were reviewed and adjudicated by an independent clinical events committee. HF hospitalization was defined as the presence of typical symptoms and signs, treatment with an intravenous diuretic, and at least an overnight hospitalization. All deaths were classified as cardiovascular, which was further categorized as secondary to progressive HF, myocardial infarction, sudden death, or other, versus non-cardiovascular. The detailed study design, entry criteria, and main results have all been previously described.7,8,9,10,11
Statistical Analysis
Baseline characteristics were stratified by sub study (Alternative and Added compared to Preserved) and history of HF hospitalization prior to enrollment. Categorical variables were compared using X2 tests and continuous variables were compared using t tests. All tests were 2 sided and a p-value of <0.05 was considered statistically significant. Event rates and 95% confidence intervals (CI) comparing prior HF hospitalization were estimated. Rates were compared using the logrank test stratified by sub study. Patients were grouped by EF (≤ or >40%) and the univariate association between EF and the primary outcome of all-cause mortality, as well as cardiovascular death or unplanned admission to hospital for the management of worsening HF were analyzed in proportional hazards regression models and displayed on a Kaplan-Meier plot according to EF and prior HF hospitalization status. Covariate adjusted hazard ratios were calculated using the baseline predictors identified in previous analyses.12 The 5,421 patients who had complete data on the timing of prior HF hospitalization were further stratified by elapsed time from prior HF hospitalization to randomization and event rates were calculated for the primary outcomes. The trend in event rates across categories of months between HF hospitalization and randomization was compared using Poisson regression. We explored the relationship between a heart failure hospitalization within the trial and subsequent re-hospitalization or death by fitting a Cox model in which participants were considered to be at risk for the event starting at the time of discharge for the first hospitalization. Stata/SE version 12.1 (StataCorp, College Station, TX) was used for all analyses.
Results
Baseline Analyses
Of the 7,599 patients enrolled in the CHARM program, Table 1 lists the baseline characteristics of the 7,593 CHARM patients with complete data (99.9%) included in this analysis broken down by ejection fraction above or below 40% and by history of prior hospitalization for heart failure. 5,421 (71%) had a heart failure hospitalization prior to enrollment in the CHARM program. Overall, those patients with a prior hospitalization were more likely to be female, with more advanced heart failure symptomatology, a longer duration of heart failure, and higher rates of hypertension, diabetes mellitus, and atrial fibrillation. Conversely, they were less likely to have had a prior myocardial infarction. There were no statistically significant differences between groups in rates of smoking, prior stroke, or assignment to candesartan.
Table 1.
Baseline Characteristics
| Overall (n=7,593) | p-value | HFrEF (n=4,572) | p-value | HFpEF (n=3,021) | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| No Prior HF Hospitalization | Prior HF Hospitalization | No Prior HF Hospitalization | Prior HF Hospitalization | No Prior HF Hospitalization | Prior HF Hospitalization | ||||
| N | 2,172 | 5,421 | 1226 | 3,346 | 946 | 2,075 | |||
| Age (y) | 66 (11) | 65 (11) | 0.88 | 65 (10) | 65 (11) | 0.53 | 66 (11) | 67 (11) | 0.29 |
| Female | 634 (29) | 1,764 (33) | 0.004 | 289 (24) | 898(27) | 0.03 | 345 (37) | 866 (42) | 0.001 |
| Current smoker | 322 (15) | 792 (15) | 0.81 | 179 (15) | 526 (16) | 0.35 | 143 (15) | 266 (13) | 0.09 |
| BMI (kg/m2) | 28.4 (5.1) | 28.2 (5.6) | 0.07 | 27.9 (4.7) | 27.6 (5.3) | 0.09 | 29.2 (5.6) | 29.2 (5.9) | 0.98 |
| Candesartan | 1,078(50) | 2,721 (50) | 0.66 | 602 (49) | 1,684 (50) | 0.46 | 476 (50) | 1,037 (50) | 0.86 |
| NYHA class | <0.001 | 0.01 | <0.001 | ||||||
| II | 1,077 (50) | 2,338 (43) | 460 (38) | 1,120 (33) | 617 (65) | 1,218 (59) | |||
| III-IV | 1,095 (50) | 3,083 (57) | 766 (62) | 2,226 (67) | 329 (35) | 857 (41) | |||
| Time since HF dx (y) | 3.1 (3.8) | 3.9 (4.6) | <0.001 | 3.4 (3.9) | 4.1 (4.5) | <0.001 | 2.7 (3.6) | 3.5 (4.6) | <0.001 |
| Hypertension | 1,125 (52) | 3,056 (56) | <0.001 | 550 (45) | 1,689 (51) | 0.001 | 575 (61) | 1,367 (66) | 0.007 |
| Prior MI | 1,315 (61) | 2,684 (50) | <0.001 | 785 (64) | 1,878 (56) | <0.001 | 530 (56) | 808 (39) | <0.001 |
| Diabetes | 483 (22) | 1,677 (31) | <0.001 | 267 (22) | 1,037 (31) | <0.001 | 216 (23) | 640 (31) | <0.001 |
| Prior stroke | 185(9) | 478 (9) | 0.68 | 100 (8) | 295 (9) | 0.48 | 85 (9) | 183 (9) | 0.88 |
| Atrial fibrillation | 434 (20) | 1,649 (30) | <0.001 | 238 (19) | 964 (29) | <0.001 | 196 (21) | 685 (33) | <0.001 |
| Etiology of HF | |||||||||
| ischemic | 1,519 (70) | 3,157 (58) | <0.001 | 853 (70) | 2,119 (63) | <0.001 | 666 (70) | 1,038 (50) | <0.001 |
| idiopathic | 290 (13) | 1,036 (19) | <0.001 | 246 (20) | 817 (24) | 0.002 | 44 (5) | 219 (11) | <0.001 |
| hypertensive | 235 (11) | 746 (14) | 0.001 | 69 (6) | 228 (7) | 0.15 | 166 (18) | 518 (25) | <0.001 |
| HR (bpm) | 71 (13) | 74 (13) | <0.001 | 72 (13) | 74 (13) | <0.001 | 69 (12) | 72 (12) | <0.001 |
| SBP (mmHg) | 131 (19) | 131 (19) | 0.22 | 128 (18) | 127 (19) | 0.048 | 135 (18) | 137 (19) | 0.05 |
| DBP(mmHg) | 77 (10) | 77 (11) | 0.12 | 76 (10) | 76 (11) | 0.14 | 78 (10) | 78 (11) | 0.97 |
Of those patients with a heart failure hospitalization prior to enrollment 3,346 had reduced ejection fraction (LVEF ≤ 40%) and 2,075 had heart failure with preserved ejection fraction (LVEF>40%). Compared to patients with reduced ejection fraction, patients with HFpEF and a prior hospitalization were slightly older, more likely to be female, and be in a lower NYHA class. Patients with HFpEF were also more likely to be hypertensive with higher blood pressures, and have hypertensive, rather than ischemic heart disease, as the etiology of their heart failure.
Heart Failure Hospitalization, Ejection Fraction, and Event Rates
For patients with both HFrEF and HFpEF, event rates for the primary endpoint of time to CV death or hospitalization for heart failure were higher among those with a hospitalization for HF prior to randomization than those without a prior HF hospitalization (Figure 1). The magnitude of increased risk associated with a prior hospitalization was similar regardless of ejection fraction (EF≤40% unadjusted HR 1.74, p<0.001, adjusted HR 1.56, 95% CI 1.38-1.76, p<0.001; EF>40% unadjusted HR 1.81, p<0.001, adjusted HR 1.59, 95% CI 1.32-1.91, p<0.001) and there was no significant interaction between ejection fraction and prior hospitalization (p =0.76).
Figure 1.
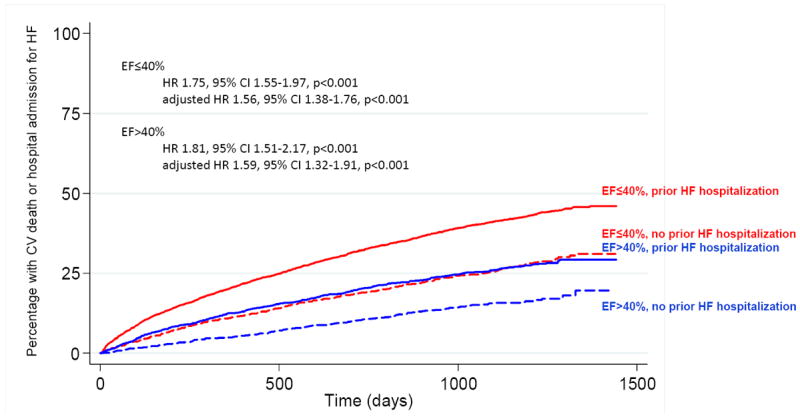
Prior hospitalization for heart failure is associated with increased risk of CV death and HF hospitalization independent of EF.
Among patients with HFpEF, annualized event rates of CV death or heart failure hospitalization for those with a HF hospitalization prior to randomization were comparable to the rate in HFrEF patients with no prior HF hospitalization [10.1 (95% C.I. 9.3-11.0) vs. 10.1 (95% C.I. 9.1-11.2) per 100 patient years] (Table 2). Overall, patients with HFpEF had lower rates of all-cause mortality (unadjusted HR 0.52, p<0.001, adjusted HR 0.86, 95% CI 0.72-1.03, p=0.10) and the composite endpoint of CV death/HF hospitalization (unadjusted HR 0.55, p<0.001, adjusted HR 0.94, 95% CI 0.80-1.09, p=0.39) compared to patients with reduced ejection fraction though the difference did not persist after adjustment for significant covariates. Event rates for HFpEF patients were similar if HFpEF was defined as an EF>50% (Table 3). When the time period between prior hospitalization and enrollment in CHARM was further broken down, patients with the shortest interval between hospitalization and randomization were at the greatest risk of death or hospitalization for heart failure during the trial (Figure 2). Event rates were higher for HFrEF patients within each time period and the trend in event rates was statistically significant for both HFpEF and HFrEF patients.
Table 2.
Event rates stratified by sub-study and prior heart failure hospitalization. Rates are per 100 patient years
| HFrEF (n=4572) | HFpEF (n=3021) | |||
|---|---|---|---|---|
| No prior HF Hospitalization | Prior HF Hospitalization | No prior HF Hospitalization | Prior HF Hospitalization | |
| Complete Study Data* | ||||
| CV death/HF hospitalization | 10.1 (9.1, 11.2) | 17.9 (17.0, 18.8) | 5.5 (4.7, 6.5) | 10.1 (9.3, 11.0) |
| CV death | 6.4 (5.6, 7.2) | 9.7 (9.1, 10.3) | 2.9 (2.3, 3.6) | 4.3 (3.8, 4.8) |
| All-cause mortality | 7.6 (6.7, 8.6) | 11.7 (11.0, 12.4) | 4.0 (3.4, 4.9) | 6.1 (5.5, 6.8) |
| All death or HF hospitalization | 11.3 (10.2, 12.5) | 19.5 (18.5, 20.5) | 6.5 (5.6, 7.5) | 11.5 (10.6, 12.4) |
| HF hospitalization | 5.4 (4.7, 6.2) | 12.3 (11.5, 13.0) | 3.2 (2.6, 4.0) | 7.9 (7.2, 8.7) |
| HF death | 1.2 (0.9, 1.7) | 3.5 (3.1, 3.9) | 0.6 (0.4, 1.0) | 1.4 (1.1, 1.7) |
| Second HF hospitalization or death after a HF hospitalization (n=1525) | 45.0 (36.5, 55.5) | 78.7 (72.7, 85.1) | 29.1 (19.7, 43.1) | 60.9 (53.8, 69.0) |
Table 3.
Event rates in HFpEF patients with EF>50%. Rates are per 100 patient years
| HFpEF (EF >50%) (n=1,949) | ||
|---|---|---|
| No prior HF Hospitalization (n=642) | Prior HF Hospitalization (n=1,307) | |
| CV death/HF hospitalization | 5.4 (4.4, 6.6) | 10.8 (9.7, 11.9) |
| CV death | 2.7 (2.0, 3.5) | 4.3 (3.7, 5.0) |
| All-cause mortality | 4.2 (3.4, 5.2) | 6.5 (5.7, 7.3) |
| All death or HF hospitalization | 6.7 (5.6, 8.0) | 12.3 (11.2, 13.6) |
| HF hospitalization | 3.1 (2.4, 4.0) | 8.5 (7.6, 9.5) |
| HF death | 0.6 (0.3, 1.0) | 1.4 (1.1, 1.8) |
| Second HF hospitalization or death after a HF hospitalization (n=311) | 21.1 (12.3, 36.4) | 61.4 (52.7, 71.6) |
Figure 2.
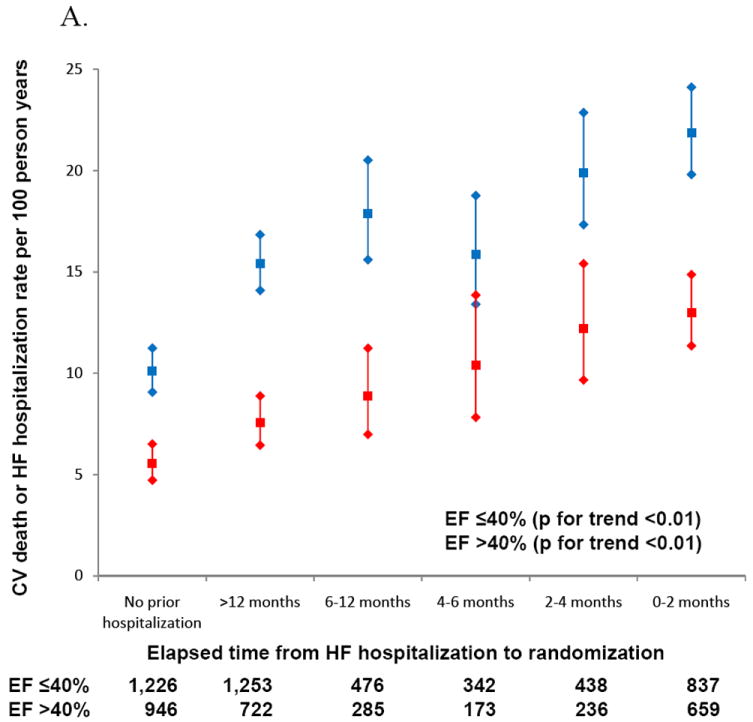
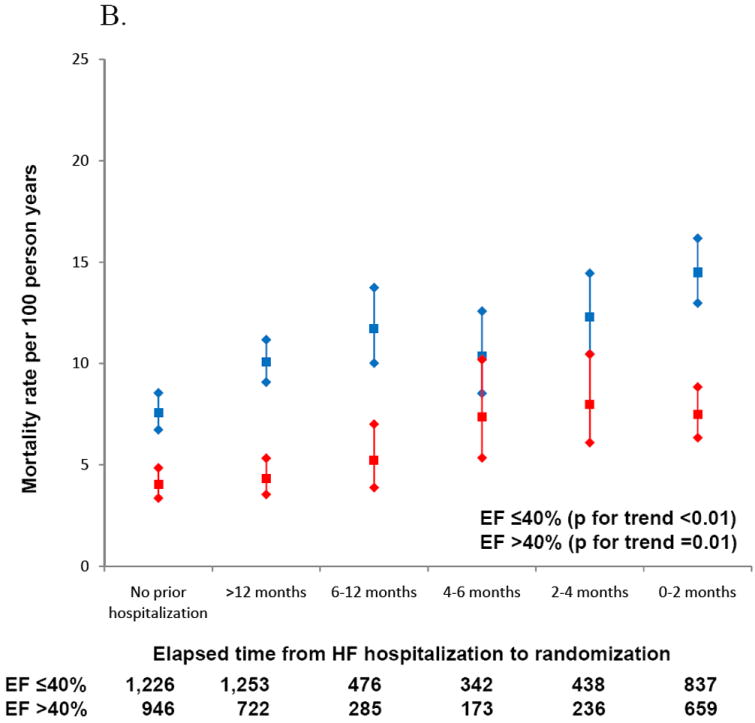
A. Increasing rates of CV death and HF hospitalization with more recent hospitalization prior to enrollment.
B. Mortality rates increase as time between prior hospitalization and enrollment decreases.
Within all four subgroups, annualized event rates for a second heart failure hospitalization or death following a hospitalization for HF within CHARM were markedly elevated compared to the rates of a first HF hospitalization or death (Table 2). Following a HF hospitalization within CHARM, those patients who were hospitalized for heart failure prior to enrollment continued to display an elevated risk for a second event (HF hospitalization or death) during the trial compared to those who were not hospitalized prior to enrollment (Figure 3). Although patients with HFpEF continued to have lower rates of a second hospitalization for heart failure or death than those with HFrEF overall (HR 0.79, 95% CI 0.68-0.90), patients with HFpEF and a prior hospitalization for HF had higher second event rates following an in-trial hospitalization for heart failure than those with a reduced ejection fraction who only experienced a HF hospitalization during the trial (HR 0.76, 95% CI 0.59-0.97, p=0.027),.
Figure 3.
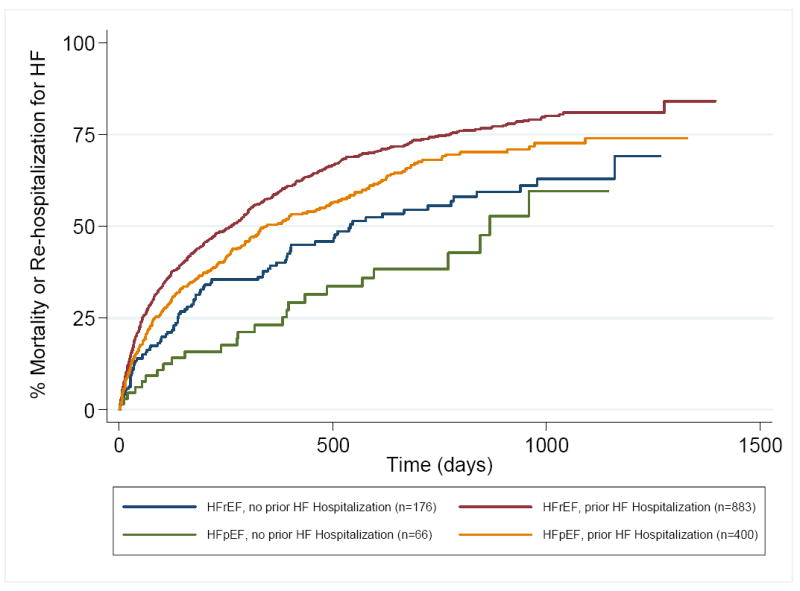
Time to death or second heart failure hospitalization following an in-trial heart failure hospitalization in CHARM in patients stratified by ejection fraction and presence or absence of a HF hospitalization prior to enrollment.
Discussion
The CHARM program enrolled patients with symptomatic heart failure across a broad spectrum of ejection fractions. The majority of patients in CHARM (71%) experienced a hospitalization for heart failure prior to enrollment and the current analysis demonstrates that this history of an acute heart failure event is a powerful predictor of adverse cardiovascular outcomes for patients with both reduced and preserved ejection fractions during the trial. Moreover, the time between the last HF hospitalization and enrollment was itself a powerful predictor of subsequent event rates. These findings have implications for the design of future clinical trials.
Previously published findings from acute and chronic heart failure populations have reported varied estimates of subsequent mortality and re-hospitalization rates for patients with HFpEF and HFrEF.13,14,15,16,17,18,19,20,21,22,23,24,25 Some studies have shown heart failure patients face a similar magnitude of risk regardless of ejection fraction13,14,19, while others have demonstrated lower event rates in patients with HFpEF compared to HFrEF.16,17,18,22,23,24 This disparate finding regarding the effect of ejection fraction on event rates is also seen when the studies are grouped into acute heart failure,13,14,15,16,17 versus chronic, prevalent heart failure.18,19,20,23The admixture of different combinations of patients into single studies is likely responsible for some of the published disparities in risk.
The current analysis which stratified CHARM patients by ejection fraction and the presence or absence of a recent hospitalization for heart failure prior to enrollment, provides clarification of the prior discrepant findings. Overall, and for both HFpEF and HFrEF patients, we observed an inverse relationship between time from HF hospitalization and randomization into the trial and event rates within the trials. Additionally, although the magnitude of increased hazard seen with a prior HF hospitalization was similar regardless of ejection fraction, patients with low EF had consistently higher event rates than those with HFpEF.
Event rates in cardiovascular clinical trials continue to decline over time as new interventions that have been tested and proven efficacious in prior trials are applied to clinical care.26 As a result, increasing numbers of patients are needed to show a difference between treatment groups because the net number of clinical events, rather than the number of patients enrolled in a trial, ultimately determines a trial’s overall power to detect a significant difference.26,27 Historically, the development of the composite endpoint was one of the first ways trial design was adapted to leverage this knowledge.28 Further innovations in the design and implementation of clinical trials, including targeted patient recruitment, can increase the efficiency of heart failure trials. The present data demonstrate that event rates for patients with HFpEF and a recent HF hospitalization approximate those of patients with HFrEF and no hospitalization prior to enrollment. This relationship persisted when HFpEF is defined more rigorously as clinical heart failure with an ejection fraction >50%. A history of recent hospitalization for heart failure therefore provides a means to identify a high risk patient population. While mitigating risk in a high risk population may not be the goal of every trial, we believe this strategy can inform enrollment strategies for some future clinical trials. It is important to design trials with a recruitment strategy that most effectively identifies the target population, for example if a therapy is designed to work in lower risk patients a recent hospitalization for HF is unlikely to identify those individuals.
The current analysis also reinforces the concept that a heart failure hospitalization is a sentinel event that can be used to identify patients who are at an increased risk for additional events. Patients who were hospitalized for heart failure prior to the trial were more likely to have recurrent hospitalizations or die during the trial, regardless of ejection fraction. During the course of a heart failure trial such as CHARM, many patients experience multiple events that are positively adjudicated by the clinical events committee. A prior analysis of CHARM demonstrated that the risk of death is directly related to the duration and frequency of HF hospitalization.1 The current analysis demonstrates that this principle holds true in patients with reduced and preserved ejection fractions and extends the period of elevated risk to include hospitalizations which occurred prior to the trial start date. Since this analysis includes only patients who survived their prior hospitalization and lived to be randomized in the CHARM program, the increased risk associated with a prior hospitalization is likely underestimated since the sickest patients likely died during or shortly after hospitalization before they could be randomized. In general, current methods of survival analysis are based on a time to first event model and do not take into account these other events which are important to both patients and providers. Future strategies in trial design such as time to multiple events may also be a novel way to utilize morel of the data collected during a trial and provide composite endpoints that are easy to interpret.29,30
Some limitations of this analysis should be noted. The heart failure hospitalizations prior to randomization in CHARM were not formally adjudicated events. It is possible that some of those events would not meet the standards of a clinical endpoints committee; however non-differential misclassification of HF hospitalization would dilute our findings and have biased the results towards the null. However, event rates at 24 months in CHARM patients who fit enrollment criteria for EVEREST,31 a trial of acute heart failure, are very similar; all-cause mortality (EVEREST 26% vs. CHARM 28%) and CV death or HF hospitalization (EVEREST 41% vs. CHARM 41%). The ejection fractions in CHARM were site reported using several techniques, and not verified by a core laboratory. Any noise created by the different techniques or imprecise measurement should be diminished In light of the large number of events. Lastly, as with other clinical trials, these findings may not be generalizable to the general population of heart failure patients.
In summary, rates of CV death or HF hospitalization are greatest in those with a prior heart failure hospitalization and decline with time between hospitalization and randomization across a broad spectrum of ejection fraction. The use of a history of prior recent heart failure hospitalization as an inclusion criterion for future clinical trials can increase trial efficiency. Acute exacerbations of heart failure requiring hospitalization signify a vulnerable time period in a patient’s life and should trigger intensified management and heightened surveillance for additional events.
Acknowledgments
Sources of Funding
Dr. Bello reports receiving grant support from NIH/NHBLI grantT32 HL007374-34. The CHARM program was funded by AstraZeneca.
Drs, McMurray, Granger, Yusuf, Swedberg, Pfeffer and Solomon have received research funding from AstraZeneca.
Footnotes
Disclosures
Drs. Desai, Claggett, and Bello report no conflicts.
References
- 1.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of Heart Failure with Preserved Ejection Fraction in a Population-Based Study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 3.Cowie MR, Mosterd A, Wood DA, Deckers JW, Poole-Wilson PA, Sutton GC, Sutton GC, Grobbee DE. The epidemiology of heart failure. Eur Heart J. 1997;18:208–25. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- 4.Krumholz HM, Chen J, Murillo JE, Cohen DJ, Radford MJ. Admission to hospitals with onsite cardiac catheterization facilities :impact on long-term costs and outcomes. Circulation. 1998;98:2010–6. doi: 10.1161/01.cir.98.19.2010. [DOI] [PubMed] [Google Scholar]
- 5.Roguin A, Behar D, Ben Ami H, Reisner SA, Edelstein S, Linn S, Edoute Y. Long-term prognosis of acute pulmonary oedema--an ominous outcome. Eur J Heart Fail. 2000;2:137–44. doi: 10.1016/s1388-9842(00)00069-6. [DOI] [PubMed] [Google Scholar]
- 6.Gurwitz JH, Magid DJ, Smith DH, Goldberg RJ, McManus DD, Allen LA. Contemporary Prevalence and Correlates of Incident Heart Failure with Preserved Ejection Fraction. Am J Med. 2013;14:393–400. doi: 10.1016/j.amjmed.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swedberg K, Pfeffer M, Granger C, Held P, McMurray J, Ohlin G, Olofsson B, Ostergren J, Yusuf S. Candesartan in heart failure--assessment of reduction in mortality and morbidity (CHARM): rationale and design. Charm-Programme Investigators. J Card Fail. 1999;5:276–82. doi: 10.1016/s1071-9164(99)90013-1. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–66. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 9.Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–6. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 10.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–71. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 12.Pocock S, Wang D, Pfeffer MA, Yusuf S, McMurray JJV, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 13.Berry C, Hogg K, Norrie J, Stevenson K, Brett M, McMurray J. Heart failure with preserved left ventricular systolic function: a hospital cohort study. Heart. 2005;91:907–13. doi: 10.1136/hrt.2004.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, Treatments, and Outcomes of Patients With Preserved Systolic Function Hospitalized for Heart Failure: A Report From the OPTIMIZE-HF Registry. Journal of the American College of Cardiology. 2007;50:768–77. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 15.Kirk V, Bay M, Parner J, Krogsgaard K, Herzog TM, Boesgaard S, Hassager C, Nielsen OW, Aldershvile J, Nielsen H. N-terminal proBNP and mortality in hospitalised patients with heart failure and preserved vs. reduced systolic function: data from the prospective Copenhagen Hospital Heart Failure Study (CHHF) Eur J Heart Fail. 2004;6:335–41. doi: 10.1016/j.ejheart.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Varadarajan P, Pai RG. Prognosis of congestive heart failure in patients with normal versus reduced ejection fractions: results from a cohort of 2,258 hospitalized patients. J Card Fail. 2003;9:107–12. doi: 10.1054/jcaf.2003.13. [DOI] [PubMed] [Google Scholar]
- 17.Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J, Komajda M. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J. 2004;25:1214–20. doi: 10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.McCullough PA, Khandelwal AK, McKinnon JE, Shenkman HJ, Pampati V, Nori D, Sullivan RA, Sandberg KR, Kaatz S. Outcomes and prognostic factors of systolic as compared with diastolic heart failure in urban America. Congest Heart Fail. 2005;11:6–11. doi: 10.1111/j.1527-5299.2005.03731.x. [DOI] [PubMed] [Google Scholar]
- 19.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 20.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KKL, Murabito JM, Vasan RS. Long-Term Trends in the Incidence of and Survival with Heart Failure. N Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 21.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 22.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 23.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–9. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–42. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 25.Gotsman I, Zwas D, Lotan C, Keren A. Heart failure and preserved left ventricular function: long term clinical outcome. PLoS One. 2012;7:19. doi: 10.1371/journal.pone.0041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antman EM. Clinical Trials in Cardiovascular Medicine. Circulation. 2001;103:e101–e4. doi: 10.1161/01.cir.103.21.e101. [DOI] [PubMed] [Google Scholar]
- 27.Collins R, MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet. 2001;357:373–80. doi: 10.1016/S0140-6736(00)03651-5. [DOI] [PubMed] [Google Scholar]
- 28.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–68. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 29.Murphy SA, Cannon CP, Wiviott SD, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid-lowering statin therapy compared with moderate lipid-lowering statin therapy after acute coronary syndromes from the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol. 2009;54:2358–62. doi: 10.1016/j.jacc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Tikkanen MJ, Szarek M, Fayyad R, Holme I, Cater NB, Faergeman O, Kastelein JJ, Olsson AG, Larsen ML, Lindahl C, Pedersen TR. Total cardiovascular disease burden: comparing intensive with moderate statin therapy insights from the IDEAL (Incremental Decrease in End Points Through Aggressive Lipid Lowering) trial. J Am Coll Cardiol. 2009;54:2353–7. doi: 10.1016/j.jacc.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Konstam MA, Gheorghiade M, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannand F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]