Muscle Ring Finger 1, but not Muscle Ring Finger 2, Regulates Cardiac Hypertrophy In Vivo (original) (raw)
. Author manuscript; available in PMC: 2014 Jul 27.
Abstract
Muscle ring finger (MuRF) proteins have been implicated in transmitting mechanical forces to cell signaling pathways through their interactions with the giant protein titin. Recent evidence has linked mechanically-induced stimuli with the control of serum response factor activity and localization through MuRF2. This observation is particularly intriguing in the context of cardiac hypertrophy, where serum response factor transactivation is a key event necessary for the induction of cardiac hypertrophy in response to increased afterload. We have previously reported that MuRF1, which is also a titin-associated protein, exerts antihypertrophic activity in vitro. In the present study, we induced cardiac hypertrophy in mice lacking MuRF1 and MuRF2 to distinguish the physiologic role of these divergent proteins in vivo. We identified for the first time that MuRF1, but not MuRF2, plays a key role in regulating the induction of cardiac hypertrophy, likely by its direct interactions with serum response factor. These studies describe for the first time distinct and nonoverlapping functional characteristics of MuRF1 and MuRF2 in response to cardiac stress in vivo.
Keywords: adenosine receptors, heat shock proteins, proteasome, serum response factor, ubiquitin
The muscle ring finger (MuRF) proteins are striated muscle-specific proteins that have been implicated in various aspects of contractile regulation and myogenic responses.1 Whereas MuRF3 is primarily microtubule-associated, both MuRF1 and MuRF2 are associated with the giant sarcomeric protein titin. Titin spans half sarcomeres and is ideally positioned to sense mechanical loading. It has been speculated that MuRF2 participates in a circuit that links mechanical force generation to transcriptional responses in cardiac myocytes by modulating the activity and localization of the transcription factor serum response factor (SRF) in response to changes in mechanical stimuli.2 In this model, titin undergoes conformational changes in response to stretch, allowing its titin kinase domain to interact with scaffolding proteins (nbr1 and p62), which in turn interact with MuRF2.2 MuRF2 subsequently associates directly with the transactivation domain of SRF, and is able to inhibit its nuclear localization and transcriptional activity.2 If the model whereby MuRF2 links titin dynamics with SRF activity is correct, then MuRF2 should play a necessary role in suppressing hypertrophic responses elicited by mechanical forces.
MuRF1 also associates with titin, although its regulation by mechanical stress has not been directly tested. Instead, MuRF1 is a well-characterized RING-finger-dependent ubiquitin ligase that is active toward the sarcomeric protein troponin I.3 In addition, MuRF1 inhibits PKC[H9255] activity through interactions with RACK1, the receptor for activated protein kinase C protein, which in turn suppresses focal adhesion kinase and ERK1/2 in cardiomyocytes.4 The inhibitory activity of MuRF1 in the setting of cardiomyocyte hypertrophy has been demonstrated in cultured cells, but cardiac phenotypes of mice deficient in MuRF1 have not been tested.4 Similarly, the role for MuRF2 as a requisite transducer of mechanical stress has never been directly tested in vivo. In the present report, we have induced cardiac hypertrophy in mice lacking MuRF1 or MuRF2 to determine the physiological role of these proteins in vivo.
Materials and Methods
Animals
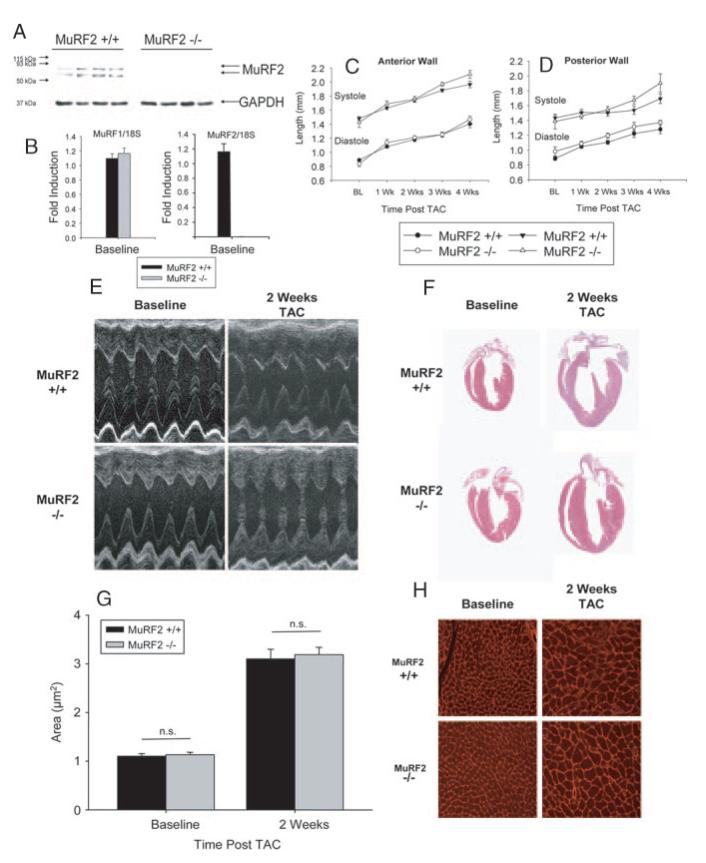
MuRF1−/− mice have been previously described and the absence of MuRF1 protein expression in MuRF1−/− mice has previously been demonstrated.5 The MuRF2−/− mice have a 15-Kb deletion from the ATG to 1 kb downstream of exon 3 replaced with a LacZ cassette inserted in its place. Immunoblot analysis of MuRF2 in cardiac tissue from MuRF2−/− mice and their wild-type (WT) controls demonstrate that MuRF2−/− mice do not express the 2 MuRF2 isoforms (see Figure 1A and 1B). MuRF2−/− mice are born in Mendelian ratios from heterozygous crosses. They have no obvious developmental defects and are not detectably different from WT littermates in size, activity, or longevity (data not shown). Mice are on a 129S/C57Bl6 background, and all experiments used sex-matched WT littermates as controls.
Figure 1.
Mice lacking MuRF2 have a cardiac hypertrophic response comparable to WT mice. MuRF2 protein is expressed in MuRF2+/+ (A) but not MuRF2−/− mice by immunoblot (N=4/ group). Hearts from MuRF2+/+ and MuRF2−/− mice (B) at baseline express the same amount of MuRF1 message by Real Time PCR (N=3/group). Mice lacking MuRF2 (MuRF2−/−) increase anterior (C) and posterior (D) wall thickness to the same extent as WT littermate controls (MuRF2+/+) in response to pressure overload. M-mode representations of MuRF2−/− and MuRF2+/+ (E) parallel increases in wall thickness by histology (F) and increases in individual cardiomyocytes area (G and H). Echocardiography results represent at least 10 mice per group. *P<0.05.
Experimental Design
The transaortic constriction (TAC) model of cardiac hypertrophy induction was performed as previously described.6,7 M-mode and 2-dimensional imaging was performed using a Vevo 660 ultrasound biomicroscopy system as previously described (VisualSonics, Inc, Toronto, Ontario, Canada).8,9 All LV dimension data are presented as the average at least 3 independent waveforms, in at least 8 independent mice at each time point. Left ventricular mass index was determined by the M-mode (cubed) method. For histology, hearts were perfused and fixed with freshly made 4% paraformaldehyde. Samples were embedded in paraffin using standard methods, cut in 5 μm sections, and stained with H&E or Masson’s Trichrome. For lectin staining, paraformaldehyde-fixed cardiac tissue was deparaffinized, hydrated, and incubated with Triticum vulgaris lectin TRITC conjugate. Sections were subsequently examined by fluorescence microscopy. For Western blot analysis, PVDF membranes were immunoblotted with goat anti-MURF2 (ab4387, AbCam Inc, Cambridge, Mass).
Statistical Analysis
Statistical analysis (Student’s t test) was performed using Sigma Stat 2.03 (Systat Software, Inc, San Jose, Calif) and Microsoft Excel 2003 (Microsoft, Seattle, Wash). Statistical significance for all analyses was defined as _P_≤0.05.
Results
At baseline, no structural or functional deficits were identified in either MuRF2−/− or MuRF1−/− mice compared with littermate control WT mice for at least the first 6 months of age (data not shown). After TAC, echocardiographic analysis indicated that anterior and posterior heart wall thickness of hearts from MuRF2+/+ mice increased progressively for up to 4 weeks of banding (Figure 1C and 1D). Surprisingly, these indices in MuRF2−/− hearts were nearly identical and statistically not different over the same time course. Neither were echocardiographic differences observed in fractional shortening, LV mass index, and relative wall thickness (data not shown). In addition, differences in hypertrophic response to TAC in MuRF2−/− were not identified by measurements of cardiomyocyte area or gross histological evaluation (Figure 1F-1H). Surprisingly, and in contrast to predictions from in vitro studies,2 these experiments indicate that MuRF2 is dispensable for normal cardiac response to mechanical stress.
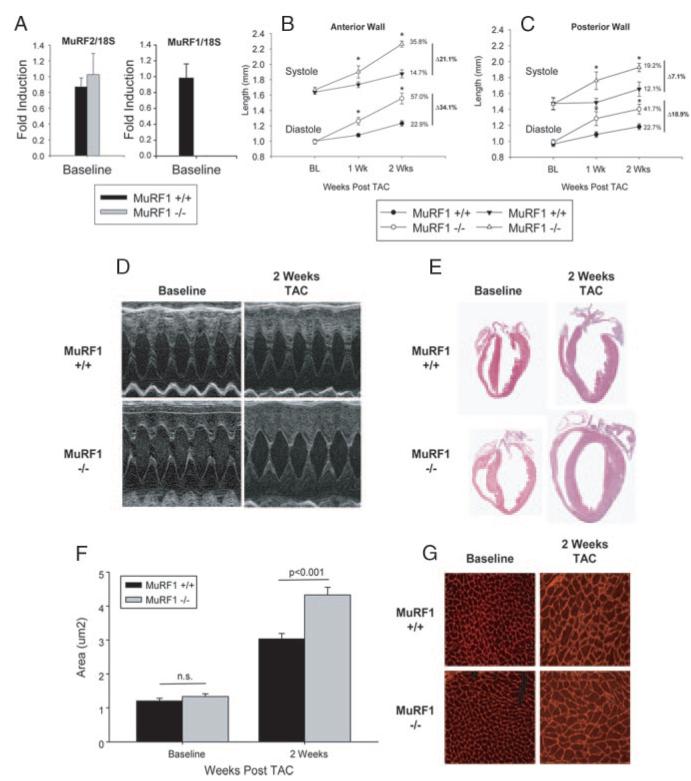
In contrast to the MuRF2−/− mice, MuRF1−/− mice exhibited a striking accentuation of the hypertrophic response after TAC. Although baseline measurements of both anterior and posterior wall thicknesses were not significantly different in hearts of MuRF1−/− mice compared with WT littermates, these dimensions were markedly increased in MuRF1−/− mice during both systole and diastole within the first week after TAC (Figure 2B and 2C). Two weeks after TAC, this difference was even greater; anterior wall thickness in systole and diastole was increased in MuRF1+/+ mice by 14.7% and 22.9% relative to baseline, respectively, whereas systolic and diastolic wall thickness in MuRF1−/− mice increased by 35.8% and 57.0%, respectively (P<0.001). The posterior wall thicknesses in MuRF1−/− mice followed similar trends. The accelerated growth in MuRF1−/− mice continued for up to 4 weeks after banding, without decompensation as determined by echocardiography (data not shown). Left ventricular mass index and heart weight/body weights were significantly greater in MuRF1−/− mice compared with MuRF1+/+ mice (Table), and gross cardiac examination revealed markedly increased heart size after TAC (Figure 2E). Similarly, enhanced myocyte hypertrophy in MuRF1−/− hearts after TAC was detected in measurements of cardiomyocyte area (Figure 2F and 2G).
Figure 2.
Mice lacking MuRF1 have an exaggerated cardiac hypertrophic response to pressure overload compared with WT mice. Hearts from MuRF1+/+ and MuRF1−/− mice at baseline express the same amount of MuRF2 message by Real Time PCR (A). Anterior (B) and posterior (C) wall thickness increases in response TAC. MuRF1−/− undergo an exaggerated hypertrophy as demonstrated by echocardiography (D), histology (E), and individual cardiomyocyte area (F) calculated from fluorescence microscopy (G). Echocardiography studies represent at least 8 mice per group. *P<0.05
Echocardiography of Dimensions and Function in MuRF1+/+ and MuRF1−/− Mice at Baseline and 2 Weeks After TAC (±SE).
| MuRF1+/+ | MuRF1−/− | MuRF1+/+ | MuRF1−/− | MuRF1+/+ | MuRF1−/− | |
|---|---|---|---|---|---|---|
| Baseline | Baseline | 1 Weeks | 1 Weeks | 2 Weeks | 2 Weeks | |
| (n=8) | (n=8) | (n=8) | (n=8) | (n=8) | (n=8) | |
| BW, g | 22.4±2.6 | 24.6±3.6 | 21.5±2.4 | 24.3±2.3 | 22±3.4 | 25.3±2.7 |
| LV mass index, mg | 86.7±5.1 | 102±16 | 91.6±5.1 | 150.1±11.5 | 108.1±6.9 | *180.3±17.8 |
| Relative Wall | 0.32±0.02/ | 0.31 ±0.02/ | 0.39±0.02/ | 0.41 ±0.02/ | 0.45±0.02/ | 0.47±0.02 |
| Thickness (Diastole/Systole) | 1.01 ±0.09 | 0.92±0.10 | 1.20±0.06 | 1.00±0.06 | 1.36±0.13 | 1.41±0.15 |
| HR, bpm | 613.3±21.5 | 608.9±16.9 | 625.4±17.3 | 622.7±20.2 | 651.8±10.0 | 603.7±27.4 |
| LVEDD, mm | 3.1±0.1 | 3.4±0.1 | 2.7±0.1 | 3.3±0.1 | 2.7±0.2 | 3.1±0.1 |
| LVESD, mm | 1.5±0.1 | 1.7±0.1 | 1.3±0.1 | 1 + o | 1.3±0.1 | 1.4±0.2 |
| FS, % | 50.9±1.8 | 50.5±2.1 | 51.9±2.0 | 46.4±3.6 | 52.9±1.7 | 52.4±3.0 |
Discussion
In the present study, we tested the hypothesis that a recently described mechanotransduction signaling pathway mediated through MuRF2’s direct effects on SRF activity and localization2 participates in regulating the magnitude of cardiac hypertrophy after TAC. Using mice deficient in MuRF2, we demonstrate that MuRF2 is dispensable for appropriate hypertrophic responses to hemodynamic stress in vivo. In contrast, we found that its paralog MuRF1 is required for an appropriate response to mechanical stress and that, in the absence of MuRF1, the cardiac hypertrophic response is exaggerated. These observations discount a major physiological role for MuRF2 in mechanically induced cardiac hypertrophy, and emphasize the importance of MuRF1 as a requisite negative regulator of cardiac hypertrophy in response to mechanical stress in vivo.
MuRF1 was first identified as a muscle-specific protein that mediates skeletal muscle atrophy.5 We subsequently demonstrated that MuRF1 has anti-hypertrophic activity in cardiomyocytes in vitro.4 In these studies, increasing MuRF1 expression inhibited the induction of cardiac hypertrophy induced by agonists that signal through G-coupled proteins such as phenylephrine, angiotensin II, and endothelin-1.4 We extend these findings by demonstrating that MuRF1 plays an endogenous role in regulation of cardiac hypertrophy in vivo, although it appears to be dispensable for normal cardiac development and physiologic function for at least the first 6 months of age.
The mechanisms through which MuRF1 participates in the effects observed in these studies remain to be determined. The exaggerated hypertrophic response in MuRF1−/− mice in our studies is accompanied by enhanced expression of selected SRF-dependent genes (ie, βMHC, smooth muscle actin, BNP), and we have found that MuRF1 directly interacts with SRF and inhibits its activity in Cos7 cells (supplemental Figure I in the online data supplement at http://circres.ahajournals.org). These observations may account in part for enhanced cardiac hypertrophy after mechanical stress observed in our studies, and suggest that MuRF1, rather than MuRF2, is the physiologic regulator of SRF in response to mechanical stress in the heart in vivo. In addition, MuRF1 may also participate in negatively regulating cardiac hypertrophy through its interactions with PKC[H9255].4 MuRF1 may also participate in regulation of sarcomere integrity through degradation of specific proteins such as troponin I.3 In any event, these studies demonstrate for the first time that MuRF1, but not MuRF2, is required for an appropriate response to mechanical stress in the development of cardiac hypertrophy in vivo.
Supplementary Material
Supplemental data
Acknowledgments
The authors wish to acknowledge the assistance of the Janice Weaver for assistance in preparing histological specimens.
Sources of Funding
This work was supported by NHLBI R01HL065619 (to CP) and support from the UNC Research Council.
Footnotes
References
- 1.Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson LG, Hughes SM, Marchand S, Sejersen T, Richard I, Edstrom L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308:1599–1603. doi: 10.1126/science.1110463. [DOI] [PubMed] [Google Scholar]
- 3.Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci U S A. 2004;101:18135–18140. doi: 10.1073/pnas.0404341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arya R, Kedar V, Hwang JR, McDonough H, Li HH, Taylor J, Patterson C. Muscle ring finger protein-1 inhibits PKCε activation and prevents cardiomyocyte hypertrophy. J Cell Biol. 2004;167:1147–1159. doi: 10.1083/jcb.200402033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 6.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 7.Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest. 2004;114:1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics. 2003;13:227–239. doi: 10.1152/physiolgenomics.00005.2003. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Xu Z, He XR, Michael LH, Patterson C. CHIP, a cochaperone/ ubiquitin ligase that regulates protein quality control, is required for maximal cardioprotection after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2005;288:H2836–H2842. doi: 10.1152/ajpheart.01122.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data