Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 5.
SUMMARY
Beige fat, which expresses the thermogenic protein UCP1, provides a defense against cold and obesity. Although a cold environment is the physiologic stimulus for inducing beige fat in mice and humans, the events that lead from the sensing of cold to the development of beige fat remain poorly understood. Here, we identify the efferent beige fat thermogenic circuit, consisting of eosinophils, type 2 cytokines interleukin (IL)-4/13 and alternatively activated macrophages. Genetic loss of eosinophils or IL-4/13 signaling impairs cold-induced biogenesis of beige fat. Mechanistically, macrophages recruited to cold-stressed subcutaneous white adipose tissue (scWAT) undergo alternative activation to induce tyrosine hydroxylase expression and catecholamine production, factors required for browning of scWAT. Conversely, administration of IL-4 to thermoneutral mice increases beige fat mass and thermogenic capacity to ameliorate pre-established obesity. Together, our findings have uncovered the efferent circuit controlling biogenesis of beige fat and provide support for its targeting to treat obesity.
INTRODUCTION
Obesity, which affects 1.4 billion adults globally, represents the greatest current threat to human health (Finucane et al., 2011). Chronic imbalance between energy intake and energy expenditure causes obesity for which there is no effective therapy (Harms and Seale, 2013; Lowell and Spiegelman, 2000). Thus, a major challenge for biomedical sciences is to identify targetable pathways that can decrease energy intake or increase energy expenditure. One of the most promising targets for treatment of human obesity is brown adipose tissue (BAT) (Enerback, 2010; Harms and Seale, 2013), but adult humans lack this thermogenic interscapular organ (Lidell et al., 2013). However, recent studies have demonstrated that adult humans harbor a separate depot of brown adipocytes that are cold inducible and interspersed amongst white adipocytes in the supraclavicular, para-aortic, and suprarenal regions (Cypess et al., 2009; Saito et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). Since these human brown adipocytes share some molecular, histologic, and functional characteristics with cold-inducible beige adipocytes found in the subcutaneous white adipose tissue (scWAT) of mice (Cypess et al., 2013; Liu et al., 2013; Sharp et al., 2012; Wu et al., 2012; Wu et al., 2013), there is great clinical interest in the therapeutic targeting of beige fat for the treatment of obesity (Enerback, 2010; Harms and Seale, 2013). However, our lack of understanding of how cold triggers the development of functional beige fat is a major barrier for its therapeutic translation.
Uncoupling protein-1 (UCP1), which dissipates the mitochondrial electrochemical gradient to stimulate cellular respiration, mediates the thermogenic activity of both brown and beige adipocytes (Cannon and Nedergaard, 2010, 2011; Feldmann et al., 2009). Despite this similarity in thermogenesis, multiple lines of evidence indicate that brown and beige adipocytes have unique expression profiles that likely contribute to their tissue-specific functions (Harms and Seale, 2013). First, unlike interscapular brown adipocytes that arise from Myf5+/Pax7+ myogenic precursors (Lepper and Fan, 2010; Seale et al., 2008; Timmons et al., 2007), beige adipocytes residing in the scWAT of mice do not have a history of Myf5+ expression (Seale et al., 2011). Second, brown adipocytes constitutively express Ucp1 after differentiation, whereas beige adipocytes specifically increase expression of thermogenic genes, such as Ucp1, in response to environmental cold, and agonists of the β-adrenergic receptor or peroxisome proliferator-activated receptor-γ (Ppar-γ) (Liu et al., 2013; Ohno et al., 2012; Wu et al., 2012). Third, a number of genes, such as Klhl13, Ear2, Tbx1, Tmem26, and CD137, are preferentially expressed in beige adipocyte precursors (Liu et al., 2013; Sharp et al., 2012; Wu et al., 2012). Together, these findings suggest that beige and brown adipocytes are likely to have complementary functions in the maintenance of energy balance and thermogenesis; however, rigorous proof for the therapeutic efficacy of beige fat in the treatment of obesity is lacking.
In the textbook view of thermogenesis, the sensing of cold by the neuronal system triggers the sympathetic efferents that promote the biogenesis and activation of beige fat (Cannon and Nedergaard, 2011; Lowell and Spiegelman, 2000). While this model works well for tissues that are densely innervated by the sympathetic nerves (Morrison and Nakamura, 2011), such as the interscapular BAT, it does not explain how cold exposure results in the rapid remodeling of the poorly innervated scWAT (Daniel and Derry, 1969; Slavin and Ballard, 1978; Trayhurn and Ashwell, 1987). In these classic studies, adrenergic nerves only innervated 2-3% of all adipocytes in WAT, leading these authors to conclude that the sympathetic nerves primarily innervate blood vessels of the WAT (Daniel and Derry, 1969; Slavin and Ballard, 1978). These older observations thus suggest that the adrenergic tone of WAT must somehow be amplified during cold stress to stimulate biogenesis of beige fat. In this regard, we recently reported that acute cold stress results in type 2 (alternative) activation of macrophages in WAT, which locally secrete catecholamines, to sustain the metabolic adaptations to environmental cold (Nguyen et al., 2011). These findings, which provide a potential explanation for this remodeling conundrum, led us to investigate the functions of type 2 cytokines, IL-4 producing cells, and alternatively activated macrophages in the biogenesis of beige fat.
Here, we report that the cold-induced remodeling of scWAT into thermogenic beige fat is dependent on eosinophils, type 2 cytokines, macrophages, and myeloid cell-derived catecholamines. Genetic disruption of IL-4/13 signaling or tyrosine hydroxylase (Th), the rate limiting step in the synthesis of catecholamines, in myeloid cells prevents cold-induced biogenesis of beige fat, whereas treatment of thermoneutral obese mice with IL-4 enhances the growth of beige fat to mitigate obesity and its attendant sequelae. These data provide fundamental insights into how the sensing of cold is translated into the biological program of beige fat differentiation and thermogenesis in mice, findings that have potential implications for increasing beige fat mass and function in humans.
RESULTS
Type 2 cytokines are required for development of functional beige fat
To investigate the role of type 2 immunity in biogenesis of cold-induced beige fat, we quantified the expression of thermogenic genes in scWAT of WT and Il4/13-/- mice housed at thermoneutrality (30°C), 22°C, or 5°C for 48 hours. Quantitative RT-PCR analysis revealed that prolonged exposure to environmental cold (5°C) induced the expression of the core set of thermogenic genes, including Ucp1, Ppargc1a, Cox8b, Cidea, Elovl3, and Cpt1b, in the scWAT, whose induction was largely absent in eWAT of WT mice (Figure 1A and S1A). This cold-induced increase in expression of thermogenic genes was reduced by ~4-9-fold in the scWAT of Il4/13-/- mice (Figure 1A). Congruent with these observations, expression of UCP1 protein, which is required for uncoupled respiration in brown and beige adipocytes (Cannon and Nedergaard, 2011; Wu et al., 2012), was increased (~4-fold) after cold exposure in the scWAT of WT but not Il4/13-/- mice (reduced by ~80%, Figure 1B). This difference in scWAT UCP1 expression was also evident at 22°C, a housing temperature that is known to impose thermal stress in young mice (Figure 1B) (Cannon and Nedergaard, 2011). In contrast to the changes in scWAT, prolonged exposure to 5°C did not alter the expression or content of UCP1 in BAT of WT and Il4/13-/- mice (Figure 1B and Table S1A). Histologic analysis further affirmed that cold-induced remodeling of scWAT, but not eWAT, into beige fat was dependent on the type 2 cytokines IL-4 and-13, as evidenced by the paucity of multilocular, UCP1+ adipocytes in the scWAT of Il4/13-/- mice (Figure 1C, D and Figure S1B,C). Furthermore, measurement of oxygen consumption in scWAT and BAT of cold exposed mice confirmed that type 2 cytokine signaling was preferentially required for the browning of scWAT, as evidenced by ~51% and ~70% reduction in the rate of oxygen of scWAT of Il4/13-/- mice housed at 22°C and 5°C, respectively (Figure 1E, F).
Figure 1. Type 2 cytokines IL-4/13 are required for biogenesis of cold-induced beige fat.
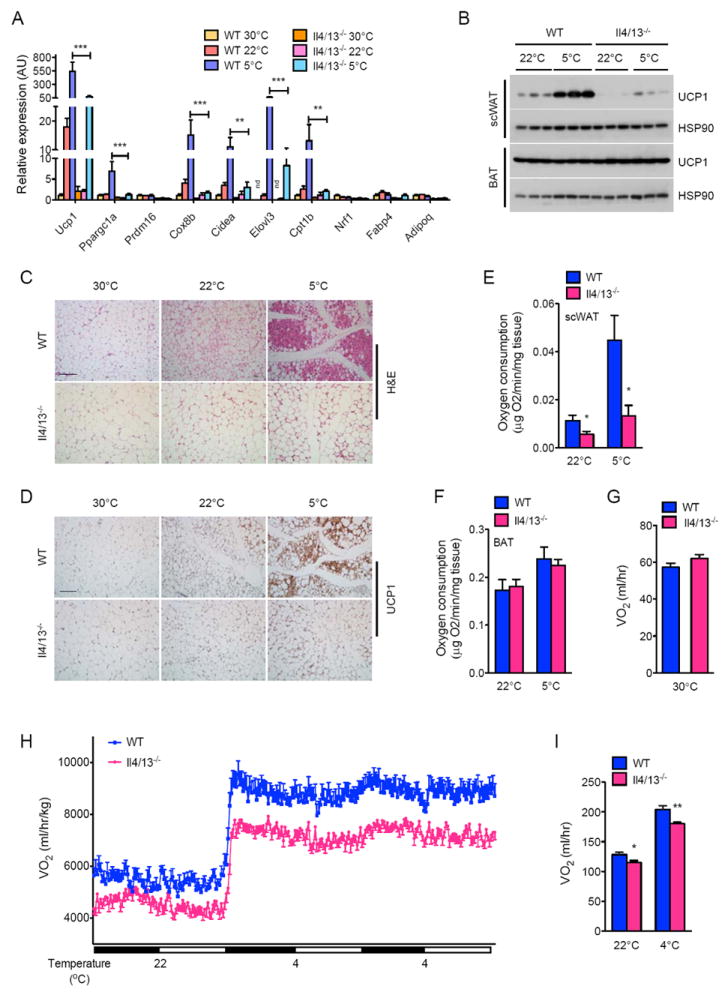
(A) Quantitative RT-PCR analysis of thermogenic genes in scWAT of WT and Il4/13-/- mice housed at 30°C, 22°C, or 5°C for 48 hours (n=5 per genotype and temperature). (B) Immunoblot analysis of UCP1 in scWAT and BAT of WT and Il4/13-/- mice housed at 22°C or 5°C for 48 hours (n=3 per genotype and temperature). (C, D) Representative scWAT sections of WT and Il4/13-/- mice housed at 30°C, 22°C, or 5°C for 48 hours were stained with hematoxylin and eosin (C) or for UCP1 (D), scale bar 100 μm. (E, F) Oxygen consumption in scWAT (E) and BAT (F) from WT and Il4/13-/- mice housed at 22°C or 5°C for 48 hours (n=7-8 per genotype and temperature). (G-I) Oxygen consumption (VO2) in WT and Il4/13-/- mice at different environmental temperatures: at thermoneutrality, 30°C (G) or at different ambient temperatures (H, I), n=5-8 per genotype. Data are represented as mean ± SEM. See also Figure S1.
Since brown/beige fat thermogenesis is stimulated by environmental cold in humans (van der Lans et al., 2013; Yoneshiro et al., 2013), we next investigated the thermogenic capacity of WT and Il4/13-/- mice under different environmental temperatures. Consistent with the human studies, total oxygen consumption was not different between WT and Il4/13-/- mice when they were housed at the thermoneutral temperature of 30°C (Figure 1G). However, exposure of these animals to progressively colder temperatures increased oxygen consumption in WT mice, a response that was blunted in Il4/13-/- mice, especially at 4°C (Figure 1H, I). This difference in energy expenditure (~11-12%) between WT and Il4/13-/- mice, which was independent of body mass (Table S1B and Figure 1I), likely reflects the thermogenic activity of beige fat because interscapular BAT UCP1 content, respiratory exchange ratio (RER), muscle thermogenic gene expression, total activity, and food intake were not different amongst the genotypes (Tables S1A, C, S2A-B, and Figure S1D, E). These data thus demonstrate that cold-induced remodeling of scWAT into functional beige fat requires the type 2 cytokines IL-4 and 13.
Signaling via the IL4Rα and STAT6 regulates biogenesis of beige fat
We next investigated the downstream signaling pathways by which IL-4/13 mediate their browning effects in the scWAT. Since IL-4Rα is the shared subunit between the type I (IL-4Rα/γc) and II (IL-4Rα/IL-13Rα1) receptors (Kelly-Welch et al., 2003), which mediate the known biologic effects of IL-4/13, we first examined the requirement of IL-4Rα in the biogenesis of beige fat. Similar to Il4/13-/- mice, cold induced increases in thermogenic gene and UCP1 protein expression were reduced (~3-16- and 10-fold, respectively) in the scWAT of Il4ra-/- mice (Figure S2A and 2A). Histological examination confirmed that the scWAT of Il4ra-/- mice housed at 5°C had fewer multilocular, UCP1+ beige adipocytes (Figure S2B and 2B). Accordingly, cold-induced increase in energy expenditure (VO2 consumption) was reduced by ~14-24% in Il4ra-/- mice (Figure 2C, D), whereas oxygen consumption was not different between WT and Il4ra-/- mice at thermoneutrality (Figure S2C). Moreover, deletion of Il4ra did not alter BAT UCP1 content, body mass, RER, muscle thermogenic gene expression, food intake, and total activity in these animals (Table S1A-C, Table S2A, B, and Figure S2D, E). These results thus demonstrate that cold-induced activation of canonical IL-4/13 signaling via IL-4Rα mediates the remodeling of scWAT into thermogenic beige fat.
Figure 2. Signaling via IL-4Rα and STAT6 controls growth of functional beige fat.
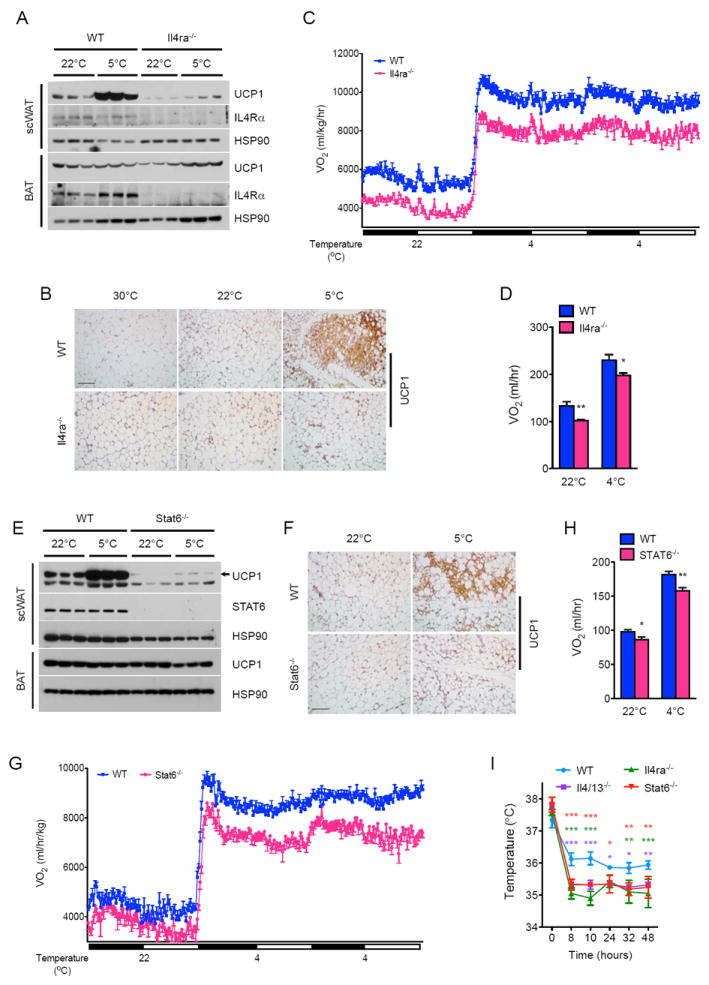
(A, E) Immunoblot analysis for UCP1 protein in the scWAT and BAT of WT, Il4/13-/- (A) and Stat6-/-(E) mice housed at 22°C or 5°C for 48 hours (n=3 per genotype and temperature). (B, F) Representative sections of scWAT from WT, Il4ra-/- (B) and Stat6-/- (F) mice housed at various temperatures were stained for UCP1, scale bar 100 μm. (C-D, G-H) Oxygen consumption (VO2) in WT, Il4ra-/- (C) and Stat6-/- (F) mice at 22°C and 4°C (n=8-10 per genotype). (I) Core body temperature of WT, Il4/13-/-, Il4ra-/-, and Stat6-/- mice during a 48 hour cold challenge (n=4-5 per genotype). Data are represented as mean ± SEM. See also Figure S2.
Although stimulation with IL-4 or IL-13 activates PI3K-AKT and MAPK/ERK signaling pathways (Heller et al., 2008), the reprogramming of cellular networks requires the transcription factor STAT6 in most cells (Goenka and Kaplan, 2011; Maier et al., 2012). This observation prompted us to investigate whether STAT6 might also be necessary for cold-induced remodeling of scWAT into beige fat. Indeed, the molecular, histologic, and metabolic phenotypes of cold-challenged Stat6-/- mice were similar to those of Il4/13-/- and Il4ra-/- mice (Figures 2E-G and S2F-J). For instance, cold-induced expression of UCP1 protein in the scWAT was decreased by ~12-fold in Stat6-/- mice (Figure 2E, F), as was the concomitant increase in oxygen consumption (Figure 2G, H). Accordingly, compared to WT mice, Il4/13-/-, Il4ra-/- and Stat6-/- mice maintained thermal homeostasis at a lower body temperature (Figure 2I). In aggregate, these findings provide strong evidence to support the hypothesis that canonical type 2 immunity, specifically the IL4/13-IL4Rα-STAT6 pathway, is required for biogenesis of functional beige fat, and raise four important questions. First, what cells secrete IL-4 to support the remodeling of scWAT into thermogenic beige fat? Second, how does activation of IL4/13 signaling promote development of beige fat? Third, is administration of IL-4 sufficient to increase beige fat mass in thermoneutral mice? And fourth, can IL-4 induced increase in beige fat mass ameliorate metabolic disease in an established model of obesity?
IL-4 producing eosinophils are required for biogenesis of beige fat
To identify cells competent for IL-4 production in scWAT, we housed 4get mice, which express green fluorescent protein (GFP) from the endogenous IL-4 locus (Mohrs et al., 2001), at 30°C and 5°C. Flow cytometric analysis revealed that the majority (~90%) of GFP+ cells in the scWAT of 4get mice housed at 5°C were eosinophils, as they expressed Siglec F and had high side scatter (Figure S3A) (Heredia et al., 2013; Wu et al., 2011). Exposure to environmental cold increased numbers of GFP+ cells in the scWAT of 4get mice (Figure 3A), a response that was reduced by ~85-95% in 4get-ΔdblGATA mice (Figure 3B-D), which lack eosinophils (Yu et al., 2002).
Figure 3. IL-4 producing eosinophils are required for development of cold-induced functional beige fat.
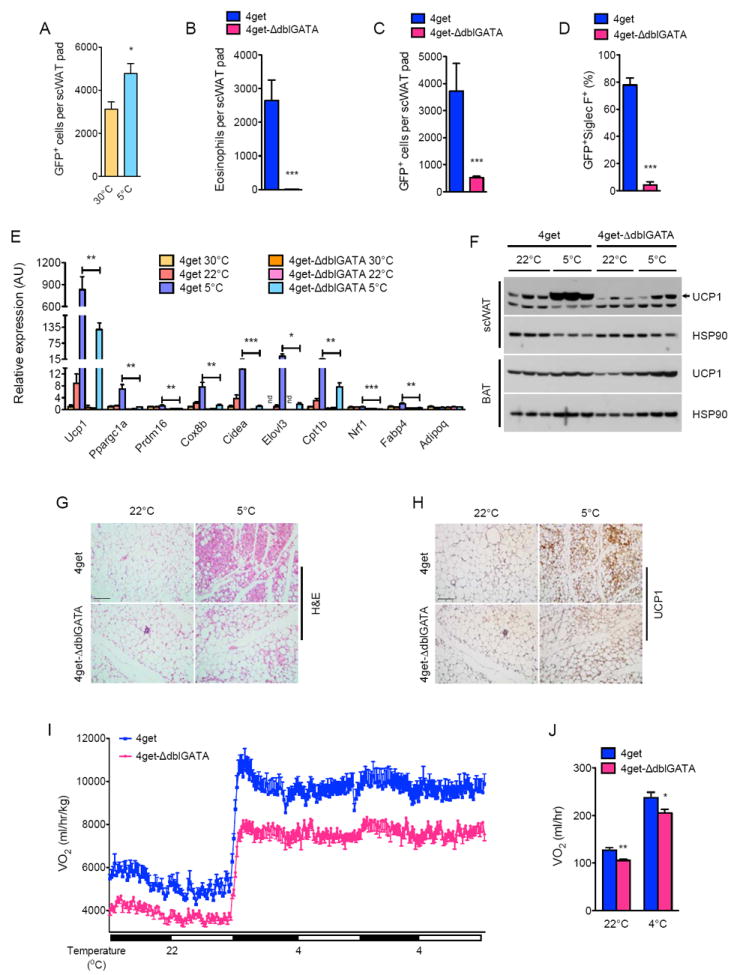
(A) Quantification of IL-4 producing cells in the scWAT of 4get mice housed at 30°C and 5°C. GFP expression marks cells competent for production of IL-4 (n=6 per temperature). (B-D) Quantification of eosinophils (B), GFP+ cells (C) and GFP+ SiglecF+ cells (eosinophils, D) in the scWAT of 4get and 4get-ΔdblGATA mice housed at 5°C (n=5-9 per genotype). (E) Quantitative RT-PCR analysis of thermogenic genes in scWAT of 4get and ΔdblGATA mice housed at 30°C, 22°C, or 5°C for 48 hours (n=5 per genotype and temperature). (F) Immunoblot analysis of UCP1 in scWAT and BAT of 4get and ΔdblGATA mice housed at 22°C or 5°C for 48 hours (n=3 per genotype and temperature). (G, H) Representative scWAT sections of 4get and ΔdblGATA mice housed at 22°C, or 5°C for 48 hours were stained with hematoxylin and eosin (G) or for UCP1 (H), scale bar 100 μm. (I, J) Oxygen consumption (VO2) in 4get and ΔdblGATA mice at different environmental temperatures (n=7-8 per genotype). Data are represented as mean ± SEM. See also Figure S3.
Using 4get and 4get-ΔdblGATA mice, we next investigated the requirement of eosinophils in cold-induced browning of scWAT. In a manner similar to Il4/13-/-, Il4ra-/- and Stat6-/- mice, exposure to 5°C for 48 hours failed to increase the expression of core set of thermogenic genes, including Ucp1, Ppargc1a, Prdm16, Cidea, Elovl3, Cpt1b, and Nrf1, in 4get-ΔdblGATA mice (Figure 3E). This was accompanied by attenuated induction of thermogenic protein UCP1 (reduced by ~70%) and a marked reduction in morphological transformation of scWAT into beige fat (Figure 3F-H). Furthermore, similar to mice impaired in type 2 cytokine signaling, cold-induced increase in energy expenditure was reduced by ~14-17% in 4get-ΔdblGATA mice (Figure 3I, J). This decrease in energy expenditure was independent of BAT UCP1 content, body mass, RER, muscle thermogenic gene expression, food intake, and total activity because these indices were similar between 4get and 4get-ΔdblGATA mice (Table S1A-C, Table S2A, B, and Figure S3B, C). Together, these results suggest that eosinophils, which are the primary IL-4 producing cells in the scWAT, are required for the cold-induced transformation of scWAT into beige fat.
Cold-induced biogenesis of beige fat requires CCR2+ macrophages
Since macrophages are an important target of IL-4/13 signaling in adipose tissue (Odegaard and Chawla, 2013), we investigated whether macrophage content of scWAT was altered by prolonged cold exposure. Exposure to 5°C progressively increased macrophage content of scWAT, with maximal numbers observed at 48 hours after initiation of cold exposure (Figure S3D). This increase in scWAT macrophage content likely resulted from recruitment of Ly6Chi monocytes because majority of the recruited macrophages continued to express Ly6C (Figure 4A) (Geissmann et al., 2010). Congruent with this idea, exposure to environmental cold failed to increase the numbers of total and Ly6Chi macrophages in Ccr2-/- mice (Figure 4A, S3D), which lack the chemokine receptor required for the recruitment of Ly6Chi monocytes to sites of tissue stress and injury (Charo and Ransohoff, 2006; Shi and Pamer, 2011).
Figure 4. Macrophage recruitment via CCR2 and alternative activation via IL-4Rα is required for biogenesis of beige fat.
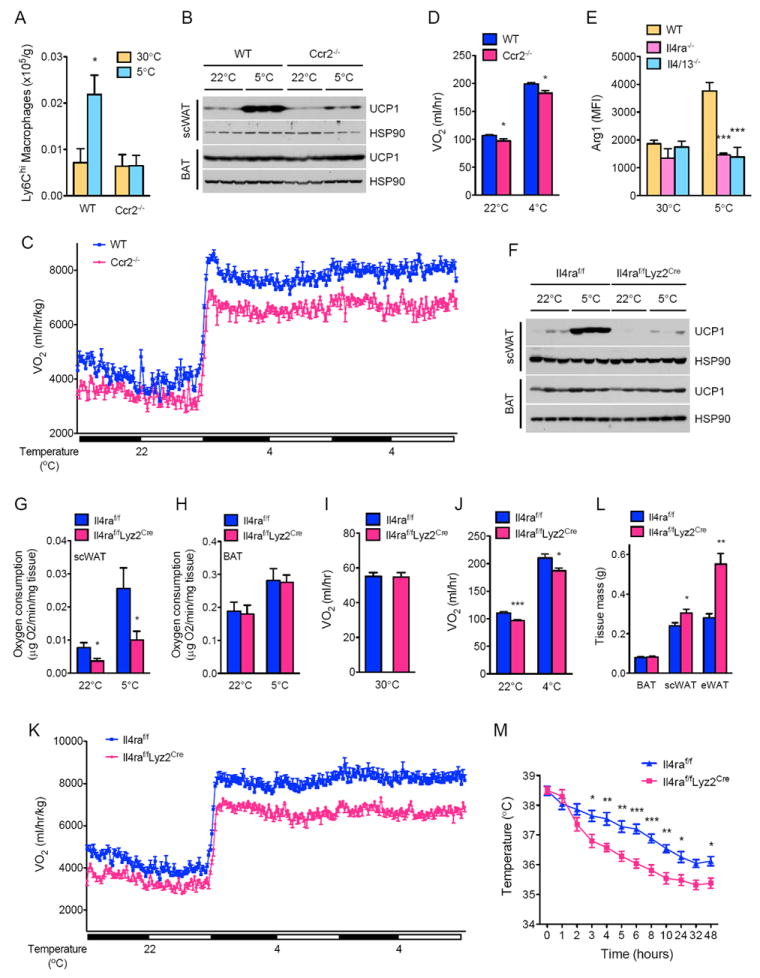
(A) Ly6Chi macrophage content of scWAT in WT and Ccr2-/- mice housed at thermoneutrality (30°C) or at 5°C for 48 hours (n=5 per genotype and temperature). (B, F) UCP1 protein expression in WT and Ccr2-/- mice (B) or Il4raf/f and Il4raf/fLyz2Cre (F) mice at 22°C or 5°C (n=3 per genotype and temperature). (C, D) Cold-induced changes in oxygen consumption (VO2) in WT and Ccr2-/- mice (n=8 per genotype). Data are represented as mean ± SEM. (E) Expression of alternative activation marker Arginase 1 in scWAT macrophages of WT, Il4/13-/-, and Il4ra-/- mice housed at 30°C or 5°C (n=4-5 per genotype and temperature). (G, H) Oxygen consumption in scWAT (G) and BAT (H) from Il4raf/f and Il4raf/fLyz2Cre mice housed at 22°C or 5°C for 48 hours (n=6 per genotype and temperature). (I-K) Oxygen consumption (VO2) in Il4raf/f and Il4raf/fLyz2Cre mice at various environmental temperatures: thermoneutrality, 30°C (n=5 per genotype) or 22°C and 4°C (n=7-9 per genotype). (L) Adipose tissue weights of Il4raf/f and Il4raf/fLyz2Cre mice after cold challenge at 5°C for 48 hours (n=5 per genotype). (M) Core body temperature of Il4raf/f and Il4raf/fLyz2Cre mice during a 48 hour cold challenge (n=5 per genotype). Data are represented as mean ± SEM. See also Figures S3 and S4.
Using Ccr2-/- mice (Charo and Ransohoff, 2006; Shi and Pamer, 2011), we next investigated whether recruitment of macrophages is necessary for the remodeling of scWAT from a site of energy storage to a site of thermogenesis. Cold-induced expression of core set of thermogenic genes, including the uncoupling protein UCP1, was reduced in the scWAT of Ccr2-/- mice (Figure S3E, 4B). This impairment in biogenesis of beige fat was also evident histologically, as the scWAT of Ccr2-/- mice had fewer multilocular, UCP1+ beige adipocytes (Figure S3F). Accordingly, Ccr2-/- mice had ~8-9% lower oxygen consumption (Figure 4C, D) without any significant differences in UCP1 BAT content, body mass, RER, muscle thermogenic gene expression, food intake, or total activity (Table S1A-C, Table S2A, B, and Figure S3G, H). This defect in beige fat thermogenesis was unlikely secondary to a developmental defect in Ccr2-/- mice because clodronate-mediated macrophage depletion in adult WT mice yielded similar results (Figures S4A-E). Together, these data support a model in which factors elaborated by recruited macrophages stimulate the growth of functional beige fat.
Signaling via IL-4Rα in macrophages is required for biogenesis of beige fat
Type 2 cytokines IL-4 and IL-13 promote the alternative activation of macrophages (Martinez et al., 2009; Odegaard and Chawla, 2011), prompting us to ask whether prolonged exposure to 5°C induces this program of macrophage activation in the scWAT. Compared to thermoneutral mice, macrophages residing in the scWAT of mice housed at 5°C had higher expression of alternative activation markers Arginase 1 and CD301 (Figures 4E, S4F). Moreover, the requirement of IL-4/13 and IL-4Rα for the induction of CD301 and Arginase 1 suggested that these were bona fide alternatively activated macrophages (Figures 4E, S4F). Together, these findings suggest that type 2 cytokine signaling via the IL-4Rα in macrophages might be required for development of functional beige fat in animals habituated to 5°C.
To test this postulate, we utilized Il4raf/f and Il4raf/fLyz2Cre, the latter genetically engineered to lack Il4ra in myeloid cells (Herbert et al., 2004). The cold-induced increases in UCP1 protein and thermogenic gene expression was reduced by ~3- and 4-10-fold, respectively, in Il4raf/fLyz2Cre (Figure 4F, S4G), indicating that myeloid cells are an important target for the browning effects of IL-4/13. Consistent with this notion, scWAT of Il4raf/fLyz2Cre mice had fewer UCP1+ beige adipocytes (Figure S4H) and lower rate of oxygen consumption at 22°C and 5°C (reduced by ~53% and 62%, respectively) (Figure 4G). In contrast, oxygen consumption rates were not different in the BAT of Il4raf/f and Il4raf/fLyz2Cre mice (Figure 4H), results that are similar to what was observed in Il4/13-/- mice (Figure 1E, F). These observations prompted us to investigate whether cold-induced increases in energy expenditure might be impaired in Il4raf/fLyz2Cre mice. Indeed, rate of oxygen consumption was ~11-13% lower in Il4raf/fLyz2Cre mice at colder temperatures but not at thermoneutrality (Figure 4I-K). Consequently, scWAT and eWAT mass was higher in Il4raf/fLyz2Cre mice (Figure 4L), and these animals maintained thermal homeostasis at a lower core body temperature than Il4raf/f mice (Figure 4M). These cold-induced changes in energy expenditure occurred without significant alterations in BAT UCP1 content, body mass, RER, muscle thermogenic gene expression, food intake, or total activity (Table S1A-C, Table S2A, B, and Figure S4I, J). These findings demonstrate for the first time that type 2 cytokines and alternatively activated macrophages mediate the long-term physiologic adaptations to environmental cold by stimulating the growth of functional beige fat. Moreover, our results suggest that factors secreted by alternatively activated macrophages likely work in trans to regulate the development and activity of beige fat.
Myeloid cell Th is required for development of beige fat
In the prevailing model of cold-induced thermogenesis, release of norepinephrine by the sympathetic nerves stimulates the development of beige fat (Cannon and Nedergaard, 2011; Lowell and Spiegelman, 2000). However, we have previously demonstrated that alternatively activated macrophages, which express all enzymes necessary for catecholamine synthesis, comprise an important accessory circuit for catecholamine production during acute cold stress (Nguyen et al., 2011). These observations led us to postulate that a similar mechanism might underlie the biogenic effects of macrophages on cold-induced browning of scWAT. To investigate this hypothesis, we first examined effects of cold-exposure on expression of tyrosine hydroxylase (TH), the rate-limiting step in biosynthesis of norepinephrine (Thomas and Palmiter, 1997; Zhou et al., 1995), in various adipose depots. As shown in Figure S5A, expression of TH protein was temperature responsive and inducible in all three adipose depots (BAT, scWAT, and eWAT). However, unlike BAT, basal expression of TH was nearly absent in scWAT and eWAT of mice housed at 22°C, but dramatically induced upon cold exposure (Figure S5A), findings that are consistent with published reports demonstrating the dense innervation of BAT but not scWAT and eWAT by the sympathetic nerves (Daniel and Derry, 1969; Morrison and Nakamura, 2011; Slavin and Ballard, 1978; Trayhurn and Ashwell, 1987). Since this change in TH expression correlated with alternative activation of scWAT macrophages (Figure S5A, S4E and 4E), we utilized intracellular flow cytometry to quantify TH protein content of scWAT macrophages. In an IL-4/13- and IL-4Rα-dependent manner, environmental cold induced expression of TH protein in the scWAT macrophages (Figure 5A and S5B), findings that implicate macrophage derived catecholamines in the biogenesis of beige fat.
Figure 5. Myeloid cell tyrosine hydroxylase is required for biogenesis of functional beige fat.
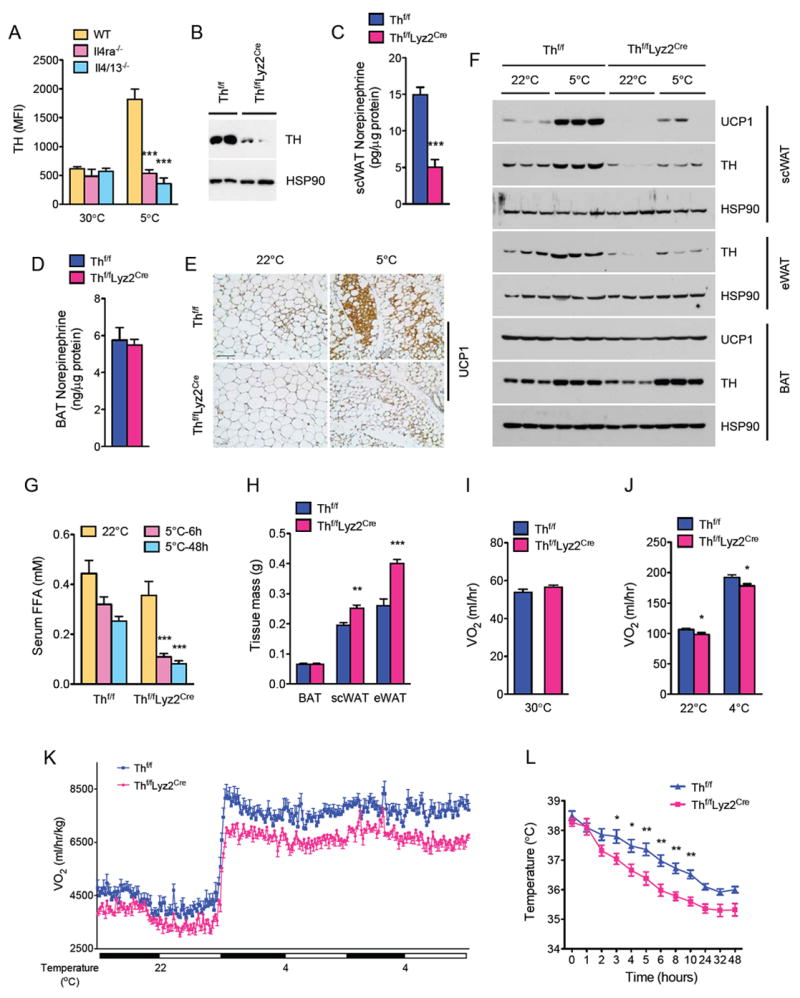
(A) Intracellular staining for TH expression in scWAT macrophages of WT, Il4/13-/- and Il4ra-/- mice housed at 30°C or 5°C. (B) Immunoblotting for TH protein in resident peritoneal macrophages of Thf/f and Thf/fLyz2Cre mice. (C, D) Norepinephrine content of scWAT (C) and BAT (D) of Thf/f and Thf/fLyz2Cre mice housed at 5°C (n=6-7 per genotype). (E) Representative sections of scWAT from Thf/f and Thf/fLyz2Cre mice stained for UCP1, scale bar 100 μm. (F) Immunoblot analysis for TH and UCP1 in scWAT, eWAT, and BAT of Thf/f and Thf/fLyz2Cre mice housed at 22°C or challenged with 5°C for 48 hours (n=3 genotype and temperature). (G) Serum concentration of free fatty acids in Thf/f and Thf/fLyz2Cre mice at various temperatures (n=6 per genotype and temperature). (H) Adipose tissue weights of Thf/f and Thf/fLyz2Cre mice after cold challenge at 5°C for 48 hours (n=5 per genotype). (I-K) Oxygen consumption (VO2) in Thf/f and Thf/fLyz2Cre mice at different environmental temperatures: at thermoneutrality, 30°C (I, n=5 per genotype) or during at different ambient temperatures (J, K), n=8 per genotype. (L) Core body temperature of Thf/f and Thf/fLyz2Cre mice during a 48 hour cold challenge (n=5 per genotype). Data are represented as mean ± SEM. See also Figure S5.
To definitively address the contribution of myeloid cell-derived catecholamines to cold-induced browning, we generated mice in which the Th gene, which is required for synthesis of all catecholamines (Zhou et al., 1995), was deleted in myeloid cells (designated Thf/fLyz2Cre) (Jackson et al., 2012). Immunoblot analysis revealed robust expression of TH protein in the peritoneal macrophages of control (Thf/f) mice, which was reduced by ~80-85% in peritoneal macrophages isolated from Thf/fLyz2Cre mice (Figure 5B). Since catecholamines mediate the browning of scWAT (Lee et al., 2012), this novel mouse model provided us a unique opportunity to dissect the contribution of myeloid cell-versus sympathetic nerve-derived catecholamines to cold-induced biogenesis of beige fat. In line with our hypothesis, steady state norepinephrine content was reduced by ~70% in the scWAT, but not BAT, of Thf/fLyz2Cre mice housed at 5°C (Figure 5C, D). Consequently, cold-induced browning was impaired in Thf/fLyz2Cre mice, as evaluated by the histological appearance of multilocular, UCP1+ beige adipocytes (Figure 5E, S5C). Immunoblot analysis of UCP1 protein in scWAT (reduced by ~3-fold) provided independent confirmation that biogenesis of beige fat but not BAT was defective in Thf/fLyz2Cre mice (Figure 5F). TH protein expression, which was induced by cold exposure in the scWAT and eWAT of Thf/f mice, was decreased (~4.5- and ~5.5-fold, respectively) in Thf/fLyz2Cre mice (Figure 5F), findings that suggest that myeloid cells rather than sympathetic nerves are the primary source of catecholamines in these cold stressed WAT depots. In contrast, TH protein expression was similar in BAT of Thf/f and Thf/fLyz2Cre mice at 22°C and 5°C, confirming that adrenergic tone of this richly innervated tissue is dependent on the sympathetic nerves. The importance of myeloid cell-derived catecholamines in the regulation of WAT function was further affirmed by the ~66-68% reduction in serum levels of free fatty acids and higher weights of WATs of Thf/fLyz2Cre mice after cold challenge (Figure 5G, H). These findings collectively suggest that cold-induced lipolysis and mobilization of stored triglycerides is dependent on myeloid cell-derived catecholamines.
Based on these results, we next tested whether myeloid cell-derived catecholamines are required for the increase in energy expenditure that is necessary to maintain thermal homeostasis. While oxygen consumption rates were similar between Thf/f and Thf/fLyz2Cre mice at thermoneutrality (30°C), indirect calorimetry revealed that oxygen consumption was reduced by ~7-8% in Thf/fLyz2Cre at different ambient temperatures (Figure 5H, I). These differences in energy expenditure between Thf/f and Thf/fLyz2Cre mice could not be accounted for by differences in BAT content of UCP1, body mass, RER, muscle thermogenic gene expression, food intake, or total activity (Table S1A-C, Table S2A, B, and Figure S5D, E). Congruent with these observations, Thf/fLyz2Cre mice maintained thermal homeostasis at a lower body temperature during prolonged cold exposure (Figure 5L). Taken together, these data support our hypothesis that myeloid cell-derived catecholamines, in particular those secreted by alternatively activated macrophages, are necessary for the molecular, histologic, and metabolic remodeling of scWAT into thermogenic beige fat.
Administration of IL-4 increases beige fat mass in thermoneutral mice
Having established a requirement for type 2 cytokines in the cold-induced remodeling of scWAT, we next investigated whether pharmacological activation of this signaling pathway was sufficient to increase the total thermogenic capacity in thermoneutral mice. To test this postulate, we administered IL-4 to WT BALB/cJ mice housed at 30°C (Figure 6A), a temperature at which mice lack the thermal drive to activate BAT or beige fat (Cannon and Nedergaard, 2011). In a dose-dependent manner, treatment with IL-4 increased UCP1 protein expression (~3-fold) in the scWAT but not interscapular BAT (Figure S6A). Of note, at the higher dose of IL-4 (2μg), even eWAT, which was resistant to cold-induced browning, developed molecular and histologic features of beige fat (Figure S6A, D, E). Based on these data, we decided to use the 2μg dose of IL-4 complexed to anti-IL-4 antibody (10 μg) for all subsequent studies (Ricardo-Gonzalez et al., 2010).
Figure 6. IL-4 induces beige fat in thermoneutral mice.
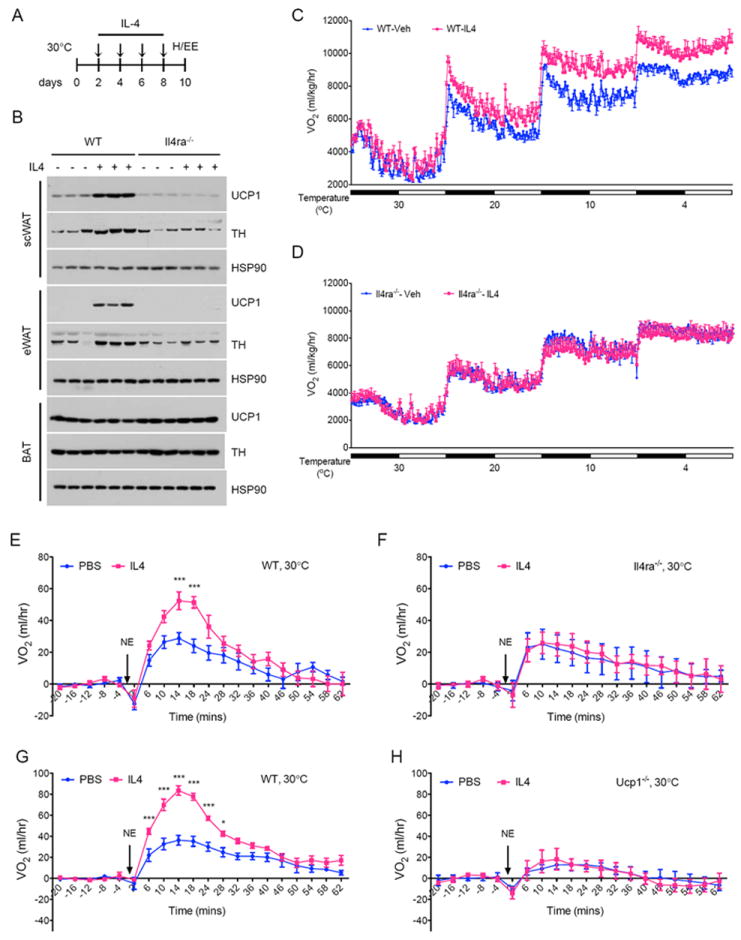
(A) Schematic for IL-4 dosing and metabolic analysis in thermoneutral mice. (B) Immunoblotting for UCP1 and TH in scWAT, eWAT, and BAT of WT and Il4ra-/- thermoneutral mice administered IL-4 complex over 8 days (n=3 per genotype and treatment). (C, D) Cold-induced changes in oxygen consumption in WT (C) and Il4ra-/- (D) thermoneutral mice administered IL-4 complex over 8 days (n=4-5 per genotype and treatment). (E-H) Norepinephrine stimulated changes in oxygen consumption (VO2) in conscious, thermoneutral mice that were pretreated with vehicle (Veh) or IL-4 complexes (IL-4): BALB/cJ (E), Il4ra-/- (F), C57BL/6J (G) and Ucp1-/- (H). All metabolic and histological analyses were performed 2 days after the last dose of IL-4 complex. Data are represented as mean ± SEM. See also Figure S6.
We next investigated the requirement of IL-4Rα in mediating the browning effects of IL-4 in thermoneutral mice. Administration of IL-4 complex increased the expression of UCP1 (~15-fold), alternative activation markers Arginase 1 and CD206, and TH (~2-3 fold) in the scWAT and eWAT of WT mice (Figures 6B and S5F-H), augmenting the steady state catecholamine content of these WAT depots by ~69-83% (Figure S5I). This increase in adrenergic tone of scWAT and eWAT was sufficient to cause these fat depots to acquire histological characteristics of beige fat (Figure S6B-E). Since IL-4-mediated browning of scWAT and eWAT was nearly absent in Il4ra-/- mice (Figure 6B and S6B-E), it suggests that the on-target effects of this type 2 cytokine control the growth of beige fat. In contrast to IL-4-mediated remodeling of scWAT and eWAT, BAT’s expression of UCP1 protein or its catecholamine content were unaffected by IL-4 administration in WT or Il4ra-/- mice (Figure 6B, Table S1A, and Figure S5J).
A hallmark of human beige/brown fat is the cold-induced increase in its thermogenic activity (van der Lans et al., 2013; Yoneshiro et al., 2013), prompting us to ask whether administration of IL-4 increases total thermogenic capacity in mice. To address this question, we first measured oxygen consumption in vehicle or IL-4 treated WT mice under different environmental temperatures. Although whole-body energy expenditure was similar between the two groups at thermoneutrality, the IL-4 treated animals had higher energy expenditure when housed at progressively cooler temperatures (Figure 6C, S6F). For instance, the total thermogenic capacity of IL-4 treated mice was 8-11% higher at the cooler environmental temperatures (Figure 6C, S6F), likely reflecting the increased beige fat mass in these animals. This is an important point because the UCP1 content of interscapular BAT was not significantly different between vehicle and IL-4 treated WT mice (Table S1A). Furthermore, IL-4-mediated increase in total thermogenic capacity was absent in Il4ra-/- mice (Figure 6D, S6G), which lacked molecular and histologic evidence of WAT browning but had similar amounts of interscapular BAT (Figures 6B, S6B-E and Table S1A). In both genotypes, chronic treatment with IL-4 did not significantly alter body mass, RER, muscle thermogenic gene expression, total activity, or food intake (Table S1B, C, Table S2A, B, and Figure S6H-K).
To definitively quantify the contribution of recruited beige fat to the total thermogenic capacity of mice, we administered norepinephrine (NE) to thermoneutral mice to maximally activate all adrenoreceptors and thermogenesis. As reported previously, injection of NE into conscious, thermoneutral mice transiently increases energy expenditure (Golozoubova et al., 2006). This observed increase in metabolic rate was greatly augmented in BALB/cJ mice that were pretreated with IL-4 (Figure 6E). Again, the thermogenic effects of IL-4 were on target because IL-4 failed to increase the rate of oxygen consumption in Il4ra-/- mice (Figure 6F). Based on the results, we next sought to determine whether this observed increase in metabolic rate was dependent on UCP1. While treatment with IL-4 augmented energy expenditure in C57BL/6J mice, this increase was completely absent in congenic Ucp1-/- mice (Figure 6G, H), indicating that IL-4 therapy results in the recruitment of beige fat to specifically increase the UCP1-dependent component of metabolic rate. Together, these findings demonstrate for the first time that IL-4-mediated increase in beige fat mass significantly contributes to the total thermogenic capacity of mice, findings that are likely to be relevant for the cold-induced recruitment of beige fat in humans.
Beige fat ameliorates metabolic dysfunction in established models of obesity
Although recruitment of beige fat has been postulated to exert negative energy balance and mitigate the metabolic complications of obesity, most studies have focused on prevention rather than treatment of diet-induced obesity (Harms and Seale, 2013). Since administration of IL-4 selectively increased beige fat mass in thermoneutral mice, we investigated whether this newly recruited beige fat can ameliorate metabolic dysfunction in the setting of pre-established obesity. For these studies, thermoneutral C57BL/6J mice were fed normal chow (NC) or high fat diet (HFD) for 10 weeks (Figure 7A). After matching for adiposity (Figure S7A), mice on HFD were treated with vehicle or IL-4 complexes over a period of 14 days (Figure 7A). Remarkably, dual-energy X-ray absorptiometry (DXA) revealed that, compared to vehicle treated animals, treatment with IL-4 decreased total body mass (~5.7g) and fat mass (~13.5%) without significantly affecting lean body mass (Figures 7B, C and S7B). This decrease in adiposity was also reflected in the smaller mass of scWAT and eWAT (Figures S7C-D). Immunoblotting analysis of adipose tissues revealed that HFD feeding decreased expression of TH (~70%) and UCP1 (~85%) proteins in the scWAT, which were restored after IL-4 therapy (Figure 7D). This was not limited to UCP1 because expression of the entire set of core thermogenic genes was restored by IL-4 therapy in the scWAT (Figure S7E). Similar increases in expression of TH and UCP1 proteins were observed in eWAT, but not BAT, of mice treated with IL-4 (Figure 7D, S7F), findings that were confirmed by the histologic examination of scWAT and eWAT of treated animals (Figure 7E and S7G).
Figure 7. Administration of IL-4 to obese thermoneutral mice reverses high fat diet-induced metabolic dysfunction.
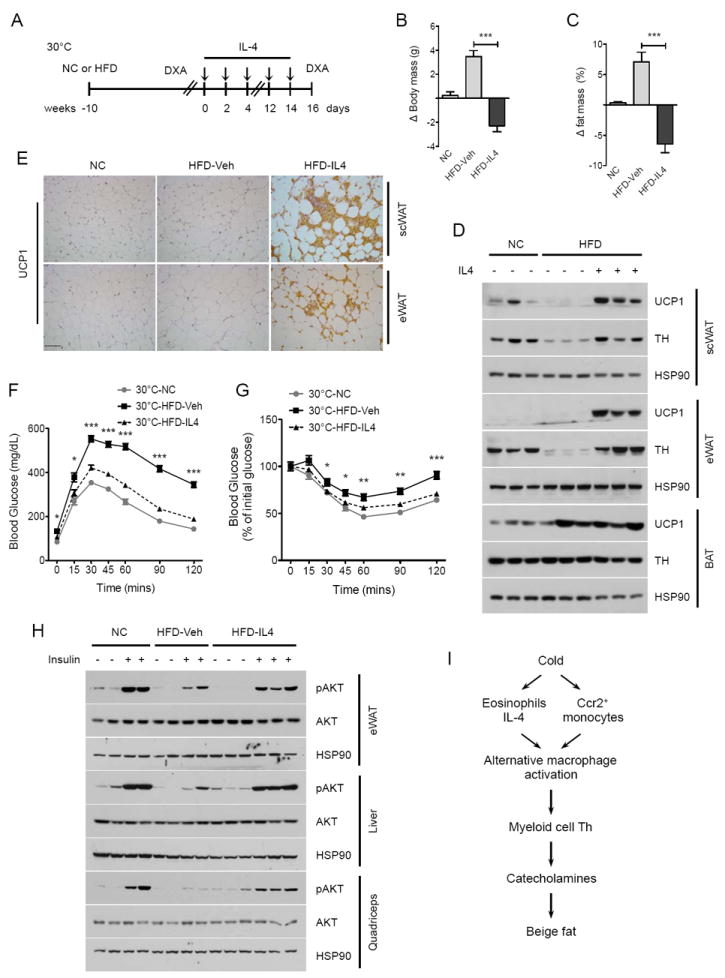
(A) Schematic for treatment of pre-established obesity with IL-4 complex in thermoneutral mice. (B, C) Changes in body mass (C) and fat mass (C) in obese C57BL/6J thermoneutral mice treated with vehicle (Veh) or IL-4 complex over 14 days (n=5 per treatment). (D) Immunoblot analysis for UCP1 and TH in scWAT, eWAT, and BAT of obese C57BL/6J thermoneutral mice administered Veh or IL-4 (n=3 per treatment). (E) Representative sections of scWAT and eWAT from thermoneutral mice treated with vehicle or IL-4 complex for 14 days were stained for UCP1, scale bar 100 μm. (F, G) Glucose (F) and insulin (G) tolerance tests in obese thermoneutral mice treated with Veh or IL-4 (n=5-7 per treatment). (H) Assessment of insulin signaling, as quantified by the phosphorylation of AKT, in obese thermoneutral mice treated with Veh or IL-4 (n=2-3 per treatment group). (I) Model for biogenesis and function of cold-induced beige fat. All metabolic and provocative testing was performed 2 days after the last dose of IL-4 complex. Data are represented as mean ± SEM. See also Figure S7.
We next investigated whether IL-4-induced increase in beige fat mass can restore insulin sensitivity in obese mice. Glucose and insulin tolerance tests demonstrated improvement in glucose disposal and insulin sensitivity, respectively, in IL-4 treated obese animals (Figure 7F, G). This was further affirmed by provocative insulin signaling studies, which revealed that IL-4 therapy improved insulin action, as quantified by the phosphorylation of AKT, in eWAT, liver, and quadriceps (Figure 7H). This improvement in insulin action was also associated with a decrease in liver triglyceride content, and in the circulating levels of triglycerides and cholesterol (Figure S7H-J). In aggregate, these results demonstrate for the first time that restoration of beige fat mass and activity can mitigate pre-established obesity and insulin resistance in mice.
DISCUSSION
Adult human BAT, which is dispersed in the supraclavicular, para-aortic, and suprarenal regions, is cold inducible, both in terms of its activity and mass (Enerback, 2010; Harms and Seale, 2013; Wu et al., 2013). However, unlike the classical brown adipocytes found in the interscapular regions of rodents and human infants, adult human BAT shares many molecular, histologic, and functional characteristics with the cold-inducible beige fat found in the scWAT of rodents (Harms and Seale, 2013; Liu et al., 2013; Sharp et al., 2012; Wu et al., 2012). For instance, rodent beige fat is derived from a group of non-myogenic progenitors that express CD137 and Tmem26 and induce their thermogenic activity upon cold exposure (Harms and Seale, 2013). Since these characteristics are in part shared by adult human BAT, cold-induced recruitment and activation of human BAT is thought to primarily involve beige adipocytes. These observations led us to ask the fundamental question of how exposure to environmental cold triggers the growth of functional beige fat, a question that previously had not been addressed.
In the classical paradigm of cold-induced thermogenesis, the central sensing of cold by the dorsomedial hypothalamus results in the activation of sympathetic outflow to stimulate BAT thermogenesis (Morrison et al., 2012). While this efferent thermogenic circuit works well for the richly innervated interscapular BAT, it fails to explain how cold exposure induces browning of the poorly innervated scWAT (Daniel and Derry, 1969; Slavin and Ballard, 1978; Trayhurn and Ashwell, 1987). Since we previously implicated alternatively activated macrophages in local production of catecholamines (Nguyen et al., 2011), we investigated here the requirement and sufficiency of type 2 immunity in supporting the browning of scWAT. Using mice impaired in type 2 immune responses, including Δ_dbl-GATA_, Il4/13-/-, Il4ra-/-, and Stat6-/- mice (Martinez et al., 2009), we demonstrate for the first time that eosinophils and type 2 cytokines comprise the efferent arm of the thermogenic circuit that regulates beige fat development (Figure 7I). Macrophages, which are recruited to the cold-stressed scWAT via the chemokine receptor CCR2, are the core integrators of these thermogenic signals. Accordingly, mice lacking CCR2 or IL-4Rα in myeloid cells are unable to remodel their scWAT into beige fat. The induction of tyrosine hydroxylase (Th), the rate limiting enzyme in the biosynthesis of catecholamines, provides a unifying mechanism by which type 2 cytokines and alternatively activated macrophages support the browning of scWAT (Figure 7I). For instance, cold stress selectively induces the expression of TH in alternatively activated scWAT macrophages, whereas myeloid specific deletion of Th impairs catecholamine production necessary for biogenesis of beige fat. Thus, in contrast to the neuronal thermogenic circuit that regulates interscapular BAT thermogenesis (Morrison and Nakamura, 2011), the development and function of beige fat requires catecholamine production by the hematopoietic circuit consisting of eosinophils, type 2 cytokines and alternatively activated macrophages, findings that reinforce another fundamental difference between beige and brown fat. Thus, in the future, it will be important to determine how neuronal sensing of cold results in the activation of the hematopoietic circuit described here to stimulate beige fat biogenesis.
A hallmark of human beige fat is its cold-induced recruitment and activation. For instance, chronic exposure to mild cold results in recruitment of new BAT in healthy young subjects, resulting in increased energy expenditure and weight loss (van der Lans et al., 2013; Yoneshiro et al., 2013). Interestingly, the contribution of this new beige/brown fat to total body energy expenditure can only be detected after mild cold exposure but not in thermoneutral subjects (Yoneshiro et al., 2013). We observe a similar dependence on environmental temperature for the thermogenic activation of beige fat. For instance, at thermoneutrality, oxygen consumption rates are similar between control and various knockout mice, including Il4/13-/-,Il4ra-/-, Stat6-/-, Il4raf/fLyz2Cre, and Thf/fLyz2Cre mice. In contrast, upon cold exposure, one observes a significant difference in the rates of oxygen consumption between control and knockout animals. For example, under colder environmental conditions, energy expenditure is reduced by ~10% in the various knockout animals, an amount that likely reflects the thermogenic activity of beige fat because UCP1 content of BAT remains unchanged. However, since myeloid cell-derived catecholamines are also required for maximal lipolysis of stored triglycerides, we cannot formally exclude the possibility that decreased delivery of fatty acids to BAT might also contribute to the reduced energy expenditure in these knockout mice.
Conversely, treatment of thermoneutral WT mice with IL-4 increases beige fat mass but not oxygen consumption. However, upon exposure to progressively colder temperatures, these IL-4 treated animals have ~8-12% higher energy expenditure, reflecting their higher thermogenic capacity. It is important to note that this IL-4-induced increase in thermogenesis is completely dependent on UCP1 and independent of BAT UCP1 content, suggesting that the recruitment of beige fat is primarily responsible for the observed increase in oxygen consumption. Finally, although pharmacological activation of all adrenoreceptors by NE results in marked increase in oxygen consumption in IL-4 treated animals, it likely represents an overestimate of beige fat thermogenic capacity because interscapular BAT mediated thermogenesis is reduced to ~20-25% when mice are housed at thermoneutrality for prolonged periods of time (Golozoubova et al., 2006). However, if one takes this into account, then the UCP1-dependent and IL-4-induced beige fat mass could account for ~15-20% of total thermogenic capacity in mice, findings that are in agreement with the recent report that suggested beige fat respiration can account for ~10-37% of interscapular BAT thermogenic capacity (Shabalina et al., 2013)
Cold and diet are the two physiological triggers known to activate the thermogenic activity of brown fat (Cannon and Nedergaard, 2011). Although the importance of cold in stimulating beige fat thermogenesis has previously been investigated (Harms and Seale, 2013), its role in diet-induced thermogenesis has not been systematically explored. By housing mice at thermoneutrality, which inactivates cold-induced thermogenesis, we found that high fat diet activates the thermogenic activity of recruited beige fat to promote negative energy balance. For example, treatment of thermoneutral obese animals with IL-4 increased beige fat mass, whose activation by high fat diet decreased adiposity and weight gain. In this experimental setting, BAT UCP1 content remained unchanged, indicating that weight loss was primarily driven by dietary activation of recruited beige fat. Not only do these findings provide strong experimental support for the therapeutic potential of beige fat in the setting of obesity, but they also outline a rigorous experimental strategy to systematically evaluate the potency and activity of other browning factors, such as irisin, Fgf21 and natriuretic peptides. Together, our results demonstrate that recruitment of new beige fat can ameliorate the established metabolic dysfunction resulting from diet-induced obesity, thereby providing strong support for the therapeutic targeting of beige fat for the treatment of human obesity and obesity-associated insulin resistance.
In summary, the studies presented here have elucidated the efferent thermogenic circuit consisting of eosinophils, type 2 cytokines, and alternatively activated macrophages, which regulates the development of cold-induced beige fat. Surprisingly, this thermogenic circuit requires local production of catecholamines by alternatively activated macrophages rather than sympathetic nerves to stimulate the conversion of scWAT into a thermogenic organ. Moreover, since this hematopoietic circuit is activated in response to the physiologic stimulus of cold, it is plausible that other factors promoting the browning of scWAT might induce or activate components of this thermogenic circuit to increase beige fat mass. If so, it will suggest that the efferent beige fat thermogenic circuit identified here is a central, common pathway by which mammals increase their thermogenic capacity to meet their physiologic needs in response to a diverse set of stimuli.
EXPERIMENTAL PROCEDURES
Animals and in vivo studies
All mice were maintained in the vivarium under a 12 hour light:dark cycle and used at the designated environmental temperature. Male mice, 8-12 week old, were used for the cold exposure and thermoneutrality experiments. The following strains were on the BALB/cJ background: WT, Il4/13-/-, Il4ra-/-, Stat6-/-, 4get, 4get-ΔdblGATA, Il4raf/f, and Il4raf/fLyz2Cre, whereas WT, _Ccr2_-/-, Ucp1-/-, Thf/f, and Thf/fLyz2Cre were on the C57BL6/J background. In addition, C57BL6/J mice were used for the obesity studies performed at thermoneutrality with IL-4. Animals were maintained at 30°C for 4 weeks prior to molecular and histologic quantification of brown or beige fat, or measurement of energy expenditure. For the cold-induced changes in energy expenditure, mice were chronically housed at 22°C and acutely shifted to 4-5°C environment for 48 hours. During the cold challenge experiments, mice were fed ad lib and housed in 5°C chamber (Powers Scientific) for 48 hours in groups of 2 mice per cage. Cages were pre-chilled overnight at 5°C and contained Enviro-dri as enrichment to allow animals to make large nests. At the end of experiments, tissues were harvested and snap frozen in liquid nitrogen for RNA and protein analysis, or fixed in 10% formalin for histology. Mice were acclimated to 30°C in laboratory incubator (Darwin Chambers) for 2-4 weeks prior to initiation of experiments at thermoneutrality. For IL-4-induced browning, 4-5 week old male mice were housed at 30°C for 3 weeks prior to intraperitoneal injection with PBS (Vehicle) or IL-4 (2 μg, Peprotech) that was complexed with anti-IL4 mAb (10 μg of clone 11B11). To deplete monocytes and tissue macrophages, mice were given 5 intraperitoneal injections of clodronate-containing or empty liposomes (100 μl) starting 4 days prior to through initiation of cold challenge. Depletion of circulating monocytes and resident macrophages was confirmed by flow cytometric analysis of blood, adipose tissues, and spleen. Cohorts of ≥ 4 mice per genotype or treatment were assembled for all in vivo studies, which were repeated 2-3 independent times.
Statistical analysis
All data are presented as mean ± s.e.m and analyzed using Prism (Graphpad). Statistical significance was determined using the unpaired two-tailed Student’s t-test for single variables and two-way ANOVA followed by Bonferroni post-tests for multiple variables. A p-value of < 0.05 was considered to be statistically significant, and is presented as * (p < 0.05), ** (p < 0.01), or *** (p < 0.001).
Extended Experimental Procedures are included in the Supplemental Information.
Supplementary Material
01
02
HIGHLIGHTS.
- Eosinophils and type 2 cytokines are required for biogenesis of beige fat
- Alternatively activated macrophages support the development of functional beige fat
- Myeloid cell-derived catecholamines mediate the growth of cold-induced beige fat
- IL-4 therapy increases beige fat mass to ameliorate obesity in thermoneutral mice
Acknowledgments
We thank members of the Chawla laboratory, A. Loh for comments on the manuscript, P. Cohen for providing the protocol to measure oxygen consumption in isolated adipose tissues, and S. Kajimura for assistance with use of their Clark electrode. The authors’ work was supported by grants from NIH (HL076746, DK094641), American Heart Association Innovative Science Award (12PILT11840038), and an NIH Director’s Pioneer Award (DP1AR064158) to A.C.. Y.Q was supported by an AHA Postdoctoral Fellowship and K.D.N. was supported by an AHA Predoctoral Fellowship.
Footnotes
AUTHOR CONTRIBUTION
Y.Q., K.D.N., J.I.O., and X.T. designed and performed the main experiments, and X.C. provided technical assistance for the main experiments. R.M.L. and R.D.P. provided essential mouse lines for the completion of the studies. Y.Q., K.D.N., J.I.O., and A.C. discussed and interpreted the results from the study. Y.Q. and A.C. conceived, supervised, and wrote the paper. All animal care and procedures were performed in accordance with UCSF’s IACUC guidelines.
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans) Int J Obes (Lond) 2010;34(Suppl 1):S7–16. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. The Journal of experimental biology. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. The New England journal of medicine. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel H, Derry DM. Criteria for differentiation of brown and white fat in the rat. Canadian journal of physiology and pharmacology. 1969;47:941–945. doi: 10.1139/y69-154. [DOI] [PubMed] [Google Scholar]
- Enerback S. Human brown adipose tissue. Cell Metab. 2010;11:248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunologic research. 2011;50:87–96. doi: 10.1007/s12026-011-8205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. American journal of physiology Endocrinology and metabolism. 2006;291:E350–357. doi: 10.1152/ajpendo.00387.2005. [DOI] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, Keegan AD. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Science signaling. 2008;1:ra17. doi: 10.1126/scisignal.1164795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM, McMahon DG. Retinal dopamine mediates multiple dimensions of light-adapted vision. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9359–9368. doi: 10.1523/JNEUROSCI.0711-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- Liu W, Shan T, Yang X, Liang S, Zhang P, Liu Y, Liu X, Kuang S. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. Journal of cell science. 2013;126:3527–3532. doi: 10.1242/jcs.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Maier E, Duschl A, Horejs-Hoeck J. STAT6-dependent and -independent mechanisms in Th2 polarization. European journal of immunology. 2012;42:2827–2833. doi: 10.1002/eji.201242433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annual review of immunology. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Frontiers in endocrinology. 2012;3 doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Frontiers in bioscience. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell reports. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin BG, Ballard KW. Morphological studies on the adrenergic innervation of white adipose tissue. The Anatomical record. 1978;191:377–389. doi: 10.1002/ar.1091910310. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature. 1997;387:94–97. doi: 10.1038/387094a0. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, Ashwell M. Control of white and brown adipose tissues by the autonomic nervous system. The Proceedings of the Nutrition Society. 1987;46:135–142. doi: 10.1079/pns19870017. [DOI] [PubMed] [Google Scholar]
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, Hansen J, Jorgensen JA, Wu J, Mottaghy FM, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes & development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. The Journal of experimental medicine. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374:640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02