Regression From Prediabetes to Normal Glucose Regulation Is Associated With Reduction in Cardiovascular Risk: Results From the Diabetes Prevention Program Outcomes Study (original) (raw)
Abstract
OBJECTIVE
Restoration of normal glucose regulation (NGR) in people with prediabetes significantly decreases the risk of future diabetes. We sought to examine whether regression to NGR is also associated with a long-term decrease in cardiovascular disease (CVD) risk.
RESEARCH DESIGN AND METHODS
The Framingham (2008) score (as an estimate of the global 10-year CVD risk) and individual CVD risk factors were calculated annually for the Diabetes Prevention Program Outcomes Study years 1–10 among those patients who returned to NGR at least once during the Diabetes Prevention Program (DPP) compared with those who remained with prediabetes or those in whom diabetes developed during DPP (N = 2,775).
RESULTS
The Framingham scores by glycemic exposure did not differ among the treatment groups; therefore, pooled estimates were stratified by glycemic status and were adjusted for differences in risk factors at DPP baseline and in the treatment arm. During 10 years of follow-up, the mean Framingham 10-year CVD risk scores were highest in the prediabetes group (16.2%), intermediate in the NGR group (15.5%), and 14.4% in people with diabetes (all pairwise comparisons P < 0.05), but scores decreased over time for those people with prediabetes (18.6% in year 1 vs. 15.9% in year 10, P < 0.01). The lower score in the diabetes group versus other groups, a declining score in the prediabetes group, and favorable changes in each individual risk factor in all groups were explained, in part, by higher or increasing medication use for lipids and blood pressure.
CONCLUSIONS
Prediabetes represents a high-risk state for CVD. Restoration of NGR and/or medical treatment of CVD risk factors can significantly reduce the estimated CVD risk in people with prediabetes.
Introduction
As the human and economic costs of type 2 diabetes have surged, focus on its prevention has intensified. Clinical trials (1–7) aimed at diabetes prevention have universally enrolled participants with “prediabetes” (i.e., impaired glucose tolerance [IGT] and/or impaired fasting glucose [IFG] levels) because of their high conversion rate to diabetes. Interventions were deemed successful if diabetes was prevented or delayed, yet many participants remained with prediabetes, with its attendant metabolic and vascular risks. Arguably, the prevention of diabetes and its complications lies in the restoration of normal glucose regulation (NGR) rather than in the maintenance of prediabetes. Indeed, our recent post hoc analysis from the Diabetes Prevention Program (DPP) Outcomes Study (DPPOS) (8) demonstrated a 56% lower risk of diabetes 10 years from randomization among those individuals who were able to achieve NGR during DPP versus those who remained with prediabetes. As a result, there is mounting interest in learning whether NGR should be the goal for people with prediabetes and, further, whether they should be monitored for relapse to prediabetes with escalating and earlier intervention instituted as needed to maintain NGR (9).
This potential shift in our clinical approach may be justified considering the higher incidence of diabetes-related complications seen in people with prediabetes (10–13). Nevertheless, enthusiasm for the medical treatment of prediabetes is currently tempered by cost related to the estimated 79 million Americans who currently have prediabetes and the risk/benefit ratio, especially in light of the many clinical trials failing to demonstrate cardiovascular disease (CVD) risk reduction from short-term glucose lowering in patients with frank diabetes (14–17). Hence, it is noteworthy to point out that several studies have shown benefit from short-term glucose-lowering interventions on CVD risk factors, surrogate markers of CVD (18,19), as well as absolute CVD event rates (20) in people with prediabetes. These data suggest that glucose lowering could have a disproportionate benefit in CVD risk reduction in prediabetes versus diabetes patients, providing further support for the pursuit of NGR.
After completion of the DPP, DPPOS was initiated and afforded a unique opportunity to examine CVD risk profiles over time in people who regressed to NGR or maintained their prediabetes, or in whom diabetes developed during the DPP. We hypothesized that, compared with individuals with persistent prediabetes or diabetes, those reaching NGR in the DPP would have a significant and enduring decreased estimated risk of CVD over the period of observation in DPPOS.
Research Design and Methods
The DPPOS is the follow-up to a randomized clinical trial performed at 27 centers involving 2,775 persons (as of data lock on 10 July 2013) who were at high risk for diabetes. The detailed methods have been reported (21), and the protocol is available at http://www.bsc.gwu.edu/dpp. Institutional review boards at each center approved the protocol, and all participants gave written informed consent prior to participation.
Participants
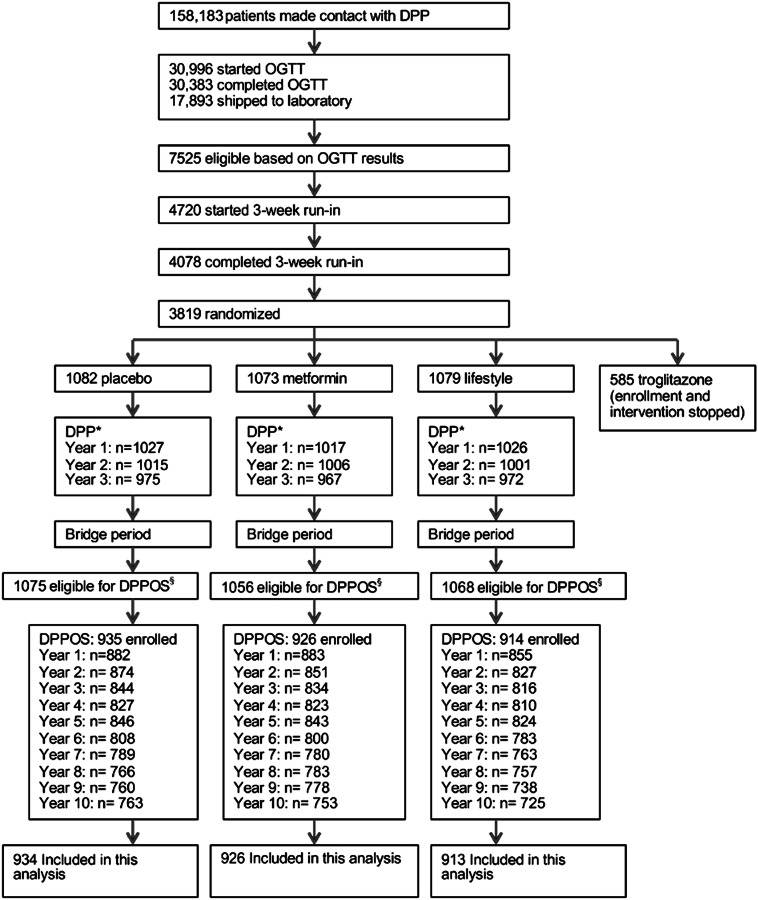
Participants were observed for a median time of 3.2 years during the masked intervention phase of the DPP, when the glycemic response was established. All surviving DPP participants were eligible for entry into the DPPOS. Of the 2,775 participants in DPPOS, 1,509 (54%) had achieved NGR at least once during the DPP, whereas 496 (18%) remained with prediabetes and diabetes developed in 770 (28%) (see Classification for group definitions). Participant flow through the DPP and DPPOS, as well as participants included in this analysis, are depicted in Fig. 1.
Figure 1.
Participant flow through DPP, DPPOS, and this study.
Interventions
All participants were offered group-implemented lifestyle sessions prior to the start of DPPOS, including those who had been randomized to the intensive lifestyle arm during the DPP (22). Open-label metformin was also continued in participants initially randomized to receive metformin (850 mg twice daily as tolerated) during the DPPOS, unless discontinued for the development of diabetes requiring management outside of the protocol, or for reasons of safety and/or tolerability.
Classification
The primary outcome of the DPP was the development of diabetes: fasting plasma glucose concentration of ≥126 mg/dL (≥7.0 mmol/L; checked semiannually), and/or a 2-h glucose concentration of ≥200 mg/dL (≥11.1 mmol/L; checked annually) after a 75-g oral glucose challenge (confirmed on repeat testing) (23). For the current analysis, participants were classified according to their glycemic status during the DPP. They were classified as having NGR if they had achieved both a fasting plasma glucose concentration of <100 mg/dL (<5.6 mmol/L) and a 2-h plasma glucose concentration of <140 mg/dL (<7.8 mmol/L) at least once during an annual oral glucose tolerance test (OGTT), and never met the criteria for the diagnosis of diabetes (as above) during the DPP period. Participants were classified as having prediabetes (23) if they consistently had fasting plasma glucose levels of 100–125 mg/dL (5.6–6.9 mmol/L) and/or 2-h plasma glucose levels of 140–199 mg/dL (7.8–11.0 mmol/L) on annual OGTT, and never met the criteria for the diagnosis of diabetes (as above) during the DPP period.
Assessments
The Framingham CVD risk score was calculated according to the method of Wilson and Morrell (24), updated in 2008 (25), to estimate the 10-year CVD risk from the time of data collection (e.g., data collected at year 1 of the DPPOS would predict CVD risk at year 11 of the DPPOS). The Framingham 2008 CVD risk estimate engine was used over alternatives risk estimation systems because it has been validated in men, women, Caucasians, and African Americans (26), collectively representing 85% of the multiethnic DPPOS cohort (27). In addition, use of this Framingham score allows the incorporation of diabetes status at each assessment—the a priori major outcome of the DPP. Furthermore, the primary end points most closely resemble the composite CVD end point adopted by the DPPOS (i.e., fatal and nonfatal CVD, including stroke, congestive heart failure, and peripheral artery disease), hence its accuracy in predicting CVD in a multiethnic cohort with prediabetes (in whom no CVD risk estimator currently exists) ultimately will be determined with the eventual publication of the CVD outcomes data from DPPOS. Blood pressure, plasma lipid levels, and medication usage were obtained on annual examination using previously published methods (28). Of note, the Framingham score does not account for the use of lipid-lowering medication (see discussion on study limitations in Conclusions).
Statistical Analyses
Comparisons among groups at baseline were made using ANOVA for quantitative variables and the χ2 test for categorical variables with nominal P values not adjusted for multiple comparisons. The outcomes evaluated were the 10-year CVD risk estimate using the Framingham score and the individual CVD risk factors (total cholesterol [TC], HDL cholesterol [HDL-C], LDL cholesterol [LDL-C]), smoking status, systolic BP [SBP], and diastolic BP [DBP]), and diabetes status, all calculated annually. The normal-errors longitudinal regression model (29) assessed differences between glycemic exposure groups in the mean of the Framingham risk score and individual CVD risk factors adjusted for multiple comparisons, adjusting for baseline components (TC, HDL-C, smoking status, and SBP), demographics (sex, age at randomization, and race/ethnicity), and treatment group. Two-way interaction terms for treatment group, year, and glycemic status were also assessed and adjusted if significant at the 0.10 level. Initial analyses revealed significant interaction between year and glycemic status, but no interaction between the glycemic exposure and treatment, hence Framingham risk scores and individual CVD risk factors by glycemic response were pooled across treatment groups except for HDL-C (interaction P value = 0.04). An additional model was used to assess whether the differences among glycemic responses can be explained by the use of the lipid-lowering medications. Measures of explained variation (_R_2) for fixed effects is used to assess the contribution of covariates on the longitudinal measures of CVD risk and components (30). The SAS system (version 9.3; SAS Institute, Cary, NC) was used for all analyses.
Results
The demographics of the DPP/DPPOS cohort, with (8) and without (5,27) stratification by the glycemic status defined in the DPP, have been previously reported. Participants who subsequently were classified into these groups exhibited some differences in CVD risk factors at DPP and DPPOS baselines (Table 1). These differences were relevant to the calculation of the Framingham score; therefore, differences at DPP baseline were used to adjust the estimated risk over the time of the DPPOS. Predictors and maintenance in the NGR group (8,31), as well as the effects of interventions during DPP and DPPOS on the CVD risk factors (32,33), have been previously published.
Table 1.
Components of the Framingham score by group
| Components | Diabetes | Prediabetes | NGR | P value |
|---|---|---|---|---|
| DPP baseline | ||||
| Age (years) | 50.9 (10.4) | 52.4 (10.4) | 50.7 (10.4) | 0.015 |
| Female (%) | 66.5 | 70.4 | 67.2 | 0.34 |
| TC (mg/dL) | 203 (35) | 206 (37) | 203 (36) | 0.38 |
| HDL (mg/dL) | 44 (11) | 46 (12) | 46 (12) | <0.001 |
| SBP (mmHg) | 125 (15) | 125 (15) | 123 (14) | <0.001 |
| Use of antihypertensive medications (%) | 20 | 18 | 14 | <0.001 |
| Current smoker (%) | 8.7 | 7.1 | 5.4 | 0.009 |
| DPPOS baseline | ||||
| Age (years) | 56.2 (10.3) | 57.4 (10.3) | 55.9 (10.3) | 0.025 |
| Female (%) | 66.5 | 70.4 | 67.2 | 0.34 |
| TC (mg/dL) | 191 (35) | 199 (51) | 195 (36) | <0.001 |
| HDL (mg/dL) | 45 (12) | 47 (13) | 49 (13) | <0.001 |
| SBP (mmHg) | 125 (15) | 124 (15) | 121 (14) | <0.001 |
| Use of antihypertensive medications (%) | 49 | 40 | 30 | <0.001 |
| Current smoker (%) | 6.8 | 7.1 | 5.8 | 0.49 |
Estimated 10-Year CVD Risk by Glycemic Category
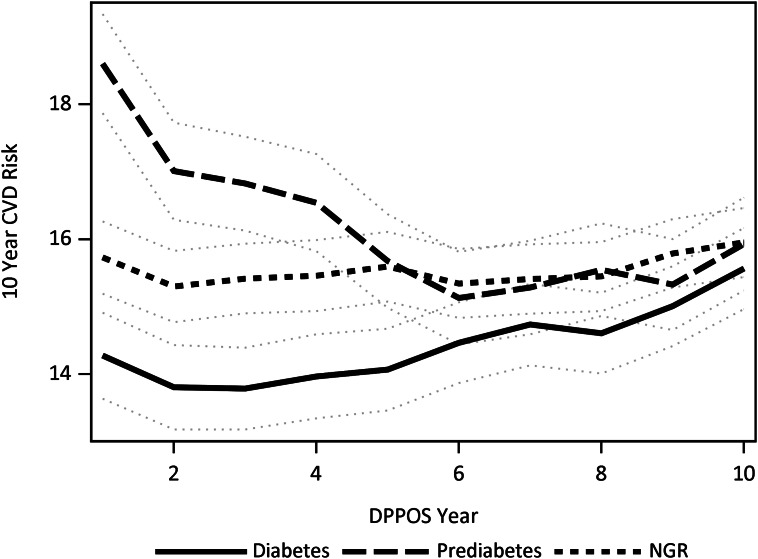
The trajectory of estimated 10-year CVD risk over the course of the DPPOS, in groups defined by glycemic status in the DPP, is depicted in Fig. 2. The mean estimated CVD risk during the follow-up was 14.4% (95% CI 13.9–15.0%) in people with diabetes, 16.2% (15.6–16.8%) in the prediabetes group, and 15.5% (15.1–16.0%) in those who reached NGR (P < 0.001, NGR vs. diabetes and prediabetes vs. diabetes; P = 0.02, NGR vs. prediabetes). Absolute differences in estimated mean CVD risk among the groups were greatest at year 1 of the DPPOS: diabetes 14.3% (13.7–14.9%), NGR 15.7% (15.2–16.3%), prediabetes 18.6% (17.8–19.3%) (P < 0.001 for all pairwise comparisons). These risk estimates converged over the period of observation because of an increase in Framingham score over the time of the DPPOS in people with diabetes (15.6% in year 10 vs. 14.3% in year 1, P < 0.001) and a decrease over time in people with prediabetes (15.9% in year 10 vs. 18.6% in year 1, P < 0.001).
Figure 2.
Trajectories of 10-year CVD risk during the DPPOS in people with diabetes (solid), prediabetes (medium dash), and NGR (short dash) represented by means (lines) and 95% CIs (gray dotted line) with adjustment for differences in treatment group, age at randomization, sex, race/ethnicity, and baseline CVD risk factors (TC concentration, SBP or use of antihypertensive medication, smoking status, diagnosis of diabetes, and/or HDL-C concentration).
CVD Risk Factors by Glycemic Category
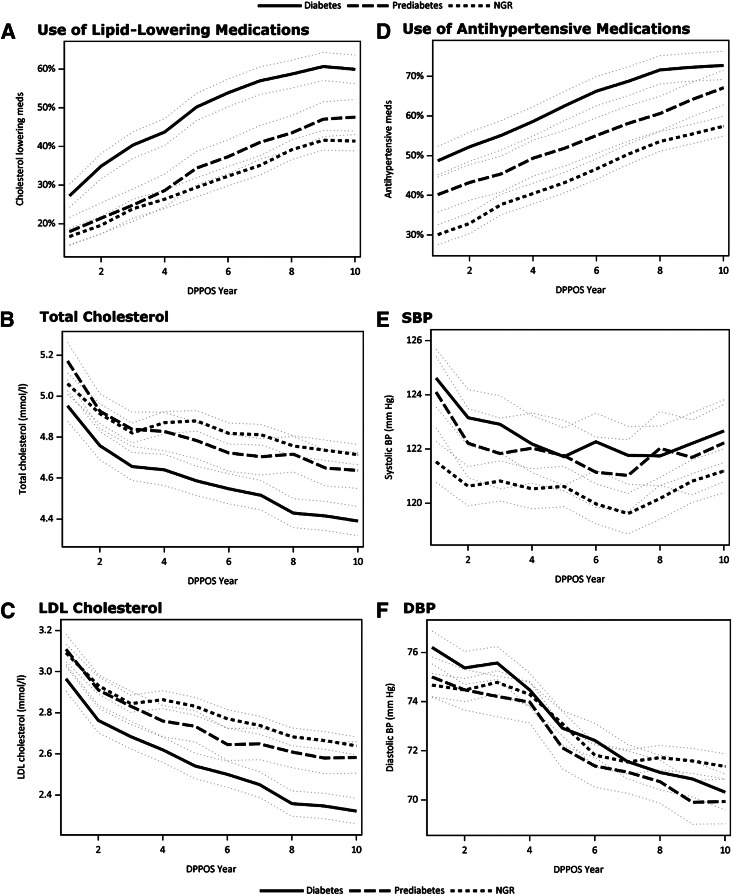
The association between glycemic exposure and four of the CVD risk factors did not differ among the treatment groups; therefore, pooled estimates by glycemic status are presented with adjustment for treatment group. Trajectories for the use of lipid-lowering medication, TC concentration, LDL-C concentration, use of blood pressure–lowering medication, SBP, and DBP over the course of the DPPOS are shown in Fig. 3_A–F_. The mean TC was lower in people with diabetes (4.58 mmol/L) versus those with prediabetes (4.79 mmol/L) or NGR (4.84 mmol/L) (P < 0.001 for both), but was not different between people with prediabetes and NGR (P = 0.31). LDL-C concentration was also lower in people with diabetes (2.56 mmol/L) versus those with persistent prediabetes (2.75 mmol/L) and NGR (2.80 mmol/L, P < 0.001 for both). In contrast, SBP was lower in the NGR group (121 mmHg) versus the diabetes group (123 mmHg, P < 0.001) and the prediabetes group (122 mmHg, P = 0.01). SBP values were similar between the diabetes and prediabetes groups (P = 0.41). DBP did not differ by glycemic exposure. Unlike the other CVD risk factors, there was a significant treatment group difference in the association between glycemic exposure and HDL-C concentration. HDL-C concentration was generally higher in NGR and lower in diabetes among the lifestyle (1.37 vs. 1.24 mmol/L) and placebo (1.32 vs. 1.27 mmol/L) groups. Specifically, in the placebo group, HDL-C concentration was 0.7 mmol/L higher in NGR versus the diabetes group (P < 0.01), and, in the lifestyle group, was 0.12 mmol/L higher in the NGR versus the diabetes group (P < 0.01). In the metformin group, there were no differences in HDL-C concentration by glycemic response (diabetes 1.32 mmol/L, prediabetes 1.37 mmol/L, and NGR 1.35 mmol/L).
Figure 3.
Trends represented by means (lines) and 95% CIs (gray dotted line) for use of lipid-lowering medications (A), TC concentration (B), LDLconcentration (C), use of blood pressure–lowering medications (D), SBP (E), and DBP (F) over 10 years of the DPPOS in people with diabetes (solid), prediabetes (medium dash), and NGR (short dash) with adjustment for treatment group.
Confounding by Medication Use
There were significant differences in blood pressure–lowering medication use among the glycemic exposure groups over the course of the DPPOS (all comparisons P < 0.001), with the greatest use in people with diabetes (63%), intermediate use in people with prediabetes (54%), and lowest use in people with NGR (45%; Fig. 3_D_). People with diabetes (49%) also had the greatest use of lipid-lowering medication compared with those with NGR (31%, P < 0.001) or prediabetes (34%, P < 0.001; Fig. 3_A_). The decline in estimated risk observed in the prediabetes group, as well as a decrease in each individual CVD risk factor for all groups, reflected an increase in their medication use (Fig. 3_A–F_). This may have been magnified by the conversion rate from prediabetes to diabetes (8), when medication is more routinely instituted for blood pressure and lipid lowering. To assess whether glycemic group differences in the Framingham risk score may be explained by the use of lipid-lowering medications, which was not included the Framingham formula, we considered further covariate adjustment for lipid-lowering medications to the model used for Fig. 2. The adjusted means were similar to Fig. 2, but the estimated effect of glycemic exposure was diminished with the covariate _R_2 reduced from 1.45 to 0.71% when all the groups were considered together (overall model _R_2 = 50%). Because the change in estimated CVD risk was greatest over time in the prediabetes group, nested mixed models were constructed to examine the contribution of lipid-lowering medication use on estimated CVD risk in the prediabetes group alone. The latter analysis revealed a reduction in the _R_2 from 16.2 to 6.5%, suggesting that lipid-lowering medication use explained 60% of the variance in Framingham score in the prediabetes group over time.
Conclusions
CVD remains the leading cause of death in the U.S. in people with and without diabetes (34). Considerable interest exists in the knowledge of whether the prevention of diabetes prevents related CVD, and hence is one of the much-anticipated outcomes from the DPPOS. Our recent analysis (8) revealed a 56% long-term reduction in diabetes incidence in people with prediabetes who were able to return to NGR. Major findings from the current study would contend that regression from prediabetes to NGR not only reduces risk of diabetes, but also that for CVD. The mean estimated 10-year risk of CVD was 16.2% in people with prediabetes—a risk level that possibly warrants treatment (35). Interestingly, the trajectory of estimated the 10-year CVD risk decreased over time in people with prediabetes due, in part, to increased medical treatment of their CVD risk factors. In summary, regression to NGR and/or medical treatment of CVD risk factors can significantly reduce the estimated risk of CVD in people with prediabetes.
Few pursuits in medicine have been as vexing as confirming a causal relationship between hyperglycemia and CVD risk. Despite the fact that many investigators consider diabetes to be a CVD equivalent (36), major clinical trials in diabetes have universally failed to demonstrate a reduction in CVD events (15–17,37,38) from glucose-lowering interventions over the short term (15–17,37,38). Therefore, it is most surprising to see data to the contrary in people with prediabetes. For example, two separate investigations (18,19) have reported slowing in the progression of carotid intima-media thickening by 30–70% in people with prediabetes or a history of gestational diabetes treated with pioglitazone for <3 years. In addition, the STOP-NIDDM Trial demonstrated a 49% reduction in CVD events in prediabetic participants randomized to acarbose (20), despite a 25% drop-out rate. These reports raise the obvious question as to why glucose lowering appears to be effective in retarding carotid intima-media thickening, and, in one study, reducing CVD events, in patients with prediabetes while at the same time it does not appear to be effective for the same in diabetes patients. One must then consider that prediabetes is a formative stage in the development of CVD, and thus may be more amenable to efforts at CVD prevention, whether by lowering risk factors or glucose itself. Results from the current analysis and from our earlier study (32) would support this contention.
All participants entering the DPP were at increased risk for CVD because all had prediabetes (39) and 53% had the metabolic syndrome (40). A recent meta-analysis by Ford et al. (12) illustrates an ∼20% increased risk of CVD in people with prediabetes, irrespective of type (IFG vs. IGT) or the criteria used to define it. In the current analysis, we observed an ∼18% per 10-year estimated CVD risk—the highest risk for any group and any time—at year 1 of the DPPOS among those individuals with persistent prediabetes during the DPP. Given the fact that the Framingham score does not differentiate prediabetes from NGR, this risk may have been underestimated. Furthermore, the mean Framingham score remained highest across the DPPOS for people with persistent prediabetes during the DPP. Importantly, the higher risk of CVD was not entirely driven by the higher conversion rate to diabetes in people with prediabetes versus NGR (8), as the estimated CVD risk was actually lowest in the diabetes group, likely because of aggressive glycemic and nonglycemic risk factor management. Regression to NGR was associated with an ∼6% reduction in the estimated 10-year risk for CVD, suggesting that treatment of dysglycemia may attend some, but not all, of the risk in people with prediabetes.
Risk factors for CVD are virtually indistinguishable in those individuals with prediabetes versus diabetes, but can significantly diminish with the restoration of NGR (32). In the DPP, intensive lifestyle modification, but not metformin therapy, was significantly associated with the restoration of NGR (31) and also had a far more favorable effect on individual CVD risk factors (32) (vs. metformin therapy) by virtue of its pleiotropic nature. Indeed, exercise and weight loss have well-known effects on lowering blood pressure (41,42) and plasma triglyceride concentration (43,44), as well as on raising plasma HDL levels (45,46), which occur independently of, but in tandem with, their impact on glucose homeostasis (47). This is noteworthy since SBP was higher and HDL was mostly lower in people with prediabetes (vs. NGR), likely contributing to higher CVD risk over the course of the DPPOS. It should be pointed out that 85% of participants enrolled in the DPP had IFG/IGT, according the American Diabetes Association; hence, overall CVD risk and the individual CVD risk factors may differ in people with isolated IFG or IGT. Unlike the case in individuals with diabetes, formal guidelines for individual CVD risk factor management in those with prediabetes have lagged.
Appreciation of diabetes as a high-risk state for CVD (36) has long been the topic of much discussion and, ultimately, resulted in diabetes-specific recommendations in formal guidelines for CVD prevention (48). Since the publication of the first National Cholesterol Education Program Adult Treatment Panel guideline in 1988 and reinforcement by the Adult Treatment Panel III in 2001, the use of antihypertensive medications has doubled and the use of lipid-lowering drugs increased 12-fold in people with diabetes (49). Thus, the lower CVD risk observed in people with diabetes (vs. those with prediabetes) in the current analysis—spanning DPPOS 2002–2012—likely reflects their increased medication usage, at least in part. Further, the declining 10-year CVD risk estimate in people with prediabetes likely reflects the same reason, either by virtue of their conversion to diabetes or a shift in physician prescribing practice. Altogether, the medical treatment of individual CVD risk factors in individuals with prediabetes can attenuate risk, perhaps as much or more than through the restoration of NGR.
It is important to note that the data presented should be interpreted in light of several limitations. First, the results and conclusions herein stem from the use of a single CVD risk assessment tool. As noted, the Framingham (2008) model was used because it has been validated in Caucasians, blacks, men, and women (26), collectively representing 85% of the DPPOS cohort (27). Additionally, the model incorporates diabetes status (the primary outcome of the DPP). The Framingham score, however, does not differentiate prediabetes from NGR status and, hence, may underestimate the risk in individuals with prediabetes. Further, it is likely that the score is affected by the unaccounted use of lipid-lowering medication, types of medications used for lipid or blood pressure lowering, and the conversion of people with prediabetes to diabetes, collectively affecting the risk score disproportionately in people with diabetes. Second, variability inherent in the glucose measures, as well as differences in the study protocol to confirm glucose status, may have contributed to misclassification. Specifically, those with a diagnosis of diabetes required a confirmatory test within 6 weeks of the initial test. Those with persistent prediabetes or NGR had their OGTT status evaluated annually during the DPP. Together, either a confirmatory OGTT or repeated-measures OGTTs over time lowered the likelihood of misclassification between the groups. A1C was not used for group classification because of the clustering of values in the low range. Last, hard CVD outcomes data are still being collected in the DPPOS and are not available for analysis. Ten-year CVD risk estimates from the current analysis will be compared with the hard CVD outcomes data when available. Comparing the eventual concordance or discordance between the risk estimates and event rates will enhance our ability to predict CVD in people with prediabetes in future studies.
In conclusion, all participants of the DPP had prediabetes, placing them at higher risk for CVD, as well as for diabetes. Importantly, however, when prediabetes was reversed, even temporarily, diabetes risk significantly diminished (8); hence, there is reason to speculate the same may be true for CVD risk. Major findings from the current study thus highlight the potential CVD risk associated with prediabetes and suggest that this risk can be attenuated through the restoration of NGR and/or medical treatment of CVD risk factors. These findings support a shift to earlier and more aggressive treatment of lipid levels and blood pressure, as well as glucose levels, in people with prediabetes.
Supplementary Material
Supplementary Data
Acknowledgments
Acknowledgments. The article is dedicated to Dr. Richard Rubin, our friend and collaborator, who died 25 March 2013. The Diabetes Prevention Program Research Group thanks the participants of the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study for their commitment and dedication.
Funding. This work was supported by National Institutes of Health (NIH) grant 5U01-DK-048375-12. During the Diabetes Prevention Program Outcomes Study (DPPOS), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study and for the collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart, Lung, and Blood Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the Diabetes Prevention Program (DPP), Lipha (Merck Santé) provided medication, and LifeScan Inc. donated materials during the DPP and DPPOS.
The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies.
Duality of Interest. L.P. served on advisory boards for Merck, Boehringer Ingelheim, and Liposcience and served as a speaker for Merck, Boehringer Ingelheim, and Novo Nordisk. E.H. served on advisory boards for Amgen, Amylin, AstraZeneca, Gilead, Janssen, Merck, Sanofi, and Vivus. T.J.O. served on advisory boards for Bristol-Myers Squibb and Lilly. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. L.P. proposed the analysis, interpreted the data, and wrote and edited the manuscript. M.T., K.J.M., E.H., A.K., M.L., M.G.M., D.T., T.J.O., R.F.H., and R.B.G. refined the analysis plan, interpreted the data, and edited the manuscript. M.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
Diabetes Prevention Program Research Group. The centers, investigators, and staff of the Diabetes Prevention Program Research Group are as follows (*Principal Investigator; **Program Coordinator): Pennington Biomedical Research Center (Baton Rouge, LA): George A. Bray, MD*; Annie Chatellier, RN, CCRC**; Crystal Duncan, LPN; Frank L. Greenway, MD; Erma Levy, RD; and Donna H. Ryan, MD. University of Chicago (Chicago, IL): David Ehrmann, MD*; Margaret J. Matulik, RN, BSN**; Kirsten Czech, MS; and Catherine DeSandre, BA. Jefferson Medical College (Philadelphia, PA): Barry J. Goldstein, MD, PhD*; Kevin Furlong, DO*; Kellie A. Smith, RN, MSN**; Wendi Wildman, RN**; and Constance Pepe, MS, RD. University of Miami (Miami, FL): Ronald B. Goldberg, MD*; Jeanette Calles, MSEd**; Juliet Ojito, RN**; Sumaya Castillo-Florez, MPH; Hermes J. Florez, MD, PhD; Anna Giannella, RD, MS; Olga Lara; and Beth Veciana. The University of Texas Health Science Center (San Antonio, TX): Steven M. Haffner, MD, MPH*; Helen P. Hazuda, PhD*; Maria G. Montez, RN, MSHP, CDE**; Carlos Lorenzo, MD, PhD; and Arlene Martinez, RN, BSN, CDE. University of Colorado (Denver, CO): Richard F. Hamman, MD, DrPH*; Dana Dabelea, MD, PhD*; Lisa Testaverde, MS**; Alexis Bouffard, MA, RN, BSN; Tonya Jenkins, RD, CDE; Dione Lenz, RN, BSN, CDE; Leigh Perreault, MD; David W. Price, MD; and Sheila C. Steinke, MS. Joslin Diabetes Center (Boston, MA): Edward S. Horton, MD*; Catherine S. Poirier, RN, BSN**; Kati Swift, RN, BSN**; Enrique Caballero, MD; Barbara Fargnoli, RD; Ashley Guidi, BS; Mathew Guido, BA; Sharon D. Jackson, MS, RD, CDE; Lori Lambert, MS, RD, LD; Kathleen E. Lawton, RN; Sarah Ledbury, MEd, RD; Jessica Sansoucy, BS; and Jeanne Spellman, RD. VA Puget Sound Health Care System and University of Washington (Seattle, WA): Steven E. Kahn, MB, ChB*; Brenda K. Montgomery, RN, BSN, CDE**; Wilfred Fujimoto, MD; Robert H. Knopp, MD (deceased); Edward W. Lipkin, MD; Anne Murillo, BS; and Dace Trence, MD. University of Tennessee (Memphis, TN): Abbas E. Kitabchi, PhD, MD, FACP*; Mary E. Murphy, RN, MS, CDE, MBA**; William B. Applegate, MD, MPH; Michael Bryer-Ash, MD; Samuel Dagogo-Jack, MD, MSc, FRCP, FACP; Sandra L. Frieson, RN; Helen Lambeth, RN, BSN; Lynne C. Lichtermann, RN, BSN; Hooman Otkaei, MD; Lily M.K. Rutledge, RN, BSN; Amy R. Sherman, RD, LD; Clara M. Smith, RD, MHP, LDN; Judith E. Soberman, MD; and Beverly Williams-Cleaves, MD. Northwestern University’s Feinberg School of Medicine (Chicago, IL): Boyd E. Metzger, MD*; Mark E. Molitch, MD*; Mariana K. Johnson, MS, RN**; Mimi M. Giles, MS, RD; Diane Larsen, BS; Charlotte Niznik, MS, RN, CDE; Samsam C. Pen, BA; and Pamela A. Schinleber, RN, MS. Massachusetts General Hospital (Boston, MA): David M. Nathan, MD*; Mary Larkin, MSN*; Charles McKitrick, BSN**; Heather Turgeon, BSN; Ellen Anderson, MS, RD; Laurie Bissett, MS, RD; Kristy Bondi, BS; Enrico Cagliero, MD; Kali D’Anna; Linda Delahanty, MS, RD; Jose C. Florez, MD, PhD; Valerie Goldman, MS, RD; Alexandra Poulos; Elyse Raymond, BS; Christine Stevens, RN; and Beverly Tseng. University of California-San Diego (San Diego, CA): Elizabeth Barrett-Connor, MD*; Mary Lou Carrion-Petersen, RN, BSN**; Lauren N. Claravall, BS; Jonalle M. Dowden, BS; Javiva Horne, RD; Diana Leos, RN, BSN; Sundar Mudaliar, MD; Jean Smith, RN; Simona Szerdi Janisch, BS; and Karen Vejvoda, RN, BSN, CDE, CCRC. St. Luke’s-Roosevelt Hospital (New York, NY): F. Xavier Pi-Sunyer, MD*; Jane E. Lee, MS**; Sandra T. Foo, MD; and Susan Hagamen, MS, RN, CDE. Indiana University (Indianapolis, IN): David G. Marrero, PhD*; Susie M. Kelly, RN, CDE**; Ronald T. Ackermann, MD; Edwin S. Fineberg, MD; Angela Hadden; Marcia A. Jackson; Marian S. Kirkman, MD; Kieren J. Mather, MD; Paris J. Roach, MD; and Madelyn L. Wheeler, RD. Medstar Research Institute (Washington, DC): Robert E. Ratner, MD*; Vanita Aroda, MD*; Sue Shapiro, RN, BSN, CCRC**; Catherine Bavido-Arrage, MS, RD, LD; Lilia Leon; Gabriel Uwaifo, MD; Debra Wells-Thayer, NP, CDE; and Renee Wiggins, RD. University of Southern California/UCLA Research Center (Alhambra, CA): Mohammed F. Saad, MD*; Karol Watson, MD*; Medhat Botrous, MD**; Sujata Jinagouda, MD**; Maria Budget; Claudia Conzues; Perpetua Magpuri; Kathy Ngo; and Kathy Xapthalamous. Washington University (St. Louis, MO): Neil H. White, MD, CDE*; Samia Das, MS, MBA, RD, LD**; Ana Santiago, RD; Angela L. Brown, MD; and Cormarie Wernimont, RD, LD. Johns Hopkins School of Medicine (Baltimore, MD): Christopher D. Saudek, MD* (deceased); Sherita Hill Golden, MD, MHS, FAHA*; Tracy Whittington, BS**; Jeanne M. Clark, MD; Alicia Greene; Dawn Jiggetts; Henry Mosley; John Reusing; Richard R. Rubin, PhD (deceased); Shawne Stephens; and Evonne Utsey. University of New Mexico (Albuquerque, NM): David S. Schade, MD*; Karwyn S. Adams, RN, MSN**; Claire Hemphill, RN, BSN**; Penny Hyde, RN, BSN**; Janene L. Canady, RN, BSN, CDE; Kathleen Colleran, MD; Ysela Gonzales; Doris A. Hernandez-McGinnis; and Carolyn King, MEd. Albert Einstein College of Medicine (Bronx, NY): Jill Crandall, MD*; Janet O. Brown, RN, MPH, MSN**; Elsie Adorno, BS; Helena Duffy, MS, C-ANP; Angela Goldstein, FNP-C, NPP, CSW; Jennifer Lukin, BA; Helen Martinez, RN, MSN, FNP-C; Dorothy Pompi, BA; Harry Shamoon, MD; Jonathan Scheindlin, MD; Elizabeth A. Walker, RN, DNSc, CDE; and Judith Wylie-Rosett, EdD, RD. University of Pittsburgh (Pittsburgh, PA): Trevor Orchard, MD*; Andrea Kriska, PhD*; Susan Jeffries, RN, MSN**; M. Kaye Kramer, BSN, MPH**; Marie Smith, RN, BSN**; Catherine Benchoff; Stephanie Guimond, BS; Jessica Pettigrew, CMA; Debra Rubinstein, MD; Linda Semler, MS, RD; Elizabeth Venditti, PhD; and Valarie Weinzierl, MPH. University of Hawaii (Honolulu, HI): Richard F. Arakaki, MD*; Narleen K. Baker-Ladao, BS**; Mae K. Isonaga, RD, MPH**; Nina E. Bermudez, MS; and Marjorie K. Mau, MD. Southwest American Indian Centers (Phoenix, AZ; Shiprock, NM; Zuni, NM): William C. Knowler, MD, DrPH*; Norman Cooeyate**; Mary A. Hoskin, RD, MS**; Camille Natewa**; Carol A. Percy, RN, MS**; Kelly J. Acton, MD, MPH; Vickie L. Andre, RN, FNP; Shandiin Begay, MPH; Brian C. Bucca, OD, FAAO; Sherron Cook; Matthew S. Doughty, MD; Justin Glass, MD; Martia Glass, MD; Robert L. Hanson, MD, MPH; Doug Hassenpflug, OD; Louise E. Ingraham, MS, RD, LN; Kathleen M. Kobus, RNC-ANP; Jonathan Krakoff, MD; Catherine Manus, LPN; Cherie McCabe; Sara Michaels, MD; Tina Morgan; Julie A. Nelson, RD; Robert J. Roy; Miranda Smart; Darryl P. Tonemah, PhD; and Charlton Wilson, MD. George Washington University Biostatistics Center (DPP Coordinating Center, Rockville, MD): Sarah Fowler, PhD*; Marinella Temprosa, PhD*; Michael D. Larsen, PhD*; Tina Brenneman**; Hanna Sherif, MS**; Sharon L. Edelstein, ScM**; Solome Abebe, MS; Julie Bamdad, MS; Melanie Barkalow; Joel Bethepu; Tsedenia Bezabeh; Jackie Callaghan; Costas Christophi, PhD; Nicole Butler; Mary Foulkes, PhD; Yuping Gao; Robert Gooding; Adrienne Gottlieb; Nisha Grover; Heather Hoffman, PhD; Kathleen Jablonski, PhD; Richard Katz, MD; Preethy Kolinjivadi, MS; John M. Lachin, ScD; Yong Ma, PhD; Qing Pan, PhD; Susan Reamer; and Alla Sapozhnikova. Lifestyle Resource Core: Elizabeth M. Venditti, PhD*; Andrea M. Kriska, PhD; Linda Semler, MS, RD; and Valarie Weinzierl, MPH. Central Biochemistry Laboratory (Seattle, WA): Santica Marcovina, PhD, ScD*; Greg Strylewicz, PhD**; and John Albers, PhD. Epidemiological Cardiology Research Center- Epicare (Winston-Salem, NC): Ronald J. Prineas, MD, PhD*; Teresa Alexander; Charles Campbell, MS; Sharon Hall; Susan Hensley; Yabing Li, MD; Margaret Mills; Elsayed Soliman, MD; and Zhuming Zhang, MD. Fundus Photo Reading Center (Madison, WI): Ronald Danis, MD*; Matthew Davis, MD*; Larry Hubbard*; Ryan Endres**; Deborah Elsas**; Samantha Johnson**; Vonnie Gama; Anne Goulding; Carotid Ultrasound; and Gregory Evans. CT Scan Reading Center: Elizabeth Stamm. Neurocognitive Assessment Group: Jose A. Luchsinger, MD, MPH. National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (Bethesda, MD): Judith Fradkin, MD, and Sanford Garfield, PhD. Centers for Disease Control and Prevention (Atlanta, GA): Edward Gregg, PhD, and Ping Zhang, PhD. University of Michigan (Ann Arbor, MI): William H. Herman, MD, MPH, and Morton B. Brown, PhD. Nutrition Coding Center (Columbia, SC): Elizabeth Mayer-Davis, PhD*, and Robert R. Moran, PhD**. Quality of Well-Being Center (La Jolla, CA): Ted Ganiats, MD*; Andrew J. Sarkin, PhD**; Naomi Katzir; and Erik Groessl, PhD. Coronary Artery Calcification Reading Center: Matthew Budoff, MD, and Chris Dailing. Genetics Working Group (1, Massachusetts General Hospital; 2, Broad Institute; 3, NIDDK; 4, University of Maryland; 5, Coordinating Center; 6, Lund University, Sweden; 7, Umeå University, Sweden; 8, Harvard School of Public Health; 9, Université de Sherbrooke): Jose C. Florez, MD, PhD1,2; David Altshuler, MD, PhD1,2; Liana K. Billings, MD1; Ling Chen, MS1; Maegan Harden, BS2; Robert L. Hanson, MD, MPH3; William C. Knowler, MD, DrPH3; Toni I. Pollin, PhD4; Alan R. Shuldiner, MD4; Kathleen Jablonski, PhD5; Paul W. Franks, PhD, MPhil, MS6,7,8; and Marie-France Hivert, MD9.
Footnotes
*
A complete list of centers, investigators, and staff of the Diabetes Prevention Program Research Group can be found in the Appendix.
References
- 1.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 2.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trial Research Group . Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–2077 [DOI] [PubMed] [Google Scholar]
- 3.Eriksson KF, Lindgärde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmö feasibility study. Diabetologia 1991;34:891–898 [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Yusuf S, Bosch J, et al. DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators . Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–161 [DOI] [PubMed] [Google Scholar]
- 7.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 8.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, Diabetes Prevention Program Research Group . Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yakubovich N, Gerstein HC. Is regression to normoglycaemia clinically important? Lancet 2012;379:2216–2218 [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group . The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng YJ, Gregg EW, Geiss LS, et al. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: Implications for diabetes diagnostic thresholds. Diabetes Care 2009;32:2027–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol 2010;55:1310–1317 [DOI] [PubMed] [Google Scholar]
- 13.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group . Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 15.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 16.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 18.Xiang AH, Hodis HN, Kawakubo M, et al. Effect of pioglitazone on progression of subclinical atherosclerosis in non-diabetic premenopausal Hispanic women with prior gestational diabetes. Atherosclerosis 2008;199:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tripathy D, Schwenke DC, et al. ACT NOW Study . Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 20.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trial Research Group . Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003;290:486–494 [DOI] [PubMed] [Google Scholar]
- 21.Murphy EF, Cotter PD, Healy S, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 2010;59:1635–1642 [DOI] [PubMed] [Google Scholar]
- 22.Pakoskey AM, Lesher EC, Scott DB. Hexokinase of Escherichia coli. Assay of enzyme activity and adaptation to growth in various media. J Gen Microbiol 1965;38:73–80 [DOI] [PubMed] [Google Scholar]
- 23.Tataranni PA, Ortega E. A burning question: does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes 2005;54:917–927 [DOI] [PubMed] [Google Scholar]
- 24.Wilson A, Morrell J. Prevention of heart disease in general practice: the use of a risk score. Health Trends 1991;23:69–73 [PubMed] [Google Scholar]
- 25.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753 [DOI] [PubMed] [Google Scholar]
- 26.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P, CHD Risk Prediction Group . Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 2001;286:180–187 [DOI] [PubMed] [Google Scholar]
- 27.Knowler WC, Fowler SE, Hamman RF, et al. Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Diabetes Prevention Program . The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diggle P, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. New York, Oxford University Press, 1994 [Google Scholar]
- 30.Edwards LJ, Muller KE, Wolfinger RD, Qaqish BF, Schabenberger O. An R2 statistic for fixed effects in the linear mixed model. Stat Med 2008;27:6137–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF, Diabetes Prevention Program Research Group . Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009;32:1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg RB, Temprosa M, Haffner S, et al. Diabetes Prevention Program Research Group . Effect of progression from impaired glucose tolerance to diabetes on cardiovascular risk factors and its amelioration by lifestyle and metformin intervention: the Diabetes Prevention Program randomized trial by the Diabetes Prevention Program Research Group. Diabetes Care 2009;32:726–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orchard TJ, Temprosa M, Barrett-Connor E, et al. Diabetes Prevention Program Outcomes Study Research Group . Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med 2013;30:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 12 November 2013. [Epub ahead of print]24222016 [Google Scholar]
- 36.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 37.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998;316:823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wing RR, Bolin P, Brancati FL, et al. Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Diabetes Prevention Program Research Group . The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 2000;23:1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orchard TJ, Temprosa M, Goldberg R, et al. Diabetes Prevention Program Research Group . The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 2005;142:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trials of Hypertension Prevention, Phase I . The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA 1992;267:1213–1220 [DOI] [PubMed] [Google Scholar]
- 42.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002;136:493–503 [DOI] [PubMed] [Google Scholar]
- 43.Chan DC, Watts GF, Ng TW, Yamashita S, Barrett PH. Effect of weight loss on markers of triglyceride-rich lipoprotein metabolism in the metabolic syndrome. Eur J Clin Invest 2008;38:743–751 [DOI] [PubMed] [Google Scholar]
- 44.Lee S, Burns SF, White D, Kuk JL, Arslanian S. Effects of acute exercise on postprandial triglyceride response after a high-fat meal in overweight black and white adolescents. Int J Obes (Lond) 2013;37:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leenen R, van der Kooy K, Meyboom S, Seidell JC, Deurenberg P, Weststrate JA. Relative effects of weight loss and dietary fat modification on serum lipid levels in the dietary treatment of obesity. J Lipid Res 1993;34:2183–2191 [PubMed] [Google Scholar]
- 46.Williams PT, Wood PD, Krauss RM, et al. Does weight loss cause the exercise-induced increase in plasma high density lipoproteins? Atherosclerosis 1983;47:173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mavros Y, Kay S, Anderberg KA, et al. Changes in insulin resistance and HbA1c are related to exercise-mediated changes in body composition in older adults with type 2 diabetes: interim outcomes from the GREAT2DO trial. Diabetes Care 2013;36:2372–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grundy SM, Howard B, Smith S, Jr, Eckel R, Redberg R, Bonow RO. Prevention Conference VI: diabetes and cardiovascular disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation 2002;105:2231–2239 [DOI] [PubMed] [Google Scholar]
- 49.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care 2013;36:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data